Abstract
The goal of this study was to identify the intrinsic links that explain the effect of a Western diet (WD) on cognitive dysfunction. Specific pathogen-free, wild-type mice were fed either a control diet (CD) or a high-fat, high-sucrose WD after weaning and were euthanized at 10 mo of age to study the pathways that affect cognitive health. The results showed that long-term WD intake reduced hippocampal synaptic plasticity and the level of brain-derived neurotrophic factor mRNA in the brain and isolated microglia. A WD also activated ERK1/2 and reduced postsynaptic density-95 in the brain, suggesting postsynaptic damage. Moreover, WD-fed mice had increased inflammatory signaling in the brain, ileum, liver, adipose tissue, and spleen, which was accompanied by microglia activation. In the brain, as well as in the digestive tract, a WD reduced signaling regulated by retinoic acid and bile acids (BAs), whose receptors form heterodimers to control metabolism and inflammation. Furthermore, a WD intake caused dysbiosis and dysregulated BA synthesis with reduced endogenous ligands for BA receptors, i.e., farnesoid X receptor and G-protein–coupled bile acid receptor in the liver and brain. Together, dysregulated BA synthesis and dysbiosis were accompanied by systemic inflammation, microglial activation, and reduced neuroplasticity induced by WD.—Jena, P. K., Sheng, L., Di Lucente, J., Jin, L.-W., Maezawa, I., Wan, Y.-J. Y. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet–induced systemic inflammation, microglial activation, and reduced neuroplasticity.
Keywords: gut microbiota, FXR, TGR5, cognition, Alzheimer’s disease
Characterized by a progressive loss of cognitive function, Alzheimer’s disease (AD) is the most common form of dementia and affects >36 million people worldwide. Aging is by far the major risk factor for dementia; however, recent studies suggest that life style, including food habits, have a critical role in the onset of dementia (1). Obesity and diabetes, which are often associated with Western diet (WD) intake, are also implicated in the development of dementia (2). In contrast, a healthy diet enriched with vegetables, grains, fish, and low-fat dairy products protects against the development of dementia (3). The current study examines the long-term effect of a high-fat and high-sucrose, i.e., table sugar, diet on pathways implicated in cognitive dysfunction.
Emerging evidence has revealed the significance of gut microbiota on brain function, behavior, and disease (4, 5); WD alters gut microbiota ecology and, therefore, has the potential to affect cognitive health. A few molecules that link gut microbes to CNS function have been uncovered. Among those, serum amyloid A, which enhances inflammatory cytokines in the gut and causes cerebral AD-like amyloid-β (Aβ) deposition, is highly linked with AD pathophysiology (4–7). Inflammation as an etiologic factor for AD is well-established. Sustained inflammation causes tissue damage and neurodegeneration (8). Bile acids (BAs), which are generated by hepatic and bacterial enzymes to aid in digestion as well as to regulate inflammatory signaling, may also explain the effect of microbiota on brain function (9, 10). In addition to facilitating absorption and metabolism of lipids, BAs also regulate sugar homeostasis and insulin sensitivity (11, 12). In the gut, bacteria-generated, taurine-conjugated lithocholic acid (TLCA) activates G protein-coupled bile acid receptor (TGR5), which controls the production of peptide YY (PYY) and glucagon-like peptide 1 (GLP1), thereby regulating food intake and insulin sensitivity, respectively (13). The anti-inflammatory effect of BAs has been revealed by the observations that BA receptor farnesoid X receptor (FXR) knockout mice spontaneously develop steatohepatitis and have increased susceptibility to dextran sodium sulfate–induced colitis (14–18). Retinoid X receptor (RXR), which forms a dimer with FXR, also has an anti-inflammatory effect in the digestive tract (19). A therapeutic dose of retinoic acid (RA) ameliorates age-related dementia and psychosis, and RA is implicated in neurogenesis, cell differentiation, synaptic connectivity, and neuronal plasticity (20). Furthermore, RA regulates BA synthesis, and RXR is a permissive partner of FXR (21). Thus, BAs and RA share common roles in regulating metabolism and inflammation (22–24). Emerging evidence shows that dysregulated BA synthesis, accompanied by dysbiosis, contributes to metabolic disease and inflammation (10, 11, 25). Thus, it is important to understand the effect of diet-associated BAs in regulating cognitive health.
In this study, we have examined the long-term effect of a WD on metabolism, inflammation, and neuroplasticity, which are implicated in the development of cognitive dysfunction in mice. Our novel data revealed protective as well as detrimental effects of BAs that can potentially affect cognitive health and dysfunction.
MATERIALS AND METHODS
Mice
Specific pathogen-free, C57BL/6, wild-type, male mice (The Jackson Laboratory, Bar Harbor, ME, USA) were housed in steel, microisolator cages at 22°C with a 12-h/12-h light/dark cycle. Mice were given a control diet (CD; 5.2% fat, 12% sucrose, and 0.01% cholesterol, w/w) or a WD (21.2% fat, 34% sucrose, and 0.2% cholesterol, w/w; Teklad diet; Envigo, East Millstone, NJ, USA) after weaning (3 wk, 3–6 mice/group). When mice were 10 mo old, they were euthanized with 2% isoflurane.
To test RA-associated signaling, 3-mo-old, wild-type mice fed with rodent chow diet (Teklad Diet; Envigo) were treated with or without RA, twice a week for 3 wk by oral gavage at a dose of 15 mg/kg body weight. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA), under protocols approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Biochemical analysis
Blood samples were collected, and serum was separated by centrifugation and stored in aliquots at −80°C until analysis. Serum cholesterol (Bioassay System, Hayward, CA, USA), and serum alanine aminotransferase (ALT; Pointe Scientific, Lincoln Park, IL, USA) levels were quantified according to the manufacturer’s instructions.
Isolation of fresh mouse microglia
Fresh microglia were isolated from CD- and WD-fed mice brains. Briefly, the brains were dissociated enzymatically with a neural tissue dissociation kit (Miltenyi Biotec, Bergisch Gladbach, Germany), followed by isolation using magnetic-activated cell sorting with anti-CD11b–coated magnetic beads (Miltenyi Biotec). Using this method, 94% of isolated cells are microglial cells, as determined by flow cytometry (26).
Electrophysiologic recording
Brains from euthanized mice were transferred to modified, artificial cerebrospinal fluid (ACSF), which contained 220 mM sucrose, 2 mM KCl, 0.2 mM CaCl2, 6 mM MgSO4, 26 mM NaHCO3, 1.3 mM NaH2PO4, and 10 mM d-glucose (pH 7.4; set by aeration with 95% O2 and 5% CO2). Coronal brain slices (300 µm) were cut in ice-cold, modified ACSF with the use of a DTK-1000 D.S.K Microslicer (TedPella, Redding, CA, USA). The sliced brain was then immersed in 10 mM d-glucose (pH 7.4; set by aeration with 95% O2 and 5% CO2) for 40 min at 35°C. After subsequent incubation for ≥1 h at room temperature, hemislices were transferred to the recording chamber, and perfused with standard ACSF at a constant flow rate of ∼2 ml/min.
Field excitatory postsynaptic potentials (fEPSPs) were obtained from the stratum radiatum of the CA1 region of the hippocampus after stimulation of the Schaffer collateral afferents. Extracellular recording electrodes were prepared from borosilicate capillaries with an outer diameter of 1.5 µm (Sutter Instruments, Novato, CA, USA) and filled with 3 M NaCl (resistance, 1–2 MΩ). Baseline stimulation rate was 0.05 Hz. The fEPSPs were filtered at 2 kHz and digitized at 10 kHz with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA). Data were collected and analyzed with pClamp software (version 10.3; Molecular Devices). Slope values of fEPSPs were considered for quantitation of the responses. After 10 min of stable baseline recording of fEPSPs evoked every 20 s, long-term potentiation (LTP) was elicited by high-frequency stimulation, consisting of 2 trains of 100-Hz (1-s) stimulation with the same intensity and pulse duration used in the sampling of baseline fEPSPs.
Quantification of BAs
Sample preparation of brain, serum, and hepatic BAs was performed based on published methods (14–16, 27). The detection of BAs was performed on a Prominence ultrafast liquid chromatography system (Shimadzu, Kyoto, Japan) coupled to an API 4000 QTRAP mass spectrometer (AB Sciex, Redwood City, CA, USA) operated in the negative ionization mode. Chromatography was performed on a Kinetex C18 column (50 × 2.1 mm, 2.6 μm; Phenomenex, Torrance, CA, USA), maintained at 40°C, preceded by a high-pressure column prefilter. The mobile phase consisted of a gradient of methanol delivered at a flow rate of 0.4 ml/min. The hydrophobicity of BAs was calculated based on other publications (28–31).
Immunohistochemistry
Paraformaldehyde-fixed, frozen sections were used for immunofluorescence staining of brain tissue, as previously described (32). Incubation with primary antibody CD11b (rat anti-CD11b IgG (1:100; MorphoSys, Martinsried, Germany) was followed by secondary Alexa 488–conjugated anti-mouse antibody (1:700; Thermo Fisher Scientific, Waltham, MA, USA). The immunostained images were observed under an Eclipse E600 microscope (Nikon, Tokyo, Japan) and photographed with a digital camera (Spot RTke; Spot Imaging, Sterling Heights, MI, USA).
Western blot analysis
To obtain protein lysates, brain tissues were washed with ice-cold, phosphate-buffered saline and incubated with a buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2% SDS, proteinase inhibitor mixture, and phosphatase inhibitor mixture (MilliporeSigma, Billerica, MA, USA). Lysates were briefly sonicated and cleared by centrifugation at 50,000 rpm for 10 min. Equivalent amounts of protein were analyzed by Tris-HCl gel electrophoresis (Ready Gel Tris-HCI Precast Gels; Bio-Rad Laboratories, Hercules, CA, USA). Proteins were transferred to PVDF membranes and probed with antibodies. Visualization was performed using ECL (GE Healthcare Life Sciences, Little Chalfont St. Giles, United Kingdom). The following primary antibodies (dilutions) were used: anti-PSD95 (1:1000), anti-phospho ERK [1:1000; primary pERK antibody recognizes both ERK1 (p44) and ERK2 (p42)], and anti-ERK (1:1000; Cell Signaling Technology, Danvers, MA, USA). Secondary antibodies were horseradish peroxidase–conjugated anti-rabbit or anti-mouse antibodies (1:1000; GE Healthcare Life Sciences).
Gene expression profiling
RNA was isolated with TRIzol (Thermo Fisher Scientific) and reverse transcribed into cDNA. quantitative RT-PCR was performed on an ABI 7900HT Fast Real-time PCR system using Power SYBR Green PCR Master Mix (Thermo Fisher Scientific). The mRNA levels were normalized to the level of Gapdh mRNA using ΔΔCt value, based on a published method (33).
Flow cytometry
Freshly isolated spleen cells (106) were first incubated with Fc block and then labeled with anti–mouse antibodies, including, phycoerythrin-Cy5–conjugated anti-CD62L, allophycocyanin (APC)-Cy7–conjugated anti-CD25, phycoerythrin-Cy5–conjugated anti-CD44, APC anti-F4/80, and APC-Cy7 anti-CD11b (BD Biosciences, Franklin Lakes, NJ, USA), and a staining buffer consisting of 1% fetal bovine serum, 1 mM EDTA, and 0.02% azide in Dulbecco’s phosphate-buffered saline (Mediatech, Manassas, VA, USA). For intracellular staining, the cells were fixed and stained using the Cytofix/Cytoperm Kit (BD Biosciences) per the manufacturer’s protocols. After staining, cells were analyzed using a custom-configured Fortessa cytometer (BD Biosciences) and FACSDiva software (BD Biosciences). All data sets were analyzed with FlowJo software (v. 10; Tree Star, Ashland, OR, USA).
16S rRNA gene sequencing of gut microbial communities
Cecum content collected by scraping the cecum was subjected to DNA extraction using a ZR Fecal DNA Miniprep Kit (Zymo Research, Irvine, CA, USA). Illumina (San Diego, CA, USA) sequencing of barcoded 16S rRNA gene amplicons of genomic DNA was done based on published methods (34). Variable region 4 of the 16S rRNA gene was amplified and sequenced. DNA sequence reads were demultiplexed and classified with a custom python application dbcAmplicons (https://github.com/msettles/dbcAmplicons) to identify and assign reads by both expected barcode and primer sequences (34). The Ribosomal Database Project Bayesian classifier (35) was used to assign sequences to phylotypes. Reads were assigned to the first Ribosomal Database Project taxonomic level with a bootstrap score ≥50.
Statistical analysis
An unpaired Student t test and 1-way ANVOA were performed using Prism software (v.6.0; GraphPad Software, La Jolla, CA, USA). Data are expressed as means ± sd. Differences among groups in microbiota family and genus level were calculated with Mann-Whitney test. The significance values were adjusted for multiple comparisons using the false-discovery rate, and P < 0.05 was considered statistically significant.
RESULTS
Biochemical and morphologic analysis
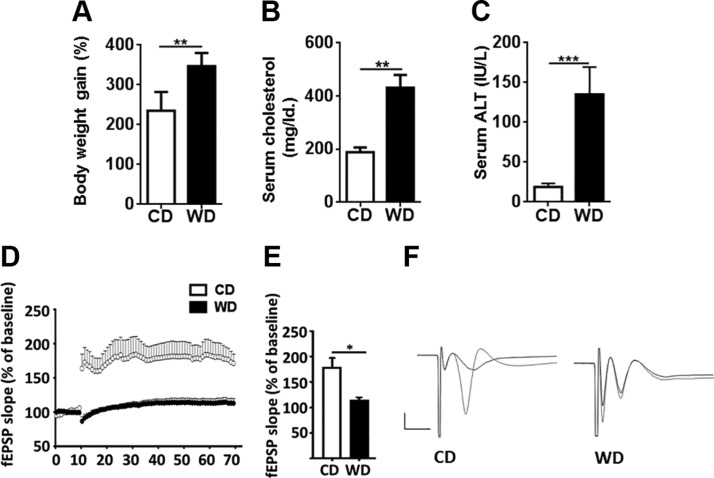
At 10 mo of age, CD- or WD-fed mice were weighed, and blood and tissue samples were analyzed biochemically and morphologically. Compared with CD-fed mice, WD intake increased body weight by ∼47% and serum cholesterol by >2-fold (Fig. 1A, B). In addition, WD elevated the serum ALT level by 7-fold, suggesting liver injury (Fig. 1C). Morphologic analysis of WD-fed mice revealed obvious hepatic steatosis as well as lymphocyte and neutrophil infiltration, indicating the presence of steatohepatitis (not shown).
Figure 1.
Phenotypic analysis and synaptic deficits present in the CA3–CA1 region of mice. A–C) Percentage of body weight gain (A), serum cholesterol (B), and serum ALT (C) of CD- and WD-fed mice. D–F) Scatter plot showing high-frequency stimulation-induced LTP (D), a bar graph showing LTP calculated by averaging the change in fEPSP slope apparent between 50 and 60 min after high-frequency stimulation (E), and representative fEPSP traces showing evoked responses before (black) and 90 min after (red) high-frequency stimulation (F) of CD- and WD-fed mice. Calibration bar, 1 mV/5 ms. All data are presented as the percentage change in fEPSP slope means ± sem from baseline [n = 6 (A, C); n = 3 for D–F). *P < 0.05, **P < 0.01, ***P < 0.001.
WD reduces synaptic plasticity and affects brain signaling
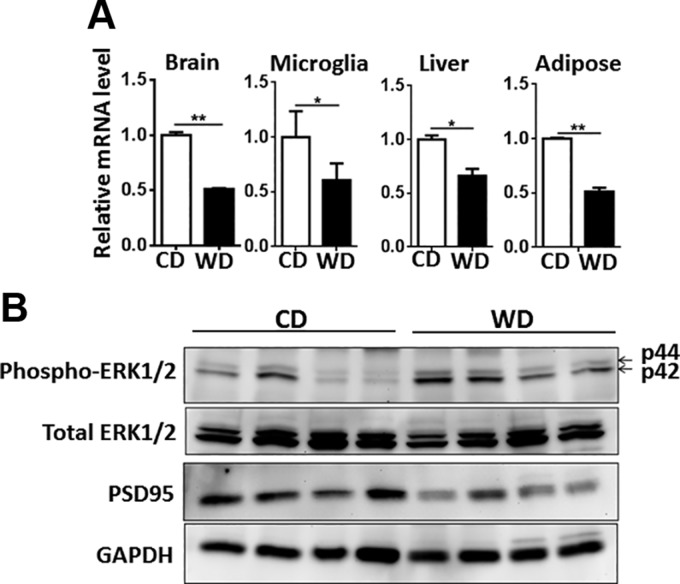
To study the effect of diet on the brain, we analyzed LTP, a widely used indicator of synaptic plasticity, and the expression of genes and proteins implicated in regulating cognition. LTP data showed that WD-fed mice had substantially reduced LTP at Schaffer collateral–CA1 synapses compared with CD-fed mice (Fig. 1D–F). Gene expression data revealed that WD-reduced synaptic plasticity was accompanied by reduced levels of brain-derived neurotrophic factor (Bdnf) in the brain, isolated microglia, liver, and adipose tissue (Fig. 2A). BDNF regulates synaptic plasticity and synaptic transmission (36). Although BDNF is the most prevalent growth factor in the CNS and essential for neuronal health, it is also expressed in many peripheral tissues, including liver and adipose tissue (37, 38). Moreover, compared with CD-fed mice, brains from WD-fed mice had activated ERK1/2, but reduced postsynaptic density protein 95 (PSD-95) as revealed by Western blots (Fig. 2B).
Figure 2.
WD diet reduces synaptic plasticity. (A) Bdnf mRNA levels in the brain, isolated microglia, liver, and adipose tissue. B) Western blot analysis of phospho-ERK1/2, total ERK1/2, and PSD-95 of CD- and WD-fed mice [n = 6 for liver, ileum, and adipose tissue; n = 3 for isolated microglia (A); n = 4 (B)]. All data are presented as means ± sd. *P < 0.05, ** P < 0.01.
WD increases proinflammatory signaling at the systemic level
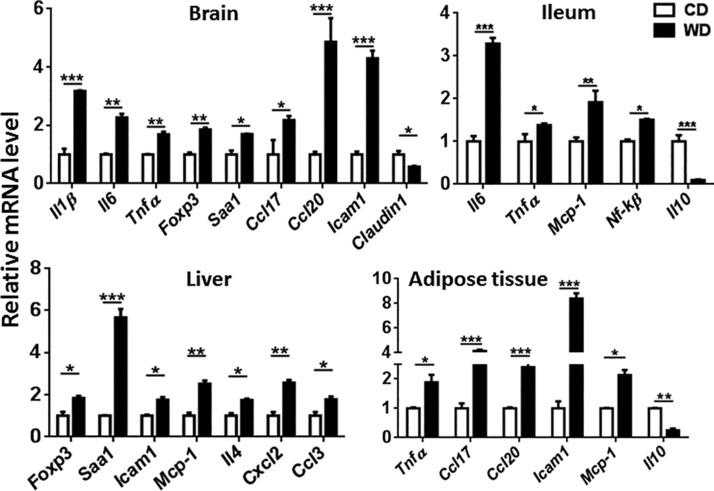
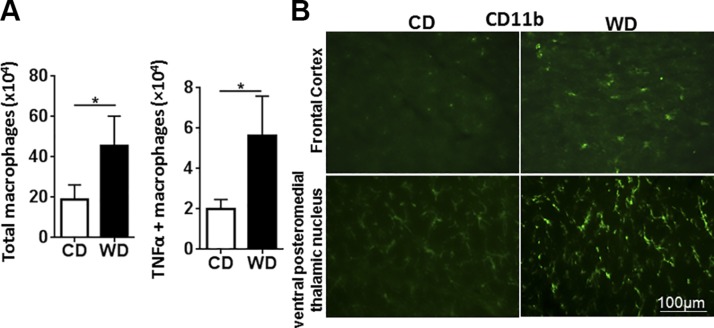
The effect of diet on the expression of genes implicated in inflammatory pathways was studied in different organs. The data showed that, compared with controls, WD-fed mice had increased expression of proinflammatory genes in the brain, ileum, liver, and adipose tissue, indicating a systemic effect (Fig. 3). Specifically, expression levels of the brain inflammatory genes Il1β, Il6, Tnf-α, Foxp3, Saa1, Ccl17, Ccl20, and Icam1 were significantly higher in WD- than in CD-fed mice (Fig. 3). In addition, brains from WD-fed mice also had reduced expression of Claudin1, which encodes a protein that seals tight junctions, suggesting increased vascular permeability (Fig. 3). Moreover, WD-fed mice had increased splenic total macrophages as well as TNF-α–positive macrophages based on flow cytometry analysis (Fig. 4A). Immunofluorescence staining data revealed that WD-fed mice had increased CD11b-positive cell immunoreactivity in the brain, highlighting the enhanced activity/number of mononuclear phagocytes, such as microglia, compared with the CD-fed mice (Fig. 4B).
Figure 3.
WD intake-induced systemic inflammation. The mRNA level of inflammatory genes in the brain, ileum, liver, and adipose tissue of CD- and WD-fed mice (n = 6/group). Data are presented as means ± sd. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4.
WD increased total and TNF-α–positive macrophage and activated microglia. A) Flow cytometry analysis of macrophages derived from spleens. B) Immunofluorescence staining of CD11b in the frontal cortex and ventral posteromedial thalamic nucleus of CD- and WD-fed mice. Data are expressed as means ± sd [n = 6 (A); n = 4 (B)]. *P < 0.05.
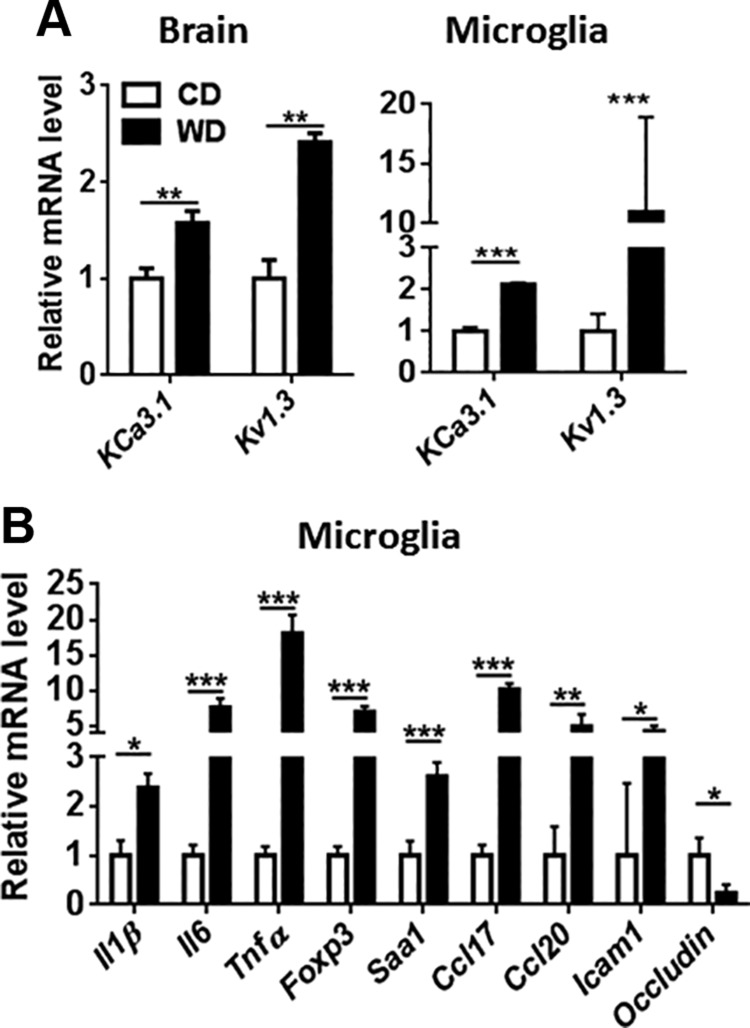
The effect of WD microglia
Ion channels are drug targets for treating CNS diseases, including AD (39). Both KCa3.1 and Kv1.3 blockers reduce inflammatory cytokines, which implicates their role in microglia activation (40, 41). Our data revealed increased transcript levels of KCa3.1, and Kv1.3, which encode major microglial potassium channels that mediate proinflammatory activation, in the brain and isolated microglia of WD-fed mice, suggesting enhanced inflammation (40) (Fig. 5A). Consistent with that finding, freshly isolated microglial cells from WD-fed mice had elevated mRNA levels of the proinflammatory genes Il1β, Il6, Tnf-α, Foxp3, and Saa1 as well as Ccl17, Ccl20, and Icam1 (Fig. 5B). Expression of those genes was much greater in isolated microglia compared with the brain. Microglia from WD-fed mice also had reduced Occludin mRNA levels compared with controls, suggesting increased tight junction permeability (Fig. 5B).
Figure 5.
WD-induced potassium channel mRNA levels and inflammatory signaling. Potassium channel mRNA level in the brain and isolated microglia (A), and mRNA level of inflammatory genes in the isolated microglia (B) of CD- and WD-fed mice. Data are expressed as means ± sd (n = 3 for isolated microglia; n = 6 for brain). *P < 0.05, **P < 0.01, ***P < 0.001.
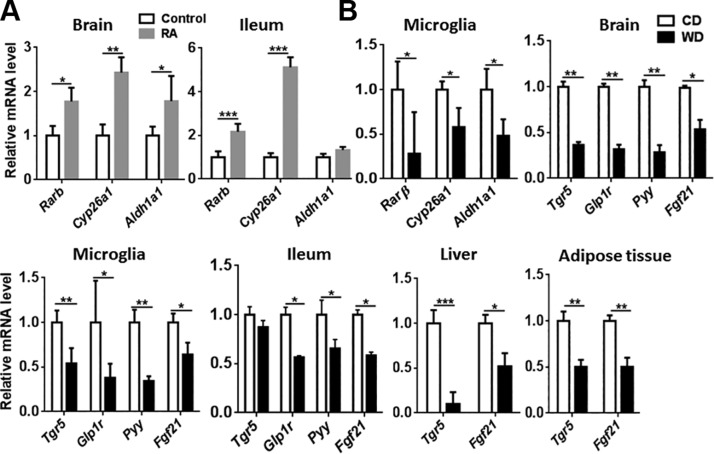
The effect of WD in regulating RA and BA signaling in the brain
Deficiency in vitamin A (retinol), a precursor of retinoic acid, causes amyloid deposition in the liver (39). Furthermore, impaired RA signaling leads to neurodegeneration and advancement of AD (42, 43). RA inhibits the expression of proinflammatory cytokines in microglia and astrocytes, which are activated in AD (44). To monitor RA-regulated signaling, we first wanted to establish the effect of RA supplementation in healthy mice. The data revealed that RA supplementation increased the expression of Rarβ and Cyp26a1 in the brain and ileum (Fig. 6A). Moreover, RA supplementation induced expression of Aldh1a1 in the brain, which catalyzes the oxidation of retinaldehyde into RA (Fig. 6A). In contrast to RA supplementation, WD intake reduced the expression of Rarβ, Cyp26a, and Aldh1a1 in isolated microglial cells, suggesting compromised RA signaling (Fig. 6B).
Figure 6.
The effect of diet on RA and TGR5-regulated signaling. A) The mRNA level of RA target genes in the brain and ileum of healthy mice supplemented with and without retinoic acid. The mRNA level of RA-regulated signaling genes in isolated microglia (B) and TGR5-regulated signaling genes in brain, isolated microglia, ileum, liver, and adipose tissue of CD- and WD-fed mice. Data are expressed as means ± sd (n = 6 for brain, ileum, liver, and adipose tissue; n = 3 for isolated microglia). *P < 0.05, **P < 0.01, ***P < 0.001.
RA and BA are necessary for common functions, such as metabolism and anti-inflammation (22, 45). We studied expression of the BA receptor TGR5 and its targeted pathways. The data revealed that WD intake also reduced BA-regulated pathways. Expression levels of Tgr5, Glp1r, and Pyy were reduced in the brain, isolated microglia, and ileum in WD-fed mice (Fig. 6). Moreover, expression of the metabolism master regulator Fgf21, which can be induced by RA and BAs (46, 47), was reduced in the brain, isolated microglia, ileum, liver, and adipose tissue of WD-fed mice (Fig. 6).
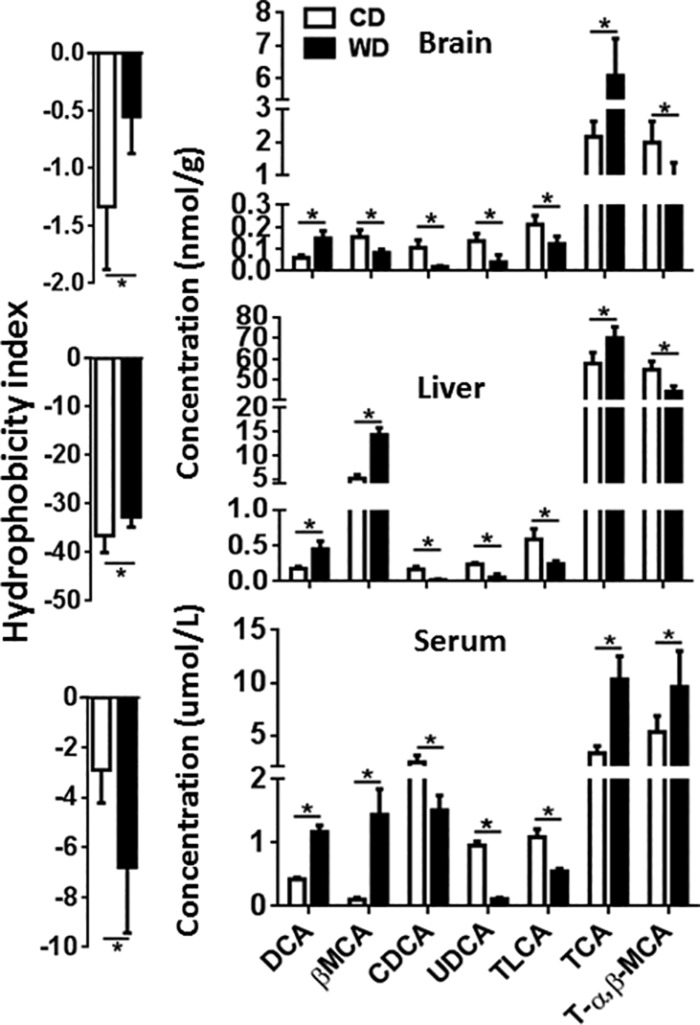
Dysregulation of BA synthesis is implicated in inflammation, immunologic diseases, and liver carcinogenesis (14–16). To understand the role of BAs in those processes, we quantified and compared BAs in the brains, livers, and sera of CD- and WD-fed mice. Compared with the controls, WD-fed mice had elevated BA hydrophobicity in the brains and livers, but a reduced BA hydrophobicity in the sera (Fig. 7). The number indicates the hydrophobicity of the conjugated BAs, and a large number indicates high hydrophobicity. Deoxycholic acid (DCA) and taurocholic acid (TCA) were consistently increased in the brains, livers, and sera of WD-fed mice compared with controls; in contrast, chenodeoxycholic acid (CDCA), ursodeoxycholic acid (UDCA), and taurolithocholic acid (TLCA) were consistently reduced in the same tissues (Fig. 7).
Figure 7.
Brain, liver, and serum BA profiles. Hydrophobicity and BA profile of CD- and WD-fed mice. Hydrophobicity was derived from summation of the values of individual BA’s hydrophobicity index multiplied by its concentration. Hydrophobicity indices of individual BAs are listed: TCA = 0, T-α–MCA = −0.84, T-β–MCA = −0.78, taurohyodeoxycholic acid = −0.35, taurine-conjugated chenodeoxycholic acid = 0.46, taurine-conjugated deoxycholic acid = 0.59, TLCA = 1, glycoursodeoxycholic acid = −0.43. Data are expressed as means ± sd (n = 6/group). *P < 0.05.
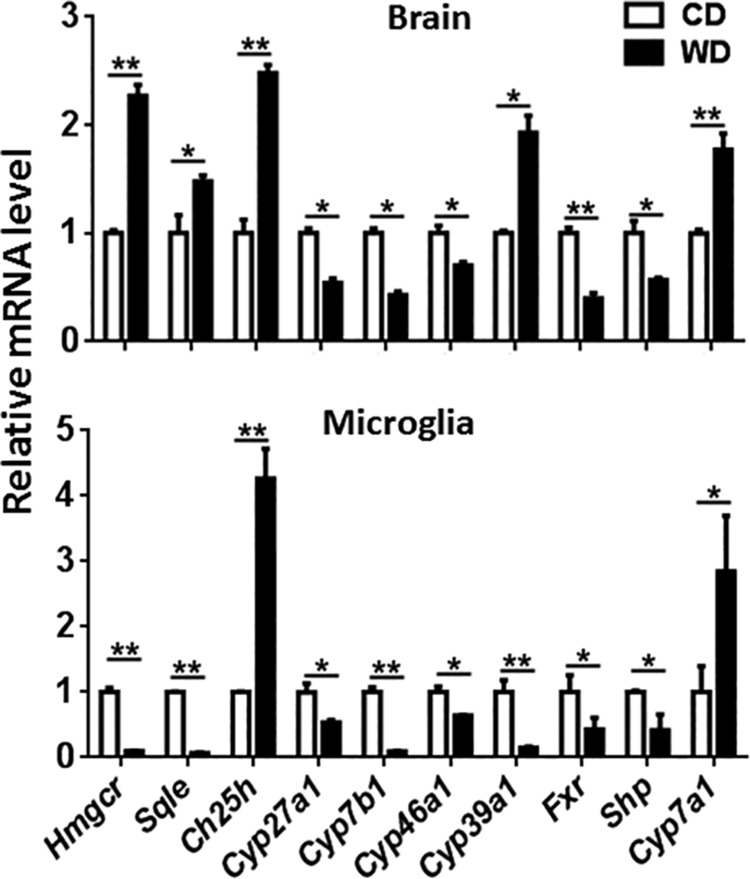
The brain contains 20% of the body’s cholesterol, the precursor of BAs. Thus, we studied the expression of genes implicated in cholesterol and BA synthesis to understand the mechanism that may explain dysregulated BA synthesis found in WD-fed mice. Our data showed that the mRNA levels of Hmgcr and Sqle genes, encoding 3-hydroxy-3-methylglutaryl-CoA reductase and squalene epoxidase, respectively, the key enzymes for cholesterol synthesis, were elevated in the brains but reduced in the isolated microglia of WD-fed mice (Fig. 8). In addition, expression of the Ch25h gene that encodes cholesterol 25-hydroxylase was consistently induced in the brains and isolated microglia of WD-fed mice. However, WD reduced expression of Cyp46a1, which encodes cholesterol 24-hydroxylase, in the brains and isolated microglia (Fig. 8). Moreover, expression of Cyp39a1, which encodes 7α-hydroxylase, was increased in the brains, but reduced in the isolated microglia of WD-fed mice (Fig. 8). WD also reduced the level of mRNA for mitochondrial sterol 27-hydroxylase (Cyp27a1), which catalyzes sterol side-chain oxidation of the acidic pathway of BA synthesis, in the brain and microglia. Moreover, WD reduced the level of mRNA encoding oxysterol and steroid 7-α-hydroxylase (Cyp7b1), which is involved in the metabolism of cholesterol and neurosteroid in both brain and microglia (Fig. 8). Activation of the BA receptor FXR has been used to treat steatohepatitis, atherosclerosis, and insulin resistance (48). Our data showed that WD disrupted FXR signaling in the brain and isolated microglia (Fig. 8). WD-fed mice had reduced Fxr and Shp mRNA in the brains and isolated microglia (Fig. 8). SHP is a negative regulator for BA synthesis and is positively regulated by FXR in the liver. Additionally, expression of the cholesterol 7α-hydroxylase (Cyp7a1), the rate-limiting enzyme for BA synthesis, was induced in the brains and microglia of WD-fed mice (Fig. 8).
Figure 8.
Expression of cholesterol and BA homeostasis genes in the brain and isolated microglia of CD- and WD-fed mice. Data are expressed as means ± sd (n = 6 for brain; n = 3 for isolated microglia). *P < 0.05, **P < 0.01.
The effect of WD in shifting gut microbiota
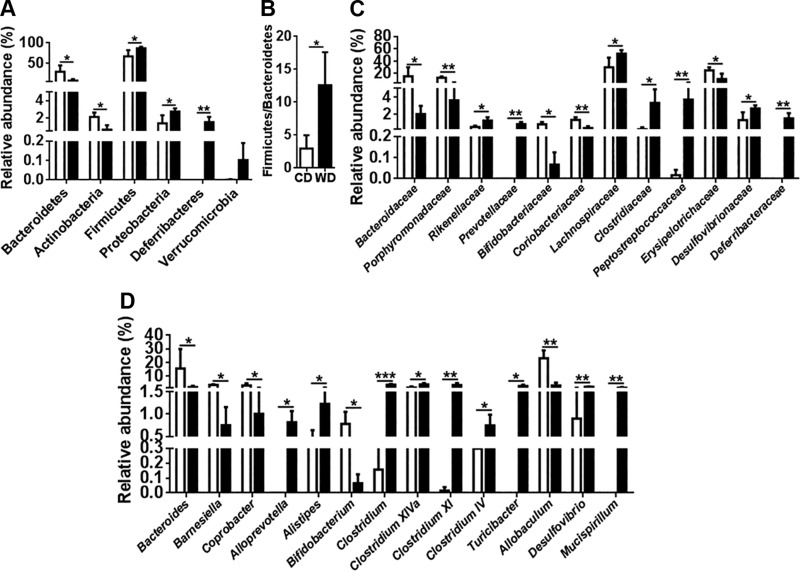
Emerging evidence suggests that the gut microbiota has a role in host cognition or AD-related pathogenesis (4, 5, 49, 50). Thus, we analyzed the effect of diet on the gut microbiota. Bacterial sequencing data showed that WD-fed mice had reduced Bacteroidetes and Actinobacteria, but increased Firmicutes, Proteobacteria, and Deferribacteres (Fig. 9A). In addition, WD-fed mice had an increased Firmicutes to Bacteroidetes ratio (Fig. 9B).
Figure 9.
Relative abundance of cecal microbiota of CD- and WD-fed mice at phylum (A), Firmicutes to Bacteroidetes ratio (B), family (C), and genus (D) levels. Data are expressed as means ± sd (n = 4/group). *P < 0.05, **P < 0.01, ***P < 0.001.
Under the Bacteroidetes phylum, WD reduced the relative abundance of the Bacteroidaceae and Porphyromonadaceae families (Fig. 9C). However, WD increased the relative abundance of Rikenellaceae and Prevotellaceae (Fig. 9C). Under the Actinobacteria phylum, WD reduced the relative abundance of Bifidobacteriaceae and Coriobacteriaceae (Fig. 9C). The WD-induced increase in Firmicutes was mainly due to increased relative abundance of Lachnospiraceae, Clostridiaceae, and Peptostreptococcaceae (Fig. 9C). Moreover, WD reduced the relative abundance of Erysipelotrichaceae (Fig. 9C). Under the Proteobacteria and Deferribacteres phyla, WD increased Desulfovibrionaceae and Deferribacteraceae (Fig. 9C).
At the genus level, WD reduced Bacteroides, Barnesiella, and Coprobacter, but increased Alloprevotella and Alistipes (Fig. 9D). Moreover, WD reduced the genus Bifidobacterium (Fig. 9D). Under the Firmicutes phylum, WD increased Clostridium; Clostridium XIVa, XI, and IV; and Turicibacter, but reduced Allobaculum (Fig. 9D). WD increased the genus Desulfovibrio, which generates genotoxic hydrogen sulfide, and the colitogenic bacteria genus Mucispirillum (Fig. 9D).
DISCUSSION
Different diets have been studied for cognitive health. High-fat diets are commonly used to study the effect of cholesterol (51, 52). A ketogenic diet induces fatty liver, but improves insulin sensitivity in rodents (53) and has beneficial effects on cognition (54). We chose to study the effects of a WD, which is high in fat and sugar because of that diet’s prevalence in modern society. The current study might be one of the first to investigate the combined effect of fat and sugar on cognitive health, which we showed occurs, in part, by dysregulating BA synthesis and inactivating BA receptor-regulated signaling in the brain and digestive tract. These events, in turn, contribute to neuroinflammation as well as systemic inflammation and compromised synaptic plasticity. We also showed here that WD reduced expression of Bdnf in the brain, microglia, liver, and adipose tissue, indicating decreased neurotrophism. Furthermore, WD activated microglia, reduced PSD-95, and activated ERK1/2 in the brain. Activated microglia can stimulate neuronal cell death and cause loss of synaptic connectivity (55). Reduced PSD-95 is associated with altered synaptic plasticity, postsynaptic degeneration, psychiatric diseases, and dementia and AD pathology (56, 57), whereas activated ERK1/2, which can phosphorylate τ protein, is found in patients with AD (58).
Inflammation is accompanied by metabolic dysregulation, which together have an effect on cognition (59). In addition to increased inflammatory gene expression found in multiple organ sites, WD-fed mice had consistently increased serum amyloid A1 (Saa1) mRNA in brains and livers. Moreover, elevated serum amyloid A is found in the brains of AD patients (60). Diet and nutrients affect the composition of gut microbiota and influence amyloid formation (4, 5, 61), suggesting a direct link between diet, microbiota and AD. Furthermore, serum amyloid A stimulates glial cell activity and increases expression of the inflammatory cytokine genes Il6, Tnf-α, Il12, and Il23 in microglia as well as in astrocytes (62). Thus, the simultaneous induction of inflammatory signaling and Saa1 indicate the pathogenesis of cognitive dysfunction induced by WD.
In a high-fat, diet-induced obese mouse model, recombinant human FGF21, which is used to treat obesity and diabetes, can improve cognitive dysfunction, restore synaptic plasticity, and ameliorate anxiety-like behavior (63, 64). Along the same lines, our data showed that WD-induced inflammatory signaling was accompanied by reduced Fgf21 gene expression in microglia and brain, as well as other organs. RA and CDCA induce the expression of FGF21 (46, 47). Consistent with this result, our data revealed that reduced RA signaling and dysregulated BA synthesis occurred because of WD intake.
Like BAs, RA regulates lipid homeostasis, insulin sensitivity, and inflammation (65, 66). Mediated through RA receptors, such as retinoic acid receptors and RXRs, RA also regulates differentiation, metabolism, and development based on genome-wide binding and gene expression profiling data (45, 67). In addition, RA has a profound effect on regulating genes involved in BA homeostasis (21). In WD-fed mice, reduced RA signaling was accompanied by dysregulated BA synthesis. BAs acting through TGR5 regulate GLP1 and PYY signaling (68). GLP1 potentiates postprandial insulin secretion (69) and is a centrally active neuropeptide produced in preproglucagon neurons located in the nucleus of the solitary tract projecting to numerous brain regions (70). GLP1 reduces amyloid deposition and neurofibrillary tangles (71); a GLP1 analog reduces brain insulin resistance in AD, and protects from oxidative damage and the formation of Aβ plaques (71). Furthermore, GLP1 production is affected by inflammatory stimuli, which can be important in the premature neurodegeneration and cognitive decline commonly seen in patients with type 2 diabetes mellitus (72). Activation of TGR5 releases PYY, which reduces gastric mobility and thus decreases food intake (73). PYY also increases phagocytosis and regulates inflammatory signaling (74). The consistent reduction in the mRNA levels of Tgr5, Glp1r, and Pyy in the brain and isolated microglia suggests compromised TGR5 signaling in the brain. The neuroprotective effect of TGR5 has also been demonstrated in stroke and hepatic encephalopathy animal models (75–77). A marked reduction of Tgr5 mRNA is found in the cerebral cortex from cirrhotic patients dying with hepatic encephalopathy when compared with the brain from noncirrhotic control patients (77). In addition, ammonia, long suspected to have a key role in the pathogenesis of hepatic encephalopathy, directly reduces TGR5 expression in cultured astrocytes (77). Thus, the role of BA receptors in cognitive health warrants further investigation.
We have shown previously that dysregulated BA and gut dysbiosis contribute to hepatic lymphocyte and neutrophil infiltration (15). Although the specific function of each BA in the brain remains to be studied, WD increases the hydrophobicity of BAs in the liver and brain with consistent induction of DCA and TCA as well as reduction of CDCA, UDCA, and TLCA in the brain, serum, and liver. BAs found in the brain parenchyma might contain BAs from the vessels because mice were not perfused before organ procurement. However, the large discrepancy between the effects of diet on serum vs. brain β-MCA suggested that vascular levels of BAs did not appreciably contaminate brain levels. Increased hydrophobicity of BAs in tissues can cause cytotoxicity and produce reactive oxygen species, resulting in DNA damage, apoptosis, and necrosis (78, 79). Secondary BAs are more hydrophobic than primary BAs, and secondary BAs such as DCA, which is elevated in WD-fed mice, are genotoxic and carcinogenic (80). DCA is also hepatotoxic and elevated in amnestic, mild cognitive impairment and patients with AD (81). TCA is implicated in the induction of proinflammatory cytokines and activates ERK1/2 by hepatocytes, indicating a role in inflammation (82, 83). Both CDCA, the endogenous ligand for FXR (84), and UDCA have neuroprotective properties and are used as N-methyl-d-aspartate receptor and γ-aminobutyric receptor antagonists to treat AD (85, 86). A reduction in CDCA accompanied by decreased FXR signaling in WD-fed mice suggests the significance of BAs in regulating cognitive function.
Although FXR is primarily expressed in the liver and gut, where BAs are produced and active, TGR5 is a membrane receptor ubiquitously expressed in endocrine glands, adipocytes, muscles, gut, liver, gall bladder, brain, spinal cord, and the enteric nervous system, as well as immune organs such as the spleen and lymph nodes (87, 88). The enteric localization of TGR5 provides neuroanatomical support to the secretory and motor effect of BAs as a component of the intramural nervous reflex (87). Our data showed that WD reduced the endogenous ligands for TGR5, that is, TLCA. That finding, along with reduced PYY and GLP1 signaling, again suggests deactivation of TGR5 in WD-fed mice.
Hydroxylated forms of cholesterols are critically important in regulating immunity and inflammatory signaling (89). Our data revealed that expression of the Hmgcr and Sqle genes in response to WD intake was opposite for the brain and microglia, suggesting that brain and microglia may have elevated and reduced cholesterol, respectively. Elevated cholesterol is implicated in the development of AD (90). The pathologic effect of reduced cholesterol in microglia warrants further investigation. In response to interferon, the expression of Ch25h is induced, which converts cholesterol into 25-hydroxycholesterol, having an anti-viral effect (91). The consistent increase of Ch25h mRNA in the brain and microglia is likely a response to inflammation. Additionally, the expression of several genes, such as Cyp27a1, Cyp7b1, and Cyp46a1, involved in cholesterol oxidation is reduced in WD-fed mouse brain and microglia, which may lead to cholesterol accumulation. In patients with AD, expression of CYP7B1 and CYP27A1 is also reduced (92, 93). Moreover, CYP27A1 mutation leads to neurologic dysfunction (94). Cholesterol is exported from the brain by converting into 24(S)-hydroxycholesterol using CYP46A1 (95). Mice deficient in CYP46A1 have impaired learning and defective hippocampal LTP (96). In the AD mouse model, amyloid-β increases because of the reduction in Cyp46a1 (97). In addition, reduced Cyp46a1 leads to neurodegeneration with progressive neuronal loss associated with an AD-like phenotype (98). Thus, WD-reduced Cyp46a1 likely has a pathologic effect. After 24(S)-hydroxycholesterol passes the blood–brain barrier, hepatic CYP39A1 converts 24-hydroxycholesterol into a 7α-hydroxylated product. Surprisingly, our data showed that the Cyp39a1 gene is expressed in the brain. Additionally, its expression was induced by WD in the brain but reduced in the isolated microglia. In addition to BA synthesis, very little information is available for CYP39A1 (99). CYP7A1 is a rate-limiting enzyme for BA synthesis, and its expression is negatively regulated by FXR (100). Although we do not know whether the low level of FXR in the brain can act as the main transcriptional factor that regulates the Cyp7a1 and Shp expression, increased Cyp7a1 and reduced Shp expression implies FXR deactivation in response to WD intake. Moreover, those changes were accompanied by reduced Fxr mRNA level in the brain and microglia of WD-fed mice. FXR knockout mice have impaired memory and reduced motor coordination, based on behavioral studies (101). Together, it is likely that WD intake via deactivation of FXR- and TGR5-regulated signaling affects cognition.
Dysregulated BA synthesis found in steatohepatitis animal models is always accompanied by dysbiosis (14, 15). Considering the difference in the relative abundance of bacteria found in CD- and WD-fed mice, the biggest changes caused by WD intake were a reduction and increase in Bacteroidetes and Firmicutes, respectively, compared with CD-fed mice. Thus, WD increased the Firmicutes to Bacteroidetes ratio, which is also elevated in irritable bowel syndrome patients, who frequently suffer from different neurologic diseases, such as depression (102, 103). WD reduced the relative abundance of many beneficial bacteria, such as those within the genera Bacteroides, Bifidobacterium, and Allobaculum, but increased the relative abundance of potentially harmful bacteria genera, such as Alistipes, Clostridium, Desulfovibrio, and Mucispirillum. Probiotic Bifidobacterium also increases hippocampal BDNF, functional synapses, and neuroplasticity (104). The butyrate producer Erysipelotrichaceae family and genus Allobaculum were reduced in WD-fed mice. Butyrate is a major energy source for colonic health (105) and also stimulates gut peptide PYY production (106). The genus Alistipes under Rikenellaceae, which was more abundant in WD-fed mice, is also increased in individuals with depressive disorder and gut inflammation (107). Moreover, Clostridium generates neurotoxins and has systemic effects (108). WD also increased the abundance of Proteobacteria, and such an increase is also noted in people who ingest low-fiber diets and have chronic inflammation (109). Colitogenic Mucispirillum bacteria, which degrade mucin, are enriched in colitis, whereas the genus Desulfovibrio produces genotoxic hydrogen sulfide during active inflammation, which may further trigger inflammation (110).
In summary, diet affects gut microbiota symbiosis, thereby altering the production of bacterial metabolites. Secondary BAs are produced by gut microbiota. Moreover, gut bacteria generate butyrate via fermentation, which, in turn, increases the production of RA in the gut dendritic cells (111). Thus, WD-associated dysbiosis, accompanied by dysregulated BA synthesis in the digestive tract, likely has significant roles in cognitive health and dysfunction.
ACKNOWLEDGMENTS
The authors thank Dr. Betty P. Guo for extensive review and editing of this manuscript, and Niki Taylor DeGeorge for editing (both from the University of California, Davis). This study was supported by U.S. National Institutes of Health (NIH) National Cancer Institute Grant U01CA179582, and NIH National Institute on Aging Grants P30AG010129 and R01AG043788. The authors declare no conflicts of interest.
Glossary
- Aβ
amyloid β
- ACSF
artificial cerebrospinal fluid
- AD
Alzheimer’s disease
- ALT
alanine aminotransferase
- APC
allophycocyanin
- BA
bile acid
- Bdnf
brain-derived neurotropic factor
- CA
cholic acid
- CD
control diet
- CDCA
chenodeoxycholic acid
- Cyp7a1
cholesterol 7 α-hydroxylase
- Cyp27a1
sterol 27-hydroxylase
- DCA
deoxycholic acid
- fEPSP
field excitatory postsynaptic potential
- FXR
farnesoid X receptor
- GLP1
glucagon-like peptide 1
- LTP
long-term potentiation
- PSD-95
postsynaptic density protein 95
- PYY
peptide YY
- RA
retinoic acid
- RXR
retinoid X receptor
- TCA
taurocholic acid
- TGR5
G protein–coupled bile acid receptor
- TLCA
taurine-conjugated lithocholic acid
- UDCA
ursodeoxycholic acid
- WD
Western diet
AUTHOR CONTRIBUTIONS
Y.-J. Y. Wan designed and supervised the implementation of the study; P. K. Jena, L. Sheng, J. Di Lucente, and I. Maezawa preformed experiments; P. K. Jena, L. Sheng, J. Di Lucente, L.-W. Jin, I. Maezawa, and Y.-J. Y. Wan analyzed and interpreted the data; P. K. Jena, L. Sheng, and Y.-J. Y. Wan wrote the manuscript; and all authors commented on and approved the final manuscript.
REFERENCES
- 1.Swerdlow R. H. (2011) Brain aging, Alzheimer’s disease, and mitochondria. Biochim. Biophys. Acta 1812, 1630–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayaraman A., Pike C. J. (2014) Alzheimer’s disease and type 2 diabetes: multiple mechanisms contribute to interactions. Curr. Diab. Rep. 14, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu N., Yu J. T., Tan L., Wang Y. L., Sun L., Tan L. (2013) Nutrition and the risk of Alzheimer’s disease. BioMed Res. Int. 2013, 524820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedland R. P. (2015) Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimers Dis. 45, 349–362 [DOI] [PubMed] [Google Scholar]
- 5.Pistollato F., Sumalla Cano S., Elio I., Masias Vergara M., Giampieri F., Battino M. (2016) Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 74, 624–634 [DOI] [PubMed] [Google Scholar]
- 6.Blanco L. P., Evans M. L., Smith D. R., Badtke M. P., Chapman M. R. (2012) Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 20, 66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puig K. L., Brose S. A., Zhou X., Sens M. A., Combs G. F., Jensen M. D., Golovko M. Y., Combs C. K. (2017) Amyloid precursor protein modulates macrophage phenotype and diet-dependent weight gain. Sci. Rep. 7, 43725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swann J. R., Want E. J., Geier F. M., Spagou K., Wilson I. D., Sidaway J. E., Nicholson J. K., Holmes E. (2011) Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl. Acad. Sci. USA 108(Suppl 1), 4523–4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuei J., Chau T., Mills D., Wan Y. J. (2014) Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp. Biol. Med. (Maywood) 239, 1489–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang J. Y. (2013) Bile acid metabolism and signaling. Compr. Physiol. 3, 1191–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T., Francl J. M., Boehme S., Ochoa A., Zhang Y., Klaassen C. D., Erickson S. K., Chiang J. Y. (2012) Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J. Biol. Chem. 287, 1861–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., Macchiarulo A., Yamamoto H., Mataki C., Pruzanski M., Pellicciari R., Auwerx J., Schoonjans K. (2009) TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng L., Jena P. K., Liu H. X., Kalanetra K. M., Gonzalez F. J., French S. W., Krishnan V. V., Mills D. A., Wan Y. Y. (2017) Gender differences in bile acids and microbiota in relationship with gender dissimilarity in steatosis induced by diet and FXR inactivation. Sci. Rep. 7, 1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jena P. K., Sheng L., Liu H.-X., Kalanetra K. M., Mirsoian A., Murphy W. J., French S. W., Krishnan V. V., Mills D. A., Wan Y. Y. (2017) Western diet-induced dysbiosis in farnesoid X receptor knockout mice causes persistent hepatic inflammation after antibiotic treatment. Am. J. Pathol. 187, 1800–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng L., Jena P. K., Hu Y., Liu H. X., Nagar N., Kalanetra K. M., French S. W., French S. W., Mills D. A., Wan Y. Y. (2017) Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation. J. Pathol. 243, 431–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang F., Huang X., Yi T., Yen Y., Moore D. D., Huang W. (2007) Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 67, 863–867 [DOI] [PubMed] [Google Scholar]
- 18.Vavassori P., Mencarelli A., Renga B., Distrutti E., Fiorucci S. (2009) The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 183, 6251–6261 [DOI] [PubMed] [Google Scholar]
- 19.Wang K., Wan Y. J. (2008) Nuclear receptors and inflammatory diseases. Exp. Biol. Med. (Maywood) 233, 496–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson C. R., Mello C. V. (2010) Significance of vitamin A to brain function, behavior and learning. Mol. Nutr. Food Res. 54, 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang F., He Y., Liu H. X., Tsuei J., Jiang X., Yang L., Wang Z. T., Wan Y. J. (2014) All-trans retinoic acid regulates hepatic bile acid homeostasis. Biochem. Pharmacol. 91, 483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y., Gong L., Fang Y., Zhan Q., Liu H. X., Lu Y., Guo G. L., Lehman-McKeeman L., Fang J., Wan Y. J. (2013) The role of retinoic acid in hepatic lipid homeostasis defined by genomic binding and transcriptome profiling. BMC Genomics 14, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan L., Liu H. X., Fang Y., Kong B., He Y., Zhong X. B., Fang J., Wan Y. J., Guo G. L. (2014) Genome-wide binding and transcriptome analysis of human farnesoid X receptor in primary human hepatocytes. PLoS One 9, e105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamoon A., Subauste A., Subauste M. C., Subauste J. (2014) Retinoic acid regulates several genes in bile acid and lipid metabolism via upregulation of small heterodimer partner in hepatocytes. Gene 550, 165–170 [DOI] [PubMed] [Google Scholar]
- 25.Xie G., Wang X., Huang F., Zhao A., Chen W., Yan J., Zhang Y., Lei S., Ge K., Zheng X., Liu J., Su M., Liu P., Jia W. (2016) Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int. J. Cancer 139, 1764–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin L. W., Horiuchi M., Wulff H., Liu X. B., Cortopassi G. A., Erickson J. D., Maezawa I. (2015) Dysregulation of glutamine transporter SNAT1 in Rett syndrome microglia: a mechanism for mitochondrial dysfunction and neurotoxicity. J. Neurosci. 35, 2516–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Cañaveras J. C., Donato M. T., Castell J. V., Lahoz A. (2012) Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS–validated method. J. Lipid Res. 53, 2231–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pathak P., Liu H., Boehme S., Xie C., Krausz K. W., Gonzalez F., Chiang J. Y. L. (2017) Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem. 292, 11055–11069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heuman D. M. (1989) Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res. 30, 719–730 [PubMed] [Google Scholar]
- 30.Fader K. A., Nault R., Zhang C., Kumagai K., Harkema J. R., Zacharewski T. R. (2017) 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-elicited effects on bile acid homeostasis: alterations in biosynthesis, enterohepatic circulation, and microbial metabolism. Sci. Rep. 7, 5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poša M., Popović K. (2017) Structure-property relationships in sodium muricholate derivative (Bile Salts) micellization: the effect of conformation of steroid skeleton on hydrophobicity and micelle formation-pattern recognition and potential membranoprotective properties. Mol. Pharm. 14, 3343–3355 [DOI] [PubMed] [Google Scholar]
- 32.Maezawa I., Swanberg S., Harvey D., LaSalle J. M., Jin L.-W. (2009) Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J. Neurosci. 29, 5051–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 34.Williams J. E., Carrothers J. M., Lackey K. A., Beatty N. F., York M. A., Brooker S. L., Shafii B., Price W. J., Settles M. L., McGuire M. A., McGuire M. K. (2017) Human milk microbial community structure is relatively stable and related to variations in macronutrient and micronutrient intakes in healthy lactating women. J. Nutr. 147, 1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q., Garrity G. M., Tiedje J. M., Cole J. R. (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu B., Nagappan G., Guan X., Nathan P. J., Wren P. (2013) BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 14, 401–416 [DOI] [PubMed] [Google Scholar]
- 37.Cassiman D., Denef C., Desmet V. J., Roskams T. (2001) Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology 33, 148–158 [DOI] [PubMed] [Google Scholar]
- 38.Ukropec J., Ukropcova B., Kurdiova T., Gasperikova D., Klimes I. (2008) Adipose tissue and skeletal muscle plasticity modulates metabolic health. Arch. Physiol. Biochem. 114, 357–368 [DOI] [PubMed] [Google Scholar]
- 39.Waszkielewicz A. M., Gunia A., Szkaradek N., Słoczyńska K., Krupińska S., Marona H. (2013) Ion channels as drug targets in central nervous system disorders. Curr. Med. Chem. 20, 1241–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen H. M., Grössinger E. M., Horiuchi M., Davis K. W., Jin L. W., Maezawa I., Wulff H. (2017) Differential Kv1.3, KCa3.1, and Kir2.1 expression in “classically” and “alternatively” activated microglia. Glia 65, 106–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maezawa I., Jenkins D. P., Jin B. E., Wulff H. (2012) Microglial KCa3.1 channels as a potential therapeutic target for Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 868972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobs S., Lie D. C., DeCicco K. L., Shi Y., DeLuca L. M., Gage F. H., Evans R. M. (2006) Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc. Natl. Acad. Sci. USA 103, 3902–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Y., Qiao A., Wang Z., Goodwin J. S., Lee E.-S., Block M. L., Allsbrook M., McDonald M. P., Fan G.-H. (2008) Retinoic acid attenuates β-amyloid deposition and rescues memory deficits in an Alzheimer’s disease transgenic mouse model. J. Neurosci. 28, 11622–11634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J., Drew P. D. (2006) 9-Cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. J. Neuroimmunol. 171, 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He Y., Tsuei J., Wan Y. J. (2014) Biological functional annotation of retinoic acid α and β in mouse liver based on genome-wide binding. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G205–G218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cyphert H. A., Ge X., Kohan A. B., Salati L. M., Zhang Y., Hillgartner F. B. (2012) Activation of the farnesoid X receptor induces hepatic expression and secretion of fibroblast growth factor 21. J. Biol. Chem. 287, 25123–25138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Solt L. A., Burris T. P. (2010) Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor α. J. Biol. Chem. 285, 15668–15673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ali A. H., Carey E. J., Lindor K. D. (2015) Recent advances in the development of farnesoid X receptor agonists. Ann. Transl. Med. 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang C., Li G., Huang P., Liu Z., Zhao B. (2017) The gut microbiota and Alzheimer’s disease. J. Alzheimers Dis. 58, 1–15 [DOI] [PubMed] [Google Scholar]
- 50.Bonfili L., Cecarini V., Berardi S., Scarpona S., Suchodolski J. S., Nasuti C., Fiorini D., Boarelli M. C., Rossi G., Eleuteri A. M. (2017) Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 7, 2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guay V., Lamarche B., Charest A., Tremblay A. J., Couture P. (2012) Effect of short-term low- and high-fat diets on low-density lipoprotein particle size in normolipidemic subjects. Metabolism 61, 76–83 [DOI] [PubMed] [Google Scholar]
- 52.Christopher-Hennings J., Kurzman I. D., Haffa A. L., Kemnitz J. W., Macewen E. G. (1995) The effect of high fat diet and dehydroepiandrosterone (DHEA) administration in the rhesus monkey. In Vivo 9, 415–420 [PubMed] [Google Scholar]
- 53.Kinzig K. P., Honors M. A., Hargrave S. L. (2010) Insulin sensitivity and glucose tolerance are altered by maintenance on a ketogenic diet. Endocrinology 151, 3105–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holland A. M., Kephart W. C., Mumford P. W., Mobley C. B., Lowery R. P., Shake J. J., Patel R. K., Healy J. C., McCullough D. J., Kluess H. A., Huggins K. W., Kavazis A. N., Wilson J. M., Roberts M. D. (2016) Effects of a ketogenic diet on adipose tissue, liver, and serum biomarkers in sedentary rats and rats that exercised via resisted voluntary wheel running. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R337–R351 [DOI] [PubMed] [Google Scholar]
- 55.Barger S. W., Basile A. S. (2001) Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J. Neurochem. 76, 846–854 [DOI] [PubMed] [Google Scholar]
- 56.Shao C. Y., Mirra S. S., Sait H. B., Sacktor T. C., Sigurdsson E. M. (2011) Postsynaptic degeneration as revealed by PSD-95 reduction occurs after advanced Aβ and τ pathology in transgenic mouse models of Alzheimer’s disease. Acta Neuropathol. 122, 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong Y., Lippa C. F. (2010) Review: disruption of the postsynaptic density in Alzheimer’s disease and other neurodegenerative dementias. Am. J. Alzheimers Dis. Other Demen. 25, 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chong Y. H., Shin Y. J., Lee E. O., Kayed R., Glabe C. G., Tenner A. J. (2006) ERK1/2 activation mediates Aβ oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J. Biol. Chem. 281, 20315–20325 [DOI] [PubMed] [Google Scholar]
- 59.Bettcher B. M., Kramer J. H. (2014) Longitudinal inflammation, cognitive decline, and Alzheimer’s disease: a mini-review. Clin. Pharmacol. Ther. 96, 464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kindy M. S., Yu J., Guo J. T., Zhu H. (1999) Apolipoprotein serum amyloid A in Alzheimer’s disease. J. Alzheimers Dis. 1, 155–167 [DOI] [PubMed] [Google Scholar]
- 61.Berti V., Murray J., Davies M., Spector N., Tsui W. H., Li Y., Williams S., Pirraglia E., Vallabhajosula S., McHugh P., Pupi A., de Leon M. J., Mosconi L. (2015) Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J. Nutr. Health Aging 19, 413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu Y., Liu J., Li S. Q., Peng L., Ye R. D. (2014) Serum amyloid a differentially activates microglia and astrocytes via the PI3K pathway. J. Alzheimers Dis. 38, 133–144 [DOI] [PubMed] [Google Scholar]
- 63.Wang Q., Yuan J., Yu Z., Lin L., Jiang Y., Cao Z., Zhuang P., Whalen M. J., Song B., Wang X. J., Li X., Lo E. H., Xu Y., Wang X. (2017) FGF21 attenuates high-fat diet-induced cognitive impairment via metabolic regulation and anti-inflammation of obese mice. [E-pub ahead of print] Mol. Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sa-Nguanmoo P., Tanajak P., Kerdphoo S., Satjaritanun P., Wang X., Liang G., Li X., Jiang C., Pratchayasakul W., Chattipakorn N., Chattipakorn S. C. (2016) FGF21 improves cognition by restored synaptic plasticity, dendritic spine density, brain mitochondrial function and cell apoptosis in obese-insulin resistant male rats. Horm. Behav. 85, 86–95 [DOI] [PubMed] [Google Scholar]
- 65.Guo C., Xie S., Chi Z., Zhang J., Liu Y., Zhang L., Zheng M., Zhang X., Xia D., Ke Y., Lu L., Wang D. (2016) Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity 45, 944. [DOI] [PubMed] [Google Scholar]
- 66.Bonet M. L., Ribot J., Palou A. (2012) Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim. Biophys. Acta 1821, 177–189 [DOI] [PubMed] [Google Scholar]
- 67.Mahony S., Mazzoni E. O., McCuine S., Young R. A., Wichterle H., Gifford D. K. (2011) Ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome Biol. 12, R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Silva A., Bloom S. R. (2012) Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver 6, 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salehi M., Aulinger B., Prigeon R. L., D’Alessio D. A. (2010) Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes 59, 1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trapp S., Richards J. E. (2013) The gut hormone glucagon-like peptide-1 produced in brain: is this physiologically relevant? Curr. Opin. Pharmacol. 13, 964–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keshava H. B., Mowla A., Heinberg L. J., Schauer P. R., Brethauer S. A., Aminian A. (2017) Bariatric surgery may reduce the risk of Alzheimer’s diseases through GLP-1 mediated neuroprotective effects. Med. Hypotheses 104, 4–9 [DOI] [PubMed] [Google Scholar]
- 72.Kappe C., Tracy L. M., Patrone C., Iverfeldt K., Sjöholm Å. (2012) GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J. Neuroinflammation 9, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bala V., Rajagopal S., Kumar D. P., Nalli A. D., Mahavadi S., Sanyal A. J., Grider J. R., Murthy K. S. (2014) Release of GLP-1 and PYY in response to the activation of G protein-coupled bile acid receptor TGR5 is mediated by Epac/PLC-ε pathway and modulated by endogenous H2S. Front. Physiol. 5, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De la Fuente M., Bernaez I., Del Rio M., Hernanz A. (1993) Stimulation of murine peritoneal macrophage functions by neuropeptide Y and peptide YY. Involvement of protein kinase C. Immunology 80, 259–265 [PMC free article] [PubMed] [Google Scholar]
- 75.Yanguas-Casás N., Barreda-Manso M. A., Nieto-Sampedro M., Romero-Ramírez L. (2017) TUDCA: an agonist of the bile acid receptor GPBAR1/TGR5 with anti-inflammatory effects in microglial cells. J. Cell. Physiol. 232, 2231–2245 [DOI] [PubMed] [Google Scholar]
- 76.McMillin M., Frampton G., Tobin R., Dusio G., Smith J., Shin H., Newell-Rogers K., Grant S., DeMorrow S. (2015) TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J. Neurochem. 135, 565–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keitel V., Görg B., Bidmon H. J., Zemtsova I., Spomer L., Zilles K., Häussinger D. (2010) The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58, 1794–1805 [DOI] [PubMed] [Google Scholar]
- 78.Yui S., Kanamoto R., Saeki T. (2009) Biphasic regulation of cell death and survival by hydrophobic bile acids in HCT116 cells. Nutr. Cancer 61, 374–380 [DOI] [PubMed] [Google Scholar]
- 79.Jolly A. J., Wild C. P., Hardie L. J. (2009) Sodium deoxycholate causes nitric oxide mediated DNA damage in oesophageal cells. Free Radic. Res. 43, 234–240 [DOI] [PubMed] [Google Scholar]
- 80.Glinghammar B., Inoue H., Rafter J. J. (2002) Deoxycholic acid causes DNA damage in colonic cells with subsequent induction of caspases, COX-2 promoter activity and the transcription factors NF-kB and AP-1. Carcinogenesis 23, 839–845 [DOI] [PubMed] [Google Scholar]
- 81.Olazarán J., Gil-de-Gómez L., Rodríguez-Martín A., Valentí-Soler M., Frades-Payo B., Marín-Muñoz J., Antúnez C., Frank-García A., Acedo-Jiménez C., Morlán-Gracia L., Petidier-Torregrossa R., Guisasola M. C., Bermejo-Pareja F., Sánchez-Ferro Á., Pérez-Martínez D. A., Manzano-Palomo S., Farquhar R., Rábano A., Calero M. (2015) A blood-based, 7-metabolite signature for the early diagnosis of Alzheimer’s disease. J. Alzheimers Dis. 45, 1157–1173 [DOI] [PubMed] [Google Scholar]
- 82.Allen K., Jaeschke H., Copple B. L. (2011) Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am. J. Pathol. 178, 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Brien K. M., Allen K. M., Rockwell C. E., Towery K., Luyendyk J. P., Copple B. L. (2013) IL-17A synergistically enhances bile acid-induced inflammation during obstructive cholestasis. Am. J. Pathol. 183, 1498–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shihabudeen M. S., Roy D., James J., Thirumurugan K. (2015) Chenodeoxycholic acid, an endogenous FXR ligand alters adipokines and reverses insulin resistance. Mol. Cell. Endocrinol. 414, 19–28 [DOI] [PubMed] [Google Scholar]
- 85.Schubring S. R., Fleischer W., Lin J. S., Haas H. L., Sergeeva O. A. (2012) The bile steroid chenodeoxycholate is a potent antagonist at NMDA and GABA(A) receptors. Neurosci. Lett. 506, 322–326 [DOI] [PubMed] [Google Scholar]
- 86.Yanovsky Y., Schubring S. R., Yao Q., Zhao Y., Li S., May A., Haas H. L., Lin J. S., Sergeeva O. A. (2012) Waking action of ursodeoxycholic acid (UDCA) involves histamine and GABAA receptor block. PLoS One 7, e42512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duboc H., Taché Y., Hofmann A. F. (2014) The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig. Liver Dis. 46, 302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., Hinuma S., Fujisawa Y., Fujino M. (2003) A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278, 9435–9440 [DOI] [PubMed] [Google Scholar]
- 89.Cyster J. G., Dang E. V., Reboldi A., Yi T. (2014) 25-Hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol. 14, 731–743 [DOI] [PubMed] [Google Scholar]
- 90.Matsuzaki T., Sasaki K., Hata J., Hirakawa Y., Fujimi K., Ninomiya T., Suzuki S. O., Kanba S., Kiyohara Y., Iwaki T. (2011) Association of Alzheimer disease pathology with abnormal lipid metabolism: the Hisayama study. Neurology 77, 1068–1075 [DOI] [PubMed] [Google Scholar]
- 91.Liu S. Y., Aliyari R., Chikere K., Li G., Marsden M. D., Smith J. K., Pernet O., Guo H., Nusbaum R., Zack J. A., Freiberg A. N., Su L., Lee B., Cheng G. (2013) Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 38, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yau J. L., Rasmuson S., Andrew R., Graham M., Noble J., Olsson T., Fuchs E., Lathe R., Seckl J. R. (2003) Dehydroepiandrosterone 7-hydroxylase CYP7B: predominant expression in primate hippocampus and reduced expression in Alzheimer’s disease. Neuroscience 121, 307–314 [DOI] [PubMed] [Google Scholar]
- 93.Brown J., III, Theisler C., Silberman S., Magnuson D., Gottardi-Littell N., Lee J. M., Yager D., Crowley J., Sambamurti K., Rahman M. M., Reiss A. B., Eckman C. B., Wolozin B. (2004) Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J. Biol. Chem. 279, 34674–34681 [DOI] [PubMed] [Google Scholar]
- 94.Cali J. J., Hsieh C. L., Francke U., Russell D. W. (1991) Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J. Biol. Chem. 266, 7779–7783 [PMC free article] [PubMed] [Google Scholar]
- 95.Lavrnja I., Smiljanic K., Savic D., Mladenovic-Djordjevic A., Tesovic K., Kanazir S., Pekovic S. (2017) Expression profiles of cholesterol metabolism-related genes are altered during development of experimental autoimmune encephalomyelitis in the rat spinal cord. Sci. Rep. 7, 2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramirez D. M., Andersson S., Russell D. W. (2008) Neuronal expression and subcellular localization of cholesterol 24-hydroxylase in the mouse brain. J. Comp. Neurol. 507, 1676–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Djelti F., Braudeau J., Hudry E., Dhenain M., Varin J., Bièche I., Marquer C., Chali F., Ayciriex S., Auzeil N., Alves S., Langui D., Potier M. C., Laprevote O., Vidaud M., Duyckaerts C., Miles R., Aubourg P., Cartier N. (2015) CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer’s disease. Brain 138, 2383–2398 [DOI] [PubMed] [Google Scholar]
- 98.Ayciriex S., Djelti F., Alves S., Regazzetti A., Gaudin M., Varin J., Langui D., Bièche I., Hudry E., Dargère D., Aubourg P., Auzeil N., Laprévote O., Cartier N. (2017) Neuronal cholesterol accumulation induced by Cyp46a1 down-regulation in mouse hippocampus disrupts brain lipid homeostasis. Front. Mol. Neurosci. 10, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lorbek G., Lewinska M., Rozman D. (2012) Cytochrome P450s in the synthesis of cholesterol and bile acids--from mouse models to human diseases. FEBS J. 279, 1516–1533 [DOI] [PubMed] [Google Scholar]
- 100.Trauner M., Boyer J. L. (2003) Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 83, 633–671 [DOI] [PubMed] [Google Scholar]
- 101.Huang F., Wang T., Lan Y., Yang L., Pan W., Zhu Y., Lv B., Wei Y., Shi H., Wu H., Zhang B., Wang J., Duan X., Hu Z., Wu X. (2015) Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Front. Behav. Neurosci. 9, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naseribafrouei A., Hestad K., Avershina E., Sekelja M., Linløkken A., Wilson R., Rudi K. (2014) Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 26, 1155–1162 [DOI] [PubMed] [Google Scholar]
- 103.Jeffery I. B., O’Toole P. W., Öhman L., Claesson M. J., Deane J., Quigley E. M., Simrén M. (2012) An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61, 997–1006 [DOI] [PubMed] [Google Scholar]
- 104.Pinto-Sanchez M. I., Hall G. B., Ghajar K., Nardelli A., Bolino C., Lau J. T., Martin F.-P., Cominetti O., Welsh C., Rieder A., Traynor J., Gregory C., De Palma G., Pigrau M., Ford A. C., Macri J., Berger B., Bergonzelli G., Surette M. G., Collins S. M., Moayyedi P., Bercik P. (2017) Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153, 448–459.e8 [DOI] [PubMed] [Google Scholar]
- 105.Donohoe D. R., Garge N., Zhang X., Sun W., O’Connell T. M., Bunger M. K., Bultman S. J. (2011) The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13, 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou J., Hegsted M., McCutcheon K. L., Keenan M. J., Xi X., Raggio A. M., Martin R. J. (2006) Peptide YY and proglucagon mRNA expression patterns and regulation in the gut. Obesity (Silver Spring) 14, 683–689 [DOI] [PubMed] [Google Scholar]
- 107.Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., Wang W., Tang W., Tan Z., Shi J., Li L., Ruan B. (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194 [DOI] [PubMed] [Google Scholar]
- 108.Parracho H. M., Bingham M. O., Gibson G. R., McCartney A. L. (2005) Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 54, 987–991 [DOI] [PubMed] [Google Scholar]
- 109.Shin N. R., Whon T. W., Bae J. W. (2015) Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33, 496–503 [DOI] [PubMed] [Google Scholar]
- 110.Attene-Ramos M. S., Wagner E. D., Plewa M. J., Gaskins H. R. (2006) Evidence that hydrogen sulfide is a genotoxic agent. Mol. Cancer Res. 4, 9–14 [DOI] [PubMed] [Google Scholar]
- 111.Schilderink R., Verseijden C., Seppen J., Muncan V., van den Brink G. R., Lambers T. T., van Tol E. A., de Jonge W. J. (2016) The SCFA butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial HDAC. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G1138–G1146 [DOI] [PubMed] [Google Scholar]