Abstract
Objectives
The purpose of this anatomic investigation is to (1) establish accuracy of dry needle placement into the medial third of the piriformis muscle as it exits the pelvis from the greater sciatic notch in unembalmed cadaveric specimens, while avoiding puncture of the sciatic nerve, and (2) establish guidelines for dry needle length selection.
Methods
Dry needles were placed in nineteen unembalmed cadaveric posterior hips. Dissection of the posterior hip musculature was performed to confirm location of the needle. A binary decision (yes/no) was made to determine whether the needle reached the piriformis muscle, went through the piriformis muscle, and/or pierced the sciatic nerve. Additionally, mean adipose tissue thickness, gluteus maximus muscle thickness, and perpendicular distance from the needle to the exiting sciatic nerve were recorded.
Results
The needle reached the medial third of the piriformis in 16 out of 19 hips (84.2% accuracy) and never punctured the sciatic nerve. There was a fair (r = 0.493) and good (r = 0.759) correlation between the needle length and the mean fat thickness for the left and right hips, respectively.
Discussion
A physical therapist was able to use bony landmark palpation to locate the piriformis muscle and use estimated adipose tissue thickness to choose a sufficient needle length to reach the medial third of the piriformis muscle. While the needle placement technique was safe and no sciatic nerve puncture occurred, the proximity of the piriformis muscle to the sciatic nerve warrants caution during needle placement.
Level of Evidence
2c
Keywords: Buttock pain, cadaver, dry needle, injection, piriformis, posterior hip pain, sciatic nerve, trigger point
Introduction
When considering dry needling (DN) application into clinical practice settings, clinicians mainly rely on their ability to use palpation to accurately and reliably identify and insert the needle into the target muscle fibers. Investigators attempting to establish the reliability of identifying upper trapezius muscle myofascial trigger points (MTrPs) through palpation has ranged from poor (10–21% concordance) [1] to sufficient (G-coef > = 80%) [2] to high (intraclass correlation coefficient 0.62–0.81) [3]. Despite initial results suggesting that palpation for identification of MTrPs in the upper trapezius may not be reliable, more recent investigations have emphasized that appropriately trained and experienced clinicians can achieve high levels of reliability [1–4]. To date, many of the studies attempting to assess the reliability of palpation have focused on more superficial muscles [5–7] vs. muscles that lie deep to multiple layers of more superficial muscles, i.e. the piriformis muscle.
The piriformis muscle has been implicated as a source of posterior buttock pain and non-discogenic sciatica in Piriformis Syndrome [8,9]. Despite differences in opinions on the pathogenesis of this condition [8,9], there is support that ultrasound-guided local anesthetic and corticosteroid injection can provide pain relief in cases resistant to conservative, non-invasive treatment [10–12]. Misirlioglu [11] concluded that much of the pain in Piriformis Syndrome may be myofascial in origin secondary to no additional pain relief with the addition of corticosteroid to a local intramuscular piriformis anesthetic injection. While there is an absence of literature investigating DN to treat Piriformis Syndrome, clinicians can be formally trained through postgraduate continuing education courses to treat the piriformis muscle without the assistance of ultrasound. Generally, there are two approaches to access the piriformis muscle with DN [13]. The medial approach attempts to access the piriformis muscle as it exits the pelvis from the greater sciatic notch, while the lateral approach attempts to access the piriformis muscle as it inserts onto the posterior greater trochanter [13]. Needle length (50–100 mm) is selected empirically by the clinician based on patient morphology without specific published guidelines.
Likely, one of the most discussed aspects of performing DN is the potential adverse events that could occur in response to the procedure. Most recently, a survey of chartered physiotherapists in Ireland found that the most common adverse event following DN is bruising [14]. The risk for a significant adverse event includes neural puncture [13]. Other authors that reviewed adverse events following acupuncture conclude that most serious adverse events can be prevented by a more thorough understanding of applied clinical anatomy in the treatment areas [15]. Despite theoretical differences between dry needling and acupuncture, the risk of an adverse event is specific to the area being treated, the relevant underlying anatomy that would potentially be punctured, and the depth of needle penetration [15–17]. Some of the specific adverse events could include pneumothorax in the thoracic spine, visceral puncture in the abdominal region, and neural puncture in the posterior pelvis/hip region [15–17].
Another anatomic consideration that will likely be unknown to the clinician is the presence of potential anatomic variation with exit of the sciatic nerve from the greater sciatic notch and the relationship to the piriformis muscle. There have been six potential routes described that the sciatic nerve can take relative to the piriformis muscle: (1) Undivided nerve below undivided muscle; (2) Divisions of nerve between and below undivided muscle; (3) Divisions above and below undivided muscle; (4) Undivided nerve between heads; (5) Divisions between and above heads; and (6) Undivided nerve above undivided muscle [18]. With the undivided sciatic nerve being considered the normal relationship between the exiting sciatic nerve and the piriformis muscle, recent cadaveric investigations have found the prevalence to range from 89 [19] to 92% [20]. These same investigators have demonstrated the presence of abnormal sciatic nerve and piriformis muscle relationships to range from 8 [20] to 11% [19], while a systematic review and meta-analysis found the prevalence to be as high as 16% [21].
Consequently, it is imperative that with instruction in DN technique, accurate identification via surface palpation is needed to not only access the target muscle(s) fibers, but avoid any unintended adverse events. Most instruction related to DN techniques is attained through postgraduate continuing education courses. Unfortunately, there is no agreed-upon technique, with many varied approaches proposed to reach target muscles. This begs the question whether the techniques taught and clinically incorporated allow the clinician to accurately reach the target muscle without threatening surrounding neural, vascular or visceral structures. To date, only three investigations have attempted to validate DN placement in the lateral pterygoid, the lumbar multifidus, and the cervical multifidus muscles [22–24]. Mesa-Jiménez et al. [22] validated a proposed dry needling approach to reach the lateral pterygoid, using two fresh cadaveric heads. Hannah, et al. [23] compared the accuracy of two proposed dry needle insertion angles to reach the lumbar multifidus in embalmed cadavers. The investigators found that an inferior medial approach would target the multifidus muscle at the lamina of the vertebra below the insertion site, while a posterior–anterior approach would target the multifidus at the lamina of the vertebra of the same level as the insertion site [23]. Most recently, Fernández-de-las-Peñas et al. utilized ultrasonography to validate dry needle placement into the cervical multifidus at C3-4 in five patients with mechanical neck pain and two fresh cadavers [24]. Mesa-Jiménez et al. [22] focused primarily on accuracy of needle placement in the lateral pterygoid muscle without specific mention of potential adverse events. Both Hannah et al. [23] and Fernández-de-las-Peñas et al. [24] briefly addressed the possibility of lumbar subarachnoid space puncture and broaching underlying cervical spinal structures, respectively, but possible adverse events were not a stated primary objective in either investigation. In light of this void in the evidence focusing on both safety and accuracy of dry needle placement, a study is needed that validates proper dry needle placement in the muscles of deep body regions that make visual inspection and manual palpation difficult.
Clinicians desiring to target the myofascial trigger point within the muscle belly to elicit a local twitch response [25–28] advocate a medial approach to target the piriformis muscle belly [13], whereas a lateral approach may increase the likelihood of hitting the piriformis tendon. While no deleterious effects have been documented of dry needling to a tendon, it increases the risk of missing the myofascial trigger point within the muscle belly. No study to date has investigated the accuracy and safety of such method. Therefore, the primary purpose of this anatomic investigation was to establish the accuracy of DN placement into the medial third of the piriformis muscle as it exits the pelvis from the greater sciatic notch in unembalmed cadaveric specimens, while avoiding puncture of the sciatic nerve. A secondary purpose was to establish guidelines for dry needle length selection.
Methods
Cadaveric specimens were handled in accordance with Texas Tech University Health Sciences Center university policy and State of Texas regulations as determined by the Texas State Anatomical Board. Using unembalmed cadavers placed in the prone position, the principal investigator (PI) used surface anatomy as described by Reichert [29] to identify the piriformis location (Figure 1). The needle insertion site was then selected immediately lateral to the most lateral border of the sacrum, over where the piriformis muscle exits the greater sciatic notch. The angle of insertion of the dry needle was perpendicular to the contour of the posterior pelvis, angling toward the symphysis pubis [13] (Figure 2). Prior to pursuing in vivo investigation, safety must be established and, consequently, only the PI inserted the dry needles. In order to ensure a secure placement of the dry needle to prevent any migration of the dry needle during tissue dissection, the following process was used. The PI, a physical therapist who is a Board Certified Orthopaedic Clinical Specialist and a Fellow of the American Academy of Orthopaedic Manual Physical Therapists with 15 years of musculoskeletal care and 7 years of dry needling experience inserted all needles. The skin surrounding each target location for needle insertion was thoroughly cleaned with isopropyl alcohol and dried. A small piece of cheese cloth was adhered to the skin using superglue. A dry needle was then inserted through the cheese cloth into the target location. The length and gauge of the needle used was chosen pragmatically by the PI based on the size of the cadaver and estimated depth of penetration required to reach the piriformis. The advancement of each needle was stopped once a depth of penetration was attained that the PI estimated was sufficient to reach the piriformis, or ceased once 10 mm of the needle remained exposed outside of the buttock. Only one needle could be used on each buttock due to the method used to fixate the needle with superglue and cheese cloth. Once the needle was placed, a plastic needle guide tube filled with superglue was placed over the needle to enhance stability. To further prevent needle disruption, the dissection of the posterior hip musculature preserved the tissue immediately surrounding the secured needle. The gluteus maximus was dissected down to the deepest layer of hip rotators and reflected laterally (Figure 3). Once this was completed, the remaining tissue that had been preserved surrounding each needle was carefully dissected until the needle was located. Once the needle was located, further dissection exposed the piriformis and sciatic nerve to validate whether the dry needle was accurately placed (i.e. reached the medial third of the piriformis muscle as intended) and whether neural puncture occurred (Figure 4).
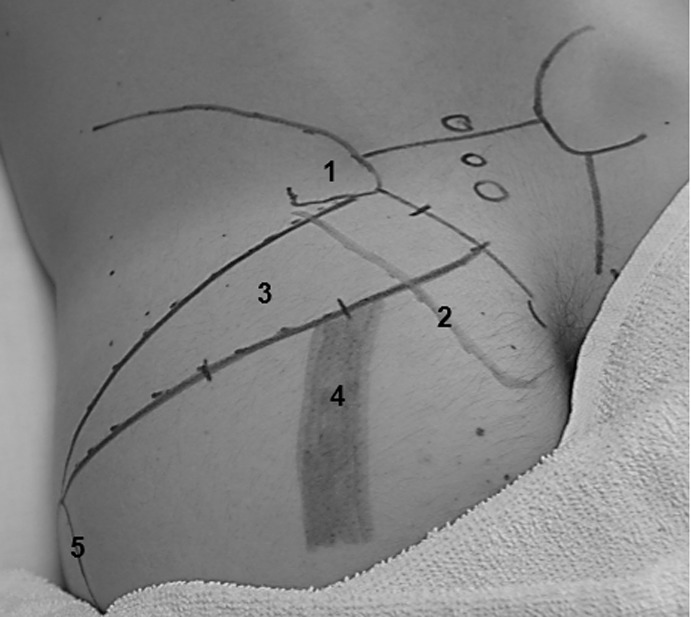
Figure 1.
Posterior hip surface anatomy. 1 – Posterior Superior Iliac Spine; 2 – Lateral border of sacrum; 3 – Piriformis; 4 – Sciatic Nerve; 5 – Greater Trochanter.
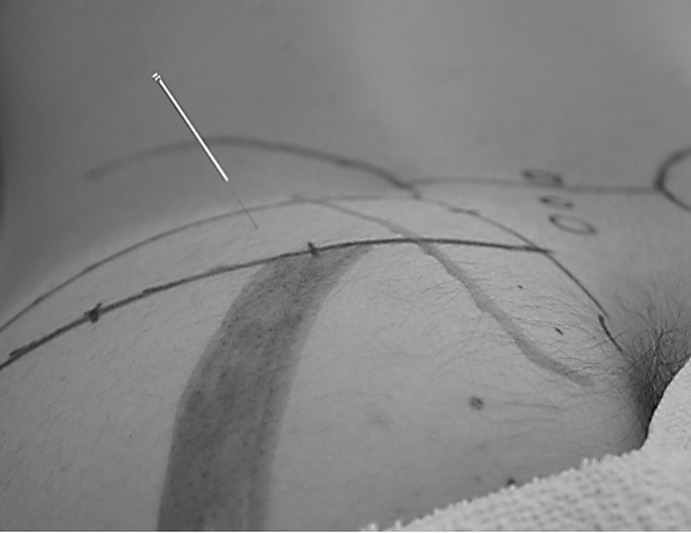
Figure 2.
In-vivo needle insertion.
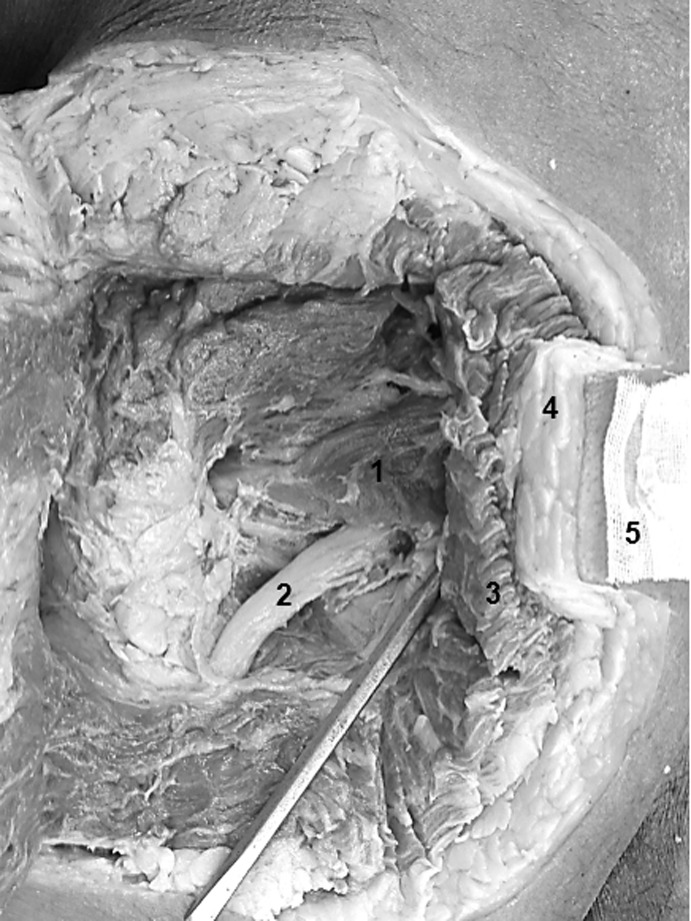
Figure 3.
Left hip with dissection down to the piriformis. 1 – Piriformis muscle, 2 – Sciatic nerve, 3 – Gluteus Maximus, 4 – Adipose tissue, 5 – Needle fixated with superglue and cheese cloth.
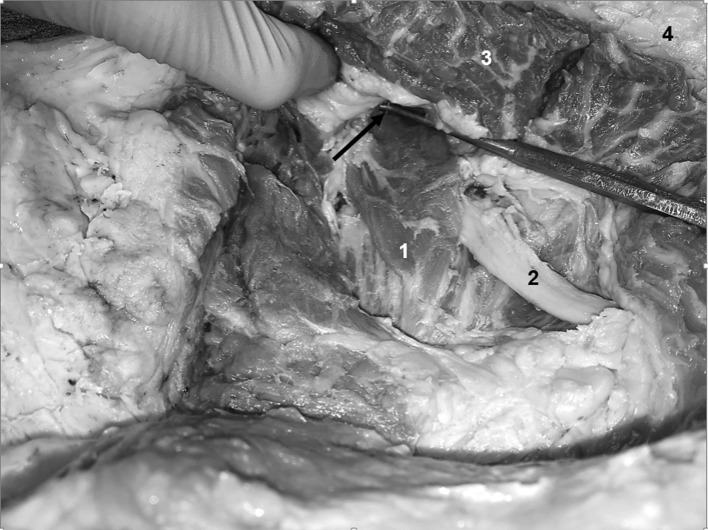
Figure 4.
Left hip with dissection (needle visible in piriformis). Arrow – pointing to needle in piriformis, 1 – Piriformis muscle, 2 – Sciatic nerve, 3 – Gluteus Maximus, 4 – Adipose tissue.
A binary decision (Yes vs. No) was used to evaluate whether the dry needle (1) reached the piriformis muscle, (2) went through the piriformis, and (3) pierced the sciatic nerve. Preferably, while dry needling, it is desired to reach the piriformis without puncturing through the piriformis or piercing the sciatic nerve. Additionally, a digital caliper was used to measure the perpendicular distance between the dry needle placed in the medial piriformis to the sciatic nerve as it exited caudal to the piriformis, the adipose tissue thickness and gluteus maximus thickness overlying the insertion point of the needle.
Results
A total of ten cadaveric specimens including five women and five men (mean age: 70.6 [SD = 13.5], mean height: 165.4 cm [SD = 7.8], mean estimated weight: 74.8 kg [SD = 11.5], and mean estimated BMI: 27.3 [SD = 3.5]) were obtained with 19 posterior hips used for data collection (Table 1). One hip was used as a pilot to ascertain the most efficacious dissection method that would best preserve the tissue surrounding the needle. The 0.35 (diameter) × 75 mm (length) needles were used in 15 hips, while 0.35 × 100 mm needles were used in the remaining four hips based on a clinical decision of the PI regarding what he would have done in the clinical setting with a patient of similar morphology. The needle reached the medial third [18] of the piriformis muscle in 16 out of 19 hips for a total accuracy of 84.2%. Of the three attempts that failed to reach the piriformis, two needles were not inserted to a sufficient depth. The final needle that did not reach the piriformis penetrated deep enough, however, missed the piriformis muscle cranially. In all three instances of missing the piriformis, a 0.35 × 75 mm needle was used.
Table 1.
Cadaveric descriptive statistics.
| Gender | Age (years) | Height (Cm) | Weight (kg) | BMI | Side | Needle (Gauge × Length) (mm) | Fat Thickness (mm) | Glut Max Thickness (mm) | Needle Went Into Piriformis | Needle Pierced Through Piriformis | Distance Piriformis To Sciatic (mm) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | F | 76 | 162.56 | 63.49 | 24.03 | R | 0.35 × 75 | 19.77 | 9.10 | Yes | No | 30.64 |
| Subject 2a | M | 76 | 167.64 | 65.76 | 23.40 | L | 0.35 × 75 | 17.37 | 17.33 | Yes | No | 27.13 |
| Subject 2b | M | 76 | 167.64 | 65.76 | 23.40 | R | 0.35 × 75 | 20.24 | 16.99 | Yes | Yes | 31.23 |
| Subject 3a | F | 75 | 167.64 | 61.22 | 21.79 | L | 0.35 × 75 | 30.77 | 14.45 | Yes | No | 19.41 |
| Subject 3b | F | 75 | 167.64 | 61.22 | 21.79 | R | 0.35 × 75 | 25.12 | 18.32 | Yes | No | 20.03 |
| Subject 4a | M | 75 | 180.34 | 90.7 | 27.89 | L | 0.35 × 100 | 28.62 | 16.67 | Yes | No | 36.76 |
| Subject 4b | M | 75 | 180.34 | 90.7 | 27.89 | R | 0.35 × 100 | 32.37 | 19.73 | Yes | No | 18.60 |
| Subject 5a | F | 94 | 152.4 | 61.22 | 26.36 | L | 0.35 × 75 | 20.26 | 13.83 | Yes | No | 33.17 |
| Subject 5b | F | 94 | 152.4 | 61.22 | 26.36 | R | 0.35 × 75 | 18.89 | 14.22 | No | No | n/a |
| Subject 6a | M | 72 | 170.18 | 81.63 | 28.19 | L | 0.35 × 75 | 18.7 | 29.8 | No | No | n/a |
| Subject 6b | M | 72 | 170.18 | 81.63 | 28.19 | R | 0.35 × 75 | 20.58 | 23.72 | Yes | No | 25.44 |
| Subject 7a | F | 72 | 165.1 | 90.7 | 33.28 | L | 0.35 × 100 | 25.38 | 19.97 | Yes | Yes | 28.23 |
| Subject 7b | F | 72 | 165.1 | 90.7 | 33.28 | R | 0.35 × 100 | 25.16 | 18.89 | Yes | Yes | 21.89 |
| Subject 8a | M | 67 | 170.18 | 81.63 | 28.19 | L | 0.35 × 75 | 22.78 | 16.65 | Yes | No | 26.80 |
| Subject 8b | M | 67 | 170.18 | 81.63 | 28.19 | R | 0.35 × 75 | 22.63 | 23.84 | Yes | No | 22.40 |
| Subject 9a | M | 62 | 157.48 | 77.1 | 31.09 | L | 0.35 × 75 | 22.52 | 24.06 | Yes | No | 35.64 |
| Subject 9b | M | 62 | 157.48 | 77.1 | 31.09 | R | 0.35 × 75 | 23.07 | 25.19 | Yes | No | 17.46 |
| Subject 10a | F | 63 | 160.02 | 74.83 | 29.23 | L | 0.35 × 75 | 22.07 | 17.67 | No | No | n/a |
| Subject 10b | F | 63 | 160.02 | 74.83 | 29.23 | R | 0.35 × 75 | 23.41 | 17.04 | Yes | No | 17.29 |
| Mean +/− SD | – | 70.6 +/− 13.45 | 165.4 +/− 7.8 | 74.8 +/− 11.46 | 27.3 +/− 3.54 | – | – | 23.2 +/− 4.02 | 18.8 +/− 4.83 | – | – | 25.8 +/− 6.47 |
There were three hips where the needle penetrated through the piriformis muscle. While not the desired outcome, the potential for penetration through the piriformis muscle increases the likelihood for sciatic nerve puncture as it can course ventral to the medial third of the piriformis muscle. In two cases, a 0.35 × 100 mm needle was utilized, whereas the other case used a 0.35 × 75 mm needle. Only the terminal tip of the needle in all three hips was visible and palpable on the deep surface of the piriformis muscle. In these three instances, the needle did not advance far enough to puncture the sciatic nerve. The location of the needle in the piriformis muscle relative to the course of the sciatic nerve would have allowed for possible neural puncture if the needles would have been advanced deeper. However, there was no instance where the needle penetrated the sciatic nerve. The perpendicular distance from the needle in the piriformis to where the sciatic nerve emerged from beneath the caudal border of the piriformis (mean distance: 25.8 mm, range: 16.8–37.6 mm) was recorded in all instances of the needle reaching the piriformis muscle.
Pearson’s correlation coefficient (r) between the needle length and mean gluteus maximus thickness (Left hip: −0.069; Right hip: 0.066), mean fat thickness (Left hip: 0.493; Right hip: 0.759), and estimated BMI (Left hip: 0.482; Right hip: 0.482), respectively, were calculated. While most of the r values indicated no correlation, there was a fair correlation (r = 0.493) and a good correlation (r = 0.759) between the needle length chosen and the mean fat thickness for the left and right hip, respectively (Table 2).
Table 2.
Descriptive statistics.
| Mean | SD | Range | 95% CI |
Skewness | Kurtosis | Shapiro–Wilk |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Statistic | df | Sig | ||||||
| Fat thickness (mm) | 23.47 | 4.51 | 17.7–32.4 | 19.70 | 27.24 | 0.524 | −0.776 | 0.95 | 8 | 0.715 |
| Glut max thickness (mm) | 16.51 | 4.41 | 16.2–33.6 | 12.82 | 20.19 | 0.086 | 0.982 | 0.972 | 8 | 0.914 |
| Perpendicular distance: Piriformis to sciatic nerve (mm) | 29.72 | 5.62 | 17.3–36.8 | 25.02 | 34.42 | −0.584 | 0.341 | 0.949 | 8 | 0.705 |
| Estimated BMI | 27.00 | 3.92 | 21.8–33.3 | 23.72 | 30.28 | 0.323 | −0.818 | 0.966 | 8 | 0.863 |
| Age | 70.60 | 13.45 | 48–94 | 60.98 | 80.22 | −0.475 | 0.867 | 0.853 | 10 | 0.062 |
| Height (cm) | 165.35 | 7.80 | 152.4–180.34 | 159.77 | 170.93 | 0.228 | 0.637 | 0.975 | 10 | 0.934 |
| Weight (kg) | 74.83 | 11.46 | 61.2–90.7 | 66.63 | 83.03 | 0.129 | −1.519 | 0.895 | 10 | 0.194 |
Discussion
Despite differing opinions as to the pathogenesis, diagnostic criteria, and most effective management of Piriformis Syndrome [8, 9, 29, 30], needle-based therapies [31–34], including dry needling [13] are commonly used to provide pain relief. While physicians utilize fluoroscopy [33], Computed Tomography [12], Ultrasound [8, 34], Electromyography [30, 33, 35], or a combination [31, 33] thereof, to guide their injections and confirm location in the piriformis while avoiding puncture of the sciatic nerve, health care providers performing dry needling frequently do not have these tools at their disposal.
The concern with needle therapies of deeper muscles that are not directly palpable is accuracy of location and, more importantly, avoiding puncture of other surrounding structures. Physical therapists implementing dry needling of the piriformis are essentially performing a blind needle insertion. Clinicians use bony landmarks [13, 35] to locate the piriformis and choose the length of needle via estimation of the depth of penetration required to reach the piriformis. Currently, there are no published accepted guidelines for choosing the correct needle length.
This is the first study investigating the accuracy and safety of dry needling targeting the piriformis as it exits the pelvis via the greater sciatic notch using a systematic surface anatomy approach [35] combined with a pragmatic approach of needle length selection based on cadaver morphology. Our investigation demonstrated that using the dry needle technique advocated by Dommerholt [13] resulted in 84.2% accuracy reaching the medial piriformis muscle and 100% rate of safety as the needle reached the sciatic nerve in no instance. Both the 0.35 × 75 mm needles and 0.35 × 100 mm needles were found to be safe in this cadaveric sample with a mean estimated BMI of 27.3 (range: 21.8–33.3). The PI inserted the needles into the buttock area leaving at least 10 mm of needle length outside the buttock as performed in clinical practice to allow retrieval of the needle post-needling treatment. Further research investigating longer needles and different depth of penetration of each needle would be valuable to standardize this process.
In two of the three instances where the needle did not reach the piriformis muscle, the needle would have reached the piriformis muscle, if the needle had been advanced deeper. Currently, there are no published guidelines to aid clinicians in selecting the proper dry needle length that would allow the necessary depth of penetration required to reach the piriformis muscle. Utilizing the technique by Dommerholt [13] allows the clinician to adopt an appropriate needle angle to potentially reach the piriformis muscle, should a sufficient needle length be used. In the one case where the piriformis muscle was missed, the orientation of the needle toward the symphysis pubis was inclined too cranially. Although the piriformis muscle was not reached, the depth of penetration would have been sufficient to penetrate the piriformis muscle should the correct angle been used. While taking a needle orientation that is too far cranial may lead to missing the piriformis muscle, it causes the needle to course away from the path of the sciatic nerve, minimizing the risk of neural puncture. Clinically, while performing dry needling on a patient, failure to elicit the local twitch response or reproduce the patient’s concordant pain would allow the clinician to be aware of missing the piriformis muscle. Additionally, while piercing the sciatic nerve is not desired as it could result in complaints of neural symptoms, if the needle is advanced slowly, the clinician would be able to sense any potential changes in tissue resistance indicating the needle is touching the sciatic nerve, which would prompt the clinician to adjust the needle insertion angle.
While most of the r values indicated no correlation, there was a fair (r = 0.495) and good correlation (r = 0.759) between the needle length chosen and the mean fat thickness for the left and right hip, respectively. These results suggest that clinicians utilizing needle therapies can use the amount of adipose tissue to guide their decision on the most appropriate length needle to use for insertion. During identification of bony landmarks to locate the needle insertion site, clinicians can obtain an estimate of adipose tissue thickness via palpation and subsequently choose an appropriate length needle. The more adipose tissue present overlying the gluteus maximus would necessitate a longer needle.
Future studies including performance of dry needling targeting the piriformis muscle in vivo are needed to increase external validity of the findings. The accuracy of reaching the piriformis muscle could be increased as clinicians rely on patient verbal feedback regarding symptom reproduction, as well as tactile feedback felt via the needle regarding tissue reactivity (twitch response) and resistance to confirm location. However, this study is a first step in pursuing in vivo research of needling technique to target the piriformis muscle, as Ethics committee requires investigations to evaluate first the safety of techniques before pursuing in vivo experimentations.
Limitations of this study include the lack of systematic measurements using various needle length sizes (50, 75, 100, and 125 mm), as the technique of securing needle placement with super glue and performing careful dissection to measure reaching/penetrating the piriformis muscle only allowed the performance of one trial with each needle insertion. Additionally, with only one investigator placing the needles, the results of this study cannot be generalized.
Conclusion
A physical therapist was able to use bony landmark palpation in order to locate the piriformis muscle and, given a sufficient needle length, reach the medial piriformis muscle with 84% accuracy as it exits the greater sciatic notch. While no puncture of the sciatic nerve occurred in this investigation, the proximity of the piriformis muscle to the sciatic nerve does allow for the possibility of neural puncture.
Notes on contributors
Gary Kearns is an assistant professor in the Doctor of Physical Therapy Program at Texas Tech University Health Sciences Center. He is a fellow in the American Academy of Orthopaedic Manual Physical Therapists (FAAOMPT) and his fellowship project entitled Medical Diagnosis of Cubital Tunnel Syndrome Ameliorated with Thrust Manipulation of the Elbow and Carpals was published in the Journal of Manual and Manipulative Therapy in 2012. He graduated with his Doctor of Science (ScD) in Physical Therapy through Texas Tech University Health Sciences Center in 2015. Most recently, she became a Board Certified Specialist in Orthopaedic Physical Therapy (OCS) in 2016.
Kerry Gilbert is a program director of the Doctor of Physical Therapy Program at Texas Tech University Health Science Center (TTUHSC) and the director of Anatomy Research and Education for the School of Health Professions and the director of the Clinical Anatomy Research Lab within the Center for Rehabilitation Research at TTUHSC. Dr. Gilbert, was awarded the ‘Young Investigator Award’ (2007) by SPINE and was a co-author of the SPINE ‘Young Investigator Award’ manuscript (2015) for his work in nerve root displacement and strain in unembalmed cadavers. He is also known for his work in intraneural fluid dynamics in response to neurodynamic mobilization.
Brad Allen is currently the assistant program director and assistant professor in the Doctor of Science Program in Physical Therapy at Texas Tech University Health Sciences Center.
Phillip S. Sizer Jr is a professor and program director of the Doctorate of Science Program in Physical Therapy, faculty in the PhD Program in Rehabilitation Science and in the Medical Pain Fellowship Program in the Department of Anesthesiology, and is a director of the Clinical Musculoskeletal Research Laboratory at the Center for Rehabilitation Research at Texas Tech University Health Science Center. Dr. Sizer,, is a fellow in the AAOMPT and has authored over 100 scientific papers in refereed journals and serves as the Associate Dean for Research for the School of Health Professions at Texas Tech University Health Sciences Center.
Jean-Michel Brismée is a professor in the Doctor of Science Program of Physical Therapy at Texas Tech University Health Sciences Center. He has authored over 60 scientific papers in refereed journals, was co-investigator in Research projects funded for more than half a million dollars, and is Editor in Chief of the Journal of Manual and Manipulative Therapy.
Timothy Pendergrass is an assistant professor in the Doctor of Physical Therapy Program at Texas Tech University Health Sciences Center. He is the co-chair of the Admissions Committee and coordinates the Clinical Exercise Physiology and Therapeutic Exercise curriculum. Dr. Pendergrass has co-authored publications investigating the anatomical relationship of the carpal bones and anatomic landmarks to locate the first rib in cadavers.
Micah Lierly is an assistant professor in the Doctor of Physical Therapy Program at Texas Tech University Health Sciences Center. She is currently completing her PhD in Rehabilitation Sciences.
Deborah York is an assistant professor in the Doctor of Physical Therapy Program at Texas Tech University Health Sciences Center. Most recently, she became a Board Certified Specialist in Orthopaedic Physical Therapy (OCS) in 2016. She is currently pursuing her Doctor of Science in Physical Therapy.
Disclosure statement
The primary investigator teaches dry needling as a guest faculty member for the North American Institute of Orthopaedic Manual Therapy.
Supplemental data
The supplementary material for this article is available online at https://doi.org/10.1080/10669817.2017.1346745
Video disclaimer
The supplemental video is intended to illustrate the techniques used in this investigation. It is not intended to be instructional on the techniques for clinical application.
Supplementary Material
Acknowledgments
The authors extend their deepest gratitude to the unselfish men, women, and family members who donate their bodies to the Willed Body Program at the Texas Tech University Health Sciences Center for educational and research purposes. Without their contribution, studies like this one would not be possible. The authors thank the Texas Tech University Health Sciences Center and the School of Health Professions for the use of Gross Anatomy and the Clinical Anatomy Research Laboratories, respectively.
References
- [1].Lew PC, Story Li. Inter-therapist reliability in locating latent myofascial trigger points using palpation. Man Ther. 1997;2(2):87–90. [DOI] [PubMed] [Google Scholar]
- [2].Sciotti VM, Mittak VL, DiMarco L, et al. Clinical precision of myofascial trigger point location in the trapezius muscle. Pain. 2001;93:259–266. [DOI] [PubMed] [Google Scholar]
- [3].Barbaro M, Bertoli P, Cescon C, et al. Intra-rater reliability of an experienced physiotherapist in locating myofascial trigger points in upper trapezius muscle. J Man Manip Ther. 2012;20(4):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Myburgh C, Lauridsen HH, Larsen AH, et al. Standardized manual palpation of myofascial trigger points in relation to neck/shoulder pain; the influence of clinical experience on inter-examiner reproducibility. Man Ther. 2011;16:136–140. [DOI] [PubMed] [Google Scholar]
- [5].Gerwin RD, Shannon S, Hong CZ, et al. Interrater reliability in myofascial trigger point examination. Pain. 1997;69:65–73. [DOI] [PubMed] [Google Scholar]
- [6].Al-Shenqiti AM, Oldham JA. Test-retest reliability of myofascial trigger point detection in patients with rotator cuff tendonitis. Clin Rehabil. 2005;19:482–487. [DOI] [PubMed] [Google Scholar]
- [7].Bron C, Franssen J, Wensing M, et al. Interrater reliability of palpation of myofascial trigger points in three shoulder muscles. J Man Manip Ther. 2007;15(4):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jankovic D, Peng P, van Zundert A. Brief review: Piriformis syndrome: etiology, diagnosis, and management. Can J Anesth. 2013;60:1003–1012. [DOI] [PubMed] [Google Scholar]
- [9].Cass SP. Piriformis syndrome: a cause of nondiscogenic sciatica. Curr Sports Med Rep. 2015;14(1):41–44. [DOI] [PubMed] [Google Scholar]
- [10].Jeong HS, Lee GY, Lee EG, et al. Long-term assessment of clinical outcomes of ultrasound-guided steroid injections in patients with piriformis syndrome. Ultrasonography. 2015;34(3):206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Misirlioglu TO, Akgun K, Palamar D, et al. Piriformis syndrome: comparison of the effectiveness of local anesthetic and corticosteroid injections: a double-blinded randomized controlled study. Pain Physician. 2015;18(2):163–171. [PubMed] [Google Scholar]
- [12].Akdemir Ozesik P, Toru M, Denk CC, et al. CT-Guided Piriformis Muscle Injection for the Treatment of Piriformis Syndrome. Turk Neurosurg. 2014;24(4):471–477. [DOI] [PubMed] [Google Scholar]
- [13].Dommerholt J, Fernández-de-las-Peñas C, eds. Trigger point dry needling – an evidenced and clinical-based approach. Churchill Livingstone Elsevier; 2013; p. 1–249. [Google Scholar]
- [14].Brady S, McEvoy J, Dommerholt J, et al. Adverse events following trigger point dry needling: a prospective study of chartered physiotherapists. J Man Manip Ther. 2014;22:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Peuker E, Grönemyer D. Rare but serious complications of acupuncture: traumatic lesions. Acupunct Med. 2001;19(2):103–108. [DOI] [PubMed] [Google Scholar]
- [16].Halle JS, Halle RJ. Pertinent dry needling considerations for minimizing adverse events – Part One. Int J Sports Phys Ther. 2016;11(4):651–662. [PMC free article] [PubMed] [Google Scholar]
- [17].Halle JS, Halle RJ. Pertinent dry needling considerations for minimizing adverse events – Part two. Int J Sports Phys Ther. 2016;11(5):810–819. [PMC free article] [PubMed] [Google Scholar]
- [18].Beaton LE, Anson BJ. The relation of the sciatic nerve and of its subdivision to the piriformis muscle. Pain. 1937;47:345–352. [Google Scholar]
- [19].Lewis S, Jurak J, Lee C, et al. Anatomic variations of the sciatic nerve, in relation to the piriformis muscle. Translational Research in Anatomy. 2016;5:15–19. [Google Scholar]
- [20].Adibatti M. V S. Study on variant anatomy of sciatic nerve. J Clin Diag Res. 2014;8(8):AC07–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Smoll NR. Variations of the piriformis and sciatic nerve with clinical consequences: a review. Clin Anat. 2010;23(1):8–17. [DOI] [PubMed] [Google Scholar]
- [22].Mesa-Jiménez JA, Sánchez-Gutiérrez J, de-la-Hoz-Aizpurua JL, et al. Cadaveric validation of dry needle placement in the lateral pterygoid muscle. J Manipulative Physiol Ther. 2015;38(2):145–150. [DOI] [PubMed] [Google Scholar]
- [23].Hannah MC, Cope J, Palermo A, et al. Comparison of two angles of approach for trigger point dry needling of the lumbar multifidus in human donors (cadavers). Man Ther. 2016;26:160–164. [DOI] [PubMed] [Google Scholar]
- [24].Fernández-de-las-Peñas C, Mesa-Jiménez JA, Paredes-Mancilla JA, Koppenhaver SL, et al. Cadaveric and ultrasonographic validation of needling placement in the cervical multifidus muscle. J Manipulative Physiol Ther . 2017. Apr 13. DOI: 10.1016/j.jmpt.2017.03.002 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [25].Lavelle ED, Lavelle W, Smith HS. Myofascial Trigger Points. Anesthesiology Clin. 2007;25:841–851. [DOI] [PubMed] [Google Scholar]
- [26].Dommerholt J, Mayoral del Moral O, Grobli C. Trigger point dry needling. J Man Manip Ther. 2006;14(4):E70–E87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huguenin LK. Myofascial trigger points: the current evidence. Phys Ther Sport. 2004;5:2–12. [Google Scholar]
- [28].Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point: The importance of the local twitch response. J Phys Med Rehabil. 1994;73:256–263. [DOI] [PubMed] [Google Scholar]
- [29].Reichert R. Palpation techniques: surface anatomy for physical therapists. Stuttgart: Thieme; 2011; p. 229–231. [Google Scholar]
- [30].Michel F, Decavel P, Toussirot E, et al. Piriformis muscle syndrome: Diagnostic criteria and treatment of a monocentric series of 250 patients. Ann Phys Rehabil Med. 2013;56:371–383. [DOI] [PubMed] [Google Scholar]
- [31].Michel F, Decavel P, Toussirot E, et al. The piriformis muscle syndrome: An exploration of anatomical context, pathophysiological hypotheses and diagnostic criteria. Ann Phys Rehabil Med. 2013;56:300–311. [DOI] [PubMed] [Google Scholar]
- [32].Childers MK, Wilson DJ, Gnatz SM, et al. Botulinum toxin type a use in Piriformis muscle syndrome: a pilot study. Am J Phys Med Rehabil. 2002;81:751–759. [DOI] [PubMed] [Google Scholar]
- [33].Fishman LM, Anderson C, Rosner B. BOTOX and physical therapy in the treatment of piriformis syndrome. Am J Phys Med Rehabil. 2002;81:936–942. [DOI] [PubMed] [Google Scholar]
- [34].Betts A. Combined fluoroscopic and nerve stimulator technique for injection of the Piriformis muscle. Pain Phys. 2004;7:279–281. [PubMed] [Google Scholar]
- [35].Blunk JA, Nowotny MN, Scharf J, et al. MRI verification of ultrasound-guided infiltration of local anesthetics into the piriformis muscle. Pain Med. 2013;14:1593–1599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.