Abstract
Parathyroid hormone (PTH) affects the skeleton by acting on osteocytes (Ots) in bone through yet unclear mechanisms. We report that matrix metalloproteinase 14 (MMP14) expression/activity are increased in bones from mice with genetic constitutive activation (ca) of the PTH receptor 1 (PTH1R) in Ots (caPTH1ROt) and in bones from mice exposed to elevated PTH levels but not in mice lacking [conditional knockout (cKO)] the PTH1R in Ots (cKOPTH1ROt). Furthermore, PTH upregulates MMP14 in human bone cultures and in Ot-enriched bones from floxed control mice but not from cKOPTH1ROt mice. MMP14 activity increases soluble receptor activator of NF-κΒ ligand production, which in turn, stimulates osteoclast differentiation and resorption. Pharmacologic inhibition of MMP14 activity reduced the high bone remodeling exhibited by caPTH1ROt mice or induced by chronic PTH elevation and decreased bone resorption but allowed full stimulation of bone formation induced by PTH injections, thereby potentiating bone gain. Thus, MMP14 is a new member of the intricate gene network activated in Ots by PTH1R signaling that can be targeted to adjust the skeletal responses to PTH in favor of bone preservation.—Delgado-Calle, J., Hancock, B., Likine, E. F., Sato, A. Y., McAndrews, K., Sanudo, C., Bruzzaniti, A., Riancho, J. A., Tonra, J. R., Bellido, T. MMP14 is a novel target of PTH signaling in osteocytes that controls resorption by regulating soluble RANKL production.
Keywords: osteoclasts, osteoblasts, antiresorptive, osteoporosis
Parathyroid hormone (PTH) has profound effects on the skeleton, and its elevation in the circulation can generate both catabolic and anabolic effects in bone. Chronic excess of PTH, as in primary hyperparathyroidism or secondary to calcium deficiency, is characterized by increased remodeling that can lead to bone loss (1). In contrast, intermittent PTH elevation, as achieved by daily injections, causes bone gain as a result of a rapid increase in osteoblast number and function (2). However, the specific contribution of different PTH-responsive cells and the molecular mechanisms underlying the actions of PTH in bone are not completely understood.
The bone catabolic actions of PTH are associated with increased expression of receptor activator of NF-κB ligand (RANKL), a master regulator of osteoclastogenesis, and result in excessive production of osteoclasts with a subsequent increase in osteoblasts, although the work within the bone-remodeling unit is unbalanced in favor of resorption (1, 3). Daily injections of PTH, leading to bone anabolism, also increase RANKL expression, which in turn, increases bone resorption, counterbalancing the anabolic effects of PTH, particularly after long-term administration (2). Thus, the understanding of the molecular mechanisms mediating the effects of PTH on bone resorption could identify new therapeutic targets to protect from bone loss induced by chronic elevation of PTH, as well as to potentiate or prolong the bone gain induced by anabolic regimens of the hormone.
Current evidence demonstrates that osteocytes (Ots) are critical target cells for the skeletal actions of PTH (4). Ots, the most abundant cells in bone, control osteoblast and osteoclast differentiation and function, thereby maintaining bone homeostasis (5, 6). Constitutive activation (ca) of the PTH receptor 1 (PTH1R) signaling in Ots (caPTH1ROt mice) is sufficient to increase bone mass and the rate of bone remodeling (7). Furthermore, the expression of the PTH1R in Ots is required for the full anabolic response to daily injections of PTH (8). Ots are major producers of RANKL (9, 10), and more recent studies demonstrated that resorption induced by PTH in the adult skeleton requires direct regulation of the Rankl gene in Ots (11). In addition, genetic deletion of RANKL from Ots blunts the increase in RANKL expression in bone and reduces osteoclast number induced by chronic elevation of PTH (12). Taken together, these pieces of evidence suggest that Ot-derived RANKL plays an important role in overall bone remodeling, as well as in PTH-induced bone resorption.
RANKL exists in both membrane-bound and soluble forms (13); however, the specific contribution of each form to the physiologic or PTH-induced bone resorption is unknown. In vitro, in cells of the osteoblastic lineage, the soluble form (sRANKL) is generated after proteolytic cleavage of membrane-bound RANKL, preferentially by matrix metalloproteinase 14 (MMP14) (14), a transmembrane MMP that degrades multiple components of the extracellular matrix, cleaves a number of cell adhesion molecules and signaling receptors, and is required for skeletal development (13, 15). Herein, we report that MMP14 is a novel target gene of PTH receptor signaling in Ots. With the use of a highly selective neutralizing antibody against MMP14 (16), we also demonstrate that inhibition of MMP14 activity decreases sRANKL production and bone resorption induced by PTH in murine and human bone, attenuates the bone loss induced by chronic elevation of endogenous PTH, and potentiates the bone gain induced by intermittent administration of the hormone. These findings identify MMP14 as a component of the network of genes regulated by PTH through actions on Ots and provide the bases for a novel therapeutic strategy to preserve bone gain induced by anabolic regimens of PTH.
MATERIALS AND METHODS
Mice
Generation and characterization of C57BL/6 dentin-matrix-protein 1-8 kb caPTH1R (caPTH1rOt) mice were previously published (7). One-month-old female and male caPTH1ROt and control wild-type (WT) littermate mice were injected with neutralizing antibody-blocking MMP14 activity (KD014; 30 mg/kg; Kadmon, New York, NY, USA) or saline, twice per week, for a total of 8 wk. Previous studies demonstrated that KD014 potently inhibits human MMP14 (Ki = 0.8 nM) and mouse (Ki = 0.3 nM) MMP14 and that neutralizes MMP14 and decreases breast cancer tumor growth compared with either control IgG1 or saline (16). No evidence of development of neutralizing anti-human antibodies has been reported in the published studies in rodents using KD014 (formerly DX-2400) (16–18). Moreover, in contrast to other broad-spectrum MMP inhibitors, KD014 specifically inhibits MMP14 and no other MMPs or TNF-α-converting enzymes (16). C57BL/6 mice with conditional deletion (knockout; cKO) of the PTH1R in Ots (cKO-PTH1ROt) and floxed (fl/fl) control littermates were generated, as described before (4). Four-month-old cKO-PTH1ROt and fl/fl female mice were injected daily with PTH (100 ng/g) for 28 d. Four-month-old C57BL/6 female mice (Harlan, Indianapolis, IN, USA) received daily injections of PTH (1–34) (100 ng/g/day; Bachem, Torrance, CA, USA) and subcutaneous injections of KD014 (30 mg/kg) or saline, three times per week for 28 d. Control animals received vehicle injections. Mice (5 per cage) were fed with a regular diet (Harlan), received water ad libitum, and were maintained on a 12-h light/dark cycle. Four-month-old C57BL/6 female mice (Harlan), fed a diet containing 0.01% calcium and 0.4% phosphorous (low calcium diet, TD.95027; Harlan), and control mice, fed a diet containing 0.6% calcium and 0.4% phosphorous (normal calcium diet, TD.97191; Harlan), received subcutaneous injections of KD014 (30 mg/kg) or saline, three times per week for 14 d. KD014 treatment was well tolerated with no evidence of toxicity and no effects on mouse survival. Mice were assigned to the experimental groups based on total bone mineral density (BMD) before treatment administration. In brief, mice were sorted by total BMD and starting from the highest BMD, randomly distributed to the treatment groups. After randomization, no statistical differences on total BMD were found between experimental groups.
Study approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine, and animal care was carried out in accordance with institutional guidelines. Studies with human samples were approved by the Institutional Clinical Research Ethics Committee of Cantabria, Spain, and all procedures were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Analysis of the skeletal phenotype
BMD measurements and micro-computed tomography (microCT) analysis were performed, as previously described (11, 19). Mechanical properties of L6 vertebrae were determined, as previously described (20).
Serum biochemistry
Procollagen type 1 N-terminal propeptide (P1NP), PTH, and C-telopeptide fragments of type I collagen (CTX) were measured using ELISAs (Biomedical Technologies, Stoughton, MA, USA, and Immunodiagnostic Systems, Fountain Hill, AZ, USA, respectively), as previously published (8). Serum levels of sRANKL were measured using an ELISA from R&D Systems (Minneapolis, MN, USA).
Bone histomorphometry
Histomorphometric analyses were performed using the OsteoMeasure High Resolution Digital Video System (OsteoMetrics, Decatur, GA, USA), as previously described (19, 21, 22). In brief, for caPTH1ROt and WT mice, dynamic and static histomorphometry analyses were performed in the cortical bone at the midfemoral diaphysis using cross-sectional and longitudinal sections, respectively. For PTH experiments, dynamic and static histomorphometry analyses were performed in an 800-μm region of the vertebrae, starting 200 μm below the growth plate using longitudinal sections.
Quantitative PCR
Calvaria, vertebra, and tibia were harvested, and total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA), and quantitative PCR (qPCR) was performed using primer probe sets from Applied Biosystems (Foster City, CA, USA) or from Roche Applied Science (Indianapolis, IN, USA), as previously reported (11, 19). For experiments administering PTH daily, bones were harvested 3 h after the last PTH injection. Relative mRNA expression levels were normalized to the housekeeping gene ribosomal protein S2 by using the comparative threshold (Ct) method (23).
Ex vivo experiments
For Ot-enriched bone preparations, bones were harvested from 1-mo-old C57BL/6 mice, cKO-PTH1ROt mice, and fl/fl littermate controls and then were subjected to serial digestions with collagenase and EDTA, as previously published (11, 19, 20, 24). Ot enrichment was demonstrated by a 30-fold increase in Sost expression compared with preparations from nondigested bones. Digested and nondigested bones were treated with PTH (100 nM) or vehicle for 24 h to detect PTH effects at the mRNA level. Tibia and femora were harvested from 1-mo-old caPTH1ROt mice and littermate controls and maintained in α-minimum essential medium containing 10% fetal bovine serum overnight. Bones were then treated with KD014 (0.6 µg/ml) or vehicle for 96 h to achieve sRANKL/CTX concentrations in the culture medium above the detection limit of the ELISA kits. For these experiments, both conditioned medium and total mRNA were collected. Gene expression was measured by qPCR, as indicated above. Levels of sRANKL (R&D Systems) and CTX (Immunodiagnostic Systems) in the conditioned medium were measured using ELISAs, as previously described (25). Human bone samples were obtained during hip arthroplasty, after obtaining informed consent (see Supplemental Methods for study approval). Trabecular bone cylinders of the central part of the femoral head were obtained with a trephine, washed extensively in PBS, and plated for experiments. Bone tissue fragments were treated for 24 h (for mRNA expression) or 96 h (for mRNA expression and sRANKL/CTX determination in conditioned medium) with PTH (1–34) (10−7 M). Gene expression was analyzed using the TATA box-binding protein as a housekeeping gene, as described before (26, 27). Earlier published work demonstrates the functionality of Ots in these bone organ cultures (11, 19, 20, 28, 29).
Western blotting
Bone protein lysates were prepared from whole tibias, as previously described (25). Immunoblots were performed by using rabbit polyclonal anti-MMP14 (1:1,000; ab38971; Abcam, Cambridge, United Kingdom), rabbit polyclonal anti-MMP2 (1:1,000, ab37150; Abcam), mouse monoclonal anti-sRANKL (1:500, NB100-56748; NovusBio, Littleton, CO, USA), mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:2000, sc-365062; Santa Cruz Biotechnology, Dallas, TX, USA), or mouse monoclonal anti-β-actin (1:2,000; A5316; Santa Cruz Biotechnology) antibodies, followed by goat anti-rabbit or goat anti-mouse antibodies conjugated to horseradish peroxidase (1:2000 in 5% milk; Santa Cruz Biotechnology). Bands were detected, and their intensity was quantified using TotalLab TL 100 software (Nonlinear Dynamics, Durham, NC, USA). Band densities for proteins of interest (MMP14, MMP2, sRANKL) were divided by band densities of the corresponding housekeeping proteins (β-actin, GAPDH) to obtain ratios corrected for total protein loaded. Fold changes induced by the treatments were then calculated, dividing by the ratio corresponding to vehicle-treated mice. Original, uncropped images of Western blots can be found in Supplemental Data 1.
Osteoclast differentiation
Bone marrow cells were isolated as described before (30). For the osteoclastogenesis assay, 2 × 105 nonadherent cells/cm2 were seeded and cultured with 20 ng/ml recombinant murine M-CSF and 80 ng/ml recombinant murine RANKL (PeproTech, Rocky Hill, NJ, USA). Medium was refreshed every 2 d for 6–8 d. Osteoclast differentiation in the presence of vehicle or KD014 (0.6 µg/ml) was evaluated after staining for tartrate-resistant acid phosphatase (TRAP) using a commercial kit (Sigma-Aldrich, St. Louis, MO, USA). The resorption activity of osteoclasts cultured in the absence or presence of KD014 (0.6 µg/ml) was evaluated, as previously described (31). Conditioned medium was collected, and CTX was measured as described above.
Mineralization assay
Primary osteoblastic cells were isolated from the neonatal calvarial bones and plated at 5000 cells/cm2 density in α-minimum essential medium, as previously described (20). Osteogenic medium, containing 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate, was used to induce osteoblast differentiation after cultures reached confluence (with/without KD014 0.6 µg/ml). Medium was replaced every 2–3 d, and mineralization was visualized by Alizarin Red staining and quantified as previously published (20).
Statistical analysis
Data were analyzed using SigmaStat (SPSS Science, Chicago, IL, USA). All values are reported as means ± sd. Differences between vehicle- and KD014-treated osteoclast and osteoblast and vehicle- and PTH-treated human bones were evaluated using 2-tailed Student’s t test. For the in vivo and ex vivo experiments, differences between group means were evaluated using 2-way ANOVA, with genotype/PTH and treatment (KD014) as independent variables. When ANOVA detected a significant interaction between the variables or a significant main effect of genotype/PTH or treatment, a Tukey’s post hoc was used to determine the significance of the effect of the genotype/PTH within each KD014 treatment group. Values of P ≤ 0.05 were considered statistically significant. Complete 2-way ANOVA and post hoc analyses can be found in Supplemental Data 2.
RESULTS
Mmp14 is a target gene of PTH1R signaling in Ots
Bones from mice expressing a constitutive active PTH1R in Ots (caPTH1ROt) exhibited increased Mmp14 mRNA expression compared with WT control littermates (Fig. 1A). The expression of Mmp14 was also elevated in bones from fl/fl mice receiving daily injections of PTH, as well as in mice with endogenous chronic elevation of PTH induced by a low calcium diet (Fig. 1B, C). In contrast, daily or chronic elevation of PTH did not increase Mmp14 expression in bones from mice with cKO of the PTH1R in Ots (cKOPTH1ROt; Fig. 1B, C). PTH also increased MMP14 mRNA expression ex vivo in organ cultures established with human bone from patients with osteoporosis (Fig. 1D). Furthermore, PTH increased MMP14 mRNA expression in calvarial bone organ cultures established from WT mice. A similar regulation of MMP14 by PTH was observed in organ cultures established from calvarial bones enriched in Ots (Fig. 1E). In contrast, MMP14 upregulation by PTH was absent in Ot-enriched bones from cKOPTH1ROt mice (Fig. 1F). These results demonstrate that Mmp14 is a target gene of the direct actions of PTH in Ots, and that is regulated in both murine and human bone.
Figure 1.
Genetic and hormonal activation of PTH receptor signaling in Ots upregulates MMP14 gene expression in murine and human bone. A–C) Mmp14 mRNA expression in whole bones (L4) from caPTH1ROt mice (n = 7) and WT control littermates (n = 7) (A), from fl/fl and cKOPTH1ROt mice receiving daily injections of vehicle (veh)/PTH (vehicle/PTH injections, n = 10/8 fl/fl; n = 9/8, cKOPTH1ROt mice) (B), or fed a normal/low-calcium diet (normal/deficient calcium diet, n = 9/9 fl/fl; n = 7/6, cKOPTH1ROt mice) (C). D) MMP14 mRNA expression in human bone fragments treated with vehicle (n = 8) or PTH (n = 8) for 24 h. E, F) Ex vivo bone organ cultures established from normal and Ot-enriched bones were treated with vehicle or PTH for 24 h (n = 4 mice/genotype/treatment). Bars represent means ± sd. *P ≤ 0.05 vs. WT/fl/fl (A), vs. vehicle (B, D–F), or vs. a normal diet (C) by Student’s t test (A, D, E) or 2-way ANOVA, Tukey’s post hoc test (B, C, F).
MMP14 is required for the increase in bone remodeling induced by genetic activation of PTH1R signaling in Ots
We first examined the role of MMP14 in the skeletal actions of PTH in a model of ca of the PTH1R in Ots (caPTH1ROt), characterized by high bone remodeling with bone gain (7, 21). Toward this end, we administered a neutralizing antibody that inhibits the proteolytic activity of MMP14 (KD014) to growing caPTH1ROt and WT littermate mice for 8 wk. caPTH1ROt mice exhibited increased MMP14 expression at mRNA and protein levels compared with WT mice, regardless of KD014 administration (Fig. 2A, B). However, KD014 decreased MMP14 activity, as demonstrated by reduced levels of the active form of MMP2, a product of MMP14 proteolytic activity (34), in both WT and caPTH1ROt mice (Fig. 2B). caPTH1ROt mice displayed the previously shown increase in total, spinal, and femoral BMD compared with WT mice (7, 21); in contrast, caPTH1ROt mice receiving KD014 exhibited an ∼10% reduction in BMD in all skeletal sites (Fig. 2C). Consistent with these findings, caPTH1ROt mice receiving KD014 had lower cortical bone area over tissue area (Ct.Ar/Tt.Ar; −25%) as a result of a lower Ct.Ar, thinner cortex (−30%), and larger marrow cavity compared with saline-injected caPTH1ROt mice (Fig. 2D). KD014 did not alter Ct.Ar or thickness in WT mice (Figs. 2D).
Figure 2.
Inhibition of MMP14 activity reduces the increase in bone gain coupled to resorption induced by ca of PTH1R signaling in Ots. A, B) Mmp14 mRNA expression (A) MMP14 and MMP2 protein levels (B) (n = 2/group) and band quantification in whole bones (L4) from WT and caPTH1ROt receiving control or KD014 for 8 wk. Fold changes relative to WT/saline-treated mice are shown. Proteins of interest were assayed on different gels. C) Total, spinal, and femoral BMD percentage change calculated over initial BMD measurements in WT and caPTH1ROt mice receiving control/KD014 injections. D) microCT analysis of cortical bone in mice receiving control/KD014 for 8 wk. Control/KD014 injections: n = 10/13, WT; n = 11/10, caPTH1ROt mice. Bars represent means ± sd. *P ≤ 0.05 vs. WT mice of each group, unless otherwise indicated by the lines, by 2-way ANOVA, Tukey’s post hoc test. Ct.Ar, cortical area; Ct.Th, cortical thickness; Ma.Ar, marrow area; Tt.Ar, tissue area.
To determine the cellular mechanism underlying the effects of KD014 on caPTH1ROt mice, we examined bone resorption and bone formation indexes. Consistent with the absence of effects on bone mass or microarchitecture, KD014 did not alter either bone resorption or bone formation in WT mice (Fig. 3A–F). In contrast, the elevated levels of the bone resorption marker CTX, the osteoclast marker TRAP5b, and the increased number/surface of osteoclasts exhibited by caPTH1ROt mice were all decreased in caPTH1ROt mice receiving KD014 (Fig. 3A, B). caPTH1ROt mice receiving KD014 exhibited a transient decrease in serum levels of the bone formation marker P1NP compared with caPTH1ROt mice receiving saline after 4 wk of treatment, which at 8 wk, returned to the levels exhibited by caPTH1ROt receiving saline (Fig. 3C). At the tissue level, caPTH1ROt mice exhibited increased periosteal and endocortical mineral apposition rate (MAR; 1.7- and 2.3-fold, respectively) and bone formation rate (BFR; 2.8- and 2.4-fold, respectively) compared with WT mice (Fig. 3D, E). Administration of KD014 did not alter periosteal MAR, BFR, or mineralizing surface per bone surface (MS/BS) in either caPTH1ROt or WT mice (Fig. 3D). In contrast, caPTH1ROt mice receiving KD014 exhibited reduced endocortical MAR and MS/BS to values undistinguishable from WT levels and displayed decreased BFR compared with saline-treated PTH1ROt mice (Fig. 3E), consistent with elevated bone formation following bone resorption on this surface. Furthermore, KD014 reduced the elevated osteoblast number/surface displayed by caPTH1ROt mice in the endocortical surface (Fig. 3F). Remarkably, the effects of MMP14 inhibition on bone mass and bone formation occurred even when Sost expression remained low, and the expression of Wnt target genes (Axin2 and Bmp1) remained elevated in caPTH1ROt mice treated with KD014 (Fig. 3G). KD014 did not affect osteoblast differentiation in vitro (Fig. 3H), suggesting that the decreased bone formation induced by KD014 in caPTH1ROt mice is secondary to decreased bone resorption. Together, these results demonstrate that MMP14 activity contributes to the high bone remodeling and increased bone gain that ensue with the genetic ca of PTH1R signaling in Ots.
Figure 3.
Inhibition of MMP14 activity decreases the elevated bone resorption and bone remodeling displayed by caPTH1ROt mice. A) Longitudinal analysis of serum CTX and TRAP5b levels (control/KD014 injections: n = 10/13, WT; n = 11/10, caPTH1ROt mice). B) Osteoclast number/surface (control/KD014 injections: n = 5/5, WT; n = 3/6, caPTH1ROt mice) and representative images of TRAP staining in endocortical bone surfaces of WT and caPTH1ROt mice after 8 wk of treatment. Arrows point to TRAP-positive osteoclasts. C–G) Serum P1NP levels (C), histomorphometric analysis of bone formation dynamic parameters in the periosteum (D) and endosteum of femoral middiaphysis (E) and representative images of labeled surfaces (control/KD014 injections: n = 10/13, WT; n = 11/10, caPTH1ROt mice), endocortical osteoblast (F) number/surface (control/KD014 injections: n = 5/5, WT; n = 3/6, caPTH1ROt mice), and Sost and Wnt target-gene mRNA expression (G) in WT and caPTH1ROt mice after 8 wk of treatment (control/KD014 injections: n = 10/13, WT; n = 11/10, caPTH1ROt mice). H) Mineralization in murine primary osteoblasts treated with/without KD014 for 14 d by Alizarin Red S staining (control/KD014, n = 4/3). Bars represent means ± sd. *P ≤ 0.05 vs. WT mice of each group, unless otherwise indicated by the lines, by 2-way ANOVA, Tukey’s post hoc test. BFR/BS, BFR per bone surface; Ec., endocortical; MS/BS, mineralizing surface per bone surface; N.Ob/B.Pm, osteoblast number per bone perimeter; N.Oc/B.Pm, osteoclast number per bone perimeter; Ob.S/BS, osteoblast surface per bone surface; Oc.S/BS, osteoclast surface per bone surface; Ps., periosteal.
Pharmacological inhibition of MMP14 partially prevents the high bone remodeling induced by chronic elevation of PTH
We next examined whether MMP14 plays a part in the bone loss induced by chronic endogenous elevation of PTH secondary to calcium deficiency, another model of high bone remodeling induced by PTH signaling. Skeletally mature mice fed a low-calcium diet exhibited a 50% increase in PTH levels, irrespective of KD014 administration (Fig. 4A). KD014 decreased circulating CTX in control mice and prevented the increase in CTX induced by chronic PTH elevation (Fig. 4B). Moreover, KD014 partially blocked the increase in P1NP in mice fed the calcium-deficient diet, although did not alter P1NP in control mice (Fig. 4C). In contrast, KD014 prevented 40% of the decrease in total BMD at 2 wk but had no effect at 4 wk or at either time point in the femur (Fig. 4D). In the spine, KD014 prevented 40 and 20% of the decrease in BMD after 2 and 4 wk, respectively, although these effects did not reach statistical significance (Fig. 4D). These results suggest that MMP14 activity modestly contributes to the high bone remodeling induced by chronic elevation of endogenous PTH, but its inhibition only has minor effects on the bone loss secondary to calcium deficiency.
Figure 4.
Pharmacological inhibition of MMP14 activity partially prevents the increase in bone remodeling and the bone loss induced by chronic elevation of PTH. A–C) Serum PTH (B), CTX (B), and P1NP (C) levels in mice fed a normal or a low-calcium diet and receiving control or KD014 injections for 4 wk. D) Total, spinal, and femoral BMD percentage change calculated over initial BMD measurements. Control/KD014 injections: n = 10/11, normal calcium diet; n = 10/11, low calcium diet. Bars represent means ± sd. *P ≤ 0.05 vs. mice fed a normal Ca diet of each group, unless otherwise indicated by the lines, by 2-way ANOVA, Tukey’s post hoc test.
Inhibition of MMP14 activity potentiates the bone gain induced by daily injections of PTH
We next investigated the role of MMP14 in the anabolic effects of PTH. Daily injections of PTH to skeletally mature mice increased Mmp14 mRNA and protein levels (Fig. 5A, B). Consistent with our results in caPTH1ROt mice, KD014 did not alter MMP14 expression, but it reduced the levels of the active form of MMP2 in PTH-treated mice (Fig. 5B). Daily PTH increased total, spinal, and femoral BMD, and coadministration of KD014 had an additive effect on spinal BMD at 2 wk and potentiated the increase in BMD in the spine (25% vs. vehicle) at 4 wk, but not in the femur (Fig. 5C). Furthermore, combined administration of PTH and KD014 potentiated the gain in spinal cancellous bone volume over tissue volume (BV/TV; 49% over PTH alone) and had an additive effect in cancellous BV/TV in distal femur (37% over mice receiving PTH alone; Fig. 5D–F). In contrast, KD014 did not alter the increase in Ct.Ar or cortical thickness induced by PTH in the femur (Fig. 5G). Moreover, KD014 administration to vehicle-treated mice significantly increased spinal BMD in this experiment and increased cancellous BV/TV in the distal femur, although femoral BMD or Ct.Ar of total area remained unchanged (Fig. 5C, F, G).
Figure 5.
Coadministration of KD014 potentiates the cancellous bone gain induced by daily injections of PTH. A, B) Mmp14 mRNA expression (L4) (A), MMP14 and MMP2 protein levels (n = 2/group) (B), and band quantification in whole bones (tibia; fold changes relative to vehicle/saline-treated mice) from mice injected with vehicle or PTH and receiving control or KD014 treatment for 4 wk. Proteins of interest were assayed on different gels. C) Effect of coadministration of KD014 on total, spinal, and femoral BMD percentage change calculated over initial BMD measurements. D) Representative von Kossa staining images of undecalcified sections of the lumbar spine. MicroCT analysis showing the increase in (E) spinal and (F) femoral cancellous BV and (G) the absence of changes in Ct.Ar or thickness in mice receiving PTH and KD014 for 4 wk (control/KD014 injections: n = 10/11, vehicle; n = 10/11, PTH). Bars represent means ± sd. *P ≤ 0.05 versus vehicle-treated mice of each group, unless otherwise indicated by the lines, by 2-way ANOVA, Tukey’s post hoc test. Ct.Th, cortical thickness; Ma.Ar, marrow area; Tb.N., trabecular number; Tb.Th., trabecular thickness.
Daily injections of PTH did not increase serum CTX or the number or surface of osteoclasts in vertebral cancellous bone but increased the levels of TRAP5b (Fig. 6A). KD014 reduced serum CTX in both vehicle- and PTH-treated mice. Coadministration of KD014 reduced TRAP5b levels (∼20%) and decreased cancellous osteoclast number/surface below the values exhibited by mice treated with PTH alone (Fig. 6A, B). In contrast, the increases induced by PTH in circulating P1NP (37%; Fig. 6C), MAR and BFR (∼20%; Fig. 6D), and osteoblast number and surface (Fig. 6E) were not altered by coadministration of KD014. Furthermore, Sost was downregulated and Wnt target genes upregulated by PTH, similarly in mice coadministered with either KD014 or saline (Fig. 6F). Likewise, coadministration of KD014 did not significantly alter a PTH-induced increase in ultimate force, energy to yield, and stiffness (Fig. 6G). Taken together, these results demonstrate that KD014 did not perturb the PTH-induced increase in bone formation and anabolic signaling but inhibited resorption, leading to an increase in bone gain induced by PTH injections.
Figure 6.
Inhibition of MMP14 decreases bone resorption but preserves the increased bone formation induced by anabolic regimes of PTH. Serum CTX and TRAP5b levels (A), cancellous osteoclast number/surface and representative images of TRAP staining, with arrows pointing to TRAP-positive osteoclasts (B), serum P1NP (C), cancellous MS/BS, MAR, and BFR (control/KD014 injections: n = 5/5, vehicle; n = 5/5, PTH) (D), cancellous osteoblast number/surface (control/KD014 injections: n = 5/5, vehicle; n = 5/5, PTH) (E), Sost downregulation and upregulation of Wnt target genes mRNA expression (F), and biomechanical properties measured by axial compression testing in vertebral bone (L6) in mice receiving vehicle/PTH in combination with control or KD014 injections (G). Control/KD014 injections: n = 10/11, vehicle; n = 10/11, PTH. Bars represent means ± sd. *P ≤ 0.05 versus vehicle-treated mice of each group, unless otherwise indicated by the lines, by 2-way ANOVA, Tukey’s post hoc test. BFR/BS, BFR per bone surface; MS/BS, mineralizing surface per bone surface; N.Oc/B.Pm, osteoclast number per bone perimeter; Ob.N/B.Pm, osteoblast number per bone perimeter; Ob.S/BS, osteoblast surface per bone surface; Oc.S/BS, osteoclast surface per bone surface.
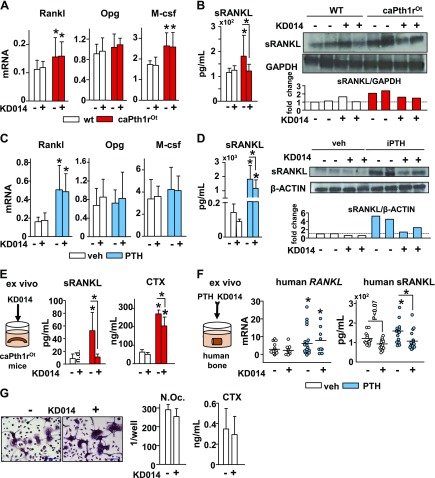
Neutralization of MMP14 activity decreases the levels of sRANKL in vitro, ex vivo, and in vivo
We next investigated the molecular mechanisms underlying the reduction in bone resorption induced by inhibition of MMP14 activity. Treatment with KD014 antibody did not reduce the elevated mRNA expression of Rankl or M-csf or change osteoprotegerin (Opg) expression in bones from caPTH1ROt mice (Fig. 7A) or in mice treated with daily PTH (Fig. 7C). The levels of circulating RANKL in serum, as well as the protein levels of sRANKL, were elevated in whole bone protein preparations from caPTH1ROt mice compared with WT mice. KD014 did not affect sRANKL levels in WT mice; however, the high sRANKL serum and bone protein levels exhibited by caPTH1ROt mice were reduced by KD014 (Fig. 7B). Daily administration of PTH also increased circulating RANKL in serum and sRANKL in whole bone preparations. KD014 decreased sRANKL protein levels in bone and serum sRANKL (by ∼20%) in vehicle-treated mice, although this result did not reach statistical significance by 2-way ANOVA. In addition, KD014 modestly decreased serum sRANKL and reduced sRANKL expression in bone in PTH-treated mice (Fig. 7D). Furthermore, sRANKL and CTX were increased in culture medium from bone organ cultures established from caPTH1ROt mouse bones, and ex vivo addition of KD014 decreased sRANKL to WT levels and reduced CTX by 23% (Fig. 7E). Consistent with these findings in murine bone, PTH addition to human bone organ cultures upregulated RANKL mRNA expression and stimulated sRANKL production, and KD014 reduced sRANKL levels to those observed in vehicle-treated samples (Fig. 7F). KD014 did not affect sRANKL-induced osteoclast differentiation in vitro from murine bone marrow precursors or their resorptive activity, as measured by CTX in the culture medium (Fig. 7G), suggesting that the reduction of bone resorption exerted by KD014 in vivo does not result from inhibition of MMP14 activity directly in osteoclasts. Taken together, these results demonstrate that MMP14 inhibition decreases sRANKL production, thereby inhibiting bone resorption.
Figure 7.
MMP14 controls bone resorption by regulating the production of sRANKL. A, B) Rankl, Opg, and M-csf mRNA levels in bone (A) and serum RANKL and sRANKL protein levels (B) (n = 2/group; fold changes relative to WT/saline-treated mice) in whole bones from WT and caPTH1ROt mice treated with control or KD014 injections for 8 wk (control/KD014 injections: n = 10/13, WT; n = 11/10, caPTH1ROt mice). C, D) Rankl, Opg, and M-csf mRNA expression (C) and serum RANKL and sRANKL protein expression (D) (n = 2/group; fold changes relative to vehicle/saline-treated mice) in whole bones from mice receiving daily PTH in combination with control or KD014 injections for 4 wk (control/KD014 injections: n = 10/11, vehicle; n = 10/11, PTH). E) sRANKL and CTX levels in 96 h conditioned medium from WT and caPTH1ROt bones treated with control or KD014 (control/KD014 injections: n = 4/4, WT; n = 6/6, caPTH1ROt mice). F) Human RANKL mRNA expression (control/KD014 injections: n = 19/10, vehicle; n = 23/10, PTH) and sRANKL levels in 96 h conditioned medium collected from human bones treated with PTH, with or without KD014 (control/KD014 injections: n = 14/15, vehicle; n = 18/21, PTH). G) N.Oc and resorption activity measured as CTX released to the medium measured in murine primary nonadherent bone marrow cells cultured with RANKL and M-CSF in the presence/absence of KD014 (control/KD014, n = 6/6). Bars represent means ± sd. *P ≤ 0.05 vs. WT mice of each group (A, E), or vs. vehicle-treated mice (C, F), unless otherwise indicated by the lines, by 2-way ANOVA, Tukey’s post hoc. Proteins of interest were assayed on different gels.
DISCUSSION
The cellular and molecular mechanisms underlying the profound effects in bone of activation of the PTH receptor are not fully understood. It is now recognized that Ots mediate at least some of the skeletal actions of PTH. With the use of in vitro, ex vivo, and in vivo approaches, we demonstrate in this study that MMP14 is a new target of the actions of the PTH receptor in Ots and that its activity contributes to the increased bone resorption and bone remodeling induced by PTH. First, we found that PTH receptor signaling in Ots, activated genetically or by PTH treatment, upregulates the expression of MMP14 in murine and human bone. Second, with the use of a pharmacologic approach, we show that MMP14 activity is required to increase osteoclasts, bone resorption, and the consequent bone formation that enables bone gain in caPTH1ROt mice, a mouse model of high bone remodeling with positive balance (7). Third, we found that MMP14 activity contributes to increase bone resorption and the rate of bone remodeling that favors bone loss in a model of chronic elevation of endogenous PTH characterized by high bone remodeling with a negative bone balance (8). Fourth, we demonstrate that MMP14 can be targeted to favor the bone gain induced by daily injections of PTH. Lastly, mechanistic studies in human and murine bone demonstrate that increased MMP14 activity stimulates sRANKL production, which in turn, increases osteoclast number and bone resorption. In concert, our findings identify MMP14 as a novel target gene of PTH receptor signaling in Ots and unravel a previously unknown function of this protein as a mediator of PTH-induced bone resorption through sRANKL production (Fig. 8).
Figure 8.
MMP14 is a novel osteocytic PTH1R target gene that stimulates bone resorption through sRANKL production. Upon binding to the PTH1R in Ots, PTH activates Wnt signaling to increase osteoblast differentiation, survival, and bone forming activity and also increases the expression of RANKL and MMP14. MMP14 cleaves membrane-bound RANKL protein and produces sRANKL, which in turn, promotes osteoclast differentiation and bone resorption. Inhibition of MMP14 activity with KD014 antibody reduces bone resorption driven by osteocytic PTH1R signaling and preserves the newly built bone.
Although both membrane-bound and sRANKL stimulate osteoclastogenesis in vitro, the contribution of each form of the cytokine for osteoclast formation and resorption in vivo remains unclear. Earlier studies suggested that sRANKL could act as a negative regulator of osteoclastogenesis by reducing the amount of membrane-bound RANKL available for osteoclast precursors (14). Opposite to these findings, Teti and collaborators (33) showed that sRANKL shed by MMP14 is sufficient to increase osteoclasts and resorption in RANKL KO mice, supporting an active role of sRANKL in vivo. More recently, O’Brien and collaborators (34) generated a mouse model with a mutation in the endogenous RANKL gene that impedes shedding of sRANKL, which exhibited decreased osteoclast number and increased cancellous BV. Our current results show that sRANKL, produced by MMP14 activity, contributes to PTH-induced bone resorption and that KD014 decreased resorption in control mice fed a normal diet or receiving vehicle injections. Taken together with the previous evidence, our study supports the notion that sRANKL enhances osteoclast differentiation and contributes to both physiologic and PTH-induced bone resorption. Nevertheless, an unresolved issue that warrants further investigation is the cellular source of sRANKL under both basal and PTH-stimulated conditions, as cells of the osteoblastic lineage at different states of differentiation and other cells in the bone marrow express membrane-bound RANKL and are responsive to PTH (35, 36). Importantly, inhibition of MMP14 does not alter RANKL-induced osteoclast differentiation or function in vitro, and it does not change the mRNA expression of Rankl, Opg, or M-Csf in vivo. This evidence strongly supports the notion that KD014 achieves its inhibitory actions on osteoclast formation and resorption by reducing sRANKL protein production, rather than by direct effects on osteoclasts or by regulating the mRNA expression of pro- or anti-osteoclastogenic cytokines.
KD014 inhibition of MMP14 (a target of osteocytic PTH1R signaling) had minor effects on the bone loss induced by chronic elevation of PTH, suggesting that sRANKL has only a minimal contribution to resorption in this condition. This is consistent with earlier findings showing that PTH1R signaling in Ots is not required for the bone loss induced by chronic PTH (8) and that cKO of Rankl from Ots only partially protects from bone loss in the same model (12). This evidence implies that the catabolic actions of chronic PTH elevation can be mediated by other PTH responsive cells not targeted by the dentin-matrix-protein 1-8 kb promoter (37–40).
Our study showed a distinct role of MMP14 activity in cancellous versus cortical bone, as the effects of KD014 combined with PTH were confined to the cancellous compartment. These findings are in line with evidence that loss of RANKL regulation by PTH (41) or removal of the osteocytic PTH1R (8) decreases the RANKL/OPG ratio and increases cancellous but not cortical bone. However, KD014 also impacted the cortical bone of growing caPTH1ROt mice, a genetic mouse model with a unique bone mass phenotype, characterized by high bone remodeling on the endocortical surface (7). Taken together, these results suggest that the osteocytic PTH1R/MMP14/sRANKL axis exerts a preferential role in cancellous bone of skeletally mature mice, as a result of its more dynamic nature compared with cortical bone. Nevertheless, further experiments are required to determine whether sRANKL has indeed differential effects in cancellous vs. cortical bone.
Our findings, showing that inhibition of MMP14 activity decreases sRANKL production in vitro, ex vivo, and in vivo, are consistent with the demonstration that osteoblastic cells derived from MMP14 null mice exhibit reduced levels of sRANKL in vitro (14). However, in contrast to our studies showing a modest effect of pharmacological inhibition of MMP14 activity postnatally, global genetic deletion of MMP14 has profound effects in skeletal development, leading to increased osteoclasts, reduced bone formation, and a progressive reduction in bone mass (14, 15, 42, 43). These findings suggest that the critical role of MMP14 during bone development might be independent of sRANKL. Indeed, the increase in resorption observed in MMP14 KO mice has been attributed to impaired remodeling of soft tissues during development, which in turn, could increase bone resorption as a compensatory mechanism to accommodate bone growth (15). Furthermore, whereas KD014 blocks MMP14 proteolytic activity, the genetic deletion of MMP14 eliminates both proteolytic and nonproteolytic activities of the protein (43–45), thus also raising the possibility that MMP14 proteolytic vs. nonproteolytic activities differentially regulate bone homeostasis.
Our results also shed light on a still-debated issue in the field, which is whether the anabolic actions of PTH require resorption. Coadministration of KD014 did not alter the PTH-induced increase in femoral BMD or cortical bone. Likewise, in cancellous bone, KD014 did not affect bone formation induced by PTH, and even more, KD014 further increased spinal BMD and vertebral and distal femur cancellous bone. These findings with KD014 in our mouse models are consistent with clinical studies in humans, demonstrating that PTH exhibits bone anabolic effects even when coadministered with bisphosphonates or neutralizing RANKL antibodies (46–48), and support the notion that bone resorption limits the gain in cancellous bone induced by PTH in the initial stages of the therapy. However, antiresorptive drugs reduce the remodeling space and can impair the bone gain induced by PTH during the second year of therapy. Yet, whether inhibition of resorption with KD014 can become a useful tool to increase the efficacy of anabolic regimens of PTH by maximizing the bone gain and/or decreasing the length of treatment remains to be determined.
Sustained suppression of bone turnover by antiresorptive drugs, such as bisphosphonates, could induce microdamage accumulation and a reduction in the energy that bone tissue absorbs before failure (49, 50), which in turn, increases the risk for low-energy fractures (51, 52). In contrast to potent antiresorptive agents, KD014 modestly inhibited bone resorption, either alone or in combination with PTH injections, as it only reduces sRANKL-dependent resorption. Therefore, whereas strong antiresorptive agents suppress bone remodeling and decrease bone formation, inhibition of resorption by KD014 did not have an impact on bone formation, which remained unchanged under physiologic conditions and still elevated by daily injections of PTH. Furthermore, KD014 did not induce negative effects on bone mechanical properties. In fact, KD014 alone increased ultimate load and energy to yield and preserved PTH-induced increases in ultimate load, energy to yield, and stiffness. As KD014 preserves physiologic bone turnover and the beneficial effects induced by PTH on bone formation and mechanical properties, it might provide advantages over strong antiresorptive agents used in the clinic.
In summary, our study identifies the osteocytic PTH1R/MMP14/sRANKL axis as a novel regulator of bone resorption that can be pharmacologically targeted to adjust PTH skeletal responses in favor of bone preservation. Whether this pathway is differentially activated by ligands of the PTH1R with distinct proresorption profiles, such as the synthetic analog of human PTH-related protein (1–34) (abaloparatide) (53), recently approved by the U.S. Food and Drug Administration for the treatment of osteoporosis, warrants further investigation.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Drs. Keith Condon, Gretel Pellegrini, and Sumana Posritong and Meloney Cregor (all from Indiana University School of Medicine) for their assistance in tissue and data collection. The authors also thank Drs. David Burr and Munro Peacock (both from the Indiana University School of Medicine) for critical reading of the manuscript. This research was supported by the U.S. Department of Veterans Affairs (1 I01 BX002104-01 to T.B.); the U.S. National Institutes of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant R01-AR059357 to T.B.; R01-AR060332 to A.B.); NIH National Heart, Lung, and Blood Institute (Grant T35 HL1 10854-01); a Scholar Award from the American Society of Hematology (to J.D-C.); and a grant from Instituto de Salud Carlos III (PI12/615), cofunded by the European Union through Fonds Européen de Développement Économique et Régional (FEDER) funds (to J.A.R.). The KD014 neutralizing antibody was obtained under a Materials Transfer Agreement with Kadmon Corp., LLC. J.R.T. was an employee of Kadmon Corp., LCC. The remaining authors declare no conflicts of interest.
Glossary
- BFR
bone formation rate
- BMD
bone mineral density
- BV/TV
bone volume over tissue volume
- ca
constitutive activation
- cKO
conditional knockout
- Ct.Ar
cortical bone area
- CTX
C-telopeptide fragments of type I collagen
- fl/fl
floxed
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MAR
mineral apposition rate
- microCT
micro-computed tomography
- MMP14
matrix metalloproteinase 14
- MS/BS
mineralizing surface per bone surface
- Opg
osteoprotegerin
- Ot
osteocyte
- P1NP
procollagen type 1 N-terminal propeptide
- PTH
parathyroid hormone
- PTH1R
parathyroid hormone receptor 1
- qPCR
quantitative PCR
- RANKL
receptor activator of NF-κB ligand
- sRANKL
soluble receptor activator of NF-κB ligand
- TRAP
tartrate-resistant acid phosphatase
- Tt.Ar
tissue area
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. Delgado-Calle and T. Bellido designed research, analyzed and interpreted data, and wrote the manuscript; J. Delgado-Calle, B. Hancock, A. Y. Sato, K. McAndrews, E. F. Likine, C. Sanudo, J. A. Riancho, J. R. Tonra, and A. Bruzzaniti performed research; and all authors reviewed the manuscript.
REFERENCES
- 1.Silva B. C., Bilezikian J. P. (2015) Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 22, 41–50 10.1016/j.coph.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jilka R. L. (2007) Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40, 1434–1446 10.1016/j.bone.2007.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Y. L., Cain R. L., Halladay D. L., Yang X., Zeng Q., Miles R. R., Chandrasekhar S., Martin T. J., Onyia J. E. (2001) Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology 142, 4047–4054 10.1210/endo.142.9.8356 [DOI] [PubMed] [Google Scholar]
- 4.Bellido T., Saini V., Pajevic P. D. (2013) Effects of PTH on osteocyte function. Bone 54, 250–257 10.1016/j.bone.2012.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado-Calle J., Bellido T. (2015) Osteocytes and skeletal pathophysiology. Curr. Mol. Biol. Rep. 1, 157–167 10.1007/s40610-015-0026-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonewald L. F. (2011) The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 10.1002/jbmr.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien C. A., Plotkin L. I., Galli C., Goellner J. J., Gortazar A. R., Allen M. R., Robling A. G., Bouxsein M., Schipani E., Turner C. H., Jilka R. L., Weinstein R. S., Manolagas S. C., Bellido T. (2008) Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One 3, e2942 10.1371/journal.pone.0002942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado-Calle J., Tu X., Pacheco-Costa R., McAndrews K., Edwards R., Pellegrini G. G., Kuhlenschmidt K., Olivos N., Robling A., Peacock M., Plotkin L. I., Bellido T. (2017) Control of bone anabolism in response to mechanical loading and PTH by distinct mechanisms downstream of the PTH receptor. J. Bone Miner. Res. 32, 522–535 10.1002/jbmr.3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong J., Onal M., Jilka R. L., Weinstein R. S., Manolagas S. C., O’Brien C. A. (2011) Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 10.1038/nm.2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J. Q., Bonewald L. F., Kodama T., Wutz A., Wagner E. F., Penninger J. M., Takayanagi H. (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 10.1038/nm.2452 [DOI] [PubMed] [Google Scholar]
- 11.Ben-awadh A. N., Delgado-Calle J., Tu X., Kuhlenschmidt K., Allen M. R., Plotkin L. I., Bellido T. (2014) Parathyroid hormone receptor signaling induces bone resorption in the adult skeleton by directly regulating the RANKL gene in osteocytes. Endocrinology 155, 2797–2809 10.1210/en.2014-1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong J., Piemontese M., Thostenson J. D., Weinstein R. S., Manolagas S. C., O’Brien C. A. (2014) Osteocyte-derived RANKL is a critical mediator of the increased bone resorption caused by dietary calcium deficiency. Bone 66, 146–154 10.1016/j.bone.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabbota A. L., Kim H. R., Zhe X., Fridman R., Bonfil R. D., Cher M. L. (2010) Shedding of RANKL by tumor-associated MT1-MMP activates Src-dependent prostate cancer cell migration. Cancer Res. 70, 5558–5566 10.1158/0008-5472.CAN-09-4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hikita A., Yana I., Wakeyama H., Nakamura M., Kadono Y., Oshima Y., Nakamura K., Seiki M., Tanaka S. (2006) Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-kappaB ligand. J. Biol. Chem. 281, 36846–36855 10.1074/jbc.M606656200 [DOI] [PubMed] [Google Scholar]
- 15.Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S. A., Mankani M., Robey P. G., Poole A. R., Pidoux I., Ward J. M., Birkedal-Hansen H. (1999) MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99, 81–92 10.1016/S0092-8674(00)80064-1 [DOI] [PubMed] [Google Scholar]
- 16.Devy L., Huang L., Naa L., Yanamandra N., Pieters H., Frans N., Chang E., Tao Q., Vanhove M., Lejeune A., van Gool R., Sexton D. J., Kuang G., Rank D., Hogan S., Pazmany C., Ma Y. L., Schoonbroodt S., Nixon A. E., Ladner R. C., Hoet R., Henderikx P., Tenhoor C., Rabbani S. A., Valentino M. L., Wood C. R., Dransfield D. T. (2009) Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 69, 1517–1526 10.1158/0008-5472.CAN-08-3255 [DOI] [PubMed] [Google Scholar]
- 17.Ager E. I., Kozin S. V., Kirkpatrick N. D., Seano G., Kodack D. P., Askoxylakis V., Huang Y., Goel S., Snuderl M., Muzikansky A., Finkelstein D. M., Dransfield D. T., Devy L., Boucher Y., Fukumura D., Jain R. K. (2015) Blockade of MMP14 activity in murine breast carcinomas: implications for macrophages, vessels, and radiotherapy. J. Natl. Cancer Inst. 107, djv017 10.1093/jnci/djv017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko K., Williams R. O., Dransfield D. T., Nixon A. E., Sandison A., Itoh Y. (2016) Selective inhibition of membrane type 1 matrix metalloproteinase abrogates progression of experimental inflammatory arthritis: synergy with tumor necrosis factor blockade. Arthritis Rheumatol. 68, 521–531 10.1002/art.39414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu X., Delgado-Calle J., Condon K. W., Maycas M., Zhang H., Carlesso N., Taketo M. M., Burr D. B., Plotkin L. I., Bellido T. (2015) Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc. Natl. Acad. Sci. USA 112, E478–E486 10.1073/pnas.1409857112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato A. Y., Cregor M., Delgado-Calle J., Condon K. W., Allen M. R., Peacock M., Plotkin L. I., Bellido T. (2016) Protection from glucocorticoid-induced osteoporosis by anti-catabolic signaling in the absence of Sost/sclerostin. J. Bone Miner. Res. 31, 1791–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee Y., Allen M. R., Condon K., Lezcano V., Ronda A. C., Galli C., Olivos N., Passeri G., O’Brien C. A., Bivi N., Plotkin L. I., Bellido T. (2011) PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J. Bone Miner. Res. 26, 1035–1046 10.1002/jbmr.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu X., Rhee Y., Condon K. W., Bivi N., Allen M. R., Dwyer D., Stolina M., Turner C. H., Robling A. G., Plotkin L. I., Bellido T. (2012) Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50, 209–217 10.1016/j.bone.2011.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25, 402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 24.Delgado-Calle J., Anderson J., Cregor M. D., Condon K. W., Kuhstoss S. A., Plotkin L. I., Bellido T., Roodman G. D. (2017) Genetic deletion of sost or pharmacological inhibition of sclerostin prevent multiple myeloma-induced bone disease without affecting tumor growth. Leukemia 31, 2686–2694 10.1038/leu.2017.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delgado-Calle J., Anderson J., Cregor M. D., Hiasa M., Chirgwin J. M., Carlesso N., Yoneda T., Mohammad K. S., Plotkin L. I., Roodman G. D., Bellido T. (2016) Bidirectional Notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 76, 1089–1100 10.1158/0008-5472.CAN-15-1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado-Calle J., Sañudo C., Bolado A., Fernández A. F., Arozamena J., Pascual-Carra M. A., Rodriguez-Rey J. C., Fraga M. F., Bonewald L., Riancho J. A. (2012) DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J. Bone Miner. Res. 27, 926–937 10.1002/jbmr.1491 [DOI] [PubMed] [Google Scholar]
- 27.Delgado-Calle J., Sañudo C., Fernández A. F., García-Renedo R., Fraga M. F., Riancho J. A. (2012) Role of DNA methylation in the regulation of the RANKL-OPG system in human bone. Epigenetics 7, 83–91 10.4161/epi.7.1.18753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suvannasankha A., Tompkins D. R., Edwards D. F., Petyaykina K. V., Crean C. D., Fournier P. G., Parker J. M., Sandusky G. E., Ichikawa S., Imel E. A., Chirgwin J. M. (2015) FGF23 is elevated in multiple myeloma and increases heparanase expression by tumor cells. Oncotarget 6, 19647–19660 10.18632/oncotarget.3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders M. M., Simmerman L. A., Reed G. L., Sharkey N. A., Taylor A. F. (2010) Biomimetic bone mechanotransduction modeling in neonatal rat femur organ cultures: structural verification of proof of concept. Biomech. Model. Mechanobiol. 9, 539–550 10.1007/s10237-010-0195-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacheco-Costa R., Hassan I., Reginato R. D., Davis H. M., Bruzzaniti A., Allen M. R., Plotkin L. I. (2014) High bone mass in mice lacking Cx37 because of defective osteoclast differentiation. J. Biol. Chem. 289, 8508–8520 10.1074/jbc.M113.529735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S., Eleniste P. P., Wayakanon K., Mandela P., Eipper B. A., Mains R. E., Allen M. R., Bruzzaniti A. (2014) The Rho-GEF kalirin regulates bone mass and the function of osteoblasts and osteoclasts. Bone 60, 235–245 10.1016/j.bone.2013.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zucker S., Drews M., Conner C., Foda H. D., DeClerck Y. A., Langley K. E., Bahou W. F., Docherty A. J., Cao J. (1998) Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP). J. Biol. Chem. 273, 1216–1222 10.1074/jbc.273.2.1216 [DOI] [PubMed] [Google Scholar]
- 33.Cappariello A., Paone R., Maurizi A., Capulli M., Rucci N., Muraca M., Teti A. (2015) Biotechnological approach for systemic delivery of membrane receptor activator of NF-κB ligand (RANKL) active domain into the circulation. Biomaterials 46, 58–69 10.1016/j.biomaterials.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong J., Cawley K., Piemontese M., Fujiwara Y., Macleod R., Goellner J., Zhao H., O’Brien C. (2017) The soluble form of RANKL contributes to cancellous bone remodeling in adult mice but is dispensable for ovariectomy-induced bone loss. J. Bone Miner. Res., http://www.asbmr.org/ItineraryBuilder/PresentationDetail.aspx?pid=6a768380-8d00-4f25-bd08-49ca4524355f&ptag=WebItinerarySearch [Google Scholar]
- 35.Pacifici R. (2016) T cells, osteoblasts, and osteocytes: interacting lineages key for the bone anabolic and catabolic activities of parathyroid hormone. Ann. N. Y. Acad. Sci. 1364, 11–24 10.1111/nyas.12969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien C. A., Nakashima T., Takayanagi H. (2013) Osteocyte control of osteoclastogenesis. Bone 54, 258–263 10.1016/j.bone.2012.08.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien C. A. (2010) Control of RANKL gene expression. Bone 46, 911–919 10.1016/j.bone.2009.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., Suda T. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 95, 3597–3602 10.1073/pnas.95.7.3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pacifici R. (2016) The role of IL-17 and TH17 cells in the bone catabolic activity of PTH. Front. Immunol. 7, 57 10.3389/fimmu.2016.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong J., Piemontese M., Onal M., Campbell J., Goellner J. J., Dusevich V., Bonewald L., Manolagas S. C., O’Brien C. A. (2015) Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One 10, e0138189 10.1371/journal.pone.0138189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galli C., Zella L. A., Fretz J. A., Fu Q., Pike J. W., Weinstein R. S., Manolagas S. C., O’Brien C. A. (2008) Targeted deletion of a distant transcriptional enhancer of the receptor activator of nuclear factor-kappaB ligand gene reduces bone remodeling and increases bone mass. Endocrinology 149, 146–153 10.1210/en.2007-0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmbeck K., Bianco P., Pidoux I., Inoue S., Billinghurst R. C., Wu W., Chrysovergis K., Yamada S., Birkedal-Hansen H., Poole A. R. (2005) The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J. Cell Sci. 118, 147–156 10.1242/jcs.01581 [DOI] [PubMed] [Google Scholar]
- 43.Gutiérrez-Fernández A., Soria-Valles C., Osorio F. G., Gutiérrez-Abril J., Garabaya C., Aguirre A., Fueyo A., Fernández-García M. S., Puente X. S., López-Otín C. (2015) Loss of MT1-MMP causes cell senescence and nuclear defects which can be reversed by retinoic acid. EMBO J. 34, 1875–1888 10.15252/embj.201490594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strongin A. Y. (2010) Proteolytic and non-proteolytic roles of membrane type-1 matrix metalloproteinase in malignancy. Biochim. Biophys. Acta 1803, 133–141 10.1016/j.bbamcr.2009.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rozanov D. V., Sikora S., Godzik A., Postnova T. I., Golubkov V., Savinov A., Tomlinson S., Strongin A. Y. (2004) Non-proteolytic, receptor/ligand interactions associate cellular membrane type-1 matrix metalloproteinase with the complement component C1q. J. Biol. Chem. 279, 50321–50328 10.1074/jbc.M409174200 [DOI] [PubMed] [Google Scholar]
- 46.Cosman F., Nieves J. W., Dempster D. W. (2017) Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J. Bone Miner. Res. 32, 198–202 10.1002/jbmr.3051 [DOI] [PubMed] [Google Scholar]
- 47.Eriksen E. F., Brown J. P. (2016) Commentary: concurrent administration of PTH and antiresorptives: additive effects or DXA cosmetics. Bone 86, 139–142 10.1016/j.bone.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 48.Leder B. Z., Tsai J. N., Burnett-Bowie S. A., Bouxsein M. L., Neer R. M. (2016) Letter to the editor in response to the commentary, “concurrent administration of PTH and antiresorptives: additive effects or DXA cosmetics.” Bone 89, 73–74 10.1016/j.bone.2016.04.026 [DOI] [PubMed] [Google Scholar]
- 49.Allen M. R., Iwata K., Phipps R., Burr D. B. (2006) Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone 39, 872–879 10.1016/j.bone.2006.04.028 [DOI] [PubMed] [Google Scholar]
- 50.Allen M. R., Burr D. B. (2007) Mineralization, microdamage, and matrix: how bisphosphonates influence material properties of bone. Bonekey Osteovision 4, 49–60 10.1138/20060248 [DOI] [Google Scholar]
- 51.Aspenberg P., Schilcher J. (2014) Atypical femoral fractures, bisphosphonates, and mechanical stress. Curr. Osteoporos. Rep. 12, 189–193 10.1007/s11914-014-0200-9 [DOI] [PubMed] [Google Scholar]
- 52.Shane E., Burr D., Abrahamsen B., Adler R. A., Brown T. D., Cheung A. M., Cosman F., Curtis J. R., Dell R., Dempster D. W., Ebeling P. R., Einhorn T. A., Genant H. K., Geusens P., Klaushofer K., Lane J. M., McKiernan F., McKinney R., Ng A., Nieves J., O’Keefe R., Papapoulos S., Howe T. S., van der Meulen M. C., Weinstein R. S., Whyte M. P. (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 29, 1–23 10.1002/jbmr.1998 [DOI] [PubMed] [Google Scholar]
- 53.Miller P. D., Hattersley G., Riis B. J., Williams G. C., Lau E., Russo L. A., Alexandersen P., Zerbini C. A., Hu M. Y., Harris A. G., Fitzpatrick L. A., Cosman F., Christiansen C.; ACTIVE Study Investigators . (2016) Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316, 722–733 10.1001/jama.2016.11136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.