Abstract
Objectives
We previously found large variations in general practitioner (GP) hypertension treatment probability in oldest-old (>80 years) between countries. We wanted to explore whether differences in country-specific cardiovascular disease (CVD) burden and life expectancy could explain the differences.
Design
This is a survey study using case-vignettes of oldest-old patients with different comorbidities and blood pressure levels. An ecological multilevel model analysis was performed.
Setting
GP respondents from European General Practice Research Network (EGPRN) countries, Brazil and New Zeeland.
Subjects
This study included 2543 GPs from 29 countries.
Main outcome measures
GP treatment probability to start or not start antihypertensive treatment based on responses to case-vignettes; either low (<50% started treatment) or high (≥50% started treatment). CVD burden is defined as ratio of disability-adjusted life years (DALYs) lost due to ischemic heart disease and/or stroke and total DALYs lost per country; life expectancy at age 60 and prevalence of oldest-old per country.
Results
Of 1947 GPs (76%) responding to all vignettes, 787 (40%) scored high treatment probability and 1160 (60%) scored low. GPs in high CVD burden countries had higher odds of treatment probability (OR 3.70; 95% confidence interval (CI) 3.00–4.57); in countries with low life expectancy at 60, CVD was associated with high treatment probability (OR 2.18, 95% CI 1.12–4.25); but not in countries with high life expectancy (OR 1.06, 95% CI 0.56–1.98).
Conclusions
GPs’ choice to treat/not treat hypertension in oldest-old was explained by differences in country-specific health characteristics. GPs in countries with high CVD burden and low life expectancy at age 60 were most likely to treat hypertension in oldest-old.
Key Points
• General practitioners (GPs) are in a clinical dilemma when deciding whether (or not) to treat hypertension in the oldest-old (>80 years of age).
• In this study including 1947 GPs from 29 countries, we found that a high country-specific cardiovascular disease (CVD) burden (i.e. myocardial infarction and/or stroke) was associated with a higher GP treatment probability in patients aged >80 years.
• However, the association was modified by country-specific life expectancy at age 60. While there was a positive association for GPs in countries with a low life expectancy at age 60, there was no association in countries with a high life expectancy at age 60.
• These findings help explaining some of the large variation seen in the decision as to whether or not to treat hypertension in the oldest-old.
Keywords: Oldest-old, hypertension, clinical decision-making, cardiovascular disease burden, life expectancy
Introduction
In the Global Burden of Disease (GBD) study (2015), elevated blood pressure was among the leading risk factors for disability-adjusted life years (DALYs) [1]. Globally, about 10% of all DALYs are lost due to hypertension. To improve management of hypertension, the Lancet Commission issued a 10-point action plan in which one of these points was to individualize antihypertensive treatment according to cardiovascular risk, cultural differences, age, etc. [2].
The group of the oldest-old (patients aged >80 years) is both the fastest growing and also the most heterogeneous age group [3]. Some are healthy with very few chronic conditions, whereas others are frail, have multimorbidity (≥2 chronic conditions), or other complex problems [4]. This heterogeneity makes it particularly challenging for general practitioners (GPs) to find the best strategy (with optimal benefit to risk ratio) when deciding whether or not elevated blood pressure should be treated in this group [5]. This clinical dilemma can lead to variation in treating hypertension in oldest-old [6–9].
In the ATTENTIVE study [10], a large variation was found in GPs’ decision to start antihypertensive treatment in oldest-old. In that study, eight case vignettes of oldest-old were presented to >2500 GPs from 29 (mainly) European countries and, for each case, they were asked whether or not they would start treatment. In the Netherlands, 34% of all cases would have been treated compared with 88% in Ukraine. Part of this variation was explained by the differences in patient characteristics, i.e. level of blood pressure, cardiovascular disease (CVD) and frailty. However, given the variation across countries, it seems feasible that country-specific health characteristics could explain part of the variation.
Therefore, the present study investigates whether country-specific health differences in CVD burden in older patients, and life expectancy at age 60 years, are related to GP treatment probability to start antihypertensive treatment. We hypothesized that there would be a positive association between CVD burden and GP treatment probability, but that life expectancy at age 60 years would modify that association.
Materials and methods
Design and setting
This was an ecological study using a multilevel model. Aggregated country-specific data were used from publicly available sources (see section ‘Variables’) and individual-level data (level of GPs) were used from the Antihypertensive TreaTmENT In Very Elderly (ATTENTIVE) study. In the ATTENTIVE study, GPs from 29 countries (including Brazil, Israel and New Zealand) were enrolled (March–July 2016) [10].
Ethical considerations
The ATTENTIVE study was conducted in compliance with the Declaration of Helsinki [11]. GPs provided informed consent by responding to the questionnaire. Since the participating GPs responded anonymously, no formal medical ethics approval was required from most of the countries. However, in Brazil and Switzerland, the research ethics committees issued a waiver, and in New Zealand the research ethics committee of the University of Auckland approved this study.
Participants
The only inclusion criterion for ATTENTIVE was that each participant had to be a practicing GP; this was established from the first question in the survey. Non-practicing GPs were excluded. GPs were invited by email without offering an incentive. For this study, only GPs that provided an answer for all eight case vignettes were included; this stipulation enabled us to calculate GP treatment probability over all the cases.
Survey
In short, the survey contained eight case vignettes of oldest-old patients (aged >80 years; males and females) that consulted their GPs for a routine visit without showing blood pressure-related symptoms or receiving antihypertensive treatment. All case vignettes differed in three primary characteristics: systolic blood pressure (SBP) of 140 or 160 mm Hg, CVD present or absent, and frailty (yes or no). For each case vignette, GPs were asked to decide if they would start antihypertensive treatment. We piloted and then translated the questionnaire into 21 languages (Additional file 1 in [10]). SurveyMonkey (www.surveymonkey.com, Palo Alto, CA) was used to build the online questionnaire. As an exception, in Ukraine (where web access was limited) a paper questionnaire was used.
Variables
The outcome of this study was the proportion of case vignettes for which GPs decided to start antihypertensive treatment, i.e. GP treatment probability. GPs were dichotomised into two groups according to the median of GP treatment probability, i.e. ≤50% ‘low’, >50% ‘high’.
The exposure was CVD burden per country. CVD burden per country was defined as: the ratio of DALYs in persons aged >70 years lost due to ischemic heart disease and/or stroke and the total DALYs lost in persons aged >70 years. These data were retrieved from the GBD database (hosted by the Institute for Health Metrics and Evaluation). Data specific for individuals >80 years were not available why we chose the next best estimate (>70). The GBD is a public database capturing national estimates on total and disease-specific DALYs [12]. The country-specific CVD burden ranged from 16% in France to 59% in Ukraine (Appendix 1). The countries were divided into two groups according to the median of CVD burden, i.e. <22.5% (‘low’) and ≥22.5% (‘high’).
Country-specific life expectancy at age 60 years was considered a possible effect modifier, and the prevalence of persons aged ≥80 years per country was considered a possible confounder for the association between CVD burden and GP treatment probability. Life expectancy at age 60 years was obtained from the 2015 Global Health Observatory data repository of the World Health Organisation [13]. Prevalence of oldest-old was available from the 2015 report of the United Nations [14]. Data specific for individuals >80 years were not available why we chose the next best estimate (>60). Both covariates were dichotomized in two quantiles according to their medians: life expectancy at age 60 years, low (<24 years) and high (≥24 years) and prevalence of oldest-old, low (<4.6%) and high (≥4.6%).
Per GP, we included gender and years of experience on an individual level from the ATTENTIVE data. Years of experience was categorized into two groups of about equal sizes: <15 years (‘low’) and ≥15 years (‘high’).
The previous ATTENTIVE study [10] showed that patient characteristics (SBP, CVD and frailty) were independently associated with the GPs’ decisions to start antihypertensive treatment. However, for the present study, we were only interested in the overall effect of CVD burden on GP treatment probability; therefore, as an outcome, we chose the proportion of all case vignettes for which GPs decided to start treatment, and neglected the case characteristics (SBP, CVD and frailty).
Statistical analysis
The ATTENTIVE dataset was visually explored and checked for missing data, outliers and inconsistencies. New dichotomized variables were generated (after visual checks) by grouping of the distributions using histograms. The exposure and all covariates were checked for multicollinearity by calculating pairwise correlation coefficients.
Chi-squared tests and unadjusted odds ratios (OR), as well as 95% confidence intervals (CI), were used to investigate whether the exposure (CVD burden) and the other independent variables (GP gender/years of experience, life expectancy at age 60, and prevalence of oldest-old) were associated with the outcome (GP treatment probability).
On a country level, continuous data of CVD burden and averaged GP treatment probability per country were visualized using scatter plots. A linear regression line with 95% CI was derived using a univariate linear regression model. In a sensitivity analysis, this analysis was restricted to those countries where >60% of the GPs responded to the survey.
Chi-squared tests were then used to investigate whether CVD burden was associated with any of the independent variables and, if not on a causal pathway, these were considered to be potential confounders.
All potential confounders were tested for the degree of confounding and/or effect modification using the Mantel–Haenszel test of homogeneity of ORs (detailed in Appendix 3). As pre-specified, the causal model presented stratum-specific ORs and 95% CI for low and high life expectancy at age 60 years. Variables that confounded the association between the exposure and the outcome were included in the final model.
A two-sided p value of .05 was considered statistically significant. All analyses were performed in STATA release 14.2 (Stata Corp, College Station, TX).
Results
In the ATTENTIVE study, 2543 GPs from 29 countries participated. The median response rate for all countries was 26% (21 countries with <60%, eight countries with ≥60%). Of those participating, 1947 GPs (76.6%), provided an answer for all eight case vignettes.
Table 1 presents the baseline characteristics of the participating GPs and the countries, stratified by GP treatment probability. There were 1160 (59.6%) GPs with a low and 787 (40.4%) GPs with a high GP treatment probability. Countries with a high CVD burden showed a positive association with GP treatment probability (OR 3.70, 95% CI 3.03, 4.52; p < .001).
Table 1.
Baseline characteristics of general practitioners (GPs) and countries, and their association with high GP treatment probability to start antihypertensive treatment in oldest-old (n = 1947).
| GP treatment probability |
||||
|---|---|---|---|---|
| Characteristics | Low (≤50%)(n = 1160) | High (>50%)(n = 787) | Crude odds ratio of highGP treatment probability (95% CI) | p Value |
| GP gender | ||||
| Female | 535 (54.6) | 445 (45.4) | 1.00 (reference) | |
| Male | 625 (64.6) | 342 (35.4) | 0.66 (0.55, 0.79) | <.001 |
| Experience as GP | ||||
| <15 years | 558 (56.7) | 427 (43.4) | 1.00 (reference) | |
| >15 years | 602 (62.7) | 358 (37.3) | 0.78 (0.65, 0.93) | .007 |
| Prevalence of oldest-old | ||||
| Low | 404 (45.0) | 493 (55.0) | 1.00 (reference) | |
| High | 756 (72.0) | 294 (28.0) | 0.32 (0.26, 0.38) | <.001 |
| Life expectancy at age 60 years | ||||
| Low | 216 (36.4) | 378 (63.6) | 1.00 (reference) | |
| High | 944 (69.8) | 409 (30.2) | 0.25 (0.20, 0.30) | <.001 |
| Cardiovascular disease burden | ||||
| Low | 930 (69.4) | 411 (30.7) | 1.00 (reference) | |
| High | 230 (38.0) | 376 (62.1) | 3.70 (3.03, 4.52) | <.001 |
p Values are from univariate logistic regression.
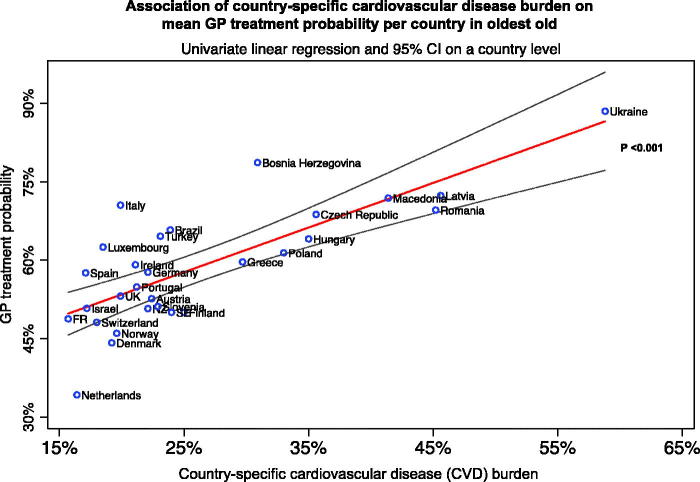
Figure 1 shows the association between CVD burden and GP treatment probability on a country level using continuous data. Strong evidence was found for an association between CVD burden and GP treatment probability (p < .001). Of all countries, the Netherlands had the lowest GP treatment probability (34%) and one of the lowest CVD burdens (16%), whereas Ukraine was among the countries with both the highest GP treatment probability (88%) and CVD burden (59%). When restricting the analysis to countries with a response rate of >60%, the sensitivity analysis confirmed this association (p = .001) (Appendix 2).
Figure 1.
Association between country-specific cardiovascular disease burden and mean general practitioner (GP) treatment probability per country in oldest-old. Univariate linear regression was used (straight line), 95% confidence intervals (outer lines) and p value. FR: France; NZ: New Zealand; SE: Sweden; UK: United Kingdom.
In countries with a high CVD burden, the ORs for treatment were higher compared to countries with a low CVD burden (3.70, 95% CI 3.00, 4.57). Country-specific prevalence of oldest-old was a significant confounder (adjusted OR 2.71, 95% CI 2.17, 3.38) while GP gender and GP years of experience were not confounders. Life-expectancy at age 60 years was an effect modifier (the Mantel–Haenszel test of homogeneity p = .005) of the association between CVD burden and GP treatment probability. Therefore, we included country-specific prevalence of oldest-old in the multivariate model and present stratum specific estimates for low and high life expectancy at age 60 years.
In the final model (Table 2), GPs working in countries with a high CVD burden and a low life expectancy at age 60 years were more likely to start antihypertensive treatment in the oldest-old (adjusted OR 2.18, 95% CI 1.12, 4.25) compared to their counterparts in countries with a low CVD burden. In countries with a high life expectancy at age 60 years, there was no evidence for such an association (adjusted OR 1.06, 95% CI 0.56, 1.98).
Table 2.
Final model including 1947 GPs for the association of cardiovascular disease (CVD) burden on GP treatment probability in oldest-old.
| Fully-adjusted odds ratio ofGP treatment probability(95% CI) | |
|---|---|
| CVD burden (stratum-specific) | |
| Low life expectancy at age 60 | 2.18 (1.12, 4.25) |
| High life expectancy at age 60 | 1.06 (0.56, 1.98) |
| Prevalence of oldest-old | 0.48 (0.39, 0.59) |
Discussion
Statement of principle findings
The clinical dilemma when deciding whether (or not) to start antihypertensive treatment in the oldest-old may not only be explained by differences in patient characteristics but also in country-specific characteristics. In the present study including 1947 GPs from 29 countries, a high country-specific CVD burden was associated with a higher probability of GPs deciding to start antihypertensive treatment in patients aged >80 years. However, the association was modified by country-specific life expectancy at age 60 years. While there was a positive association for GPs in countries with a low life expectancy at age 60 years, there was no association for GPs in countries with a high life expectancy at age 60 years. These findings (partly) explain some of the large variation seen in the decision as to whether or not to treat hypertension in the oldest-old [10].
Strengths and limitations
The inclusion of a large number of GPs from a large number of countries (in Europe and beyond) is a strength of this study; this allowed us to study the relation between country-specific health characteristics and GP decisions in an ecological analysis. Also, we could describe GP treatment probabilities in countries that are not usually included in international studies.
This study also has limitations. First, GP treatment probability was self-reported and based on fictive cases stories and not on, for example, chart reviews. Second, the overall response rate was only 26% across all countries, which is not uncommon in surveys involving GPs [15]. However, our response rate was not lower than in other GP survey studies [16,17] and low response rates of GPs do not necessarily result in selection bias [18,19]. In addition, when restricting our analysis to countries where the GPs responded for ≥60%, the results remained unchanged. Third, we can only report associations and not causation as this was an observational study with limitations such as residual confounding. However, we explored and reported patient-related factors associated with GP treatment probability in an earlier study [10].
Findings in relation to other studies
The results from this study suggest that GPs in countries where their 60-year-old patients will die (on average) before the age of 84 years, base their decision to start antihypertensive treatment in the oldest-old not only on the individual risk or prevalence of oldest-old, but also on the CVD burden of their country. In our opinion, the daily experience and case load provide GPs with sufficient knowledge to assess CVD burden and country-specific DALY of the patients that they see and treat, even without knowing the exact burden. DALYs due to CVD burden are not only a problem in high-income countries but mostly in low- and middle-income countries (LMIC) [20]. The inequity in cardiovascular health in LMIC compared to high-income countries, calls for empowering GPs with the knowledge/skills to meet the requirements in these countries [21]. While our study shows that, in countries with a lower life-expectancy, GPs are more inclined to treat hypertension when CVD burden is high, the effects of such treatment on e.g. mortality or patient-relevant outcomes such as quality of life, remain unclear. Treatment goals for hypertension (especially in older patients) are constantly changing [22]. Although trials including oldest-old show a clear benefit of lowering blood pressure [23,24], the generalizability of these studies is still debated [22,25–27]. In this clinical dilemma, prognosis and life expectancy are issues that GPs relate to in the decision-making process in older patients [6].
Meaning of the study
Future high-quality observational studies, or new trials including the otherwise excluded frail patients with multimorbidity, should be conducted to provide more evidence for decision-making with respect to hypertension treatment in the oldest-old. With evidence that can be generalized for GP patients that are frail and multimorbid, the implementation into daily practice should be thoughtfully planned. Our study found also a crude association of female GPs and GPs with a shorter than 15-year experience to treat more often hypertension in oldest-old. Future studies could further investigate if this association is real. These steps are needed to overcome inequities in treatment decisions across countries with different CVD burdens and life expectancies.
Conclusions
The clinical dilemma when deciding whether (or not) to start antihypertensive treatment in the oldest-old appears not only to be explained by differences in patient characteristics but also in country-specific health characteristics. In this ecological comparative study, GPs living in countries with a high CVD burden and low life expectancy at age 60 years were more likely to start antihypertensive treatment in the oldest-old than GPs in countries with a low CVD burden and a high life expectancy at age 60 years.
Acknowledgements
The authors thank all the general practitioners who participated in this study.
Appendix 1
Characteristics of all 29 included countries (number of GPs included per country)

Appendix 2
Sensitivity analysis including only countries with a response rate of >60% (n = 8). Association between countryspecific cardiovascular disease (CVD) burden on mean general practitioner (GP) treatment probability per country in oldestold. Univariate linear regression was used (red line), 95% confidence intervals (grey lines) and p-value. UK = United Kingdom

Appendix 3
Assessing confounding and testing for effect modification on the association of cardiovascular disease (CVD) burden on GP treatment probability in oldest-old.
| Odds ratio of GP treatment probability (95% CI) | P-value | |
|---|---|---|
| Unadjusted effect of CVD burden | 3.70 (3.00, 4.57) | |
| Effect of CVD burden adjusted for … | ||
| Gender | 3.55 (2.87, 4.41) | 0.19 |
| Female | 4.01 (3.01, 5.34) | |
| Male | 3.01 (2.18, 4.16) | |
| High experience (15 years) | 3.73 (3.02, 4.60) | 0.87 |
| Low | 3.79 (2.81, 5.12) | |
| High | 3.66 (2.71, 4.93) | |
| Life expectancy at age 60 | 1.48 (0.97, 2.29) | 0.005 |
| Low | 2.96 (1.53, 5.72) | |
| High | 0.82 (0.44, 1.53) | |
| Prevalence of oldest old | 2.71 (2.17, 3.38) | 0.57 |
| Low | 2.59 (1.96, 3.41) | |
| High | 2.96 (2.06, 4.24) |
P-values are from Mantel-Haenszel test of homogeneity of odds ratios. Variables in grey were chosen for the final model.
Funding Statement
Dr. Streit’s research is supported by grants (P2BEP3_165353) from the Swiss National Science Foundation (SNF) and the Gottfried and Julia Bangerter-Rhyner Foundation, Switzerland. This study was supported by the Swiss University Conference and the State Secretariat for Education, Research and Innovation (SUC project P-10).
Ethical approval
This study was conducted in compliance with the Declaration of Helsinki [11]. Since the participating GPs responded anonymously, no formal medical ethics approval was required from most of the countries. However, in Brazil and Switzerland, the research ethics committees issued a waiver, and in New Zealand the research ethics committee of the University of Auckland approved this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Sven Streit, MD, MSc, is a general practitioner and researcher at the Institute of Primary Health Care (BIHAM) at the University of Bern, Switzerland.
Sven Streit, MD, MSc, is a general practitioner and researcher at the Institute of Primary Health Care (BIHAM) at the University of Bern, Switzerland.
Robert Burman, MD, PhD is a general practitioner at Vennesla Primary Health Care Center and Chief Medical Officer at Kristiansand Casualty Clinic.
Claire Collins, PhD, is the director of the Research Unit of the Irish College of General Practice, Ireland.
Biljana Gerasimovska Kitanovska, MD, Msc, PhD, Specialist in Internal medicine, Subspecialist in nephrology, Specialist in Family medicine, is employed at Department of nephrology, University Clinical Centre Skopje, Macedonia also teaching at Department of family medicine, Medical faculty, University Sts. Cyril and Methodius, Skopje, Macedonia.
Sandra Gintere, MD PhD, from the Faculty of Medicine, Department of Family Medicine, Riga Stradiņs University, Riga, Latvia.
Raquel Gómez Bravo, MD, PhD Student at the Institute for Health and Behaviour, Research Unit INSIDE, University of Luxembourg. Member of the WONCA Europe Communications Advisory Board. Member of the Executive of the WONCA WORLD Special Interest Group on Family Violence SIGFV.
Kathryn Hoffmann, MD, MPH, Assoc. Prof. Priv.-Doz. is the interim head of the department of General Practice and Family Medicine at the Medical University of Vienna.
Claudia Iftode, MD, from the Timis Society of Family Medicine, Sano Med West Private Clinic, Timisoara, Romania.
Kasper L Johansen, MD, from the Danish College of General Practice, Denmark.
Ngaire Kerse, PhD, MBChB, FRNZCGP, is a general practitioner and researcher at the University of Auckland leads the School of Population Health.
Tumoas H Koskela, MD PhD, from the Department of General Practice, University of Tampere, Tampere, Finland.
Sanda Kreitmayer Pestic, MD MSc is a specialist in Family medicine and researcher at the Family Medicine Department, Medical School at the University of Tuzla, Bosnia and Herzegovina.
Donata Kurpas, MD, PhD, Assoc. Prof. is a family practitioner, public health specialist and researcher at the Family Medicine Department, Wroclaw Medical University, Poland.
Christian Mallen is Professor of General Practice and Deputy Director of the Institute for Primary Care and Health Sciences, Keele University.
Hubert Maisonneuve is primary care physician and senior lecturer, Primary Care Unit, Faculty of Medicine, University of Geneva, Geneva, Switzerland.
Christoph Merlo, MD is a general practitioner and head of the Institute of Primary and Community Care Lucerne, Switzerland and lecturer at the Universities of Lucerne and Berne, Switzerland.
Yolanda Mueller, MD MIH, from the Institute of Family Medicine Lausanne (IUMF), Switzerland.
Christiane Muth, MD, MPH is a general internist and senior researcher at the Institute of General Practice at Goethe University, Frankfurt / Main, Germany.
Rafael Herrera Ornelas, MD, from the Hospital Israelita Albert Einstein, São Paulo, Brazil.
Marija Petek Šter, MD, PhD is a general practitioner and associated professor of family medicine at the University of Ljubljana, Slovenia.
Ferdinando Petrazzuoli, MD, MSc is a researcher at the Center for Primary Health Care Research of the Department of Clinical Research Center at Lund University, Malmö, Sweden.
Thomas Rosemann, MD, PhD, full Professor for Primary Care at the University of Zurich.
Martin Sattler, MD is a General Practitioner in Luxembourg. Member of the Executive of the SSLMG, Societé Scientifique Luxembourgois en Medicine Generale. WONCA Europe Council representative for Luxembourg.
Zuzana Švadlenková, MUDr is a general practitioner Prague, Czech Republic.
Athina Tatsioni, MD, PhD is Assistant Professor at the Faculty of Medicine at the School of Health Sciences in the University of Ioannina, Greece.
Hans Thulesius is an associate professor in family medicine and part time family physician. He is involved in a diversity of primary care research fields.
Tkachenko Victoria I, MD, PhD, DrMSc is a general practitioner, associate professor, senior lecturer and researcher at the Institute of Family Medicine at the Shupyk National Medical Academy of Postgraduate Education, Kiev, Ukraine.
Peter Torzsa, MD, PhD, from the Department of Family Medicine, Semmelweis University, Budapest, Hungary.
Rosy TSOPRA, MD, PhD works at LIMICS INSERM U1142, University of Paris 13, and AP-HP in Paris, France.
Canan Tuz, MD is a family medicine specialist and assistant professor at the Family Medicine Department of Erzincan University, Turkey.
Marjolein Verschoor, MD, is a general practitioner from Switzerland.
Rita Viegas, MD is a graduated general practitioner and an invited assistant at the Department of Family Medicine, NOVA Medical School, Portugal.
Shlomo Vinker, MD, MHA is a full professor in family medicine, vice dean and chair of the department of family medicine, Sackler Faculty of Medicine, Tel Aviv University. Family physician and the chief medical director of Leumit Health Services, Israel.
Margot WM de Waal, PhD, is from the Department of Public Health and Primary Care, Leiden University Medical Center, the Netherlands.
Andreas Zeller, MD, MSc is a general practitioner and head of the Centre for Primary Health Care at the University of Basel, Switzerland.
Nicolas Rodondi, MD, MAS is Full Professor and Director of primary care and Head of Ambulatory Care, University of Bern, Switzerland.
Rosalinde K.E. Poortvliet, MD, PhD is a general practitioner, epidemiologist and senior researcher at the Department of Public Health and Primary Care at the Leiden University Medical Center, Leiden, the Netherlands.
References
- 1.Collaborators GBDRF. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen MH, Angell SY, Asma S, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388:2665–2712. [DOI] [PubMed] [Google Scholar]
- 3. The United Nations: world population prospects: the 2012 revision; [cited 2017 Nov 6]. Available from: http://esa.un.org/unpd/wpp.
- 4.World Health Organisation (WHO) Active ageing: a policy framework; 2002; [cited 2017 Nov 7]. Available from: http://apps.who.int/iris/bitstream/10665/67215/1/WHO_NMH_NPH_02.8.pdf [Google Scholar]
- 5.Materson BJ, Garcia-Estrada M, Preston RA.. Hypertension in the frail elderly. J Am Soc Hypertens. 2016;10:536–541. [DOI] [PubMed] [Google Scholar]
- 6.Jansen J, McKinn S, Bonner C, et al. General practitioners’ decision making about primary prevention of cardiovascular disease in older adults: a qualitative study. PLoS One. 2017;12:e0170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Peet PG, Drewes YM, Gussekloo J, et al. GPs’ perspectives on secondary cardiovascular prevention in older age: a focus group study in the Netherlands. Br J Gen Pract. 2015;65:e739–e747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bog-Hansen E, Merlo J, Gullberg B, et al. Survival in patients with hypertension treated in primary care. A population-based follow-up study in the Skaraborg Hypertension and Diabetes Project. Scand J Prim Health Care. 2004;22:222–227. [DOI] [PubMed] [Google Scholar]
- 9.Getz L, Kirkengen AL, Hetlevik I, et al. Ethical dilemmas arising from implementation of the European guidelines on cardiovascular disease prevention in clinical practice. A descriptive epidemiological study. Scand J Prim Health Care. 2004;22:202–208. [DOI] [PubMed] [Google Scholar]
- 10.Streit S, Verschoor M, Rodondi N, et al. Variation in GP decisions on antihypertensive treatment in oldest-old and frail individuals across 29 countries. BMC Geriatr. 2017;17:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 12.Institute for Health Metrics and Evaluation (IHME) GBD compare data visualization. Seattle (WA): IHME, University of Washington; 2016; [cited 2017 Feb 24]. Available from: http://vizhub.healthdata.org/gbd-compare [Google Scholar]
- 13. Global Health Observatory data repository of the World Health Organisation (WHO); [cited 2017 Nov 7]. Available from: http://apps.who.int/gho/data.
- 14. World Population Prospects: The 2015 Revision by the United Nations (UN); [cited 2017 Nov 7]. Available from: https://esa.un.org/unpd/wpp/Download/Standard/Population.
- 15.McAvoy BR, Kaner EF.. General practice postal surveys: a questionnaire too far? BMJ. 1996;313:732–733. discussion 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyman DJ, Pavlik VN.. Self-reported hypertension treatment practices among primary care physicians: blood pressure thresholds, drug choices, and the role of guidelines and evidence-based medicine. Arch Intern Med. 2000;160:2281–2286. [DOI] [PubMed] [Google Scholar]
- 17.Tomasik T, Windak A, Seifert B, et al. The self-perceived role of general practitioners in care of patients with cardiovascular diseases. A survey in Central and Eastern European countries following health care reforms. Int J Cardiol. 2013;164:327–333. [DOI] [PubMed] [Google Scholar]
- 18.Kellerman SE, Herold J.. Physician response to surveys. A review of the literature. Am J Prev Med. 2001;20:61–67. [DOI] [PubMed] [Google Scholar]
- 19.Asch DA, Jedrziewski MK, Christakis NA.. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–1136. [DOI] [PubMed] [Google Scholar]
- 20.Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. [DOI] [PubMed] [Google Scholar]
- 21.Joshi R, Jan S, Wu Y, et al. Global inequalities in access to cardiovascular health care: our greatest challenge. J Am Coll Cardiol. 2008;52:1817–1825. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer MA, McMurray JJ.. Lessons in uncertainty and humility – clinical trials involving hypertension. N Engl J Med. 2016;375:1756–1766. [DOI] [PubMed] [Google Scholar]
- 23.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years. A randomized clinical trial. JAMA. 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 25.Messerli FH, Sulicka J, Gryglewska B.. Treatment of hypertension in the elderly. N Engl J Med. 2008;359:972–973. author reply 3–4. [PubMed] [Google Scholar]
- 26.Oparil S, Lewis CE.. Should patients with cardiovascular risk factors receive intensive treatment of hypertension to <120/80 mm Hg target? A protagonist view from the SPRINT Trial (Systolic Blood Pressure Intervention Trial). Circulation. 2016;134:1308–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonn EM, Yusuf S. Should patients with cardiovascular risk factors receive intensive treatment of hypertension to <120/80 mm Hg target? An antagonist view from the HOPE-3 Trial (Heart Outcomes Evaluation-3). Circulation. 2016;134:1311–1313. [DOI] [PubMed] [Google Scholar]