Abstract
Patients with hepatitis C virus (HCV) who have virological failure (VF) after treatment containing a nonstructural protein 5A (NS5A) inhibitor have limited retreatment options. MAGELLAN‐1 Part 2 was a randomized, open‐label, phase 3 study to evaluate the efficacy and safety of ribavirin (RBV)‐free glecaprevir and pibrentasvir (G/P; 300 mg/120 mg) in patients with chronic HCV and past VF on at least one NS3/4A protease and/or NS5A inhibitor‐containing therapy. Patients with compensated liver disease, with or without cirrhosis, and HCV genotype (GT) 1, 4, 5, or 6 were randomized 1:1 to receive 12 or 16 weeks of G/P. The primary endpoint was sustained virological response (SVR) at 12 weeks posttreatment (SVR12). Among 91 patients treated, 87 had GT1 and 4 had GT4 infection. SVR12 was achieved by 89% (39 of 44) and 91% (43 of 47) of patients who received 12 and 16 weeks of G/P, respectively. Virological relapse occurred in 9% (4 of 44) of patients treated with 12 weeks of G/P; there were no relapses with 16 weeks of treatment. Past treatment history with one class of inhibitor (protease or NS5A) had no impact on SVR12, whereas past treatment with both classes of inhibitors was associated with lower SVR12 rate. The most common adverse event (AE) was headache (≥10% of patients), and there were no serious AEs assessed as related to study drugs or AEs leading to discontinuation. Conclusion: Sixteen weeks of G/P treatment achieved a high SVR12 rate in patients with HCV GT1 infection and past failure to regimens containing either NS5A inhibitors or NS3 protease inhibitors. (Hepatology 2018;67:1253‐1260)
Abbreviations
- AEs
adverse events
- ALT
alanine aminotransferase
- APRI
aspartate aminotransferase to platelet ratio index
- AST
aspartate aminotransferase
- CI
confidence interval
- DAA
direct‐acting antiviral
- DCV
daclatasvir
- DSV
dasabuvir
- EC50
half‐maximal effective concentration
- GLE
glecaprevir
- G/P
glecaprevir and pibrentasvir
- GT
genotype
- HCV
hepatitis C virus
- kPa
kilopascals
- LDV
ledipasvir
- LLOD
lower limit of detection
- LLOQ
lower limit of quantification
- NS5A
nonstructural protein 5A
- OBV
ombitasvir
- PI
protease inhibitor
- PIB
pibrentasvir
- PTV/r
ritonavir boosted paritaprevir
- RASs
resistance‐associated substitutions
- RBV
ribavirin
- SIM
simeprevir
- SOF
sofosbuvir
- SVR
sustained virological response
- SVR12
sustained virological response at posttreatment week 12
- TE
transient elastography
- VEL
velpatasvir
- VF
virological failure
Most currently available direct‐acting antiviral (DAA) treatment regimens for chronic infection with hepatitis C virus (HCV) include nonstructural protein 5A (NS5A) inhibitors and are highly effective. However, patients that have virological failure (VF) with NS5A inhibitor‐containing regimens commonly develop resistance‐associated substitutions (RASs) that decrease the efficacy of subsequent retreatment.1, 2, 3 Additionally, in contrast to treatment‐emergent substitutions in NS3, which disappear within the first year posttreatment, RASs in NS5A can persist for several years.4, 5 Therefore, effective retreatment of patients who have had VF on NS5A inhibitor‐containing regimens has been challenging, and retreatment options for this population are currently limited.6, 7 Addition of ribavirin (RBV) and/or extended treatment durations using combinations of three to four DAAs have been strategies utilized to provide retreatment options for patients2, 8, 9, 10, 11, 12; however, clinical data from large studies are not available for most of these regimens, and sustained virological response (SVR) rates have generally been suboptimal. Recently, the three DAA fixed‐dose combination of sofosbuvir (SOF)/velpatasvir (VEL)/voxilaprevir demonstrated an overall 96% SVR12 rate in patients with past NS5A inhibitor experience.13, 14 However, the use of SOF may not be suitable for important subpopulations, such as those with advanced renal disease.15 Therefore, effective retreatment options using RBV‐free DAA combinations without SOF, which retain antiviral activity against common NS5A RASs, are needed.
Glecaprevir (GLE) is an HCV NS3/4A protease inhibitor (PI), and pibrentasvir (PIB) is an NS5A inhibitor. They have been coformulated (G/P) in clinical trials for the treatment of all six major HCV genotypes. GLE has demonstrated in vitro half‐maximal effective concentration (EC50) values ≤5 nanomolar across all major HCV genotypes (GTs), and has <5‐fold loss of activity against most GT1 RASs (such as those at positions 36, 56, 80, 155, and 156) that may be selected during treatment with currently available NS3/4A PIs.16, 17 PIB has EC50 values ≤5 picomolar across all major HCV GTs and has demonstrated minimal loss in potency against known NS5A RASs (such as those at positions 24, 28, 30, 31, 58, 92, and 93), including GT1a Y93H (6.7‐fold increase in EC50),18 which substantially reduces susceptibility to other NS5A inhibitors, such as VEL (609‐fold increase in EC50), ledipasvir (LDV; 3,294‐fold increase in EC50), and daclatasvir (DCV; 1,600‐fold increase in EC50).19 This suggests that G/P could provide an effective retreatment regimen, including for patients with past experience with NS5A inhibitor‐containing regimens.
MAGELLAN‐1 Part 1 was a phase 2 dose‐ranging study that evaluated the efficacy and safety of GLE and PIB for 12 weeks, with or without RBV, in GT1‐infected patients with past treatment failure on DAA regimens containing an NS5A inhibitor and/or NS3/4A PI with or without NS5B inhibitors. The modified intent‐to‐treat (modified intent‐to‐treat analysis excludes patients that failed because of nonvirological reasons) sustained virological response (SVR) at 12 weeks posttreatment (SVR12) rate was 95%, regardless of RBV coadministration, and was not affected by past treatment regimen or presence of baseline RASs.20
In this study, MAGELLAN‐1 Part 2, the efficacy and safety of RBV‐free G/P for 12 or 16 weeks was planned to be evaluated in patients with chronic HCV GT1, 4, 5, or 6 infection, including those with compensated cirrhosis, and past treatment failure on NS3/4A protease and/or NS5A inhibitor‐containing regimens.
Patients and Methods
STUDY OVERSIGHT
All patients signed informed consent, and the study was conducted in accord with the International Conference on Harmonization guidelines and the ethics set forth by the Declaration of Helsinki.
STUDY DESIGN
MAGELLAN‐1 (NCT02446717) Part 2 was a randomized, open‐label, multicenter, phase 3 study that assessed the efficacy and safety of G/P in patients with chronic HCV GT1 or GT4 infection, including those with compensated cirrhosis, who had past failure on at least one NS3/4A protease and/or NS5A inhibitor‐based regimen. The trial design schematic is shown in Fig. 1, and a flow diagram showing patient recruitment and enrollment is depicted in http://onlinelibrary.wiley.com/doi/10.1002/hep.29671/suppinfo. S1. Patients were randomized 1:1 to receive either 12 or 16 weeks of all‐oral coformulated G/P (without RBV; GLE was discovered by AbbVie and Enanta). G/P was dosed as 3 pills (100 mg/40 mg each) for a total dose of 300 mg/120 mg once‐daily.
Figure 1.
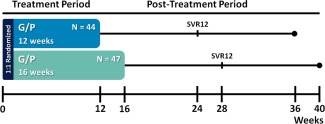
MAGELLAN‐1, Part 2 study design. Patients were randomized 1:1 and stratified by HCV GT (1 or 4) and past treatment experience (NS5A inhibitor‐naïve or ‐experienced) to receive either 12 or 16 weeks of once‐daily G/P (300 mg/120 mg). All patients had past VF, virological failure to at least one HCV treatment regimen containing an approved NS3/4A protease and/or NS5A inhibitor allowed by study protocol. Patients were followed for 24 weeks posttreatment to monitor safety and SVR.
PATIENT POPULATION
Patients were screened at 31 sites in Australia, France, Spain, the United Kingdom, and the United States (including Puerto Rico). Patients at least 18 years old (no upper limit) were eligible if they had chronic HCV GT1, 4, 5, or 6 infection with HCV RNA >1,000 IU/mL at screening. HCV genotype and subtype were assessed with the Versant HCV Genotype Inno LiPA Assay, version 2.0 or higher, or Sanger sequencing of the NS5B region if indeterminate initially by LiPA. Patients had to have past VF failure to an approved DAA‐containing regimen that included an NS5A inhibitor (limited to DCV, LDV, or ombitasvir [OBV]) and/or NS3/4A PI (limited to paritaprevir, simeprevir [SIM], asunaprevir, telaprevir, or boceprevir). NS5B inhibitors (SOF or dasabuvir [DSV]) could have been present in any past treatment regimen, and patients could have had failure to multiple past regimens. Patients with sequential exposures to multiple regimens (i.e., PI‐containing regimen followed by an NS5A inhibitor‐containing regimen) were considered to have past experience to both. Median time since patients' last previous VF is reported in the http://onlinelibrary.wiley.com/doi/10.1002/hep.29671/suppinfo. Patients could have compensated cirrhosis (Child‐Pugh score of 6 or less) or no cirrhosis. Absence of cirrhosis (e.g., METAVIR score ≤3, Ishak score ≤4) was determined by liver biopsy within 24 months before (or during) screening, transient elastography (TE; FibroScan) score of <12.5 kilopascals (kPa) within 6 months before (or during) screening, or a screening FibroTest score of ≤0.48 and an aspartate aminotransferase (AST) to platelet ratio index (APRI) <1. Presence of cirrhosis was determined by past histological diagnosis of cirrhosis on liver biopsy (e.g., METAVIR [or equivalent] score of >3 [including 3/4], Ishak score of >4), past TE (FibroScan) score of ≥14.6 kPa, or a screening FibroTest score of ≥0.75 and an APRI >2. Patients with indeterminate FibroScan were required to have liver biopsy and indeterminate FibroTest or conflicting FibroTest, and APRI scores were required to have TE or liver biopsy to determine cirrhosis status. Complete inclusion and exclusion criteria are included in http://onlinelibrary.wiley.com/doi/10.1002/hep.29671/suppinfo.
ASSESSMENT OF EFFICACY, SAFETY, AND VIROLOGICAL RESISTANCE
The primary endpoint was the percentage of patients who achieved SVR at 12 weeks after the last dose of study drug (SVR12 defined as HCV RNA below the lower limit of quantification [LLOQ; 15 IU/mL]). Secondary endpoints included the percentage of patients with on‐treatment VF and relapse. Plasma samples were collected at screening, days 1 and 3, weeks 1 and 2, 4, 6, 8, 10, 12, and 16 (only for patients treated for 16 weeks), and at post‐treatment weeks 2, 4, 8, 12, and 24. In the event of premature discontinuation, a plasma sample was taken at the time of discontinuation. Plasma HCV‐RNA levels were determined using the Roche (Basel, Switzerland) AmpliPrep COBAS TaqMan real‐time reverse transcriptase PCR assay v2.0 with a high pure system. The lower limit of detection (LLOD) and LLOQ of HCV RNA for this assay were both 15 IU/mL.
Safety analyses were conducted on adverse events (AEs), vital signs, physical examinations, electrocardiograms, and laboratory tests and included all patients receiving at least 1 dose of study drug. Treatment‐emergent AEs were collected from study drug initiation until 30 days after study drug discontinuation. Each AE was classified using the MedDRA version 19.0 system organ class and preferred term, and, subsequently, causality of each AE with respect to study drugs was determined by the study physician. Changes from baseline in laboratory tests and vital sign measurements were also assessed.
Next‐generation sequencing was used with a 15% detection threshold to identify the presence of baseline substitutions in NS3 and NS5A relative to subtype‐specific reference sequences. The amino acid positions included in the analysis of baseline substitutions were 36, 56 (GT1a only), 155, 156, and 168 in NS3, and 24, 28, 30, 31, 58, 92, and 93 in NS5A. Positions known to commonly harbor polymorphisms that do not confer significant in vitro resistance to GLE or PIB (e.g., Q80 in NS3) were not included in the analysis in order to focus analysis on relevant resistance positions. Treatment‐emergent substitutions in NS3 and NS5A were analyzed for patients who had VF.
STATISTICAL ANALYSES
The study did not test a formal hypothesis. The percentage of patients who achieved SVR12 in each arm was summarized with a two‐sided 95% confidence interval (CI) using the Wilson score method for binomial proportions. The difference in SVR12 rates between treatment arms was analyzed using the stratum‐adjusted Mantel‐Haenszel proportion with a continuity correction for variance, adjusted for each randomization stratum. Statistical analyses were performed using SAS software.
Results
BASELINE PATIENT DEMOGRAPHICS
A total of 122 patients were screened between January 12, 2016 and March 14, 2016; 31 patients failed screening, and 91 with HCV GT1 (n = 87) or GT4 (n = 4) infection were enrolled and treated. No patients with GT5 or GT6 infection were enrolled. Patients were randomized (1:1): 44 patients received 12 weeks of G/P and 47 were treated for 16 weeks. Demographics across the two treatment arms were generally well balanced. A majority of patients were male (70%), white race (76%), and had subtype 1a HCV (74%); 30% (27 of 91) of patients had compensated cirrhosis (34% and 26% of patients in the 12‐ and 16‐week arms, respectively). In both the 12‐ and 16‐week treatment arms, approximately one third of patients had past experience with an NS3/4A PI alone, an NS5A inhibitor alone, or both (Table 1). Nineteen (21%) patients had previously taken SOF + LDV (NS5A inhibitor‐only experienced) as their only past treatment. Twenty (22%) patients had past treatment with more than one course of DAA‐containing regimen.
Table 1.
Baseline Demographics and Clinical Characteristics
| Characteristic | 12 Weeks N = 44 | 16 Weeks N = 47 |
|---|---|---|
| Male, n (%) | 31 (70) | 33 (70) |
| White race, n (%) | 34 (77) | 35 (75) |
| Black race, n (%) | 9 (20) | 11 (23) |
| Age, median years (range) | 57 (22‐67) | 56 (36‐70) |
| BMI, median kg/m2 (range) | 28 (21‐41) | 29 (20‐52) |
| IL28B non‐CC genotype, n (%) | 38 (86) | 42 (89) |
| HCV RNA, median log10 IU/mL (range) | 6.1 (4.7‐7.2) | 6.3 (4.7‐7.1) |
| Compensated cirrhosis, n (%) | 15 (34) | 12 (26) |
| HCV subtypea, n (%) | ||
| 1a | 35 (80) | 32 (71) |
| 1b | 8 (18) | 11 (23) |
| 1c | — | 1 (2) |
| 4 | 1 (2) | 3 (6) |
| Previous DAA regimen classb, n (%) | ||
| NS3/4A PI only (NS5A inhibitor‐naïve) | 14 (32) | 13 (28) |
| NS5A inhibitor only (PI‐naïve) | 16 (36) | 18 (38) |
| N3/4A PI + NS5A inhibitor | 14 (32) | 16 (34) |
| Past DAA treatment response, n (%) | ||
| On‐treatment failure | 14 (32) | 13 (28) |
| Virological relapse | 30 (68) | 34 (72) |
| Time since last treatment, median (range) months | 19 (2‐94) | 10 (3‐61) |
| Presence of key baseline substitutionsc, n (%) | ||
| None | 13 (30) | 13 (30) |
| NS3 only | 2 (5) | 4 (9) |
| NS5A only | 24 (55) | 23 (52) |
| NS3 + NS5A | 5 (11) | 4 (9) |
Genotype and subtype determined by the Versant HCV Genotype Inno LiPA Assay, version 2.0 or higher, or Sanger sequencing of NS5B if LiPA result was indeterminate.
SOF (NS5B inhibitor) could be included in any previous treatment regimen.
Sequencing data were available in 44 patients in each arm; percentages are based on N = 44; substitutions detected by next‐generation sequencing using 15% detection threshold at positions 36, 56 (GT1a only), 155, 156, and 168 in NS3, and 24, 28, 30, 31, 58, 92, and 93 in NS5A. Abbreviation: BMI, body mass index.
Across both treatment arms, 30% of patients had no baseline substitutions in either NS3 or NS5A, whereas 66% (29 of 44) and 61% (27 of 44) had substitution(s) in NS5A in the 12‐ and 16‐week treatment arms, respectively (Table 1). Seventeen percent (15 of 88) of patients had substitutions in NS3 at baseline. Baseline substitutions in both NS3 and NS5A had similar prevalence in the 12‐ and 16‐week treatment arms. In patients with past experience with only NS5A inhibitors (NS3/4A PI‐naïve), 84% (27 of 32) had baseline substitutions in NS5A; none of the patients had baseline NS3 substitutions. In contrast, 27% (8 of 30) of patients with past experience with both classes of inhibitors had baseline substitutions in both NS3 and NS5A, whereas 3% (1 of 30) and 50% (15 of 30) of those patients had baseline substitutions in NS3 or NS5A alone, respectively. Detailed information on prevalence of baseline substitutions is shown in http://onlinelibrary.wiley.com/doi/10.1002/hep.29671/suppinfo.
EFFICACY
Patients treated with 12 weeks of G/P had an SVR12 rate of 89% (39 of 44; 95% CI, 76‐95), with one on‐treatment VF and four relapses, whereas those treated for 16 weeks had an SVR12 rate of 91% (43 of 47; 95% CI, 80‐97), with four on‐treatment VFs (Table 2). All 4 patients with GT4 infection achieved SVR12; details for the 9 GT1 patients that had VF (four relapses and five on‐treatment failures) are shown in http://onlinelibrary.wiley.com/doi/10.1002/hep.29671/suppinfo. Of the 5 patients with on‐treatment failure, all 5 ended their past treatment within 1 year of initiating G/P, with a median time since last treatment of 4.6 (range, 2.6‐8.7) months. Median time from past treatment failure to retreatment with G/P for the entire enrolled population was 17 (range, 2.2‐94) months, whereas those with relapse had a median time since last treatment of 26 (range, 5.7‐60) months.
Table 2.
SVR and Efficacy Outcomes
| Event, n/N (%, CIa) | 12 Weeks N = 44 | 16 Weeks N = 47 |
|---|---|---|
| Overall SVR12 | 39/44 (89; 76‐95) | 43/47 (91; 80‐97) |
| On‐treatment VF | 1/44 (2) | 4/47 (9) |
| Virological relapse | 4/44 (9) | 0/47 |
| Past DAA class | ||
| NS3/4A PI only | 14/14 (100; 79‐100) | 13/13 (100; 77‐100) |
| NS5A inhibitor only | 14/16 (88; 64‐97) | 17/18 (94; 74‐99) |
| NS3/4A PI + NS5A inhibitor | 11/14 (79; 52‐92) | 13/16 (81; 57‐93) |
| Past DAA regimen | ||
| SOF/LDV | 8/9 (89) | 9/10 (90) |
| SOF + SIM | — | 3/3 (100) |
| OBV/PTV/r ± DSV ± RBV | 5/5 (100) | 5/6 (83) |
| Other DAAb + pegIFN/RBV | 16/17 (94) | 16/16 (100) |
| Multiple past regimens | 4/5 (80) | 4/6 (67) |
| Otherc | 6/8 (75) | 6/6 (100) |
| Baseline substitutionsd | ||
| None | 13/13 (100; 77‐100) | 13/13 (100; 77‐100) |
| NS3 only | 2/2 (100) | 4/4 (100) |
| NS5A only | 20e/24 (83; 64‐93) | 22f/23 (96; 79‐99) |
| NS3 + NS5A | 4g/5 (80) | 1h/4 (25) |
CI calculated at 95% with the normal approximation to the binomial distribution.
Includes DCV, telaprevir, or boceprevir.
Any other combination of DAA regimens allowed by study protocol.
Sequencing data were available in 44 patients in each arm; substitutions detected by next‐generation sequencing using 15% detection threshold at positions 36, 56 (GT1a only), 155, 156, and 168 in NS3, and 24, 28, 30, 31, 58, 92, and 93 in NS5A.
Three relapses and one on‐treatment failure.
One on‐treatment failure.
One relapse.
Three on‐treatment failures.
Abbreviation: pegIFN/RBV, pegylated interferon plus ribavirin.
Patients with past treatment experience with only NS3/4A PIs (NS5A inhibitor‐naïve) had 100% SVR12 regardless of treatment duration. For patients with past experience with only NS5A inhibitors, SVR12 rates were 88% (14 of 16; 95% CI, 64‐97) and 94% (17 of 18; 95% CI, 74‐99) for 12 and 16 weeks of treatment, respectively. For patients with past experience with both classes of inhibitors (NS3/4A and NS5A), SVR12 rates were 79% (11 of 14; 95% CI, 52‐92) and 81% (13 of 16; 95% CI, 57‐93) in those treated for 12 and 16 weeks, respectively. Three of six patients with past experience to both classes of inhibitors that did not achieve SVR12 had on‐treatment VF.
Patients with no baseline substitutions in either NS3 or NS5A, or with substitutions in NS3 alone, had a 100% SVR12 rate. Patients with baseline substitutions in NS5A alone had SVR12 rates of 83% (20 of 24; 95% CI, 64‐93) and 96% (22 of 23; 95% CI, 79‐99) with 12 and 16 weeks of G/P treatment, respectively. Among these patients, three of four VFs in the 12‐week arm were relapses; there were no relapses in patients treated for 16 weeks (Table 2). Of those patients with baseline substitutions in both NS3 and NS5A, three of nine (33%) had on‐treatment VF in the 16‐week arm of G/P treatment. These 3 patients all failed their last previous treatment regimen within 9 months of starting G/P. Furthermore, all 3 of these patients had past experience with NS5A, NS5B, and PIs, including OBV/paritaprevir/ritonavir and DSV (OBV/PTV/r [ritonavir boosted paritaprevir] + DSV) with or without additional regimens (http://onlinelibrary.wiley.com/doi/10.1002/hep.29671/suppinfo). SVR12 rates by all past DAA regimens are listed in Table 2.
SAFETY, AEs, AND LABORATORY ABNORMALITIES
The majority of AEs were classified as mild (65%), and no patient prematurely discontinued study drug because of AEs (Table 3). There were three treatment‐emergent serious AEs reported in the study: a gastrointestinal viral infection, back pain, and wound infection; none were assessed by the study investigators as related to the study drugs. Headache was the only AE reported in ≥10% of patients. There were no clinically significant alanine aminotransferase (ALT), AST, or bilirubin elevations in the study (Table 3). Safety was comparable between those with and without cirrhosis. Three patients experienced non‐treatment‐emergent AEs of hepatocellular carcinoma, all detected during the posttreatment period (37, 71, and 105 days after last treatment), and all considered not related to the study drugs by investigators.
Table 3.
AEs and Laboratory Abnormalitiesa
| Event, n (%) | 12 Weeks N = 44 | 16 Weeks N = 47 |
|---|---|---|
| Adverse events | ||
| Any AE | 33 (75) | 32 (68) |
| Grade 1 (mild) AE, n/N (%) | 24/33 (73) | 18/32 (56) |
| Serious AEb | 1 (2) | 2 (4) |
| Serious AE related to study drugs | 0 | 0 |
| AEs leading to study drug discontinuation | 0 | 0 |
| AEs occurring in ≥ 10% of patients | ||
| Headache | 6 (14) | 11 (23) |
| Laboratory abnormalities | ||
| ALTc, Grade ≥3 (>5 × ULN) | 0 | 0 |
| ASTc, Grade ≥3 (>5 × ULN) | 0 | 0 |
| Total bilirubin, Grade ≥3 (>3 × ULN) | 0 | 0 |
| Hemoglobin, Grade ≥3 (<8 g/dL) | 0 | 0 |
Grades as per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Serious AEs included gastrointestinal viral infection, back pain, and wound infection.
Postnadir increase in grade to Grade ≥3.
Discussion
In this study, RBV‐free coformulated G/P for 16 weeks was safe and demonstrated 100% and 94% SVR12 rates, with no virological relapses, in patients with past failure to NS3/4A PI only– and NS5A inhibitor only–containing regimens, respectively. Those with past experience with both of these classes of inhibitors had a lower SVR12 rate.
NS5A inhibitor‐naïve patients with past failure with NS3/4A PI–containing regimens (i.e., boceprevir, telaprevir, and SIM) had 100% SVR12 rate, regardless of treatment duration. The median time from past treatment failure to retreatment with G/P in the enrolled population was 17 months, and the majority of patients had no substitutions in NS3 at baseline. This is consistent with the observation that RASs in NS3 selected by past PI therapy are generally undetectable within 1 year posttreatment,21 making successful retreatment of this patient population more likely over time.
Patients with past failure to NS5A inhibitor‐containing regimens (but PI‐naïve) had a 94% (17 of 18) SVR12 rate after 16 weeks of G/P treatment, compared to 88% (14 of 16) when treated for 12 weeks. Consistent with these results, patients with baseline substitutions in NS5A alone had a 96% (22 of 23) SVR12 rate when treated for 16 weeks, compared to 83% (20 of 24) when treated for 12 weeks. Among those with chronic HCV GT1 infection and past failure with NS5A inhibitor‐containing regimens, the lack of virological relapses in patients treated for 16 weeks (compared with one relapse in the 12‐week treatment arm), indicates that a 16‐week duration may be required to minimize virological relapse. With only 4 GT4‐infected patients enrolled in this study, all whom achieved SVR12, the optimal treatment duration could not be identified in this population.
The presence of NS5A substitutions at baseline was observed in the majority of patients with past NS5A inhibitor experience (50 of 64; 78%); however, there was no consistent pattern of substitutions associated with VF to G/P. Treatment failure in this study was generally associated with the presence of baseline NS5A substitutions in conjunction with NS3 substitutions. All 3 patients that had on‐treatment VF after past exposure to both NS3/4A and NS5A classes of inhibitors and had detectable combinations of Y56H and D168A/E substitutions in NS3 at baseline, in addition to substitutions in NS5A. All 3 patients were randomized to the 16‐week treatment arm, had past failure to OBV/PTV/r ± DSV with or without additional regimens, and had recent failure on their previous course of DAA therapy (within 9 months of enrollment). These factors explain the presence of multiple baseline NS3 substitutions that decrease susceptibility to GLE in these patients.22 Thus, these data suggest that patients with past experience to OBV/PTV/r ± DSV ± RBV, or those with multiple past courses of therapy that included NS3/4A protease and NS5A classes of inhibitors, may have an increased rate of VF.
One limitation of this study was the small number of GT4 patients available for enrollment, which prevented a robust assessment of efficacy in this genotype. Another limitation was the lack of patients with failure to more recently approved DAA regimens, such as elbasvir/grazoprevir and SOF/VEL; these regimens were approved after study initiation. However, SOF/LDV‐ or DCV‐containing regimens are currently the most commonly prescribed HCV treatment regimens for patients with GT1 infection; 27% and 24% of patients enrolled in this study were SOF/LDV‐ and DCV‐experienced, respectively. Another limitation was the small number of patients enrolled with compensated cirrhosis. Finally, few patients were enrolled with past failure to both an NS3/4A PI and NS5A inhibitor, because these patients are less common given the high efficacy of current generation regimens. In this study, patients with past experience to both classes of DAAs had lower SVR12 rates, at 79% and 81% after 12 or 16 weeks of G/P treatment, respectively. In contrast, 30 of 31 (97%) patients with past experience to either NS3/4A protease or NS5A inhibitor DAA classes achieved SVR12 after 16 weeks of G/P treatment.
In this study, the RBV‐free combination of G/P was safe and well tolerated, regardless of treatment duration (12 or 16 weeks), and there were no AEs that led to study drug discontinuation. Furthermore, G/P demonstrated a low rate of serious AEs (none of which were assessed as related to study drugs), and there were no clinically relevant elevations in ALT, AST, or total bilirubin.
In summary, patients with NS3/4A PI experience alone had a 100% SVR12 rate regardless of 12‐ or 16‐week treatment duration, whereas 16‐week treatment duration may be required for patients with NS5A inhibitor experience alone (94% SVR12 with no virological relapses). Those with past experience with both of these DAA classes had lower SVR12 rates. Overall, RBV‐free G/P demonstrated high SVR12 rates in patients with past failure with approved NS3/4A PIs or NS5A inhibitors.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.29671/suppinfo.
Supporting Information 1
Ackowledgments
AbbVie and the authors express their gratitude to the patients who participated in this study, and their families. They also thank all of the participating study investigators and coordinators, particularly Traci Baker, of AbbVie. Medical writing was provided by Ryan J Bourgo, Ph.D., of AbbVie.
Potential conflict of interest: Dr. Poordad consults for, advises for, is on the speakers' bureau for, and received grants from Gilead, Merck, GlaxoSmithKline, and Vertex. He consults for, advises for, and received grants from AbbVie, Achillion, Anadys, Biolex, Boehringer Ingelheim, Bristol‐Myers Squibb, GlobeImmune, Idenix, and Novartis. He consults for and advises for Tibotec/Janssen and Theravance. He is on the speakers' bureau for and received grants from Genentech. He is on the speakers' bureau for Kadmon, Onyx/Bayer for, and Salix. He received grants from Idera, Intercept, Janssen, Medarex, Medtronic, Santaris, Scynexis, and ZymoGenetics. Prof. Pol is on the speakers' bureau for and received grants from Bristol‐Myers Squibb, Gilead, Roche, AbbVie, and MSD. He is on the speakers' bureau for Boehringer Ingelheim and Janssen. Dr. Bernstein consults for and is on the speakers' bureau for AbbVie. Dr. Reindollar advises for and is on the speakers' bureau for AbbVie and Gilead. Dr. Fried consults for and received grants from AbbVie, Bristol‐Myers Squibb, and Merck. He received grants from Gilead. He owns stock in Target PharmaSolutions. Dr. Pianko advises for, is on the speakers' bureau for, and received grants from AbbVie, Gilead, and MSD. Dr. Hezode consults for AbbVie, Bristol‐Myers Squibb, Gilead, Janssen, and MSD. Dr. Felizarta is on the speakers' bureau for and received grants from AbbVie, Gilead, Janssen, and Merck. Dr. Gallant advises for, received grants from, and is employed by Gilead. He advises for and received grants from Bristol‐Myers Squibb, Merck, ViiV, and GlaxoSmithKline. He advises for Thera‐technologies. He received grants from AbbVie, Janssen, and Sangamo. Dr. Buti consults for and is on the speakers' bureau for Gilead, AbbVie, and MSD. Dr. Gordon advises for and received grants from AbbVie, Bristol‐Myers Squibb, Intercept, Gilead, and Merck. He advises for CVS Caremark. He received grants from Conatus, CymaBay, and Exalenz. He received royalties from UpToDate. Dr. Kort is employed by and owns stock in AbbVie. Dr. Ng is employed by and owns stock in AbbVie. Dr. Lin is employed by and owns stock in AbbVie. Dr. Asatryan is employed by and owns stock in AbbVie. Dr. Mensa is employed by and owns stock in AbbVie. Dr. Krishnan is employed by and owns stock in AbbVie. Dr. Lei is employed by and owns stock in AbbVie. Dr. Kopecky‐Bromberg owns stock in AbbVie.
REFERENCES
- 1. Jacobson IM, Asante‐Appiah E, Wong P, Black TA, Howe AY, Wahl J, et al. Prevalence and impact of baseline NS5A resistance‐associated variants (RAVs) on the efficacy of elbasvir/grazoprevir (EBR/GZR) against GT1a infection. Hepatology 2015;62(Suppl 6):1393A‐1394A. [Google Scholar]
- 2. Lawitz E, Flamm S, Yang JC, Pang PS, Zhu Y, Svarovskaia E, et al. Retreatment of patients who failed 8 or 12 weeks of ledipasvir/sofosbuvir‐based regimens with ledipasvir/sofosbuvir for 24 weeks. J Hepatol 2015;62(Suppl 2):S192. [Google Scholar]
- 3. Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, et al. Grazoprevir‐elbasvir combination therapy for treatment‐naive cirrhotic and noncirrhotic patients with chronic HCV genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med 2015;163:1‐13. [DOI] [PubMed] [Google Scholar]
- 4. Dvory‐Sobol H, Wyles D, Ouyang W, Chodavarapu K, McNally J, Cheng W, et al. Long‐term persistance of HCV NS5A variants after treatment with NS5A inhibitor ledipasvir. J Hepatol 2015;62(Suppl 2):S221. [Google Scholar]
- 5. Krishnan P, Tripathi R, Schnell G, Reisch T, Beyer J, Dekhtyar T, et al. Long‐term follow‐up of treatment‐emergent resistance‐associated variants in NS3, NS5A, and NS5B with paritaprevir/r‐, ombitasvir‐, and dasabuvir‐based regimens. J Hepatol 2015;62(Suppl 2):S220. [Google Scholar]
- 6. AASLD‐IDSA . Recommendations for testing, managing, and treating hepatitis C. 2017. http://www.hcvguidelines.org. Accessed July 16, 2017.
- 7. European Association for the Study of the Liver . Electronic address eee. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol 2017;66:153‐194. [DOI] [PubMed] [Google Scholar]
- 8. Buti M, Gordon SC, Zuckerman E, Lawitz E, Calleja JL, Hofer H, et al. Grazoprevir, Elbasvir, and Ribavirin for Chronic Hepatitis C Virus Genotype 1 Infection After Failure of Pegylated Interferon and Ribavirin With an Earlier‐Generation Protease Inhibitor: Final 24‐Week Results From C‐SALVAGE. Clin Infect Dis 2016;62:32‐36. [DOI] [PubMed] [Google Scholar]
- 9. Gane E, Shiffman ML, Etzkorn K, Morelli G, Stedman CA, Davis MN, et al. Sofosbuvir/Velpatasvir in combination with ribavirin for 24 weeks is effective retreatment for patients who failed prior NS5A containing DAA regimens: results of the GS‐US‐342‐1553 study. J Hepatol 2016;64(Suppl 2):S147‐S148. [Google Scholar]
- 10. Lawitz E, Poordad F, Gutierrez JA, Wells JT, Landaverde CE, Reiling JR, et al. C‐SWIFT Retreatment (Part B): 12 Weeks of Elbasvir/Grazoprevir with Sofosbuvir and Ribavirin Successfully Treated G1‐Infected Subjects who Failed Short‐Duration All‐Oral Therapy. Paper presented at The Liver Meeting 2015: American Association for the Study of Liver Diseases (AASLD) 2015, November 13‐17, San Francisco, CA.
- 11. Poordad F, Bennett M, Sepe TE, Cohen E, Reindollar RW, Everson GT, et al. QUARTZ‐I: Retreatment of HCV Genotype 1 DAA‐failures With Ombitasvir/Paritaprevir/r, Dasabuvir, and Sofosbuvir. Paper presented at The Liver Meeting 2015: American Association for the Study of Liver Diseases (AASLD) 2015, November 13‐17, San Francisco, CA.
- 12. Hezode C, Fourati S, Chevaliez S, Scoazec G, Soulier A, Varaut A, et al. Sofosbuvir‐Daclatasvir‐Simeprevir Plus Ribavirin in Direct‐Acting Antiviral‐Experienced Patients With Hepatitis C. Clin Infect Dis 2017;64:1615‐1618. [DOI] [PubMed] [Google Scholar]
- 13. VOSEVI (sofosbuvir/velpatasvir/voxilaprevir) [package insert] . Foster City, CA: Gilead Sciences; 2017.
- 14. Bourliere M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, et al. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med 2017;376:2134‐2146. [DOI] [PubMed] [Google Scholar]
- 15. SOVALDI (sofosbuvir) tablets [package insert]. Foster City, CA: Gilead Sciences. Revised on November 2017.
- 16. Ahmed A, Felmlee DJ. Mechanisms of Hepatitis C Viral Resistance to Direct Acting Antivirals. Viruses 2015;7:6716‐6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ng TI, Krishnan P, Pilot‐Matias T, Kati W, Schnell G, Beyer J, et al. ABT‐493, a potent HCV NS3/4A protease inhibitor with broad genotype coverage. 2014. http://www.croiconference.org/sites/default/files/posters/636.pdf. Accessed July 25, 2016.
- 18. Ng TI, Krishnan P, Pilot‐Matias T, Kati W, Schnell G, Beyer J, et al. In vitro antiviral activity and resistance profile of the next generation hepatitis C virus NS5A inhibitor pibrentasvir. Antimicrob Agents Chemother 2017;61. pii: e02558‐16. doi: 10.1128/AAC.02558-16. Print 2017 May. [DOI] [PMC free article] [PubMed]
- 19. Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol 2013;3:514‐520. [DOI] [PubMed] [Google Scholar]
- 20. Poordad F, Felizarta F, Asatryan A, Sulkowski MS, Reindollar RW, Landis CS, et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct‐acting antiviral treatment. Hepatology 2017;66:389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 2014;146:1176‐1192. [DOI] [PubMed] [Google Scholar]
- 22. Pilot‐Matias T, Krishnan P, Schnell G, Tripathi R, Beyer J, Reisch T, et al. Resistance Analysis in the MAGELLAN‐I Study (Part 2): Glecaprevir/Pibrentasvir Therapy in HCV‐Infected Patients who had Failed Prior DAA Regimens Containing NS3/4A Protease and/or NS5A Inhibitors (SAT‐204). Paper presented at International Liver Congress (EASL) 2017, April 19‐23, Amsterdam, The Netherlands.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.29671/suppinfo.
Supporting Information 1


