Abstract
Autosomal-dominant polycystic kidney disease (ADPKD) is associated with progressive formation of renal cysts, kidney enlargement, hypertension, and typically end-stage renal disease. In ADPKD, inherited mutations disrupt function of the polycystins (encoded by PKD1 and PKD2), thus causing loss of a cyst-repressive signal emanating from the renal cilium. Genetic studies have suggested ciliary maintenance is essential for ADPKD pathogenesis. Heat shock protein 90 (HSP90) clients include multiple proteins linked to ciliary maintenance. We determined that ganetespib, a clinical HSP90 inhibitor, inhibited proteasomal repression of NEK8 and the Aurora-A activator trichoplein, rapidly activating Aurora-A kinase and causing ciliary loss in vitro. Using conditional mouse models for ADPKD, we performed long-term (10 or 50 wk) dosing experiments that demonstrated HSP90 inhibition caused durable in vivo loss of cilia, controlled cystic growth, and ameliorated symptoms induced by loss of Pkd1 or Pkd2. Ganetespib efficacy was not increased by combination with 2-deoxy-d-glucose, a glycolysis inhibitor showing some promise for ADPKD. These studies identify a new biologic activity for HSP90 and support a cilia-based mechanism for cyst repression.—Nikonova, A. S., Deneka, A. Y., Kiseleva, A. A., Korobeynikov, V., Gaponova, A., Serebriiskii, I. G., Kopp, M. C., Hensley, H. H., Seeger-Nukpezah, T. N., Somlo, S., Proia, D. A., Golemis, E. A. Ganetespib limits ciliation and cystogenesis in autosomal-dominant polycystic kidney disease (ADPKD).
Keywords: PKD1, heat shock protein 90, polycystins, renal cyst, cilia
Approximately 1 in 500 individuals are affected with autosomal-dominant polycystic kidney disease (ADPKD), a disease in which cysts accumulate in the kidney and renal function declines during middle age (1). ADPKD is also associated with liver bile ductal cysts, hypertension, chronic pain, and potential extrarenal problems such as cerebral aneurysms. ADPKD arises from inherited mutations in the PKD1 or PKD2 genes, which encode polycystin proteins (PC1 and PC2) that heterodimerize and signal from cell cilia; in ADPKD, this signaling is anomalous, resulting in downstream activation of a large number of proproliferative proteins including S6, RAF, AKT, mTOR, HER2, and STAT (2). Signaling through these and other proteins results in increasing renal fibrosis and inflammation. Most ADPKD patients eventually experience end-stage renal disease in late middle age.
There is at present no cure for ADPKD, with therapies focused on limiting the rate of decline and ameliorating the symptoms (3, 4). Among numerous preclinical and clinical strategies to treat ADPKD, most have focused either on controlling ADPKD-linked systemic pathologies, such as hypertension (5, 6), or targeting proproliferative signaling effectors activated by defective forms of PKD1 or PKD2 (7–11). Heat shock protein 90 (HSP90) inhibition has attracted significant interest as a target for cancer and other diseases because of the requirement of this chaperone for protein stabilization and full activation of a large number of client proteins that support rapid cell growth under conditions of intracellular and environmental stress. In earlier work, we determined that HSP90 expression is specifically and highly elevated in renal cystic epithelia, and that the HSP90 inhibitor STA-2842 limited the activity of a number of clients activated as downstream ADPKD effectors (12). Suggesting an alternative strategy to control ADPKD symptoms, Ma et al. (2) used a genetic approach to demonstrate that loss of cilia ameliorated the symptoms of ADPKD by reducing disease-inducing signaling from cilia-localized defective polycystin complexes. On the basis of this model, drugs with activity destabilizing cilia would result in reduced disease symptoms, while drugs stabilizing cilia might be expected to exacerbate symptoms. Prior work from our group (8, 13) generally supported this idea, in particular demonstrating that inhibition of Aurora-A (AURKA), a kinase promoting ciliary disassembly (14, 15), accelerated cystogenesis in mouse models for ADPKD.
Our analysis of extensive online resources has for the first time identified multiple HSP90 clients among proteins that regulate ciliogenesis and ciliary resorption. This result raised the unexpected possibility that HSP90 inhibition may influence cystogenesis by targeting the integrity of the polycystin ciliary signaling platform. We have now examined the tolerability and efficacy of ganetespib in very long term (10 and 50 wk) in vivo experiments using mouse models of ADPKD. Results of this study for the first time directly demonstrate that ganetespib is a regulator of ciliary dynamics, thus identifying a new mode of action for a compound that has been evaluated in over 35 clinical trials involving hundreds of patients (16) and that has a generally manageable profile of adverse effects (17). The results also demonstrate a promising and durable activity of this compound in limiting the growth of renal cysts and in controlling ADPKD-associated phenotypes such as weight gain.
MATERIALS AND METHODS
Analysis of cilia-associated HSP90 clients
Data were assembled from public databases reporting RNA interference–defined regulators of ciliogenesis (18, 19), genes linked to morbid ciliopathies (20), and a compendium of genes linked to ciliary dynamics and function (21, 22). HSP90 interactors were retrieved using the metasearch engines Ingenuity Pathway Analysis (Qiagen, Germantown, MD, USA; http://www.qiagen.com/ingenuity) and String (http://string.embl.de/) (23), and from the HSP90 interaction database HSP90Int.db (https://www.picard.ch/Hsp90Int/index.php) (24, 25).
Mouse strains and drug treatment
Conditional Pkd1−/− mice in which tamoxifen induction of the Cre-flox regulatory system permits targeted inactivation of the Pkd1 gene in vivo have been previously described (13, 26, 27), and for our study, they were kindly provided by G. Germino [National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH), Bethesda, MD, USA]. Pkd1fl/fl;Cre/Esr1+ (referred to as Pkd1−/−), Pkd2fl/fl;Cre/Esr+ (referred to as Pkd2−/−), and control mice lacking an intact Cre-flox system (Pkd1fl/fl;Cre/Esr1− and Pkd2fl/fl;Cre/Esr1−) mice were injected intraperitoneally with tamoxifen (250 mg/kg body weight, formulated in corn oil) on postnatal days P35 and P36 for late cyst induction to induce Pkd1 deletion in the test group, as described elsewhere (26). Mice were randomly assigned to experimental groups, with male and female animals used for experiments at a 1:1 ratio. The Institutional Animal Care and Use Committee of Fox Chase Cancer Center approved all animal experiments. Ganetespib or STA-2842 (Synta Pharmaceuticals, Lexington, MA, USA) was formulated in 10% Cremophor and 5% dextrose (vehicle) (Sigma-Aldrich, St. Louis, MO, USA) and administered via tail vein intraveneous injection at a dose of 100 mg/kg. 2-Deoxy-d-glucose (2DG; Sigma-Aldrich) was formulated in 0.9% saline and dosed intraperitoneally at 0.5 or 1 g/kg. Mice were humanely killed at the times indicated in the Results to collect blood and kidneys for analysis. Blood samples were collected 24 h after the last dose of the drugs. Blood serum analysis was performed using a commercial service at Antech Diagnostics (Irvine, CA, USA).
MRI protocol and image analysis
Degree of cystogenesis and renal volume were established by MRI before treatment initiation and at the subsequent times indicated in the Results. MRI was performed exactly as described elsewhere (12, 13, 28). Briefly, mice were anesthetized with 1% to 2% isoflurane in O2 and then imaged using a vertical bore 7 T magnet on a Bruker DRX300 spectrometer with ParaVision 3.0.2 software (Bruker, Billerica, MA, USA). Kidney and cyst volume were quantified using ImageJ software (Image Processing and Analysis in Java; NIH; http://imagej.nih.gov/) (29). Estimation of kidney volume was performed as described elsewhere (30). Isolated kidney areas were subsequently prepared using defined settings for background subtraction and band passing, with a threshold set for each kidney on the basis of the original images by targeting threshold values designating the transition between parenchyma and cyst at the border of the larger cysts in the kidneys. Cyst volume was estimated using a semiautomatic threshold approach (31, 32).
Tissue preparation, histology, immunohistochemical analysis, and cystic index
All tissues were collected and fixed in 10% phosphate-buffered formaldehyde (formalin) for 24 to 48 h, dehydrated, and embedded in paraffin. Hematoxylin and eosin (H&E)-stained 5-µm sections were used for morphologic evaluation. Masson trichrome staining (Electron Microscopy Sciences, Hatfield, PA, USA) was performed according to kit directions. H&E-stained sections were used for morphologic evaluation and cystic index analysis. Unstained sections were used for fluorescent immunohistochemical analysis. After deparaffinization and rehydration, sections were subjected to heat-induced epitope retrieval by steaming in 0.01 M citrate buffer (pH 6.0) for 20 min. After blocking nonspecific protein binding with 10% goat serum, sections were incubated overnight with primary monoclonal antibodies against anti-acetylated tubulin (mouse, clone 6-11B-1, T6793; Sigma-Aldrich), after which secondary antibodies labeled with Alexa Fluor 488 were applied for an hour and sections were mounted with Prolong Gold with DAPI (Life Technologies, Carlsbad, CA, USA) to stain DNA. Sections were imaged using a Leica SP8 confocal system (Leica, Wetzlar, Germany) equipped with an oil-immersion ×63 objective with NA 1.4. Images were acquired at room temperature using LAS AF (Leica Application Suite Advanced Fluorescence) from Leica Microsystems. For cystic index analysis, a grid was placed over representative images of H&E-stained kidney sections, and the cystic index was calculated as the percentage of grid intersection points that bisected cystic or noncystic areas, as previously described (33).
Cell culture
Human cell lines derived from ADPKD cyst-lining epithelia, WT9-7 [ATCC CRL-2830; American Type Culture Collection (ATCC), Manassas, VA, USA] and WT9-12 (ATCC CRL-2833), were cultured in DMEM media containing 10% fetal bovine serum (FBS; vol/vol) plus penicillin/streptomycin. Human retinal epithelial hTERT-RPE1 cells were maintained in DMEM/F-12 with 10% FBS plus penicillin/streptomycin and 0.01 mg/ml hygromycin B. The immortalized human kidney proximal tubular cell line (HK-2; ATCC CRL-219) was grown in keratinocyte media (Life Technologies). Cell lines were sent for authentication to IDEXX BioResearch (Westbrook, ME, USA). Short tandem repeat analysis using the Promega Cell ID System (8 short tandem repeat markers + amelogenin) (Promega, Madison, WI, USA) verified that the genetic profile of the sample matched the known profile of the cell line. Nonimmortalized primary kidney cells derived from P10 Pkd1fl/fl;Cre/Esr1+ or Pkd1fl/fl;Cre/Esr1− mice induced by treatment with tamoxifen on days P2 and P3 were used to generate Pkd1−/− and wild-type (WT) cultures (3 animals per genotype). Cells were maintained in low-calcium media containing 5% (vol/vol) chelated horse serum. Only cells between passages 3 and 8 were used for experiments. Lack of mycoplasma contamination in cell cultures was confirmed using Hoechst dye to stain DNA and assessing after 3 d.
Immunofluorescence
For ciliary disassembly, cells were plated at 50% to 70% confluence on coverslips coated with collagen (StemCell Technologies, Vancouver, BC, Canada) in Opti-MEM medium without serum. In some experiments, cells were starved of serum for 72 h with drug or vehicle present in the medium for the final 48 h. For other experiments, after 72 h of serum starvation, ciliary disassembly was induced by adding medium containing 10% FBS, and ciliary status was assessed after 0, 2, 8, or 24 h with and without drug. Vehicle (0.01% DMSO), 100 nM ganetespib (Synta Pharmaceuticals), 200 nM alisertib (MedchemExpress, Monmouth Junction, NJ, USA), 5 mM 2DG (Sigma-Aldrich), or drug combinations were added in the presence or absence of 10% FBS. Cells were fixed with 4% (vol/vol) paraformaldehyde for 10 min and then cold methanol for 5 min, permeabilized with 1% Triton X-100 in PBS, blocked in PBS with 3% (v/v) bovine serum albumin, and incubated with antibodies using standard protocols. Primary antibodies included anti-acetylated α-tubulin mAb [mouse, clone 6-11B-1 (T6793; Sigma-Aldrich), γ-tubulin (T5192 and ab191114, rabbit; Sigma-Aldrich), anti-phospho AURKA (T288) (IHC-00067; Bethyl Laboratories, Montgomery, TX, USA), and anti-total AURKA (3092S; Cell Signaling Technology, Danvers, MA, USA). Secondary antibodies labeled with Alexa Fluor 488, Alexa Fluor 568, Alexa Fluor 647, and mounting medium Prolong Gold with DAPI to stain DNA were obtained from Life Technologies. Samples were imaged using a Leica SP8 confocal system equipped with an oil-immersion ×63 objective with NA 1.4. Images were acquired at room temperature by LAS AF (Leica Application Suite Advanced Fluorescence) software.
Glycolytic function determination
A bioenergetic function assay (34) and an XF96 extracellular flux analyzer (Seahorse Bioscience, North Billerica, MA, USA) were used to determine the glycolytic function in murine-derived WT and mutant Pkd1 cells and human renal cell lines bearing mutant, deficient alleles of PKD1, and in hTERT-RPE1 human retinal pigmented epithelial cells. After they had been seeded in the amount of 20K per well on an assay plate, cells were treated with vehicle (0.01% DMSO), 100 nM ganetespib (Synta Pharmaceuticals), 5 mM 2DG (Sigma-Aldrich), or a combination for 12 h. Further, cells were washed with an unbuffered assay medium, and the functions were measured using standard Seahorse Bioscience protocols. Both the oxygen consumption rate and the extracellular acidification rate were measured over 1 h after the cells had been conditioned in the assay medium. Three replicates were used for each group, and the experiments were repeated 3 times to confirm the results.
Western blot analysis
To analyze the expression levels of individual proteins, cells were lysed and resolved by SDS-PAGE. Western blot analysis was performed using standard procedures. Blots were developed by chemiluminescence using Luminata Western horseradish peroxidase (HRP) substrates (Classico, Crescendo, and Forte) (EMD Millipore, Billerica, MA, USA) and Immun-Star AP Substrate (Bio-Rad, Hercules, CA, USA). Quantitative analysis of signals on Western blots was performed by ImageJ with signaling intensity normalized to loading control (β-actin or β-tubulin). Primary antibodies included anti-FBXW7 (ab109617, 1:1000; Abcam, Cambridge, MA, USA), anti-trichoplein (TCHP; 25931-1-AP, 1:1000; PTG Labs, Rosemont, IL, USA), anti-NEK8 (#ab175320, 1:700; Abcam), anti–PP2A-B (2290S, 1:1000; Cell Signaling Technology), anti–β-tubulin conjugated to HRP (5346, 1:10,000; Cell Signaling Technology), and mouse anti–β-actin conjugated to HRP (ab49900, 1:50,000; Abcam). Secondary anti-mouse and anti-rabbit HRP-conjugated antibodies (GE Healthcare, Bensalem, PA, USA) were used at a dilution of 1:10,000, and secondary anti-mouse and anti-rabbit AP-conjugated antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) were used at a dilution of 1:5000.
Statistical analysis
No statistical method was used to predetermine sample size. Sample sizes were estimated from previous experience and common knowledge of animal studies. We generally used Wilcoxon rank-sum tests for pairwise comparisons, and 1-way ANOVA for 2 or more group comparisons. Survival curves analysis was performed by the log-rank (Mantel-Cox) test unless otherwise noted. Analyses were performed by GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA).
RESULTS
HSP90 clients include multiple proteins linked to ciliary maintenance and ganetespib causes rapid loss of cilia in cultured cells
We analyzed a set of resources including public databases reporting results from functional small interfering RNA screens to identify regulators of ciliogenesis (18, 19), a database of genes linked to morbid ciliopathies (20), and additional resources reporting genes linked to ciliary dynamics (18, 21, 22). We evaluated the intersection of genes noted in these resources with genes that had been defined as HSP90 targets or that were regulated by HSP90 inhibition (23–25, 35) (Supplemental Table S1). This suggested that many proteins linked to formation, maintenance, and disassembly of cilia were bound, stabilized, and/or activated by HSP90, including, among others, GSK3β, VHL, NEK8 and multiple components of the proteasome (Fig. 1A).
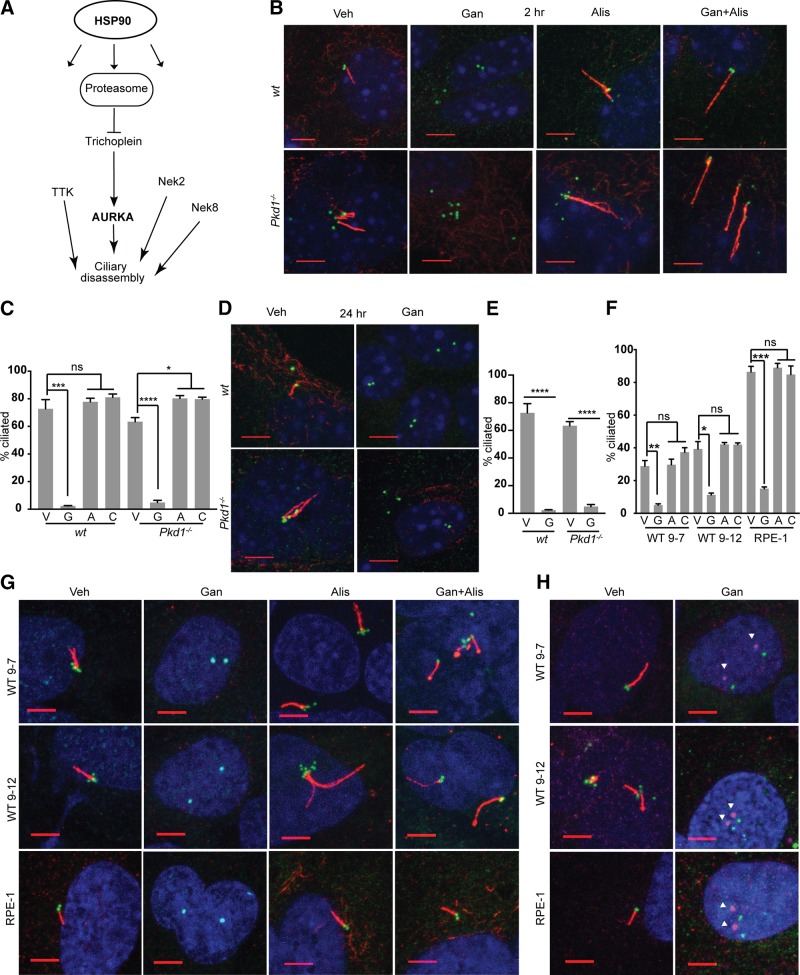
Figure 1.
HSP90 is required for in vitro maintenance of cilia. A) Selected subset of defined proteins that influence ciliary dynamics or structural integrity, and are HSP90 clients or regulated by HSP90 inhibition. B–E) Representative immunofluorescence (IF) image and graph with frequency of ciliated WT or Pkd1−/− murine renal epithelial cells at 2 h (B, C) or 24 h (D, E) after treatment with vehicle (V), ganetespib (G), or alisertib (A) to inhibit AURKA, or combination (C) of alisertib and ganetespib. On IF, acetylated α-tubulin (red); γ-tubulin (green); DAPI (blue). Scale bars, 5 μm; original magnification, ×240. F, G) Analysis as in B and C for human PKD1-mutant WT9-7 and WT9-12 cells or for hTERT-RPE1 (RPE1) cells, 2 h after drug treatment. H) IF for phospho-T288 (activated) AURKA (magenta, indicated by white arrowheads) at ciliary basal body (γ-tubulin, green) with acetylated α-tubulin (red); images of ph-AURKA and basal body are offset. No antibodies currently available for phospho-T288 are reactive with mouse AURKA. Scale bars, 5 μm. Data are expressed as means ± sem; n/s, not significant. IF experiments were performed in triplicate; ≤750 cells were scored per genotype. *P ≤ 0.05, ***P ≤ 0.001, ****P = 0.0001.
We therefore directly tested the idea that ganetespib may affect ciliation. Treatment of primary renal epithelial cells in vitro with ganetespib caused near-complete loss of ciliation of primary murine renal epithelial cells within 2 h of drug addition, independent of the addition of serum or growth factors to induce ciliary resorption (Fig. 1B, C). This effect was seen in cells derived from the kidneys of both Pkd1−/− and WT mice, and was sustained for at least 24 h after treatment (Fig. 1D, E). Ganetespib similarly triggered rapid loss of cilia in 2 human renal cell lines bearing mutant, deficient alleles of PKD1 (WT9-7, WT9-12), and in immortalized human retinal pigmented epithelial cells (hTERT-RPE1) (Fig. 1F, G). Ganetespib treatment was associated with the rapid and potent induction of AURKA kinase activity at the ciliary basal body (Fig. 1H), and the ability of ganetespib to induce ciliary loss was inhibited by prior treatment with the AURKA inhibitor alisertib in all cell types (Fig. 1B–E).
To gain more insight into the mechanism by which ganetespib induced ciliary resorption, we performed a number of experiments, focusing first on the rapid 2 h time point after administration of ganetespib (Fig. 2). We first focused on the relationship between ganetespib and proteasomal activity, as the proteasome inhibits expression of AURKA in the context of cytokinesis (36) but also supports ciliogenesis (37) based on a mechanism of inhibiting the AURKA activator TCHP at the ciliary basal body while not affecting total AURKA expression in this context (38). We first used immunofluorescence to total vs. phosphorylated AURKA to compare the effect of ganetespib and 2 inhibitors of the proteasome, MG-132 and bortezomib, alone and in combination. We found that MG-132 and bortezomib were as effective as ganetespib in inducing AURKA activation at the basal body but did not affect total levels of AURKA at the basal body; and that combination of the proteasome inhibitors and ganetespib was not better than either alone (Fig. 2A). In parallel, we compared the efficacy of ganetespib and the proteasomal inhibitors, alone and in combination, on resorption of cilia, finding that all resulted in a comparable degree of ciliary resorption (Fig. 2B). We also confirmed that all treatments result in a similar induction of the E3 ligase FBXW7 (Fig. 2C), which is induced when the proteasome is inhibited (39), providing further evidence that ganetespib is inhibiting the proteasome.
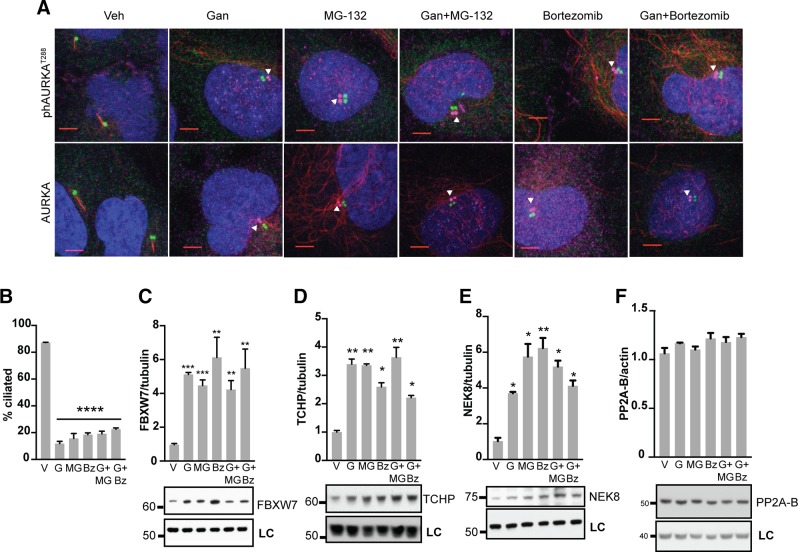
Figure 2.
Mechanism of ganetespib regulation of AURKA activation in ciliary disassembly. A) Immunofluorescence analysis of hTERT-RPE1 cells treated for 2 h with vehicle (Veh), ganetespib (Gan), and/or proteasomal inhibitors MG-132 and bortezomib. DAPI (blue), γ-tubulin (green), acetylated α-tubulin (red), activated ph-T288-AURKA (top row, magenta) or total AURKA (bottom row, magenta). Scale bars, 5 μm; original magnification, ×240. Images of ph-AURKA or total AURKA are offset from basal body. B) Frequency of ciliated cells in populations of cells 2 h after treatment with vehicle (V), ganetespib (G), and/or MG-132 (MG) and bortezomib (Bz). C–F) Normalized quantification and representative data for Western blots showing expression of FBXW7 (C), TCHP (D), NEK8 (E), or PP2A-B (F), 2 h after treatment with indicated drugs. LC, loading control. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Mechanistically, we found that ganetespib was as effective as proteasomal inhibitors in inducing the AURKA activator TCHP (Fig. 2D). In addition, ganetespib and the proteasomal inhibitors each significantly induced NEK8 (Fig. 2E), another protein linked to ciliary disassembly, although not yet directly connected to AURKA (40, 41), but this did not affect expression of PP2A (Fig. 2F), a phosphatase that negatively regulates AURKA activation (36). In sum, these new data indicate that ganetespib treatment inhibits the proteasome and results in rapidly increased expression of the AURKA activators TCHP and NEK8, increasing AURKA activity but not expression.
Long-term administration of ganetespib ameliorates pathologic features induced by conditional loss of Pkd1 and reduces ciliation
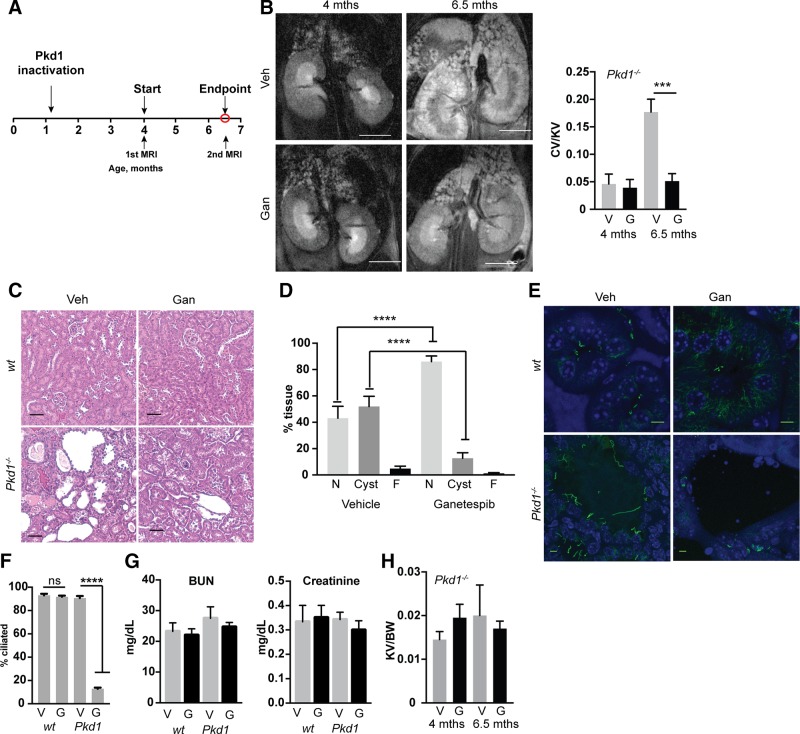
In Pkd1fl/fl;Cre/Esr1+ mice, intraperitoneal administration of tamoxifen induces the Cre-flox regulatory system, causing targeted inactivation of the Pkd1 gene (13, 26, 27). In a first assessment of ganetespib in vivo (Fig. 3A), we treated Pkd1fl/fl;Cre/Esr1+ (referred to as Pkd1−/−) mice and control mice lacking an intact Cre-flox system (Pkd1fl/fl;Cre/Esr1−) with tamoxifen at postnatal days P35 and P36 to induce sporadic late cyst induction. After MRI confirmed initial formation of cysts at 4 mo of age, we treated mice with 100 mg/kg ganetespib or vehicle for 10 wk from 4 to 6.5 mo of age. Ganetespib completely controlled the growth of renal cysts (Fig. 3B–D). This was accompanied by striking reduction in the ciliation of the cystic lining of renal tissue in Pkd1−/− mice but had no effect on the ciliation of normal renal tissue (Fig. 3E, F and Supplemental Fig. S1). This selective activity on ciliation was also seen in cysts arising in the liver and pancreas, but not normal cells in these organs (Supplemental Fig. S1); this likely reflects the selective expression of and dependence on HSP90 in the cyst lining (12), as well as the selective up-regulation of AURKA in these cells (13, 28, 42). In contrast, ganetespib treatment induced modest but not statistically significant decreases in serum markers of renal dysfunction [blood urea nitrogen (BUN), creatinine] (Fig. 3G), and because of heterogeneity of rate of kidney expansion, did not significantly influence kidney size by the experimental end point (Fig. 3H).
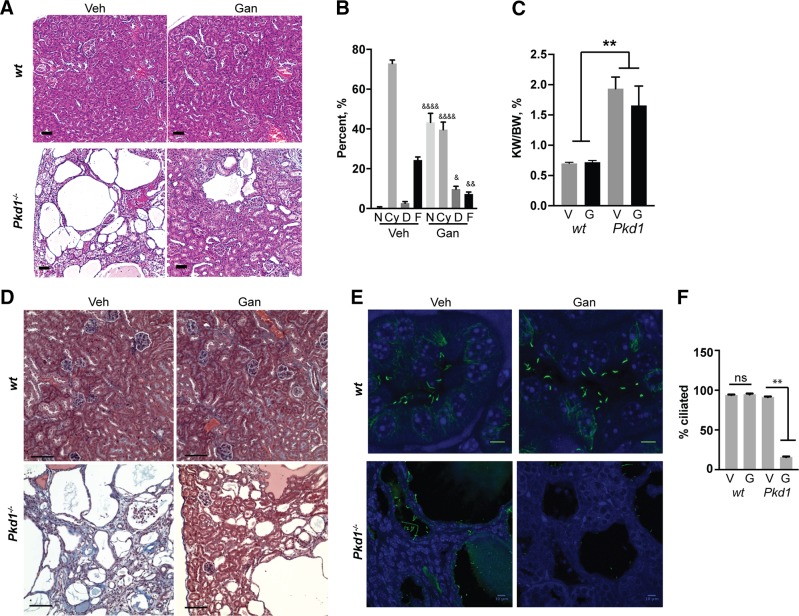
Figure 3.
Ganetespib limits growth and in vivo ciliation in established Pkd1−/− cysts. A) Experimental outline. N = 5 for vehicle (veh)- and ganetespib (gan)-treated WT; 13 for Pkd1−/− with vehicle; and 14 for Pkd1−/− with ganetespib. B) Left, representative MRI images for indicated treatment groups of Pkd1−/− mice at 4 and 6.5 mo of age. Right, quantification of cyst volume as percentage kidney volume (CV/KV) for each group. V, vehicle; G, ganetespib. Scale bars, 500 μm. C) Representative H&E-stained kidney of vehicle- or ganetespib-treated Pkd1−/− mice. Scale bars, 50 μm. D) Cystic index quantified from H&E images in Fig. 1C. N, normal renal tissue; C, cystic; F, fibrotic. E, F) Representative images (E) and quantification (F) of cilia in kidneys of Pkd1−/− and WT mice treated with vehicle or ganetespib for 10 wk. Cilia are visualized with antibody to acetylated α-tubulin (green), with DAPI (blue) indicating nuclei. Scale bars, 5 μm; original magnification, ×240. G) Serum levels of BUN and creatinine in Pkd1−/− and WT mice treated with vehicle (V) or ganetespib (G). N = 6 for WT mice and n = 13 for Pkd1−/− mice. H). Kidney volume (cm³) to body weight (KV/BW) calculated from analysis of MRI images. Data are expressed as means ± sem. *P ≤ 0.05, ***P ≤ 0.001, ****P = 0.0001.
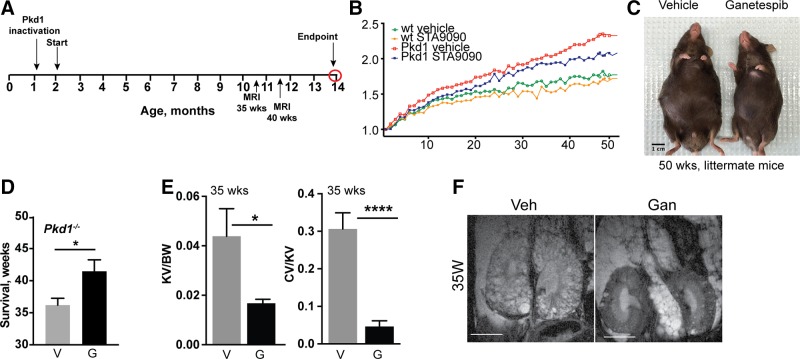
To more rigorously establish long-term tolerability and efficacy of ganetespib treatment as well as the consequences for ciliation, we established cohorts of Pkd1−/− and control mice by tamoxifen induction at P35 and P36 (Fig. 4A). Mice were then dosed weekly starting from ∼3 mo of age before cysts were first detectable (1.5–4 mo of age), then biweekly (4 mo until death) with 100 mg/kg ganetespib or vehicle. Development of cysts and kidney growth were monitored by MRI in mice at 35 and 40 wk of age; mice were maintained until the appearance of symptoms suggesting discomfort, up to a maximum end point of 50 wk treatment, after which they were humanely killed, and organs and blood collected for analysis.
Figure 4.
Continuous dosing with ganetespib controls cystogenesis and reduces ADPKD-associated symptoms over 50 wk. A) Experimental outline and dosing schedule. Cohorts evaluated included: n = 8 for WT mice treated with vehicle; n = 8 for WT/ganetespib; n = 12 for Pkd1−/−/vehicle; and n = 12 for Pkd1−/−/ganetespib. B) Quantification of body weight differences at 35 and 40 wk of treatment with vehicle (V) or ganetespib (G). C) Representative images of surviving Pkd1−/− mice after 50 wk of treatment. D) Average length of survival of indicated treatment cohorts at time after beginning of treatment. E, F) Kidney volume (cm³) to body weight (KV/BW) and cyst volume to body weight (CV/BW) calculated from MRI data collected after 35 wk of treatment (E); representative MRI images are shown (F). Numbers in bars indicate number of mice surviving after 35 wk of treatment. Data are expressed as means ± sem; n/s, not significant. *P ≤ 0.05, ****P ≤ 0.0001.
Ganetespib treatment reduced the typical weight accumulation and bloating due to inadequate renal function in treated Pkd1−/− mice, although it did not reduce weight to that of WT mice (Fig. 4B, C). Kaplan-Meier analysis indicated a borderline significant survival benefit of ganetespib treatment, with the average survival of vehicle-treated mice 42 wk (36 wk of treatment) vs. 48 wk (42 wk of treatment, limited in some cases by the predetermined end point at 50 wk) (Fig. 4D). On the basis of analysis of MRI data collected at 35 and 40 wk of dosing, kidney volume and cyst formation were both significantly reduced by ganetespib treatment (Fig. 4E, F and Supplemental Fig. S2A–C). This was more notable at 35 than 40 wk of treatment because of the heterogeneity of the disease, vehicle-treated mice with more severe phenotypes had died by 40 wk of treatment (Supplemental Fig. S2D).
Histopathologic assessment confirmed striking control of cyst formation in the kidney in ganetespib-treated Pkd1−/− mice (Fig. 5A, B). We also observed a trend toward reduction in the size of kidney (Fig. 5C) and a trend toward less impaired organ function (Supplemental Fig. S2E). This was accompanied by marked reduction in fibrosis in ganetespib-treated Pkd1−/− mice (Fig. 5D). Further, many vehicle-treated Pkd1−/− mice had significant myeloid infiltration into renal tissue; in contrast, few ganetespib-treated Pkd1−/− mice had such infiltration, and myeloid cells were more localized when observed (Supplemental Fig. S2F). Strikingly, kidney tissue from Pkd1−/− mice treated with ganetespib for 50 wk continued to show absence of cilia compared to vehicle-treated controls, while no such difference was observed with ganetespib treatment of comparably aged WT controls (Fig. 5E, F and Supplemental Fig. S1). This likely reflects the fact that HSP90 is overexpressed in cysts relative to normal renal tissue, which affects ganetespib accumulation.
Figure 5.
Pathologic analysis of tissue and serum biomarkers after long-term ganetespib dosing. A, B) Representative H&E-stained images of kidney (A), and quantitation of cystic index for kidney (B) of vehicle (veh)- or ganetespib (gan)-treated Pkd1−/− mice at time of euthanasia. Cohorts evaluated included: n = 8 for WT mice treated with vehicle; n = 8 for WT/ganetespib; n = 12 for Pkd1−/−/vehicle; and n = 12 for Pkd1−/−/ganetespib. N, normal; Cy, cystic; D, dilated; F, fibrotic. C) Kidney weight to body weight (KW/BW) ratios for mice of indicated genotypes and treatment groups. D) Representative trichrome staining for vehicle vs. ganetespib-treated mice; blue areas are fibrotic. Scale bars, 200 μm; original magnification, ×10. E, F) Representative images (E) and quantification (F) of cilia in kidneys of Pkd1−/− and WT mice treated with vehicle or ganetespib for 50 wk. Cilia are visualized with antibody to acetylated α-tubulin (green), with DAPI (blue) indicating nuclei. Scale bars, 5 μm; original magnification, ×240 (E); scale bars, 10 μm; original magnification, ×63 (F). Data are expressed as means ± sem; ns, not significant. *P ≤ 0.05, **P ≤ 0.01 compared to WT mice; &P ≤ 0.05, &&P ≤ 0.01, &&&&P ≤ 0.0001 compared to vehicle-treated Pkd1−/− mice.
HSP90 inhibition is beneficial in Pkd2−/− mice
While ∼85% of clinical cases of ADPKD arise from mutations in the PKD1 gene, the remainder arise from mutations in PKD2. We used a tamoxifen-induced mouse model for ablation of the Pkd2 gene (43, 44) as an independent system to gauge the effect of HSP90 inhibition. Using a similar experimental design to that shown in Fig. 1A, mice were dosed with HSP90 inhibitor or with vehicle once weekly for 10 wk from ages 4 to 6.5 mo. MRI and histopathologic assessment of mice showed a trend toward separation in kidney size and number of renal cysts (Supplemental Fig. S3A–E) between HSP90 inhibitor-treated and non–drug-treated groups, which was statistically significant for cyst volume measurements. Analysis of BUN and creatinine indicated that HSP90 inhibition caused a trend toward reduction in these markers of renal dysfunction (Supplemental Fig. S3F, G). Finally, as with the Pkd1 mouse model, treatment with ganetespib resulted in significant loss of ciliation in the lining of renal cysts (Supplemental Fig. S3H).
Analysis of ganetespib in comparison and in combination with 2DG
2DG, an inhibitor of glycolysis, has shown some efficacy in controlling cystogenesis in rapidly progressing Ksp-Cre;Pkd1flox/− and Pkd1V/V mouse models for polycystic kidney disease (9, 45). 2DG and glycolysis have no known link to control of ciliation, raising the possibility that the combination of ganetespib with 2DG might result in a synergistic effect that is based on orthogonal action. To evaluate the activity of ganetespib in contrast to that of 2DG, and in combination with 2DG, we treated Pkd1−/− and control mice induced with tamoxifen at P35 and P36 with ganetespib (100 mg/kg), 2DG (at 0.5 or 1 g/kg), or both from 2.5 to 6.5 mo of age (Fig. 6A). In an analysis of the ratio of MRI-assessed kidney volume (Fig. 6B) or kidney to body weight (Fig. 6C), 0.5 g/kg 2DG had little effect, and 1 g/kg 2DG and ganetespib offered comparable control of kidney size. In general, the combination of ganetespib with 2DG did not result in improved phenotype over either agent alone. For control of cyst formation (Fig. 6D), both concentrations of 2DG showed similar effects, which was comparable to that of 100 mg/kg ganetespib (Fig. 6E). Finally, use of an independent inhibitor of HSP90, STA-2842, in combination with 2DG resulted in qualitatively similar results in control of cystogenesis and kidney growth (Supplemental Fig. S4).
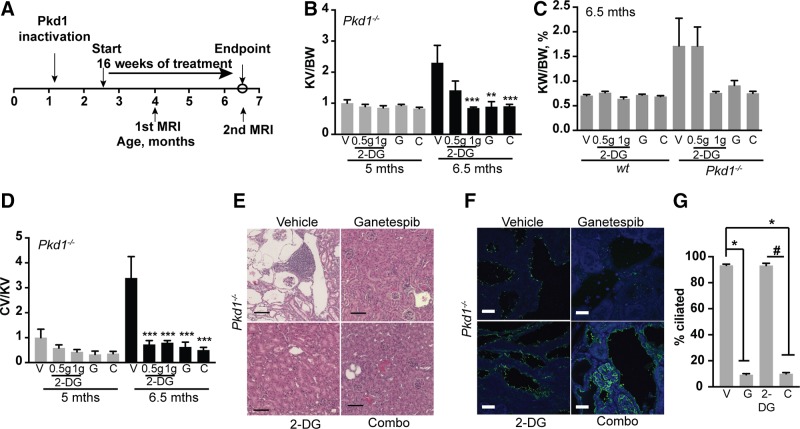
Figure 6.
Benchmarking and combination of ganetespib with glycolysis inhibitor 2DG. A) Experimental outline. Cohorts evaluated included: n = 8 for WT mice treated with vehicle; n = 5 for WT/ganetespib; n = 6 for WT mice/2DG 0.5 g; n = 5 for WT mice/2DG 1 g; n = 6 for WT mice/combination of ganetespib and 2DG; n = 10 for Pkd1−/−/vehicle; n = 11 for Pkd1−/−/ganetespib; n = 8 for Pkd1−/−/2DG 0.5 g; n = 7 for Pkd1−/−/2DG 1 g; n = 8 for Pkd1−/−/combination of ganetespib and 2DG. B) Quantification of kidney volume to body weight (KV/BW) from MRI images. V, vehicle; G, ganetespib; C, combined ganetespib and 2DG. C) Kidney weight to body weight (KW/BW) ratio for indicated treatment groups. D) Cystic index quantified from H&E-stained images for indicated groups. E) Representative images of H&E-stained kidney of Pkd1−/− mice treated with vehicle, ganetespib, 2DG (1 g), and combination of 2DG and ganetespib. Scale bars, 250 μm; original magnification, ×10. F, G) Representative images (F) and quantitation (G) of ciliation in kidney of mice treated with vehicle, ganetespib, 2DG, and combination for 16 wk. Cilia are visualized with antibody to acetylated α-tubulin (green) and nucleus is stained with DAPI (blue). Scale bars, 20 μm; original magnification, ×63. Data are expressed as means ± sem; n/s, not significant. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 relative to vehicle control; #P ≤ 0.05 relative to 2DG.
To confirm anticipated mechanisms of action, we also investigated whether treatment with 2DG affected ciliation or whether ganetespib affected glycolysis. Analysis of ciliation in kidneys of mice treated for 16 wk with 2DG showed no effect on ciliation (Fig. 6F). In vitro, treatment of either human cell lines or murine primary renal cells with WT or PKD1-mutant genotypes with 5 μΜ 2DG was sufficient to almost eliminate glycolysis (Supplemental Fig. S5A, B). However, 2DG did not affect ciliary resorption in human cells; although it very weakly increased ciliation in primary murine lines, this was not observed in an immortalized murine NIH3T3 cells and is unlikely to be biologically significant (Supplemental Fig. S6A, B). Conversely, treatment of these cell lines with a dose of ganetespib caused rapid ciliary resorption (Supplemental Fig. S6A, B), but it had no statistically significant effect on glycolytic capacity and active glycolysis (Supplemental Fig. S5A, B).
DISCUSSION
The preclinical studies summarized here indicate that ganetespib, delivered once every 1 or 2 wk, provides a sustained treatment benefit that is manifested by reduced kidney growth, reduced formation of renal cysts, reduced fibrosis, and reduced fluid retention, with a trend toward increased survival in mouse models simulating PKD1- and PKD2-dependent forms of ADPKD. Mechanistically, this is consistently associated with both rapid and durable loss of cilia on renal epithelial cells, mediated through ganetespib inhibition of the proteasome, and thus stimulation of TCHP and NEK8, representing a novel mechanism of action of HSP90 inhibition.
A number of therapeutic strategies are under investigation for ADPKD. One group of therapies targets systemic disease-associated processes relevant to the decline in renal function, such as hypertension. These include investigation of the angiotensin-converting enzyme inhibitors lisinopril and valsartan; tolvaptan, to control vasopressin-dependent cAMP signaling; and somatostatin receptor inhibitors such as octreotide and related compounds (6, 46–49). To date, perhaps the most successful clinical trial with ADPKD patients has been with the vasopressin V(2) receptor antagonist tolvaptan (46, 50), although adverse effects (notably decreased ability to concentrate urine) have posed challenges, and there have been concerns about the cost-effectiveness of treatment (51) as well as hepatic toxicity.
Other therapeutic approaches that have been investigated have focused on controlling the signaling changes that occur in ADPKD-associated kidneys downstream of the initiating PKD1 and PKD2 lesions. Many of these changes involve activation of proteins that have also been implicated as therapeutic targets in controlling cancer proliferation (12, 13, 28, 43, 44, 52, 53). Many of the proproliferative signaling proteins are also HSP90 clients, and we have previously shown these are repressed in ADPKD by HSP90 inhibition with the compound STA-2842 both in vitro and in vivo (12). However, the data in this study raise the interesting possibility that some of the suppression of activity of the HSP90 signaling clients may be in part a consequence of activity of HSP90 in limiting cell ciliation, influencing the ability of cells to interact with many extracellular cues (54, 55), and quenching the ability of mutated alleles of PKD1 to activate these proproliferative pathways. Given the large number of cilia-associated candidates that have been associated with HSP90 (Supplemental Table S1), and the multiple stimuli shown to activate AURKA (36, 56), it is possible that HSP90 control of additional factors than TCHP and NEK8 may contribute to ciliary resorption. Independent of our study, an intriguing kinome analysis indicated that while HSP90 inhibition reduced activity of >90% of kinases, this inhibition up-regulated a very limited subset of kinases including AURKA, each of which has separately been shown to induce ciliary resorption (14, 57, 58). It is also striking that while ganetespib induces loss of cilia regardless of genotype in vitro, it only induces this loss in vivo in the context of ADPKD cysts. Together with earlier data indicating up-regulation of HSP90 in cysts (12), this suggests HSP90 is not as critical for AURKA activity in quiescent cells (e.g., in WT kidneys in vivo) but is in cultured cells or in renal epithelial cells growing under stress in vivo.
Overall, given the role of HSP90 in supporting expression and activity of many proteins associated with stress resistance and proliferation, the beneficial effect of ganetespib that we observed in ADPKD is likely to involve a pleiotropic targeting of disease-associated processes. Further, the degree to which the morbidity of ADPKD arises from cystogenesis eliminating functional kidney tissue, as opposed to PKD1 or PKD2 mutations causing defective renal hormonal signaling and metabolism [e.g., renin-angiotensin-aldosterone homeostasis (4)], as well as extrarenal actions of these mutations in affecting the vasculature (5, 59), are topics of ongoing debate and affect the development of predictive biomarkers for clinical trials (60). Finally, it is possible that some of the beneficial activity of ganetespib in the ADPKD models reflects additional activities beyond targeting cystic signaling and the ciliary cycle: for instance, this agent has been shown to reduce lung inflammation in one mouse model (61), and our data indicate reduced immune cell infiltration in the long-term dosing study (Supplemental Fig. S2). Further investigation of ganetespib for ADPKD, potentially in combination with lisinopril (6) or other agents targeting vascular symptoms of this disease, are warranted.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors express sincere gratitude to J. Calvet (University of Kansas Cancer Center, Kansas City, KS, USA) and J. Grantham (Kansas University Medical Center, Kansas City, KS, USA) for helpful conversations and encouragement. The authors thank G. Germino [National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)] for providing the Pkd1 mutant mouse strains, S. Tarpinian and A. Lerro (Fox Chase Laboratory Animal Facility, Institute for Cancer Research, Fox Chase Cancer Center) for help with mice, and B. Egleston (Fox Chase Cancer Center Biostatistics Facility) for consultation on analysis. This work was supported by NIH, NIDDK R01 DK108195, by DOD PRMRP W81XWH-12-1-0437/PR110518, and by an award from the PKD Foundation (to E.A.G.); the Russian Government Program for Competitive Growth of Kazan Federal University (to A.Y.D. and A.A.K.); and by NIH National Cancer Institute Core Grant CA006927 (to Fox Chase Cancer Center). The authors declare no conflicts of interest.
Glossary
- 2DG
2-deoxy-d-glucose
- ADPKD
autosomal-dominant polycystic kidney disease
- AURKA
Aurora-A
- BUN
blood urea nitrogen
- FBS
fetal bovine serum
- H&E
hematoxylin and eosin
- HRP
horseradish peroxidase
- HSP90
heat shock protein 90
- hTERT-RPE1
immortalized human retinal pigmented epithelial cells
- PKD1/2
polycystic kidney disease 1/2
- TCHP
trichoplein
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. S. Nikonova took part in all experiments with mouse and cell culture for PKD1, coordinating efforts of A. Y. Deneka, V. Korobeynikov, A. Gaponova, and M. C. Kopp, and performed analysis of data; A. Y. Deneka, V. Korobeynikov, A. Gaponova, and M. C. Kopp contributed to analysis of mice for PKD1; A. Y. Deneka performed Seahorse Bioscience analyses; S. Somlo developed Pkd2 mouse models, and T. N. Seeger-Nukpezah performed studies with Pkd2 mice; H. H. Hensley performed MRI experiments and related data analysis; I. G. Serebriiskii performed bioinformatics analysis of S. Somlo, and D. A. Proia contributed suggestions for experimental design, study concepts, and interpretation of data; E. A. Golemis conceived and organized the project, contributed to experimental design, and obtained funding; and A. S. Nikonova and E. A. Golemis drafted the article with critical input from A. Y. Deneka, T. N. Seeger-Nukpezah, S. Somlo, and D. A. Proia.
REFERENCES
- 1.Torres V. E., Harris P. C. (2009) Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 76, 149–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma M., Tian X., Igarashi P., Pazour G. J., Somlo S. (2013) Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat. Genet. 45, 1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris P. C., Torres V. E. (2009) Polycystic kidney disease. Annu. Rev. Med. 60, 321–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman A. B., Torres V. E., Perrone R. D., Steinman T. I., Bae K. T., Miller J. P., Miskulin D. C., Rahbari Oskoui F., Masoumi A., Hogan M. C., Winklhofer F. T., Braun W., Thompson P. A., Meyers C. M., Kelleher C., Schrier R. W. (2010) The HALT polycystic kidney disease trials: design and implementation. Clin. J. Am. Soc. Nephrol. 5, 102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrone R. D., Malek A. M., Watnick T. (2015) Vascular complications in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 11, 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrier R. W., Abebe K. Z., Perrone R. D., Torres V. E., Braun W. E., Steinman T. I., Winklhofer F. T., Brosnahan G., Czarnecki P. G., Hogan M. C., Miskulin D. C., Rahbari-Oskoui F. F., Grantham J. J., Harris P. C., Flessner M. F., Bae K. T., Moore C. G., Chapman A. B.; HALT-PKD Trial Investigators . (2014) Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2255–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres V. E., Sweeney W. E., Jr., Wang X., Qian Q., Harris P. C., Frost P., Avner E. D. (2003) EGF receptor tyrosine kinase inhibition attenuates the development of PKD in Han:SPRD rats. Kidney Int. 64, 1573–1579 [DOI] [PubMed] [Google Scholar]
- 8.Nikonova A. S., Deneka A. Y., Eckman L., Kopp M. C., Hensley H. H., Egleston B. L., Golemis E. A. (2015) Opposing effects of inhibitors of Aurora-A and EGFR in autosomal-dominant polycystic kidney disease. Front. Oncol. 5, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowe I., Chiaravalli M., Mannella V., Ulisse V., Quilici G., Pema M., Song X. W., Xu H., Mari S., Qian F., Pei Y., Musco G., Boletta A. (2013) Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 19, 488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serra A. L., Poster D., Kistler A. D., Krauer F., Raina S., Young J., Rentsch K. M., Spanaus K. S., Senn O., Kristanto P., Scheffel H., Weishaupt D., Wüthrich R. P. (2010) Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 820–829 [DOI] [PubMed] [Google Scholar]
- 11.Sweeney W. E., Jr., von Vigier R. O., Frost P., Avner E. D. (2008) Src inhibition ameliorates polycystic kidney disease. J. Am. Soc. Nephrol. 19, 1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeger-Nukpezah T., Proia D. A., Egleston B. L., Nikonova A. S., Kent T., Cai K. Q., Hensley H. H., Ying W., Chimmanamada D., Serebriiskii I. G., Golemis E. A. (2013) Inhibiting the HSP90 chaperone slows cyst growth in a mouse model of autosomal dominant polycystic kidney disease. Proc. Natl. Acad. Sci. USA 110, 12786–12791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikonova A. S., Plotnikova O. V., Serzhanova V., Efimov A., Bogush I., Cai K. Q., Hensley H. H., Egleston B. L., Klein-Szanto A., Seeger-Nukpezah T., Golemis E. A. (2014) Nedd9 restrains renal cystogenesis in Pkd1−/− mice. Proc. Natl. Acad. Sci. USA 111, 12859–12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pugacheva E. N., Jablonski S. A., Hartman T. R., Henske E. P., Golemis E. A. (2007) HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129, 1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plotnikova O. V., Nikonova A. S., Loskutov Y. V., Kozyulina P. Y., Pugacheva E. N., Golemis E. A. (2012) Calmodulin activation of Aurora-A kinase (AURKA) is required during ciliary disassembly and in mitosis. Mol. Biol. Cell 23, 2658–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jhaveri K., Modi S. (2015) Ganetespib: research and clinical development. Onco Targets Ther. 8, 1849–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Socinski M. A., Goldman J., El-Hariry I., Koczywas M., Vukovic V., Horn L., Paschold E., Salgia R., West H., Sequist L. V., Bonomi P., Brahmer J., Chen L. C., Sandler A., Belani C. P., Webb T., Harper H., Huberman M., Ramalingam S., Wong K. K., Teofilovici F., Guo W., Shapiro G. I. (2013) A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non–small cell lung cancer. Clin. Cancer Res. 19, 3068–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheway G., Schmidts M., Mans D. A., Szymanska K., Nguyen T. T., Racher H., Phelps I. G., Toedt G., Kennedy J., Wunderlich K. A., Sorusch N., Abdelhamed Z. A., Natarajan S., Herridge W., van Reeuwijk J., Horn N., Boldt K., Parry D. A., Letteboer S. J. F., Roosing S., Adams M., Bell S. M., Bond J., Higgins J., Morrison E. E., Tomlinson D. C., Slaats G. G., van Dam T. J. P., Huang L., Kessler K., Giessl A., Logan C. V., Boyle E. A., Shendure J., Anazi S., Aldahmesh M., Al Hazzaa S., Hegele R. A., Ober C., Frosk P., Mhanni A. A., Chodirker B. N., Chudley A. E., Lamont R., Bernier F. P., Beaulieu C. L., Gordon P., Pon R. T., Donahue C., Barkovich A. J., Wolf L., Toomes C., Thiel C. T., Boycott K. M., McKibbin M., Inglehearn C. F., Stewart F., Omran H., Huynen M. A., Sergouniotis P. I., Alkuraya F. S., Parboosingh J. S., Innes A. M., Willoughby C. E., Giles R. H., Webster A. R., Ueffing M., Blacque O., Gleeson J. G., Wolfrum U., Beales P. L., Gibson T., Doherty D., Mitchison H. M., Roepman R., Johnson C. A.; UK10K ConsortiumUniversity of Washington Center for Mendelian Genomics . (2015) An siRNA-based functional genomics screen for the identification of regulators of ciliogenesis and ciliopathy genes. Nat. Cell Biol. 17, 1074–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J. H., Ki S. M., Joung J. G., Scott E., Heynen-Genel S., Aza-Blanc P., Kwon C. H., Kim J., Gleeson J. G., Lee J. E. (2016) Genome-wide screen identifies novel machineries required for both ciliogenesis and cell cycle arrest upon serum starvation. Biochim. Biophys. Acta 1863(6, 6 Pt A), 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaheen R., Szymanska K., Basu B., Patel N., Ewida N., Faqeih E., Al Hashem A., Derar N., Alsharif H., Aldahmesh M. A., Alazami A. M., Hashem M., Ibrahim N., Abdulwahab F. M., Sonbul R., Alkuraya H., Alnemer M., Al Tala S., Al-Husain M., Morsy H., Seidahmed M. Z., Meriki N., Al-Owain M., AlShahwan S., Tabarki B., Salih M. A., Ciliopathy Working Group , Faquih T., El-Kalioby M., Ueffing M., Boldt K., Logan C. V., Parry D. A., Al Tassan N., Monies D., Megarbane A., Abouelhoda M., Halees A., Johnson C. A., Alkuraya F. S. (2016) Characterizing the morbid genome of ciliopathies. Genome Biol. 17, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J. E., Gleeson J. G. (2011) A systems-biology approach to understanding the ciliopathy disorders. Genome Med. 3, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Dam T. J., Wheway G., Slaats G. G., SYSCILIA Study Group , Huynen M. A., Giles R. H. (2013) The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen L. J., Kuhn M., Stark M., Chaffron S., Creevey C., Muller J., Doerks T., Julien P., Roth A., Simonovic M., Bork P., von Mering C. (2009) STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 37(Database), D412–D416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Echeverría P. C., Bernthaler A., Dupuis P., Mayer B., Picard D. (2011) An interaction network predicted from public data as a discovery tool: application to the Hsp90 molecular chaperone machine. PLoS One 6, e26044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taipale M., Krykbaeva I., Koeva M., Kayatekin C., Westover K. D., Karras G. I., Lindquist S. (2012) Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 150, 987–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piontek K., Menezes L. F., Garcia-Gonzalez M. A., Huso D. L., Germino G. G. (2007) A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 13, 1490–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piontek K. B., Huso D. L., Grinberg A., Liu L., Bedja D., Zhao H., Gabrielson K., Qian F., Mei C., Westphal H., Germino G. G. (2004) A functional floxed allele of Pkd1 that can be conditionally inactivated in vivo. J. Am. Soc. Nephrol. 15, 3035–3043 [DOI] [PubMed] [Google Scholar]
- 28.Smithline Z. B., Nikonova A. S., Hensley H. H., Cai K. Q., Egleston B. L., Proia D. A., Seeger-Nukpezah T., Golemis E. A. (2014) Inhibiting heat shock protein 90 (HSP90) limits the formation of liver cysts induced by conditional deletion of Pkd1 in mice. PLoS One 9, e114403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasband W. S. (1997–2009) ImageJ. National Institutes of Health, Bethesda, Maryland [Google Scholar]
- 30.Reichardt W., Romaker D., Becker A., Buechert M., Walz G., von Elverfeldt D. (2009) Monitoring kidney and renal cyst volumes applying MR approaches on a rapamycin treated mouse model of ADPKD. MAGMA 22, 143–149 [DOI] [PubMed] [Google Scholar]
- 31.Lee Y. R., Lee K. B. (2006) Reliability of magnetic resonance imaging for measuring the volumetric indices in autosomal-dominant polycystic kidney disease: correlation with hypertension and renal function. Nephron Clin. Pract. 103, c173–c180 [DOI] [PubMed] [Google Scholar]
- 32.Shults J., Ratcliffe S. J., Leonard M. (2007) Improved generalized estimating equation analysis via xtqls for implementation of quasi-least squares in Stata. Stata J. 7, 147–166 [Google Scholar]
- 33.Shillingford J. M., Murcia N. S., Larson C. H., Low S. H., Hedgepeth R., Brown N., Flask C. A., Novick A. C., Goldfarb D. A., Kramer-Zucker A., Walz G., Piontek K. B., Germino G. G., Weimbs T. (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 103, 5466–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholls D. G., Darley-Usmar V. M., Wu M., Jensen P. B., Rogers G. W., Ferrick D. A. (2010) Bioenergetic profile experiment using C2C12 myoblast cells. J. Vis. Exp. 46, 2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z., Gholami A. M., Kuster B. (2012) Systematic identification of the HSP90 candidate regulated proteome. Mol. Cell Proteomics 11, M111.016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikonova A. S., Astsaturov I., Serebriiskii I. G., Dunbrack R. L., Jr., Golemis E. A. (2013) Aurora A kinase (AURKA) in normal and pathological cell division. Cell. Mol. Life Sci. 70, 661–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasahara K., Kawakami Y., Kiyono T., Yonemura S., Kawamura Y., Era S., Matsuzaki F., Goshima N., Inagaki M. (2014) Ubiquitin–proteasome system controls ciliogenesis at the initial step of axoneme extension. Nat. Commun. 5, 5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoko A., Matsuyama M., Goto H., Ohmuro-Matsuyama Y., Hayashi Y., Enomoto M., Ibi M., Urano T., Yonemura S., Kiyono T., Izawa I., Inagaki M. (2012) Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J. Cell Biol. 197, 391–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cizmecioglu O., Krause A., Bahtz R., Ehret L., Malek N., Hoffmann I. (2012) Plk2 regulates centriole duplication through phosphorylation-mediated degradation of Fbxw7 (human Cdc4). J. Cell Sci. 125, 981–992 [DOI] [PubMed] [Google Scholar]
- 40.Zalli D., Bayliss R., Fry A. M. (2012) The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis. Hum. Mol. Genet. 21, 1155–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding X. F., Zhou J., Hu Q. Y., Liu S. C., Chen G. (2015) The tumor suppressor pVHL down-regulates never-in-mitosis A–related kinase 8 via hypoxia-inducible factors to maintain cilia in human renal cancer cells. J. Biol. Chem. 290, 1389–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plotnikova O. V., Pugacheva E. N., Golemis E. A. (2011) Aurora A kinase activity influences calcium signaling in kidney cells. J. Cell Biol. 193, 1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spirli C., Okolicsanyi S., Fiorotto R., Fabris L., Cadamuro M., Lecchi S., Tian X., Somlo S., Strazzabosco M. (2010) Mammalian target of rapamycin regulates vascular endothelial growth factor–dependent liver cyst growth in polycystin-2–defective mice. Hepatology 51, 1778–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spirli C., Okolicsanyi S., Fiorotto R., Fabris L., Cadamuro M., Lecchi S., Tian X., Somlo S., Strazzabosco M. (2010) ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology 138, 360–371 e367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiaravalli M., Rowe I., Mannella V., Quilici G., Canu T., Bianchi V., Gurgone A., Antunes S., D’Adamo P., Esposito A., Musco G., Boletta A. (2016) 2-Deoxy-d-glucose ameliorates PKD progression. J. Am. Soc. Nephrol. 27, 1958–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres V. E., Chapman A. B., Devuyst O., Gansevoort R. T., Grantham J. J., Higashihara E., Perrone R. D., Krasa H. B., Ouyang J., Czerwiec F. S.; TEMPO 3:4 Trial Investigators . (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 367, 2407–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson M. L., Macnicol A. M., Allan P. L., Wright A. F. (1992) Effects of angiotensin converting enzyme inhibition in adult polycystic kidney disease. Kidney Int. 41, 206–210 [DOI] [PubMed] [Google Scholar]
- 48.Torres V. E., Abebe K. Z., Chapman A. B., Schrier R. W., Braun W. E., Steinman T. I., Winklhofer F. T., Brosnahan G., Czarnecki P. G., Hogan M. C., Miskulin D. C., Rahbari-Oskoui F. F., Grantham J. J., Harris P. C., Flessner M. F., Moore C. G., Perrone R. D.; HALT-PKD Trial Investigators . (2014) Angiotensin blockade in late autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2267–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wüthrich R. P., Mei C. (2014) Pharmacological management of polycystic kidney disease. Expert Opin. Pharmacother. 15, 1085–1095 [DOI] [PubMed] [Google Scholar]
- 50.Chang M. Y., Ong A. C. (2012) Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron. Clin. Pract. 120, c25–c34; discussion c35 [DOI] [PubMed] [Google Scholar]
- 51.Erickson K. F., Chertow G. M., Goldhaber-Fiebert J. D. (2013) Cost-effectiveness of tolvaptan in autosomal dominant polycystic kidney disease. Ann. Intern. Med. 159, 382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takiar V., Nishio S., Seo-Mayer P., King J. D., Jr., Li H., Zhang L., Karihaloo A., Hallows K. R., Somlo S., Caplan M. J. (2011) Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl. Acad. Sci. USA 108, 2462–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seeger-Nukpezah T., Geynisman D. M., Nikonova A. S., Benzing T., Golemis E. A. (2015) The hallmarks of cancer: relevance to the pathogenesis of polycystic kidney disease. Nat. Rev. Nephrol. 11, 515–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan J., Seeger-Nukpezah T., Golemis E. A. (2013) The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies. Cell. Mol. Life Sci. 70, 1849–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seeger-Nukpezah T., Golemis E. A. (2012) The extracellular matrix and ciliary signaling. Curr. Opin. Cell Biol. 24, 652–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plotnikova O. V., Pugacheva E. N., Dunbrack R. L., Golemis E. A. (2010) Rapid calcium-dependent activation of Aurora-A kinase. Nat. Commun. 1, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majumder S., Fisk H. A. (2013) VDAC3 and Mps1 negatively regulate ciliogenesis. Cell Cycle 12, 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim S., Lee K., Choi J. H., Ringstad N., Dynlacht B. D. (2015) Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nat. Commun. 6, 8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Outeda P., Huso D. L., Fisher S. A., Halushka M. K., Kim H., Qian F., Germino G. G., Watnick T. (2014) Polycystin signaling is required for directed endothelial cell migration and lymphatic development. Cell Rep. 7, 634–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grantham J. J., Torres V. E. (2016) The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat. Rev. Nephrol. 12, 667–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lilja A., Weeden C. E., McArthur K., Nguyen T., Donald A., Wong Z. X., Dousha L., Bozinovski S., Vlahos R., Burns C. J., Asselin-Labat M. L., Anderson G. P. (2015) HSP90 inhibition suppresses lipopolysaccharide-induced lung inflammation in vivo. PLoS One 10, e0114975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.