Abstract
Ecotypic differentiation in the tussock‐forming sedge Eriophorum vaginatum has led to the development of populations that are locally adapted to climate in Alaska's moist tussock tundra. As a foundation species, E. vaginatum plays a central role in providing topographic and microclimatic variation essential to these ecosystems, but a changing climate could diminish the importance of this species. As Arctic temperatures have increased, there is evidence of adaptational lag in E. vaginatum, as locally adapted ecotypes now exhibit reduced population growth rates. Whether there is a physiological underpinning to adaptational lag is unknown. Accordingly, this possibility was investigated in reciprocal transplant gardens. Tussocks of E. vaginatum from sites separated by ~1° latitude (Coldfoot: 67°15′N, Toolik Lake: 68°37′, Sagwon: 69°25′) were transplanted into the Toolik Lake and Sagwon sites and exposed to either an ambient or an experimental warming treatment. Five tussocks pertreatment combination were measured at each garden to determine photosynthetic capacity (i.e., V cmax and J max) and dark respiration rate (R d) at measurement temperatures of 15, 20, and 25°C. Photosynthetic enhancements or homeostasis were observed for all ecotypes at both gardens under increased growth temperature, indicating no negative effect of elevated temperature on photosynthetic capacity. Further, no evidence of thermal acclimation in R d was observed for any ecotype, and there was little evidence of ecotypic variation in R d. As such, no physiological contribution to adaptational lag was observed given the increase in growth temperature (up to ~2°C) provided by this study. Despite neutral to positive effects of increased growth temperature on photosynthesis in E. vaginatum, it appears to confer no lasting advantage to the species.
Keywords: adaptational lag, Eriophorum vaginatum, moist tussock tundra, photosynthetic capacity, respiration, temperature acclimation
1. INTRODUCTION
Current and projected increases in global temperature are disproportionately affecting the Arctic (ACIA 2004; Diffenbaugh & Field, 2013), and ecosystem‐level changes in response to increased temperature include altered community composition, diversity, and productivity (Chapin, Shaver, Giblin, Nadelhoffer, & Laundre, 1995; Elmendorf et al., 2012; Oberbauer et al., 2007; Walker et al., 2006). Vegetation in northwestern North America's moist tundra is comprised of a mixture of graminoids, forbs, deciduous and evergreen shrubs, mosses, and lichens. The tussock‐forming sedge, Eriophorum vaginatum, is a foundation species for these ecosystems, providing much of the moist tundra's topographic structure, as well as microclimatic variation exploited by other plant species (Figure 1; Chapin & Shaver, 1985). Given E. vaginatum's central role in structuring these ecosystems, there is much interest in understanding the species’ response to a warming climate. Accordingly, these responses have been studied in reciprocal transplant gardens at six sites along a latitudinal gradient in Alaska, and it is evident that populations of this species form ecotypes that are locally adapted to climate (Bennington et al., 2012; Fetcher & Shaver, 1990; McGraw et al., 2015; Souther, Fetcher, Fowler, Shaver, & McGraw, 2014).
Figure 1.

Moist tussock tundra at Toolik Lake, Alaska. The tussock‐forming sedge, Eriophorum vaginatum, is a foundation species in these ecosystems
Differentiation of E. vaginatum into ecotypes has conferred “home‐site” advantage to populations, such that enhanced survival, tiller biomass, and light‐saturated photosynthesis have been found for ecotypes transplanted back into their home‐sites, relative to ecotypes from different latitudes (Bennington et al., 2012; Souther et al., 2014). While ecotypic differentiation is common in plants and often allows a species to occupy large and climatically variable niche space (Linhart & Grant, 1996), it may lead to adaptational lag (i.e., the rate of adaptation in a population may fall behind the rate of environmental change) if climate changes rapidly (Anderson, Panett, & Mitchell‐Olds, 2012; Etterson & Shaw, 2001). Because E. vaginatum is a long‐lived species (Mark, Fetcher, Shaver, & Chapin, 1985), ecotypes may have limited genetic variation and/or low gene flow between ecotypes, making the species susceptible to adaptational lag in a rapidly warming Arctic (Aitken, Yeaman, Holliday, Wang, & Curtis‐McLane, 2008). Evidence of adaptational lag is accumulating for E. vaginatum, as conditions for optimal population growth rates are now shifting north of a given ecotype's present location (Chandler et al., 2015; McGraw et al., 2015). This puts the species at risk for replacement by deciduous woody shrubs and could lead to substantial changes in ecosystem function (Heskel et al., 2013; Walker et al., 2006).
At present, little is known about possible physiological underpinnings to adaptational lag in E. vaginatum. Photosynthetic adjustments to growth temperature can manifest in a variety of ways (Way & Yamori, 2014), but the focus of this study is on whether variation in photosynthetic capacity contributes to adaptational lag in E. vaginatum. Measurement of the maximum carboxylation rate of Rubisco (V cmax) and maximum rate of electron transport (J max) allow for the assessment of photosynthetic capacity, as each are rate‐limiting photosynthetic processes (Sage & Kubien, 2007). Plants grown in cool climates acclimate by increasing the capacity of enzymes such as Rubisco, thereby enhancing photosynthetic capacity at low temperatures (Berry & Björkman, 1980; Yamori, Hokosaka, & Way, 2014; Yamori, Noguchi, & Terashima, 2005).
As growth temperature rises, it is unclear how photosynthetic acclimation may manifest in E. vaginatum; maintenance of low‐temperature enzymatic capacity would increase photosynthetic capacity at higher temperature, whereas a decline in enzyme concentration could lead to consistent or lower photosynthetic capacity across growth temperatures (Way & Yamori, 2014). Evidence of temperature homeostasis of photosynthesis between low and high growth temperatures in perennial herbs and woody evergreens adapted to cooler environments (Yamori et al., 2014) indicates that the latter option is likely, and “detractive adjustments” (after Way & Yamori, 2014) in photosynthesis with increased temperature sometimes occur (Berry & Björkman, 1980; Yamori et al., 2014). Additionally, declines in photosynthesis have been observed for a single E. vaginatum ecotype exposed to increased growth temperature (Heskel et al., 2013).
This study seeks to determine how the responses of V cmax and J max to increased measurement temperature vary for E. vaginatum ecotypes grown in transplant gardens under ambient and experimentally warmed conditions. Indications of adaptational lag in the species suggest that southern ecotypes will out‐perform locally adapted northern ecotypes as growth temperature increases. Variation in leaf morphology and leaf nitrogen content will also be investigated, as both can affect photosynthetic capacity. Additionally, the temperature response of dark respiration (R d) will be examined, as it has the potential to affect the overall carbon balance of E. vaginatum. Prior research indicates that while E. vaginatum R d acclimates to higher growth temperature, R d does not vary among ecotypes growing in common gardens (Heskel, Bitterman, Atkin, Turnbull, & Griffin, 2014; Kornfeld et al., 2013; Souther et al., 2014; van de Weg, Fetcher, & Shaver, 2013). This leads to an expectation that growing temperature‐induced changes in R d will not differentially affect ecotypes, and any alterations in E. vaginatum carbon balance will be related to changes in photosynthetic processes. As a consequence, the following research questions were addressed: (1) Do V cmax and J max show evidence of detractive adjustments in photosynthesis as growth temperature increases? (2) Are detractive adjustments in photosynthesis more pronounced in northern ecotypes, relative to southern ecotypes? (3) Are responses of R d to growth temperature uniform across ecotypes?
2. MATERIALS AND METHODS
2.1. Study species
Eriophorum vaginatum L. is a sedge that forms tussocks comprised of ~300–600 live tillers when mature (Fetcher & Shaver, 1982). Individual tillers produce 1–4 new leaves per year, exhibit deciduous root growth originating from tiller rhizomes, and typically live fewer than 8 years (Fetcher & Shaver, 1983). Tussocks grow vegetatively, with each tiller producing 1–3 daughter tillers per growing season (Fetcher & Shaver, 1983). Tussocks are thought to develop vegetatively from a single individual, and individual tussocks can persist for upwards of 100 years (Mark et al., 1985).
2.2. Experimental design
In 2014, tussocks of E. vaginatum from three ecotypes growing at the sites Coldfoot (CF, 67°15′32″N, 150°10′12″W), Toolik Lake (TL, 68°37′44″N, 149°35′0″W), and Sagwon (SAG, 69°25′26″N, 148°42′49″W) were transplanted into gardens located at TL and SAG. Coldfoot is located on the south slope of the Brooks Range, while both TL and SAG are north of the range and at a latitude above treeline. Ecotypes from these sites were selected because they represent a range of latitudes, each separated by ~1°.
Tussocks from each site were sliced from the ground beneath rhizomes to prevent significant damage during transplantation (Bennington et al., 2012; Parker, Tang, Clark, Moody, & Fetcher, 2017). Sixty tussocks per ecotype were transplanted into each garden. Tussocks from a given ecotype were transplanted in groups of three and assigned to either an ambient or an open‐top chamber (OTC) treatment, yielding 10 replicates per treatment (Figure 2). The OTC treatment was designed to passively warm tussocks, and chambers were constructed of fiberglass glazing (Sun‐Lite HP, Kalwall Corp., Manchester, NH) in an open‐ended cone shape. Chamber diameter was 1.23 m at the base and 0.84 m at the top. Chambers were 0.70 m in height and were secured with rope and tent stakes.
Figure 2.
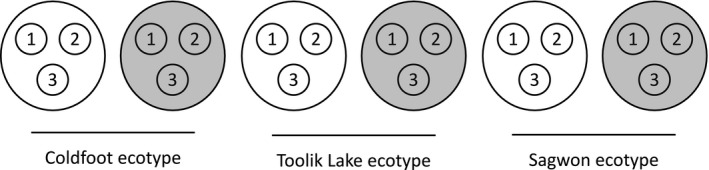
Schematic of transplant garden study design showing sets of three transplanted Eriophorum vaginatum tussocks (circles containing numbers) per ecotype and treatment (ambient—large white circles or open‐top chamber—large gray circles) combination. The pattern shown here was replicated 10 times at both the Toolik Lake and Sagwon gardens
2.3. Field and laboratory measurements
To assess differences in light availability between treatments, photosynthetically active radiation (PAR, LI‐190SA quantum sensor, LI‐COR, Inc., Lincoln, NE) was measured every minute from 22 June to 23 August 2016 within one ambient and one OTC plot at the TL garden and recorded with a data logger (CR5000, Campbell Scientific, Inc., Logan, UT). The SAG garden was not instrumented for PAR measurements. Air temperature (T air) was measured hourly with shielded iButtons (DS1921G‐F5#, Maxim Integrated, San Jose, CA) mounted 20 cm above the ground surface at both gardens from 4 June to 27 August 2016. At TL, one sensor was located in an ambient plot and three were within OTCs, while at SAG, two sensors were in ambient plots and three were within OTCs.
To examine changes in photosynthetic rate (A) as a function of leaf intercellular CO2 concentration (C i; i.e., to develop A/C i curves), gas exchange measurements were made on five tussocks per ecotype and treatment (i.e., ambient or OTC) combination at both the TL and SAG transplant gardens. A total of 30 tussocks were measured in each garden, and all measurements were made over a four‐week period from late June to late July 2016 between 0900 and 1700 Alaska Daylight Time (ADT, with one exception where a measurement ended at 1820 ADT). Alaska Daylight Time is 2 hr later than solar time. Measurements of gas exchange were made with two separate LI‐6400XT infrared gas analyzers (LI‐COR, Inc.), each using a 6400‐02B LED light source and 6400‐01 CO2 injector. Both analyzers were fitted with water jackets (6400‐88) on either side of the sensor head's Peltier coolers. Warmed or cooled water was circulated through the jackets as needed to help achieve target measurement temperatures of 15, 20, and 25°C within the LI‐6400's cuvette.
Prior to each measurement, six leaves per tussock were aligned parallel to one another and clamped into the cuvette. Given the small width of E. vaginatum leaf blades, leaf temperature (T leaf) within the cuvette was computed using the energy balance method during all gas exchange measurements. Leaves were cooled to 15°C, and measurement of an A/C i curve was initiated with PAR set at 1,500 μmol m−2 s−1 for the measurement's duration. Twenty‐eight years of PAR data from TL show that mean hourly PAR exceeded 1,500 μmol m−2 s−1 for fewer than 0.6% of all hours in June and July (EDCT 2017), while light response curves showed that E. vaginatum leaves were light saturated, but showed no sign of photoinhibition at 1,500 μmol m−2 s−1 (data not shown). Photosynthetic rate was measured sequentially at the following ambient CO2 concentrations (C a): 400, 400, 300, 200, 100, 50, 400, 400, 600, 800, 1,000, 1,200 ppm, and measurements were initiated after stability was achieved in the cuvette (i.e., the rate of change in CO2 concentration, water vapor concentration, and flow rate over 15 s had a slope <1). The LI‐6400's block temperature was adjusted as needed to maintain T leaf for the duration of each measurement, and cuvette relative humidity was maintained as close to 50% as possible. During A/C i curve measurements, mean vapor pressure deficit (D) was 1.00 kPa at 15°C, 1.30 kPa at 20°C, and 1.89 kPa at 25°C. Following completion of each A/C i curve, a measurement of R d was made at the same temperature once stability had been reached, following ~5–10 min of darkness. Leaf temperature was next raised to 20°C and subsequently 25°C, and the above protocol was repeated at each measurement temperature on the same six leaf blades. When all gas exchange measurements were completed for a given tussock, the width of each leaf was measured in the field with an optical comparator, and gas exchange rates were recomputed based on the leaf area within the cuvette. Gas exchange data were not leak corrected.
In late July one to two tillers were clipped from an E. vaginatum tussock in each ecotype by treatment combination per garden, although at TL six of the 60 groups of tussocks were not sampled because of ongoing measurements that precluded removal of foliage. Collected leaves were stored at 4.5°C in plastic bags containing a damp paper towel for no longer than 48 hr. All leaves were scanned on a calibrated flatbed scanner and analyzed for leaf area with the program WinFOLIA (Regent Instruments, Inc., Montreal, QC, Canada). Leaves were then dried to constant mass at 70°C and weighed to determine specific leaf area (SLA). Dried leaves were ground into a fine powder with an oscillating ball mill (MM 200, Retsch, Haan, Germany) and packed into tin capsules for percent nitrogen (% N) analyses. Samples were analyzed at the Washington State University Stable Isotope Core Laboratory, and error analysis of duplicate samples representing 18% of all samples had a mean difference of 0.07% N. The % N and SLA data were used to compute nitrogen content per unit leaf area (Narea, g N/m2).
Data from this study are archived at the Arctic LTER Data Archive, maintained by the Marine Biological Laboratory's Ecosystems Center (http://arc-lter.ecosystems.mbl.edu/content/ecotypic-variation-tundra-plants).
2.4. Data analyses
With the exception of the modeling described below, all analyses were carried out with R (v. 3.3.2, R Core Team 2016). Specific leaf area and Narea data were analyzed separately for each study site with ANOVA where the main effects were ecotype, treatment, and the ecotype × treatment interaction. To meet assumptions of normality, TL SLA data were 1/y transformed, while SAG SLA data were y−2 transformed. At SAG, two outliers >2 SD from the mean were removed prior to analysis of Narea data.
Individual A/C i curves were fit using the R package plantecophys (v. 1.1.8, Duursma, 2015, 2016) based on the Farquhar, von Caemmerer, and Berry (1980) photosynthesis model where V cmax and J max were estimated at each measurement temperature, and the measurement of R d at each temperature was used as an input variable. The Farquhar et al. (1980) model was fit with the parameterization of Medlyn et al. (2002), using the temperature functions of Bernacchi, Singsaas, Pimentel, Portis, and Long (2001) to determine the Michaelis‐Menten coefficients of Rubisco activity for CO2 (Kc) and O2 (Ko), as well as the CO2 compensation point in the absence of mitochondrial respiration (Γ*). The default fit method, employing nonlinear regression, was attempted for all A/C i data. When the default method did not converge, a bilinear method was used to estimate V cmax and J max. After curves were fit, the model fits and output data were examined to determine whether any data should be excluded from further analysis. Data were excluded if two of the four following criteria were met: (1) maximum C i < 700 ppm, (2) photosynthesis did not plateau at high C i, (3) model RMSE > 6, or (4) the bilinear method was used to fit the curve. Criterion 1 ensured a reliable estimate of J max, while criterion 2 ensured that A had saturated. Criteria 3 and 4 were indicators of the quality of model fits. This process led to the removal of six of 90 A/C i curves from the data set at the TL garden and three of 90 curves at the SAG garden. One additional tussock at SAG from the TL‐ambient treatment was removed from further analyses, as it was measured during an atypically cold period with T air ~8°C, and residuals of V cmax, J max, and R d measurements made for this tussock were at or beyond model standard error (see modeling details below).
The terms V cmax, J max, and R d were modeled as a function of temperature with SigmaPlot (v. 11.0, Systat Software, Inc. 2008) as described below. Because V cmax and J max were measured over a relatively small temperature range, below the optimal temperature for each parameter, both V cmax and J max were modeled in relation to temperature with an Arrhenius equation (after Medlyn et al., 2002),
| (1) |
where f(T k) represents either V cmax or J max at a given measurement temperature, k25 is the value of either V cmax or J max at 25°C (hereafter referred to as V cmax(25) or J max(25)), E a is the activation energy (exponential rate of increase) for either V cmax or J max, R is the gas constant (8.314 J mol−1 K−1), and T k is measurement temperature in Kelvin. Modeling was carried out separately for each ecotype by treatment combination at each garden.
The ratio of J max to V cmax at 25°C was computed for each tussock and analyzed with a three‐way ANOVA to determine whether the ratio varied across gardens, ecotypes, and/or treatments. The ANOVA yielded no significant differences in any of the main effects or in any of the interaction terms (p > .05); therefore, data were pooled across gardens, ecotypes, and treatments to examine the relationship between J max and V cmax with linear regression.
To assess the effect of the largest difference in growing season temperature imposed by this study on net photosynthesis (A net), data from the TL garden were examined. As described below, the OTC treatment effect at TL was stronger than at SAG, and both common gardens had nearly identical ambient growing environments in 2016. Therefore, separate ANOVAs were performed for each ecotype growing at the TL garden to determine the effect of treatment, measurement temperature, and their interaction on A net. Data for these analyses were extracted from A/C i curves using the final measurement made at a CO2 concentration of 400 ppm. Data for the TL ecotype were y−1/2 transformed to meet assumptions of normality.
Variation in R d as a function of temperature was modeled for each ecotype by treatment combination within a garden with the equation,
| (2) |
where a and b are modeled coefficients (Lloyd & Taylor, 1994). The temperature responsiveness (i.e., Q 10) of each ecotype by treatment combination was determined with the equation,
| (3) |
where b is the coefficient determined in Equation (2). Dark respiration was modeled using an exponential function rather than a variable Q 10 function because measurements were made over a small temperature range (i.e., 10°C) and were made below the optimal temperature for R d (Atkin, Bruhn, & Tjoelker, 2005).
Finally, because photosynthetic temperature responses can be confounded by stomatal closure as temperature, thus D, rises, stomatal conductance (g s) was compared to D using separate linear regressions for each ecotype. These data were extracted from A/C i curves as described above.
3. RESULTS
3.1. Meteorological site comparison
Fifteen years of T air data for the months of June, July, and August show that, of the three sites from which E. vaginatum ecotypes were collected, CF had consistently warmer mean, minimum, and maximum T air than TL or SAG (Table 1). Mean monthly T air was 3.2–6.1°C warmer at CF than at TL or SAG, depending on month and site (Table 1). Over the last 15 years, growing season T air was similar for TL and SAG, with mean T air slightly warmer (0.6°C) at TL in June, nearly identical in July, and slightly warmer (0.4°C) at SAG in August (Table 1). During the 2016 growing season, patterns in T air were similar to those over the last 15 years, with CF as the warmest site and a high degree of similarity between TL and SAG (Table 1). Mean thawing degree days (TDD, the sum of daily mean T air >0°C between May and September, after Shaver, Fetcher, & Chapin, 1986) computed for the past 15 years indicate that CF had the highest TDD (1606 ± 37 (SE)), followed by TL and SAG, which were statistically indistinguishable (TL: 944 ± 27, SAG: 971 ± 37) (EDCT 2017, NRCS 2017).
Table 1.
Mean, minimum, and maximum temperature (T mean, T min, T max) averaged monthly ±SE for June, July, and August at the study sites from which tussocks were transplanted. Data for the 15 year period from 2002 to 2016 are presented along with those from just the 2016 growing season. Data from Coldfoot and Sagwon are from SNOTEL stations (NRCS 2017), while those from Toolik Lake are from the Toolik Field Station meteorological tower (EDCT 2017)
| Month | June | July | August | June | July | August |
|---|---|---|---|---|---|---|
| Parameter | Coldfoot: 2002–2016 | Coldfoot: 2016 | ||||
| T mean | 14.3 ± 0.2 | 14.2 ± 0.2 | 10.9 ± 0.2 | 13.0 ± 0.6 | 15.2 ± 0.6 | 12.2 ± 0.4 |
| T min | 7.1 ± 0.1 | 8.1 ± 0.1 | 5.1 ± 0.2 | 5.9 ± 0.5 | 9.0 ± 0.6 | 7.1 ± 0.6 |
| T max | 21.0 ± 0.2 | 20.5 ± 0.2 | 17.2 ± 0.2 | 19.6 ± 0.9 | 21.6 ± 0.8 | 18.0 ± 0.5 |
| Toolik Lake: 2002–2016 | Toolik Lake: 2016 | |||||
|---|---|---|---|---|---|---|
| T mean | 8.8 ± 0.2 | 10.8 ± 0.2 | 7.3 ± 0.2 | 6.5 ± 1.2 | 11.3 ± 0.8 | 8.5 ± 0.7 |
| T min | 3.1 ± 0.2 | 5.1 ± 0.2 | 2.1 ± 0.2 | 1.2 ± 1.0 | 5.2 ± 0.7 | 3.5 ± 0.5 |
| T max | 13.8 ± 0.3 | 15.4 ± 0.2 | 12.1 ± 0.3 | 11.9 ± 1.3 | 16.2 ± 1.0 | 13.1 ± 0.8 |
| Sagwon: 2002–2016 | Sagwon: 2016 | |||||
|---|---|---|---|---|---|---|
| T mean | 8.2 ± 0.2 | 10.6 ± 0.2 | 7.7 ± 0.2 | 7.7 ± 1.0 | 11.4 ± 0.9 | 8.3 ± 0.8 |
| T min | 3.1 ± 0.2 | 5.7 ± 0.2 | 3.8 ± 0.2 | 3.0 ± 0.8 | 6.5 ± 0.8 | 4.2 ± 0.6 |
| T max | 13.3 ± 0.3 | 15.4 ± 0.2 | 11.8 ± 0.3 | 12.6 ± 1.2 | 16.5 ± 1.0 | 12.8 ± 0.9 |
3.2. Characterization of ambient and OTC growing environments
During the 2016 growing season, PAR data from the TL garden indicated that light availability was consistently lower in the OTC treatment (data not shown). For the nine measured days in June, the percent difference in PAR between treatments was 25%, while it was 16% and 9% in July and August, respectively. These values represent hours of the day when PAR was >500 μmol m−2 s−1 for at least one of the treatments.
Over a 24‐hr period, mean T air in the OTCs was higher than in the ambient treatment at both the TL and SAG gardens, although the degree of warming was greater at TL (Table 2). The OTC treatments at TL were 1.0–1.9°C warmer than ambient treatments depending on the month, while at SAG, OTCs produced temperatures 0.3–0.8°C warmer than ambient (Table 2). The OTC treatment was likely less effective at the SAG garden because the site is windier than TL (J. L. Schedlbauer, personal observation).
Table 2.
Comparison of temperature environments between ambient and open‐top chamber (OTC) treatments at the Toolik Lake and Sagwon transplant gardens in June, July, and August 2016. Mean ± SE air temperature (T air) data for 24 hr are shown, as well as mean temperature differences between treatments
| 24‐hr Mean T air (°C) | |||
|---|---|---|---|
| Ambient | OTC | Difference | |
| Toolik Lake | |||
| June | 9.2 ± 0.30 | 11.0 ± 0.35 | 1.8 |
| July | 12.1 ± 0.25 | 14.0 ± 0.30 | 1.9 |
| August | 8.6 ± 0.22 | 9.6 ± 0.25 | 1.0 |
| Sagwon | |||
| June | 9.9 ± 0.29 | 10.7 ± 0.32 | 0.8 |
| July | 12.8 ± 0.26 | 13.4 ± 0.26 | 0.6 |
| August | 8.7 ± 0.23 | 9.1 ± 0.24 | 0.3 |
Given that the TL and SAG gardens did not differ dramatically in growing season temperature and the degree of warming provided by the OTC was lower at the SAG garden, relative to the TL garden, the data collected in this study reflect a gradient of warming. Specifically, the TL and SAG garden ambient treatments were nearly identical (SAG was slightly warmer), moderate warming was provided by the SAG OTC treatment, and the greatest warming was provided by the TL‐OTC treatment.
3.3. Leaf morphology and leaf nitrogen content
Within both the TL and SAG transplant gardens, SLA did not vary by ecotype or treatment, and there was no interaction between the main effects (p > .05, Table 3). Specific leaf area varied between 91.71 and 103.96 cm2/g (Table 3). Similarly, Narea was invariant across ecotypes and treatments at each garden, and no ecotype by treatment interaction was detected (p > .05, Table 3). Narea ranged from 1.59 to 1.90 g N/m2 (Table 3).
Table 3.
Mean ± SE specific leaf area (SLA) and leaf nitrogen content per unit leaf area (Narea) for Eriophorum vaginatum foliage. No significant differences in SLA or Narea were found among ecotypes (CF—Coldfoot, TL—Toolik Lake, SAG—Sagwon) or between treatments (ambient or OTC—open‐top chamber) within either garden (p > .05)
| SLA (cm2/g) | Narea (g N/m2) | ||||
|---|---|---|---|---|---|
| Ecotype | Treatment | Toolik Lake Garden | Sagwon Garden | Toolik Lake Garden | Sagwon Garden |
| CF | Ambient | 100.37 ± 3.06 | 103.96 ± 3.59 | 1.90 ± 0.09 | 1.59 ± 0.09 |
| OTC | 97.19 ± 2.83 | 96.44 ± 4.44 | 1.88 ± 0.10 | 1.66 ± 0.07 | |
| TL | Ambient | 95.74 ± 1.78 | 96.28 ± 2.60 | 1.88 ± 0.11 | 1.88 ± 0.11 |
| OTC | 99.39 ± 4.28 | 94.00 ± 2.88 | 1.81 ± 0.06 | 1.72 ± 0.10 | |
| SAG | Ambient | 91.85 ± 3.34 | 91.71 ± 2.69 | 1.74 ± 0.08 | 1.87 ± 0.10 |
| OTC | 96.80 ± 1.40 | 93.81 ± 4.08 | 1.72 ± 0.08 | 1.64 ± 0.05 | |
3.4. Temperature response of V cmax, J max, A net, and R d
At the TL garden, treatment did not differentially affect the relationship between V cmax or J max and temperature for the CF and TL ecotypes (Figures 3a,b, and 4a,b). However, for the SAG ecotype, the OTC treatment had higher V cmax and J max values at measurement temperatures of 20 and 25°C, as there was little overlap in the models’ 95% CIs (Figures 3c and 4c). Accordingly, the OTC treatment had somewhat higher values for E a, a term that reflects the exponential rate of increase in enzyme activity with temperature (Table 4). At the SAG garden, both the CF and SAG ecotypes had similar V cmax versus T leaf and J max versus T leaf relationships, regardless of treatment (Figures 3d,f and 4d,f), while the TL ecotype exhibited different responses by treatment (Figures 3e and 4e). Specifically, V cmax and J max in the ambient treatment were higher at temperatures of 20 and 25°C (Figures 3e and 4e). Use of the Arrhenius function to model V cmax or J max in relation to measurement temperature generally yielded significant relationships (p < .05) with model SE <30 (in 19 or 24 cases, Table 4). Overall, the Arrhenius function performed better when modeling V cmax than J max, as there was higher variation in the J max data (Figures 3 and 4, Table 4).
Figure 3.
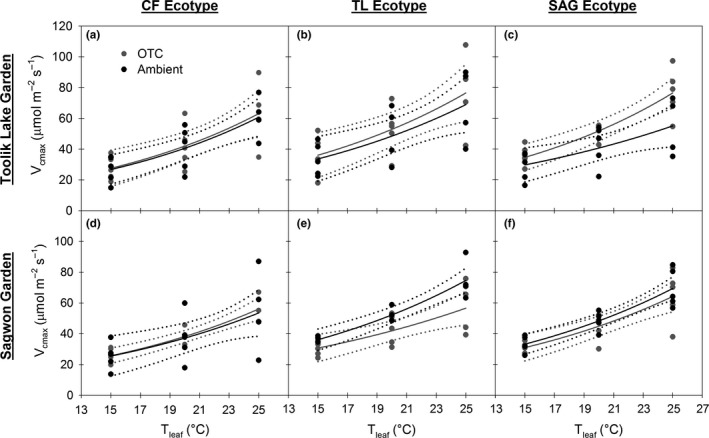
Maximum carboxylation rate of Rubisco (V cmax) versus leaf temperature (T leaf) for each ecotype of Eriophorum vaginatum (CF—Coldfoot, TL—Toolik Lake, SAG—Sagwon) within each garden (a–f). Each panel shows data and models for both the ambient (black) and open‐top chamber (OTC) treatments (gray). Solid lines show the Arrhenius function used to model these relationships; dotted lines are 95% confidence intervals
Figure 4.
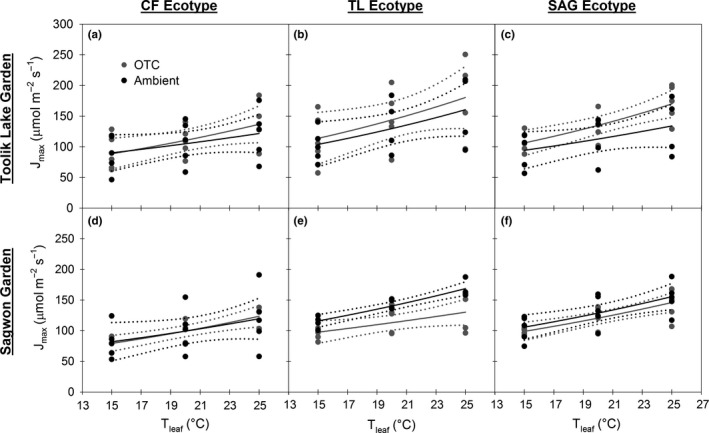
Maximum rate of electron transport (J max) versus leaf temperature (T leaf) for each ecotype of Eriophorum vaginatum (CF—Coldfoot, TL—Toolik Lake, SAG—Sagwon) within each garden (a–f). Each panel shows data and models for both the ambient (black) and open‐top chamber (OTC) treatments (gray). Solid lines show the Arrhenius function used to model these relationships; dotted lines are 95% confidence intervals
Table 4.
Outputs of an Arrhenius function used to model the maximum carboxylation rate of Rubisco (V cmax) and maximum rate of electron transport (J max) versus leaf temperature for each ecotype of Eriophorum vaginatum (CF—Coldfoot, TL—Toolik Lake, SAG—Sagwon) and treatment (ambient or OTC—open‐top chamber) within each transplant garden. Parameter estimates ±SE include V cmax and J max at 25°C (V cmax(25) and J max(25), respectively), as well as E a, the activation energy for either V cmax or J max
| Ecotype | Treatment | V cmax(25) (μmol m−2 s−1) | E a (kJ/mol) | Model SE | R 2 adj | F | p |
|---|---|---|---|---|---|---|---|
| Toolik Lake Garden | |||||||
| CF | Ambient | 60.8 ± 5.7 | 56.8 ± 14.2 | 11.9 | .57 | 18.2 | .0011 |
| OTC | 63.1 ± 7.0 | 57.3 ± 16.9 | 14.7 | .48 | 13.0 | .0036 | |
| TL | Ambient | 68.9 ± 8.2 | 49.6 ± 17.1 | 17.2 | .41 | 9.4 | .0106 |
| OTC | 76.5 ± 8.6 | 52.1 ± 16.4 | 18.0 | .44 | 11.2 | .0058 | |
| SAG | Ambient | 55.0 ± 6.3 | 42.7 ± 15.6 | 13.3 | .36 | 8.2 | .0142 |
| OTC | 76.5 ± 4.5 | 54.9 ± 9.5 | 10.4 | .75 | 40.0 | <.0001 | |
| Sagwon Garden | |||||||
| CF | Ambient | 53.5 ± 7.0 | 51.3 ± 20.2 | 16.3 | .32 | 7.5 | .0171 |
| OTC | 56.1 ± 3.3 | 54.0 ± 8.1 | 6.1 | .79 | 46.5 | <.0001 | |
| TL | Ambient | 74.7 ± 3.6 | 50.2 ± 7.4 | 7.5 | .83 | 53.1 | <.0001 |
| OTC | 56.6 ± 5.0 | 42.4 ± 12.1 | 10.7 | .48 | 13.2 | .0034 | |
| SAG | Ambient | 69.4 ± 3.5 | 51.0 ± 7.8 | 8.2 | .78 | 49.6 | <.0001 |
| OTC | 64.2 ± 4.6 | 50.5 ± 11.0 | 10.8 | .62 | 23.8 | .0003 | |
| Ecotype | Treatment | J max(25) (μmol m−2 s−1) | E a (kJ/mol) | Model SE | R 2 adj | F | p |
|---|---|---|---|---|---|---|---|
| Toolik Lake Garden | |||||||
| CF | Ambient | 121.5 ± 14.5 | 20.8 ± 14.6 | 34.6 | .07 | 2.1 | .1705 |
| OTC | 136.6 ± 13.8 | 30.0 ± 12.6 | 29.6 | .27 | 5.8 | .0328 | |
| TL | Ambient | 160.3 ± 19.6 | 30.1 ± 15.1 | 41.7 | .21 | 4.2 | .0662 |
| OTC | 180.2 ± 23.3 | 32.0 ± 16.3 | 49.9 | .19 | 4.0 | .0691 | |
| SAG | Ambient | 133.9 ± 16.3 | 24.4 ± 14.6 | 35.2 | .13 | 2.9 | .1139 |
| OTC | 169.9 ± 10.2 | 32.5 ± 8.0 | 24.0 | .56 | 17.5 | .0013 | |
| Sagwon Garden | |||||||
| CF | Ambient | 119.6 ± 15.5 | 26.1 ± 16.5 | 36.9 | .10 | 2.6 | .1295 |
| OTC | 123.6 ± 7.9 | 30.7 ± 7.5 | 15.0 | .56 | 16.5 | .0019 | |
| TL | Ambient | 168.7 ± 5.0 | 26.0 ± 3.8 | 10.6 | .82 | 50.0 | <.0001 |
| OTC | 130.3 ± 9.7 | 19.9 ± 8.7 | 21.1 | .26 | 5.5 | .0377 | |
| SAG | Ambient | 155.6 ± 9.9 | 26.7 ± 8.1 | 23.6 | .43 | 11.4 | .0050 |
| OTC | 146.0 ± 7.4 | 26.9 ± 6.5 | 17.6 | .55 | 18.2 | .0009 | |
Given the gradient of warming provided by treatments in this study (see above), estimates of the parameters V cmax(25), J max(25), and E a were examined in relation to this gradient. The CF and TL ecotypes did not exhibit strong variation in V cmax(25) or J max(25) with increased growth temperature, as evidenced by a high degree of overlap in the SE of these parameter estimates (Figure 5a,c, Table 4). However, the SAG ecotype exhibited increases in both parameters with increased growth temperature, such that both parameters were much higher in the TL garden OTC treatment than in the TL‐ambient treatment (Figure 5a,c, Table 4). When examining just the warmest treatment (i.e., TL‐OTC), estimates of V cmax and J max were higher for the TL and SAG ecotypes, relative to the CF ecotype (Figure 5a,c, Table 4). A plot of J max(25) versus V cmax(25) for each measured tussock yielded a positive linear relationship between the two variables, with a slope of 2.09 (R 2 adj = .91, Figure 6). Estimates of E a for both V cmax and J max were largely invariant within and across ecotypes because of the magnitude of the standard errors associated with these parameter estimates (Figure 5b,d, Table 4).
Figure 5.
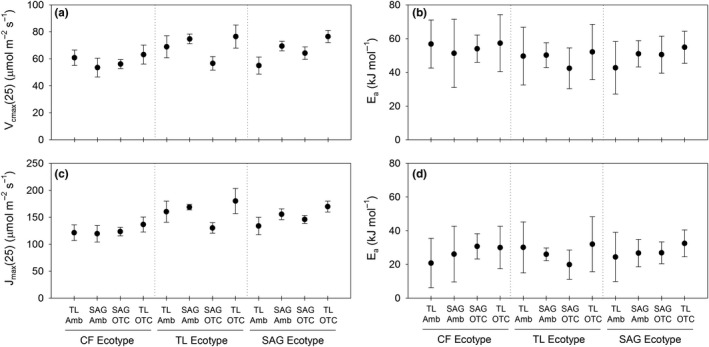
Plots of parameter estimates ± SE for the Arrhenius functions used to model the maximum carboxylation rate of Rubisco (V cmax) and maximum rate of electron transport (J max) versus leaf temperature for each ecotype of Eriophorum vaginatum (CF—Coldfoot, TL—Toolik Lake, SAG—Sagwon) and treatment (ambient—Amb or open‐top chamber—OTC) within each transplant garden. Parameter estimates are arranged within each ecotype along the temperature gradient provided by treatments, where TL ambient was the coolest treatment and TL‐OTC was the warmest treatment. Shown are estimates of (a) V cmax at 25°C (V cmax(25)), (b) activation energy (E a) for V cmax, (c) J max at 25°C (J max(25)), and (d) E a for J max
Figure 6.
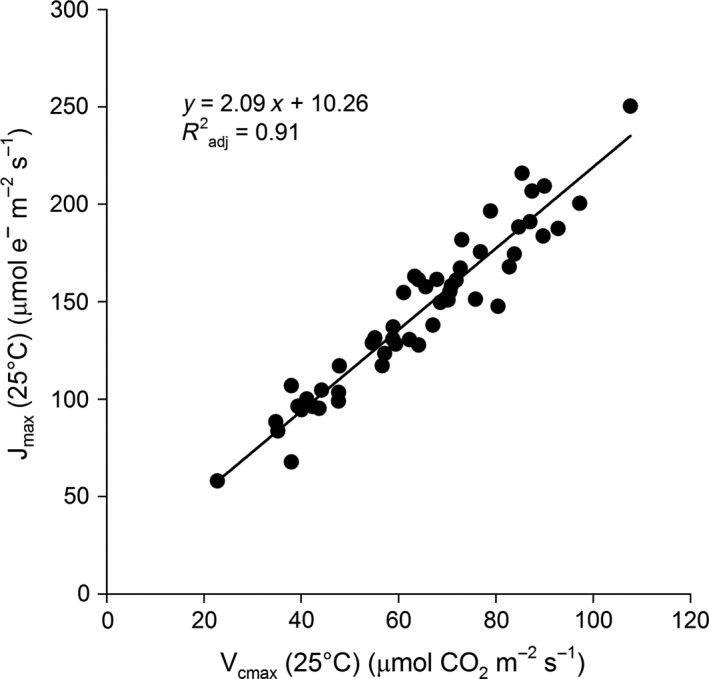
Linear regression of the maximum rate of electron transport at 25°C (J max(25)) against the maximum carboxylation rate of Rubisco at 25°C (V cmax(25)) for E. vaginatum. Because the ratio of these terms did not vary significantly by garden, ecotype, or treatment (p > .05), all data were pooled for this analysis
Despite moderate differences in temperature between treatments at the TL garden (Table 2), A net did not vary significantly for any of the ecotypes with treatment or measurement temperature (p > .05, Figure 7, Table 5). However, the patterns reported above for V cmax(25) and J max(25) are reflected in mean A net values, such that the OTC treatment tended to produce marginally higher A net in TL and SAG ecotypes across measurement temperatures. This pattern was particularly notable for the SAG ecotype (p = .068) and contrasts with nearly identical mean A net values across treatments for the CF ecotype (p = .763). Rates of A net ranged from 9.6 to 13.6 μmol m−2 s−1 (Figure 7).
Figure 7.
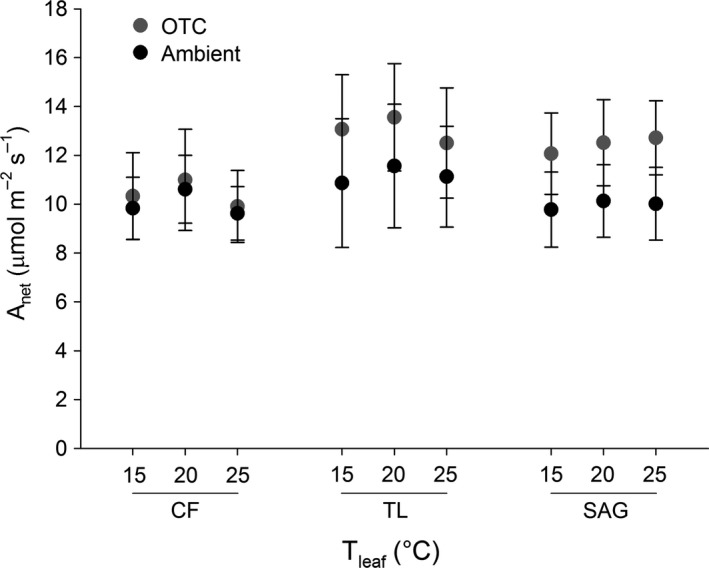
Mean ± SE net photosynthesis (A net) for each E. vaginatum ecotype (CF—Coldfoot, TL—Toolik Lake, SAG—Sagwon) and leaf measurement temperature (T leaf). Data are shown for the Toolik Lake garden only, as the treatment effect (i.e., degree of warming) was most effective at this site. Separate ANOVAs for each ecotype indicated no significant differences in A net with treatment or T leaf (p > .05)
Table 5.
Results of two‐way ANOVAs run separately for each ecotype (CF—Coldfoot, TL—Toolik Lake, SAG—Sagwon) at the TL transplant garden. Treatment, leaf temperature (T leaf), and their interaction were analyzed to determine their effect on Eriophorum vaginatum net photosynthesis (A net)
| Toolik Lake Garden | |||
|---|---|---|---|
| Ecotype | Main effect | F‐value | p |
| CF | Treatment | 0.0932 | .7628 |
| T leaf | 0.2360 | .7916 | |
| Treatment × T leaf | 0.0024 | .9976 | |
| TL | Treatment | 1.7947 | .1929 |
| T leaf | 0.0766 | .9265 | |
| Treatment × T leaf | 0.0754 | .9276 | |
| SAG | Treatment | 3.6488 | .0681 |
| T leaf | 0.0479 | .9533 | |
| Treatment × T leaf | 0.0090 | .9911 | |
In evaluating the relationship between R d and temperature, all but one comparison yielded comparable model fits between treatments within each garden (Figure 8a–f). The exception was for the CF ecotype at the TL garden, where the OTC treatment had higher rates of R d across all measurement temperatures, relative to the ambient treatment (Figure 8a). The Q 10 of R d computed for each curve ranged from 1.23 to 1.70 (Table 6). Examining Q 10 values for each ecotype along the gradient of warming described above did not yield any positive or negative trends (Table 6). The exponential function used to model the R d versus T leaf relationship yielded significant relationships (p < .05) in seven of 12 cases, but overall model fits were of poor quality (i.e., model SE > 30 and R 2 adj < .40 in the majority of cases) given high variance in the data (Figure 8, Table 6).
Figure 8.
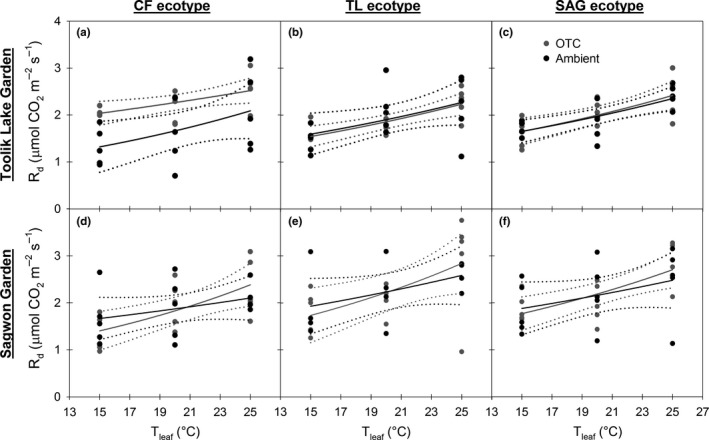
Dark respiration (R d) versus leaf temperature (T leaf) for each ecotype of Eriophorum vaginatum (CF—Coldfoot, TL—Toolik Lake, SAG—Sagwon) within each garden (a–f). Each panel shows data and models for both the ambient (black) and open‐top chamber treatments (gray). Solid lines show the exponential function used to model these relationships and dotted lines are 95% confidence intervals
Table 6.
Outputs of an exponential function used to model dark respiration (R d) versus leaf temperature for each ecotype (CF—Coldfoot, TL—Toolik Lake, SAG—Sagwon) of E. vaginatum and treatment (ambient or OTC—open‐top chamber) within each transplant garden. Also included is the computed Q 10 value for each model that was fit
| Ecotype | Treatment | Q 10 | Model SE | R 2 adj | F | p |
|---|---|---|---|---|---|---|
| Toolik Lake Garden | ||||||
| CF | Ambient | 1.58 | 0.65 | .15 | 3.5100 | .0837 |
| OTC | 1.23 | 0.30 | .28 | 6.5668 | .0236 | |
| TL | Ambient | 1.42 | 0.53 | .19 | 4.3130 | .0582 |
| OTC | 1.45 | 0.26 | .53 | 16.7464 | .0013 | |
| SAG | Ambient | 1.42 | 0.29 | .49 | 14.4374 | .0022 |
| OTC | 1.46 | 0.34 | .47 | 13.2927 | .0030 | |
| Sagwon Garden | ||||||
| CF | Ambient | 1.26 | 0.52 | .05 | 1.7321 | .2109 |
| OTC | 1.70 | 0.50 | .39 | 10.0976 | .0073 | |
| TL | Ambient | 1.34 | 0.60 | .11 | 2.3898 | .1532 |
| OTC | 1.63 | 0.70 | .27 | 6.0926 | .0282 | |
| SAG | Ambient | 1.32 | 0.66 | .07 | 2.1001 | .1710 |
| OTC | 1.52 | 0.42 | .43 | 11.6807 | .0046 | |
3.5. Relationship between g s and D
Negative relationships between g s and D were found for all three ecotypes, although only 11%–15% of the variation in the g s was explained by D (Figure 9). For CF and TL ecotypes, linear regression revealed similar slopes (−60.67 and −62.97, respectively), while the SAG ecotype had a shallower slope (−40.48), indicating less responsiveness of g s to changes in D (Figure 9). Of the measurements made when D was >1.5 kPa, 91% were made at a T leaf of 25°C.
Figure 9.
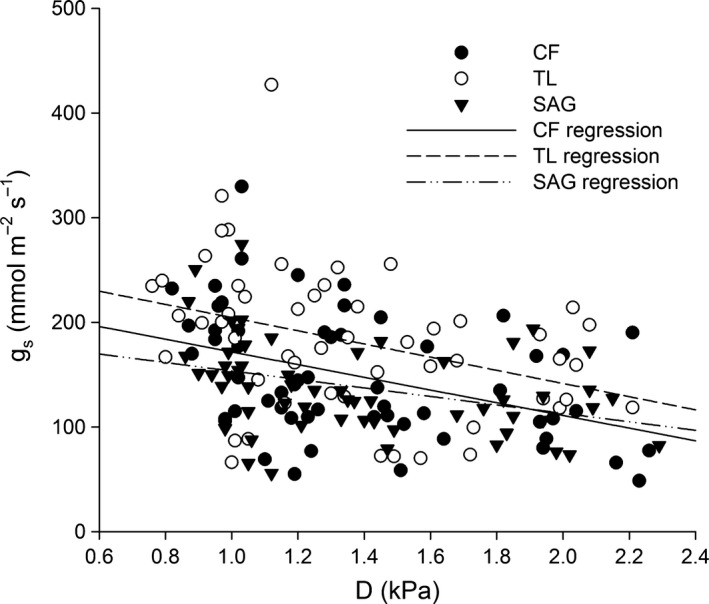
Linear regressions showing the relationship between stomatal conductance (g s) and vapor pressure deficit (D) for each ecotype of Eriophorum vaginatum (CF—Coldfoot, TL—Toolik Lake, SAG—Sagwon). Data within each ecotype were pooled across treatments and gardens and were extracted from gas exchange measurements made at 400 ppm CO 2. The equation for the CF ecotype (solid black circles, solid black line) was y = −60.67x + 232, R 2 adj = .15, for the TL ecotype (open circles, dashed line) was y = −62.97x + 267, R 2 adj = .11, and for the SAG ecotype (black triangles, dash‐dot line) was y = −40.48x + 194, R 2 adj = .12
4. DISCUSSION
4.1. Photosynthetic temperature responses
Contrary to expectations of detractive adjustments in photosynthesis for E. vaginatum given increased growth temperature, there was evidence of enhanced or no change in photosynthetic capacity with higher growth temperature. Enhancements were most pronounced for the northern ecotype (SAG), with substantial increases in both V cmax(25) and J max(25), between the coolest and warmest growing conditions. The SAG ecotype also had a marginally significant increase in A net with increased growth temperature. The most southern ecotype, CF, displayed evidence of photosynthetic homeostasis across growth temperatures, both in terms of photosynthetic capacity and A net. Responses of the mid‐latitude TL ecotype to increased growth temperature fell between that of the northern and southern ecotypes. These findings suggest that ecotypic specialization affects photosynthetic responses to growth temperature, although not in the ways initially expected. Over the range of warming provided in this experiment, there was no evidence of a photosynthetic underpinning to adaptational lag in E. vaginatum.
In many ways, photosynthetic responses to the treatments imposed in the present study showed a graduated response to increased temperature as a function of an ecotype's latitude of origin. While the range of temperatures to which tussocks were exposed was likely within or slightly below the historic range of variation for the CF ecotype, the OTC treatments provided warmer than average conditions for both the TL and SAG ecotypes. Although mean growing season temperatures over the past 15 years were similar between the Toolik Lake and Sagwon sites, photosynthetic data suggest that the SAG E. vaginatum ecotype is adapted to a local climate that has historically been different (possibly cooler and/or more variable) than at Toolik Lake. Unfortunately, historical temperature data from Alaska's north slope are limited to 2–4 years of data from the 1970s, and they provide an insufficient baseline for the region's climate given the short time period and high interannual variation (Haugen, 1982).
The shift in photosynthetic responses to increased growth temperature from homeostasis in the south to temperature acclimation in the north must be interpreted in light of the south to north decline in growing season temperature coupled with the degree of warming provided by the OTC treatments. In context, it is probable that photosynthetic enhancements with increased growth temperature exist for all three ecotypes given a moderate (at least up to ~2°C) temperature increase. However, the methodological limitations of the present study made it impossible to assess the effect of warming above historical conditions on the CF ecotype. Evidence of photosynthetic acclimation to increased growth temperature is consistent with much of the literature, although it was unexpected for a species adapted to a relatively cool climate (Berry & Björkman, 1980; Kattge & Knorr, 2007; Way & Yamori, 2014; Yamori et al., 2014). If the SAG E. vaginatum ecotype in the present study serves as a model for the species when exposed to moderately elevated growth temperature, relative to historical conditions, modest enhancements in carbon uptake should be expected. Certainly, further research is required to determine whether this is the case. It should also be noted that a moderate decline in g s with increased D was found for all E. vaginatum ecotypes. This indicates that photosynthesis may have been limited at a measurement temperature of 25°C, thereby masking additional photosynthetic enhancement. However, because little of the variation in g s was explained by D across ecotypes (R 2 adj = .11–.15), the effect is likely to be minor.
Potential enhancements in carbon gain contrast with the lower population growth rates observed for northern ecotypes transplanted into warmer, southern gardens (Chandler et al., 2015; McGraw et al., 2015). Additionally, Heskel et al. (2013) found that an increase in growth temperature of 5°C negatively affected A net for the TL E. vaginatum ecotype. Further, Souther et al. (2014) compiled data from six reciprocally transplanted E. vaginatum ecotypes and found that A net was maximized when TDD was lower (i.e., growing conditions were cooler) than at the home‐site, thereby suggesting the negative effects of a warming climate on E. vaginatum. These contrasting findings raise two possibilities: (1) Ecotypes of E. vaginatum do not allocate fixed carbon from enhanced photosynthesis to aboveground growth. Instead, respiratory costs, root or rhizome growth, and storage are potential avenues of carbon allocation; (2) The degree of warming provided by the present study was within the tolerance limits of photosynthesis for these E. vaginatum ecotypes, but warmer temperatures do lead to negative effects on growth. These possibilities can be investigated in part by considering findings related to the R d growth temperature response.
4.2. Respiratory temperature responses
Respiratory responses to increased growth temperature typically show evidence of thermal acclimation, such that warm‐grown plants have lower respiration rates than cold‐grown plants when measured at the same temperature, and these responses have previously been observed for E. vaginatum (Heskel et al., 2014; Kornfeld et al., 2013; Souther et al., 2014; van de Weg et al., 2013). Acclimation can result from either a decreased Q 10 (i.e., Type I acclimation—lower temperature responsiveness at high temperature) or a decrease in R d at both low and high temperature (i.e., Type II acclimation), and both of these responses are thought to be driven by substrate or adenylate availability (Atkin & Tjoelker, 2003). The present study shows that, with the exception of the CF ecotype grown at the TL garden, responses of R d to increased measurement temperature did not differ substantially between ambient and OTC treatments. In the case of the CF ecotype at the TL garden, R d was lower under ambient conditions, relative to the OTC treatment. Because TL‐ambient conditions were cooler than typical for the CF ecotype, the decrease in R d may represent an enzymatic limitation not experienced by the more cold‐adapted TL and SAG ecotypes.
Overall, there was no evidence of thermal acclimation in R d given the growth temperatures provided by this experiment. Because photosynthetic capacity was not negatively affected by increased growth temperature, respiratory substrate and adenylate availability likely remained high enough to maintain R d across treatments. However, multiple reports of respiratory acclimation to increased growth temperature in E. vaginatum (Heskel et al., 2014; Kornfeld et al., 2013; Souther et al., 2014) raises the likelihood that a sustained growth temperature 4–5°C warmer than ambient (as in Kornfeld et al., 2013; Heskel et al., 2014) is an important threshold for E. vaginatum that leads to physiological adjustment. These conclusions are consistent with the second hypothesis described above.
Across ecotypes, there was a high degree of overlap in modeled responses of R d to measurement temperature, as well as Q 10 values, suggesting little ecotypic variation in R d. These uniform responses are unlikely to differentially affect overall tussock carbon balance for different ecotypes. The Q 10 values for E. vaginatum across ecotypes and treatments were exceptionally low (1.23–1.70) relative to typical values for Arctic plants (2.56, Atkin & Tjoelker, 2003). This suggests that E. vaginatum is less responsive to increased growth temperature than many Arctic plants, an attribute likely to be beneficial in terms of plant carbon balance as Arctic temperatures continue to rise.
4.3. Photosynthetic capacity of E. vaginatum in context
While E. vaginatum V cmax(25) and J max(25), as well as E a of V cmax were within the range of values reported across functional types comprised of both temperate and Arctic plants (i.e., crops, deciduous tree, coniferous trees, herbs, grasses, sedges, deciduous shrubs), the E a of J max was at the lower end of reported values (Kattge & Knorr, 2007; Medlyn et al., 2002; Rogers, Serbin, Ely, Sloan, & Wullschleger, 2017). Eriophorum vaginatum's consistent ratio of J max(25) to V cmax(25), regardless of garden, ecotype, or treatment, indicated that J max and V cmax were similarly responsive to changes in growth temperature. Onoda, Hikosaka, and Hirose (2005) suggested that species without J max/V cmax plasticity in response to growth temperature may have photosynthetic rates that are limited by Rubisco carboxylation (V cmax) over the range of growth temperatures. Additionally, the slope of the relationship between J max(25) and V cmax(25) for E. vaginatum was 2.09, a value somewhat greater than those reported in synthesis studies focused principally on temperate species (slopes of 1.67 and 1.89, Medlyn et al., 2002; Kattge & Knorr, 2007). Recent work on seven species from Alaskan coastal tundra yielded a mean slope of 2.53, indicating that Arctic species likely maintain a higher ratio than temperate species (Rogers, Serbin et al., 2017).
Differences between temperate and Arctic plants show that Arctic species, including E. vaginatum, allocate more resources to light harvesting and electron transport, relative to temperate species (Rogers, Serbin et al., 2017). This may be an adaptation to life in the relatively low‐light growing conditions of the Arctic. Further, a high J max(25)/V cmax(25) should confer a physiological advantage under elevated CO2 because ribulose‐1,5‐bisphosphate (RuBP) regeneration will not limit A over a relatively wide range of C i (Rogers, Medlyn et al., 2017; Rogers, Serbin et al., 2017). Photosynthetic rates of Arctic plants should therefore experience high responsiveness to rising CO2, although further study is required to assess the magnitude of elevated J max/V cmax in the Arctic. As noted by Medlyn et al. (2002) and Rogers, Serbin et al. (2017), additional research is needed to develop a more robust understanding of photosynthetic capacity in plants from cold climates. Such data are of key importance, as Rogers, Serbin et al. (2017) recently demonstrated that terrestrial biosphere models underestimate Arctic plant photosynthetic capacity, largely due to lack of data.
4.4. Leaf morphology and nitrogen content
In addition to physiology, growth temperature often affects leaf morphology, such that plants grown under cooler temperature regimes have lower SLA, which may enhance photosynthesis, as it is often coupled with higher N content (Campbell et al., 2007; Yamori et al., 2005). Further, because approximately half a leaf's N content is associated with the photosynthetic apparatus (Evans, 1989; Evans & Seemann, 1989), an assessment of variation in Narea among ecotypes and treatments may explain some of the observed variation in photosynthetic capacity. Both SLA and Narea were invariant for E. vaginatum among ecotypes and between treatments. These findings indicate that any photosynthetic responses to growth temperature within each garden were not related to underlying variation in leaf morphology. However, the maintenance of low‐temperature enzyme capacity, as indicated by constant Narea with increased growth temperature, should support enhanced photosynthetic capacity and A net, although this response was only observed for the SAG ecotype. Overall, the Narea analysis suggests a lack of plasticity in enzyme concentration among the three ecotypes of E. vaginatum, and further research is merited to determine how leaf N affects photosynthetic capacity in this species.
5. CONCLUSIONS
Over the range of growth temperatures examined in the present study, no evidence of a physiological underpinning to adaptational lag was observed for E. vaginatum. Instead of detractive adjustments in photosynthesis with increased growth temperature, photosynthetic homeostasis or enhancement was observed, depending on an ecotype's latitude of origin. Contrary to expectations, the greatest enhancement in photosynthetic capacity and rate occurred in the northernmost ecotype. Further, no evidence of temperature acclimation was observed in measurements of R d, and all ecotypes responded similarly to variation in growth temperature. Together, these findings suggest that moderate enhancements in growth temperature (up to ~2°C) do not negatively affect the physiological performance of E. vaginatum. Despite neutral to modest enhancements in photosynthesis given moderate warming, this appears to confer no lasting advantage to E. vaginatum as growth temperature rises. Other experimental warming treatments indicate that warming of ~5°C above ambient over the course of 20 years is enough to shift the species composition of Alaska's tussock tundra toward a more shrub‐dominated ecosystem (Heskel et al., 2013), a pattern that has been documented throughout the rapidly warming Arctic (Tape, Sturm, & Racine, 2006). Within the moist tussock tundra, it is likely that E. vaginatum will eventually be unable to effectively compete with shrubs, although the present study shows there remains a window of time before negative physiological effects are evident.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
JLS designed and carried out the research, data analysis, and data interpretation, drafted the manuscript, and approved the final manuscript. NF conceived of the experimental design that made this study possible, maintained transplant gardens, generated the underlying idea for this study, aided in developing methods, provided comments on the manuscript, and approved the final manuscript. KH contributed substantially to data collection, provided comments on the manuscript, and approved the final manuscript. MLM and JT conceived of the experimental design that made this study possible, maintained transplant gardens, provided comments on the manuscript, and approved the final manuscript.
ACKNOWLEDGMENTS
Thanks for invaluable assistance in the field and lab to Tom Parker, Jen Chandler, and Katie Thompson. Thanks also to the Toolik Field Station staff for logistics support and to Salvatore Curasi for sharing meteorological data. Funding for this research was provided through the National Science Foundation (NSF/PLR 1418010 to NF, NSF/PLR 1417645 to MLM, NSF/PLR 1417763 to JT, and a Research Opportunity Award supplement), as well as a Cullen Fund Award from West Chester University's Department of Biology. Three anonymous reviewers provided useful comments on an earlier version of this manuscript.
Schedlbauer JL, Fetcher N, Hood K, Moody ML, Tang J. Effect of growth temperature on photosynthetic capacity and respiration in three ecotypes of Eriophorum vaginatum . Ecol Evol. 2018;8:3711–3725. https://doi.org/10.1002/ece3.3939
REFERENCES
- ACIA [Arctic Climate Impact Assessment] (2004). Impacts of a warming Arctic. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Aitken, S. N. , Yeaman, S. , Holliday, J. A. , Wang, T. , & Curtis‐McLane, S. (2008). Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evolutionary Applications, 1, 95–111. https://doi.org/10.1111/j.1752-4571.2007.00013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. T. , Panett, A. M. , & Mitchell‐Olds, T. (2012). Evolutionary and ecological responses to anthropogenic climate change. Plant Physiology, 160, 1728–1740. https://doi.org/10.1104/pp.112.206219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin, O. K. , Bruhn, D. , & Tjoelker, M. G. (2005). Response of plant respiration to changes in temperature: Mechanisms and consequences of variations in Q10 values and acclimation In Lambers H., & Ribas‐Carbo M. (Eds.), Plant respiration: From cell to ecosystem (pp. 95–135). Dordrecht, the Netherlands: Springer; https://doi.org/10.1007/1-4020-3589-6 [Google Scholar]
- Atkin, O. K. , & Tjoelker, M. G. (2003). Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science, 8, 343–351. https://doi.org/10.1016/S1360-1385(03)00136-5 [DOI] [PubMed] [Google Scholar]
- Bennington, C. C. , Fetcher, N. , Vavrek, M. C. , Shaver, G. R. , Cummings, K. J. , & McGraw, J. B. (2012). Home site advantage in two long‐lived arctic plant species: Results from two 30‐year reciprocal transplant studies. Journal of Ecology, 100, 841–851. https://doi.org/10.1111/j.1365-2745.2012.01984.x [Google Scholar]
- Bernacchi, C. J. , Singsaas, E. L. , Pimentel, C. , Portis, A. R. Jr , & Long, S. P. (2001). Improved temperature response functions for models of Rubisco‐limited photosynthesis. Plant, Cell and Environment, 24, 253–259. https://doi.org/10.1111/j.1365-3040.2001.00668.x [Google Scholar]
- Berry, J. , & Björkman, O. (1980). Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology, 31, 491–543. https://doi.org/10.1146/annurev.pp.31.060180.002423 [Google Scholar]
- Campbell, C. , Atkinson, L. , Zaragoza‐Castells, J. , Lundmark, M. , Atkin, O. , & Hurry, V. (2007). Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. New Phytologist, 176, 375–389. https://doi.org/10.1111/j.1469-8137.2007.02183.x [DOI] [PubMed] [Google Scholar]
- Chandler, J. L. , McGraw, J. B. , Bennington, C. , Shaver, G. R. , Vavrek, M. C. , & Fetcher, N. (2015). Tiller population dynamics of reciprocally transplanted Eriophorum vaginatum L. ecotypes in a changing climate. Population Ecology, 57, 117–126. https://doi.org/10.1007/s10144-014-0459-9 [Google Scholar]
- Chapin, F. S. III , & Shaver, G. R. (1985). Individualistic growth responses of tundra plant species to environmental manipulations in the field. Ecology, 66, 564–576. https://doi.org/10.2307/1940405 [Google Scholar]
- Chapin, F. S. III , Shaver, G. R. , Giblin, A. E. , Nadelhoffer, K. J. , & Laundre, J. A. (1995). Responses of Arctic tundra to experimental and observed changes in climate. Ecology, 76, 694–711. https://doi.org/10.2307/1939337 [Google Scholar]
- Diffenbaugh, N. S. , & Field, C. B. (2013). Changes in ecologically critical terrestrial climate conditions. Science, 341, 486–492. https://doi.org/10.1126/science.1237123 [DOI] [PubMed] [Google Scholar]
- Duursma, R. A. (2015). Plantecophys ‐ An R package for analysing and modelling leaf gas exchange data. PLoS ONE, 10, e0143346 https://doi.org/10.1371/journal.pone.0143346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duursma, R. A. (2016). plantecophys: Modelling and analysis of leaf gas exchange data, R package version 1.1‐8. Retrieved from https://cran.r-project.org/package=plantecophys
- EDCT [Environmental Data Center Team] (2017). Meteorological monitoring program at Toolik, Alaska. Toolik Field Station, Institute of Arctic Biology, University of Alaska, Fairbanks, AK. Retrieved from http://toolik.alaska.edu/edc/abiotic_monitoring/data_query.php
- Elmendorf, S. C. , Henry, G. H. R. , Hollister, R. D. , Björk, R. G. , Bjorkman, A. D. , Callaghan, T. V. , … Wookey, P. A. (2012). Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecology Letters, 15, 164–175. https://doi.org/10.1111/j.1461-0248.2011.01716.x [DOI] [PubMed] [Google Scholar]
- Etterson, J. R. , & Shaw, R. G. (2001). Constraint to adaptive evolution in response to global warming. Science, 294, 151–154. https://doi.org/10.1126/science.1063656 [DOI] [PubMed] [Google Scholar]
- Evans, J. R. (1989). Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia, 78, 9–19. https://doi.org/10.1007/BF00377192 [DOI] [PubMed] [Google Scholar]
- Evans, J. R. , & Seemann, J. R. (1989). The allocation of protein nitrogen in the photosynthetic apparatus: Costs, consequences and control In Briggs W. R. (Ed.), Photosynthesis (pp. 183–205). New York, NY: Alan R. Liss. [Google Scholar]
- Farquhar, G. D. , von Caemmerer, S. , & Berry, J. A. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149, 78–90. https://doi.org/10.1007/BF00386231 [DOI] [PubMed] [Google Scholar]
- Fetcher, N. , & Shaver, G. R. (1982). Growth and tillering patterns within tussocks of Eriophorum vaginatum . Holarctic Ecology, 5, 180–186. [Google Scholar]
- Fetcher, N. , & Shaver, G. R. (1983). Life histories of tillers of Eriophorum vaginatum in relation to tundra disturbance. Journal of Ecology, 71, 131–147. https://doi.org/10.2307/2259967 [Google Scholar]
- Fetcher, N. , & Shaver, G. R. (1990). Environmental sensitivity of ecotypes as a potential influence on primary productivity. The American Naturalist, 136, 126–131. https://doi.org/10.1086/285085 [Google Scholar]
- Haugen, R. K. (1982). Climate of remote areas in north‐central Alaska 1975–1979 summary. Hanover, NH: Cold Regions Research and Engineering Lab. [Google Scholar]
- Heskel, M. A. , Bitterman, D. , Atkin, O. K. , Turnbull, M. H. , & Griffin, K. L. (2014). Seasonality of foliar respiration in two dominant plant species from the Arctic tundra: Response to long‐term warming and short‐term temperature variability. Functional Plant Biology, 41, 287–300. https://doi.org/10.1071/FP13137 [DOI] [PubMed] [Google Scholar]
- Heskel, M. , Greaves, H. , Kornfeld, A. , Gough, L. , Atkin, O. K. , Turnbull, M. H. , … Griffin, K. L. (2013). Differential physiological responses to environmental change promote woody shrub expansion. Ecology and Evolution, 3, 1149–1162. https://doi.org/10.1002/ece3.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattge, J. , & Knorr, W. (2007). Temperature acclimation in a biochemical model of photosynthesis: A reanalysis of data from 36 species. Plant, Cell and Environment, 30, 1176–1190. https://doi.org/10.1111/j.1365-3040.2007.01690.x [DOI] [PubMed] [Google Scholar]
- Kornfeld, A. , Heskel, M. , Atkin, O. K. , Gough, L. , Griffin, K. L. , Horton, T. W. , & Turnbull, M. H. (2013). Respiratory flexibility and efficiency are affected by simulated global change in Arctic plants. New Phytologist, 197, 1161–1172. https://doi.org/10.1111/nph.12083 [DOI] [PubMed] [Google Scholar]
- Linhart, Y. B. , & Grant, M. C. (1996). Evolutionary significance of local genetic differentiation in plants. Annual Review of Ecology and Systematics, 27, 237–277. https://doi.org/10.1146/annurev.ecolsys.27.1.237 [Google Scholar]
- Lloyd, J. , & Taylor, J. A. (1994). On the temperature dependence of soil respiration. Functional Ecology, 8, 315–323. https://doi.org/10.2307/2389824 [Google Scholar]
- Mark, A. F. , Fetcher, N. , Shaver, G. R. , & Chapin, F. S. III (1985). Estimated ages of mature tussocks of Eriophorum vaginatum along a latitudinal gradient in central Alaska, U.S.A. Arctic and Alpine Research, 17, 1–5. https://doi.org/10.2307/1550957 [Google Scholar]
- McGraw, J. B. , Turner, J. B. , Souther, S. , Bennington, C. C. , Vavrek, M. C. , Shaver, G. R. , & Fetcher, N. (2015). Northward displacement of optimal climate conditions for ecotypes of Eriophorum vaginatum L. across a latitudinal gradient in Alaska. Global Change Biology, 21, 3827–3835. https://doi.org/10.1111/gcb.12991 [DOI] [PubMed] [Google Scholar]
- Medlyn, B. E. , Dreyer, E. , Ellsworth, D. , Forstreuter, M. , Harley, P. C. , Kirschbaum, M. U. F. , … Loustau, D. (2002). Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell and Environment, 25, 1167–1179. https://doi.org/10.1046/j.1365-3040.2002.00891.x [Google Scholar]
- NRCS [National Resource Conservation Service] (2017). Daily SNOTEL Data Report ‐ Historic, Coldfoot (SNOTEL 958) and Sagwon (SNOTEL 1183). National Water and Climate Center, U.S. Department of Agriculture. Retrieved from https://www.wcc.nrcs.usda.gov/snow/snotel-data.html
- Oberbauer, S. F. , Tweedie, C. E. , Welker, J. M. , Fahnestock, J. T. , Henry, G. H. R. , Webber, P. J. , … Starr, G. (2007). Tundra CO2 fluxes in response to experimental warming across latitudinal and moisture gradients. Ecological Monographs, 77, 221–238. https://doi.org/10.1890/06-0649 [Google Scholar]
- Onoda, Y. , Hikosaka, K. , & Hirose, T. (2005). The balance between RuBP carboxylation and RuBP regeneration: A mechanism underlying the interspecific variation in acclimation of photosynthesis to seasonal change in temperature. Functional Plant Biology, 32, 903–910. https://doi.org/10.1071/FP05024 [DOI] [PubMed] [Google Scholar]
- Parker, T. C. , Tang, J. , Clark, M. B. , Moody, M. M. , & Fetcher, N. (2017). Ecotypic differences in the phenology of the tundra species Eriophorum vaginatum reflect sites of origin. Ecology and Evolution, 7, 9775–9786. https://doi.org/10.1002/ece3.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2016). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rogers, A. , Medlyn, B. E. , Dukes, J. S. , Bonan, G. , von Caemmerer, S. , Dietze, M. C. , … Zaehle, S. (2017). A roadmap for improving the representation of photosynthesis in Earth system models. New Phytologist, 213, 22–42. https://doi.org/10.1111/nph.14283 [DOI] [PubMed] [Google Scholar]
- Rogers, A. , Serbin, S. P. , Ely, K. S. , Sloan, V. L. , & Wullschleger, S. D. (2017). Terrestrial biosphere models underestimate photosynthetic capacity and CO2 assimilation in the Arctic. New Phytologist, 216, 1090–1103. https://doi.org/10.1111/nph.14740 [DOI] [PubMed] [Google Scholar]
- Sage, R. F. , & Kubien, D. S. (2007). The temperature response of C3 and C4 photosynthesis. Plant, Cell and Environment, 30, 1086–1106. https://doi.org/10.1111/j.1365-3040.2007.01682.x [DOI] [PubMed] [Google Scholar]
- Shaver, G. R. , Fetcher, N. , & Chapin, F. S. III (1986). Growth and flowering in Eriophorum vaginatum: Annual and latitudinal variation. Ecology, 67, 1524–1535. https://doi.org/10.2307/1939083 [Google Scholar]
- Souther, S. , Fetcher, N. , Fowler, Z. , Shaver, G. R. , & McGraw, J. B. (2014). Ecotypic differentiation in photosynthesis and growth of Eriophorum vaginatum along a latitudinal gradient in the Arctic tundra. Botany‐Botanique, 92, 551–561. https://doi.org/10.1139/cjb-2013-0320 [Google Scholar]
- Tape, K. , Sturm, M. , & Racine, C. (2006). The evidence for shrub expansion in Northern Alaska and the Pan‐Arctic. Global Change Biology, 12, 686–702. https://doi.org/10.1111/j.1365-2486.2006.01128.x [Google Scholar]
- van de Weg, M. J. , Fetcher, N. , & Shaver, G. R. (2013). Response of dark respiration to temperature in Eriophorum vaginatum from a 30‐year‐old transplant experiment in Alaska. Plant Ecology and Diversity, 6, 377–381. [Google Scholar]
- Walker, M. D. , Wahren, C. H. , Hollister, R. D. , Henry, G. H. R. , Ahlquist, L. E. , Alatalo, J. M. , … Wookey, P. A. (2006). Plant community responses to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences of the United States of America, 103, 1342–1346. https://doi.org/10.1073/pnas.0503198103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way, D. A. , & Yamori, W. (2014). Thermal acclimation of photosynthesis: On the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynthesis Research, 119, 89–100. https://doi.org/10.1007/s11120-013-9873-7 [DOI] [PubMed] [Google Scholar]
- Yamori, W. , Hokosaka, K. , & Way, D. A. (2014). Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynthesis Research, 119, 101–117. https://doi.org/10.1007/s11120-013-9874-6 [DOI] [PubMed] [Google Scholar]
- Yamori, W. , Noguchi, K. , & Terashima, I. (2005). Temperature acclimation of photosynthesis in spinach leaves: Analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant, Cell and Environment, 28, 536–547. https://doi.org/10.1111/j.1365-3040.2004.01299.x [Google Scholar]


