Abstract
Objective
To assess the impact of patient characteristics, patient-professional engagement, communication and context on the probability that healthcare professionals will discuss goals or priorities with older patients.
Design
Secondary analysis of cross-sectional data from the 2014 Commonwealth Fund International Health Policy Survey of Older Adults.
Setting
11 western countries.
Subjects
Community-dwelling adults, aged 55 or older.
Main outcome measure
Assessment of goals and priorities.
Results
The final sample size consisted of 17,222 respondents, 54% of whom reported an assessment of their goals and priorities (AGP) by healthcare professionals. In logistic regression model 1, which was used to analyse the entire population, the determinants found to have moderate to large effects on the likelihood of AGP were information exchange on stress, diet or exercise, or both. Country (living in Sweden) and continuity of care (no regular professional or organisation) had moderate to large negative effects on the likelihood of AGP. In model 2, which focussed on respondents who experienced continuity of care, country and information exchange on stress and lifestyle were the main determinants of AGP, with comparable odds ratios to model 1. Furthermore, a professional asking questions also increased the likelihood of AGP.
Conclusions
Continuity of care and information exchange is associated with a higher probability of AGP, while people living in Sweden are less likely to experience these assessments. Further study is required to determine whether increasing information exchange and professionals asking more questions may improve goal setting with older patients.
Key points
- A patient goal-oriented approach can be beneficial for older patients with chronic conditions or multimorbidity; however, discussing goals with these patients is not a common practice.
- The likelihood of discussing goals varies by country, occurring most commonly in the USA, and least often in Sweden.
- Country-level differences in continuity of care and questions asked by a regularly visited professional affect the goal discussion probability.
- Patient characteristics, including age, have less impact than expected on the likelihood of sharing goals.
Keywords: Goal setting, determinants, continuity of care, older patients, chronic condition, multimorbidity, logistic regression model
Introduction
Multimorbidity, the coexistence of two or more chronic morbidities, is highly prevalent among older people. A cross-sectional study of about one-third of the Scottish population concluded that half of them suffered from at least one morbidity by the age of 50 and most were multimorbid by the age of 65 [1]. The 2014 Commonwealth Fund International Health Policy Survey of Older Adults, which surveyed adults aged 55 and above in 11 countries, confirmed these results. For respondents aged 65 or older, the percentage with one chronic disease varied from 63% (New Zealand) to 87% (USA), and the percentage with two or more diseases varied from 33% (UK) to 68% (USA) [2].
Globally, multimorbidity rates are rising due to urbanisation, industrialisation and population aging, increasing the demands on the healthcare work force and resources [3]. In daily practice, the presence of chronic multimorbidity presents a challenge for the decision-making processes between practitioners and patients; applying disease-specific guidelines to patients with multiple conditions is difficult, and this is compounded by the fact that the patients’ health-related goals arise from a variety of dimensions [4–7]. An assessment of patient goals and preferences could be helpful for overcoming this challenge [5,8–10]. For individual patients, a goal-oriented approach to healthcare can contribute to their well-being and quality of life, by changing the focus from a disease-specific orientation to the patient’s individual health goals. For societies, this approach and change in focus could also contribute to the long-term quality, accessibility and affordability of the healthcare system [1,3,5,8–11].
The 2014 Commonwealth Fund survey found that, for adults aged 65 or older who have a chronic condition, the rate of patients sharing their goals with a professional varied from 23% (Sweden) to 59% (UK), with nine of the 11 countries having rates lower than 50% [2]. Sharing goals is clearly not yet a common care practice; therefore, the aim of this secondary analysis of the 2014 Commonwealth Fund data is to assess which factors determine whether healthcare professionals engage in an assessment of the goals or priorities associated with medical care in older patients with one or more chronic diseases.
Materials and methods
Study design, setting and subjects
This empirical analysis was designed and conducted based on the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) Statement [12,13]. The Commonwealth Fund’s 2014 International Health Policy Survey of Older Adults had a cross-sectional design and surveyed community-living adults aged 55 or older. This computer-assisted telephone survey was conducted between March and May 2014 in 11 countries: Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, the UK and the USA. The questionnaire was developed, translated, adapted to local health system contexts, and pre-tested by The Commonwealth Fund and SSRS, a market and survey research firm, in co-operation with researchers from the participating countries [2,14].
Telephone surveys were conducted among nationally representative samples of adults aged 55 or older, based on a random-digit-dialling method. Sample generation was performed by Sample Solutions Europe (SSE) (Australia, France, the Netherlands, New Zealand and the UK); SM research (Canada); GESIS, Leibniz-Institut für Sozialwissenschaften (Germany); Norstat (Norway); PAR Konsument registry (Sweden); the Swiss Federal Statistical Office (Switzerland) and SSRS (USA). Both mobile phone and landline numbers were used, except for Canada where only landline phone numbers were used. Telephone numbers were dialled on average nine times in the case of non-response. Prior to conducting the interviews, interviewers received written material on the interviews and were formally trained. Survey topics were access to care, chronic conditions and care co-ordination, patient engagement, social care needs and end-of-life care planning. On average, interviews lasted 20 minutes [2,14].
The response rates varied from 16% to 60% across the countries (Appendix) [2], and those with a response rate of less than 20% were dropped from the analysis. The respondents assessed whether they had one or more chronic conditions by answering whether a doctor had ever told them they had any of the medical conditions on a pre-specified list [14]. The surveys for two countries had additional possible chronic diseases: stroke for France and dementia for Switzerland. They were not included in the analyses, as questions about these additional diseases were limited to those two countries.
Outcome
Having one or more chronic conditions was a prerequisite for answering the outcome question. The outcome variable ‘assessment of goals and priorities’ (AGP) had two categories: ‘yes’ and ‘no.’ It was based on the following survey question: ‘During the past year, when you received care, has any healthcare professional you see for your (diabetes OR high blood pressure OR heart disease OR chronic lung problems OR depression, anxiety, or another mental health problem OR cancer OR joint pain or arthritis) discussed with you your main goals or priorities in caring for this condition?’ In the context of this question, ‘OR’ means having one or more of these chronic conditions and seeing a healthcare professional for any of them.
Possible determinants
We clustered three groups of questionnaire-based variables as possible determinants for AGP. The first group consisted of Patient Characteristics and included categorical variables on age (Age), gender (Gender) and health status; for health status, we created two variables with accompanying groupings: Number_of_Chronic_Conditions and Chronic_Disease_Type.
The second group considered Patient-Professional Engagement and Communication. Earlier research defined variables (i.e. a professional asking questions, medical staff explaining things in a way that is easy to understand when explanations about care or treatment are required, and a patient’s assessment of time spent in a consultation) that capture patient engagement [15]. We created the categorical variables Asking_Questions, Explaining and Time_Spent, respectively. In addition, we created categorical variables about the professional’s knowledge of a patient’s history (History_Knowledge), and the exchange of information about stress (Information_Exchange_on_Stress) and lifestyle (Lifestyle_Information_Exchange) to build this cluster of factors.
The third group of possible determinants consisted of Context variables, including the Country variable (10 categories) and a Continuity of Care (CoC) variable (whether respondents have a regular doctor/healthcare professional, a regular place for medical care, or neither). Table 1 depicts the variables considered to be potential determinants.
Table 1.
Univariate analysis of sharing goals and priorities in the Commonwealth Fund International Health Policy Survey of Older Adults.
| Sharing goals and priorities n (%) |
|||
|---|---|---|---|
| No | Yes | Total | |
| n | |||
| Sample | 7903 (45.9) | 9319 (54.1) | 17,222 |
| Population characteristics | |||
| Age (years) | |||
| 55–64 (1959–1950) | 2457 (41.6) | 3447 (58.4) | 5904 |
| 65–74 (1949–1940) | 2903 (45.3) | 3506 (54.7) | 6409 |
| 75 + (1939–1906) | 2543 (51.8) | 2366 (48.2) | 4909 |
| Gender | |||
| Female | 4899 (48.1) | 5290 (51.9) | 10,189 |
| Male | 3004 (42.7) | 4029 (57.3) | 7033 |
| Number_of_Chronic_Diseases | |||
| One chronic condition | 3631 (50.7) | 3525 (49.3) | 7156 |
| Two chronic conditions | 2520 (45.8) | 2979 (54.2) | 5499 |
| Three chronic conditions | 1752 (38.4) | 2815 (61.6) | 4567 |
| Chronic_Disease_Type | |||
| Mental disease only | 235 (46.8) | 267 (53.2) | 502 |
| Mental and somatic disease | 1099 (43.3) | 1438 (56.7) | 2537 |
| Somatic disease only | 6546 (46.3) | 7594 (53.7) | 14,140 |
| Patient-professional engagement and communication | |||
| Asking_Questions | |||
| Sometimes/rarely/never | 3096 (60.5) | 2020 (39.5) | 5116 |
| Always/often | 3976 (36.9) | 6787 (63.1) | 10,763 |
| Explaining | |||
| Sometimes/rarely/never | 1024 (61.8) | 634 (38.2) | 1658 |
| Always/often | 6473 (43.2) | 8497 (56.8) | 14,970 |
| History_Knowledge | |||
| Sometimes/rarely/never | 1248 (61.2) | 791 (38.8) | 2039 |
| Always/often | 5959 (41.9) | 8248 (58.1) | 14,207 |
| Time_Spent | |||
| Sometimes/rarely/never | 1427 (60.1) | 949 (39.9) | 2376 |
| Always/often | 6055 (42.6) | 8175 (57.4) | 14,230 |
| Information_Exchange_on_Stress | |||
| No | 6588 (52.3) | 6006 (47.7) | 12,594 |
| Yes | 1201 (27.1) | 3234 (72.9) | 4435 |
| Lifestyle_Information_Exchange | |||
| No exchange | 3901 (67.2) | 1902 (32.8) | 5803 |
| Exchange on diet or exercise | 2080 (47.9) | 2266 (52.1) | 4346 |
| Exchange on diet and exercise | 1782 (26.0) | 5069 (74.0) | 6851 |
| Context | |||
| Continuity of care (CoC) | |||
| Has regular doctor/GP/NP/PA | 6624 (43.3) | 8686 (56.7) | 15,310 |
| Has regular healthcare organisation | 1103 (66.5) | 555 (33.5) | 1658 |
| No regular doctor/GP/NP/PA or regular healthcare organisation | 176 (69.3) | 78 (30.7) | 254 |
| Country | |||
| Australia | 726 (34.2) | 1398 (65.8) | 2124 |
| Canada | 1596 (38.6) | 2534 (61.4) | 4130 |
| France | 490 (47.2) | 549 (52.8) | 1039 |
| Germany | 257 (36.7) | 443 (63.3) | 700 |
| Netherlands | 333 (46.8) | 379 (53.2) | 712 |
| New Zealand | 167 (39.7) | 254 (60.3) | 421 |
| Sweden | 3169 (65.7) | 1652 (34.3) | 4821 |
| Switzerland | 551 (44.3) | 692 (55.7) | 1243 |
| United Kingdom | 186 (32.9) | 379 (67.1) | 565 |
| United States of America | 428 (29.2) | 1039 (70.8) | 1467 |
GP: general practitioner; NP: nurse practitioner; PA: physician assistant.
Statistical analysis
Only respondents who had a regular professional or organisation for medical care (indicating CoC) were asked to answer questions about Asking_Questions, Explaining, History_Knowledge and Time_Spent. We considered this to be an important aspect for AGP, and decided to analyse the two populations in a logistic regression analysis. In Model 1, we explored all potential determinants except Asking_Questions, Explaining, History_Knowledge and Time_Spent. In Model 2, we used all potential determinants, resulting in an analysis of the subpopulation of respondents who experience CoC. For the interpretation of the results, an odds ratio (OR) can be seen as a measure of the association between an exposure and an outcome. An OR of 1 means that the determinant does not affect the likelihood of the outcome. For the interpretation of our results, we considered determinants with OR ≤0.5 or ≥1.5, as having relevant (decreasing or increasing) effects on the probability of AGP.
For both models, we analysed the ‘missing’ data. When considering all respondents and all variables (except Asking_Questions, Explaining, History_Knowledge and Time_Spent), 2.0% of the data were missing; however, when the respondents who answered ‘not applicable’ were removed, this declined to 1.4%. For the subpopulation of respondents who experienced CoC, the overall ‘missing’ percentage was 13.6% (6.6% without the ‘not applicable’ responses). Further analysis suggested that the missing data were randomly distributed; therefore, as a high number of respondents remained, multiple imputation was not necessary and we could focus on a complete case analysis.
Results
Respondent characteristics
The original sample size was 25,530 respondents. We excluded all respondents without one or more chronic condition(s) or who had a missing value for the outcome question of the analysis. In addition, Norwegian respondents were excluded based on the 16% response rate for that country. These adjustments resulted in a final sample size of 17,222 respondents.
Figure 1 presents a flow diagram of respondents, while Table 1 describes the variables considered to be potential determinants in relation to the outcome variable, AGP. Of the respondents, 34% were 55–64 years old, 37% were 65–74 years old and 29% were 75 or older; 41% were male. Overall, 42% reported having one chronic condition, 32% reported having two chronic conditions and 27% reported having three or more chronic conditions.
Figure 1.
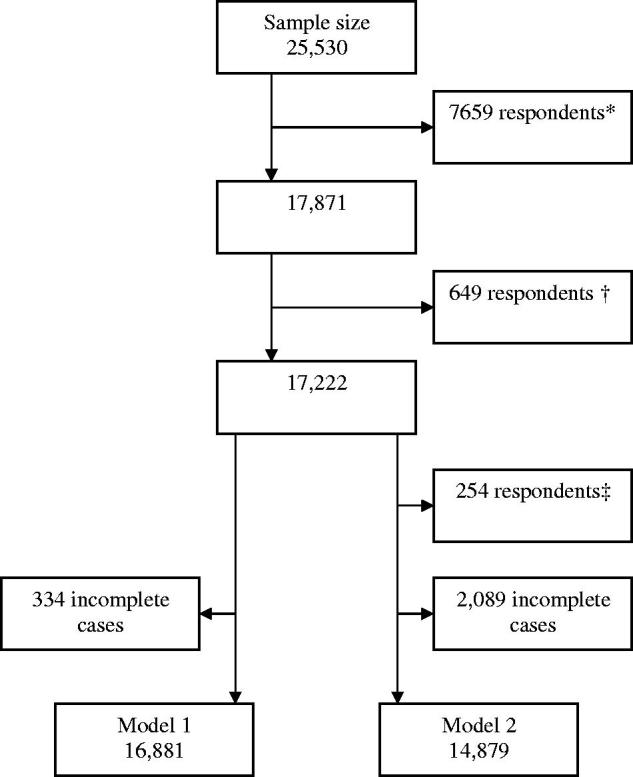
Flow diagram of respondents. *Respondents with no chronic disease or a missing value on the outcome; †respondents from Norway; ‡subject without a regular doctor/general practitioner/nurse practitioner/physician assistant or a regular health care organisation.
Model 1
The prerequisite of a complete case analysis led to a final population for this analysis of 16,881 respondents (98%). Table 2 shows the results for Models 1 and 2. Model 1, including all previously determined factors, was statistically significant overall (p < .001). For Model 1, the area under the curve was 0.746 (95%CI: 0.739–0.753).
Table 2.
Logistic regression Model 1 and 2 of possible determinants of the assessment of goals and priorities outcome.
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| OR | p value (95%CI) | OR | p value (95%CI) | |
| Constant | 0.77 | .001 | 0.38 | .000 |
| Age (years) | ||||
| 55–64 (1959–1950)a | .000 | .000 | ||
| 65–74 (1949–1940) | 1.02 | .583 (0.94–1.11) | 1.00 | .948 (0.92–1.09) |
| 75 + (1939–1906) | 0.82 | .000 (0.75–0.90) | 0.82 | .000 (0.75–0.90) |
| Gender | ||||
| Femalea | ||||
| Male | 1.23 | .000 (1.14–1.31) | 1.21 | .000 (1.13–1.31) |
| Number_of_Chronic_Diseases | .000 | .000 | ||
| One chronic conditiona | ||||
| Two chronic conditions | 1.13 | .005 (1.04–1.22) | 1.11 | .018 (1.02–1.22) |
| Three chronic conditions | 1.30 | .000 (1.18–1.43) | 1.33 | .000 (1.20–1.47) |
| Chronic_Diseases_Type | .000 | 0.000 | ||
| Somatic disease onlya | ||||
| Mental and somatic disease | 0.67 | .000 (0.60–0.74) | 0.71 | .000 (0.64–0.80) |
| Mental disease only | 1.02 | .854 (0.83–1.25) | 1.03 | .774 (0.83–1.28) |
| Asking_Questions | NAb | |||
| Sometimes/rarely/nevera | ||||
| Always/often | 1.60 | .000 (1.47–1.75) | ||
| Explaining | NAb | |||
| Sometimes/rarely/nevera | ||||
| Always/often | 1.19 | .016 (1.03–1.37) | ||
| History_Knowledge | NAb | |||
| Sometimes/rarely/nevera | ||||
| Always/often | 1.18 | .009 (1.04–1.34) | ||
| Time_Spent | NAb | |||
| Sometimes/rarely/nevera | ||||
| Always/often | 1.18 | .007 (1.05–1.33) | ||
| Information_Exchange_on_Stress | ||||
| Noa | ||||
| Yes | 1.82 | .000 (1.67–1.99) | 1.74 | .000 (1.59–1.91) |
| Lifestyle_Information_Exchange | .000 | .000 | ||
| No exchangea | ||||
| Exchange on diet or exercise | 1.98 | .000 (1.82–2.16) | 1.89 | .000 (1.73–2.07) |
| Exchange on diet and exercise | 4.20 | .000 (3.86 –4.57) | 3.75 | .000 (3.43–4.10) |
| Continuity of care (CoC) | .000 | NAc | ||
| Has regular doctor/GP/NP/PAa | ||||
| Has regular healthcare organisation | 0.77 | .000 (0.68–0.88) | ||
| No regular professional or healthcare organisation | 0.51 | .000 (0.38–0.69) | ||
| Country | .000 | .000 | ||
| Australia | 0.82 | .012 (0.70–0.96) | 0.77 | .002 (0.66–0.91) |
| Canada | 0.80 | .001 (0.69–0.92) | 0.81 | .005 (0.70–0.94) |
| France | 0.57 | .000 (0.48–0.68) | 0.52 | .000 (0.43–0.63) |
| Germany | 0.77 | .012 (0.62–0.94) | 0.76 | .015 (0.61–0.95) |
| Netherlands | 0.75 | .005 (0.61–0.92) | 0.79 | .034 (0.64–0.98) |
| New Zealand | 0.71 | .006 (0.56–0.91) | 0.69 | .005 (0.53–0.89) |
| Sweden | 0.32 | .000 (0.28–0.37) | 0.36 | .000 (0.31–0.42) |
| Switzerland | 0.71 | .000 (0.60–0.85) | 0.68 | .000 (0.57–0.82) |
| United Kingdom | 0.99 | .954 (0.79–1.25) | 0.93 | .548 (0.74–1.18) |
| United States of Americaa | ||||
CI: confidence interval; GP: general practitioner; NP: nurse practitioner; PA: physician assistant; NA: not applicable.
Reference category.
The variables on Professional Attitude and Communication are not applicable to model 1 because they were not used as potential determinants in this analysis.
This variable was not included in model 2 because it was not significant.
As shown in Table 2, for the whole population (Model 1), there were only three independent variables with an OR ≤0.5 or ≥1.5: Country (living in Sweden), Information_Exchange_on_Stress and Information_ Exchange_ on_ Diet_and_Exercise. Having no regular professional or organisation had a borderline impact, while the variables with smaller effects on the probability of AGP were Age (>75 years), Gender (male), having three or more chronic diseases, having a combination of mental and one or more somatic diseases, and only having a regular organisation (not a regular professional) for medical care. All countries except for the UK had a lower probability rate for assessing patient goals than the reference country (USA).
Model 2
For Model 2, 14,879 (86%) respondents were included in the complete case analysis. Model 2 (see Table 2) was significant overall (p < .001) and the area under the curve was 0.743 (95%CI 0.735–0.751).
As shown in Table 2, for the subpopulation of respondents who experience CoC, the main determinants with OR ratios of ≤0.5 or ≥1.5 were comparable to the determinants of Model 1. The only exception was the inclusion of Asking_Questions.
The remaining variables with smaller effects also had a comparable direction and size of effects to Model 1 (summarised in Table 2). In addition to Asking_Questions, the variables Explaining, History_Knowledge and Time_Spent (specifically explored in Model 2) were statistically significant (p < .05).
Discussion
Our study reveals that CoC and information exchange on lifestyle and/or stress are strong determinants of the probability that goals and priorities will be assessed by healthcare professionals and patient. Patients living in Sweden were less likely to receive AGP than those living in the USA. For respondents who experienced CoC, a professional asking questions was found to be a relevant factor.
Our research has several limitations. First, the response rates differed among countries and were relatively low in general, potentially introducing response bias. Research in Korea using random digit dialling in 2012 and 2014 with a target population of 9600 community-dwelling adults aged 19–79 years reported response rates of 19% and 16% for landline telephones and 14% and 12% for mobile phones [16]. The response rates for The Commonwealth Fund’s survey were higher than in the Korean study, indicating that relatively low response rates are probably to be expected when using this randomisation method; however, the direction of this potential bias is unknown [2]. The weighting of data was not considered to be contributory to our research aim of demonstrating potential associations. Our results provide first insights into the relevant determinants for AGP across countries, but cannot be used to draw conclusions for individual countries.
Secondly, the complexity of the concept of goals must be considered when interpreting the results. In the survey, sharing goals is related to a specific chronic condition(s), with a lack of differentiation between the types of goals; however, as argued in the introduction, disease-specific guidelines are often not applicable to older patients with multiple conditions, and their health-related goals can arise from a variety of dimensions. Moreover, care-related goals for community-dwelling frail older adults are highly individual and relate to well-being as much as to health and functioning [4–7]. This could have led to an underestimation of the sharing of goals.
Furthermore, this is a secondary analysis of the Commonwealth Fund dataset. Ideally, we would have had additional data on the healthcare professionals involved and on the complexity of care required by the respondents. Finally, the determinants of the AGP originate from different levels, the macro level, the meso level and the micro level; however, the meso level (the organisational perspective) was not part of this analysis.
Our study also has several strengths. The underlying survey is an international project with a high level of standardisation in content and execution. In addition, although the survey had relatively low response rates per country, the overall population that could be analysed is large. Moreover, despite the widespread advocation of shared decision-making (SDM), there is a general lack of details about goal setting as an autonomous element of an SDM approach, as well as its accompanying barriers and facilitators. This is one of the few studies to address this knowledge gap.
To the best of our knowledge, there is a lack of research specifically focussed on goal setting with community-dwelling older patients with multimorbidity. Other research has generally focussed on goal setting with seriously ill (hospitalised) patients [17,18] or goal setting in relation to shared decision-making [19–21].
A wide range of patient characteristics influence the demands on healthcare. Although having more than three chronic diseases was associated with an increase in sharing goals and priorities, while having a combination of a mental and somatic disease was associated with a decrease in sharing goals and priorities, variables in the Patient Characteristics group had less of an impact than expected. For analytical reasons, multimorbidity was defined as having two or more chronic conditions; however, in defining multimorbidity, disease severity and the burden of physiological dysfunctions resulting from the multiple conditions should also be incorporated [22]. These factors together are indicators of the actual complexity of patient healthcare needs. Although the impact is relatively low, our findings on Number_of_Chronic_Conditions and Chronic_Disease_Type are consistent with multimorbidity as described by Zulman et al. [22]. Age (75+) only had a slight impact. The survey was targeted at community-dwelling adults aged 55 or older; therefore, the survey respondents, including those aged 75+, were probably relatively capable of engaging in their own healthcare. This may explain why, for this population, age had less of an impact on the probability of AGP than expected.
Variables in the Patient-Professional Engagement and Communication cluster appeared to be relevant determinants for this analysis. Although we cannot draw conclusions on causality, these findings provide initial insights into possible future engagement points, especially when focusing on potential barriers. Information exchange regarding lifestyle and/or stress has a large impact on AGP; however, a study on lifestyle consultations by Dutch general practitioners and practice nurses found that information about lifestyle is mostly given in generic terms and not tailored to the specific patient [23].
Country is a strong contextual determinant. This could be an effect of certain characteristics of different healthcare systems, cultures or other factors, which complicates the interpretation of this finding; for example, Swedish clinicians mentioned that the remuneration system does not allow them to spend enough time on communication, instead emphasising easy accessibility, rapid turnover and reduced performance time [24].
From our analysis, it appears that CoC is a relevant determinant, which is in line with the findings of Kohnke and Zielinski [25] on the association between CoC and the utilisation of Swedish primary care emergency services. Incidence rate ratios suggested that patients with the lowest CoC had a higher number of emergency services visits compared with those experiencing the highest CoC [25]. Furthermore, Hultberg and Rudebeck [26] investigated patient participation in decision-making about cardiovascular preventive drug treatments through the resistance to treatment proposals in Sweden, concluding that the decision-making process extends beyond single encounters, which underpins the importance of CoC. Other studies found that CoC with a general practitioner is associated with lower healthcare costs, higher patient satisfaction and improvements in patient health [27,28]. CoC in general practice is also associated with reduced hospital admissions, especially among heavy users of primary care [29]. The potential contribution of goal setting to these effects is not yet clear.
Other studies have found that time constraints are an important barrier to shared decision-making and goal setting [19,30,31]. In this analysis, Time_Spent was an assessment of whether the respondents thought healthcare professionals spent enough time with them. In this sense, in line with Osborn et al. [15], Time_Spent is a variable of patient engagement rather than a contextual factor.
Our research has several implications. To facilitate the consideration of different types of goals in future research, survey questions about different types of goals and specific healthcare professionals could be added to increase the representation of a goal setting focus in daily practice. Furthermore, survey questions on the complexity of healthcare needs should be added to increase the representation of patients who would probably benefit most from goal setting. Further research based on our findings could consider the determinants and their underlying causal relationships to provide healthcare professionals and policymakers with engagement points for realising patient goal-oriented healthcare.
In conclusion, our analysis shows that patient-professional engagement and communication and contextual factors are related to the probability of AGP. It also indicates that AGP is most likely to occur in consultations where a healthcare professional asks questions and exchanges information about stress and lifestyle with a patient, though this still varies greatly by country. Considering the context, CoC differences between countries appear to be a relevant factor in explaining the likelihood of AGP, while patient characteristics have less of an impact than might be expected. Quality of care projects may be stimulated to reduce the substantial international variation in this very relevant aspect of setting healthcare goals and priorities with older adults.
Acknowledgements
We thank Janine Liefers and Reinier Akkermans for providing statistical support. The ‘Council for Health and Society’ provided financial support for this research, as described under Funding. Radboud in’to Languages provided language assistance. This research was supported by The Commonwealth Fund, a private foundation based in New York City that supports independent research on healthcare issues and provides grants to improve healthcare practice and policy. The views presented here are those of the authors and not necessarily those of The Commonwealth Fund, its directors, officers or staff.
Appendix. Response rates by country [2]
| Australia | 31% |
| Canada | 28% |
| France | 29% |
| Germany | 26% |
| Netherlands | 25% |
| New Zealand | 27% |
| Norway | 16% |
| Sweden | 23% |
| Switzerland | 60% |
| UK | 23% |
| USA | 24% |
Funding Statement
This work was supported by the ‘Council for Health and Society’, a strategic advisory council for the Dutch government and the employer of the first author. The council provided financial support (not a grant) for the conduct of this research and the preparation of this article, but had no role in the study design; the collection, analysis and interpretation of the data; writing the report; nor the decision to submit the article for publication.
Disclosure statement
The authors confirm that there has been no financial support for this work that could have influenced its outcome and that there are no other potential conflicts of interest associated with this publication.
Notes on contributors
Neeltje Vermunt, MD, MSc, is PhD student at Radboud University Medical Center, Radboud Institute for Health Sciences, Scientific Center for Quality of Healthcare (IQ healthcare), Nijmegen, The Netherlands, and senior advisor at the Council for Health and Society, The Hague, The Netherlands. She initiated the conception and design of the study, performed the statistical analysis, and drafted and wrote the manuscript.
Gert Westert, PhD, is Professor of Health Services Research and theme leader Health Care Improvement Science at Radboud University Medical Center, Radboud Institute for Health Sciences, Scientific Center for Quality of Healthcare (IQ healthcare), Nijmegen, The Netherlands and director of IQ healthcare. He contributed to the conception and design of the study, to the interpretation of the data and analysis and revisions of the manuscript.
Marcel Olde Rikkert, MD, PhD, is Professor of Geriatric Medicine, head of the Department of Geriatrics and coordinator of the Radboudumc Alzheimer Center, Radboud University Medical Center, Nijmegen, The Netherlands. He contributed to the conception and design of the study, to the interpretation of the data and analysis and revisions of the manuscript.
Marjan Faber, PhD, is senior researcher at Radboud University Medical Center, Radboud Institute for Health Sciences, Scientific Center for Quality of Healthcare (IQ healthcare), Nijmegen, The Netherlands. She contributed to the conception and design of the study, to the interpretation of the data and analysis and revisions of the manuscript.
References
- 1.Barnett K, Mercer SW, Norbury M, et al. . Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. [DOI] [PubMed] [Google Scholar]
- 2.Osborn R, Moulds D, Squires D, et al. . International survey of older adults finds shortcomings in access, coordination, and patient-centered care. Health Aff. 2014;33:2247–2255. [DOI] [PubMed] [Google Scholar]
- 3.Bergman H, Karunananthan S, Robledo LMG, et al. . Understanding and meeting the needs of the older population: a global challenge. Can Geriatr J. 2013;16:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried TR, Tinetti ME, Iannone L.. Primary care clinicians’ experiences with treatment decision-making for older persons with multiple conditions. Arch Intern Med. 2011;171:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuben DB, Tinetti ME.. Goal-oriented patient care – an alternative health outcomes paradigm. N Engl J Med. 2012;366:777–779. [DOI] [PubMed] [Google Scholar]
- 6.Reuben DB. Better care for older people with chronic diseases: an emerging vision. JAMA. 2007;298:2673–2674. [DOI] [PubMed] [Google Scholar]
- 7.Robben SHM, Perry M, Olde Rikkert MGM, et al. . Care-related goals of community-dwelling frail older adults. J Am Geriatr Soc. 2011;59:1552–1554. [DOI] [PubMed] [Google Scholar]
- 8.Mulley AG, Trimble C, Elwyn G.. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345:e6572–e6575. [DOI] [PubMed] [Google Scholar]
- 9.Tinetti ME, Esterson J, Ferris R, et al. . Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261–275. [DOI] [PubMed] [Google Scholar]
- 10.Tinetti ME, Fried TR, Boyd CM.. Designing health care for the most common chronic condition – multimorbidity. JAMA. 2012;307:2493–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangin D. Beyond diagnosis: rising to the multimorbidity challenge. BMJ. 2012;344:e3526. [DOI] [PubMed] [Google Scholar]
- 12.Vandenbroucke JPJ, von Elm E, Altman DG, et al. . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524. [DOI] [PubMed] [Google Scholar]
- 13.Von Elm E, Altman DG, Egger M, et al. . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. [DOI] [PubMed] [Google Scholar]
- 14.Commonwealth Fund Social Science Research Solutions – 2014. 2014 International Health Policy Survey of Older Adults (cited 2016 May 1). Available from: http://www.commonwealthfund.org/∼/media/files/surveys/2014/2014-ihp-questionnaire_final.pdf [Google Scholar]
- 15.Osborn R, Squires D.. International perspectives on patient engagement: results from the 2011 Commonwealth Fund Survey. J Ambul Care Manage. 2012;35:118–128. [DOI] [PubMed] [Google Scholar]
- 16.Oh G-J, Moon J, Lee Y-M, et al. . Public awareness of stroke and its predicting factors in Korea: a national public telephone survey, 2012 and 2014. J Korean Med Sci. 2016;31:1703–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You JJ, Downar J, Fowler RA, et al. . Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175:549–556. [DOI] [PubMed] [Google Scholar]
- 18.Bernacki RE, Block SD, Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;1: 1994–2003. [DOI] [PubMed] [Google Scholar]
- 19.Schulman-Green DJ, Naik AD, Bradley EH, et al. . Goal setting as a shared decision making strategy among clinicians and their older patients. Patient Educ Couns. 2006;63:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bynum JPW, Barre L, Reed C, et al. . Participation of very old adults in health care decisions. Med Decis Making. 2014;34:216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards M, Davies M, Edwards A.. What are the external influences on information exchange and shared decision-making in healthcare consultations: a meta-synthesis of the literature. Patient Educ Couns. 2009;75:37–52. [DOI] [PubMed] [Google Scholar]
- 22.Zulman DM, Asch SM, Martins SB, et al. . Quality of care for patients with multiple chronic conditions: the role of comorbidity interrelatedness. J Gen Intern Med. 2014;29:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noordman J, Koopmans B, Korevaar JC, et al. . Exploring lifestyle counselling in routine primary care consultations: the professionals' role. Fam Pract. 2013;30:332–340. [DOI] [PubMed] [Google Scholar]
- 24.Ekdahl AW, Hellström I, Andersson L, et al. . Too complex and time-consuming to fit in! Physicians’ experiences of elderly patients and their participation in medical decision making: a grounded theory study. BMJ Open. 2012;2:e001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohnke H, Zielinski A.. Association between continuity of care in Swedish primary care and emergency services utilization: a population-based cross-sectional study. Scan J Prim Health Care. 2017;35:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hultberg J, Rudebeck CE.. Patient participation in decision-making about cardiovascular preventive drugs – resistance as agency. Scan J Prim Health Care. 2017;35:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sans-Corrales M, Pujol-Ribera E, Gené-Badia J, et al. . Family medicine attributes related to satisfaction, health and costs. Fam Pract. 2006;23:308–316. [DOI] [PubMed] [Google Scholar]
- 28.De Maeseneer JM, De Prins L, Gosset C, et al. . Provider continuity in family medicine: does it make a difference for total health care costs? Ann Fam Med. 2003;1:144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker I, Steventon A, Deeny SR, Association between continuity of care in general practice and hospital admissions for ambulatory care sensitive conditions: cross sectional study of routinely collected, person level data. BMJ. 2017;3:j84. [DOI] [PubMed] [Google Scholar]
- 30.Légaré F, Ratté S, Gravel K, et al. . Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals' perceptions. Patient Educ Couns. 2008;73:526–535. [DOI] [PubMed] [Google Scholar]
- 31.Joseph-Williams N, Elwyn G, Edwards A.. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94:291–309. [DOI] [PubMed] [Google Scholar]


