Abstract
Although yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), nuclear transducers of the Hippo pathway, are mostly silent in adult organs, aberrant activation of YAP/TAZ promotes tumorigenesis and abnormal tissue repair. The extent of involvement of TAZ in chronic kidney disease (CKD) is unknown. In our study, increased TAZ nuclear accumulation and expression in the tubulointerstitium was readily evident in 3 models of renal injury including obstructive, aristolochic acid (AA), and diabetic nephropathy, correlating with fibrosis progression. Stable TAZ overexpression in human kidney (HK)-2 epithelial cells promoted connective tissue growth factor (CTGF), fibronectin, vimentin, and p21 expression, epithelial dedifferentiation, and growth inhibition, in part, via Sma mothers against decapentaplegic homologue (SMAD)-3–dependent CTGF induction. CTGF secretion by TAZ-overexpressing epithelium also triggered proliferative defects in nonengineered HK-2 cells confirming a nonautonomous role of TAZ (via a paracrine mechanism) in orchestrating kidney epithelial cell–cell communication. Renal tubular-specific induction of TGF-β1 in mice and TGF-β1 stimulation of HK-2 cells resulted in TAZ protein up-regulation. TAZ stable silencing in HK-2 cells abrogated TGF-β1–induced expression of target genes without affecting SMAD3 phosphorylation, which is also crucial for fibrotic reprogramming. Thus, TAZ was activated in fibrosis through TGF-β1–dependent mechanisms and sustained TAZ signaling promotes epithelial maladaptive repair. TAZ is also a novel non-SMAD downstream effector of renal TGF-β1 signaling, establishing TAZ as a new antifibrosis target for treatment of CKD.—Anorga, S., Overstreet, J. M., Falke, L. L., Tang, J., Goldschmeding, R. G., Higgins, P. J., Samarakoon, R. Deregulation of Hippo-TAZ pathway during renal injury confers a fibrotic maladaptive phenotype.
Keywords: CTGF, TGF-β1, CKD, UUO, AAN
Tissue fibrosis, which often culminates in end-stage organ failure, accounts for nearly half of all mortalities (1). Renal injury resulting from diabetes, obstruction, hypertension, ischemia, and nephrotoxins leads to tubulointerstitial fibrosis characterized by tubular epithelial cell cycle arrest and cell death, secretion of profibrotic factors, inflammation, and interstitial expansion (2–7). Progressive fibrosis predisposes to chronic kidney disease (CKD) which affects 8–16% of the world population (8–10). Adding to this major public health burden are inadequacies in renal replacement therapy, to meet the needs of patients with end-stage kidney disease worldwide (7–11). Effective treatments to halt CKD progression are currently lacking and, in this regard, uncovering novel mechanisms that mediate tubulointerstitial fibrosis is a significant step in developing future treatments (1, 8).
Hippo pathway involvement in the progression of CKD remains relatively unexplored. Activated Hippo signaling maintains cellular polarity and organ size by suppressing the nuclear function of transcriptional coactivator with PDZ-binding motif (TAZ) and its paralogue yes-associated protein (YAP) (reviewed in refs. 12–14). Establishment of cell polarity activates core components of the Hippo pathway [e.g., large tumor suppressor homologue (Lats)-1/2 kinases, mammalian sterile-20-like protein kinase], which suppress the nuclear translocation while promoting cytoplasmic retention of YAP/TAZ via Lats1/2 kinase-mediated YAP/TAZ phosphorylation (12–14). LATS1/2, mammalian sterile 20-like protein kinase, and YAP/TAZ form a complex in confluent cells where YAP/TAZ remains phosphorylated and inactive. Loss of cell–cell junctions disrupts this complex to promote YAP/TAZ signaling (12–15). Increased mechanical forces and soluble factors also promote YAP/TAZ activation (evident from the increased protein stability, nuclear accumulation, and decreased phosphorylation), YAP/TAZ-dependent gene expression [e.g., connective tissue growth factor (CTGF), Cyr61] and context-dependent phenotypic modifications, including cell proliferation, epithelial plasticity, transformation, and migration (12–18). However, TAZ/YAP do not bind to DNA and must have cofactors, such as TEA domain-containing (TEAD) and Sma mothers against decapentaplegic homologue (SMAD)-2/3 for their nuclear transduction function (12, 13, 19).
The YAP/TAZ pathway is relatively inactive in adults, to maintain organ homeostasis, but are robustly induced during neoplastic disorders (14, 17, 18, 20). Increased TAP/TAZ expression and core Hippo pathway deregulation are indeed linked to cancer progression in various organs (15–20). YAP/TAZ recently emerged as mechanotransducers during lung, liver, and renal disease progression, as well (21–23). Elevated TAZ expression in lung injury contributes to pulmonary disease progression, given that TAZ-deficient mice are protected from bleomycin-induced lung fibrosis compared to wild-type littermates (21, 24).
Loss of epithelial cell polarity and integrity and increased tissue mechanical forces are hallmarks of renal injury (2, 25–27). It is not known, however, whether TAZ regulates maladaptive repair responses and epithelial dysfunction critical for progressive tubulointerstitial disease. Therefore, using 3 distinct and well-characterized mouse models of nephropathy (originating from obstructive, diabetic, and toxin-induced renal injuries), we determined the extent of TAZ deregulation in CKD and tested whether TGF-β1, a master regulator of renal fibrosis (28, 29), is an upstream inducer of TAZ activation using transgenic mice that conditionally up-regulates renal epithelial TGF-β1 expression.
MATERIALS AND METHODS
Creation of stable cell lines and reagents
Human proximal tubular epithelial HK-2 cells were grown in DMEM supplemented with 5–10% fetal bovine serum (FBS). To generate stable TAZ-expressing cultures, semiconfluent HK-2 cells were infected with lentiviruses bearing a cytomegalovirus (CMV) promoter–driven TAZ cDNA construct (CMV-TAZ) or an empty vector (CMV-Con) (GeneCopoeia, Rockville, MD, USA) in 5 μg/ml Polybrene in 10% FBS/DMEM for 24 h. The cells were allowed to recover for 24 h before selection in 5 μg/ml puromycin in 10% FBS/DMEM; media were changed every 3 d. TAZ induction was confirmed by immunoblot analysis. To generate stable double-transgenic epithelial cell lines with SMAD3 or CTGF-silencing in the context of TAZ up-regulation, low-density (40%) CMV-TAZ–expressing HK-2 cells were reinfected (for 1 d) with control, SMAD3, or CTGF short hairpin RNA (shRNA) lentiviral constructs (Santa Cruz Biotechnology, Dallas, TX, USA) followed by stable selection, as previously described. SMAD3 or CTGF depletion in TAZ-overexpressing double-transgenic cultures were confirmed by Western blot analysis. To create HK-2 cells with TAZ and SMAD3 stable depletion, semiconfluent cultures growing in serum-containing medium were infected with TAZ, SMAD3 or control shRNA lentiviral particles (Santa Cruz Biotechnology) in 5 μg/ml Polybrene + 5% FBS/DMEM for 24 h. Stable clones were selected in 5 μg/ml puromycin, as previously described. TAZ stable knockdown was confirmed by Western blot analysis. For studies involving rescue of TAZ expression in TAZ-silenced cells, TAZ shRNA stably expressing HK-2 cells at semiconfluence were infected with either CMV-driven TAZ (CMV-TAZ) or empty vector (CMV-Con) expressing lentiviral constructs (GeneCopoeia) for 3 d before serum deprivation for 24 h and TGF-β1 stimulation. A SMAD3 inhibitor, SIS3, and a YAP/TAZ inhibitor, verteporfin, were purchased from EMD Chemicals (San Diego, CA, USA) and Millipore-Sigma (Billerica, MA, USA), respectively.
Mouse model of unilateral ureteral obstruction
C57Bl/6 mice (both male and female) were anesthetized by isoflurane inhalation. After a small incision made in the flank under aseptic conditions, the left ureter was exposed and ligated with two 5-0 silk sutures. The right contralateral kidney served as a control, as did mice undergoing sham surgery, but not ureteral ligation. On d 14 after surgery, all animals were euthanized with ketamine-xylazine-atropine and the obstructed (UUO), contralateral (contra), and sham procedure kidneys were harvested. Animal Ethics Committee of the University of Utrecht approved all animal protocols.
Streptozotocin-induced renal injury
C57Bl/6 mice received a single intraperitoneal injection of 200 mg/kg streptozotocin (STZ; 30 mg/ml dissolved in 100 mM sodium citrate buffer; pH 4.5) (Millipore-Sigma) or sodium citrate buffer alone (controls). Hyperglycemia was determined 3 d after injection by measurement of blood glucose levels (Medisense Precision Xtra; Abbott Laboratories). Nonresponders were injected with a second dose of STZ. Animals had continuous access to standard laboratory chow with daily addition of mash food. Slow-release insulin pellets (Linshin, Scarborough, ON, Canada) were implanted in the animals 5 d after STZ injection. The mice were euthanized with ketamine-xylazine-atropine 26 wk after induction of a diabetic state. This procedure was approved by the Experimental Animal Ethics Committee of the University of Utrecht.
Aristolochic acid-induced nephropathy
C57Bl/6 male mice received an intraperitoneal injection of aristolochic acid sodium salt (AA; 5 mg/kg body weight dissolved in distilled water, A9451; Millipore-Sigma) once a day for 5 consecutive days or NaCl vehicle (control animals) alone. Urine creatinine levels served to confirm renal injury in response to AA (J2L Elitech, Labarthe-Inard, France). Day 25 after the initial injections, mice in both groups were euthanized with ketamine-xylazine-atropine. The Animal Ethics Committee of the University of Utrecht approved these studies.
Investigation of paracrine factors mediating renal epithelial–epithelial and epithelial–fibroblast cross-talk
Conditioned media derived from equally dense CMV-Con– or CMV-TAZ-expressing HK-2 cells maintained in 5 μg/ml puromycin + DMEM + 2.5% FBS were directly added to HK-2 cells and NRK-49F renal fibroblasts stably expressing a control shRNA construct; cell cultures (initially at similar densities) were allowed to grow for an additional 3–5 d. Cell counts were determined with a Sceptor 2.0 Handheld Automated Cell Counter (Millipore-Sigma) or by direct cell count measurements of randomly chosen 5 fields for each experimental condition. Transfer of conditioned media from CMV-TAZ + Con shRNA and CMV-TAZ + CTGF shRNA double transductants to control shRNA-expressing HK-2 cultures (as previously described) served to determine the influence of CTGF expression as a consequence of TAZ up-regulation of the growth of proximal tubular epithelial cells.
Western blot analysis
Cultured cells were lysed in sample buffer containing 5% 2-ME and renal tissue lysates prepared in 2–4% SDS in PBS; samples were vortexed, homogenized, and boiled for 5 min. After electrophoretic separation, proteins were transferred to nitrocellulose membranes and blocked in 5% milk in 0.05% Triton-X 100/PBS before incubation overnight with the following primary antibodies; rabbit anti-TAZ (1:1000), rabbit anti-YAP/TAZ (1:1000), rabbit anti-YAP (1:1000), rabbit anti-SMAD3 (1:1000), and rabbit anti-p21 (1:1000), all from Cell Signaling Technology (Danvers, MA, USA). Rabbit anti-phospho-SMAD3 (1:1,000), rabbit anti-fibronectin (1:10,000), and rabbit anti-α-smooth muscle actin (α-SMA; 1:2,000) were products of Abcam Inc (Cambridge, MA, USA). Rabbit anti-vimentin (1:5,000), rabbit anti-TAZ (1:1000), rabbit anti-GAPDH (1:5,000), goat anti-CTGF (1:500), and rabbit anti p21 (1:1000) were from Santa Cruz Biotechnology. Mouse anti-E-cadherin (1:1000) was purchased from BD Biosciences (San Jose, CA, USA). Membranes were washed 3 times in 0.05% Triton-X 100/PBS before incubation with appropriate secondary antibodies for 45 min. After three 15-min washes in 0.05% Triton-X 100/PBS, immunoreactive proteins were visualized with ECL reagent and quantitated by densitometry.
Immunohistochemistry
Following deparaffinization, kidney sections were subjected to antigen retrieval and quenching of endogenous peroxidase activity. Sections were blocked in 10% normal goat serum and incubated (30 min) with primary rabbit antibodies to TAZ (1:200; Santa Cruz Biotechnology) in 1% bovine serum albumin followed by appropriate secondary biotinylated antibodies (Vector Laboratories, Burlingame, CA, USA) for 30 min. Vectastain Elite ABC (Vector Laboratories) was added for 30 min and the reactions developed with 3,3′-diaminobenzidine DAB peroxidase substrate before counterstaining with hematoxylin.
Morphometric analysis
Tissue sections were scanned with a semiautomated digital microscope (NanoZoomer 2.0-RS; Hamamatsu Photonics Corporation, Hamamatsu, Japan) and images analyzed with the Nanozoomer Digital Pathology viewer software (NDP.view; Hamamatsu Photonics Corp., Hamamatsu City, Japan). Representative UUO and control (Con) renal images were saved in JPEG format. A grid consisting of 56 (100 µm each) squares was overlaid on the immunostained sections before importing into ImageJ (National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/). DAB staining intensity was assessed using color threshold analysis (YUV colorspace, V-filter bypass) for conversion to black and white, such that positive staining appeared black and absence of stain was white. Image type was converted to 8-bit and a new binary threshold established to calculate the percentage area of total positive DAB staining for each 100 µm2. A second threshold analysis provided the percentage area within each of the dilated tubules. By subtracting dead space within each tubule from the total tissue area (100 µm2), the percentage of actual tissue area that stained positively for each of the 56 squares was calculated. All squares were included in-tissue analysis, and used for calculation of mean, standard diviations, and significance values.
Subcellular fractionation
HK-2 cultures were scraped in PBS, pelleted by centrifugation and resuspended in hypertonic Solution A [10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, and 0.1 mM EGTA] supplemented with protease and phosphatase inhibitor cocktail and shaken on ice for 20 min. Solution B (3% NP-40) was added, followed by centrifugation (8000 rpm: 60 s) to isolate the cytoplasmic fraction. The remaining pellet was resuspended in Solution C [20 mM HEPES (pH 7.9), 0.42 M NaCl, 1 mM EDTA, 1 mM EGTA, and 1 mM DTT] supplemented with protease and phosphatase inhibitor cocktail, subjected to several freeze–thaw cycles and incubated overnight in the cold room. The nuclear (supernatant) fraction was separated at 8000 rpm for 5 min. Lamin A/C provided a marker for nuclear loading.
Cell-cycle analysis
CMV-Con- or CMV-TAZ-expressing HK-2 cells were grown in complete medium supplemented with puromycin to 70–80% confluence. Cells were harvested in trypsin, incubated with soybean trypsin inhibitor (5 min), washed with PBS, and fixed in 95% ethanol (1 h). After 2 washes in PBS, cells were incubated with RNaseA (20 μl/ml) and propidium iodide (2.5 μg/ml) in PBS/Triton-X 100 for 2 h in the dark. Cell cycle distributions were measured with a FACS Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
A 2-tailed Student’s t test and ANOVA with Tukey’s post hoc analysis were used to assess significant differences. Results were significant at P < 0.05.
RESULTS
TAZ activation in multiple models of renal fibrosis
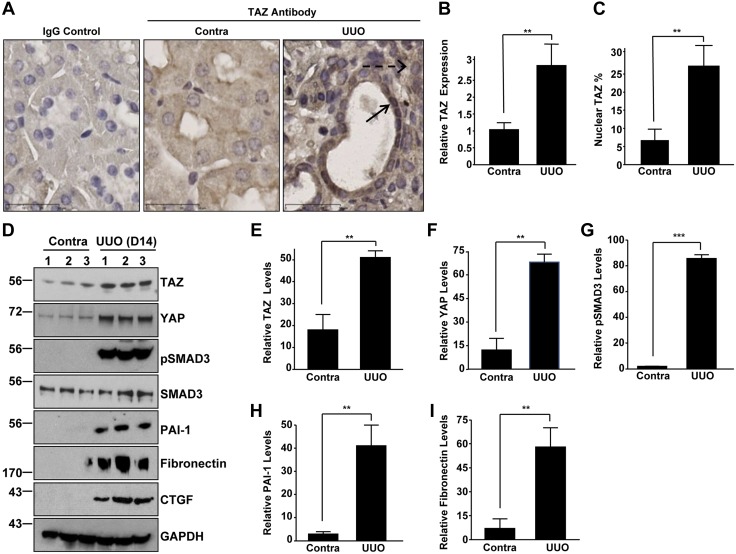
Three established mouse models were used to assess the role of TAZ in the development of CKD. UUO is a highly reproducible animal model for inducing renal fibrosis (30). Increased expression (Fig. 1A, B; 2.5-fold) and nuclear staining (Fig. 1A, C; >5-fold) of TAZ were observed in both the tubular and interstitial compartments of the obstructed (UUO) kidney compared to contralateral controls. Western blot analysis also confirmed the up-regulation of TAZ protein (Fig. 1D, E), TAZ paralogue, and YAP (Fig. 1D, F), as well as increased pSMAD3 (Fig. 1D, G), plasminogen activator inhibitor (PAI)-1 (Fig. 1D, H), fibronectin (Fig. 1D, I), and CTGF expression in the fibrotic UUO kidney, relative to levels in the nonligated controls.
Figure 1.
TAZ activation in a murine model of obstructive nephropathy. A) Immunohistochemical staining of sections of contralateral (Contra) and UUO (d 14) kidneys with a rabbit TAZ antibody and an IgG control. Scale bars, 30 μm. B) Quantitative image analysis of DAB signal (mean ± sd) illustrating differences in TAZ expression between fibrotic (UUO) and contralateral kidneys is shown in the histogram, with the staining intensity of the contralateral kidney set at 1. C) Percentages of cells with nuclear TAZ expression relative to total cells in randomly chosen contralateral and UUO kidneys sections (n = 5–10). D–I ) Western blot analysis for TAZ (D, E), YAP (D, F), pSMAD3 (D, G), PAI-1 (D, H), fibronectin (D, I), CTGF, SMAD3, and GAPDH (a loading marker) expression levels in both contralateral (Contra) and UUO kidney extracts were performed 14 d after surgery E–I) Summary of the relative expression of indicated markers in UUO and contralateral kidneys. Data in all histograms are expressed as means ± sd. (n = 5 mice/group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. contra.
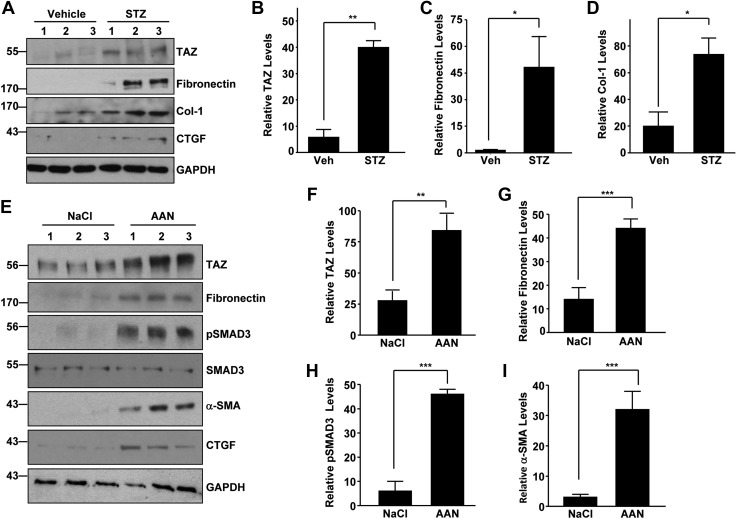
STZ-induced renal injury is a widely used rodent model for inducing diabetic nephropathy, a major cause of CKD in the United States (31, 32). Western blot analysis of kidney lysates derived from vehicle (Veh) and STZ-treated (a dose of 200 mg/kg) mice also indicated >5-fold increase in TAZ expression (Fig. 2A, B) in the STZ-treated kidneys compared to vehicle-treated controls (at 26 wk). Consistent with the state of fibrosis, STZ-treated kidneys have higher levels of expression of fibronectin (Fig. 2A, C), collagen-1 (Fig. 2A, D), and CTGF (Fig. 2A).
Figure 2.
TAZ deregulation and overexpression in diabetic nephropathy and AAN. A–D) Immune blot analysis of kidney lysates derived from Na citrate vehicle- (Veh) and STZ-treated (200 mg/kg for 26 wk) mice for TAZ (A, B), fibronectin (A, C), collagen-1 (A, D), CTGF, and GAPDH expression. B–D) Relative levels of the indicated proteins between Na citrate (Veh) or diabetic (STZ) kidneys (n = 3–4). A, E) Lanes 1–3: individual mice for each experimental group. E–I) Western blot evaluations of the relative TAZ (E, F), fibronectin (E, G), pSMAD3 (E, H), α-SMA (E, I) and CTGF protein levels between NaCl vehicle- or AA-treated (5 mg/kg; 25 d) kidneys. F–I) Relative expression of indicated markers between NaCl (Veh) or AAN kidneys (n = 3–5 animals/group). Data in all histograms are expressed as means ± sd. *P < 0.05, **P < 0.01, ***P < 0.001, vs. vehicle treated kidneys.
AA nephropathy (AAN), evident in Chinese and Balkan populations, causes tubulointerstitial disease after acute kidney injury (33). AA-mediated kidney injury in mice (at d 25) is also associated with elevated TAZ (Fig. 2E, F; >3-fold), fibronectin (Fig. 2E, G), pSMAD3 (Fig. 2E, H), α-SMA (Fig. 2E, I), and CTGF (Fig. 2E) protein levels compared to the expression levels of NaCl-treated control kidneys. These findings reveal a strong correlation between TAZ activation and fibrogenesis induced by various etiologies.
Renal epithelial TAZ overexpression promotes fibrotic factor induction, dedifferentiation, and growth arrest
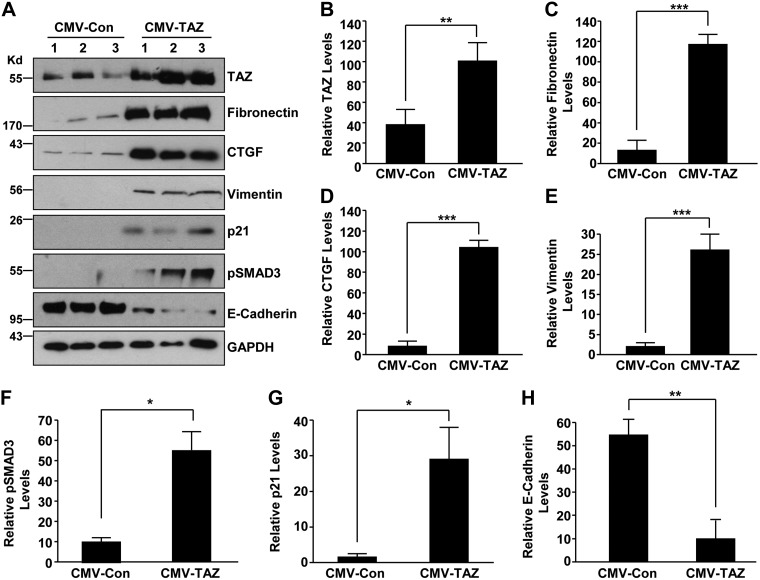
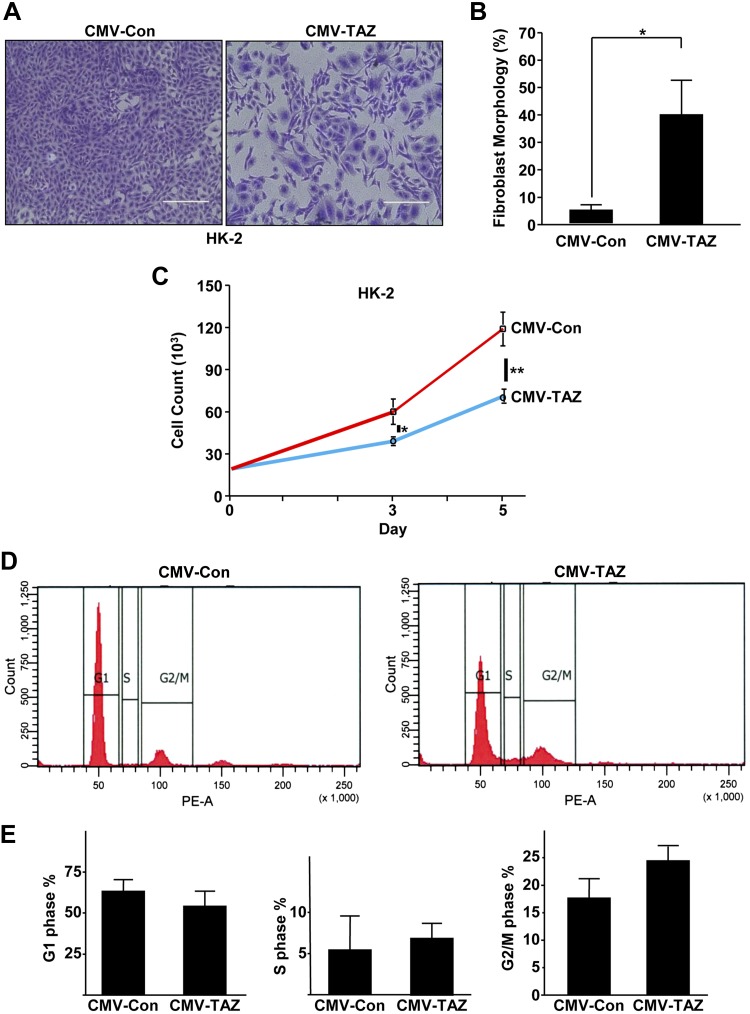
To mimic the elevated TAZ expression in the injured renal epithelium (Fig. 1), we stably expressed TAZ in HK-2 cells via lentiviral transduction (CMV-TAZ cells) which resulted in >2.5-fold increase in TAZ expression, relative to control vector–transduced (CMV-Con) cultures (Fig. 3A, B). Prolonged stable TAZ expression (7 d) resulted in increased expression of fibronectin (Fig. 3A, C; 11-fold), CTGF (Fig. 3A, D; >13-fold), vimentin (Fig. 3A, E; 6-fold), pSMAD3 (Fig. 3A, F; 5-fold), and the growth-arrest gene p21 (Fig. 3A, G; 13-fold), whereas levels of an epithelial cell adhesion molecule, E-cadherin (Fig. 3A, H), were significantly decreased (>75%) relative to CMV-Con–expressing cultures. Alterations in epithelial morphology, reflected by a 6-fold increase in cells with fibroblast-like phenotype (Fig. 4A, B), increased mesenchymal marker vimentin expression and decreased E-cadherin levels (Fig. 3), indicative of TAZ-driven epithelial dedifferentiation, were also evident in TAZ-overexpressing epithelial cells, compared with CMV-Con cultures. Cell growth analysis of equally seeded monolayers also revealed that TAZ ectopic expression resulted in epithelial growth inhibition (Fig. 4A, C vs. CMV-Con at d 5) and G2/M cell cycle arrest (Fig. 4D, E) compared with CMV-Con–expressing cells, consistent with enhanced p21 expression by TAZ up-regulation (Fig. 3).
Figure 3.
Ectopic expression of TAZ leads to fibrotic factor induction in HK-2 renal epithelial cells. HK-2 cells engineered to stably express either empty vector (CMV-Con) or TAZ construct (CMV-TAZ) were grown for 7 d before Western blot assessments for TAZ (A, B), fibronectin (A, C), CTGF (A, D), vimentin (A, E), pSMAD3 (A, F), p21 (A, G), E-cadherin (A, H), and GAPDH expression. Three independent experiments are shown (lanes 1–3; A). B–H) Relative protein levels for CMV-Con and CMV-TAZ stable transductants. Data in all histograms are expressed as means ± sd (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 vs. CMV-Con.
Figure 4.
Epithelial TAZ up-regulation is associated with dedifferentiation and G2/M proliferative arrest. A) Similarly seeded CMV-Con– or CMV-TAZ–expressing HK-2 cells, maintained in complete medium for 5 d, were stained with crystal violet. Scale bars, 400 μm. B) Percentage of cells assuming a fibroblast morphology in CMV-Con– or CMV-TAZ–expressing HK-2 cultures. Means ± sd. *P < 0.05 vs. CMV-Con cells. C) The actual number of cells recovered from identically seeded (20,000 each) CMV-Con and CMV-TAZ cultures (n = 3) at d 3 and 5. *P < 0.05 at d 3 vs. CMV-Con cell count, **P < 0.001 at d 5 vs. CMV-Con cell count. D) Flow cytometry of propidium iodide–stained CMV-Con– and CMV-TAZ–expressing cells served to determine cell cycle distributions. E) The percentages of cells in the G1, S, and G2/M phases for each population for 3 separate studies. Data are expressed as means ± sd (B, C, E).
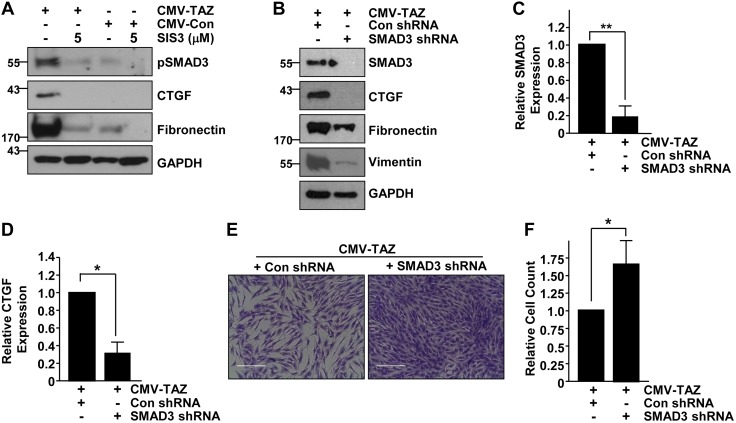
SMAD3-CTGF involvement in TAZ-induced epithelial dysfunction
Increased SMAD3 phosphorylation in epithelial cells with TAZ up-regulation warranted an investigation of potential SMAD3 involvement in TAZ-induced fibrotic programming (Fig. 3). Blockade of SMAD3 activity with the specific inhibitor, SIS3 (5 μM) in TAZ-overexpressing cultures not only reduced, as expected, pSMAD3 levels but also dramatically attenuated expression of CTGF and fibronectin expression compared to DMSO vehicle-treated, identically confluent, CMV-TAZ–expressing cells (Fig. 5A). Stable SMAD3 suppression via shRNA lentiviral transduction in TAZ-overexpressing HK-2 cells (Fig. 5B, C) similarly led to a dramatic decrease (>70%) in CTGF (Fig. 5B, D), fibronectin, and vimentin expression in comparison to CMV-TAZ cells stably transduced with control shRNA lentiviral particles (Fig. 5B). Crystal violet staining (Fig. 5E) and cell count analysis (Fig. 5F) revealed that CMV-TAZ + SMAD3 shRNA double-transduced cells attain greater cell density (>60%) relative to the similarly seeded CMV-TAZ + control shRNA cultures (Fig. 5F). These studies collectively establish that TAZ-driven epithelial fibrogenesis requires SMAD3 signaling.
Figure 5.
SMAD3 mediates the TAZ-driven epithelial fibrotic phenotype. A) Extracts from HK-2 cells stably expressing CMV-Con or CMV-TAZ treated with or without the SMAD3 inhibitor SIS3 (5 μM) were analyzed by Western blot for pSMAD3, CTGF, fibronectin, and GAPDH expression. B–D) CMV-TAZ + Con shRNA and CMV-TAZ + SMAD3 shRNA double-transductant protein extracts were similarly probed for SMAD3 (B, C), CTGF (B, D), fibronectin, vimentin, and GAPDH expression (B). E) Crystal violet staining of similarly seeded CMV-TAZ + Con shRNA and CMV-TAZ + SMAD3 shRNA stably transduced cell monolayers after 5 d of growth. Scale bars, 200 μm. F) Cell counts of parallel cultures setting the relative number of CMV-TAZ + Con shRNA–transduced cells as 1. Means ± sd. *P < 0.05, **P < 0.01.
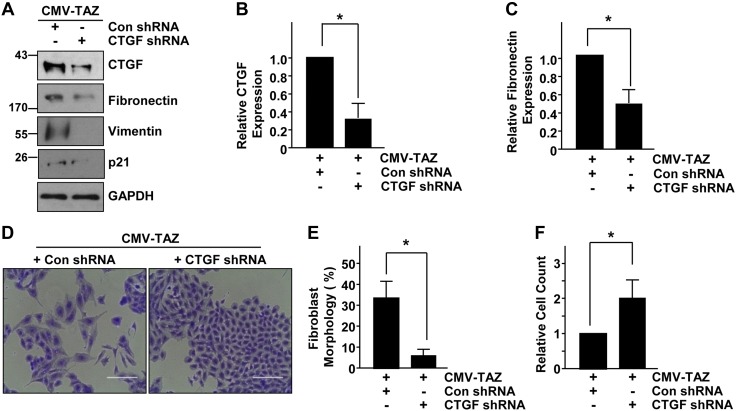
Because CTGF is a direct, well-known target of the YAP/TAZ pathway (Fig. 3) (12–14), gene-silencing approaches were used to investigate CTGF involvement in TAZ-induced epithelial dysfunction. Stable expression of CTGF shRNA in TAZ-expressing HK-2 cells (CMV-TAZ + CTGF shRNA cells) maintained under serum deprivation have significantly diminished CTGF (Fig. 6A, B), fibronectin (Fig. 6A, C), vimentin, and p21 expression relative to TAZ clones stably infected with a control shRNA lentivirus (CMV-TAZ + control shRNA) (Fig. 6A). Moreover, the mesenchymal appearance (Fig. 6D, E) and growth defects (Fig. 6D, F) evident in CMV-TAZ + control shRNA cells are largely reversed in CMV-TAZ + CTGF shRNA cells, which appear cuboidal and attain higher cell densities. The reversal of epithelial plasticity and growth inhibition are also accompanied by reductions in vimentin and p21 expression in CMV-TAZ + CTGF shRNA cells relative to CMV-TAZ + con shRNA-Con (Fig. 6A). Thus, these studies demonstrate that CTGF up-regulation (via the SMAD3 pathway) downstream of TAZ leads to fibrosis gene induction, dedifferentiation, and growth inhibition via autocrine mechanisms.
Figure 6.
CTGF is a crucial downstream transducer of the TAZ-driven epithelial maladaptive response. A) Protein lysates derived from cells stably expressing CMV-TAZ + Con shRNA and CMV-TAZ + CTGF shRNA were analyzed by immunoblot for expression of CTGF, fibronectin, vimentin, p21, and GAPDH. B, C) The relative levels of CTGF and fibronectin expression for 3 independent studies, with expression levels in CMV-TAZ + Con shRNA cells set at 1. D) Crystal violet staining of HK-2 cells stably expressing CMV-TAZ + Con shRNA and CMV-TAZ + CTGF shRNA after 3 d of growth. Original magnification, ×20. Scale bars, 200 μm. E, F) Relative percentage of cells with fibroblast-like morphology and final population density (set at 1 for CMV-TAZ + Con shRNA cultures) for 3 separate studies. Data in all histograms are expressed as means ± sd. *P < 0.05 vs. CMV-TAZ + Con shRNA.
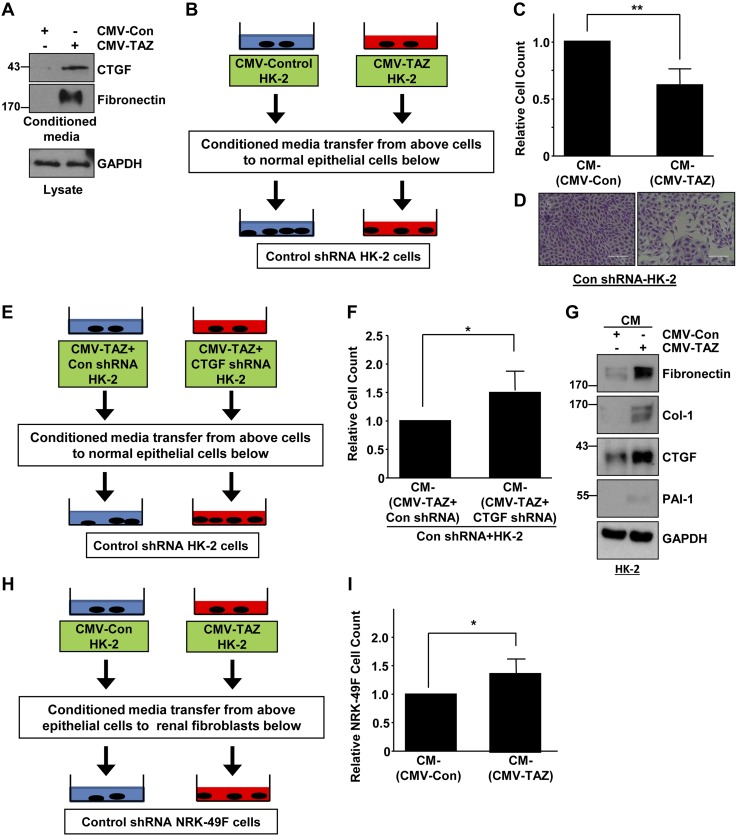
TAZ-induced soluble factors mediate renal epithelial–epithelial and epithelial–fibroblast communications
Paracrine factors (i.e., CTGF and TGF-β1) secreted by G2/M arrested epithelium after renal damage could promote fibroblast growth during CKD progression (2, 3). It is not known, however, whether soluble factors secreted by dysfunctional and fibrotic epithelial cells affect the normal epithelial phenotype. HK-2 cells overexpressing TAZ also promoted secretion of CTGF and fibronectin, compared with conditioned medium derived from CMV-Con transductants (Fig. 7A). To investigate the possible paracrine influence of such secreted factors on the renal epithelium, conditioned media isolated from CMV-Con– and CMV-TAZ–expressing cells (at similar density) were directly added to HK-2 cells stably transduced with control shRNA lentiviral particles (Fig. 7B). Incubation with conditioned medium isolated from TAZ-expressing HK-2 cells resulted in a >35% decline in normal epithelial population density relative to HK-2 cells treated with conditioned medium from CMV-Con–transduced cells (Fig. 7C, D). Furthermore, inhibition of renal epithelial cell growth triggered by conditioned medium from TAZ-overexpressing cells can be rescued by treatment of conditioned medium derived from TAZ-expressing HK-2 cells engineered to stably suppress CTGF expression CMV-TAZ + CTGF shRNA (Fig. 7E, F). In these experiments, TAZ-induced CTGF secretion by dysfunctional epithelial cells attenuated normal epithelial cell growth. Such crosstalk likely exacerbates renal injury. HK-2 cells stably expressing control shRNA treated with conditioned medium from CMV-TAZ–expressing HK-2 clones also assumed a fibrotic phenotype (i.e., increased expression of fibronectin, collagen-1, and CTGF) compared to Con-shRNA–expressing epithelial cultures similarly receiving conditioned medium from empty vector (CMV-Con)–transduced HK-2 cells (Fig. 7G). To test whether TAZ-induced paracrine factors orchestrate renal epithelial–fibroblast crosstalk, conditioned media isolated from HK-2 cells expressing TAZ or empty vector were transferred to control shRNA-expressing NRK-49F fibroblasts maintained at a similar density (Fig. 7H). Cell count analysis revealed that conditioned medium isolated from CMV-TAZ–expressing cells triggered NRK-49F growth (Fig. 7I; >30%) compared to fibroblasts receiving conditioned medium from vector-transduced HK-2 cultures. Thus, secreted factors consequent to TAZ epithelial cell up-regulation could also promote pathologic epithelial cell–fibroblast communication.
Figure 7.
TAZ-induced paracrine factors promote growth inhibition of normal kidney epithelial cells and trigger renal fibroblast growth. A) Conditioned media isolated from HK-2 cells stably expressing CMV-Con and CMV-TAZ were probed for CTGF and fibronectin expression by Western blot analysis. GAPDH expression of lysates of the cells provided an assessment of protein loading. B) Experimental design for assessing the potential influence of soluble factors in epithelial cell–cell crosstalk. Similarly confluent control shRNA-expressing HK-2 cells were treated with conditioned media isolated from CMV-Con– and CMV-TAZ–expressing HK-2 cells at a similar density for 3–5 d. C) Final cell densities were determined setting the number of cells in CM-CMV-Con treated HK-2 cells as 1 (means ± sd). *P < 0.01 vs. CM-CMV-Con. D) Crystal violet staining cell monolayers. Scale bars, 200 μm. E) Approach for investigating the potential paracrine role of CTGF in epithelial cell–cell communications. Conditioned media isolated from double-transgenic CMV-TAZ + Con shRNA– and CMV-TAZ + CTGF shRNA–expressing HK-2 cells at equal densities were directly added to similarly seeded control shRNA transduced HK-2 cells. F) Relative cell counts 3 d after addition of conditioned media for triplicate studies. *P < 0.05 vs. CM-CMV-TAZ + Con shRNA cell count (arbitrarily set at 1). G) Control shRNA-expressing epithelial cells were stimulated with conditioned media isolated from CMV-Con– or CMV-TAZ–expressing HK-2 cells, and lysate extracts were probed for fibronectin, collagen-1, CTGF, and PAI-1 protein expression. H) Conditioned media isolated from HK-2 cells stably expressing Con-CMV or TAZ-CMV maintained at a similar density were directly added to NRK-49F fibroblasts (stably expressing Con- shRNA) seeded at similar initial densities. I) Cell counts for each condition were assessed 3 d later. The plot illustrates cell counts for triplicate cultures, arbitrarily setting the number of NRK-49F cells receiving conditioned media from vector-expressing HK-2 cultures at 1. Means ± sd (C, F, I). *P < 0.05, **P <0.01 vs. CMV-Con.
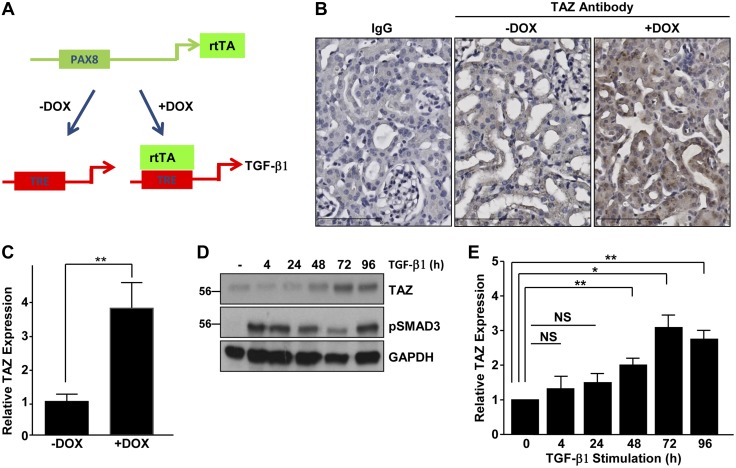
TGF-β1 promotes renal TAZ abundance in vivo and in vitro
Precise mechanisms of TAZ up-regulation in tissue fibrosis, in general, remain unknown. We investigated whether TGF-β1 is a potential upstream regulator of TAZ expression, because this cytokine is causatively linked to kidney fibrosis progression by numerous etiologies (1, 4, 28, 29). Double-transgenic animals (referred to as Pax8-rtTA-tet-o-TGF-β1 mice) specifically expressing TGF-β1 in the renal tubular epithelium spontaneously develop renal injury and peritubular fibrosis (34). In the current study, Pax8 promoter–driven expression of reverse tetracycline-dependent transactivator activates TGF-β1 expression only upon doxycycline treatment (Fig. 8A; +DOX). This animal model provides a direct method to test the upstream role of TGF-β1 in regulating Hippo signaling and minimizes the potential influence of additional fibrotic growth factors/cytokines (which contribute to the progression of renal injury) on the Hippo-TAZ pathway. Immunohistochemistry revealed that TAZ expression was significantly elevated (Fig. 8B, C; >3.5-fold) in tubules and interstitial cells of renal sections of Pax8-rtTA-tet-o-TGF-β1 mice stimulated with doxycycline to induce TGF-β1 expression for 3 d (Fig. 8B; termed +Dox ) relative to kidneys derived from double-transgenic animals not receiving doxycycline (Fig. 8B; −Dox). These studies demonstrated that conditional renal tubular TGF-β1 induction up-regulated kidney TAZ protein expression in vivo. Similarly, treatment of serum-deprived HK-2 cells with TGF-β1 promoted progressive TAZ protein accumulation, reaching >3-fold at 3 d after stimulation, relative to levels in untreated controls (Fig. 8D, E). The canonical SMAD3 pathway remains activated throughout the period of TGF-β1 stimulation (Fig. 8D).
Figure 8.
TGF-β1 promotes TAZ protein expression both in vivo and in vitro. A) Double-transgenic Pax8-rtTA-tet-o-TGF-β1 mice were generated by crossing Pax8-rtTA mice with tet-o-TGF-β1 single-transgenic animals to specifically express TGF-β1 in the tubular epithelium only when doxycycline (Dox) is administered. B) Immunohistochemical analysis was used to assess TAZ levels of kidney sections derived from Pax8-rtTA-tet-o-TGF-β1 double-transgenic mice, either noninduced (termed −Dox) or stimulated with Dox for 3 d (+Dox) to conditionally express TGF-β1 in the proximal tubular compartment. Scale bars, 90 μm. C) DAB signal quantitation demonstrating differences in TAZ expression between the 2 experimental groups is shown in the histogram, with the staining intensity of the −Dox kidney set at 1. D) Extracts of HK-2 cells, untreated or treated with TGF-β1 for various times, were processed by Western analysis for TAZ (D, E) and pSMAD3 and GAPDH (D) expression. E) Relative expression of TAZ at the indicated times, arbitrarily setting relative levels of TAZ in unstimulated control cells at 1. NS, not significant. Means ± sd (C, E). *P < 0.05, **P < 0.01.
TAZ is necessary for TGF-β1–induced fibrogenesis
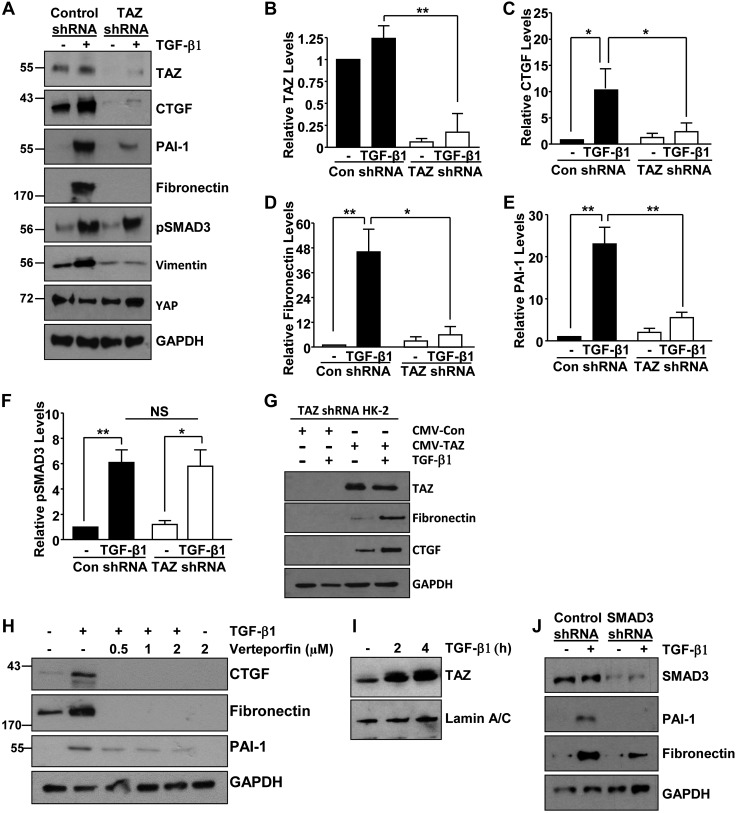
Concurrent activation of TAZ and pSMAD3 in the injured kidneys (Figs. 1–3) suggests their involvement in progression of CKD. TGF-β1 promotes interactions between YAP/TAZ and SMAD2/3 transcription factors in embryonic stem cell renewal and cancer progression (20, 35). Because of the tissue specificity and context dependency of TGF-β1 signaling (28, 29), we investigated potential TAZ involvement in the TGF-β1-mediated renal fibrogenic response. Lentiviral mediated stable expression of TAZ shRNA in HK-2 renal epithelial cells resulted in a >90% decrease in TAZ protein levels relative to control shRNA-expressing cells (Fig. 9A, B) whereas the expression of YAP (a paralogue of TAZ) remains relatively similar (Fig. 9A). Stable depletion of TAZ in HK-2 renal epithelial cells virtually eliminated TGF-β1−stimulated CTGF (Fig. 9A, C), fibronectin (Fig. 9A, D), PAI-1 (Fig. 9A, E), and vimentin (Fig. 9A) expression compared to HK-2 cultures stably expressing control shRNA. Furthermore, rescue of TAZ expression in TAZ-depleted HK-2 cells restores CTGF and fibronectin expression, which is further augmented by TGF-β1 stimulation (Fig. 9G). Pretreatment of HK-2 cells with the YAP/TAZ inhibitor verteporfin also dramatically decreased TGF-β1-induced fibronectin, CTGF, and PAI-1 expression, suggesting that both YAP/TAZ are involved in the fibrogenic response driven by TGF-β1 (Fig. 9H). Nuclear fractionation studies revealed that TGF-β1 stimulation (2–4 h) increased nuclear accumulation of TAZ compared with untreated control HK-2 cells, further confirming TAZ activation by this cytokine (Fig. 9I). SMAD3 activation by TGF-β1, in contrast, is not affected by TAZ silencing (Fig. 9A, F). Silencing of SMAD3 via RNA interference; however, similarly attenuated TGF-β1–induced fibrogenic responses, including fibronectin and PAI-1 up-regulation (Fig. 9J). Our results demonstrate independent yet cooperative roles of TAZ and SMAD3 in TGF-β1–directed renal fibrotic responses. Indeed, functional cooperation between SMAD3 and non-SMAD3 elements (e.g., p22phox, ataxia telangiectasia mutated, p53, epidermal growth factor receptor, and MAPK) is necessary for TGF-β1–driven fibrogenesis in renal epithelial cells, and fibroblasts (28, 29, 36–39).
Figure 9.
TGF-β1 mediated fibrotic gene induction requires TAZ signaling and SMAD3-TAZ cooperation. A–F) Serum-deprived HK-2 cells at similar density, stably expressing confluent control (Con) shRNA or TAZ shRNA, were stimulated with TGF-β1 (2 ng/ml) for 24 h, and lysate extracts were immunoblotted for TAZ (A, B), CTGF (A, C), fibronectin (A, D), PAI-1 (A, E), pSMAD3 (A, F), vimentin, YAP, and GAPDH (A) expression. B–F) Summary of relative TAZ, CTGF, fibronectin, PAI-1, and pSMAD3 protein levels for 3 independent experiments, setting expression levels in the untreated controls shRNA to 1 in each case. G) HK-2 cells, stably expressing TAZ shRNA and infected with either CMV-TAZ– or vector CMV-control–expressing lentiviral particles, remained unstimulated or treated with TGF-β1 for 24 h. Cell extracts were processed by immunoblotting for TAZ, fibronectin, CTGF, and GAPDH expression. H) HK-2 cells were incubated with the YAP/TAZ inhibitor verteporfin for 18 h at the indicated doses before addition of TGF-β1 at 24 h. Lysate extracts were analyzed by Western blot for expression of CTGF, fibronectin, PAI-1, and GAPDH. I) Nuclear extracts of untreated control or TGF-β1-stimulated HK-2 cells were immune blotted for TAZ, and lamin A/C (a marker of nuclear loading) expression. J) Serum-deprived HK-2 cultures expressing SMAD3- or Control shRNA were stimulated with TGF-β1 for 24 h before immune blot analysis for SMAD3, PAI-1, fibronectin, and GAPDH expression. A representative blot is shown. Data in all histograms are means ± sd. NS, not significant. *P < 0.05, **P < 0.01.
DISCUSSION
This study demonstrates that TAZ protein levels are markedly elevated during obstructive, AAN, and diabetic nephropathy in mice, suggestive of Hippo pathway deregulation in the progression of fibrotic lesions. Besides, TAZ protein induction, notable increases in TAZ nuclear accumulation (particularly in renal tubules) in the fibrotic kidneys also provide another line of direct evidence for TAZ activation (Fig. 1). These observations prompted an assessment of TAZ involvement in renal epithelial dysfunction. Indeed, sustained TAZ expression in renal epithelial cells promoted fibrotic factor expression (including CTGF, fibronectin, and PAI-1), dedifferentiation (as evident by altered morphology, increased mesenchymal marker expression, and decreased expression of epithelial characteristics), and growth inhibition. The TAZ-activated maladaptive epithelial response requires SMAD3-mediated CTGF up-regulation as SMAD3 and CTGF stable suppression in TAZ-overexpressing cells attenuated mesenchymal gene expression and rescued epithelial growth defects imposed by TAZ ectopic expression.
Renal epithelial–fibroblast crosstalk mediated by paracrine factors secreted by damaged epithelium exacerbates kidney injury and CKD (3). Another novel aspect of this study is the demonstration that paracrine factors secreted by fibrotic TAZ-expressing renal epithelial cells promote epithelial–epithelial cell communication, an area that remains less understood in the aggravation of renal fibrotic lesions. In this respect, CTGF secretion by TAZ-activated cells triggers growth inhibition of normal HK-2 cultures, establishing CTGF as a novel soluble factor that facilitates crosstalk between dysfunctional and normal renal epithelial cells. Indeed, CTGF expression is highly induced in kidney cells in response to renal injury in mice and humans (40). TGF-β1, angiotensin II, and the loss of expression of fibrotic effectors, such as phosphatase and tensin homolog and protein phosphatase magnesium-dependent A in the kidney, are contributors to increased CTGF expression during the development of kidney disease (36–43).
TAZ is critical, moreover, for TGF-β1–mediated renal gene expression (including CTGF, fibronectin, and PAI-1). Whereas pSMAD3 levels are not affected by TAZ depletion, SMAD3 is also obligatory for the fibrogenic response orchestrated by TGF-β1 in HK-2 cells consistent with previous findings (36, 38). SMAD3 and TAZ are therefore both necessary for the induction of a battery of fibrogenic proteins by TGF-β1 and promoter binding elements of SMAD2/3, and TEA domain-containing transcription factors often lie in proximity to many genes (44, 45). TAZ/YAP and SMAD2/3, furthermore, can form a transcription complex in response to TGF-β1 stimulation during stem cell differentiation and cancer progression (20, 35), and YAP gene silencing effectively inhibits TGF-β1–mediated gene activation in NRK-49F renal fibroblasts (23).
Recent studies documenting YAP/TAZ involvement in tissue fibrosis in other organ systems are in line with our finding that renal TAZ induction confers a fibrotic phenotype. Elevated YAP and TAZ expression during lung injury are causatively linked to progression of experimental lung fibrosis (21, 24). YAP activation after liver injury promotes stellate cell activation and fibrosis (22), and YAP is a downstream target of the epidermal growth factor receptor in diabetic nephropathy (46). YAP/TAZ relay mechanical signals induced by stiff matrices in the settings of lung, kidney, and liver injury, and pharmacological inhibition of YAP/TAZ-inhibit fibrosis progression (21–23). Our demonstration that TGF-β1 elevated renal TAZ protein abundance in tubular and interstitial cells in transgenic mice with conditional renal tubular induction of TGF-β1 are consistent with those in a recent study that demonstrated that TGF-β1 stimulates TAZ expression via myocardin-related transcription factor (MRTF) transcription factor–dependent pathways in C3H/10T1/2 pericyte-like fibroblast cells (47). Another recent study with mice with YAP/TAZ depletion in the glioma-associated protein–positive fibroblast population further confirmed the crucial role of these Hippo nuclear transducers in obstructive nephropathy (48).
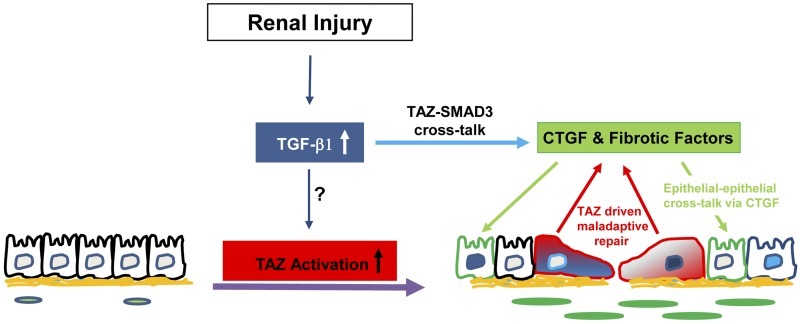
In summary, our study establishes TAZ as a novel fibrotic effector. Persistent TAZ signaling resulted in renal epithelial dysfunction marked by dedifferentiation and fibrotic factor induction, in part, via autonomous pathways involving SMAD3-dependent CTGF up-regulation. Secretion of CTGF by TAZ-activated cells, moreover, inhibited growth in normal epithelial cells via nonautonomous mechanisms (Fig. 10). TAZ is also a novel non-SMAD element that is critical for signal transmission by TGF-β1 in renal epithelial cells. Our demonstration that TAZ is also absolutely critical for TGF-β1-driven renal gene induction not only represents another level of TAZ contribution to fibrogenesis but also highlights emerging cooperation between hippo and TGF-β1 pathways in renal fibrosis. Thus, targeting TAZ activation may be a novel and plausible strategy for halting CKD progression.
Figure 10.
Model for TAZ involvement in CKD progression. Increased TGF-β1 expression in response to renal injury (e.g., UUO or AA) serves as an upstream regulator of renal TAZ activation. Elevated epithelial TAZ expression promotes fibrotic factor induction/secretion (e.g., CTGF, fibronectin), epithelial plasticity and growth inhibition via SMAD3-dependent CTGF up-regulation. CTGF secretion downstream of TAZ induction contributes to renal epithelial cell–cell crosstalk via nonautocrine mechanisms. TAZ is also necessary for TGF-β1-mediated induction of profibrotic genes, suggesting multiple modes of TAZ involvement in maladaptive repair pathways linked to CKD progression.
ACKNOWLEDGMENTS
The authors thank Dr. Wilhelm Kriz (University of Heidelberg, Heidelberg, Germany) for generously providing us with kidney tissue from Pax8-rtTA-tet-o-TGF-β1 mice for IHC studies. Portions of this study were presented as an abstract at the American Society of Nephrology Annual Meeting in November 3–5, 2015, San Diego, CA, USA. This work was supported by U.S. National Institutes of Health (NIH) General Medicine Grant GM057242 (to P.J.H.), and a Capital Region Medical Research Institute grant (to R.S.). R.G.G. was employed (2008–2009) by, performs contract research for, and receives reagents for CTGF-related research from FibroGen, Inc., a company involved in development of anti-CTGF therapies. The remaining authors declare no conflicts of interest.
Glossary
- α-SMA
α-smooth muscle actin
- AA
aristolochic acid
- AAN
AA nephropathy
- CKD
chronic kidney disease
- CMV
cytomegalovirus
- CTGF
connective tissue growth factor
- DAB
3,3′-diaminobenzidine
- ECM
extracellular matrix
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HK
human kidney
- Lats
large-tumor–suppressor homologue
- PAI
plasminogen activator inhibitor
- shRNA
short hairpin RNA
- SMAD
Sma mothers against decapentaplegic homolog 3
- STZ
streptozotocin
- TAZ
transcriptional coactivator with PDZ-binding motif
- UUO
unilateral ureteral obstruction
- YAP
yes-associated protein
AUTHOR CONTRIBUTIONS
R. Samarakoon conceived and designed the experiments; S. Anorga, J. M. Overstreet, L. L. Falke, J. Tang, and R. Samarakoon performed the experiments; all authors were involved in data analyses; R. G. Goldschmeding, and P. J. Higgins contributed key reagents and materials; R. Samarakoon wrote and R. Samarakoon and P. J. Higgins edited the manuscript; and all authors agreed on the manuscript before submission.
REFERENCES
- 1.Duffield J. S., Lupher M., Thannickal V. J., Wynn T. A. (2013) Host responses in tissue repair and fibrosis. Annu. Rev. Pathol. 8, 241–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferenbach D. A., Bonventre J. V. (2015) Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 11, 264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L., Besschetnova T. Y., Brooks C. R., Shah J. V., Bonventre J. V. (2010) Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543, 1p, 143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macconi D., Remuzzi G., Benigni A. (2014).Key fibrogenic mediators: old players. Renin- angiotensin system. Kidney Int. Suppl. (2011) 4, 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boor P., Ostendorf T., Floege J. (2010) Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 6, 643–656 [DOI] [PubMed] [Google Scholar]
- 6.Kramann R., DiRocco D. P., Humphreys B. D. (2013) Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J. Pathol. 231, 273–289 [DOI] [PubMed] [Google Scholar]
- 7.Strutz F., Neilson E. G. (2003) New insights into mechanisms of fibrosis in immune renal injury. Springer Semin. Immunopathol. 24, 459–476 [DOI] [PubMed] [Google Scholar]
- 8.Friedman S. L., Sheppard D., Duffield J. S., Violette S. (2013) Therapy for fibrotic diseases: nearing the starting line. Sci. Transl. Med. 5, 167, 167sr1. [DOI] [PubMed] [Google Scholar]
- 9.Couser W. G., Remuzzi G., Mendis S., Tonelli M. (2011) The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 80, 1258–1270 [DOI] [PubMed] [Google Scholar]
- 10.Jha V., Garcia-Garcia G., Iseki K., Li Z., Naicker S., Plattner B., Saran R., Wang A. Y., Yang C. W. (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 [DOI] [PubMed] [Google Scholar]
- 11.Perico N., Remuzzi G. (2012) Chronic kidney disease: a research and public health priority. Nephrol. Dial. Transplant. 27 (Suppl 3), iii19–iii26 [DOI] [PubMed] [Google Scholar]
- 12.Piccolo S., Dupont S., Cordenonsi M. (2014) The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 94, 1287–1312 [DOI] [PubMed] [Google Scholar]
- 13.Yu F. X., Guan K. L. (2013) The hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu F. X., Zhao B., Guan K. L. (2015) Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordenonsi M., Zanconato F., Azzolin L., Forcato M., Rosato A., Frasson C., Inui M., Montagner M., Parenti A. R., Poletti A., Daidone M. G., Dupont S., Basso G., Bicciato S., Piccolo S. (2011) The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 [DOI] [PubMed] [Google Scholar]
- 16.Yu F. X., Zhao B., Panupinthu N., Jewell J. L., Lian I., Wang L. H., Zhao J., Yuan H., Tumaneng K., Li H., Fu X. D., Mills G. B., Guan K. L. (2012) Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanconato F., Cordenonsi M., Piccolo S. (2016) YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan D. (2010) The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauviel A., Nallet-Staub F., Varelas X. (2012) Integrating developmental signals: a Hippo in the (path)way. Oncogene 31, 1743–1756 [DOI] [PubMed] [Google Scholar]
- 20.Yatabe Y., Sato A., Matsudaira Y., Ito H., Murakami H., Kondo Y., Kondo E., Hida T., Tsujimura T., Osadav H., Sekido Y. (2012) TGF-β synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J. Exp. Med. 209, 479–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F., Lagares D., Choi K. M., Stopfer L., Marinković A., Vrbanac V., Probst C. K., Hiemer S. E., Sisson T. H., Horowitz J. C., Rosas I. O., Fredenburgh L. E., Feghali-Bostwick C., Varelas X., Tager A. M., Tschumperlin D. J. (2015) Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L344–L357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannaerts I., Leite S. B., Verhulst S., Claerhout S., Eysackers N., Thoen L. F., Hoorens A., Reynaert H., Halder G., van Grunsven L. A. (2015) The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J. Hepatol. 63, 679–688 [DOI] [PubMed] [Google Scholar]
- 23.Szeto S. G., Narimatsu M., Lu M., He X., Sidiqi A. M., Tolosa M. F., Chan L., De Freitas K., Bialik J. F., Majumder S., Boo S., Hinz B., Dan Q., Advani A., John R., Wrana J. L., Kapus A., Yuen D. A. (2016) YAP/TAZ are mechanoregulators of TGF-β-smad signaling and renal fibrogenesis. J. Am. Soc. Nephrol. 27, 3117–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitani A., Nagase T., Fukuchi K., Aburatani H., Makita R., Kurihara H. (2009) Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am. J. Respir. Crit. Care Med. 180, 326–338 [DOI] [PubMed] [Google Scholar]
- 25.Rohatgi R., Flores D. (2010) Intratubular hydrodynamic forces influence tubulointerstitial fibrosis in the kidney. Curr. Opin. Nephrol. Hypertens. 19, 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tschumperlin D. J., Liu F., Tager A. M. (2013) Biomechanical regulation of mesenchymal cell function. Curr. Opin. Rheumatol. 25, 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genevet A., Tapon N. (2011) The Hippo pathway and apico-basal cell polarity. Biochem. J. 436, 213–224 [DOI] [PubMed] [Google Scholar]
- 28.Meng X. M., Nikolic-Paterson D. J., Lan H. Y. (2016) TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 [DOI] [PubMed] [Google Scholar]
- 29.Samarakoon R., Overstreet J. M., Higgins P. J. (2013) TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell. Signal. 25, 264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevalier R. L., Forbes M. S., Thornhill B. A. (2009) Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 75, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 31.Tesch G. H., Nikolic-Paterson D. J. (2006) Recent insights into experimental mouse models of diabetic nephropathy. Nephron, Exp. Nephrol. 104, e57–e62 [DOI] [PubMed] [Google Scholar]
- 32.Kanwar Y. S., Sun L., Xie P., Liu F. Y., Chen S. (2011) A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 6, 395–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gökmen M. R., Cosyns J. P., Arlt V. M., Stiborová M., Phillips D. H., Schmeiser H. H., Simmonds M. S., Cook H. T., Vanherweghem J. L., Nortier J. L., Lord G. M. (2013) The epidemiology, diagnosis, and management of aristolochic acid nephropathy: a narrative review. Ann. Intern. Med. 158, 469–477 [DOI] [PubMed] [Google Scholar]
- 34.Koesters R., Kaissling B., Lehir M., Picard N., Theilig F., Gebhardt R., Glick A. B., Hähnel B., Hosser H., Gröne H. J., Kriz W. (2010) Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am. J. Pathol. 177, 632–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varelas X., Sakuma R., Samavarchi-Tehrani P., Peerani R., Rao B. M., Dembowy J., Yaffe M. B., Zandstra P. W., Wrana J. L. (2008) TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 10, 837–848 [DOI] [PubMed] [Google Scholar]
- 36.Samarakoon R., Dobberfuhl A. D., Cooley C., Overstreet J. M., Patel S., Goldschmeding R., Meldrum K. K., Higgins P. J. (2013) Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species. Cell. Signal. 25, 2198–2209 [DOI] [PubMed] [Google Scholar]
- 37.Samarakoon R., Overstreet J. M., Higgins S. P., Higgins P. J. (2012) TGF-β1→ SMAD/p53/ USF2→PAI transcriptional axis in UUO-induced renal fibrosis. Cell Tissue Res. 347, 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overstreet J. M., Samarakoon R., Meldrum K. K., Higgins P. J. (2014) Redox control of p53 in the transcriptional regulation of TGF-β1 target genes through SMAD cooperativity. Cell. Signal. 26, 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overstreet J. M., Samarakoon R., Cardona-Grau D., Goldschmeding R., Higgins P. J. (2015) Tumor suppressor ataxia telangiectasia mutated functions downstream of TGF-β1 in orchestrating profibrotic responses. FASEB J. 29, 1258–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito Y., Aten J., Bende R. J., Oemar B. S., Rabelink T. J., Weening J. J., Goldschmeding R. (1998) Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 53, 853–861 [DOI] [PubMed] [Google Scholar]
- 41.Samarakoon R., Helo S., Dobberfuhl A. D., Khakoo N. S., Falke L., Overstreet J. M., Goldschmeding R., Higgins P. J. (2015) Loss of tumour suppressor PTEN expression in renal injury initiates SMAD3- and p53-dependent fibrotic responses. J. Pathol. 236, 421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samarakoon R., Rehfuss A., Khakoo N. S., Falke L. L., Dobberfuhl A. D., Helo S., Overstreet J. M., Goldschmeding R., Higgins P. J. (2016) Loss of expression of protein phosphatase magnesium-dependent 1A during kidney injury promotes fibrotic maladaptive repair. FASEB J. 30, 3308–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rüster C., Wolf G. (2011) Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J. Am. Soc. Nephrol. 22, 1189–1199 [DOI] [PubMed] [Google Scholar]
- 44.Beyer T. A., Weiss A., Khomchuk Y., Huang K., Ogunjimi A. A., Varelas X., Wrana J. L. (2013) Switch enhancers interpret TGF-β and Hippo signaling to control cell fate in human embryonic stem cells. Cell Reports 5, 1611–1624 [DOI] [PubMed] [Google Scholar]
- 45.Preisser F., Giehl K., Rehm M., Goppelt-Struebe M. (2016) Inhibitors of oxygen sensing prolyl hydroxylases regulate nuclear localization of the transcription factors Smad2 and YAP/TAZ involved in CTGF synthesis. Biochim. Biophys. Acta 1863, 2027–2036 [DOI] [PubMed] [Google Scholar]
- 46.Chen J., Harris R. C. (2016) Interaction of the EGF receptor and the Hippo pathway in the diabetic kidney. J. Am. Soc. Nephrol. 27, 1689–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miranda M. Z., Bialik J. F., Speight P., Dan Q., Yeung T., Szászi K., Pedersen S. F., Kapus A. (2017) TGF-β1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism. J. Biol. Chem. 292, 14902–14920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang M., Yu M., Xia R., Song K., Wang J., Luo J., Chen G., Cheng J. (2017) Yap/Taz deletion in Gli+ cell-derived myofibroblasts attenuates fibrosis. J. Am. Soc. Nephrol. 28, 3278–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]