Abstract
Stroke continues to be a leading cause of death and disability worldwide, yet effective treatments are lacking. Previous studies have indicated that stem-cell transplantation could be an effective treatment. However, little is known about the direct impact of transplanted cells on injured brain tissue. We wanted to help fill this knowledge gap and investigated effects of hematopoietic stem/progenitor cells (HSPCs) on the cerebral microcirculation after ischemia–reperfusion injury (I/RI). Treatment of HSPCs in I/RI for up to 2 wk after cerebral I/RI led to decreased mortality rate, decreased infarct volume, improved functional outcome, reduced microglial activation, and reduced cerebral leukocyte adhesion. Confocal microscopy and fluorescence-activated cell sorting analyses showed transplanted HSPCs emigrate preferentially into ischemic cortex brain parenchyma. We isolated migrated HSPCs from the brain; using RNA sequencing to investigate the transcriptome, we found metallothionein (MT, particularly MT-I) transcripts were dramatically up-regulated. Finally, to confirm the significance of MT, we exogenously administered MT-I after cerebral I/RI and found that it produced neuroprotection in a manner similar to HSPC treatment. These findings provide novel evidence that the mechanism through which HSPCs promote repair after stroke maybe via direct action of HSPC-derived MT-I and could therefore be exploited as a useful therapeutic strategy for stroke.—Smith, H. K., Omura, S., Vital, S. A., Becker, F., Senchenkova, E. Y., Kaur, G., Tsunoda, I., Peirce, S. M., Gavins, F. N. E. Metallothionein I as a direct link between therapeutic hematopoietic stem/progenitor cells and cerebral protection in stroke.
Keywords: brain, cerebrovascular disease, neuroprotectants, ischemia–reperfusion injury
The pathophysiology of ischemic stroke (IS) is prolonged throughout reperfusion of blood to injured brain tissue (1) through a process known as ischemia–reperfusion injury (I/RI). Although the exact mechanisms responsible for postischemic cerebral damage are unclear, the inflammation after I/RI could be a contributing factor (2). This injurious process occurs over hours and days after stroke onset and thus provides an extended window for intervention beyond excitotoxic cell death, initiating within just minutes.
Experimentally, stem-cell (SC) treatments for IS have shown great success by improving both survival and functional recovery (3–5). These results have been observed after administration of SCs from various lineages, most frequently adipose and other mesenchymal SCs (6, 7), neural SCs (8), and hematopoietic SCs (HSCs) (9), as well as after administration of induced pluripotent SCs that were reverse engineered from fibroblasts (10). [Embryonic or fetal SCs are now rarely used (3).]
Clinically, trials are tentatively progressing on a large body of data reporting the secondary effects of SCs that is rarely underpinned by evidence of direct mechanisms through which SCs elicit protection. Recently, CD34+ HSCs successfully passed a phase 1 clinical trial that assessed the safety and feasibility of the treatment (11). Patients in this trial had reduced brain lesions at the 6 mo end point of the study and showed no significant treatment-related adverse effects. Despite its success, this trial and the majority of related studies have not demonstrated how transplanted cells are directly of benefit to injured brain tissue, thus preventing optimization of therapy (12).
Several lines of evidence indicate the antiinflammatory nature of transplanted SCs: SC treatment appears to correlate with increased antiinflammatory IL-10 and TGF-β, as opposed to proinflammatory IL-1β and TNF-α (13). In addition, some evidence indicates the ability of SCs to promote growth and survival of surrounding tissue via secretion of VEGF (14) or other growth factors (15). Despite these findings, treatment with these elements individually is not able to replicate the success of SCs to any significant degree in clinical trials. Gaining further insight into mechanisms of SC therapy, as well as improving the migratory properties of transplanted cells, will provide huge potential for optimizing their use. It may also pave the way for their replacement with pharmaceuticals (16). Although autologous bone marrow–derived cells from the patients would remain the optimal option, the current practice of collecting an autologous population of cells from the bone marrow of patients after stroke is both time- and cost-ineffective and involves subjecting frail stroke patients to an invasive surgical procedure.
Populations of lineage negative (Lin−) hematopoietic stem/progenitor cells (HSPCs) were assessed for their potential in limiting brain damage after cerebral I/RI (Fig. 1). We demonstrated a novel role of murine HSPCs in regulating leukocyte–endothelial interactions in the cerebral microvasculature after I/RI, coupled with decreasing mortality, infarct volume (IV), and neurologic score (NS), when administered as late as 24 h after stroke. The HSPCs migrated readily and without cotreatment with migration-enhancing cytokines such as granulocyte macrophage colony-stimulating factor. We also demonstrated increased levels of metallothioneins (MTs, low MW antioxidative proteins) transcripts, especially MT-I, in explanted HSPCs as determined using RNA sequencing (RNA-Seq) analysis. Last, treatment of mice with MT-I significantly reduced IV and NS. Our studies could further advance HSPCs as a promising therapeutic strategy for promoting repair in cerebral I/RI.
Figure 1.
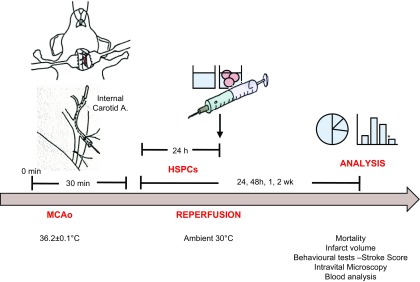
Overview of experimental design. Male C57BL/6J mice underwent 30 min middle cerebral artery occlusion (MCAo) followed by reperfusion. Mice were treated with HSPCs or saline (vehicle) 24 h after MCAo, and analyses were conducted for up to 2 wk.
MATERIALS AND METHODS
All studies were done in a blinded manner and were performed on adult male mice. Wild-type C57BL/6 mice weighing 25 to 29 g were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). C57BL/6 LysM-eGFP (LyZM) mouse strain [constitutively expressing enhanced green fluorescent protein (eGFP) in myeloid cells] weighing 15 to 17 g (4–5 wk old) were a generous gift from P. Kubes (University of Calgary, AB, Canada) and bred on site. Mice were maintained on a 12-h light–dark cycle during which room temperature was maintained at 21 to 23°C. Animals had access to a standard chow pellet diet and tap water ad libitum. All animal experiments were approved by the Louisiana State University Health Sciences Center–Shreveport Institutional Animal Care and Use Committee and were in accordance with the guidelines of the American Physiological Society.
Middle cerebral artery occlusion and reperfusion
As a cerebral I/RI model, middle cerebral artery occlusion and reperfusion (MCAo) was performed as previously reported (17). Briefly, mice were anesthetized with intraperitoneal injection of ketamine (150 mg/kg) and xylazine (7.5 mg/kg), and middle cerebral artery occlusion was occluded for 30 min using a 6-nylon intraluminal filament (Doccol, Sharon, MA, USA), followed by 24 h, 48 h, 1 wk, or 2 wk reperfusion. Sham-treated mice were subject to anesthesia and other surgical procedures without middle cerebral artery occlusion.
Cerebral intravital fluorescence microscopy
Intravital fluorescence microscopy (IVM) was performed as previously described (2). Briefly, mice were reanesthetized with intraperitoneal injection of ketamine (150 mg/kg) and xylazine (7.5 mg/kg). The jugular artery and vein were cannulated to monitor mean arterial blood pressure, as well as for intravenous administration of rhodamine 6G. The head of each mouse was fixed in a frame in sphinx position and the left parietal bone exposed by a midline skin incision, followed by a craniectomy (diameter, 2.5 mm). A 12 mm glass coverslip was placed over the craniectomy, and the space between the glass and the dura mater was filled with artificial cerebrospinal fluid (Na+ 147.8 mEq/L, K+ 3.0 mEq/L, Mg2+ 2.3 mEq/L, Ca2+ 2.3m Eq/L, Cl− 135.2 mEq/L, HCO3− 19.6 mEq/L, lactate− 1.67 mEq/L, phosphate 1.1 mM, glucose 3.9 mM; all Sigma-Aldrich, St. Louis, MO, USA). A Zeiss Axioskop microscope (Carl Zeiss GmbH, Jena, Germany) with a mercury lamp was used to observe the pial venules in the cerebral cortex. Two-minute videos were captured with a CoolSnap HQ2 (Photomerics, Tucson, AZ, USA) black-and-white camera and recorded for off-line analysis.
Confocal IVM
MCAo was performed as described above in LyZM mice. Twenty-four hours into reperfusion, mice were treated with Cell Tracker Red–labeled HSPCs. Twenty-four hours after treatment (48 h after MCAo), mice were placed on an Olympus BX51WI upright microscope (Olympus, Tokyo, Japan) with a ×20 (LUCPlanFLN) objective and equipped with a 3i LaserStack laser launch (3i, Denver, CO, USA), Yokogawa CSU-X1-A1N-E spinning disk confocal unit (Yokogawa Electric, Tokyo, Japan), and electron multiplier CCD camera (C9100-13; Hamamatsu, Bridgewater, NJ, USA). Mice were treated with PECAM-1 antibody (viewed at 594 nm, 20 μg/mouse, i.v.; eBioscience, San Diego, CA, USA) to visualize vessels.
Video analysis for IVM studies
Three to 5 randomly selected vessels, 30 to 70 μm in diameter and 100 μm long, were observed for each mouse after treatment. Adherent leukocytes were defined as cells remaining stationary for ≥30 s, expressed as the number of cells per square millimeter of the vessel surface and calculated from diameter and length, assuming a cylindrical shape (2).
Infarct volume
After a 24 h reperfusion period, mice were humanely killed; brains were removed and placed into 4°C PBS (Sigma-Aldrich) for 15 min, sectioned (2 mm), and stained with 2% 2,3,5-triphenyltetrazolium chloride in PBS at 37°C for 15 min, then fixed by immersion in 10% formaldehyde. Stained sections were photographed, and the digitized images of each brain section (and the infarcted area) were quantified using NIH 1.57 Image software (National Institutes of Health, Bethesda, MD, USA), a computerized image analysis program.
Mortality rate
Mortality rate was a binary evaluation, calculated as the percentage of animals alive in each group after MCAo (24 h after treatment with either HSPCs or vehicle).
Neurologic score
The functional consequences of cerebral I/RI were evaluated by assessing general, sensory, motor, and proprioceptive deficits that were assessed in a blinded fashion (Table 1). The 18-point score was compiled from a previously published scoring system to provide objective (yes or no) criteria for assessment (18, 19). A maximal score of 18 could be assigned to each experimental animal.
TABLE 1.
Eighteen-point neurologic score (1 point where any of the following apply)
| Sense assessed | Test |
|---|---|
| General | Irritability/piloerection |
| Immobility/staring | |
| Seizures/monoclonus/tremor | |
| Motor | Flexion of forelimb |
| Flexion of hind limb | |
| Head tilt 10° | |
| Some circling | |
| Circling over 50% of the time while moving | |
| Inability to walk straight | |
| Falling down | |
| Sensory | Corneal reflex |
| Pinna reflex (response to pinch of ear lobe) | |
| Startle reflex | |
| Proprioception | Grasps side of beam |
| Hugs beam, 1 limb down | |
| Hugs beam, 2 limbs down/spins about the beam | |
| Falls down after 5 s attempt to balance | |
| No attempt to balance, falls down |
Blood and tissue collection
Blood was taken by cardiac puncture and centrifuged at 4°C and 450g for 5 min to yield plasma. Brains were dissected and either snap frozen in liquid nitrogen or perfused with 10 ml saline followed by 10 ml 4% paraformaldehyde, then transferred into increasing concentrations of sucrose (20–30%) over 4 d. Fixed tissue was cryopreserved in Optimal Cutting Temperature compound (Thermo Fisher Scientific, Waltham, MA, USA); then both sets of samples were stored at −80°C until required.
Bone marrow extraction
Four to 5 wk old male mice (15–17 g) were humanely killed, and femurs and tibias were removed. Bones were flushed with sterile Hank buffered saline solution using a 25-gauge needle. Bone marrow was dissociated mechanically, filtered through a 70 μm gauze, centrifuged for 10 min at 450g, resuspended in 10 ml PBS, then counted using a cell counter (Thermo Fisher Scientific).
Selection of HSPCs
After bone marrow mononuclear cells (BMCs) were extracted, Lin− cells were negatively selected using magnetic beads, according to the manufacturer’s instructions of a hematopoietic cell selection kit (StemCell Technologies, Vancouver, BC, Canada). The lineage cocktail used to removed unwanted or differentiated cells consisted of the following: CD45R (B cells), CD11b (granulocytes, macrophages, and NK cells), CD3e (T cells), Ly-6G (lymphocytes), TER119 (erythrocytes).
Labeling HSPCs with carboxyfluorescein succinimidyl ester
HSPCs were reconstituted at 1 × 106 cells in 0.1% bovine serum albumin and incubated with 2 μl of 5 mM carboxyfluorescein succinimidyl ester (CFSE)/ml cells at 37°C for 10 min. After incubation, cells were washed 3 times by centrifuging for 10 min at 450 g and reconstituted at the required concentration in PBS. After staining, cell viability was 99 to 100%, as observed with Trypan blue staining.
Labeling HSPCs with Cell Tracker Red
HSPCs were reconstituted 5 × 106 cells/ml in PBS. Cell Tracker Red dye (Thermo Fisher Scientific) was added to make a final concentration of 5 μM at 37°C for 10 min (20). After incubation, cells were washed 3 times by centrifuging for 10 min at 450 g and reconstituted at the required concentration in PBS. After staining, cell viability was 99 to 100%, as observed with Trypan blue staining.
Administration of HSPCs
Under isoflurane anesthesia, mice injected intravenously with either 1 × 106 cells in 100 μl PBS, 1 × 107 cells in 200 μl PBS, or vehicle (PBS). Cell viability was checked using Trypan blue stain before injection (viability = 98–100%), and care was taken to dissociate cells thoroughly before administration. All experiments were double blinded, whereby the investigator administering treatments was unaware of the treatment type (HSPCs or vehicle), and an investigator unaware of the treatment mice conducted assessments of mice both before and after death.
Treatment with metallothionein I
Mice were subjected cerebral I/RI (30 min ischemia, followed by 48 h reperfusion) and treated with metallothionein (MT)-I, i.p. 5 μg/g body weight (21) (Enzo Life Sciences, Farmingdale, NY, USA) at the start of reperfusion. Control mice were injected with PBS.
Immunohistochemistry
Immunofluorescence staining for neuroinflammation
Cryopreserved brains were sectioned (18 μm) and stained for activated microglia. Nonspecific binding sites were blocked with 10% normal serum (Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. Sections were incubated with primary antibodies (diluted in 10% normal serum in PBS) at 4°C overnight. Microglia were detected by anti–Iba-1 antibody (1:1000; Wako BioProducts, Richmond, VA, USA). Secondary antibody used was goat anti-rabbit IgG (Life Technologies, Carlsbad, CA, USA) for 1 h at room temperature, Alexa Fluor 488 (Vector Laboratories) for 30 min at room temperature.
Staining for localized CFSE-positive HSPCs
3,3′-Diaminobenzidine (DAB) staining was used to identify localized CFSE-positive (HSPC) cells. Slices were rinsed in PBS and covered with 0.5% fish serum gelatin in PBS for 5 min. The fish serum gelatin was aspirated and incubated with a horseradish peroxidase–conjugated anti-fluorescein antibody (Abcam, Cambridge, MA, USA) 1:200, for 1 h. The antibody was washed 3 times with PBS. The slide was incubated with 0.05% DAB and 0.015% H2O2 for 5 min, then counterstained with hematoxylin.
Fluorescence-activated cell sorting analysis of SC markers and cell sorting of CFSE-positive cells from whole brain
Analysis of CD34-, Sca-1–, c-kit–, and CD31-positive cells was performed on an LSRII flow cytometer (BD Biosciences, San Jose, CA, USA), and CFSE-positive cells were sorted on a FacsAriaIII device (BD Biosciences). Where fluorescence-activated cell sorting was used to retrieve CFSE-positive transplanted HSPCs, experiments were conducted 24 h after administration of HSPCs in order to ensure that cells had time to migrate into the brain.
Western blot analysis
Total protein was extracted in RIPA buffer (Sigma-Aldrich) by homogenization and sonication. Sonication followed by centrifugation at 2000g for 15 min at 4°C was repeated until a clear solution was obtained. The concentration of protein in lysate was measured by the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Thirty micrograms of protein was mixed with loading buffer (2× Laemmli buffer; Bio-Rad, Hercules, CA, USA). The mix was loaded on 12% SDS–polyacrylamide gel with the appropriate MW markers and transferred to polyvinylidene fluoride membranes. Reversible protein staining of the membranes with 0.1% Ponceau S in 5% acetic acid was used to verify even protein transfer. Membranes were blocked with 5% nonfat dry milk, followed by overnight incubation with 2 µg/ml MT-I primary antibody (Biomatik, Wilmington, DE, USA). This was followed by 30 min washing with Tris-buffered saline containing Tween 20 and incubation for 60 min at room temperature with diluted horseradish peroxidase–conjugated secondary antibody (1:2500; Sigma-Aldrich). Membranes were washed for 30 min and proteins detected by an ECL detection kit (Bio-Rad) using film (Bio-Rad). Stripping was done using Reblot Plus (EMD Millipore, Billerica, MA, USA) and blocked with 5% nonfat dry milk. Membranes were incubated for 60 min at room temperature with 1:2000 diluted β-tubulin primary antibody and suitable secondary antibody (Cell Signaling Technology, Danvers, MA, USA). Relative band intensity was quantified using NIH 1.63 Image software.
RNA sequencing
RNA library preparation, sequencing reactions, alignment, and read count were conducted at Genewiz (South Plainfield, NJ, USA). Briefly, cDNA was directly synthesized from cell lysate and amplified by PCR, using Smartr Ultra Low Input RNA kit for sequencing-v3 (Clontech Laboratories, Mountain View, CA, USA). The Illumina Nextera XT DNA sample Prep Kit (Illumina, San Diego, CA, USA) was used to generate sequencing libraries from the cDNA. Sequencing libraries were validated using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), and quantified using the Qubit 2.0 Fluorometer (Thermo Fisher Scientific) as well as real-time PCR (Thermo Fisher Scientific). Sequencing libraries were sequenced on the Illumina HiSeq 2500 according to the manufacturer’s instructions. Sequencing was performed using a 1 × 50 Single Read configuration. The raw sequences obtained were subject to quality trim and adaptor removal with Trimmomatic 0.32. Within the CLCbio software environment (CLC genomics workbench 8.0.3, CLC Genomics Server 7.0.3), the trimmed sequences were aligned to the reference genome, Mus musculus GRCm38.75, downloaded from ENSEMBLE (http://www.ensembl.org/). RNA sequencing data have been deposited into the Gene Expression Omnibus at the National Center for Biotechnology Information (Bethesda, MD, USA; accession no. GSE95853).
RNA-Seq data analyses
Exon read count data of 12 samples given by the company were normalized with 2 methods using R software (22): read counts per kilobase and tag count comparison. Read counts per kilobase was conducted to compare the read count data among the different genes in each sample by normalizing to read counts per kilobase exon length (23), while tag count comparison was conducted to compare the read count data among the 12 samples by normalizing whole read count data, using read counts of nondifferentially expressed genes (24).
Volcano plot
A volcano plot was drawn using OriginPro 8.1 (OriginLab, Northampton, MA, USA) to visualize statistical significance together with log ratio of transcriptome data (25). Log ratios of gene expression in treated samples compared to controls were used as the x axis, and the logarithms of P values to base 10 were used as the y axis.
Functional clustering
To determine what kinds of genes were differentially expressed, we entered a list of genes that were differentially expressed (P < 0.05, more than 2-fold up- or down-regulated between the control and treated groups) for the Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/). Enrichment score was calculated by Fisher’s exact test on the basis of the number of differentially expressed genes in the sample, matching with the total number of genes that are included in each canonical pathway in the database.
Principal component analysis
Principal component analysis (PCA) can reduce the dimensionality of a data set (e.g., RNA-Seq data) consisting of a large number of interrelated variables while retaining as much as possible of the variation present in the data set (26). PCA was conducted as an unsupervised analysis to clarify the variance among RNA-Seq data among the samples, using a Q-mode PCA package “prcomp” of R (24). The proportions of variance and factor loading were also calculated (27, 28).
Statistical analysis
Data were analyzed by Student’s t test (2 groups), ANOVA with Bonferroni posttests (more than 2 groups), or χ 2 analysis (NS only). Analysis was performed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA). Data are shown as means ± sem. Differences were considered statistically significant at P < 0.05.
RESULTS
Freshly collected HSPCs are a phenotypically stable source of SCs
While CD34 is an established marker of undifferentiated BMCs in humans, in mice, undifferentiated BMCs express CD34 only in low amounts until they are 5 wk old and cease to express CD34 after 10 wk (29). Thus, we collected BMCs from 4 to 5 wk old mice, and—rather than CD34-positive selection—we used negative selection to isolate the Lin− population of progenitors from whole bone marrow. These cells were phenotypically highly consistent and were therefore selected for use in this study (Fig. 2A).
Figure 2.
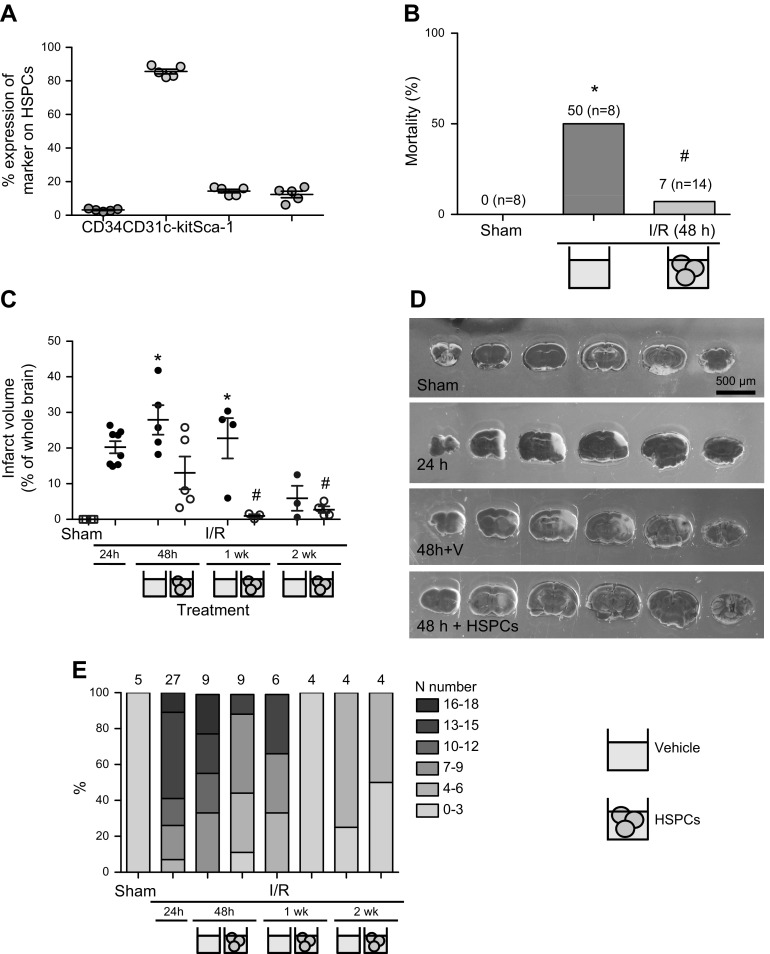
HSPCs improve outcome in brain after I/RI. Male C57BL/6J mice underwent 30 min middle cerebral artery occlusion (MCAo) followed by reperfusion. Mice were treated with HSPCs or saline (vehicle) 24 h after MCAo, and analyses were conducted up to 2 wk. Phenotypic analysis of HSPCs cells shows consistent expression of CD34, CD31, c-kit, and Sca-1, indicating HSPCs extracted on day of treatment to be phenotypically stable source of cells for use as treatment, n = 5 (A). Various outcome parameters were assessed throughout and at termination of experiments in order to assess efficacy of HSPC treatment, including mortality rates (B), IV (C, D) for representative images, and NS (E). *P < 0.05 vs. sham-treated mice, #P < 0.05 vs. saline-treated mice for respective time after MCAo; n ≥ 6 mice per group.
HSPCs afford protection for up to 2 wk after I/RI-induced cerebrovascular injury
Mortality rates after 30 min MCAo and 48 h reperfusion showed HSPC-treated mice to be protected against I/RI (Fig. 2B); the incidences of death 24 h after treatment (48 h reperfusion) in saline-treated control animals and HSPC-treated mice were 50 and 7%, respectively. No further mortality was observed in either group after 48 h up until 2 wk after ischemia, which was the termination of the experiment.
IV of saline-treated or HSPC-treated mice subjected to 30 min MCAo and 24 h, 48 h, 1 wk, or 2 wk reperfusion are presented in Fig. 2C, D. Saline-treated mice displayed larger IVs than HSPC-treated mice at all time points (Fig. 2C). At 48 h after MCAo (24 h after treatment), IV in HSPC-treated mice were reduced by 50% vs. saline-treated mice (10 vs. 27%, respectively). Furthermore, at 1 wk, no infarct was visible after HSPC treatment, whereas saline-treated mice retained infarcts of equivalent volumes (22%) to untreated mice 24 h after MCAo (20%).
HSPCs improve neurologic function up to 1 wk after cerebral I/RI
We evaluated neurologic function in animals after cerebral I/RI using an 18-point NS, which assessed various aspects of functional recovery, including changes in sensory, motor, and proprioceptive function in a blinded and binary fashion (Table 1) (17–19). NS reached a score of 13 at 24 h after stroke (mice with a score of 16+ accounted for 90% of the mortality rate). Mice treated with HSPCs showed 50% reduction in NS (on a 0–18 scale, with 0 representing no signs of stroke) at 48 h (P < 0.05), and 10% in saline treatment groups (Fig. 2). Furthermore, at 1 wk, HSPC-treated mice exhibited near sham-treated–level NS compared to saline-treated mice. All mice were almost fully recovered at 2 wk, with the only consistent sign of I/RI (in both groups) being a persistent head tilt more than 10 degrees from the vertical axis, indicating some prolonged motor dysfunction.
Transplanted HSPCs home to ischemic cortex after cerebral I/RI
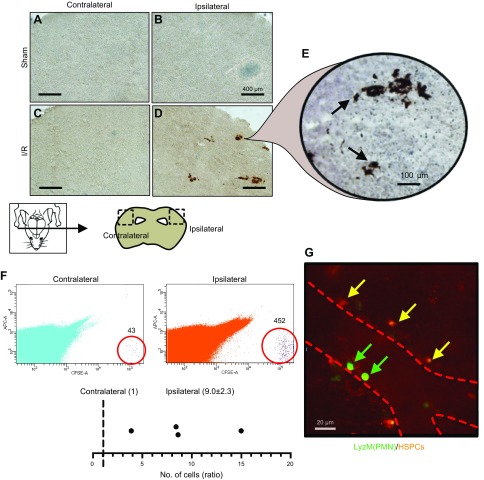
To determine whether HSPCs homed to the injured brain in HSPC-treated mice, we detected carboxyfluorescein succinimidyl ester (CSFE)-labeled HSPCs in the ipsilateral hemispheres, indicating localization of HSPCs to the periinfarct region (Fig. 3D). No positive staining was observed in the liver, lung, spleen, or muscle (gastrocnemius) (data not shown), indicating that transplanted cells homed to the infarcted region. To determine whether transplanted cells had emigrated into the parenchyma (rather than remain adhered to the luminal endothelia), we used confocal IVM to identify fluorescently labeled HSPCs that had migrated into the brain parenchyma. LyZM mice were subjected to 30 min MCAo and 48 h reperfusion, and at 24 h were treated with saline or HSPCs stained with Cell Tracker Red (Fig. 3G and Supplemental Movie 1). HSPCs were detected outside of blood vessels in the cerebral vasculature, while eGFP-positive endogenous polymorphonuclear cells were detected inside of blood vessels. At this time point (possibly due to HSPCs having already emigrated), interactions between HSPCs and polymorphonuclear cells were not observed. In addition, we also corroborated both the above findings in fluorescence-activated cell sorting experiments designed to extract transplanted cells from the brains of HSPC-treated mice, thereby identifying the migratory preference of HSPCs toward either the ipsilateral or contralateral cerebral hemisphere. This confirmed that CFSE-labeled HSPCs migrated to the ipsilateral vs. contralateral hemisphere at a ratio of 10:1 (Fig. 3F).
Figure 3.
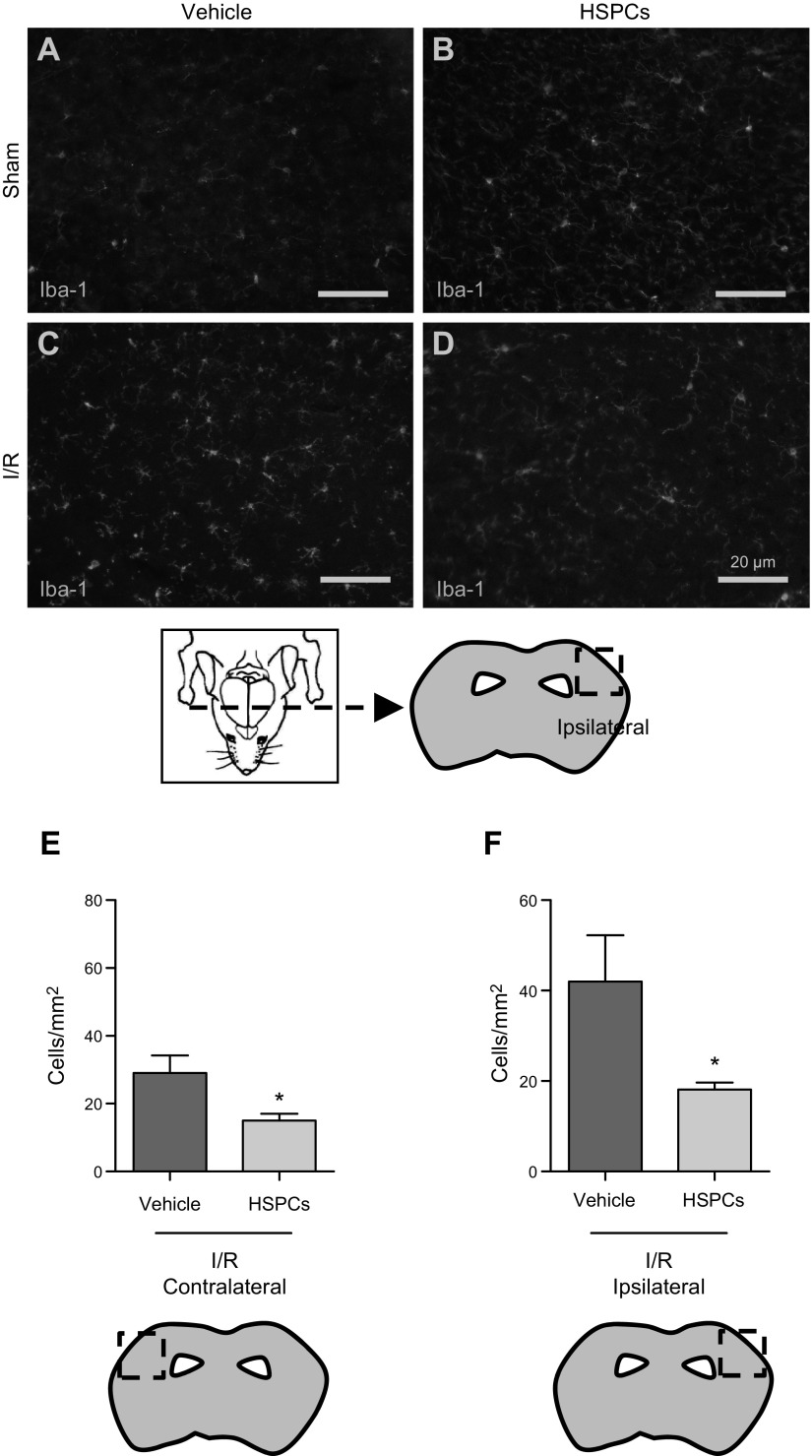
HSPCs administered i.v. migrate to ipsilateral hemisphere after cerebral I/RI. DAB staining (A–E) and sorting of CFSE-positive HSPCs from whole brain (F) indicated that HSPCs localized to ischemic brain after cerebral I/RI (graph shows ratio of HSPCs emigrated to ipsilateral vs. contralateral hemispheres, where number of HSPCs in contralateral hemisphere has been normalized to 1), and confocal intravital microscopy of ischemic brain demonstrated that once localized, HSPCs emigrated from blood vessels into surrounding parenchyma (G). Polymorphonuclear cells (PMNs) were detected in vessels (dotted line). No HSPCs were observed in contralateral cortex.
HSPCs reduce cellular responses for up to 1 wk in cerebral microcirculation of mice after I/RI
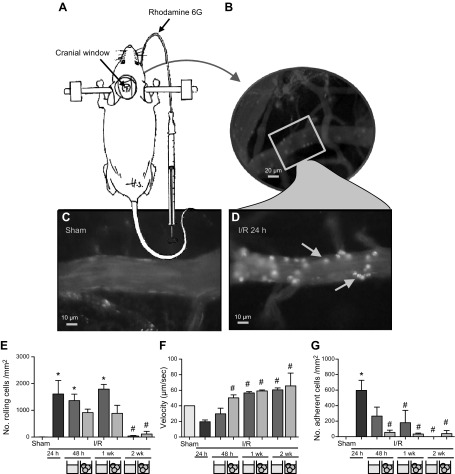
Leukocyte–endothelial interactions are required for immune cell infiltration after cerebral I/RI, with high levels of interactions leading to parenchymal inflammation and correlating with poor outcome. To investigate the impact by HSPCs treatment on these interactions, IVM of cortical venules of the ipsilateral cortex was used to film the interactions in real time (Fig. 4A). In accordance with our previous findings (1, 2), MCAo for 30 min followed by 24 h reperfusion induced interactions within the cerebral microcirculation of mice compared to sham-treated animals (Fig. 4C, D). Mice treated with HSPCs displayed reduced leukocyte rolling, increased velocity, and decreased adhesion at 48 h vs. saline-treated mice, whose interactions continued for a further week (Fig. 4). By 2 wk, both saline-treated and HSPC-treated mice displayed similar effects to prestroke levels.
Figure 4.
Leukocyte–endothelial interactions in cerebral microcirculation are reduced after HSPC treatment. Intravital microscopy was performed on mice after MCAo (A), and leukocytes were visualized using Rhodamine 6G (selectively absorbed by leukocytes) (B). Marked increases in leukocyte–endothelial interactions were observed after cerebral I/RI vs. sham-treated animals (C, D; stills from representative videos). Videos of 3 to 5 venular microvessels (30–70 μm diameter) were recorded for 2 min, then analyzed with respect to number of rolling leukocytes (mm2/min) (E) and their velocity (μm/min) (F), as well as number of leukocytes adherent to cells wall for ≥30 s (mm2/min) (G). No interactions were observed in arterioles (data not shown). *P < 0.05 vs. sham-treated mice, #P < 0.05 vs. saline (vehicle)-treated mice for respective time after MCAo (n ≥ 6 mice/group).
HSPCs protect against augmented neuroinflammation after I/RI
To investigate whether HSPCs could affect neuroinflammation, we monitored microglial activation in saline-treated and HSPC-treated mice. At 48 h reperfusion, using Iba-1 staining (Fig. 5), as expected, Iba-1 expression was substantially elevated in both the ipsilateral (injured) and contralateral side in mice after I/RI, with greater expression in the ipsilateral side. Furthermore, compared to saline-treated mice, HSPC-treated mice showed a significant decrease in Iba-1 expression, indicating a reduction in microglia activation. No Iba-1 expression was found in sham-treated mice or in sham-treated mice treated with HSPCs (data not shown).
Figure 5.
Neuroinflammation was suppressed after HSPC treatment after cerebral I/RI. Activated microglia were stained (Iba-1) (A–D) and counted within three 100 × 100 μm cortical segments along ipsilateral and contralateral hemispheres. Increase in activated microglia after MCAo was greater in ipsilateral cortex, which was reduced by 50% in both cortices after HSPC treatment (E, F). *P < 0.05 vs. saline (vehicle)-treated mice (n = 4 mice/group).
Explanted HSPCs display regulation of MT-I gene profile
To identify potential mechanisms through which HSPCs might directly affect their milieu once transplanted and migrated, we determined the transcriptome profile of HSPCs extracted from the brain after their transplantation 24 h previously, after I/RI. Analysis of transcriptomes showed that 562 genes and 47 genes were significantly up- or down-regulated more than 2-fold, respectively, in isolated vs. naive HSPCs (Supplemental Fig. 1 and Table 1). Among the differentially expressed genes, we examined the expression levels of genes associated with inflammation and angiogenesis (Supplemental Table 2), in which IL-10 receptor α subunit (Il10ra) and epiregulin (Ereg) were significantly up-regulated (Il10ra: 4.2-fold, P < 0.05; Ereg: 6.9-fold, P < 0.05), respectively.
To identify molecular pathways that are potentially associated with HSPC activity after their homing to injured tissue, we performed functional clustering with DAVID 6.8 (Supplemental Table 3). Among the pathways, the differentially expressed in normal and neoplastic cells (DENN) domain-related pathway, oligoadenylate synthetase–related pathway, and steroid hormone receptor signaling pathway were listed as the top 3 pathways on the basis of enrichment scores.
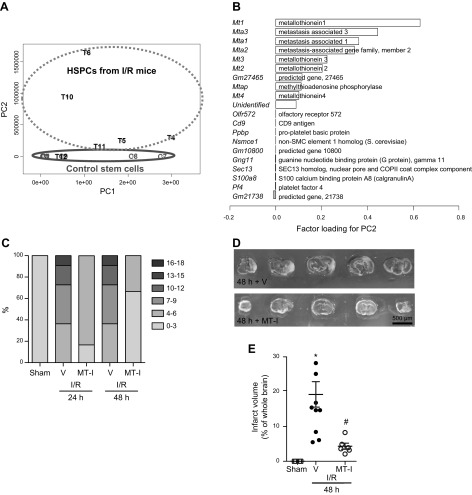
We conducted PCA to see overall gene expression patterns among the samples. Principal component (PC) 2, but not PC1, separated samples between controls and treated groups except 1 sample (sample T12) (Fig. 6A). Factor loadings for PC2 showed that up-regulation of several genes contributed to the PC2 value positively, while contribution by down-regulated genes to the PC2 value was negligible (Fig. 6B and Supplemental Fig. 1). The factor loadings in PCA are the correlation coefficients (r) between the variables (read numbers of molecules) and factors (PC2 values); the squared factor loadings (r2) are the amount of explained variation. Among the genes that positively contributed to the PC2 value, only the Mt1 gene had moderately high r2 (= 0.38), while the top 10 genes, including other MT genes (Mt2, Mt3, and Mt4), MTa genes (Mta1, Mta2, and Mta3), and other genes (Fig. 6B) had low r2 (<0.2). On the basis of these findings, we decided to investigate the role of MT-I.
Figure 6.
PCA of RNA sequencing transcriptome data from stem cells before (controls; samples C1-3, C7-9) and 24 h after (treated; samples T4-6, T10-12) transfer. A) PCA of 12-transcriptome data showed overall gene expression patterns. On the basis of PC2 values, PCA separated most samples between control vs. treated groups, with exception of sample T12. B) Factor loading for PC2 ranked genes that contributed to PC2 values positively (top half; Mt1, most significant) and negatively (bottom half). C–E) Male C57BL/6 mice underwent 30 min MCAo followed by reperfusion. Mice were treated with MT-1 or PBS (vehicle) 24 h after MCAo and neurological score (C) and infarct volume (D, E) were quantified.
MT-I attenuates I/RI-induced cerebrovascular injury
MT-I has been shown to be a secreted protein (30). Having demonstrated that explanted HSPCs possess high levels of MT-I when in the post-I/RI cerebral environment, we ascertained whether protein levels of MT-I are up-regulated in the contralateral and ipsilateral cerebral regions after HSPC administration. Supplemental Fig. 2 shows that the expression of MT-I is up-regulated in ipsilateral cerebral regions after HSPC administration vs. vehicle administration. To further build on these findings, we next tested the potential of MT-I treatment to afford protection after cerebral I/RI. Treatment of mice with MT-I markedly attenuated the inflammatory response vs. saline-treated mice, as assessed by NS (reduced from 10–12 to 0–3 over 48 h) and IV (Fig. 6C–E).
DISCUSSION
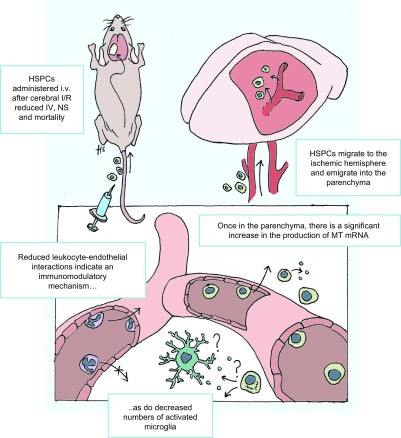
This study presents several key novel findings regarding the administration of HSPCs to mice after cerebral I/RI. We demonstrate that: 1) HSPCs mobilize and home to the injured brain without pharmacologic intervention, 2) HSPCs exert protection within the cerebral microvasculature and recovery of neurologic function, 3) HSPCs emigrate into the brain parenchyma where they produce MT mRNA, and 4) the protective effects of HSPCs in stroke can be recapitulated by the administration of MT-I (Figs. 6C–E and 7).
Figure 7.
Schematic overview of important protective role HSPCs play in abrogating I/RI-induced cerebrovascular injury.
Among the many potential regenerative medicine strategies tailored toward cerebral injury repair, SC-based therapeutics have shown the most promise. Despite the encouraging results suggesting SC therapy, including CD34+ SC, as a stroke treatment, mechanisms of action warrant additional investigations. Endogenous CD34+ SCs are mobilized into the peripheral blood after stroke (31), and enhancing their migration using granulocyte macrophage colony-stimulating factor is highly neuroprotective (32). Results observed in a phase 1 trial treating 5 IS patients with 1 × 108 CD34+ HSPCs indicated that transplantation of these cells might also induce neuroprotection, although the mechanism of action remained unknown. We demonstrate herein that mice treated with HSPCs displayed an abrogated neuroinflammation with neuroprotection after I/RI. This was observed by decreased adherent leukocytes, which is consistent with the cerebral effects observed in other models [e.g., systemic administration of LPS (33)], and decreased microglial activation, along with decreased IV (which has been linked to levels of inflammation during cerebral reperfusion), decreased NS, and increased survival. Our results are in line with experimental and clinical findings supporting the use of SCs as a therapeutic in IS. Furthermore, here we show that HSPCs may be a promising source of therapeutic SCs, supported by our findings that the injected HSPCs mobilized and preferentially homed to the ischemic hemisphere after cerebral I/RI.
Route of administration of SCs for treatment has long been under debate. Recent studies have shown that there is little or no difference in benefit between cells administered either intravenously or intraarterially. (34), Thus, we chose to administer 1 × 106 [within the range of HSC numbers that have been used in previous publications, from 5 × 105 (33) to 8 × 106 (35)] HSPCs, intravenously; by using a venous access point for the treatment rather than the carotid artery, patients need not be excluded on the basis of carotid stenosis. In addition, we administered HSPCs 24 h after stroke to represent a treatment regimen that can be applied to patients (i.e., after the onset of stroke) (36), in contrast to previous prophylactic studies that, while demonstrating efficacy of CD34+ HSC, administered cells as early as 48 h before stroke (37).
In this study, although HSPCs were found to be present in both the contralateral and ipsilateral (infarcted) hemispheres, considerably greater numbers were found in the ipsilateral hemisphere of experimental mice. Differences in the integrity and pathophysiologic status of the blood–brain barrier (2) may facilitate HSPCs into the ischemic hemisphere more selectively (38), while some studies have shown that cells fail to localize to an infarct at all (although in some cases are still protective) (39).
In addition, with respect to HSPC migration, we were able to achieve efficient migration when administering cells intravenously Clinical trials have commonly opted for intraarterial administration via the common carotid artery ipsilateral to the infarct because intuitively it is the most efficient way to deliver the largest number of cells rapidly to the infarct area while avoiding the considerable invasiveness of intracerebroventricular injection. Preclinically, recent work has shown little or no improvement when using intraarterial; and intracerebroventricular approaches compared to intravenous (40), with cells able to migrate in significant numbers to an infarct region having been administered intravenously [although some studies describe large numbers of cells becoming lodged in the lungs (39, 41)]. This less invasive route is preferable in a clinical setting when dealing with patients who are both frail and immunocompromised after stroke. Moreover, intraarterial; administration may be additionally deleterious because of the potential formation of microemboli and decreased cerebral blood flow (42).
The homing and migratory ability of HSPCs in our study is perhaps unsurprising, as hematopoietic progenitors ultimately differentiate into blood cells that themselves have migratory abilities, as the machinery with which to respond to chemokine and cytokine gradients. In fact, HSPCs have previously been shown to express both VCAM-1 and its receptor, VLA-4 (43, 44), both of which would aid in the migratory process into the brain (45, 46). Interestingly, our findings demonstrated a vast improvement between HSPC- and saline-treated mice just 24 h after administration, suggesting that the protective mechanism of the cells was unlikely to involve direct replacement of infarcted brain tissue. In fact, it has yet to be observed in humans that any type of SCs applied as a therapy replace lost neuronal circuitry (47,48).
Despite many studies focusing on the clarification of which signaling molecules attract SCs and direct their migration to damaged areas, little is known regarding what HSPCs do in the brain after stroke. We have demonstrated here that transcriptome in naive HSPCs vs. those that had been transplanted and had emigrated into the brain parenchyma showed marked differences in MT transcripts, in particular MT-I. RNA-Seq analyses also uncovered increases in inflammation-associated transcripts and other transcripts. Up-regulation of IL-10ra mRNA suggested activity of anti-inflammatory IL-10 pathway, which may inhibit the homing of inflammatory cells, but not that of HSPCs. The DENN domain–related pathway included up-regulation of DENN/MAP kinase-activating death domain containing 1A (Dennd1a), DENN/MAP kinase-activating death domain containing 3 (Dennd3), and SET binding factor 1 (Sbf1), which has been associated with Rab-mediated processes or regulation of MAPK signaling (47). The oligoadenylate synthetase-related pathway included up-regulation of 2'-5′ oligoadenylate synthetase 1D (Oas1d), 2'-5′ oligoadenylate synthetase 3 (Oas3), and 2'-5′ oligoadenylate synthetase [1H] (Oas1h), which has been associated with innate immune response (49). The steroid hormone receptor signaling pathway included up-regulation of peroxisome proliferative activated receptor γ coactivator 1β (Ppargc1b), RNA binding protein, fox-1 homolog 2 (Rbfox2), and estrogen receptor 2 (Esr2). Because the factors released by HSPCs are broad, we have not ruled out the notion that these factors may also be changeable depending on the evolving microenvironment within the brain. Further experiments will shed light on this.
Although we checked the differentially expressed genes whose fold changes were more than 2-fold and values were P < 0.05 in treated HSPCs compared to naive controls (Supplemental Table 1), using DAVID, we found neither proinflammatory nor antiinflammatory cytokines (Supplemental Table 3).
Finally, to test whether MT-I can be used as a pharmacologic strategy for the treatment of stroke, we determined its impact in our model of I/RI. MT (MTI to MTIV) are small, free-radical scavenging proteins, ubiquitously expressed and with both intra- and extracellular functions (50, 51). Notably, they are highly inducible, and dramatically increased transcription is observed during ischemia (52). Various proinflammatory mediators such as IL-6 and reactive oxygen species (53) promote growth and angiogenesis, neurogenesis, and expression of antiinflammatory cytokines (54). While few studies investigate MT activity, those that do indicate their protective effects: in one study, therapeutic effects of MT administered i.p. were observed (MT-II) in a rat model of cerebral I/RI) (55). Our study upholds this trend, as we have showed that MT-I (the best-studied MT along with MT-II, and the most significantly increased transcript in transplanted HSPCs) administered to mice with stroke could produce neuroprotection and attenuate I/RI-induced cerebrovascular injury.
In summary, our results demonstrate that administration of HSPCs leads to neuroprotection in stroke. It is likely that the mechanisms providing therapeutic benefit in this study are multidimensional. However, our findings shed light on a previously unidentified mechanism of MT-I up-regulation through which HSPCs may modulate inflammation and augment the detrimental effects of cerebral I/RI. Furthermore, this was confirmed by the administration of MT-I, which was able to successfully protect against stroke. Therefore, this study demonstrates that HSPCs are an attractive treatment option for patients with stroke, and we urge the establishment of further larger-scale clinical trials investigating their therapeutic potential.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank R. Jin (Louisiana State University Health Sciences Center–Shreveport) for his help with immunofluorescence staining, U. Cvek (Louisiana State University) for her help with statistical analysis, and P. Kubes (University of Calgary, Calgary, AB, Canada) for the LyZM mice. F.N.E.G. acknowledges the financial support of the U.S. National Institutes of Health/National Heart, Lung, and Blood Institute (HL125572-01A1). H.K.S. and S.O. acknowledge the financial support of the Malcolm Feist Cardiovascular Fellowship program. I.T. was supported by a NIH/National Institute of General Medical Sciences Centers of Biomedical Research Excellence (COBRE) grant (P30-GM110703), and the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI; 16H07356). The authors declare no conflicts of interest.
Glossary
- BMC
bone marrow mononuclear cell
- CFSE
carboxyfluorescein succinimidyl ester
- DAB
3,3′-diaminobenzidine
- DENN
differentially expressed in normal and neoplastic cells
- eGFP
enhanced green fluorescent protein
- HSC
hematopoietic stem cell
- HSPC
hematopoietic stem/progenitor cell
- I/RI
ischemia–reperfusion injury
- IS
ischemic stroke
- IV
infarct volume
- IVM
intravital fluorescence microscopy
- Lin−
lineage negative
- LyZM
LysM-eGFP
- MCAo
middle cerebral artery occlusion and reperfusion
- MT
metallothionein
- NS
neurologic score
- PC
principal component
- PCA
principal component analysis
- RNA-Seq
RNA sequencing
- SC
stem cell
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
H. K. Smith, S. Omura, F. Becker, E. Y. Senchenkova, G. Kaur, I. Tsunoda, and F. N. E. Gavins designed and performed research, analyzed data, and wrote the article; S. A. Vital performed research and wrote the article; and S.M.P. wrote the article.
REFERENCES
- 1.Smith H. K., Gil C. D., Oliani S. M., Gavins F. N. (2015) Targeting formyl peptide receptor 2 reduces leukocyte–endothelial interactions in a murine model of stroke. FASEB J. 29, 2161–2171 [DOI] [PubMed] [Google Scholar]
- 2.Vital S. A., Becker F., Holloway P. M., Russell J., Perretti M., Granger D. N., Gavins F. N. (2016) Formyl-peptide receptor 2/3/lipoxin A4 receptor regulates neutrophil–platelet aggregation and attenuates cerebral inflammation: impact for therapy in cardiovascular disease. Circulation 133, 2169–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith H. K., Gavins F. N. (2012) The potential of stem cell therapy for stroke: is PISCES the sign? FASEB J. 26, 2239–2252 [DOI] [PubMed] [Google Scholar]
- 4.Tornero D., Wattananit S., Grønning Madsen M., Koch P., Wood J., Tatarishvili J., Mine Y., Ge R., Monni E., Devaraju K., Hevner R. F., Brüstle O., Lindvall O., Kokaia Z. (2013) Human induced pluripotent stem cell–derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 136, 3561–3577 [DOI] [PubMed] [Google Scholar]
- 5.Tatarishvili J., Oki K., Monni E., Koch P., Memanishvili T., Buga A. M., Verma V., Popa-Wagner A., Brüstle O., Lindvall O., Kokaia Z. (2014) Human induced pluripotent stem cells improve recovery in stroke-injured aged rats. Restor. Neurol. Neurosci. 32, 547–558 [DOI] [PubMed] [Google Scholar]
- 6.Honmou O., Houkin K., Matsunaga T., Niitsu Y., Ishiai S., Onodera R., Waxman S. G., Kocsis J. D. (2011) Intravenous administration of auto serum–expanded autologous mesenchymal stem cells in stroke. Brain 134, 1790–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutiérrez-Fernández M., Rodríguez-Frutos B., Ramos-Cejudo J., Teresa Vallejo-Cremades M., Fuentes B., Cerdán S., Díez-Tejedor E. (2013) Effects of intravenous administration of allogenic bone marrow– and adipose tissue–derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res. Ther. 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroemer P., Patel S., Hope A., Oliveira C., Pollock K., Sinden J. (2009) The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil. Neural Repair 23, 895–909 [DOI] [PubMed] [Google Scholar]
- 9.Schwarting S., Litwak S., Hao W., Bähr M., Weise J., Neumann H. (2008) Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke 39, 2867–2875 [DOI] [PubMed] [Google Scholar]
- 10.Oki K., Tatarishvili J., Wood J., Koch P., Wattananit S., Mine Y., Monni E., Tornero D., Ahlenius H., Ladewig J., Brüstle O., Lindvall O., Kokaia Z. (2012) Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells 30, 1120–1133 [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S., Bentley P., Hamady M., Marley S., Davis J., Shlebak A., Nicholls J., Williamson D. A., Jensen S. L., Gordon M., Habib N., Chataway J. (2014) Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl. Med. 3, 1322–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei X., Yang X., Han Z. P., Qu F. F., Shao L., Shi Y. F. (2013) Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol. Sin. 34, 747–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao L., Zou Z., Tian H., Zhang Y., Zhou H., Liu L. (2014) Stem cell–based therapies for ischemic stroke. Biomed Res. Int. 2014, 468748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horie N., Pereira M. P., Niizuma K., Sun G., Keren-Gill H., Encarnacion A., Shamloo M., Hamilton S. A., Jiang K., Huhn S., Palmer T. D., Bliss T. M., Steinberg G. K. (2011) Transplanted stem cell–secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 29, 274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X. L., Liu K. D., Li F. C., Jiang X. M., Jiang L., Li H. L. (2013) Human mesenchymal stem cells increases expression of α-tubulin and angiopoietin 1 and 2 in focal cerebral ischemia and reperfusion. Curr. Neurovasc. Res. 10, 103–111 [DOI] [PubMed] [Google Scholar]
- 16.Gavins F. N., Smith H. K. (2015) Cell tracking technologies for acute ischemic brain injury. J. Cereb. Blood Flow Metab. 35, 1090–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith H. K., Russell J. M., Granger D. N., Gavins F. N. E. (2015) Critical differences between two classical surgical approaches for middle cerebral artery occlusion–induced stroke in mice. J. Neurosci. Methods 249, 99–105 [DOI] [PubMed] [Google Scholar]
- 18.Chen J., Zhang C., Jiang H., Li Y., Zhang L., Robin A., Katakowski M., Lu M., Chopp M. (2005) Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J. Cereb. Blood Flow Metab. 25, 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Chopp M., Chen J., Wang L., Gautam S. C., Xu Y. X., Zhang Z. (2000) Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J. Cereb. Blood Flow Metab. 20, 1311–1319 [DOI] [PubMed] [Google Scholar]
- 20.Swamydas M., Lionakis M. S. (2013) Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J. Vis. Exp. 77, e50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giralt M., Penkowa M., Lago N., Molinero A., Hidalgo J. (2002) Metallothionein-1+2 protect the CNS after a focal brain injury. Exp. Neurol. 173, 114–128 [DOI] [PubMed] [Google Scholar]
- 22.Yanagida K., Liu C. H., Faraco G., Galvani S., Smith H. K., Burg N., Anrather J., Sanchez T., Iadecola C., Hla T. (2017) Size-selective opening of the blood–brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc. Natl. Acad. Sci. USA 114, 4531–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortazavi A., Williams B. A., McCue K., Schaeffer L., Wold B. (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 [DOI] [PubMed] [Google Scholar]
- 24.Sun J., Nishiyama T., Shimizu K., Kadota K. (2013) TCC: an R package for comparing tag count data with robust normalization strategies. BMC Bioinformatics 14, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omura S., Kawai E., Sato F., Martinez N. E., Chaitanya G. V., Rollyson P. A., Cvek U., Trutschl M., Alexander J. S., Tsunoda I. (2014) Bioinformatics multivariate analysis determined a set of phase-specific biomarker candidates in a novel mouse model for viral myocarditis. Circ Cardiovasc Genet 7, 444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jolliffe I. T. (2011) Principal Component Analysis, 2nd ed., Springer, New York [Google Scholar]
- 27.Chaitanya G. V., Omura S., Sato F., Martinez N. E., Minagar A., Ramanathan M., Guttman B. W., Zivadinov R., Tsunoda I., Alexander J. S. (2013) Inflammation induces neuro-lymphatic protein expression in multiple sclerosis brain neurovasculature. J. Neuroinflammation 10, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato F., Omura S., Kawai E., Martinez N. E., Acharya M. M., Reddy P. C., Chaitanya G. V., Alexander J. S., Tsunoda I. (2014) Distinct kinetics of viral replication, T cell infiltration, and fibrosis in three phases of myocarditis following Theiler’s virus infection. Cell. Immunol. 292, 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa M., Tajima F., Ito T., Sato T., Laver J. H., Deguchi T. (2001) CD34 expression by murine hematopoietic stem cells. Developmental changes and kinetic alterations. Ann. N. Y. Acad. Sci. 938, 139–145 [DOI] [PubMed] [Google Scholar]
- 30.Chung R. S., Penkowa M., Dittmann J., King C. E., Bartlett C., Asmussen J. W., Hidalgo J., Carrasco J., Leung Y. K., Walker A. K., Fung S. J., Dunlop S. A., Fitzgerald M., Beazley L. D., Chuah M. I., Vickers J. C., West A. K. (2008) Redefining the role of metallothionein within the injured brain: extracellular metallothioneins play an important role in the astrocyte–neuron response to injury. J. Biol. Chem. 283, 15349–15358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paczkowska E., Larysz B., Rzeuski R., Karbicka A., Jałowiński R., Kornacewicz-Jach Z., Ratajczak M. Z., Machaliński B. (2005) Human hematopoietic stem/progenitor-enriched CD34(+) cells are mobilized into peripheral blood during stress related to ischemic stroke or acute myocardial infarction. Eur. J. Haematol. 75, 461–467 [DOI] [PubMed] [Google Scholar]
- 32.England T. J., Abaei M., Auer D. P., Lowe J., Jones D. R., Sare G., Walker M., Bath P. M. (2012) Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke 43, 405–411 [DOI] [PubMed] [Google Scholar]
- 33.Gavins F. N., Hughes E. L., Buss N. A., Holloway P. M., Getting S. J., Buckingham J. C. (2012) Leukocyte recruitment in the brain in sepsis: involvement of the annexin 1-FPR2/ALX anti-inflammatory system. FASEB J. 26, 4977–4989 [DOI] [PubMed] [Google Scholar]
- 34.Vasconcelos-dos-Santos A., Rosado-de-Castro P. H., Lopes de Souza S. A., da Costa Silva J., Ramos A. B., Rodriguez de Freitas G., Barbosa da Fonseca L. M., Gutfilen B., Mendez-Otero R. (2012) Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: is there a difference in biodistribution and efficacy? Stem Cell Res. (Amst.) 9, 1–8 [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz G., Vital S., Yilmaz C. E., Stokes K. Y., Alexander J. S., Granger D. N. (2011) Selectin-mediated recruitment of bone marrow stromal cells in the postischemic cerebral microvasculature. Stroke 42, 806–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad K., Sharma A., Garg A., Mohanty S., Bhatnagar S., Johri S., Singh K. K., Nair V., Sarkar R. S., Gorthi S. P., Hassan K. M., Prabhakar S., Marwaha N., Khandelwal N., Misra U. K., Kalita J., Nityanand S.; InveST Study Group . (2014) Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke 45, 3618–3624 [DOI] [PubMed] [Google Scholar]
- 37.Taguchi A., Soma T., Tanaka H., Kanda T., Nishimura H., Yoshikawa H., Tsukamoto Y., Iso H., Fujimori Y., Stern D. M., Naritomi H., Matsuyama T. (2004) Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest. 114, 330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shyu W. C., Lin S. Z., Yang H. I., Tzeng Y. S., Pang C. Y., Yen P. S., Li H. (2004) Functional recovery of stroke rats induced by granulocyte colony-stimulating factor–stimulated stem cells. Circulation 110, 1847–1854 [DOI] [PubMed] [Google Scholar]
- 39.Goldmacher G. V., Nasser R., Lee D. Y., Yigit S., Rosenwasser R., Iacovitti L. (2013) Tracking transplanted bone marrow stem cells and their effects in the rat MCAO stroke model. PLoS One 8, e60049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitkari B., Nitzsche F., Kerkelä E., Kuptsova K., Huttunen J., Nystedt J., Korhonen M., Jolkkonen J. (2014) Human bone marrow mesenchymal stem/stromal cells produce efficient localization in the brain and enhanced angiogenesis after intra-arterial delivery in rats with cerebral ischemia, but this is not translated to behavioral recovery. Behav. Brain Res. 259, 50–59 [DOI] [PubMed] [Google Scholar]
- 41.Detante O., Moisan A., Dimastromatteo J., Richard M. J., Riou L., Grillon E., Barbier E., Desruet M. D., De Fraipont F., Segebarth C., Jaillard A., Hommel M., Ghezzi C., Remy C. (2009) Intravenous administration of 99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: in vivo imaging and biodistribution. Cell Transplant. 18, 1369–1379 [DOI] [PubMed] [Google Scholar]
- 42.Cui L. L., Kerkelä E., Bakreen A., Nitzsche F., Andrzejewska A., Nowakowski A., Janowski M., Walczak P., Boltze J., Lukomska B., Jolkkonen J. (2015) The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res. Ther. 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulyanova T., Scott L. M., Priestley G. V., Jiang Y., Nakamoto B., Koni P. A., Papayannopoulou T. (2005) VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood 106, 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imai Y., Shimaoka M., Kurokawa M. (2010) Essential roles of VLA-4 in the hematopoietic system. Int. J. Hematol. 91, 569–575 [DOI] [PubMed] [Google Scholar]
- 45.Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. (1992) Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 356, 63–66 [DOI] [PubMed] [Google Scholar]
- 46.Tsunoda I., Terry E. J., Marble B. J., Lazarides E., Woods C., Fujinami R. S. (2007) Modulation of experimental autoimmune encephalomyelitis by VLA-2 blockade. Brain Pathol. 17, 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janowski M., Wagner D. C., Boltze J. (2015) Stem cell–based tissue replacement after stroke: factual necessity or notorious fiction? Stroke 46, 2354–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levivier E., Goud B., Souchet M., Calmels T. P., Mornon J. P., Callebaut I. (2001) uDENN, DENN, and dDENN: indissociable domains in Rab and MAP kinases signaling pathways. Biochem. Biophys. Res. Commun. 287, 688–695 [DOI] [PubMed] [Google Scholar]
- 49.Witt P. L., Spear G. T., Helgeson D. O., Lindstrom M. J., Smalley R. V., Borden E. C. (1990) Basal and interferon-induced 2′,5′-oligoadenylate synthetase in human monocytes, lymphocytes, and peritoneal macrophages. J. Interferon Res. 10, 393–402 [DOI] [PubMed] [Google Scholar]
- 50.Lee S. J., Koh J. Y. (2010) Roles of zinc and metallothionein-3 in oxidative stress–induced lysosomal dysfunction, cell death, and autophagy in neurons and astrocytes. Mol. Brain 3, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito Y., Tanaka H., Hara H. (2013) The potential roles of metallothionein as a therapeutic target for cerebral ischemia and retinal diseases. Curr. Pharm. Biotechnol. 14, 400–407 [DOI] [PubMed] [Google Scholar]
- 52.Trendelenburg G., Prass K., Priller J., Kapinya K., Polley A., Muselmann C., Ruscher K., Kannbley U., Schmitt A. O., Castell S., Wiegand F., Meisel A., Rosenthal A., Dirnagl U. (2002) Serial analysis of gene expression identifies metallothionein-II as major neuroprotective gene in mouse focal cerebral ischemia. J. Neurosci. 22, 5879–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pedersen M. O., Jensen R., Pedersen D. S., Skjolding A. D., Hempel C., Maretty L., Penkowa M. (2009) Metallothionein-I+II in neuroprotection. Biofactors 35, 315–325 [DOI] [PubMed] [Google Scholar]
- 54.Santos C. R., Martinho A., Quintela T., Gonçalves I. (2012) Neuroprotective and neuroregenerative properties of metallothioneins. IUBMB Life 64, 126–135 [DOI] [PubMed] [Google Scholar]
- 55.Eidizadeh A., Khajehalichalehshtari M., Freyer D., Trendelenburg G. (2015) Assessment of the therapeutic potential of metallothionein-II application in focal cerebral ischemia in vitro and in vivo. PLoS One 10, e0144035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.