Abstract
Aims
Bone morphogenetic protein (BMP) signalling plays a key role in regulating the development of the atrioventricular (AV) septum and valves; however, the molecules that mediate the complex activities of BMP signalling are not fully understood. The major goal of this study is to identify the critical downstream regulatory targets of BMP signalling in AV cushions, which are precursors of the AV septum and valves.
Methods and results
We established a conditional immortal AV cushion mesenchymal cell line, tsA58-AVM. Using this line, we observed that the expression of Sema6D is upregulated by BMP stimulation through microarray analysis. Sema6D is required for BMP-upregulated migration of tsA58-AVM cells. To reveal the in vivo role of Sema6D , we established the Sema6D loxp/loxp mouse line and specifically inactivated Sema6D in endocardial cells (by Nfatc1 Cre ) when cushion mesenchymal cells started to form. We observed a hypocellular AV cushion defect in mutant hearts at early stages (E9.25, E9.5). The defect was resolved at a later stage, most likely due to compensation by increased Sema6C in mutant AV cushions. Furthermore, our ex vivo culturing and in vivo transgenic studies collectively suggest that SEMA6D activates Rho through PLXNA1-FARP1 to promote cushion mesenchymal cell formation.
Conclusions
We demonstrate for the first time that Sema6D is a target of BMP signalling and that Semaphorin signalling is essential for the initiation of cushion mesenchymal cell formation in the AV canal. Our study reveals a novel BMP- Sema6D -Rho axis regulating AV cushion development.
Keywords: Sema6D, Cushion morphogenesis, Heart development, BMP
1. Introduction
Congenital heart diseases (CHDs) are the most common birth defects, affecting as many as 1–5% of newborns, and remain the most common non-infectious cause of infant morbidity and mortality in developed countries. 1–4 Malformation of valves accounts for up to 30% of CHDs. 5 Valvulogenesis in the atrioventricular canal (AVC) region is initiated with cushion formation by regional expansion of extracellular matrix (ECM) at ∼E9.0 in mouse embryos. Shortly thereafter, a group of endocardial cells in the AVC are mesenchymalized through the epithelial-mesenchyme-transition (EMT) and invade into the ECM in AV cushions. 6–11 Cellularized cushions undergo complicated remodelling processes, including condensation, elongation and ECM remodelling, and eventually mature into thin valve leaflets. 9–12 The endocardial-derived mesenchymal cells are differentiated into interstitial cells in valves, which help maintain valve homeostasis. Genetic manipulation of pre-EMT endocardial cells will lead to permanent genetic alterations in nearly all cells in the valve leaflets.
Bone morphogenetic protein (BMP) signalling pathways are essential for AV cushion formation, cellularization, and remodelling. In mouse embryos, myocardial-inactivation of BMP ligand genes (including Bmp2 or Bmp4 ) or endothelial/endocardial inactivation of BMP receptor genes (including Alk2 and Alk3 ) causes severe defects in the AVC. 13–18 However, the downstream molecules that mediate the complex activities of BMP signalling remain largely undetermined. Compared with cardiomyocytes in embryonic hearts, the number of cells in AV cushions is very low, making it challenging to apply molecular approaches to identify BMP target genes in developing AV cushions. To overcome this limitation, we developed a conditional immortal cell line that is derived from AV cushion mesenchymal cells. To the best of our knowledge, no similar cell line has been reported in the literature. Using this novel tool, we identified Sema6D as regulatory target of BMP signalling during AV cushion development.
SEMA6D belongs to the Semaphorin family, which is composed of more than 20 members. 19–21 Based on their sequence similarity, Semaphorins are classified into eight classes. The SEMA and Plexin–Semaphorin–Integrin (PSI) domains constitute the major ectodomain of SEMA6D, while its intracellular region does not contain any known functional motifs. The extracellular region of SEMA6D (SEMA + PSI domains) can be released from the cell surface through an unknown mechanism to act as a secreted cytokine. 22,23 Thus, SEMA6D may act through cell–cell interaction or at a long distance through diffusion of its cleaved ectodomain. The primary receptors of SEMA6D are PLXNA1 and PLXNA4, which belong to the Plexin receptor family. The SEMA6D-PLXNA1 or four complexes may activate various intracellular signalling cascades in a cell type-dependent fashion.
Semaphorin–Plexin signalling was initially recognized for its role in guiding axon growth, but has now been implicated in regulating numerous cellular activities including cell morphology, proliferation, adhesion, and migration. 19–21,24,25 The functions of Semaphorin signalling during cardiogenesis have been most thoroughly studied for class 3 Semaphorins. Mutations in Sema3A and C or their receptors ( PlxnA2 , PlxnD1 ) lead to severe defects in the outflow tract (OFT) region of mouse embryos 26–30 ; however, no AV cushion defect has been reported. SEMA6D was previously shown to regulate the outgrowth of endocardial cells from chicken explants 22 ; however, its role in regulating AV cushion development in mammals has not been examined. This study provides both in vitro and in vivo evidence suggesting that SEMA6D acts downstream of BMP signalling to regulate the initiation of mesenchyme formation in AV cushions.
2. Methods (detailed information is provided in supplementary methods)
2.1 Mouse maintenance, tissue preparation, and laser capture microdissection
This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 2011). All protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. Euthanasia of mice was achieved through inhalation of CO 2 followed by cervical dislocation. Generation and genotyping of H-2K b - tsA58 and Nfatc1 Cre mice have been described previously. 31,32 Embryo preparation, Haematoxylin–Eosin (HE) staining and Laser capture microdissection (LCM) were performed as described previously. 33–35
2.2 Derivation of a conditional immortal AV cushion mesenchymal cell line
The flowchart of deriving AV cushion mesenchymal cells is shown in Supplementary material online, Figure S1 . We named our cell line tsA58-AVM.
2.3 Microarray, quantitative Reverse-Transcription-Polymerase-Chain-Reaction, northern, western, and immunostaining analyses
tsA58-AVM cells were treated with BMP4 or BSA for 8 h followed by total RNA isolation. RNA samples were subjected to microarray analysis using the Affymetrix GeneChip-Mouse-Genome-430-2.0 Array. Data were averaged from three independent biological samples for each condition. Northern, western, and immunostaining analyses were performed as described previously. 33,34,36 The primers RT-PCR are listed in Supplementary material online, Table S1 .
2.4 Cell culture, transfection, conditioned medium preparation, transwell cell migration analysis, and detection of active Rho
The plasmid expressing the Myc-tagged ectodomain of SEMA6D was transfected into HEK293T cells to acquire conditioned medium containing the secreted SEMA6D. Transwell cell migration analysis was performed as described previously. 37 Pre-made short interference RNAs (siRNAs) against Sema6D, PlxnA1 , and Farp1 were purchased from IDT. The Rho Activation Assay kit (Millipore) was used to detect active Rho as instructed by the manufacturer.
2.5 Ex vivo AV explant analysis
Ex vivo AV explant analysis was performed as previously described 38 with a minor modification.
2.6 Generation of Sema6D loxp/loxp mice
The targeting strategy is shown in Supplementary material online, Figure S2 . Sema6D loxp/+ mice were backcrossed with C57BL/6 mice for ≥10 generations and were intercrossed to acquire Sema6D loxp/loxp mice.
2.7 Generation of transgenic embryos
The plasmid which expresses the dominant negative (DN) hRHOA was submitted to the UAB Transgenic Core Facility for pronuclear injection. The receiver mice were sacrificed to acquire embryos at ∼E9.25.
2.8 Echocardiography analysis
Echocardiography analysis was performed using the Vevo3100 ultrasound machine (VisualSonics, Fujifilm).
2.9 Statistical analysis
For all quantitative analyses, other than when specifically noted, data were averaged from ≥6 independent biological samples. Student’s t -test and ANOVA were performed to compare two and multiple groups, respectively. P < 0.05 is considered significant. All analyses were performed using GraphPad prism.
3. Results
3.1 Generation of the tsA58-AVM conditional immortal cell line derived from AV cushion mesenchymal cells
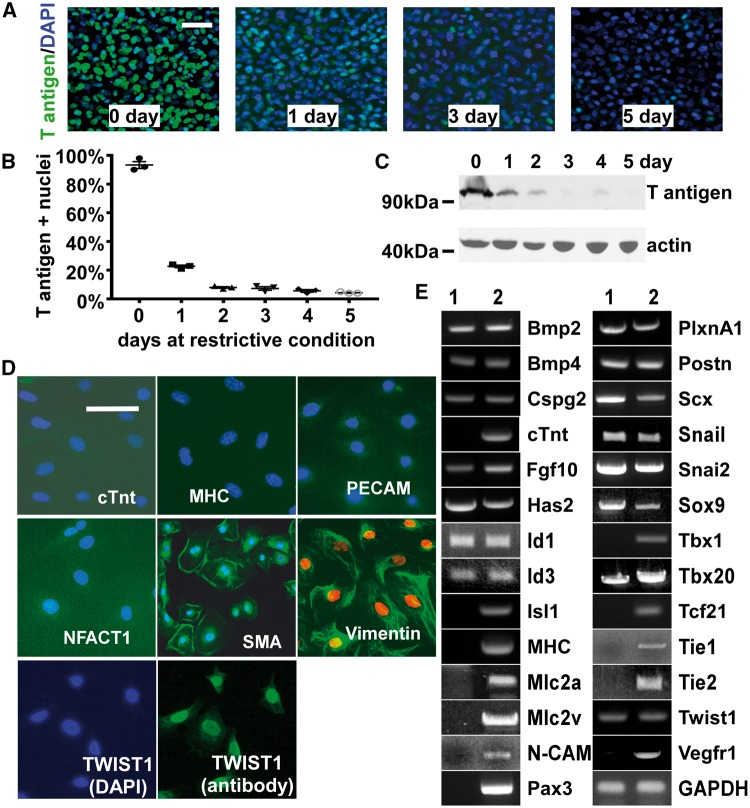
To facilitate the application of molecular/cellular strategies to study AV cushion development, we established the tsA58-AVM cell line derived from AV cushions of H-2K b - tsA58 embryos at E9.5. In H-2K b - tsA58 animals, expression of the temperature sensitive SV40 large T antigen mutant gene (tsA58) is driven by the γ-interferon inducible promoter of the H-2K b gene. 31 Under the permissive condition (33 °C, with γ-interferon), cells derived from H-2K b - tsA58 mice (Refs. 31 , 39 ) proliferate continuously, as do immortalized cells. However, under the restrictive condition (37 °C, no γ-interferon), the large T antigen is degraded and these cells cease to proliferate in this unlimited manner and more closely resemble primary cell cultures. We were able to pass these cells under the permissive condition for >30 passages without an observable reduction in their growth rate. Expression of the large T antigen was dramatically reduced within 3 days after shifting tsA58-AVM cells to the restrictive condition ( Figure 1 A–C ). Under the restrictive condition, these cells can grow without obvious abnormalities for 3 weeks. By the 4th week, some cells begin to detach from the plate, and by the end of the 4th week, many cells die. In all of our experiments, we only used tsA58-AVM cells between 3 and 14 days after shifting them to the restrictive condition.
Figure 1.
Characterization of tsA58-AVM cells. ( A , B ) tsA58-AVM cells were cultured under the permissive condition and shifted to the restrictive condition for various days as indicated. Cells were stained with an antibody against the large T antigen (green) and DAPI (blue). In panel B , the percentage of large T antigen positive nuclei was quantified. Data were averaged from three independent cultures with error bars indicating standard error (SEM). ( C ) Total protein was isolated from tsA58-AVM cells at various days after being shifted to the restrictive condition followed by western analysis. Actin was used as the loading control. ( D ) tsA58-AVM cells were stained with various antibodies as indicated. ( E ) Total RNA was isolated from tsA58-AVM cells (lane 1) or E11.5 hearts (lane 2, as a positive control for RT-PCR) and was then subjected to RT-PCR analysis. The bars in A and D represent 100 µm.
To test whether tsA58-AVM cells retain the molecular properties of AV cushion mesenchymal cells, we performed marker examination ( Figure 1 D, E ). tsA58-AVM cells do not express myocardial specific genes [including cardiac troponin T ( cTnt ), cardiac myosin heavy chain (MHC), myosin light chain (MLC) 2a, or MLC2v), endothelial/endocardial specific genes (including Nfatc1 , PECAM, N-CAM, Tie1 , Tie2 , or Vegfr1 ), second heart field (SHF) markers ( Isl1 , Tbx1 ), a pre-migratory neural crest marker ( Pax3 ), or an epicardial marker ( Tcf21 ). Furthermore, these cells express genes that are known to be present in cushion mesenchymal cells, including Bmp2, Bmp4, Cspg2, Has2, Id1, Id3, PlxnA1, Postn, SMA, Snai1, Snai2, Sox9, Tbx20, Twist1 , and Vimentin. Fgf10 was unexpectedly detected in tsA58-AVM cells. Since these cells do not express other SHF markers ( Isl1, Tbx1 ), expression of Fgf10 is likely caused by adaptive responses of cells during immortalization rather than contamination of SHF cells.
3.2 Expression of Sema6D is upregulated by BMP stimulation in tsA58-AVM cells and AV cushion mesenchymal cells
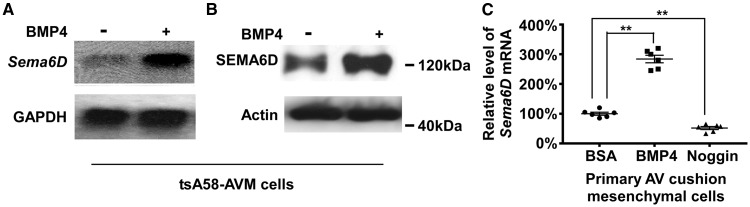
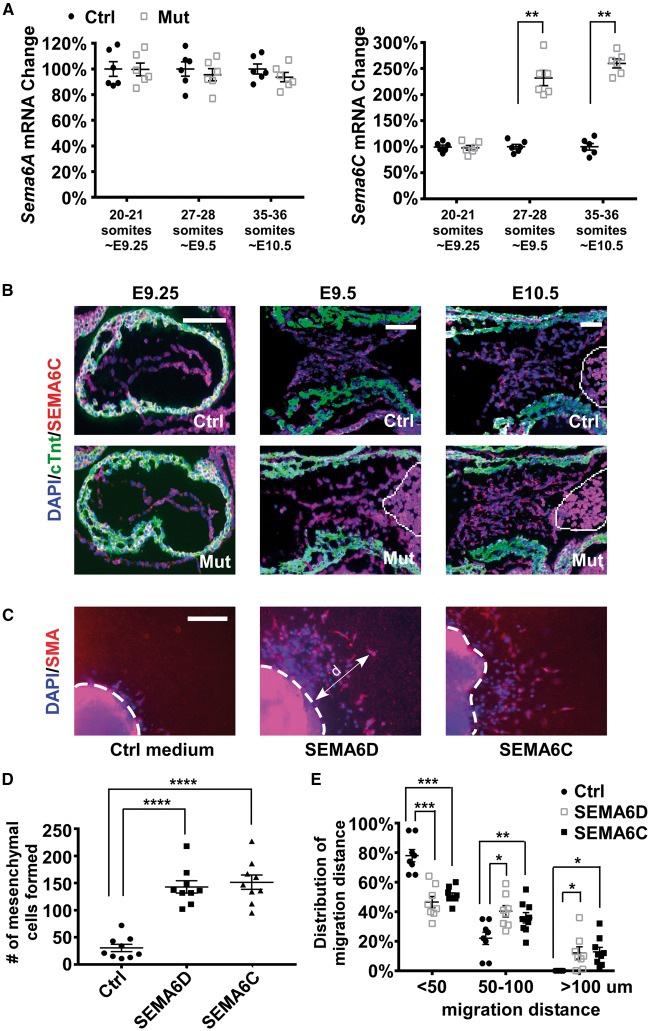
We tested whether tsA58-AVM cells can respond to BMP stimulation. BMP4 treatment led to the activation (phosphorylation) of BMP R-SMADs in tsA58-AVM cells (see Supplementary material online, Figure S3A ), suggesting that the BMP signalling cascade is intact. We then performed microarray analysis on cells treated with or without BMP4. The expression of 58 genes was significantly increased ≥1.5-fold in BMP treated cells (see Supplementary material online, Table S2 ). Many known BMP downstream targets including Id1-4 , Tbx20 , Smad6 , and Smad7 were identified. Among the upregulated genes, Sema6D expression is increased approximately two-fold by BMP4 stimulation ( P < 0.01). A previous in ovo study showed that exogenous SEMA6D in chicken embryos dramatically increased the size of AV cushions, 22 suggesting the potential function of Sema6D in regulating cushion development. The role of SEMA6D during mammalian AV cushion development remains unclear. Our northern and western analyses confirmed that BMP4 stimulation upregulates the expression of Sema6D in tsA58-AVM cells ( Figure 2 A, B ). Furthermore, BMP4 treatment significantly increased, while Noggin treatment significantly reduced the expression of Sema6D in primarily cultured AV cushion cells ( Figure 2 C ). BMP treatment also increased Sema6D expression in an endocardial cell line (ECC-1), but not in P19 teratocarcinoma cells (see Supplementary material online, Figure S4 ), suggesting that the effect of BMP is cell type dependent. We therefore identified Sema6D as a novel regulatory target of BMP signalling in AV cushion cells.
Figure 2.
Expression of Sema6D in AV cushion mesenchymal cells is upregulated by BMP4 stimulation. ( A , B ) tsA58-AVM cells were treated with BMP4 (100 ng/ml) for 24 h followed by Northern ( A ) and Western analysis ( B ). GAPDH and Actin were used as loading controls. ( C ) AV cushion mesenchymal cells at E10.5 were treated with BSA, BMP4 (100 ng/ml) or NOGGIN (100 ng/ml) for 24 h followed by qRT-PCR analysis to detect Sema6D . Hprt was included as the loading control. The Sema6D expression level in cells treated with BSA was set at 100%. Data were averaged from six independent cultures with error bars indicating SEM. ** P < 0.01, Student’s t -test.
3.3 SEMA6D promotes migration of tsA58-AVM cells
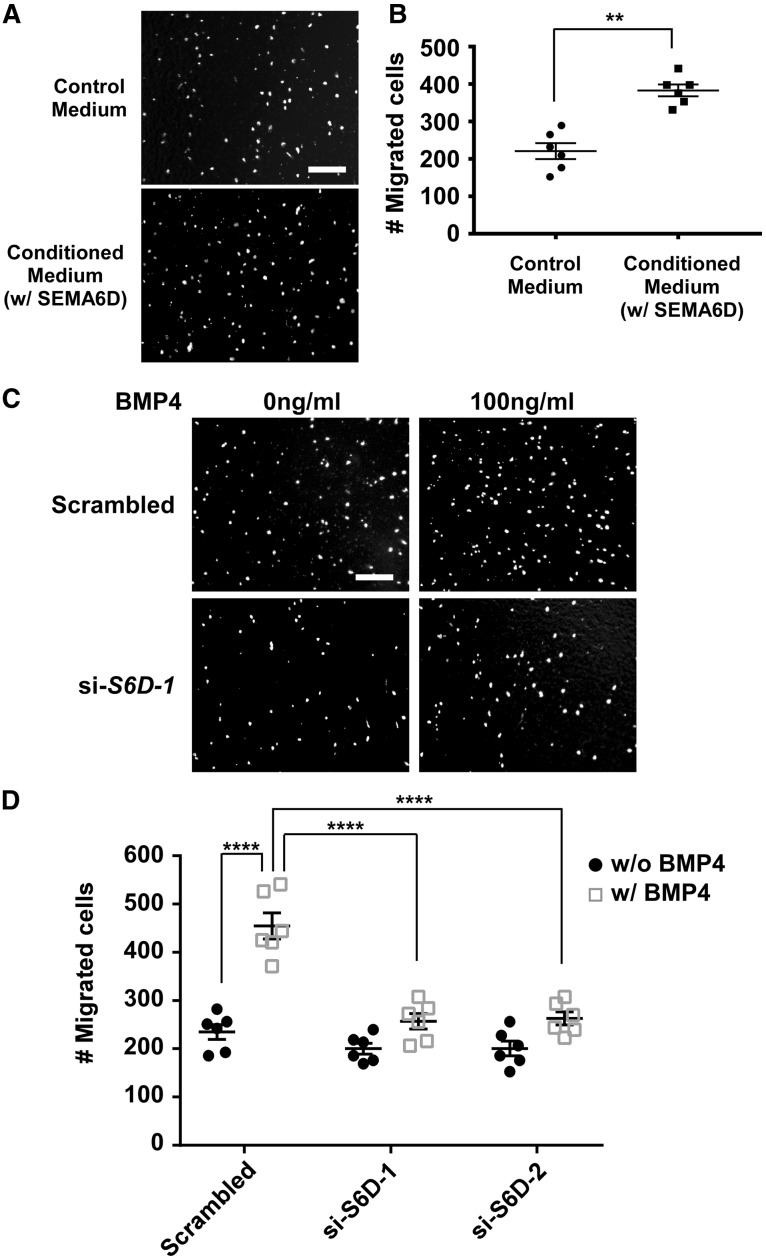
To facilitate the study of SEMA6D, we generated a cell line that expresses and secrets the ectodomain of SEMA6D (with a Myc tag) into the medium. The presence of the ectodomain of SEMA6D in the conditioned medium was confirmed with western analysis (see Supplementary material online, Figure S5 ). Treatment of tsA58-AVM cells with the conditioned medium significantly increased cell migration in a transwell assay ( Figure 3 A, B ). The SEMA6D ectodomain had no effect on cell proliferation and apoptosis. It was well established that BMP signalling promotes cushion mesenchymal cell migration. 40 Our results in Figure 3 C, D confirmed that BMP stimulation enhanced migration of tsA58-AVM cells and further revealed that knocking down SEMA6D expression by two independent siRNAs (see Supplementary material online, Figure S6 ) significantly reduced BMP-promoted migration of tsA58-AVM cells. Knocking down SEMA6D expression had a minor effect on the basal level of migration (without BMP4 stimulation).
Figure 3.
SEMA6D promotes tsA58-AVM cell migration. ( A , B ) tsA58-AVM cells were starved overnight and re-suspended in either conditioned medium (containing the ectodomain of SEMA6D) or control medium and subjected to the transwell migration test. The migrated nuclei were visualized with DAPI staining. For each filter, the number of migrated cells was determined as the sum of cells under three random fields (under a 10× objective). For each condition, data were averaged from six independent experiments performed in triplicate. The mean value of migrated cells treated with control medium is 221. ** P < 0.01, Student’s t -test. ( C , D ) tsA58-AVM cells were transfected with a scrambled siRNA control or two independent siRNAs against Sema6D . Two days after transfection, cells were treated with 0 ng/ml or 100 ng/ml BMP4 for 48-h followed by transwell migration analysis ( C ). The results from only one siRNA (si-S6D-1) treatment are shown in panel C . Panel D shows the quantification results averaged from six independent samples. The mean value of cells transfected with the scrambled control and without BMP4 treatment is 235. Two-way ANOVA reveals the effect of siRNAs and BMP4 (see Supplementary material online, Table S3 ). **** P < 0.0001, by post hoc Sidak’s multiple comparisons test. The bars in A and C represent 200 µm. Error bars in B and D indicate SEM.
3.4 Sema6D is required for the proper initiation of AV cushion mesenchyme formation in mouse embryos
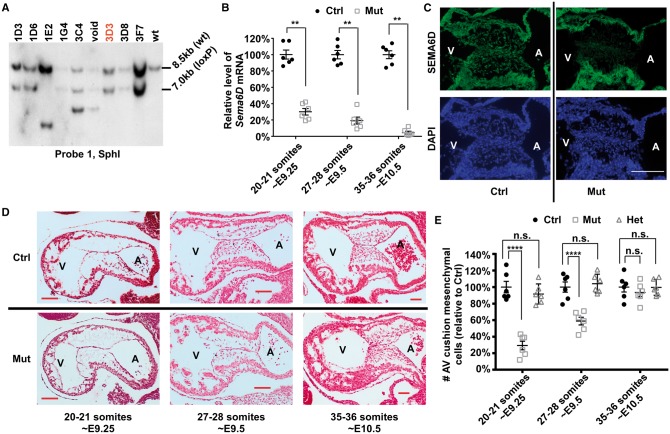
To reveal the in vivo role of Sema6D , we generated a conditional knockout mouse line following the strategy shown in Supplementary material online, Figure S2 . Our southern and PCR analyses confirmed proper homologous recombination between the targeting construct and the genomic DNA in the ES cell clone 3D3 ( Figure 4 A , see Supplementary material online, Figure S7 ), which was used to generate the Sema6D loxp/loxp mouse line. We decided to inactivate Sema6D in AV cushions using the Nfatc1 Cre mouse line, which specifically inactivates target genes in endocardial cells upon EMT initiation. 32 Our reporter analysis confirmed the specificity/efficiency of this Cre line (see Supplementary material online, Figure S8 ). We crossed Nfatc1 Cre mice with Sema6D loxp/loxp mice to acquire Nfatc1 Cre / Sema6D loxp/+ mice (male), which were then crossed with female Sema6D loxp/loxp mice to acquire mutant embryos ( Nfatc1 Cre / Sema6D loxp/loxp ). The littermate Sema6D loxp/loxp embryos were included as controls. Efficient inactivation of Sema6D was confirmed with qRT-PCR and immunostaining analyses ( Figure 4 B, C ).
Figure 4.
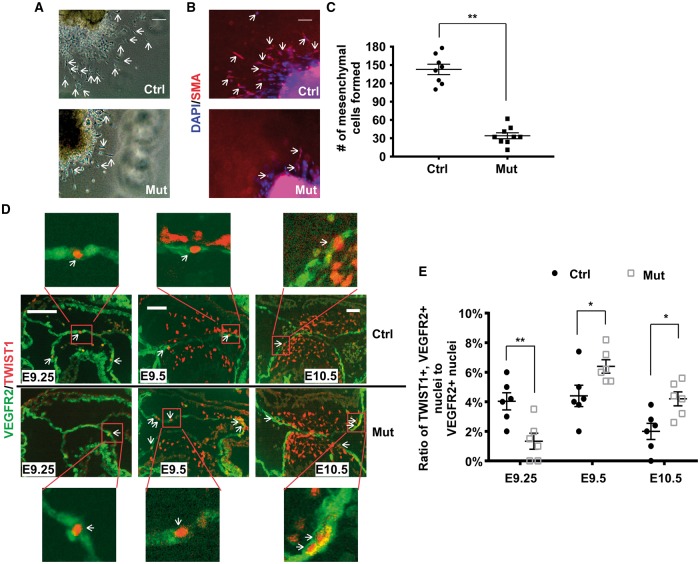
Endocardial inactivation of Sema6D leads to the hypocellular AV cushion defect. ( A ) Genomic DNA was isolated from various ES cell candidates or wild type (wt) ES cells and digested with SphI followed by Southern analysis using probe 1 (see Supplementary material online, Figure S2 ). Candidate 3D3 displayed the expected pattern. ( B ) AV cushion cells from control (ctrl, Sema6D loxp/loxp ) and mutant (mut, Nfatc1 Cre / Sema6D loxp/loxp ) embryos at various stages were isolated through LCM and subjected to qRT-PCR analysis to examine the expression of Sema6D . The level from control embryos at each stage was set at 100%. ** P < 0.01, Student’s t -test; n.s., no significant difference. ( C ) Control and mutant embryos at E9.5 were stained with an antibody against SEMA6D (green) and DAPI. ( D ) Control and mutant embryos at various stages were sagittally sectioned and HE stained. ( E ) The total number of cushion mesenchymal cells in AV cushions of control, mutant, and heterozygous (het, Nfatc1 Cre /Sema6D loxp/+ ) embryos was counted under a light microscope. Data were averaged from six embryos of each genotype. The number of control embryos at each stage was set at 100%. The raw number of cushion mesenchymal cells are shown in see Supplementary material online, Table S4 . One-way ANOVA was performed to compare different genotypes at each stage (see Supplementary material online, Table S5 ). **** P < 0.0001, by post hoc Dunnett’s multiple comparisons test. In both B and E , error bars represent SEM. The bars in C and D represent 100 µm.
To test whether Sema6D is required for normal AV cushion development, we sectioned developmental stage-matched mutant and control embryos. At the initial stage of cushion mesenchymal cell formation (∼E9.25), the number of mesenchymal cells in mutant AV cushions is reduced to ∼30% of the control level ( Figure 4 D, E , see Supplementary material online, Figure S9 ). A significant difference between mutants and controls persists at E9.5. However, at E10.5, no significant difference was observed between control and mutant hearts. Mutant animals were isolated at the expected Mendelian ratio in newborn pups. No morphological defect was observed in adult valves (see Supplementary material online, Figure S10 ). Our further echocardiography analysis did not reveal any functional abnormalities in the AV valves of adult mutants (see Supplementary material online, Figure S10 ). Therefore, we concluded that cushion mesenchymal cell formation at the initial stage is impaired by Sema6D inactivation and that the defect is resolved at later stages.
We next examined cell apoptosis and proliferation (see Supplementary material online, Figure S11 ). Few, if any, apoptotic cells were identified in either control or mutant hearts at E9.25 and 9.5. We started to observe apoptotic cells at E10.5 and no significant difference was observed between control and mutant samples. No defect in cell proliferation was observed mutant AV cushions at all stages examined. Next, collagen gel analysis confirmed that the number of mesenchymal cells formed from mutant explants is significantly reduced ( Figure 5 A–C ). Immunostaining with an anti-SMA antibody confirmed the identity of the mesenchymal cells formed in the collagen gel ( Figure 5 B ). These results suggest that the hypocellular AV cushion at E9.25 is caused by reduced mesenchyme formation. To further test this idea, we examined expression of TWIST1 and VEGFR2, which are mesenchymal and endothelial markers, respectively ( Figure 5 D, E ). Some cells in the endocardial layer of AV cushions express both markers, indicating that those cells are undergoing EMT, as previously reported. 41,42 The percentage of endocardial cells (VEFGR2 positive) that are undergoing EMT (TWIST1/VEGFR2 double positive) in mutant AV cushions is significantly reduced at E9.25 and is significantly increased at later stages (E9.5 and E10.5) ( Figure 5 D, E ). Our quantitative reverse-transcription-polymerase-chain-reaction (qRT-PCR) analysis confirmed that expression of EMT genes, including Twist1 , Snai1 , and Snai2 , is significantly reduced in the endocardial layer of mutant AV cushions at E9.25 and significantly increased at later stages (see Supplementary material online, Figure S12 ). The above data thus collectively suggest that the observed hypocellular AV cushion defect in mutants at E9.25 is due to the reduced formation of mesenchymal cells rather than abnormal cell proliferation/death. The resolution of the defect in mutants at later stages is due to increased EMT.
Figure 5.
Cushion mesenchymal cell formation is impaired by endocardial inactivation of Sema6D . ( A – C ) Explants from control (ctrl, Sema6D loxp/loxp ) and mutant (mut, Nfatc1 Cre /Sema6D loxp/loxp ) AVC regions at E9.25 were cultured on the surface of type I collagen gel for 48 h. Panel C shows representative results as seen under the light microscope. In panel B , the explants were stained with an antibody against SMA (red), which is a mesenchymal cell marker. Total nuclei were stained with DAPI (blue). The arrows in panels A and B indicate examples of mesenchymal cells. The number of mesenchymal cells formed is plotted in C . Data were averaged from 7 to 9 independent cultures for each genotype with error bars representing SEM. ( D , E ) Control and mutant embryonic sections at various stages were co-immunostained with antibodies against VEGFR2 (green) and TWIST1 (red). The ratio of TWIST1+/VEGF2+ (double positive) nuclei to total VEGFR2+ nuclei in AV endocardial cells is plotted in E . Data were averaged from six independent embryos of each genotype at each stage with error bars representing SEM. * P < 0.05; ** P < 0.01, Student’s t -test. The bars in A , B and D represent 50 µm.
3.5 Loss of Sema6D is likely compensated by upregulation of Sema6C in mutant AV cushions
To test whether the loss of Sema6D in AV cushions is compensated by other members of the SEMA6 family, we compared the expression of Sema6A , B , and C between mutant and control samples at different stages ( Figure 6 A ). Expression of Sema6B was undetectable in AV cushions (data not shown). Expression of Sema6A was not significantly altered by inactivation of Sema6D . Expression of Sema6C was significantly increased in mutant samples starting from E9.5. Increased expression of SEMA6C was observed in both endocardial and mesenchymal cells of mutant AV cushions at E9.5 and 10.5 ( Figure 6 B ). We therefore speculate that increased Sema6C compensated the loss of Sema6D in mutant AV cushions. To test this idea, we performed ex vivo explanting analysis and showed that addition of SEMA6C rescued the mesenchyme formation and migration defects in mutant AV explants ( Figure 6 C–E ).
Figure 6.
Increased expression of Sema6C may compensate for the loss of Sema6D expression in promoting cushion mesenchymal cell formation. ( A ) AV cushion cells from control (ctrl, Sema6D loxp/loxp ) and mutant (mut, Nfatc1 Cre / Sema6D loxp/loxp ) embryos at different stages were isolated through LCM and subjected to qRT-PCR analysis to examine the expression of Sema6A–C . Expression of Sema6B is undetectable and therefore is not displayed. Data were averaged from six independent embryos of each genotype with error bars representing SEM. Hprt was used as the loading control. ** P < 0.01, Student’s t -test; n.s., no significant difference. ( B ) E9.25–10.5 embryonic sections were stained with antibodies against cTnt (green) and SEMA6C (red). The cells in the circled area are blood cells exhibiting autofluorescence. ( C ) Collagen gel analysis was performed using mutant embryos that were treated with control medium, the SEMA6D conditioned medium (containing the ectodomain of SEMA6D) or the ectodomain of SEMA6C. The explants were stained with an anti-SMA antibody (red) and DAPI (blue). The dotted white lines indicate the origin of the explants. ( D ) The number of mesenchymal cells formed under each condition was counted under a phase-contrast microscope. One-way ANOVA was performed to compare different conditions (see Supplementary material online, Table S6 ). **** P < 0.0001, by post hoc Dunnett’s multiple comparisons test. ( E ) The migration distance (d) of a mesenchymal cell was measured as the shortest distance between the nuclei and the explant origin (dotted lines in panel C ). One example is shown in C . For each explant, mesenchymal cells were divided into three groups based on their migration distance (<50 µm, between 50 and 100 µm, >100 µm). The percentage of cells within each group was then calculated. Within each group (based on migration distances of cells), one-way ANOVA was performed to compare different conditions (treated with control, SEMA6D or SEMA6C) (see Supplementary material online , Table S7 ). *** P < 0.001, ** P < 0.01, * P < 0.05, by post hoc Dunnett’s multiple comparisons test. In panels D and E , data were averaged from 7 to 9 independent cultures for each condition with error bars indicating SEM. The bars in B and C represent 100 µm.
3.6 SEMA6D promotes cushion mesenchyme formation and migration through activating Rho
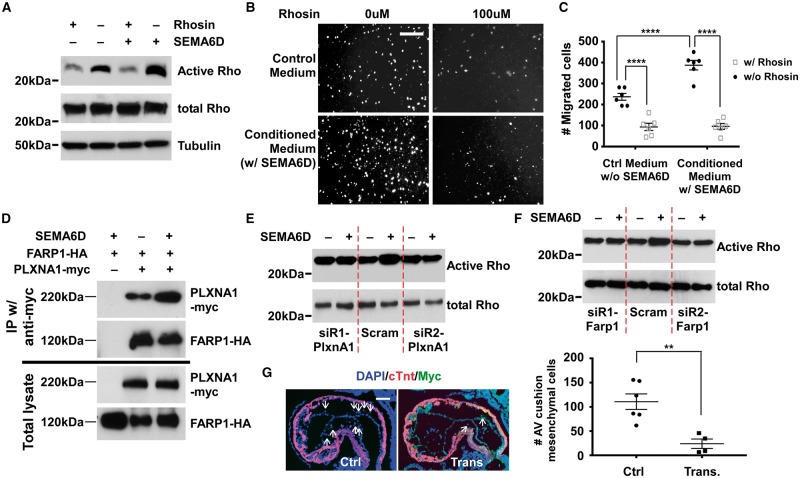
Various Semaphorin ligands can enhance Rho activity in a cell-dependent fashion. 19–21,24,25 We therefore decided to test whether Rho activation is involved in mediating functions of SEMA6D during AV cushion development. Treatment of tsA58-AVM cells with SEMA6D increased Rho activity and activation of Rho was efficiently blocked by Rhosin, a Rho specific inhibitor 43 ( Figure 7 A ). Rhosin treatment blocked SEMA6D-upregulated tsA58-AVM cell migration ( Figure 7 B, C ). PLXNA1 is the major receptor of SEMA6D 19–21 , and is expressed in tsA58-AVM cells (see Supplementary material online, Figure S13 ). It was previously reported that FARP1, a Rho guanine nucleotide exchange factor (GEF) can relay Semaphorin signalling to regulate dendritic growth. 44 We detected interaction between FARP1 and PLXNA1 by co-immunoprecipitation (co-IP) in tsA58-AVM cells and determined that this interaction is enhanced by SEMA6D treatment ( Figure 7 D ). We knocked down expression of PLXNA1 or FARP1 through siRNAs (see Supplementary material online, Figure S14 ) and found that SEMA6D-promoted RhoA activation is reduced by the siRNAs ( Figure 7 E, F ).
Figure 7.
SEMA6D activates Rho to promote AV cushion mesenchymal formation. ( A ) tsA58-AVM cells were treated with various conditions followed by the detection of the active and total Rho level. Tubulin was used as the loading control. ( B , C ) tsA58-AVM cells were treated with various conditions and were subjected to transwell migration analysis. The bar in panel B represents 200 µm. Panel C shows the quantified results. For each filter, the number of migrated cells was calculated as the sum of cells under three random fields (under a 10× objective). Data were averaged from six independent cultures performed in triplicate. The raw mean number of cells treated with the control medium without Rhosin treatment (the first column) is 237. The bar in B represents 200 µm. Two-way ANOVA was performed to examine the effects of SEMA6D and Rhosin (see Supplementary material online , Table S8 ). **** P < 0.0001 , by post hoc Tukey’s multiple comparisons test. ( D ) tsA58-AVM cells were co-transfected with plasmids expressing PLXNA1-Myc and FARP1-HA. Cells were treated with control or conditioned medium (containing the ectodomain of SEMA6D) for 30 min followed by co-IP analysis using an anti-Myc antibody. The cells transfected with FARP1-HA alone (lane 1) were used as the negative control. Western analysis was then performed using antibodies as indicated. ( E , F ) tsA58-AVM cells were transfected with siRNAs against PlxnA1 or Farp1 and then treated with control medium or conditioned medium (containing SEMA6D ectodomain). The active and total Rho levels then were determined. Cells transfected with the scrambled siRNA were used as controls. ( G ) All embryos were isolated 9 days after transferring microinjected zygotes to the receiver female mice. Embryos were at ∼E9.25 (20–25 somites). Embryos were PCR genotyped, sectioned and stained with an anti-Myc antibody. We acquired six PCR positive embryos, among which four express the Myc-dnRHOA transgene. The two embryos that do not express Myc-dnRHOA were discarded. Arrows indicate examples of cushion mesenchymal cells. Total AV cushion mesenchymal cells formed were determined for each embryo. The raw mean number of AV cushion mesenchymal cells in non-transgenic embryos ( n = 6) is 112. ** P < 0.01, Student’s t -test. The bar in G represents 50 µm. Error bars in C and G represent SEM.
The in vivo role of Rho activation in regulating AV cushion development has not been determined. We therefore decided to apply a DN approach to test how blocking Rho activation in endocardial cells affects AV cushion mesenchymal cell formation. In transgenic embryos, the dn RHOA gene is driven by the ∼1 kb mouse Tie1 promoter (see Supplementary material online, Figure S15 ), which was previously used to drive endothelial specific expression of multiple transgenes in mouse embryos. 45–48 This promoter activates its downstream transgenes starting at the stage when EMT initiates at the AV cushions. Among the 19 embryos at E9.25 (20–25 somites), six contain the transgene, as determined from PCR genotyping. We further performed immunostaining analysis using the Myc antibody on embryo sections and found that four transgenic embryos expressed Myc-tagged dnRHOA. Our results in Figure 7 G show that the number of AV cushion mesenchymal cells in embryos that express the transgene (Myc+) is significantly lower than the non-transgenic embryos at the same stage. Our data from cell culture and transgenic studies collectively suggest that SEMA6D activates RhoA through PLXNA1/FARP1 to promote AV cushion mesenchymal cell formation in mouse embryos.
4. Discussion
In this study we describe a new BMP-SEMA6D-RhoA axis that promotes AV cushion cellularization. In this axis, expression of Sema6D is activated by BMP ligands and SEMA6D promotes cushion mesenchymal cell formation (at least partially) through activation of RhoA. Our conclusion is collectively supported by evidence from in vitro , ex vivo , and in vivo studies.
Developing AV cushions contain a limited number of mesenchymal cells. It is difficult to purify these cells from surrounding cardiomyocytes. We developed the first AV cushion mesenchymal cell line, tsA58-AVM. These cells can propagate and be stored as immortal cells under the permissive condition. Under the restrictive condition they behave similar to primarily cultured cells with a limited proliferation capacity. A similar approach has been successfully applied to establish immortal cell lines for cells that are difficult to culture. 31,39 tsA58-AVM cells express known mesenchymal markers but not genes expressed in other types of cells. Furthermore, similar to primary cushion mesenchymal cells, the migration of tsA58-AVM cells is significantly upregulated by BMP stimulation. We thus provide a valuable tool for molecular and cellular studies of AV cushion mesenchymal cells under defined conditions.
During mammalian cardiogenesis, septum formation coupled with valvulogenesis occurs at both the OFT and AVC regions. Sema3C is required for normal OFT septation. Global inactivation of Sema3C led to persistent truncus arteriosus, an obligatory ventricular septal defect and aortic arch interruption. 27 A further conditional gene inactivation study revealed that SEMA3C released from neural crest cells acts on endocardial cells in the OFT region to activate EMT and promote cellularization of the OFT cushions. 29 This study provides strong evidence supporting that Sema6D plays a critical role in promoting cushion mesenchyme formation in AV cushions. Because no abnormal cell proliferation/death is observed in mutant hearts, the hypocellular defect in Nfatc1 Cre /Sema6D loxp/loxp embryos at E9.25–E9.5 is likely caused by impaired EMT. This conclusion is further supported by our collagen gel analysis and EMT marker examination. Therefore, Semaphorin signalling is important for the formation of mesenchyme in both OFT and AV regions, with different members acting in different regions. Our results are consistent with the previous discovery that SEMA6D promotes outgrowth of AV explants in avian. 22 . Therefore the role of SEMA6D during AV cushion development appears to be evolutionally conserved. In avian explants, SEMA6D also promotes migration of endocardial cells from the OFT region while inhibits endocardial cell migration from the ventricle region. 22 We did not observe any endocardial defect other than the AV canal cushion region in mouse mutant hearts. It is possible that the role of SEMA6D in ventricular and OFT endocardial cells is compensated by other Semaphorins.
We showed that the initial cushion defect in Sema6D mutants is resolved at E10.5, likely due to increased expression of Sema6C . A similar discovery was reported in Snai2 mutants. 41 Inactivation of Snai2 led to hypocellular AV cushions at E9.5 and this defect was resolved at E10.5 by increased expression of Snai1 . 41 The results from both studies suggest the existence of a monitory mechanism in AV cushions; if not enough mesenchymal cells are produced in AV cushions at E9.5, a certain compensatory mechanism is activated to promote EMT, ensuring sufficient mesenchyme generation. Our marker examination showed that at later stages (E9.5 and E10.5), expression of EMT marker genes was increased in the endocardial layer of AV cushions. We therefore provide direct evidence to support that the resolution of AV cushion defect is due to increased EMT.
Our transgenic study provides the first in vivo evidence supporting the role of Rho in promoting cushion mesenchyme formation. This result is consistent with previous in vitro tests on RhoA as well as ROCK (which is a direct target of Rho). 49,50 The BMP and TGFβ pathways act synergistically to promote cushion mesenchymal cell formation, 51 and the expression of RhoA is activated by TGFβ signalling AV cushions. 49 Therefore, RhoA may acts as a common target for both TGFβ and BMP signalling; with TGFβ regulation at the transcriptional level and BMP/SEMA6D regulation at the post-translational level. We thus provide a new mechanism for the synergistic effect between BMP and TGFβ in AV cushion development.
In conclusion, our newly identified BMP-SEMA6D-Rho axis plays a key role in the initial stages of AV cushion mesenchyme formation. Our results will help to characterize congenital heart diseases involving abnormal development of the AV septum and valves.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
We thank R.W. (Vanderbilt University Medical Center) for providing H-2K b - tsA58 mice. We thank K.Y. (Cincinnati Children’s Medical Center) and T.C. (University of Arizona) for suggestions on various techniques. We thank R.R. (University of Arizona) for sharing unpublished results. We thank Dr H.W. in K.J. laboratory for helping Echocardiography analysis. We thank the UAB Heflin Genetics Core Facility for performing microarray analysis. We thank B.H. (Duke University) and the Duke Transgenic Core Facility for making the Sema6D loxp mouse line. We thank the UAB Transgenic Core Facility for making the transgenic embryos. We thank Dr. S.S. (Johns Hopkins University) for providing necessary reagents.
Conflict of interest: none declared.
Funding
This project is supported by an R01 grant (R01HL095783) and an R03 grant (R03HD082634) from the NIH awarded to K.J.
References
- 1. Hoffman JI. Incidence of congenital heart disease: II. Prenatal incidence . Pediatr Cardiol 1995. ; 16 : 155 – 165 . [DOI] [PubMed] [Google Scholar]
- 2. Hoffman JI, Kaplan S. The incidence of congenital heart disease . J Am Coll Cardiol 2002. ; 39 : 1890 – 1900 . [DOI] [PubMed] [Google Scholar]
- 3. Onuzo OC. How effectively can clinical examination pick up congenital heart disease at birth? Arch Dis Child Fetal Neonatal Ed 2006. ; 91 : F236 – F237 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clark KL, Yutzey KE, Benson DW. Transcription factors and congenital heart defects . Annu Rev Physiol 2006. ; 68 : 97 – 121 . [DOI] [PubMed] [Google Scholar]
- 5. Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation . Circ Res 2004. ; 95 : 459 – 470 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective . Birth Defects Res C Embryo Today 2003. ; 69 : 58 – 72 . [DOI] [PubMed] [Google Scholar]
- 7. Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development . Int Rev Cytol 2005. ; 243 : 287 – 335 . [DOI] [PubMed] [Google Scholar]
- 8. Wagner M, Siddiqui MA. Signal transduction in early heart development (II): ventricular chamber specification, trabeculation, and heart valve formation . Exp Biol Med (Maywood) 2007. ; 232 : 866 – 880 . [PubMed] [Google Scholar]
- 9. Lin CJ, Lin CY, Chen CH, Zhou B, Chang CP. Partitioning the heart: mechanisms of cardiac septation and valve development . Development 2012. ; 139 : 3277 – 3299 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hinton RB, Yutzey KE. Heart valve structure and function in development and disease . Annu Rev Physiol 2011. ; 73 : 29 – 46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Markwald RR, Norris RA, Moreno-Rodriguez R, Levine RA. Developmental basis of adult cardiovascular diseases: valvular heart diseases . Ann N Y Acad Sci 2010. ; 1188 : 177 – 183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease . Circ Res 2009. ; 105 : 408 – 421 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning . Development 2005. ; 132 : 5601 – 5611 . [DOI] [PubMed] [Google Scholar]
- 14. Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart . Genes Dev 2003. ; 17 : 2362 – 2367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song L, Fassler R, Mishina Y, Jiao K, Baldwin HS. Essential functions of Alk3 during AV cushion morphogenesis in mouse embryonic hearts . Dev Biol 2007. ; 301 : 276 – 286 . [DOI] [PubMed] [Google Scholar]
- 16. Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation . Development 2006. ; 133 : 3473 – 3484 . [DOI] [PubMed] [Google Scholar]
- 17. Rivera-Feliciano J, Tabin CJ. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field . Dev Biol 2006. ; 295 : 580 – 588 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart . Dev Biol 2005. ; 286 : 299 – 310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yazdani U, Terman JR. The semaphorins . Genome Biol 2006. ; 7 : 211 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology . Annu Rev Cell Dev Biol 2007. ; 23 : 263 – 292 . [DOI] [PubMed] [Google Scholar]
- 21. Casazza A, Fazzari P, Tamagnone L. Semaphorin signals in cell adhesion and cell migration: functional role and molecular mechanisms . Adv Exp Med Biol 2007. ; 600 : 90 – 108 . [DOI] [PubMed] [Google Scholar]
- 22. Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2 . Genes Dev 2004. ; 18 : 435 – 447 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Yabuki M, Harada K, Hori M, Kikutani H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling . Nat Cell Biol 2004. ; 6 : 1204 – 1211 . [DOI] [PubMed] [Google Scholar]
- 24. Ahmed A, Eickholt BJ. Intracellular kinases in semaphorin signaling . Adv Exp Med Biol 2007. ; 600 : 24 – 37 . [DOI] [PubMed] [Google Scholar]
- 25. Jongbloets BC, Pasterkamp RJ. Semaphorin signalling during development . Development 2014. ; 141 : 3292 – 3297 . [DOI] [PubMed] [Google Scholar]
- 26. Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart . Nature 1996. ; 383 : 525 – 528 . [DOI] [PubMed] [Google Scholar]
- 27. Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, Mombaerts P, Epstein JA, Raper JA. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption . Development 2001. ; 128 : 3061 – 3070 . [DOI] [PubMed] [Google Scholar]
- 28. Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. PlexinA2 and semaphorin signaling during cardiac neural crest development . Development 2001. ; 128 : 3071 – 3080 . [DOI] [PubMed] [Google Scholar]
- 29. Plein A, Calmont A, Fantin A, Denti L, Anderson NA, Scambler PJ, Ruhrberg C. Neural crest-derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation . J Clin Invest 2015. ; 125 : 2661 – 2676 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development . Dev Cell 2004. ; 7 : 107 – 116 . [DOI] [PubMed] [Google Scholar]
- 31. Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice . Proc Natl Acad Sci U S A 1993. ; 90 : 587 – 591 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O'Rourke BP, Sharp DJ, Zheng D, Lenz J, Baldwin HS, Chang CP, Zhou B. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling . Cell 2012. ; 151 : 1083 – 1096 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peng Y, Song L, Zhao M, Harmelink C, Debenedittis P, Cui X, Wang Q, Jiao K. Critical roles of miRNA-mediated regulation of TGFbeta signalling during mouse cardiogenesis . Cardiovasc Res 2014. ; 103 : 258 – 267 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y, Harmelink C, Peng Y, Chen Y, Wang Q, Jiao K. CHD7 interacts with BMP R-SMADs to epigenetically regulate cardiogenesis in mice . Hum Mol Genet 2014. ; 23 : 2145 – 2156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiao K, Langworthy M, Batts L, Brown CB, Moses HL, Baldwin HS. Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development . Development 2006. ; 133 : 4585 – 4593 . [DOI] [PubMed] [Google Scholar]
- 36. Jiao K, Nau JJ, Cool M, Gray WM, Fassler JS, Malone RE. Phylogenetic footprinting reveals multiple regulatory elements involved in control of the meiotic recombination gene, REC102 . Yeast 2002. ; 19 : 99 – 114 . [DOI] [PubMed] [Google Scholar]
- 37. Shelton EL, Yutzey KE. Twist1 function in endocardial cushion cell proliferation, migration, and differentiation during heart valve development . Dev Biol 2008. ; 317 : 282 – 295 . [Database]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis . Dev Biol 2002. ; 248 : 170 – 181 . [DOI] [PubMed] [Google Scholar]
- 39. Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis . Cancer Res 2003. ; 63 : 2971 – 2976 . [PubMed] [Google Scholar]
- 40. Inai K, Norris RA, Hoffman S, Markwald RR, Sugi Y. BMP-2 induces cell migration and periostin expression during atrioventricular valvulogenesis . Dev Biol 2008. ; 315 : 383 – 396 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Niessen K, Fu Y, Chang L, Hoodless PA, McFadden D, Karsan A. Slug is a direct Notch target required for initiation of cardiac cushion cellularization . J Cell Biol 2008. ; 182 : 315 – 325 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors . Nat Med 2002. ; 8 : 850 – 855 . [DOI] [PubMed] [Google Scholar]
- 43. Shang X, Marchioni F, Sipes N, Evelyn CR, Jerabek-Willemsen M, Duhr S, Seibel W, Wortman M, Zheng Y. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases . Chem Biol 2012. ; 19 : 699 – 710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhuang B, Su YS, Sockanathan S. FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling . Neuron 2009. ; 61 : 359 – 372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Korhonen J, Lahtinen I, Halmekyto M, Alhonen L, Janne J, Dumont D, Alitalo K. Endothelial-specific gene expression directed by the tie gene promoter in vivo . Blood 1995. ; 86 : 1828 – 1835 . [PubMed] [Google Scholar]
- 46. Iljin K, Dube A, Kontusaari S, Korhonen J, Lahtinen I, Oettgen P, Alitalo K. Role of ets factors in the activity and endothelial cell specificity of the mouse Tie gene promoter . Faseb J 1999. ; 13 : 377 – 386 . [DOI] [PubMed] [Google Scholar]
- 47. Gustafsson E, Brakebusch C, Hietanen K, Fassler R. Tie-1-directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice . J Cell Sci 2001. ; 114 : 671 – 676 . [DOI] [PubMed] [Google Scholar]
- 48. Iljin K, Petrova TV, Veikkola T, Kumar V, Poutanen M, Alitalo K. A fluorescent Tie1 reporter allows monitoring of vascular development and endothelial cell isolation from transgenic mouse embryos . Faseb J 2002. ; 16 : 1764 – 1774 . [DOI] [PubMed] [Google Scholar]
- 49. Tavares AL, Mercado-Pimentel ME, Runyan RB, Kitten GT. TGF beta-mediated RhoA expression is necessary for epithelial-mesenchymal transition in the embryonic chick heart . Dev Dyn 2006. ; 235 : 1589 – 1598 . [DOI] [PubMed] [Google Scholar]
- 50. Zhao Z, Rivkees SA. Rho-associated kinases play a role in endocardial cell differentiation and migration . Dev Biol 2004. ; 275 : 183 – 191 . [DOI] [PubMed] [Google Scholar]
- 51. Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) . Anat Rec 2000. ; 258 : 119 – 127 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.