Abstract
Aim
To assess gender differences in ankylosing spondylitis (AS) patients in relation to tumor necrosis factor alpha inhibitor (TNFi) drug survival and occurrence of adverse events in daily practice in a large peripheral hospital.
Method
Retrospective data were collected from AS patients treated with etanercept, infliximab and adalimumab between January 2004 and January 2014. Kaplan–Meier survival curves were conducted to describe the drug survival and occurrence of adverse events in time.
Results
Overall, 122 AS patients (60.7% male) were included over a 10‐year time period, with a mean treatment period of 51 months (1–127 months). In total, 21 (17.2%) patients stopped the TNFi, mainly due to inefficacy (52.4%). Female patients showed a significant shorter treatment period compared to males (33.4 vs. 44.9 months). In addition, female patients switched more between TNFi compared to males (26.9% vs. 16.3%) and had a significantly higher risk at developing infections compared to male patients (26% vs.19%).
Conclusion
Females stayed on the same TNFi for a significantly shorter period compared to males (33.4 vs. 44.9 months) and the most important reason to stop or switch the drug was inefficacy. Moreover, females seemed to be more prone to infections during TNFi treatment than males.
Keywords: ankylosing, spondylitis, gender, survival, tumor necrosis factor
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease, which belongs to a spectrum of diseases named axial spondyloarthritis (SpA) with a prevalence of 0.1–1.4%.1 AS manifests between the 15th and 40th year of life with a male to female ratio of 3 : 1.2 The most characteristic symptom is inflammatory back pain. AS can lead to structural and functional impairments and work disability.3
Treatment of AS mainly consists of non‐steroidal anti‐inflammatory drugs (NSAIDs) combined with exercise treatment.4 In case of persistent disease activity, disease‐modifying anti‐rheumatic drugs (DMARDs) have limited effect on axial manifestations,5 but tumor necrosis factor alpha inhibitors (TNFi) are very effective. Responses with TNFi were achieved in more than 60% of patients, and over a third of the AS patients achieved partial remission.6, 7, 8, 9, 10
However, these results indicate that 40% of the patients have an insufficient response to TNFi, which could be due to patient characteristics such as age and gender. A previous meta‐analysis of several clinical randomized trials with etanercept revealed that female AS patients not only had a lower level of response (AS Disease Activity Score) to TNFi, but also showed a higher percentage of non‐responders compared to male patients.11 In addition, females stopped the TNFi more often compared to male AS patients.12 The question arises whether these gender differences in response and non‐adherence to TNFi are also found in daily clinical practice. Furthermore, the risk of side effects, such as infections, is well known and seems more prevalent in female AS patients.13, 14
In the Netherlands, all AS patients are treated in the same way according to the guideline of the Dutch Society of Rheumatologists,15 but complex AS patients are mainly treated in academic hospitals. In addition, most clinical trials, which have stricter inclusion criteria for TNFi treatment are, mainly, conducted in academic centers, in contrast to the peripheral hospitals. Therefore, the AS population treated in general hospitals will differ from the university medical centers. Further, data on gender differences in infection rates during TNFi treatment in AS in daily practice in non‐academic hospitals are lacking.16, 17
The primary aim of this study was to compare gender differences in long‐term drug survival of TNFi treatment in AS patients in daily practice in a large peripheral center.The secondary aim was to assess the occurrence of side effects, such as infections and malignancies within this population.
Materials and Methods
For this retrospective study, all AS patients (fulfilling the modified New York criteria)18 in a large peripheral hospital, ‘Kennemer Gasthuis’, who received TNFi treatment between January 2004 and January 2014 were eligible. Patients classified as AS were selected from the hospital diagnoses registry and checked in the Hospital Pharmacy database for TNFi treatment. The diagnosis of AS was verified by an independent rheumatologist using the electronic patient records.
Data of patients on the TNFi etanercept, adalimumab and infliximab were included, since these biologicals were used for many years and sufficient data were available for a reliable analysis, in contrast to other types of TNFi. Patients were excluded when data on the start of treatment with TNFi were missing.
The following data were collected from the electronic patient files and the Hospital Pharmacy database: (i) patient characteristics (age, sex, disease duration, presence of human leukocyte antigen [HLA]‐B27); and (ii) characteristics of TNFi treatment (dosage, type of TNFi used start/stop or switch date of the drug and the reasons for stopping or switching). In addition, data on side effects such as infections (including the use of antibiotics), the occurrence of malignancies and co‐medication (NSAIDs, DMARDS or phenylbutazone) were obtained.
Patients who used TNFi during the 10‐year observation period (2004–2014) could have had multiple treatment episodes, since they could have switched between several TNFi. A treatment episode was defined as the start and stop date of a single TNFi treatment. For instance, patients who used in total of two types of TNFi had two starting dates and therefore two treatment episodes. The data for survival time were calculated per drug as cumulative treatment survival. Treatment interruptions and associated reasons for interruptions were recorded. In case of treatment interruptions > 6 months, the drug survival data were used until the date of treatment interruption.
Statistics
Kaplan–Meier survival curves were used to display the drug survival and adverse events during the treatment period of the patients. Log rank tests and Cox regression analysis were performed to compare the three treatments. To compare gender differences, independent t‐tests and χ2 tests were used. Possible confounders and effect modifiers, such as age and gender, were tested with a multiple Cox regression analysis. Based on the literature, risk factors for drug survival such as gender and age (≤ 40 years/> 41 years) were assessed along with side effects by comparison of the dichotomous variables.
Results
Population
In total 223 consecutive AS patients (60.5% male) were identified from the hospital registration database, of whom 122 patients (54.7%) used TNFi (Table 1) and 95 patients did not (36 ([38%] female and 59 [62%] male). Six patients had insufficient data for follow‐up.
Table 1.
Baseline characteristics of the included AS patients (N = 122), specified for gender
| Overall | Female | Male | P‐value (if possible 95% CI) | |
|---|---|---|---|---|
| Population, n (%) | 122 (100) | 48 (39.3) | 74 (60.7) | |
| Mean age in years (range) | 43.5 (17–75) | 43.7 (17–75) | 43.4 (20–68) | 0.88 (−4.8–4.1) |
| HLA‐B27+ (%) | 91.7 | 89.6 | 93.2 | 0.24 |
| TNFi treatment, n (%) | 0.02a | |||
| Adalimumab | 95 (59.7) | 19 (28.4) | 27 (29.3) | |
| Etanercept | 46 (28.9) | 35 (52.2) | 60 (65.2) | |
| Infliximab | 18 (11.3) | 13 (19.4) | 5 (5.4) | |
| Co‐medication used n (%) | 0.69 | |||
| NSAIDs | 51 (41.8) | 19 (39.6) | 32 (43.2) | |
| DMARDs | 12 (9.8) | 5 (10.4) | 7 (9.5) | |
| Phenylbutazone | 6 (4.9) | 2 (1.6) | 4 (3.3) |
Except indicated otherwise, values were presented as n (%); TNFI, tumor necrosis factor α inhibitor; HLA‐B27+, presence of human leukocyte antigen B27; AS, ankylosing spondylitis; NSAIDs, non‐steroidal anti‐inflammatory drugs; DMARDs, disease‐modifying anti‐rheumatic drugs, methotrexate and sulfasalazine.
Significant difference.
Demographic baseline characteristics showed a male predominance (60.7%) and a mean age of 43.5 years (17–75 years; Table 1). More male patients were HLA‐B27 positive (83.6%). Significantly more female patients used infliximab (19.4%) and male patients used etanercept more often (65.2%; Table 1).
Drug survival
After starting TNFi treatment, the mean follow‐up was 5.1 years (0.1–10.6 years). Censored data showed that 119 patients (97.5%) had 6 months of follow‐up, 114 patients (93.4%) 1 year, 84 patients (68.9%) 3 years, 61 patients (50%) 5 years, 34 patients (27.9%) 8 years and four patients (3.3%) had 10 years of follow‐up data.
The retrospective data analysis over 10 years showed the highest survival rate in etanercept (85.3% after 3.7 years), followed by adalimumab (76.1% after 2.6 years). Infliximab showed the lowest survival rate. However, if patients responded to the treatment, they stayed on the drug for a long time (4.7 years).
Of the 122 patients, 101 patients (82.8%) were still treated with a TNFi at the end of the observation time, of whom 90 patients were still on their first TNFi. In total, 21 patients (17.2%) stopped the treatment without starting a new one. Overall, 32 patients (26.2%) switched to another TNFi, of whom 20 patients continued the second TNFi (62.5%), four patients switched to a third TNFi (12.5%) and eight patients stopped the first TNFi treatment without starting a new one. Among the patients who started a third TNFi, three patients continued the treatment and one patient stopped the treatment with TNFi.
The most important reason for discontinuation was inefficacy (in 21 patients; 52.4%). The second reason was the occurrence of side effects, of which infections (mostly recurrent) were the most frequent. Malignancies were not reported. Furthermore, there were no significant gender differences in reasons for discontinuation.
Female patients had a significantly lower treatment survival: 33.4 versus 44.9 months (P = 0.031; 95%CI: 1.1–22; Fig. 1). At approximately 2.5 years of TNFi treatment a rapid decrease in drug survival was shown in female patients, while male patients stayed on treatment. Although not significant, females stopped TNFi more frequently compared to male patients (20.8% vs. 14.9%).
Figure 1.
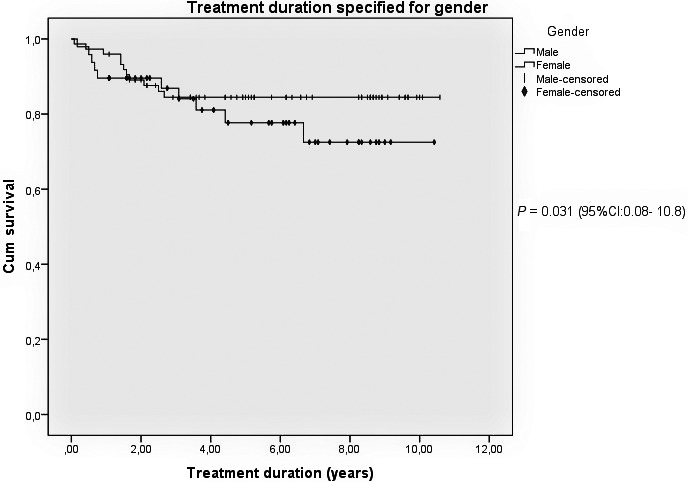
Kaplan–Meier survival curve for anti‐tumor necrosis factor (anti‐TNF) treatment duration specified for gender. Overview of treatment adherence, including switches, specified for gender.
In total 32 patients (26.2%) switched treatment, of which female patients switched more frequently compared to male patients (26.9% vs. 16.3%). The most important reasons for switching in both males and females were inefficacy (72.7%) and side effects (21.2%).
Patients on etanercept showed the highest number of switches within the first 20 months (12%), while the number of patients on adalimumab and infliximab who switched were more dispersed. The most common switch was from etanercept to adalimumab (17.2%).
Logistic regression analysis indicated that being positive for HLA‐B27 and the use of co‐medication were predictors for switching.
During the three different TNFi treatments, 31 treatment interruptions shorter than 6 months and four treatment interruptions longer than 6 months occurred. Interruptions shorter than 6 months were mainly caused by mild infections, like ear, nose and throat infections.
Interruptions longer than 6 months were mainly caused by attempts to stop the drug permanently (in consultation between patient and physician), because the patients did not experience any disease‐related symptoms any more, or because of pregnancy (one case).
In case of attempts to stop the drug, all three patients experienced an exacerbation of the disease after a median period of 8 months (6–10 months). The mean interruption duration was 7.7 months (6–10 months).
Side effects
During the different treatment episodes, 40 infections (25%) were registered in 38 patients, of which 19 were marked as serious infections, because they necessitated antibiotic treatment (based on patient‐reported outcome) mainly of the respiratory tract. In only one case hospitalization was needed due to a Staphylococcus aureus infection of a knee prosthesis.
Side effects which required the permanent discontinuation of TNFi treatment were mainly recurrent infections of the throat, nose and ears (patient‐reported outcomes). Some gastro‐intestinal infections and skin reactions, mainly local reactions, but also a total body skin rash, occurred less often.
Both sex and age were risk factors associated with infection risk. Females had a 26.1% chance on developing infections compared to 18.7% for males (hazards ratio [HR] females 2.15; 95%CI: 1.1–4.0). The age group > 41 years showed a significantly higher risk for infections (HR 1.1; 95%CI: 1.0–1.1) compared to younger patients.
Although not significant, 15 out of the 36 patients (41.7%) using NSAIDs as co‐medication developed an infection during TNFi treatment compared to 21 out of 75 patients (28%) who did not use NSAIDs. In this study no malignancies were diagnosed during TNFi treatment.
Discussion
Analysis of AS patients treated with TNFi in a peripheral hospital in daily clinical practice showed that the majority of patients (81.3%) still used one of the three TNFi after 4 years. Interestingly, females showed a significantly shorter treatment duration compared to males (33.4 vs. 44.9 months) and switched and stopped the drugs more often.
Some studies found similar results on treatment discontinuation, but not with specific details about the gender differences in treatment survival as in our study. Several studies described female gender only as a baseline predictor for early TNFi treatment discontinuation.12, 19, 20 However, these studies, as in our own study, could not give a clear answer for the exact reason for this gender difference in treatment survival.
In addition female AS patients switched more often (27%) to a second or third TNFi than male patients (16%), but this difference was not significant, most likely due to the low number of patients. This finding corresponds with the results of Glintborg et al., who also described that female patients switched between TNFi more often than male patients (33% vs. 22%). This might imply that TNFi treatment is less effective in female AS patients compared to male patients.21 Overall, in this cohort 21% of the AS patients switched between types of TNFi, which is low in comparison to the DANBIO registry, where 30% of the 1432 patients treated with the same TNFi treatments switched. This discrepancy in switches could be explained by the smaller size of our study.21
However, these studies did not specifically assess gender differences. The most important reason found for switching in our study was inefficacy, which is in line with several other studies21, 22, 23 and not related in this study to gender differences.
Interestingly, at baseline more female patients were treated with infliximab compared to male patients and infliximab had in this study the lowest survival rate of the three TNFi. We also found that females had a shorter treatment survival compared with males, which may imply an association between female gender and the lower survival rate of infliximab, but the number of patients in this cohort was too small to make reliable analysis of a probable causal relation.
Female AS patients showed an increased risk for infections during treatment compared to males. This finding corresponds with other studies in which female AS patients had a doubled increased risk of developing infections in comparison with males.13, 14 This observation is in contrast with studies in the general healthy population, where male gender was described to be a risk factor for infections.14, 24, 25, 26, 27, 28 There are no clear explanations for this result. Further investigation is needed to clarify the relation between AS, TNFi treatment infections and gender.
Overall, the majority (81.3%) of AS patients treated with TNFi showed a good drug survival over a long follow‐up period (5.1 years), which was higher compared to other studies with the same TNF blockers12, 29 which described 63% and 74% survival rates. Our higher drug survival rate could be explained by the difference in population size (842/243 vs. 122 patients) and the fact that our study was conducted in a peripheral hospital instead of a secondary referral center with probably a selection of less severe patients.
The most important reasons for drug discontinuation were inefficacy (52.4%) and side effects (47.6%), mainly due to mild infections, which corresponds with other studies on the same TNFi.12
Overall, the most important adverse events reported in this study were infections, which was expected since TNFi treatment increases the risk of mild infections.10
Another finding was that patients older than 40 years of age had a significantly increased risk of developing infections, although the reported effect was small, which corresponds with infection rates in many other studies.30, 31, 32 Age was equally distributed for gender, so in this case gender was not considered a confounder.
The retrospective design was a limitation of our study, since the data collection was dependent on information used for clinical practice. However, the retrospective design was also a strength, since we had data access over a long follow‐up period. Furthermore, the data were collected from a peripheral daily practice, instead of a more controlled environment, like secondary referral centers, which provide, in our opinion, a different perspective of TNFi treatment use in AS patients.
It would be interesting for further research to collect more data on smoking and co‐morbidities, which can make patients more vulnerable to infections, but even more important is to assess possible associations of infection rates with gender in order find strategies to increase TNFi drug survival.33, 34, 35, 36, 37, 38
In conclusion, over a mean treatment period of 5.1 years with TNFi, female AS patients showed significantly shorter treatment periods compared to males (33.4 vs. 44.9 months) and permanently stopped the drug more often and seemed to be more prone to infections. Overall, the majority of AS patients (81%) continued TNFi for many years and the most important reason to stop treatment, in both males and females, was inefficacy.
Author contributions
MSc T. Rusman: performed the study, analysis, text preparation. Dr. I.E. van der Horst‐Bruinsma: design, text preparation. Dr. S. ten Wolde: designed, text preparation. Dr. S.M. Euser: advised, reviewed. MSc O. van Hall: advised, reviewed. MSc T. van der Ploeg: analysis, reviewed.
Acknowledgments
Not applicable. There was no financial support for this study.
References
- 1. Calin A, Fries JF (1975) Striking prevalence of ankylosing spondylitis in “healthy” w27 positive males and females. N Engl J Med 293, 835–9. [DOI] [PubMed] [Google Scholar]
- 2. Will R, Edmunds L, Elswood J, Calin A (1990) Is there sexual inequality in ankylosing spondylitis? A study of 498 women and 1202 men. J Rheumatol 17, 1649–52. [PubMed] [Google Scholar]
- 3. Braun J, Sieper J (2007) Ankylosing spondylitis. Lancet 369, 1379–90. [DOI] [PubMed] [Google Scholar]
- 4. Thompson B (2011). Education and learning for people with ankylosing spondylitis. University of Newcastle, Newcaslte: Available from URL: http://hdl.handle.net/10443/1590 [Google Scholar]
- 5. van der Horst‐Bruinsma IE, Clegg DO, Dijkmans BA (2002) Treatment of ankylosing spondylitis with disease modifying antirheumatic drugs. Clin Exp Rheumatol 20(6 Suppl 28), S67–70. [PubMed] [Google Scholar]
- 6. Revicki DA, Luo MP, Wordsworth P et al (2008) Adalimumab reduces pain, fatigue, and stiffness in patients with ankylosing spondylitis: results from the adalimumab trial evaluating long‐term safety and efficacy for ankylosing spondylitis (ATLAS). J Rheumatol 35, 1346–53. [PubMed] [Google Scholar]
- 7. Lord PA, Farragher TM, Lunt M et al (2010) Predictors of response to anti‐TNF therapy in ankylosing spondylitis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 49, 563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paccou J, Bacle‐Boutry MA, Solau‐Gervais E, Bele‐Philippe P, Flipo RM (2012) Dosage adjustment of anti‐tumor necrosis factor‐alpha inhibitor in ankylosing spondylitis is effective in maintaining remission in clinical practice. J Rheumatol 39, 1418–23. [DOI] [PubMed] [Google Scholar]
- 9. van der Heijde D, Schiff MH, Sieper J et al (2009) Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long‐term results from the ATLAS trial. Ann Rheum Dis 68, 922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baraliakos X, van den Berg R, Braun J, van der Heijde D (2012) Update of the literature review on treatment with biologics as a basis for the first update of the ASAS/EULAR management recommendations of ankylosing spondylitis. Rheumatology (Oxford) 51, 1378–87. [DOI] [PubMed] [Google Scholar]
- 11. van der Horst‐Bruinsma IE, Zack DJ, Szumski A, Koenig AS (2013) Female patients with ankylosing spondylitis: analysis of the impact of gender across treatment studies. Ann Rheum Dis 72, 1221–4. [DOI] [PubMed] [Google Scholar]
- 12. Glintborg B, Ostergaard M, Krogh NS, Dreyer L, Kristensen HL, Hetland ML (2010) Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti‐tumour necrosis factor: results from 8 years’ surveillance in the Danish nationwide DANBIO registry. Ann Rheum Dis 69, 2002–8. [DOI] [PubMed] [Google Scholar]
- 13. Zochling J, Bohl‐Buhler MH, Baraliakos X, Feldtkeller E, Braun J (2006) The high prevalence of infections and allergic symptoms in patients with ankylosing spondylitis is associated with clinical symptoms. Clin Rheumatol 25, 648–58. [DOI] [PubMed] [Google Scholar]
- 14. Germano V, Cattaruzza MS, Osborn J et al (2014) Infection risk in rheumatoid arthritis and spondyloarthropathy patients under treatment with DMARDs, corticosteroids and TNF‐alpha antagonists. J Transl Med 12, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NVvR (NVR) . (2011) Richtlijn verantwoord gebruik van biologicals. [PubMed]
- 16. Machado MA, Barbosa MM, Almeida AM et al (2013) Treatment of ankylosing spondylitis with TNF blockers: a meta‐analysis. Rheumatol Int 33, 2199–213. [DOI] [PubMed] [Google Scholar]
- 17. Kvien TK, Heiberg MS, Lie E et al (2005) A Norwegian DMARD register: prescriptions of DMARDs and biological agents to patients with inflammatory rheumatic diseases. Clin Exp Rheumatol 23(5 Suppl 39), S188–94. [PubMed] [Google Scholar]
- 18. van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27, 361–8. [DOI] [PubMed] [Google Scholar]
- 19. Arends S, Brouwer E, van der Veer E et al (2011) Baseline predictors of response and discontinuation of tumor necrosis factor‐alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther 13, R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lorenzin M, Ortolan A, Frallonardo P, Oliviero F, Punzi L, Ramonda R (2015) Predictors of response and drug survival in ankylosing spondylitis patients treated with infliximab. BMC Musculoskelet Disord 16, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glintborg B, Ostergaard M, Krogh NS et al (2013) Clinical response, drug survival and predictors thereof in 432 ankylosing spondylitis patients after switching tumour necrosis factor alpha inhibitor therapy: results from the Danish nationwide DANBIO registry. Ann Rheum Dis 72, 1149–55. [DOI] [PubMed] [Google Scholar]
- 22. Plasencia C, Pascual‐Salcedo D, Garcia‐Carazo S et al (2013) The immunogenicity to the first anti‐TNF therapy determines the outcome of switching to a second anti‐TNF therapy in spondyloarthritis patients. Arthritis Res Ther 15, R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pradeep DJ, Keat AC, Gaffney K, Brooksby A, Leeder J, Harris C (2008) Switching anti‐TNF therapy in ankylosing spondylitis. Rheumatology (Oxford) 47, 1726–7. [DOI] [PubMed] [Google Scholar]
- 24. Klein SL (2000) The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev 24, 627–38. [DOI] [PubMed] [Google Scholar]
- 25. Pennell LM, Galligan CL, Fish EN (2012) Sex affects immunity. J Autoimmun 38 (2–3), J282–91. [DOI] [PubMed] [Google Scholar]
- 26. Bouman A, Heineman MJ, Faas MM (2005) Sex hormones and the immune response in humans. Hum Reprod Update 11, 411–23. [DOI] [PubMed] [Google Scholar]
- 27. Fish EN (2008) The X‐files in immunity: sex‐based differences predispose immune responses. Nat Rev Immunol 8, 737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghazeeri G, Abdullah L, Abbas O (2011) Immunological differences in women compared with men: overview and contributing factors. Am J Reprod Immunol 66, 163–9. [DOI] [PubMed] [Google Scholar]
- 29. Kristensen LE, Karlsson JA, Englund M, Petersson IF, Saxne T, Geborek P (2010) Presence of peripheral arthritis and male sex predicting continuation of anti‐tumor necrosis factor therapy in ankylosing spondylitis: an observational prospective cohort study from the South Swedish Arthritis Treatment Group Register. Arthritis Care Res (Hoboken) 62, 1362–9. [DOI] [PubMed] [Google Scholar]
- 30. Shames RS (2002) Gender differences in the development and function of the immune system. J Adolesc Health 30 (4 Suppl, 59–70. [DOI] [PubMed] [Google Scholar]
- 31. Dorshkind K, Montecino‐Rodriguez E, Signer RA (2009) The ageing immune system: is it ever too old to become young again? Nat Rev Immunol 9 (1), 57–62. [DOI] [PubMed] [Google Scholar]
- 32. Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM (2010) Aging of the innate immune system. Curr Opin Immunol 22, 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raychaudhuri SP, Nguyen CT, Raychaudhuri SK, Gershwin ME (2009) Incidence and nature of infectious disease in patients treated with anti‐TNF agents. Autoimmun Rev 9 (2), 67–81. [DOI] [PubMed] [Google Scholar]
- 34. Feldman C, Anderson R (2013) Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect 67, 169–84. [DOI] [PubMed] [Google Scholar]
- 35. Huttunen R, Heikkinen T, Syrjanen J (2011) Smoking and the outcome of infection. J Intern Med 269, 258–69. [DOI] [PubMed] [Google Scholar]
- 36. Braun J, Sieper J, Zink A (2012) The risks of smoking in patients with spondyloarthritides. Postgrad Med J 88, 617–8. [DOI] [PubMed] [Google Scholar]
- 37. Videm V, Cortes A, Thomas R, Brown MA (2014) Current smoking is associated with incident ankylosing spondylitis – the HUNT population‐based Norwegian health study. J Rheumatol 41, 2041–8. [DOI] [PubMed] [Google Scholar]
- 38. Sakellariou GT, Anastasilakis AD, Kenanidis E et al (2015) The effect of smoking on clinical and radiographic variables, and acute phase reactants in patients with ankylosing spondylitis. Rheumatol Int 35, 2109–14. [DOI] [PubMed] [Google Scholar]


