Abstract
Background
At a group level, hangover severity during the day has been described to follow an inverted U‐shaped curve, with gradually increasing severity scores that, after reaching a peak, gradually decrease toward zero. The aim of this study was to examine if and how individual drinkers' hangover severity scores vary during the day.
Methods
Data from a survey (Penning et al., 2012) in which 727 drinkers reported on their latest alcohol hangover were reanalyzed. The temporal pattern of each individual's hangover was first categorized as belonging to 1 of 6 types based on predefined temporal characteristics.
Results
Three dominant hangover patterns emerged as comprising more than 95% of the sample: (i) a continuous decline hangover (Severity Type 1 hangover, 54.5%), (ii) a steady state hangover (Severity Type 2 hangover, 19.1%), and (iii) an inverted U‐shaped curve hangover (Severity Type 3 hangover, 21.8%). Of these 3 patterns, Severity Type 2 hangovers are associated with significantly less alcohol consumption and with having the lowest severity scores of individual hangover symptoms. Severity Type 1 hangovers are associated with having the highest severity of individual hangover symptoms. In line with significantly lower levels of alcohol consumption, Severity Type 2 hangovers were significantly more often observed in women when compared to men. Severity Type 1 hangovers were significantly more common in men than in women. Severity Type 3 hangovers, characterized by the increased presence of gastrointestinal complaints, were equally commonly experienced in men and women.
Conclusions
This study revealed that the temporal pattern of hangover severity can follow marked interindividual variability. Three common temporal patterns were identified, which are uniquely related to the amount of alcohol consumed and the presence and severity of different individual hangover symptoms. Better understanding of individual differences in hangover typology may help to delineate mechanisms underlying alcohol hangover.
Keywords: Hangover, Severity, Patterns, Temporality, Symptoms, Alcohol
The alcohol hangover refers to the combination of mental and physical symptoms, experienced the day after a single episode of heavy drinking, starting when blood alcohol concentration approaches zero (Van Schrojenstein Lantman et al., 2016). Research has identified as many as 47 different symptoms that can be experienced during the alcohol hangover state (Penning et al., 2012). A recent study investigated the presence and severity of the most common hangover symptoms among 1,837 social drinkers (Van Schrojenstein Lantman et al., 2017). Sleepiness, being tired, thirst, and concentration problems were the most frequently reported hangover symptoms and also reached the highest severity scores. The 4 symptoms with the biggest combined impact on mood, cognitive performance, and physical functioning were being tired, sleepiness, concentration problems, and headache.
Overall hangover severity is commonly assessed using 1 of 3 currently used scales (Penning et al., 2013; Rohsenow et al., 2007; Slutske et al., 2003). In all 3 scales, individual symptoms are scored with the sum or average representing the overall hangover severity score. Alternatively, single item scales can be used that directly assess overall hangover severity. For example, a participant rates their overall hangover on a scale ranging from 0 (absent) to 10 (extreme) (e.g., Hogewoning et al., 2016).
Single time assessments are an effective way to provide instant, ongoing assessments of the hangover state that can be related to biomarkers or cognitive performance outcomes. Usually, the presence and severity of hangover symptoms are assessed at a fixed time point such as directly upon waking, or immediately prior to collecting cognitive performance data and/or bioassay sampling. “Time locking” data in this way allow examination of the interindividual relationship between hangover, performance, and biomarkers. One limitation of this approach, however, is that it does not provide information regarding intraindividual temporal fluctuations of hangover (symptom) severity scores across the day.
In Ylikahri et al.1974 conducted one of the few studies assessing overall hangover severity at multiple time points throughout the day. Data from 23 healthy male subjects who consumed alcohol (1.5 g/kg body weight) were analyzed. The overall mean hangover severity score was highest 12 to 14 hours after cessation of alcohol consumption and then gradually deceased. The hangover was no longer present 16 to 21 hours after drinking. On average, overall hangover severity was elevated during the first hours after waking and then gradually returned to zero. It should be taken into account, however, that this inverted U‐shaped curve represents an average for the 23 participants. It is unknown whether some drinkers had different severity patterns throughout the hangover day.
Theoretically, several hangover severity patterns can be proposed. The different severity types are graphically represented in Fig. 1.
Figure 1.
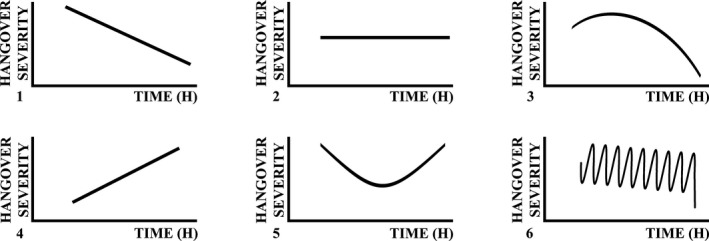
The 6 predicted hangover severity patterns. Note: If an observed pattern did not match Severity Types 1 to 5, it was allocated to Severity Type 6.
Ylikahri and colleagues (1974) found that on average, hangover severity scores followed the temporal pattern of Severity Type 3 hangover. However, other patterns are possible. Based on anecdotal evidence, it may also be possible that, in other drinkers, severity scores remain stable throughout the hangover day (Severity Type 2 hangover) or are very high on waking and then gradually decrease (Severity Type 1 hangover). As the time since stopping drinking increases throughout the hangover day, it is less likely that severity scores will gradually intensify with time (Severity Types 4 and 5).
The aim of this study was to examine possible differences in the temporal patterns of overall hangover severity across the day. To that end, a large database was examined to identify possible different types of severity patterns. Subsequent analyses aimed to determine in what respect(s) drinkers with different severity patterns differed from each other (e.g., in the amount of alcohol consumed, or the type and severity of individual hangover symptoms experienced).
Materials and Methods
Students from Utrecht University were invited to complete a survey on alcohol consumption and their latest alcohol hangover (for a detailed description of the methodology, see Penning et al., 2012). Informed consent was obtained from all subjects; no formal ethics approval was required to conduct this type of survey research, according to the Central Committee on Research Involving Human Subjects (CCMO).
Dutch students (N = 1,410) completed the original survey on alcohol consumption (Penning et al., 2012). Of them, 791 (56.1%) reported having had a hangover during the past month.
Data were collected on the number of alcoholic drinks consumed per week (a drink contains 10 g of alcohol in the Netherlands), number of alcoholic drinks consumed before the last day with a hangover in the last month, and how many hangovers usually experienced per month. In addition, total hours of sleep and hangover severity for the last hangover in the past month were assessed. The severity of each of 47 individual hangover symptoms was scored on a scale ranging from 0 (absent) to 10 (extreme). For these 47 individual symptoms, the score reflected a single overall severity score for the hangover day. Using the symptoms present in the Alcohol Hangover Severity Scale (Penning et al., 2013), an overall hangover severity was computed.
More pertinent to this study, in addition to the above measures, hangover severity was measured on a single scale and reported for every 2 hours after waking up (starting 4 am until midnight). At each time point, a participant could rate his hangover severity on a scale ranging from 0 (absent) to 10 (extreme).
Allocating Individual Hangover Severity Pattern to Severity Types 1 to 6
As the gathered data do not allow for a statistical approach to cluster drinkers with different hangover severity type patterns, an alternative methodology was developed. For each subject, their overall hangover severity scores over time were plotted. Scores reported before 8 am were seldom made and, therefore, omitted from the graphs. By visual inspection, each individual pattern was allocated to 1 of 6 possible severity types, described below (see Fig. 1).
Severity Type 1 hangover comprises a pattern of steadily declining hangover severity scores. To be allocated to the Severity Type 1 hangover, the pattern should be present for at least 5 subsequent time points, and the difference between the highest and lowest severity score should be at least 3 (of 10).
Severity Type 2 hangover is characterized by a steady stable severity score over time. To be allocated to the Severity Type 2 hangover, the difference between the highest and lowest severity score should not be >3 (of 10).
Severity Type 3 hangover follows an inverted U‐shaped curve. Hangover severity scores gradually increase, reach a maximum, and then gradually decrease. Patterns are allocated to Severity Type 3 hangover if (i) both before and after the maximum score at least 2 severity scores are 2 or more points lower than the maximum severity score, or if (ii) both before and after the maximum score if there is at least 1 severity score 3 points lower than the maximum score.
Severity Type 4 hangover is the opposite as Severity Type 1 hangover. Severity Type 4 hangover comprises a pattern of steadily increasing hangover severity scores. To be allocated to the Severity Type 4 hangover, the pattern should be present for at least 5 subsequent time points, and the difference between the highest and lowest severity score should be at least 3 (of 10).
Severity Type 5 hangover follows a U‐shaped curve, that is, the opposite of Severity Type 3 hangover. Hangover severity scores gradually decrease, reach a minimum, and then gradually increase. Patterns are allocated to Severity Type 5 hangover if (i) both before and after the minimum score at least 2 severity scores are at least 2 or more points higher than the minimum severity score, or if (ii) both before and after the maximum score if there is at least 1 severity score 3 points higher than the minimum score.
Severity Type 6 hangover comprises all patterns that do not fit the definition of Severity Types 1 to 5.
Statistical Analysis
Subjects were included in the analyses if they were 18 to 30 years old and did not use drugs on their latest heavy drinking occasion that resulted in a hangover. Statistical analyses were conducted using SPSS (version 24.0; IBM Corp., Armonk, NY). First, the percentage of occurrence of the different severity types was computed (see Table 1). Demographics, drinking characteristics, and the severity of individual hangover symptoms of the 3 most dominant hangover severity types were compared (see Table 2). Data from the 3 severity type groups were compared with ANOVA, or in case the data were not normally distributed, an independent sample Mann–Whitney U‐test was used. Percentages were compared using an “N‐1” chi‐squared test. The “N‐1” chi‐squared test is comparable to Pearson's chi‐squared test, however, with “N‐1” instead of “N” used in the formula, and no Yates's adjustment for continuity. With larger sample sizes, the results of the 2 tests are very similar; however, in contrast to the Pearson's chi‐squared test, the “N‐1” chi‐squared test also allows analyzing small total sample sizes (<20). Differences were considered significant if p < 0.05.
Table 1.
Frequency of Occurrence of Hangover Severity Types 1 to 6
| Severity Type | Frequency | Percent | Cumulative percent | Men (%) N = 224 | Women (%) N = 501 |
|---|---|---|---|---|---|
| 1 (a continuous decline hangover) | 396 | 54.5 | 54.5 | 64.7 | 49.9* |
| 2 (a steady state hangover) | 139 | 19.1 | 73.6 | 11.6 | 22.4* |
| 3 (an inverted U‐shaped curve hangover) | 159 | 21.8 | 95.4 | 19.6 | 23.0 |
| 4 (a continuous increase hangover) | 17 | 2.3 | 97.7 | 1.3 | 2.8 |
| 5 (a U‐shaped curve hangover) | 12 | 1.7 | 99.4 | 2.2 | 1.4 |
| 6 (other patterns) | 4 | 0.6 | 100.0 | 0.4 | 0.6 |
| Overall | 727 | 100.0 | 100.0 | 100.0 | 100.0 |
Significant differences (p < 0.05) between men and women are indicated by *.
Table 2.
Characteristics of Social Drinkers with Hangover Severity Types 1, 2, or 3
| Overall (n = 727) | Severity Type 1 (n = 396) (a continuous decline hangover) | Severity Type 2 (n = 139) (a steady state hangover) | Severity Type 3 (n = 159) (an inverted U‐shaped curve hangover) | |
|---|---|---|---|---|
| Male/female ratio (%) | 30.9/59.1 | 36.7/63.3 | 18.8/81.2 | 27.7/72.3 |
| Age (years) | 20.0 (2.1) | 20.1 (2.1) | 19.7 (2.0) | 20.2 (2.2) |
| Height (m) | 1.74 (0.12) | 1.75 (0.14) | 1.73 (0.08) | 1.75 (0.08) |
| Weight (kg) | 66.5 (9.8) | 67.3 (10.1) | 64.9 (9.6) | 66.2 (9.1) |
| Number of cigarettes (per day) | 1.2 (3.5) | 1.3 (3.7) | 1.2 (3.4) | 1.2 (3.3) |
| Number of alcoholic drinks (per week) | 17.1 (14.7) | 18.7 (16.1) | 12.0 (8.8)* | 17.3 (13.9)** |
| Number of hangovers (per month) | 2.5 (2.2) | 2.7 (2.6) | 1.9 (1.6)* | 2.5 (1.8)** |
| Number of alcoholic drinks consumed on evening before the hangover day | 10.7 (5.6) | 11.4 (5.8) | 8.7 (3.9)* | 10.5 (5.6)** |
| Total sleep time (h) | 6.4 (2.0) | 6.5 (2.0) | 6.3 (2.0) | 6.5 (2.0) |
| Alcohol Hangover Severity Scale score | 3.1 (1.6) | 2.2 (1.7)* | 3.2 (1.4)** |
Significant differences (p < 0.05) between Severity Types 1 and 2 are indicated by *.
Significant differences (p < 0.05) between Severity Types 2 and 3 are indicated by **.
No significant differences (p < 0.05) were found between Severity Types 1 and 3.
Applying the Penning and colleagues' (2012) symptom categorization, mean (SD) factor scores were computed for Severity Types 1, 2, and 3 (see Table 3). Ten of the original factors were considered; factor 11 (suicidal thoughts, 1 item) was omitted as this is seldom reported. Mean (SD) of individual symptoms is listed in Table 4. Severity Types 1, 2, and 3 were statistically compared using an independent sample Mann–Whitney U‐test. To correct for multiple comparisons, the significance level was set at p < 0.005.
Table 3.
Mean (SD) Score of Hangover Symptom Factors for Hangover Severity Types 1, 2, and 3
| Factor | Severity Type 1 (a continuous decline hangover) | Severity Type 2 (a steady state hangover) | Severity Type 3 (an inverted U‐shaped curve hangover) |
|---|---|---|---|
| 1 (drowsiness) | 5.4 (2.3) | 4.3 (2.6)* , ** | 5.5 (2.3) |
| 2 (cognitive problems) | 2.6 (2.0) | 1.7 (1.9)* , ** | 2.8 (2.1) |
| 3 (disturbed water balance) | 2.4 (1.7) | 1.7 (1.6)* , ** | 2.4 (1.5) |
| 4 (mood disturbances) | 0.8 (1.2) | 0.6 (0.9) | 0.9 (1.2) |
| 5 (balance problems) | 1.6 (2.0) | 1.1 (1.8)* , ** | 1.7 (1.8) |
| 6 (gastrointestinal problems) | 2.6 (2.0) | 1.5 (1.8)* , ** | 3.0 (2.1) |
| 7 (respiratory and cardiovascular problems) | 0.6 (1.3) | 0.4 (1.5)** | 0.7 (1.5) |
| 8 (impulsivity and blunted affect) | 1.2 (1.6) | 0.7 (1.3)* , ** | 1.3 (1.5) |
| 9 (vomiting and feeling guilty) | 1.7 (1.8) | 1.3 (2.0)* , ** | 1.9 (2.0) |
| 10 (headache) | 2.7 (1.9) | 1.7 (1.7)* , ** | 2.9 (2.0) |
Significant differences (p < 0.005) between Severity Types 1 and 2 are indicated by *.
Significant differences (p < 0.005) between Severity Types 2 and 3 are indicated by **.
No significant differences (p < 0.005) were found between Severity Types 1 and 3.
Symptom severity scores were grouped and averaged according to the factor analysis conducted by Penning and colleagues (2012). Severity scores between hangover Severity Types 1, 2, and 3 were statistically compared using an independent sample Mann–Whitney U‐test. To correct for multiple comparisons, the significance level was set at p < 0.005.
Table 4.
Mean (SD) Score of Individual Hangover Symptoms for Hangover Severity Types 1, 2, and 3
| Factor | Symptoms | Severity Type 1 (a continuous decline hangover) | Severity Type 2 (a steady state hangover) | Severity Type 3 (an inverted U‐shaped curve hangover) |
|---|---|---|---|---|
| 1 (drowsiness) | Drowsiness | 5.9 (2.8) | 4.4 (3.1)* , ** | 6.0 (3.0) |
| Fatigue | 6.6 (2.5) | 5.5 (2.8)* , ** | 6.9 (2.5) | |
| Sleepiness | 5.7 (3.0) | 4.8 (3.0)* , ** | 5.9 (3.0) | |
| Apathy | 4.3 (3.3) | 3.2 (3.2)* , ** | 4.5 (3.3) | |
| Weakness | 4.6 (3.2) | 3.4 (3.0)* , ** | 4.6 (3.0) | |
| 2 (cognitive problems) | Confusion | 1.0 (2.1) | 0.8 (2.1) | 1.0 (2.1) |
| Disorientation | 1.5 (2.4) | 0.9 (2.1) | 1.5 (2.4) | |
| Increased reaction time | 3.5 (2.8) | 2.3 (2.6)* , ** | 3.8 (2.8) | |
| Reduced alertness | 3.8 (2.8) | 2.7 (2.7)* , ** | 4.0 (2.8) | |
| Concentration problems | 4.1 (3.1) | 2.9 (2.9)* , ** | 4.2 (3.1) | |
| Memory problems | 2.3 (2.9) | 1.3 (2.5)* , ** | 2.8 (3.3) | |
| Clumsiness | 2.3 (2.8) | 1.3 (2.3)* , ** | 2.5 (2.7) | |
| 3 (disturbed water balance) | Muscle pain | 1.4 (2.6) | 0.8 (2.0) | 1.4 (2.5) |
| Dry mouth | 5.3 (3.2) | 4.0 (3.4)* , ** | 5.1 (3.1) | |
| Thirst | 6.0 (2.9) | 4.6 (3.2)* , ** | 6.1 (3.1) | |
| Tremor | 2.0 (2.8) | 1.0 (2.3)* , ** | 2.1 (2.8) | |
| Shivering | 1.5 (2.5) | 0.9 (2.1)* | 1.7 (2.7) | |
| Sweating | 1.4 (2.4) | 0.7 (1.6)** | 1.6 (2.3) | |
| Hot/cold flashes | 0.9 (2.1) | 0.7 (1.9) | 1.1 (2.2) | |
| Nystagmus | 0.7 (1.8) | 0.5 (1.6) | 0.6 (1.6) | |
| 4 (mood disturbances) | Depression | 0.7 (1.8) | 0.4 (1.5)* | 0.9 (1.9) |
| Anxiety | 0.2 (0.8) | 0.1 (0.7) | 0.3 (1.2) | |
| Anger | 0.3 (1.3) | 0.2 (0.8) | 0.3 (0.9) | |
| Agitation | 2.1 (2.6) | 1.5 (2.2) | 2.2 (2.7) | |
| 5 (balance problems) | Balance problems | 1.6 (2.5) | 1.1 (2.1) | 1.8 (2.6) |
| Dizziness | 2.3 (2.9) | 1.4 (2.5)** | 2.2 (2.8) | |
| Vertigo | 2.1 (2.9) | 1.4 (2.5)* , ** | 2.4 (2.9) | |
| Tinnitus | 0.7 (1.7) | 0.5 (1.5) | 0.5 (1.3) | |
| 6 (gastrointestinal problems) | Nausea | 5.0 (3.2) | 3.0 (3.2)* , ** | 5.6 (3.1)*** |
| Stomach pain | 2.1 (2.8) | 1.2 (2.2)* , ** | 2.1 (2.9) | |
| Gastrointestinal complaints | 2.3 (3.0) | 1.3 (2.3)* , ** | 2.8 (3.1) | |
| Gastritis | 1.0 (2.2) | 0.6 (1.7)** | 1.4 (2.4)*** | |
| 7 (respiratory and cardiovascular problems) | Palpitations | 0.7 (1.8) | 0.5 (1.8)** | 0.9 (2.0) |
| Heart pounding | 0.8 (1.8) | 0.5 (1.8) | 0.9 (2.0) | |
| Respiratory problems | 0.3 (1.1) | 0.3 (1.2) | 0.4 (1.2) | |
| 8 (impulsivity and blunted affect) | Restlessness | 1.7 (2.6) | 1.1 (2.4) | 1.5 (2.5) |
| Blunted affect | 1.3 (2.4) | 0.5 (1.4)* | 1.1 (2.2) | |
| Impulsivity | 0.8 (2.0) | 0.6 (1.8) | 1.1 (2.2) | |
| Loss of taste | 1.1 (2.1) | 0.6 (1.7) | 1.3 (2.3) | |
| 9 (vomiting and feeling guilty) | Vomiting | 1.2 (2.7) | 0.9 (2.7) | 1.5 (3.1) |
| Regret | 1.3 (2.5) | 0.9 (2.2) | 1.1 (2.2) | |
| Guilt | 1.0 (2.1) | 0.7 (2.1) | 1.2 (2.4) | |
| Reduced appetite | 3.3 (3.4) | 2.6 (3.2) | 3.8 (3.5) | |
| 10 (headache) | Headache | 5.4 (3.0) | 3.6 (2.9)* , ** | 5.5 (3.1) |
| Photo‐sensitivity | 1.3 (2.3) | 0.8 (1.7)** | 1.7 (2.6) | |
| Audio‐sensitivity | 1.3 (2.3) | 0.9 (1.8) | 1.6 (2.4) |
Symptoms are grouped according to the factor analysis conducted by Penning and colleagues (2012).
Severity scores between hangover Severity Types 1, 2, and 3 were statistically compared using an independent sample Mann–Whitney U‐test. To correct for multiple comparisons, statistical significance was assumed if p < 0.005.
Significant differences (p < 0.005) between Severity Types 1 and 2 are indicated by *.
Significant differences (p < 0.005) between Severity Types 2 and 3 are indicated by **.
Significant differences (p < 0.005) between Severity Types 1 and 3 are indicated by ***.
Results
Students (N = 727) with a past month hangover (91.9%) completed the questionnaire. Figure 2 A shows the average overall hangover severity over time for all drinkers together. Table 1 summarizes the frequency of occurrence of hangover Severity Types 1 to 6.
Figure 2.
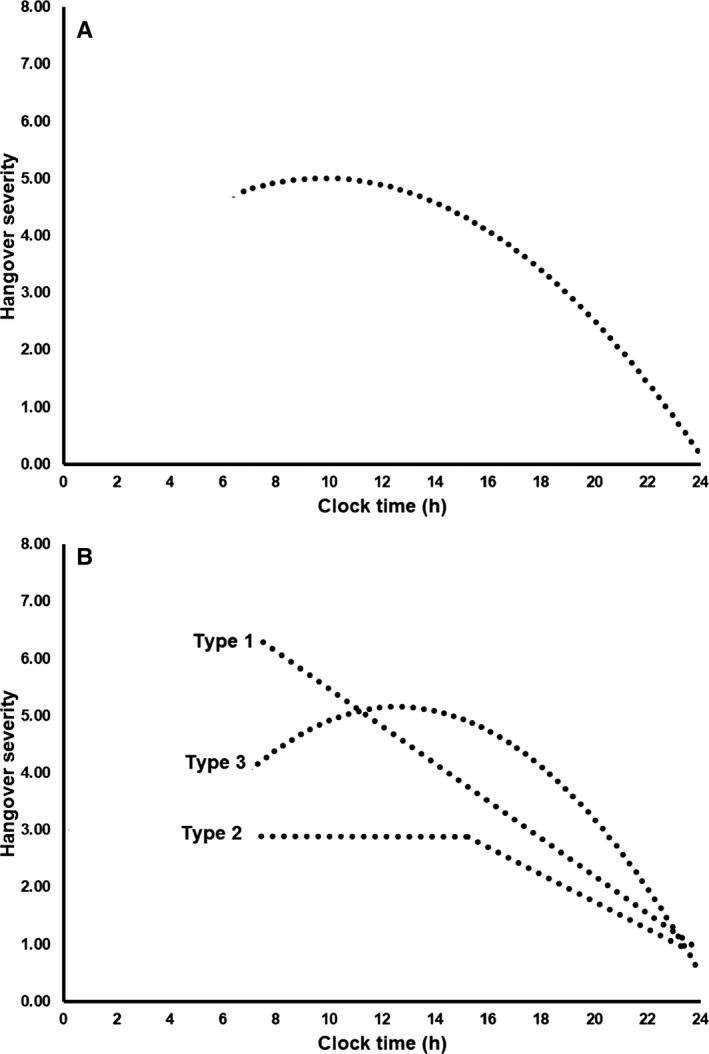
Variability in hangover severity during the day. Best fitting lines are shown. Panel A shows the overall average hangover severity score for all 727 drinkers. Panel B gives a graphical representation of the 3 most common hangover severity patterns over time. Lines represent Severity Type 1 hangover “continuous decline”; Severity Type 2 hangover “steady state hangover”; and (3) Severity Type 3 hangover “inverted U‐shaped curve hangover.”
As is evident from Table 1, Severity Type 1, a continuous decline hangover (54.5%), Severity Type 2, a steady state hangover (19.1%), and Severity Type 3, an inverted U‐shaped curve hangover (21.8%) were most commonly observed. A graphical representation of these 3 Severity Types is given in Fig. 2 B. Together, 95.5% of the participants were allocated to 1 of these 3 Severity Types. Given this, we did not further analyze Severity Types 4, 5, and 6.
The severity of 3 predominant hangover patterns converges at 24 hours on the drinking day and shows similar patterns of reduced severity in the final 6 to 8 hours. Prior to this, there are marked temporal differences (Fig. 2 B).
Severity Type 1 hangovers were significantly more frequently reported by men than women (χ 2 = 13.653, df = 1, p = 0.0002). Severity Type 2 hangovers were significantly more frequently reported by women when compared to men (χ 2 = 11.686, df = 1, p = 0.0006). The area under the curve in Severity Type 2 hangovers is clearly lower, consistent with the observation that, on average, women consumed significantly less alcohol than men (means of 9.0 vs. 14.5 alcoholic drinks on the evening before their latest hangover, t = 13.3, df = 1,723, p = 0.0001). No significant gender differences were found for the other severity types.
Table 2 summarizes the demographics of participants that were allocated to hangover Severity Types 1, 2, and 3. Participants who were allocated to Severity Type 2 hangover reported consuming significantly less alcohol on the evening before the hangover, and a significantly lower overall hangover severity score when compared to participants that were allocated to hangover Severity Types 1 or 3. They further reported significantly lower amounts of weekly alcohol consumption and experienced significantly fewer hangovers per month. No significant differences were found between hangover Severity Types 1 and 3.
The 47 hangover symptoms were grouped according to the factor analysis by Penning and colleagues (2012). Mean (SD) factor scores are summarized in Table 3, and the mean (SD) for each individual hangover symptom is summarized in Table 4. Tables 3 and 4 compare the scores of the 3 dominant Severity Types 1, 2, and 3, applying a cutoff value of p < 0.005 for statistical significance to correct for multiple comparisons.
Overall, individual symptom scores were lower for drinkers with Severity Type 2 hangovers than those with Severity Type 1 and Severity Type 3 hangovers. As is evident from Tables 3 and 4, across factors, these differences were often statistically significant. Significant differences between drinkers with Severity Type 1 and Severity Type 3 hangovers were not found. For both symptoms, the severity scores were higher among drinkers with Severity Type 3 hangovers.
Discussion
The current study showed that hangover severity during the day can show marked interindividual differences. Three predominant patterns were identified which together comprise more than 95% of the sample: (i) a continuous decline hangover (Severity Type 1 hangover), (ii) a steady state hangover (Severity Type 2 hangover), and (iii) an inverted U‐shaped curve hangover (Severity Type 3 hangover). Of these 3 patterns, Severity Type 2 hangovers are associated with significantly less alcohol consumption and with having the lowest severity scores of individual hangover symptoms. Severity Type 1 hangovers are associated with having the highest severity of individual hangover symptoms. In line with significantly lower levels of alcohol consumption, Severity Type 2 hangovers were observed significantly more often in women when compared to men. Conversely, Severity Type 1 hangovers were significantly more common in men than in women. The reasons for the observed gender differences warrant further investigation. There may be underlying causes other than the fact that in general, women consume less alcohol than men. Severity Type 3 hangovers, characterized by the increased presence of gastrointestinal complaints, were equally commonly experienced in men and women.
Finally, our overall severity data (see Fig. 2 A) followed a similar inverted U‐shaped curve as the group average severity scores presented by Ylikahri and colleagues (1974). Indeed, averaging the severity scores of Severity Types 1, 2, and 3 hangovers (see Fig. 2 B) yields the overall average severity score pattern presented in Fig. 1.
The question why there is variability in hangover patterns is an important one. Several factors, such as congeners, drinking pace, tolerance to alcohol, or a residual blood alcohol concentration at waking, may be relevant in this context. In this study, we found that total alcohol consumption is an important factor influencing the temporal pattern of hangover severity. Also, specific symptoms or classes of symptoms may be related to different severity patterns. For example, it may be that particular symptom classes (e.g., gastritis in Severity Type 3) potentially follow unique time courses, such that individual‐ or event‐level differences in risk for those symptoms determine whether they influence overall hangover severity (or alter the overall time course). Research is underway to investigate the impact of individual symptoms, sample demographics, and alcohol‐related individual‐ or event‐level differences on overall hangover severity and temporal characteristics.
A limitation of this study is that data were collected in a retrospective manner, and thus, recall bias may have influenced outcomes. On the other hand, a hangover day stands out among other past month days, which makes it more likely that participants remember key issues such as the point in time when the hangover was over, or whether hangover severity was stable or followed a certain pattern throughout the day. To prevent a possible impact of recall bias, this study should be replicated using prospective design making real‐time assessments on the day that the hangover is actually present. Another limitation is the fact that we made 1 assessment only: hangover severity on the most recent hangover. It has been hypothesized that severity of hangovers may vary from occasion to occasion (Verster et al., 2010). It was not asked whether the target hangover was typical, or whether specific circumstances played a role that could have affected hangover severity patterns. On the other hand, the sample size was relatively large, allowing some confidence that the data are representative of the sample, and for the general 18‐ to 30‐year‐old drinking population. Also, data from drinkers who reported behaviors that could have influenced hangover severity, such as drug use and the use of medicinal drugs, were excluded from the analyses.
To analyze the data, hangover patterns were allocated to different severity types based on visual inspection. At the start of this project, statisticians were consulted to determine whether it was possible to use an objective statistical approach, for example, latent class analysis, to differentiate between different patterns. A statistical method to identify different patterns of hangover severity, that is, a “factor analysis for graphical patterns” would be preferable to visual inspection. Unfortunately, given some limitations of our specific data set (e.g., many zero values or missing values), applying these statistical approaches was not possible. Therefore, the temporal severity patterns were allocated to the 6 different groups by visual inspection. To do this, however, as described in the Method section of the article, objective rules were applied to allocate each individual pattern. Hopefully, in the future, a statistical methodology can be developed to allocate data from individual drinkers to the different hangover severity types.
Knowledge on how overall alcohol hangover severity changes during the day and which individual symptoms are associated with the different severity patterns is important to better understand the pathophysiology of the alcohol hangover. Moreover, identifying variability in hangover severity may aid the development of an effective hangover cure by showing at which time period(s) such a product may be most effective. Finally, insight into which symptoms are uniquely associated with different severity patterns has implications for the translation of these findings by helping drug development efforts to target hangover treatments specifically at these symptoms. This could allow the active time course of any investigational product to be timed appropriately to the hangover dynamics of the drinker. Future research should therefore also assess the presence and severity of individual hangover symptoms throughout the day.
Taken together, this study revealed that hangover severity differs between individuals throughout the day. Three common temporal severity patterns were identified, which are uniquely related to the amount of alcohol consumed and the presence and severity of different individual hangover symptoms.
Declaration of Interest
This study was funded by Utrecht University. Joris Verster has received grants/research support from the Dutch Ministry of Infrastructure and the Environment, Janssen Research and Development, Nutricia, Red Bull, Sequential, and Takeda and has acted as a consultant for the Canadian Beverage Association, Centraal Bureau Drogisterijbedrijven, Clinilabs, Coleman Frost, Danone, Deenox, Eisai, Janssen, Jazz, Purdue, Red Bull, Sanofi‐Aventis, Sen‐Jam Pharmaceutical, Sepracor, Takeda, Transcept, Trimbos Institute, and Vital Beverages. Andrew Scholey has held research grants from Abbott Nutrition, Arla Foods, Bayer Healthcare, Cognis, Cyvex, GlaxoSmithKline, Naturex, Nestlé, Martek, Masterfoods, Wrigley, and has acted as a consultant/expert advisor to Abbott Nutrition, Barilla, Bayer Healthcare, Danone, Flordis, GlaxoSmithKline Healthcare, Masterfoods, Martek, Novartis, Unilever, and Wrigley. Johan Garssen is part‐time employee of Nutricia Research and received research grants from Nutricia Research Foundation, Top Institute Pharma, Top Institute Food and Nutrition, GSK, STW, NWO, Friesland Campina, CCC, Raak‐Pro, and EU. The other authors have no potential conflict of interests to disclose.
References
- Hogewoning A, Van de Loo AJAE, Mackus M, Raasveld SJ, De Zeeuw R, Bosma ER, Bouwmeester NH, Brookhuis KA, Garssen J, Verster JC (2016) Characteristics of social drinkers with and without a hangover after heavy alcohol consumption. Subst Abuse Rehabil 7:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning R, McKinney A, Bus LD, Olivier B, Slot K, Verster JC (2013) Measurement of alcohol hangover severity: development of the alcohol hangover severity scale (AHSS). Psychopharmacology 225:803–810. [DOI] [PubMed] [Google Scholar]
- Penning R, McKinney A, Verster JC (2012) Alcohol hangover symptoms and their contribution to the overall hangover severity. Alcohol Alcohol 47:248–252. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Minsky SJ, Greece J, Almeida A, Roehrs TA (2007) The acute hangover scale: a new measure of immediate hangover symptoms. Addict Behav 32:1314–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Piasecki TM, Hunt‐Carter EE (2003) Development and initial validation of the Hangover Symptoms Scale: prevalence and correlates of hangover symptoms in college students. Alcohol Clin Exp Res 27:1442–1450. [DOI] [PubMed] [Google Scholar]
- Van Schrojenstein Lantman M, Mackus M, van de Loo AJAE, Verster JC (2016) Development of a definition for the alcohol hangover: consumer descriptions and expert consensus. Curr Drug Abuse Rev 9:148–154. [DOI] [PubMed] [Google Scholar]
- Van Schrojenstein Lantman M, Mackus M, van de Loo AJAE, Verster JC (2017) The impact of alcohol hangover symptoms on cognitive and physical functioning, and mood. Hum Psychopharmacol Clin Exp 32:e2623 Available at: https://doi.org/10.1002/hup.2623. Accessed February 16, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster JC, Stephens R, Penning R, Rohsenow D, McGeary J, Levy D, McKinney A, Finnigan F, Piasecki TM, Adan A, Batty GD, Fliervoet LAL, Heffernan T, Howland J, Kim D‐J, Kruisselbrink LD, Ling J, McGregor N, Murphy RJL, van Nuland M, Oudelaar AM, Parkes A, Prat G, Reed N, Slutske WS, Smith G, Young M, on behalf of the Alcohol Hangover Research Group (2010) The alcohol hangover research group consensus statement on best practice in alcohol hangover research. Curr Drug Abuse Rev 3:116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikahri RH, Huttumen MO, Eriksson CJP, Nikkila EA (1974) Metabolic studies on the pathogenesis of hangover. Eur J Clin Invest 42:577–582. [DOI] [PubMed] [Google Scholar]


