Abstract
Objectives
In multiple sclerosis (MS), magnetic resonance imaging (MRI) is a sensitive tool for detecting white matter lesions, but its diagnostic specificity is still suboptimal; ambiguous cases are frequent in clinical practice. Detection of perivenular lesions in the brain (the “central vein sign”) improves the pathological specificity of MS diagnosis, but comprehensive evaluation of this MRI biomarker in MS‐mimicking inflammatory and/or autoimmune diseases, such as central nervous system (CNS) inflammatory vasculopathies, is lacking. In a multicenter study, we assessed the frequency of perivenular lesions in MS versus systemic autoimmune diseases with CNS involvement and primary angiitis of the CNS (PACNS).
Methods
In 31 patients with inflammatory CNS vasculopathies and 52 with relapsing–remitting MS, 3‐dimensional T2*‐weighted and T2–fluid‐attenuated inversion recovery images were obtained during a single MRI acquisition after gadolinium injection. For each lesion, the central vein sign was evaluated according to consensus guidelines. For each patient, lesion count, volume, and brain location, as well as fulfillment of dissemination in space MRI criteria, were assessed.
Results
MS showed higher frequency of perivenular lesions (median = 88%) than did inflammatory CNS vasculopathies (14%), without overlap between groups or differences between 3T and 1.5T MRI. Among inflammatory vasculopathies, Behçet disease showed the highest median frequency of perivenular lesions (34%), followed by PACNS (14%), antiphospholipid syndromes (12%), Sjögren syndrome (11%), and systemic lupus erythematosus (0%). When a threshold of 50% perivenular lesions was applied, central vein sign discriminated MS from inflammatory vasculopathies with a diagnostic accuracy of 100%.
Interpretation
The central vein sign differentiates inflammatory CNS vasculopathies from MS at standard clinical magnetic field strengths. Ann Neurol 2018;83:283–294
Multiple sclerosis (MS) is characterized by recurrent neurological symptoms beginning in young adulthood, associated with focal lesions scattered in the central nervous system (CNS).1 Pathologically, white matter (WM) lesions correspond to inflammatory infiltrates that develop around venules.2, 3 These infiltrates (widely known as perivascular cuffs) mainly comprise mononuclear cells that dynamically accumulate, distribute, and evolve in the CNS following recurrent waves of invasion from peripheral blood.4
The CNS of young adults may also be targeted by chronic inflammatory vasculopathies. When these vasculopathies involve small vessels, they can result in a range of “MS‐like” chronic neurological symptoms and syndromes, characterized by relapsing–remitting or progressive course.5, 6 The parenchymal WM lesions associated with these neurological phenotypes can be visualized by magnetic resonance imaging (MRI) and frequently meet the topographic MRI diagnostic criteria of MS,7 which cannot distinguish the underlying pathology of the lesions.8, 9 When such cases occur in the context of systemic inflammatory or autoimmune diseases (SADs), MS is excluded on the basis of the diagnostic criterion of “better explanation.”7 However, an acute neurological syndrome can be the first clinical presentation in SADs, and sometimes MS and SAD can coexist in the same patient.10 In all these cases, differentiation from MS may be problematic, and a “better explanation” of the diagnosis cannot be invoked.11, 12 In addition, the current standard for diagnosis of inflammatory vasculopathies is brain/meningeal biopsy or angiography,11, 12 but these, unlike MRI, are substantially invasive procedures and are not always diagnostic.13
The physical relationship between WM lesions and venules can now be visualized by susceptibility‐based MRI sequences, taking advantage of the T2*‐shortening effect of deoxyhemoglobin14, 15 in venous blood.16 Several studies conducted with 3T and 7T MRI clearly showed that in MS, the association between brain WM venules and lesions (perivenular lesions), also named the “central vein sign,”17 can be efficiently visualized; in MS, the proportion of lesions that have clear central veins is high.14, 15 These data suggest that high frequency of perivenular lesions is pathologically specific to MS, and therefore this marker is an important candidate for improving MRI diagnostic criteria14 and for reducing the still too high rate of MS misdiagnosis, with its considerable clinical consequences.18, 19
Thus far, the central vein sign has been compared primarily between MS and a limited set of other neurological diagnosis, such as migraine and ischemic small vessel disease of the elderly,20, 21 which do not involve inflammation or autoimmunity similar to MS.11, 12 Proper validation of an MRI marker to improve differential diagnosis between MS and other MS‐like neurological syndromes in young adults requires comparison with other inflammatory diseases with CNS involvement. Thus, in this study, the frequency of brain WM perivenular lesions visualized by MRI was compared between MS and SADs, encompassing Behçet disease, systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), Sjögren syndrome, and primary angiitis of the CNS (PACNS).
Patients and Methods
Patients
Eigthy‐three consecutive patients diagnosed with either SADs and clinical/MRI evidence of brain involvement, or with PACNS (hereafter both termed “inflammatory vasculopathies”), or with relapsing–remitting MS according to the 2010 McDonald revised criteria,7 were recruited between January 2015 and June 2017 at 4 academic research hospitals: the Careggi University Hospital (Florence, Italy), the Erasme University Hospital and Brugmann University Hospital (Brussels, Belgium), and the San Raffaele University Hospital (Milan, Italy). The study received approval from ethical standards committees on human experimentation at all centers. Written informed consent was obtained from all participants.
Patients were excluded if contraindicated for MRI or intravenous injection of gadolinium‐based contrast material. To avoid problems related to assignment of central veins, patients with extremely high lesion loads (>100 lesions) as well as patients with diffuse leukoencephalopathy with no or few discrete lesions were excluded a priori based on previously available MRI.
Included patients with inflammatory vasculopathies encompassed: (1) SLE, diagnosed according to the Systemic Lupus International Collaborating Clinics classification criteria22; (2) APS, diagnosed according to the Miyakis criteria23; (3) Behçet disease, diagnosed according to the International Study Group for Behçet's Disease24; (4) Sjögren disease, diagnosed according to the American College of Rheumatology/European League against Rheumatism criteria25; and (5) PACNS (imaging or biopsy proven), diagnosed according to Schuster et al.13
MRI Acquisition Protocol
All patients underwent a single brain MRI acquisition. MRI studies were performed on two 3T Philips (Best, the Netherlands) Intera MRI scanners (Brussels and Milan) and a 1.5T Philips Achieva MRI scanner (Florence). For all scans, 3‐dimensional (3D) T2*‐weighted echo‐planar imaging (EPI) and 3D T2–fluid‐attenuated inversion recovery (FLAIR) images were acquired during or after intravenous injection of a single dose (0.1mmol/kg) of gadolinium‐based contrast material, as previously described.26 3D T2*‐weighted EPI and 3D T2‐FLAIR sequences were identical for the 3T scanners in Brussels and Milan, but adapted and optimized for the 1.5T scanner in Florence, as previously described (Table 1).27 Additional routine magnetic resonance images were acquired for clinical use, including T1‐weighted sequences.
Table 1.
MRI Sequence Parameters of 1.5T and 3T MRI Scanners
| Sequence | 3D T2*‐EPI | 3D T2‐FLAIR | ||
|---|---|---|---|---|
| Magnet strength, T | 1.5 | 3 | 1.5 | 3 |
| Manufacturer | Philips | Philips | Philips | Philips |
| Model | Achieva | Intera | Achieva | Intera |
| Receive channels | 8 | 8 | 8 | 8 |
| Imaging plane | Sagittal | Sagittal | Sagittal | Sagittal |
| Imaging resolution, mm | 0.8 | 0.55 | 1 | 1 |
| Slices, No. | 200 | 336 | 180 | 180 |
| Repetition time, ms | 41 | 53 | 4,800 | 4,800 |
| Echo time, ms | 22 | 29 | 297 | 373 |
| Inversion time, ms | — | — | 1,660 | 1,600 |
| Flip angle | 10 ° | 10 ° | 90 ° | 90 ° |
| Averages | 2 | 2 | 1 | 1 |
| Acquisition time, min:s | 4:24 | 4:40 | 5:55 | 6:00 |
3D = 3‐dimensional; EPI = echo‐planar imaging; FLAIR = fluid‐attenuated inversion recovery; MRI = magnetic resonance imaging.
MRI Postprocessing and Analysis
Four neurologists, with research training experience in MS imaging and training in central vein sign assessment according to the consensus criteria of the North American Imaging in Multiple Sclerosis (NAIMS) Cooperative,14 analyzed the data blinded to the diagnosis. Two of them worked on 3T data (P.M., M.A.) and 2 on 1.5T data (L.V., M.G.). Data were collected as DICOM images and processed and visualized using Medical Image Processing, Analysis, and Visualization (MIPAV; NIH; http://mipav.cit.nih.gov). FLAIR* images were generated with the following steps26: (1) coregistration between T2‐FLAIR and T2* images, (2) upsampling of the T2‐FLAIR image to match the T2* resolution, and (3) voxelwise multiplication.
For the central vein sign assessment, only discrete brain lesions with a diameter of ≥3mm in at least 1 plane were included in the analysis. Small (<3mm), confluent, and poorly visible lesions were excluded. On T2* and FLAIR* images, lesions were defined as “perivenular” by raters' consensus agreement (2 reviewers for 3T and 2 reviewers for 1.5T MRI data), according to the NAIMS guidelines,14 as follows: “(1) the lesion contains a thin hypointense line (<2 mm diameter) or small hypointense dot that is visible in at least two perpendicular MRI planes; and (2) the vein, running partially or entirely through the lesion, appears as positioned approximately in the center of the lesion.” Lesions that did not meet these criteria were considered nonperivenular. The frequency of perivenular lesions per patient was expressed as a percentage of the total number of analyzed lesions.
For each patient, additional lesion morphological features were recorded: (1) brain location (periventricular, juxtacortical/leukocortical, subcortical/deep WM, or infratentorial), (2) lesion volume (manual segmentation using MIPAV), and (3) gadolinium enhancement.
Finally, for each patient, fulfillment of MS MRI criteria for dissemination in space was assessed, respectively, according to Polman et al7 and Filippi et al.28 We dichotomized patients as overall perivenular positive versus perivenular negative based on the highest frequency of perivenular lesions observed in the vasculopathy group. Similarly, we also dichotomized patients based on 3 previously published suggested criteria: (1) the “40% rule,” whereby a threshold of 40% perivenular lesions distinguishes MS from non‐MS17; (2) the “6‐lesion rule,” whereby 10 lesions are randomly assessed and MS is diagnosed if at least 6 lesions are perivenular20; and (3) the “3‐lesion rule,” whereby 3 lesions are randomly assessed and MS is diagnosed if these 3 lesions are perivenular.29
Statistical Analysis
Demographic, clinical, and MRI differences in patients with inflammatory vasculopathies versus MS were assessed with Mann–Whitney U test and Fisher exact test, when appropriate. Mean differences in lesion location proportion between groups were assessed with a 2‐way repeted measures analysis of variance with interaction, where brain lesion location was the within‐subject factor and group was the between‐subject factor; probability values were adjusted for post hoc comparisons (Bonferroni).
Results
Clinical Data
Clinical and demographic characteristics of inflammatory vasculopathy (n = 31) and relapsing–remitting MS (n = 52) patients are reported in Table 2. The 2 groups were comparable for sex and disease duration. As expected, in patients with inflammatory vasculopathies, there was a higher frequency of seizures, systemic vascular events/stroke, and headache (Fisher exact test, p < 0.0001). Cerebrospinal fluid data were available in 10 of the 31 inflammatory vasculopathy cases and in 47 of 52 MS cases; as expected, oligoclonal bands were more frequently detected in MS patients (see Table 2). None of the patients with inflammatory vasculopathies presented with spinal cord (or optic nerve) syndromes or with radiological signs typical of neuromyelitis optica (NMO) spectrum disorder (NMOSD). NMO IgG was tested in 1 of these patients (a case of Sjögren disease), resulting negative.
Table 2.
Demographic and Clinical Features
| Feature | Inflammatory Vasculopathies | MS | Statistical Comparison |
|---|---|---|---|
| Patients, No. | 31 | 52 | — |
| Median age (range) | 45 (27–70) | 41 (20–65) | Mann–Whitney p = 0.02 |
| Sex, F/M | 20/11 | 34/18 | n.s. |
| Clinical data | |||
| Clinical diagnosis | 9 SLE, 10 Behçet, 2 Sjögren, 7 APS, 3 PACNS | 52 RRMS | — |
| Median disease duration, yr (range) | 10 (0.5–22) | 7.7 (0.5–39) | n.s. |
| Median EDSS (range) | 1 (0–3) | 2 (0–4) | Mann–Whitney p = 0.003 |
| Median mRS (range) | 1 (0–2) | 1 (0–2) | n.s. |
| MMSE < 24 | 0% | 0% | n.s. |
| Focal neurological symptoms | 39% | 67% | Fisher p = 0.0001 |
| History of seizures | 29% | 4% | Fisher p < 0.0001 |
| History of systemic vascular events or stroke | 39% | 0% | Fisher p < 0.0001 |
| History of headache | 55% | 12% | Fisher p < 0.0001 |
| OCB presence, No. (%) | 1/10 available (10%) | 46/47 available (98%) | Fisher p < 0.0001 |
SLE= systemic lupus erythematosus; APS = antiphospholipid antibody syndrome; PACNS = primary angiitis of the central nervous system; RRMS = relapsing–remitting MS; EDSS = Expanded Disability Status Scale; F = female; M = male; MMSE = Mini‐Mental State Examination; mRS = modified Rankin Scale; n.s. = not significant; OCB = oligoclonal band.
Lesion Counts and Brain Location
The median number of brain WM lesions per patient did not differ between inflammatory vasculopathies and MS patients (median number = 15, range = 1–93 vs median number = 15, range = 2–66, respectively; Mann–Whitney U test, p = 0.6), whereas the median lesion volume was 34% smaller in inflammatory vasculopathies than in MS (122mm3, range = 15–734 vs 186mm3, range = 29–943; Mann–Whitney U test, p = 0.048). Fewer patients with inflammatory vasculopathies (2 of 31 patients, 6%) had contrast‐enhancing lesions than MS patients (11 of 52 patients, 21%).
The topographical distribution of brain lesions is shown in Figure 1. As expected, a significantly higher relative frequency of subcortical/deep WM lesions was observed in inflammatory vasculopathies than in MS (p < 0.0001); conversely, periventricular lesions were more frequent in MS than in inflammatory vasculopathies (p < 0.0001). However, there was a great deal of overlap between diagnostic groups. No differences in juxtacortical/leukocortical and infratentorial lesion frequency were observed between the 2 groups. The regional lesion distribution among patients with at least 1 lesion per brain location did not significantly differ between groups (chi‐square test, p = 0.18).
Figure 1.
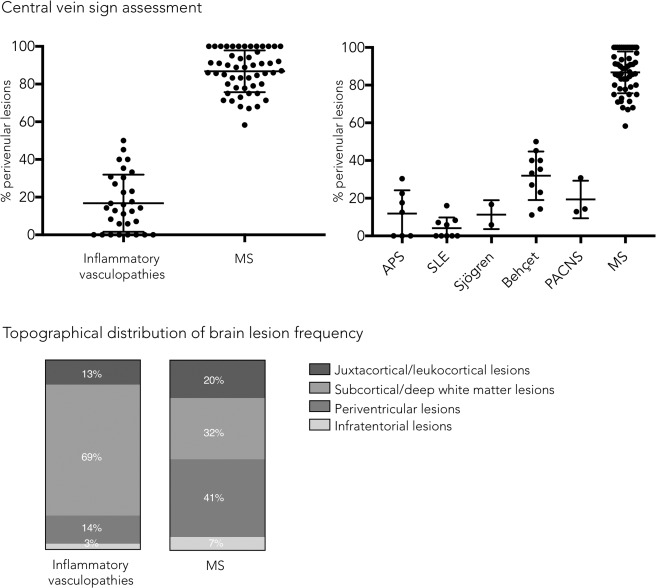
Frequency of perivenular lesions and topographical distribution of brain lesions in inflammatory vasculopathies and multiple sclerosis (MS). APS = antiphospholipid syndrome; PACNS = primary angiitis of the central nervous system; SLE = systemic lupus erythematosus.
Central Vein Sign Assessment
The frequency of perivenular lesions was remarkably higher in MS (median = 88%, range = 58–100%) versus inflammatory vasculopathies (14%, 0–50%; Mann–Whitney U test, p < 0.0001; see Fig 1). The separation between the 2 groups based on perivenular lesion frequency was complete (>50% perivenular lesions threshold, hereafter referred as the “50% rule”).
The frequency of perivenular lesions within groups did not differ significantly between 1.5T and 3T MRI (median = 88%, range = 67–100% and median = 85%, range 58–100%, respectively, in MS; median = 18%, range = 0–50% and median = 14%, range 0–40%, respectively, in inflammatory vasculopathies).
Among inflammatory vasculopathies, Behçet disease showed the highest frequency of perivenular lesions (median = 34%, range = 11–50%), followed by PACNS (median = 14%, range = 13–31%), APS (median = 12%, range = 0–30%), Sjögren (median = 11%, range = 6–17%), and SLE (median = 0%, range = 0–16%; Figs 1, 2, 3, 4, 5).
Figure 2.
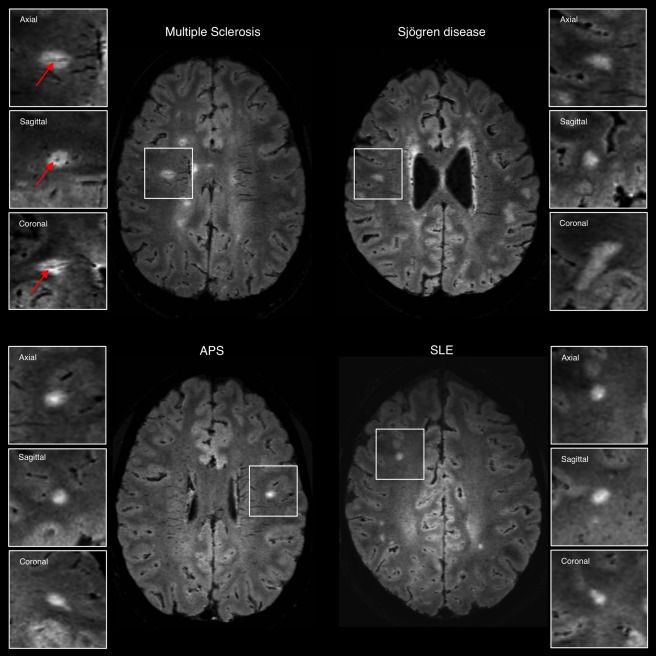
Representative axial 3T FLAIR* images from individuals with relapsing–remitting multiple sclerosis (MS; 27‐year‐old woman), Sjögren disease (46‐year‐old woman), antiphospholipid antibody syndrome (APS; 37‐year‐old man), and systemic lupus erythematosus (SLE; 38‐year‐old woman). The central vein sign (arrows) is present in the majority of MS lesions but is not typical of white matter lesions in inflammatory vasculopathies. Boxes show magnified views of lesions in the 3 orthogonal planes for central vein assessment. [Color figure can be viewed at http://www.annalsofneurology.org]
Figure 3.
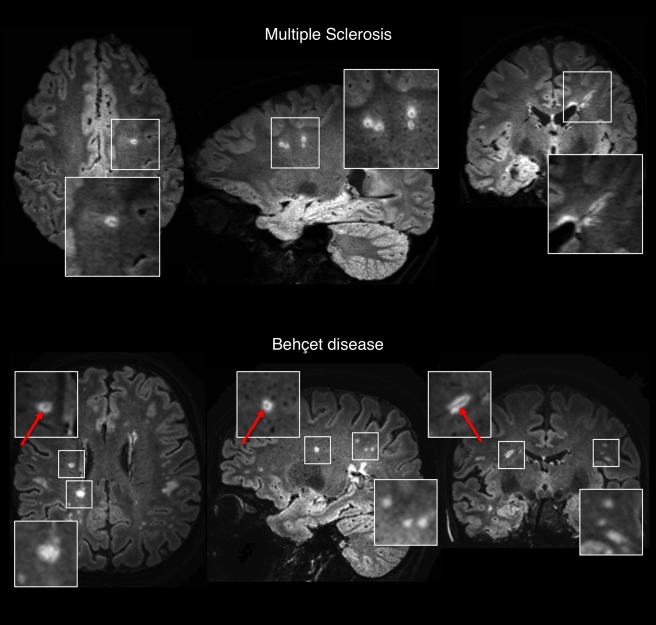
Axial, sagittal, and coronal 3T FLAIR* images from individuals with relapsing–remitting multiple sclerosis (MS; 30‐year‐old woman; top) and Behçet disease (42‐year‐old woman; bottom), respectively. Perivenular MS‐like lesions (arrows) can be seen in Behçet disease. Magnified views of representative lesions are displayed in the boxes. [Color figure can be viewed at http://www.annalsofneurology.org]
Figure 4.
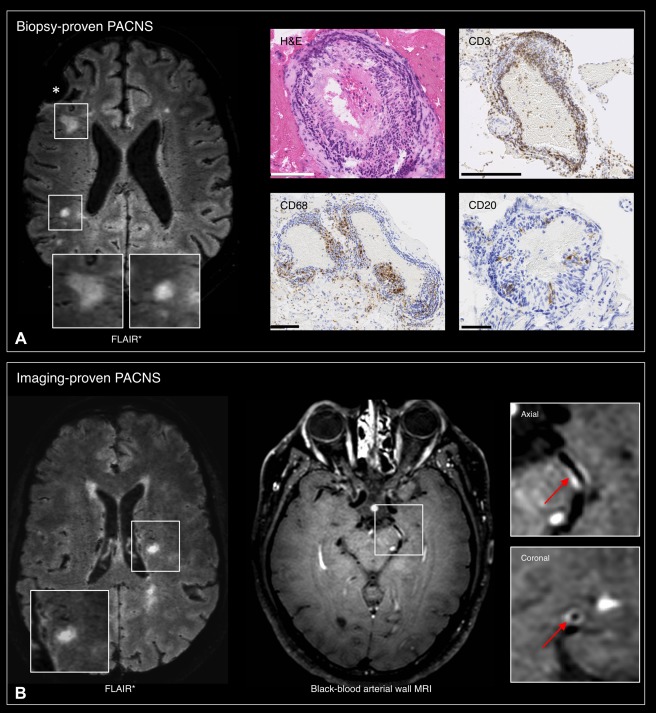
Axial 3T FLAIR* images showing the presence of nonperivenular parenchymal lesions in 2 patients with primary angiitis of the central nervous system (PACNS). (A) Biopsy‐proven PACNS (57‐year‐old man; biopsy of the right frontal lobe and overlaying leptomeninges, asterisk). The histopathology shows the presence of a vasculocentric, transmural, multilayer inflammatory infiltrate (predominantly T lymphocytes) involving both the leptomeningeal and parenchymal arterioles. Scale bars: hematoxylin & eosin (H&E), 100 µm; CD3 (T lymphocytes), 250 µm; CD68 (macrophages), 100 µm; CD20 (B lymphocytes), 50 µm. (B) Imaging‐proven PACNS (48‐year‐old man). Vessel‐wall enhancement (arrows) of the left posterior and left middle cerebral artery is demonstrated using black‐blood arterial wall magnetic resonance imaging (MRI).
Figure 5.
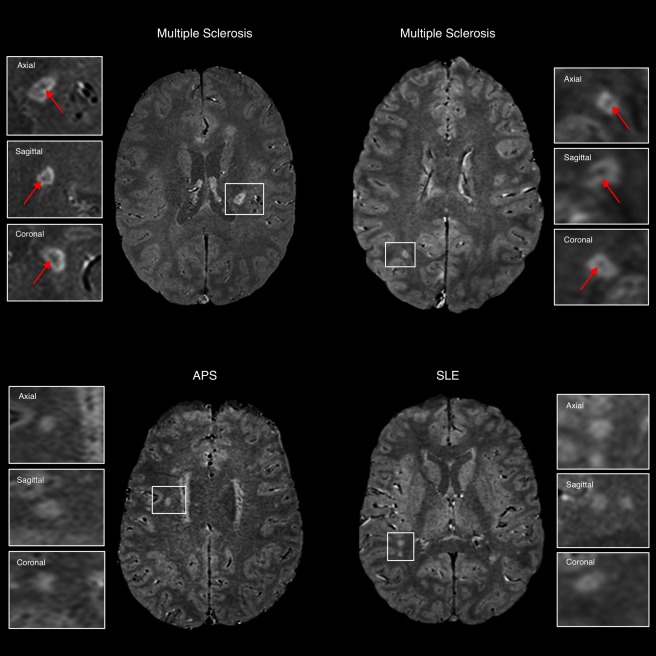
Representative axial 1.5T T2* echo‐planar images from individuals with relapsing–remitting multiple sclerosis (MS; 55‐year‐old and 24‐year‐old women), antiphospholipid antibody syndrome (APS; 51‐year‐old woman), and systemic lupus erythematosus (SLE; 40‐year‐old woman). The central vein sign (arrows) is present in the majority of MS lesions but is not typical of white matter lesions in inflammatory vasculopathies. Boxes show magnified views of lesions in the 3 orthogonal planes for central vein assessment. [Color figure can be viewed at http://www.annalsofneurology.org]
When patients were dichotomized based on the 40% rule (presence of ≥40% perivenular lesions),17 all MS patients were perivenular‐positive versus only 4 patients with vasculitis (all Behçet disease cases; Fisher exact test, p < 0.0001; Table 3). The 50% rule and the 40% rule showed higher diagnostic accuracy in comparison to the 6‐lesion rule20 and the 3‐lesion rule.29 Diagnostic specificity, sensitivity, and accuracy are shown in Table 3.
Table 3.
Fulfillment of Different MRI Criteria and Diagnostic Test Evaluation
| Variables | Inflammatory Vasculopathies, No. (%) of Patients Fulfilling Criteria | Multiple Sclerosis, No. (%) of Patients Fulfilling Criteria | Diagnostic Test Evaluation | ||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | |||
| Perivenular lesion criteria | |||||
| 50% perivenular rule | 0/31 (0%) | 52/52 (100%) | 100% | 100% | 100% |
| 40% perivenular rule | 4/31 (13%) | 52/52 (100%) | 100% | 94% | 95% |
| 6‐lesion rule | 9/31 (29%) | 44/52 (85%) | 85% | 71% | 79% |
| 3‐lesion rule | 15/31 (48%) | 51/52 (98%) | 98% | 52% | 81% |
| Dissemination in space MRI criteria | |||||
| Polman 20117 | 16/31 (52%) | 49/52 (94%) | 94% | 48% | 77% |
| FIlippi 201628 | 8/31 (26%) | 47/52 (90%) | 90% | 74% | 84% |
| Combined criteria | |||||
| Both Polman and 40% perivenular rule | 3/31 (10%) | 49/52 (94%) | 94% | 90% | 93% |
| Both Filippi and 40% perivenular rule | 0/31 (0%) | 47/52 (90%) | 90% | 100% | 94% |
| Both Polman and 6‐lesion rule | 7/31 (23%) | 42/52 (81%) | 81% | 77% | 76% |
| Both Polman and 3‐lesion rule | 11/31 (36%) | 48/52 (92%) | 92% | 64% | 82% |
| Both Filippi and 6‐lesion rule | 3/31 (10%) | 41/52 (79%) | 79% | 90% | 83% |
| Both Filippi and 3‐lesion rule | 6/31 (19%) | 46/52 (88%) | 88% | 81% | 85% |
MRI = magnetic resonance imaging.
Fulfillment of MRI Dissemination in Space MS Diagnostic Criteria
Table 3 compares the fulfillment of MRI dissemination in space MS diagnostic criteria, according to Polman et al7 and the more recent MAGNIMS criteria (Filippi et al).28 As a caveat, the analysis focused only on brain lesions, neglecting the contribution of both spinal cord (cord MRI was not available for all patients) and optic nerve (relevant for the MAGNIMS criteria) lesions. The addition of the 40% rule to Polman and to Filippi criteria, respectively, increased the specificity, without decreasing the sensitivity, of current dissemination in space MRI criteria (see Table 3). Similarly, the addition of the 6‐lesion rule or 3‐lesion rule to Polman 2011 and to Filippi 2016 criteria, respectively, increased the specificity, but did not dramatically change the overall diagnostic accuracy, of current dissemination in space MRI criteria (see Table 3).
Central Vein Sign Assessment in MS‐Mimicking Inflammatory Vasculopathies
Among the 31 patients with inflammatory vasculopathies, 15 satisfied the MRI dissemination in space MS diagnostic criteria (Polman et al7) and had no history of previous stroke. When this MS‐mimicking inflammatory vasculopathy population was compared to the MS group, the results were overall similar to those reported above. In particular, the frequency of perivenular lesions in the MS‐mimicking inflammatory vasculopathy population remained significantly lower compared to MS, maintaining no distribution overlap between groups (median = 23%, range = 0–50%; Mann–Whitney U test, p < 0.0001).
Discussion
The main finding of this multicenter study is that central vein assessment, provided by susceptibility‐based MRI, significantly improves the differential diagnosis between MS and inflammatory vasculopathies involving the CNS. Specifically, the central vein sign alone or in combination with available MS diagnostic MRI criteria7, 28 improves the diagnostic accuracy and specificity, without lowering the sensitivity, of MS diagnosis. Assessments were performed on standard clinical magnetic field strength systems, and results at 3T and the more widely available 1.5T were indistinguishable.
Our findings are particularly relevant considering that CNS inflammatory vasculopathies can present with a chronic relapsing or progressive (MS‐like) clinical course and often feature brain WM abnormalities indistinguishable from those observed in MS.10 Moreover, the diagnosis of CNS inflammatory vasculopathies remains challenging due to the lack of well‐defined diagnostic criteria and to the relatively high risk and limited accuracy of the available diagnostic techniques (biopsy and/or angiography), especially when involvement is limited to small vessels. Remarkably, despite some differences in brain lesion size (usually smaller) and location (mainly subcortical), the two disease groups cannot be efficiently discriminated by these radiological markers. A large proportion of our inflammatory vasculopathy patients fulfilled the dissemination in space MRI criteria for MS (52% and 26% using Polman and Filippi 2016, MAGNIMS criteria, respectively7, 28).
What is the value of adding the central vein assessment to the diagnostic workup? We found that the percentage of perivenular lesions was higher in every MS case than in any of the inflammatory vasculopathy cases, rendering the diagnostic accuracy of this marker outstanding. However, although the previously proposed 40% rule can differentiate MS from small vessel ischemic disease with high specificity and accuracy,17 in our cohort 4 inflammatory vasculopathy patients (all with Behçet disease) had ≥40% perivenular lesions. Based on these results, we propose a new 50% rule for the workup of MS versus vasculitis. In this setting, the 50% rule performed better than other proposed criteria, namely the 6‐lesion and 3‐lesion rules,20, 29 which avoid the requirement for analysis of every single lesion. In our cohort, those criteria, relative to the Polman or Filippi 2016 MAGNIMS criteria for MS,7, 28 had better specificity but worse sensitivity for MS diagnosis.
Although the perivenular topography of MS lesions is well known and is considered a pathological hallmark of the disease, much less is known about the perivascular nature of parenchymal WM lesions in inflammatory vasculopathies. The immunopathogenesis of MS lesion formation is believed to follow a classical inflammatory cascade, where primed lymphocytes and monocytes crawl and extravasate at the venular side of the microcirculation, perhaps due in part to lower hemodynamic shear pressure on the venous side. As a consequence, inflammatory demyelination spreads in the parenchyma surrounding small parenchymal venules.30, 31 Conversely, in both CNS‐isolated and systemic inflammatory vasculopathies, the pathogenic mechanism of the parenchymal WM lesions is different from MS. These inflammatory conditions affect medium and small vessels, usually arteries, and are pathologically characterized by inflammatory infiltrates of the vessel wall, fibrinoid necrosis, and thrombosis with ischemic damage of the CNS parenchyma.32 Microthromboembolic events and accelerated small vessel disease have also been extensively described and are thought to contribute to chronic ischemic damage occurring at the arteriolar side of cerebral microcirculation.11, 33, 34
In our cohort, among all the inflammatory vasculopathies, patients with Behçet disease showed the highest frequency of perivenular brain lesions (range = 11–50%). This is not surprising; previous pathological observations reported that inflammation, in Behçet disease, can involve both arteries and veins and that, similarly to MS, brain parenchymal damage can be associated with perivenular lymphocyte cuffing.35 Based on the limited available data, a differential diagnosis based on the presence of perivenular lesions should be applied cautiously in Behçet disease.36
Of note, the perivenular lesion frequency observed in the subgroup of inflammatory vasculopathies fulfilling the McDonald MRI criteria for MS7 and without any history of stroke events, was significantly lower compared to MS. Thus, even in this challenging clinical scenario, the separation between the two groups based on the perivenular imaging biomarker remained complete. This is particularly relevant because misdiagnosis is frequent in this subset of patients and becomes even more frequent when the vasculopathic process is confined to the CNS (PACNS).5, 6, 9 Based on our results, we can speculate that the central vein sign may help in the clinical workup of PACNS patients presenting with an MS‐like clinical course.
This study presents some limitations. Our analysis was limited to the brain and did not consider the spinal cord in the assessement of dissemination in space MRI criteria for MS. Of note, in vivo imaging reports of the central vein sign in the spinal cord are lacking, due in part to the challenge of obtaining high‐quality T2*‐weighted images of the cord.14 Patients with primarily confluent lesions were excluded from the study, and therefore it may not be possible to generalize our results to such patients. In addition, in most of the inflammatory vasculopathy patients, possible co‐occurrence of NMOSD was excluded only by the absence of typical syndromes or MRI characteristics and not by NMO‐Ig testing. Another limitation relies on the clinical applicability of the 50% rule proposed here. Although in our cohort the diagnostic accuracy was outstanding, applying this rule requires time‐consuming lesion counting and frequency estimation, both of which are difficult to implement in a clinical setting. In addition, our results concern a specific clinical setting (ie, MS vs CNS inflammatory vasculopathies), and the 50% rule proposed here may apply differently in terms of diagnostic accuracy when dealing with other specific clinical situations (incidental WM lesions, small vessel disease, migraine).
In conclusion, the central vein assessment provided by susceptibility‐based MRI is a useful tool when attempting to differentiate MS from inflammatory vasculopathies involving the CNS. These latter conditions are sometimes difficult to diagnose accurately, as they can have clinical and radiological presentations very similar to MS. Thus, when evaluating patients with chronic brain inflammatory conditions, the addition of the central vein sign assessment to the existing clinical and radiological workup can reduce the risk of misdiagnosis and aid therapeutic strategies. Moreover, considering the availability of this kind of assessment at clinical field strength (including 1.5T MRI scanners), future implementation of automated imaging postprocessing techniques (ie, automated FLAIR* reconstruction and central vein sign detection) should allow direct translation of the central vein sign into the everyday clinical practice.
Author Contributions
Conception and design of the study: P.M., M.A., L.M. Acquisition and analysis of data: P.M., M.A., M.G., L.V., G.E., G.C., G.S., A.B., A.M.R., L.E., D.P., V.M., R.S., N.S., G.P., P.S., B.D. Drafting the text and/or preparing the figures: P.M., M.A., L.V., D.S.R., M.F., L.M.
Potential Conflicts of Interest
Nothing to report.
Acknowledgment
P.M. is supported by the ECTRIMS Clinical Training Fellowship Program. M.A. is supported by a National Multiple Sclerosis Society fellowship award (#FG 2093‐A‐1) and holds a Marilyn Hilton Award for Innovation in MS Research from the Conrad N. Hilton Foundation. The MRi activities of the Florence Center were supported by the Magentic Resonance Interdipartimental Center (CIRM) of the University of Florence.
We thank P. S. Kamgang and M. Ferreira for patient recruitment (Brussels), J. Absil and T. Methens for helping with MRI sequence implementation (Brussels), I. Salmon and L. Lebrun for helping with pathological interpretation (Brussels), G. Norato for helping with statistical analysis (NIH National Institute of Neurological Disorders and Stroke), and A. Iadanza for assistance during MRI data acquisition (Milan).
References
- 1. Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–1517. [DOI] [PubMed] [Google Scholar]
- 2. Love S, Louis DN, Ellison DW. Greenfield's neuropathology. 8th ed. Boca Raton, FL: CRC Press, 2008. [Google Scholar]
- 3. Adams CW, Poston RN, Buk SJ. Pathology, histochemistry and immunocytochemistry of lesions in acute multiple sclerosis. J Neurol Sci 1989;92:291–306. [DOI] [PubMed] [Google Scholar]
- 4. Hohlfeld R, Londei M, Massacesi L, Salvetti M. T‐cell autoimmunity in multiple sclerosis. Immunol Today 1995;16:259–261. [DOI] [PubMed] [Google Scholar]
- 5. Scolding NJ, Wilson H, Hohlfeld R, et al. The recognition, diagnosis and management of cerebral vasculitis: a European survey. Eur J Neurol 2002;9:343–347. [DOI] [PubMed] [Google Scholar]
- 6. Scolding N. The differential diagnosis of multiple sclerosis. J Neurol Neurosurg Psychiatry 2001;71(suppl 2):ii9–ii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet 2017;389:1336–1346. [DOI] [PubMed] [Google Scholar]
- 9. Kim SS, Richman DP, Johnson WO, et al. Limited utility of current MRI criteria for distinguishing multiple sclerosis from common mimickers: primary and secondary CNS vasculitis, lupus and Sjogren's syndrome. Mult Scler 2014;20:57–63. [DOI] [PubMed] [Google Scholar]
- 10. Charil A, Yousry TA, Rovaris M, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation.” Lancet Neurol 2006;5:841–852. [DOI] [PubMed] [Google Scholar]
- 11. Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol 2009;66:704–709. [DOI] [PubMed] [Google Scholar]
- 12. Giannini C, Salvarani C, Hunder G, Brown RD. Primary central nervous system vasculitis: pathology and mechanisms. Acta Neuropathol 2012;123:759–772. [DOI] [PubMed] [Google Scholar]
- 13. Schuster S, Bachmann H, Thom V, et al. Subtypes of primary angiitis of the CNS identified by MRI patterns reflect the size of affected vessels. J Neurol Neurosurg Psychiatry 2017;88:749–755. [DOI] [PubMed] [Google Scholar]
- 14. Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol 2016;12:714–722. [DOI] [PubMed] [Google Scholar]
- 15. Campion T, Smith RJ, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol 2017;27:4257–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haacke EM, Mittal S, Wu Z, et al. Susceptibility‐weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol 2009;30:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra‐high‐field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 2011;76:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solomon AJ, Klein EP, Bourdette D. "Undiagnosing" multiple sclerosis: the challenge of misdiagnosis in MS. Neurology 2012;78:1986–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solomon AJ, Corboy JR. The tension between early diagnosis and misdiagnosis of multiple sclerosis. Nat Rev Neurol 2017;13:567–572. [DOI] [PubMed] [Google Scholar]
- 20. Mistry N, Abdel‐Fahim R, Samaraweera A, et al. Imaging central veins in brain lesions with 3‐T T2*‐weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Mult Scler 2016;22:1289–1296. [DOI] [PubMed] [Google Scholar]
- 21. Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol 2015;3:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 24. International Study Group for Behcet's Disease . Criteria for diagnosis of Behcet's disease. Lancet 1990;335:1078–1080. [PubMed] [Google Scholar]
- 25. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome: a consensus and data‐driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 26. Sati P, George IC, Shea CD, et al. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology 2012;265:926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vuolo L, Sati P, Massacesi L, Reich DS. Efficiency of FLAIR* at 1.5T, 3T, and 7T for detecting perivenular lesions in multiple sclerosis (MS). [abstract P479]. Presented at the 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis (2015).
- 28. Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 2016;15:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three‐lesion algorithm. Mult Scler 2017. Aug 1:1352458517726383. doi: 10.1177/1352458517726383. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gaitan MI, Shea CD, Evangelou IE, et al. Evolution of the blood‐brain barrier in newly forming multiple sclerosis lesions. Ann Neurol 2011;70:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maggi P, Macri SM, Gaitan MI, et al. The formation of inflammatory demyelinated lesions in cerebral white matter. Ann Neurol 2014;76:594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuhlmann T, Lassmann H, Brück W. Diagnosis of inflammatory demyelination in biopsy specimens: a practical approach. Acta Neuropathol 2008;115:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bot JC, Barkhof F, Lycklama à Nijeholt G, et al. Differentiation of multiple sclerosis from other inflammatory disorders and cerebrovascular disease: value of spinal MR imaging. Radiology 2002;223:46–56. [DOI] [PubMed] [Google Scholar]
- 34. Bhattacharyya S, Helfgott SM. Neurologic complications of systemic lupus erythematosus, Sjogren syndrome, and rheumatoid arthritis. Semin Neurol 2014;34:425–436. [DOI] [PubMed] [Google Scholar]
- 35. Hirohata S. Histopathology of central nervous system lesions in Behcet's disease. J Neurol Sci 2008;267:41–47. [DOI] [PubMed] [Google Scholar]
- 36. Uygunoglu U, Zeydan B, Ozguler Y, et al. Myelopathy in Behcet's disease: the bagel sign. Ann Neurol 2017;82:288–298. [DOI] [PubMed] [Google Scholar]


