Abstract
Background
Neurogenic detrusor overactivity after spinal cord injury (SCI) causes urinary incontinence and reduces bladder capacity. Surface electrical genital nerve stimulation (GNS) acutely inhibits reflex bladder contractions. The stimulation amplitude selected for GNS is typically twice the amplitude that is required to evoke the pudendal-anal reflex. There is concern about the ability of persons with sensation to comfortably tolerate effective levels of GNS. The objective of this work is to determine if persons with incomplete SCI are able to tolerate acute GNS for bladder inhibition.
Methods
Twenty-four subjects with neurogenic detrusor overactivity, SCI, and pelvic sensation were enrolled in this case series. The setting was the Spinal Cord Injury Service of a Veterans Affairs Medical Center. Primary outcome measures were sensation threshold and tolerable stimulation amplitude; secondary outcome measures were bladder capacity and bladder contraction inhibition.
Results
GNS was tolerable up to 30±16 mA (range 8 mA to ≥60 mA) at amplitudes greater than twice the pudendal-anal (PA) reflex threshold, which was 8±5 mA (range 4 mA to 20 mA). Twelve subjects tolerated GNS at greater than twice the PA, six tolerated 1–1.5 times the PA, and five had no identifiable PA. GNS at tolerable amplitudes inhibited reflexive bladder contractions or increased bladder capacity 135±109 mL (n=23). GNS did not cause autonomic dysreflexia or intolerable spasticity.
Conclusions
GNS is tolerable at amplitudes that effectively inhibit neurogenic detrusor overactivity in individuals with pelvic sensation. GNS therefore is a tool with potential clinical applications for persons with preserved sensation.
Keywords: Electrical stimulation, Spinal cord injury, Neurogenic bladder, Genital nerve stimulation
Introduction
Neurogenic bladder dysfunction is among the most challenging medical complications faced in spinal cord injury (SCI) medical practice, and it is among the most damaging to the health and quality of life of persons with SCI. Neurogenic bladder dysfunction can cause uninhibited bladder contractions, which can reduce urinary continence, reduce bladder capacity, contribute to episodes of autonomic dysreflexia, and significantly impact an individual's quality of life. Neurogenic bladder dysfunction has remained difficult to treat from the perspective of persons with SCI. Persons with SCI rank pelvic dysfunction as one of the most important areas to obtain neural restoration, even when compartmentalized into its components of erectile function and bladder/bowel dysfunction.1 Current management methods of neurogenic detrusor overactivity, including medical and surgical treatment options, are often limited by excessive invasiveness, lack of adequate effect, and troublesome side effects, including growing concerns about the cognitive side effects of chronic anticholinergic medication use.2,3
Surface electrical stimulation of the genital nerves (GNS) can acutely inhibit reflexive bladder contractions and has been evaluated for use in people with SCI in multiple studies.4–12 In men, the technique typically involves surface electrical stimulation applied over the base of the penis to stimulate the genital nerves (Fig. 1), causing reflex inhibition of the bladder through both sympathetic and parasympathic sensory pathways.13–15 In women, the first electrode is placed above the clitoris and the second electrode is placed on the labia majora or inner thigh. GNS may be applied continuously to prevent contractions. Alternatively, it may be applied conditionally and turned on only when a bladder contraction is detected so as to arrest the event.
Figure 1.
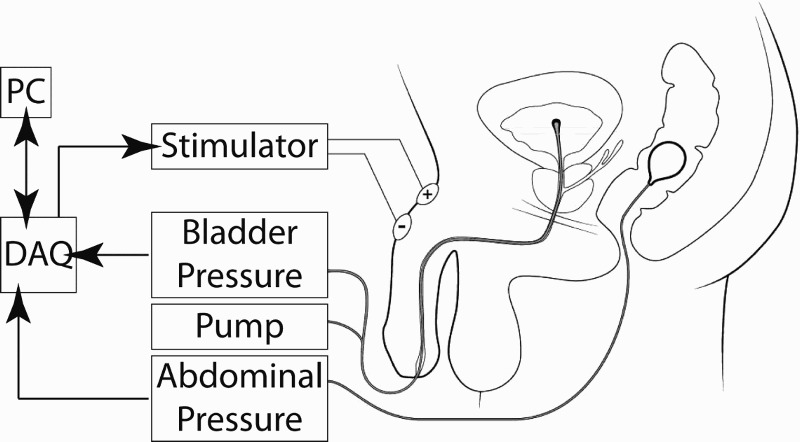
Urodynamic testing with GNS. A dual lumen catheter is inserted through the urethra into the bladder, with one lumen used for measuring bladder pressure and the other lumen for filling with saline. A rectal balloon catheter measures abdominal pressure. The stimulator provides electrical stimulation across two surface electrodes placed on the dorsum of the penis, targeting the genital nerves. A host computer (PC) communicates with a data acquisition board (DAQ), which acquires the pressure signals and also triggers the stimulator device on command.
A stimulation amplitude that is twice the threshold required to evoke a pudendal-anal reflex is typically chosen for effective bladder inhibition with surface GNS.4–12 However, the effect of stimulation amplitude on GNS effectiveness has not been well examined. GNS acutely improved bladder capacity at twice the amplitude to evoke the pudendal-anal reflex but not at the amplitude to evoke the pudendal-anal reflex in 10 subjects with SCI.5 Therefore, stimulation amplitudes below twice the pudendal-anal reflex may be effective.
Concern over subject tolerance to GNS can limit application in individuals with complete SCI. Persons with incomplete SCI, who comprise almost half of new spinal cord injuries,16 commonly report hyperalgesia and neuropathic pain14 and are able to sense electrical stimulation such as GNS. Two studies testing GNS found that uncomfortable sensations from GNS limited the stimulus amplitude in two of nine subjects17 and in one of seven subjects,18 respectively. GNS has the potential to be irritating or uncomfortable and some subjects may have difficulty tolerating the intervention.17,19 Surface electrical neuromodulation at non-genital locations for bladder inhibition in sensory intact children produced uncomfortable sensation that limited the amount of amplitude that was usable, particularly in subjects using a higher stimulation frequency.20 Percutaneous GNS in women with overactive bladder reported limitations in tolerable amplitudes of stimulation, although the tolerable stimulation level increased over one week of use.21 Feedback to the authors from SCI clinicians following GNS presentations at multiple scientific and clinical meetings has raised concern regarding the ability of persons with incomplete SCI to comfortably tolerate electrical stimulation applied to the genital nerves. The purpose of this study was to determine the GNS amplitudes that were tolerable for subjects with pelvic sensation, and then determine if GNS was effective at tolerable amplitudes.
Methods
Subjects
This study was conducted at the Louis Stokes Cleveland VA Medical Center and approved by their Institutional Review Board (IRB). Subjects with incomplete SCI and neurogenic detrusor overactivity (NDO), which was diagnosed with urodynamics (UDS), were analyzed in this work. The inclusion criteria included: suprasacral spinal cord injury with detrusor overactivity on UDS; no reported change in motor or sensory function in the last month; no active sepsis; no active pressure sores in the pelvic region; no documented history of ureteric reflux in the medical record; and no history of urethral tear in the medical record. All subjects were enrolled at least 6 months after the occurrence of their spinal cord injury. Of thirty-nine subjects screened, nine subjects did not have neurogenic detrusor overactivity or meet the inclusion and exclusion criteria, and six more were found to be sensory-complete, and were therefore not included in this analysis. Thus, twenty-four subjects with NDO and incomplete SCI with sensation were included in this analysis (Table 1).
Table 1.
Subject characteristics.
| Subject | Injury level | ASIA score | Age | Years since injury | Bladder management |
|---|---|---|---|---|---|
| 1 | L1 | D | 68 | 1 | VV |
| 2 | T10 | C | 63 | 3 | EC |
| 3 | C1 | D | 60 | 32 | IC/EC |
| 4 | C5 | B | 64 | 25 | IC |
| 5 | C1 | D | 58 | 13 | VV |
| 6 | C7 | D | 65 | 15 | VV |
| 7 | T12 | D | 61 | 13 | IC |
| 8 | C7 | D | 59 | 1 | IC |
| 9 | T12 | A | 54 | 29 | EC/VV |
| 10 | T12 | C | 59 | 3 | VV |
| 11 | T9 | D | 45 | 2 | IC |
| 12 | C6 | D | 53 | 11 | IC |
| 13 | C5 | D | 65 | 4 | EC/VV |
| 14 | T2 | C | 65 | 4 | FC |
| 15 | C6 | D | 76 | 53 | VV |
| 16 | L1 | D | 70 | 1 | VV |
| 17 | C7 | D | 71 | 14 | VV |
| 18 | C1 | D | 63 | 1 | FC |
| 19 | L1 | D | 43 | 16 | IC |
| 20 | L1 | D | 47 | 14 | VV |
| 21 | T6 | B | 23 | 4 | EC |
| 22 | C7 | D | 62 | 1 | VV |
| 23 | C1 | D | 63 | 32 | VV |
| 24 | C5 | C | 73 | 30 | EC/IC |
| Summary | 60±11 | 13±14 | |||
Injury level refers to ASIA neurologic level of injury; ASIA score is recorded in the standard A through E format. All subjects had upper motor neuron spinal cord injuries. Bladder management strategies include volitional voiding (VV), external catheterization (EC), intermittent catheterization (IC), Foley catheterization (FC), or some combination of these strategies. Subjects 14, 19, 20, 21 and 23 had non-traumatic, autoimmunite spinal cord injuries. Subject 19 had a spinal cord injury secondary to a leukodystrophy-related cord lesion, while subjects 14, 20, 21 and 23 had multiple sclerosis-related spinal cord lesions.
Urodynamics and data acquisition
In the clinic, subjects lay on a padded testing table with a catch bucket beneath them to collect and measure fluid expressed from the bladder. Figure 1 illustrates the data acquisition instrumentation. A 7-French dual lumen urethral catheter was inserted, with one lumen used for filling the bladder with room temperature normal saline and the other lumen used for measuring intravesicular pressure. Abdominal pressure was measured using a rectal balloon catheter, and detrusor pressure was calculated as the difference between intravesicular and abdominal pressures. Pressure was measured from these catheters using inline fluidic transducers (Utah Medical Inc., Midvale, UT, USA). A data acquisition board acquired pressure data and triggered an electrical stimulator (DS7A, Digitimer, Welwyn Garden City, UK) while a host computer ran the custom LabVIEW software that managed these tasks (National Instruments, Austin, TX, USA). Subjects first received a control fill without stimulation to verify the diagnosis of neurogenic detrusor overactivity (NDO). A clinical urodynamics system (Dorado, Laborie, Williston, VT, USA) was used and set to a flow rate of 50 mL/min for artificial bladder filling.
Electrical stimulation
GNS was applied across two round surface electrodes (Natus, Middleton, WI, USA) that were 2 cm in diameter. The cathode was placed on the proximal, dorsal penile shaft and the anode placed approximately 2 cm distally. Alternatively, a 5×10 cm surface electrode (Natus, Middleton, WI, USA) was sometimes used as the anode, placed on the mons pubis. In our female subject, the cathode was placed directly superior to the clitoris and the anode was placed on the left lateral labia majora. Biphasic, charge-balanced, cathodic-leading pulses were delivered at 20 Hz. Stimulation amplitudes above 60 mA were not tested. Both continuous and conditional GNS were tested. Conditional GNS was tested to determine if we could inhibit bladder contractions, and continuous GNS was tested to determine if we could significantly increase bladder capacity.
Stimulation amplitudes
The sensation threshold, tolerance limit, and pudendal-anal reflex threshold were determined. Sensation threshold was determined by first setting the stimulation amplitude to 1 mA and applying stimulation lasting a few seconds in duration, and then increasing the amplitude in increments of about 1 mA and retesting until the subject could perceive stimulation.
Stimulation was increased above the sensation threshold until the tolerance limit was achieved. The tolerance limit was defined by the study participant. Subjects were told to report when stimulation was still comfortable, but they did not want it to increase to avoid discomfort. This amplitude was defined as the tolerance limit. The tolerance amplitude was retested after pudendal-anal reflex testing and adjusted if necessary because some subjects became accustomed to the sensation of stimulation after initially encountering it and were then better able to tolerate it.
The threshold to evoke the pudendal-anal reflex was determined. The stimulation amplitude was increased above the sensation threshold until an amplitude that evoked a visible or palpable reflexive external anal sphincter contraction was reached.
Genital nerve stimulation effectiveness testing
The stimulation amplitude for effectiveness testing was set to twice the pudendal-anal reflex threshold if that amplitude was tolerable and the pudendal-anal reflex was observed. These subjects consented to test GNS at home after this study. Therefore, the expected home use amplitudes were also used for effectiveness testing acutely. In subjects who could not tolerate twice the pudendal-anal reflex or who had no pudendal-anal reflex, the stimulation amplitude was set near the subject's tolerance limit.
Subjects received an initial cystometrogram to confirm neurogenic detrusor overactivity and then a series of cystometric fill trials randomizing whether GNS was applied during the fill trial; 6±2 (range 2–8) fills were conducted in each subject. A time of 10–20 minutes was spent waiting between each trial to limit carry over effects. The number of fill trials was limited by subject availability for a testing session, up to 4 hours. Stimulation occurred in both operator-controlled stimulation, in which investigators adjusted stimulation in response to subject tolerance, and subject-controlled stimulation, in which the subjects themselves adjusted the amplitude of stimulation directly, through the use of a hand-held knob that allowed for self-titration of the stimulus amplitude.
Data analysis
Three stimulation levels were measured, including sensation threshold, pudendal-anal reflex threshold, and tolerance limit. In addition, twice the pudendal-anal reflex threshold was calculated and stimulation amplitudes for administering GNS were determined. Intravesical and abdominal pressure data were recorded to determine if stimulation was effective to inhibit bladder contractions and increase bladder capacity. Baseline capacity was determined by averaging bladder capacity from all trials without stimulation, and stimulation capacity was similarly determined by averaging bladder capacities from all trials in which stimulation was applied. Stimulation data was pooled for all subjects and compared using an analysis of variance (ANOVA) with a significance level of 0.05 to determine if stimulation levels were significantly different from each other. Stimulation amplitudes are presented as (median ± standard deviation) and bladder capacities are presented as (mean ± standard deviation).
Results
Tolerance and stimulation amplitudes
The amplitude at which individuals could sense stimulation (sensation threshold) was 6±4 mA (Table 2). The amplitude required to elicit the pudendal-anal reflex was 8±5 mA (Table 1). The tolerance limit was 30±16 mA (Table 2).
Table 2.
Subject responses to GNS.
| Subject | Sensation threshold (mA) | Pudendal-anal threshold (mA) | Tolerance limit (mA) | GNS amplitude (mA) | Bladder contraction inhibited? | Baseline bladder volume (mL) | Bladder capacity increase (mL) |
|---|---|---|---|---|---|---|---|
| 1 | 9 | 8 | 15 | 15 | Y | 249 | 0 |
| 2 | 6 | 10 | 30 | 20 | Y | 282 | 0 |
| 3 | 3 | 6 | 8 | 7 | Y | 222 | 127 |
| 4 | 8 | 8 | 35 | 16 | NA | 148 | NA |
| 5 | 2 | NA | >60 | 30 | N | 479 | 113 |
| 6 | 3 | 6 | 12 | 12 | Y | 445 | 0 |
| 7 | 5 | 9 | 15 | 15 | Y | 201 | 110 |
| 8 | 6 | NA | 40 | 30 | Y | 455 | 320 |
| 9 | 8 | 17 | 60 | 50 | Y | 572 | 109 |
| 10 | 6 | NA | 20 | 20 | N | 295 | 55 |
| 11 | 7 | NA | 22 | 20 | Y | 409 | 84 |
| 12 | 14 | 14 | 22 | 22 | Y | 343 | 187 |
| 13 | 2 | 17 | >60 | 40 | Y | 90 | 203 |
| 14 | 15 | NA | 30 | 30 | Y | 47 | 0 |
| 15 | 3 | 8 | 9 | 8 | Y | 245 | 170 |
| 16 | 4 | 8 | 30 | 28 | Y | 560 | 65 |
| 17 | 9 | 14 | 36 | 28 | Y | 122 | 162 |
| 18 | 2 | 4 | 9 | 8 | N | 180 | 130 |
| 19 | 20 | 4 | 40 | 30 | Y | 485 | 283 |
| 20 | 4 | 10 | 14 | 14 | N | 297 | 91 |
| 21 | 6 | 4 | 45 | 30 | Y | 181 | 408 |
| 22 | 5 | 6 | 35 | 30 | Y | 165 | 302 |
| 23 | 5 | 20 | 25 | 25 | Y | 389 | 72 |
| 24 | 3 | 7 | 30 | 30 | Y | 353 | 117 |
| Summary | 6±4 | 8±5 | 30±16 | 25±11 | 19/23 | 301±149 | 135±109 |
No pudendal-anal reflex was observed in subjects 5, 8, 10, 11, or 14. Subject 4 demonstrated symptoms of autonomic dysreflexia, due to filling the bladder during urodynamics, before collecting data on effectiveness of GNS. In subject 4 GNS was applied with the bladder empty and no AD occurred at 35 mA, and therefore sensation and tolerance data were collected.
All subjects with a pudendal-anal reflex tolerated stimulation above the level required to evoke the pudendal-anal reflex. The tolerance threshold was larger than both twice the pudendal-anal reflex threshold and the sensation threshold (ANOVA, P < 0.01, Fig. 2). The tolerance amplitude for individual subjects was 3.5±2.8 (range 1.1–11.3) times the pudendal-anal reflex threshold. Twelve of 19 subjects who had a pudendal-anal reflex were able to tolerate stimulation greater than or equal to twice the amplitude required to elicit the pudendal-anal reflex (Table 2). Seven subjects were able to tolerate stimulation at amplitudes between one and two times the pudendal-anal reflex threshold. Subject 4 had autonomic dysreflexia related symptoms upon bladder filling and therefore stimulation during bladder filling was not tested (NA in Table 2). Nineteen of twenty four subjects demonstrated a pudendal-anal reflex; the remaining five of which had no identifiable pudendal-anal reflex.
Figure 2.
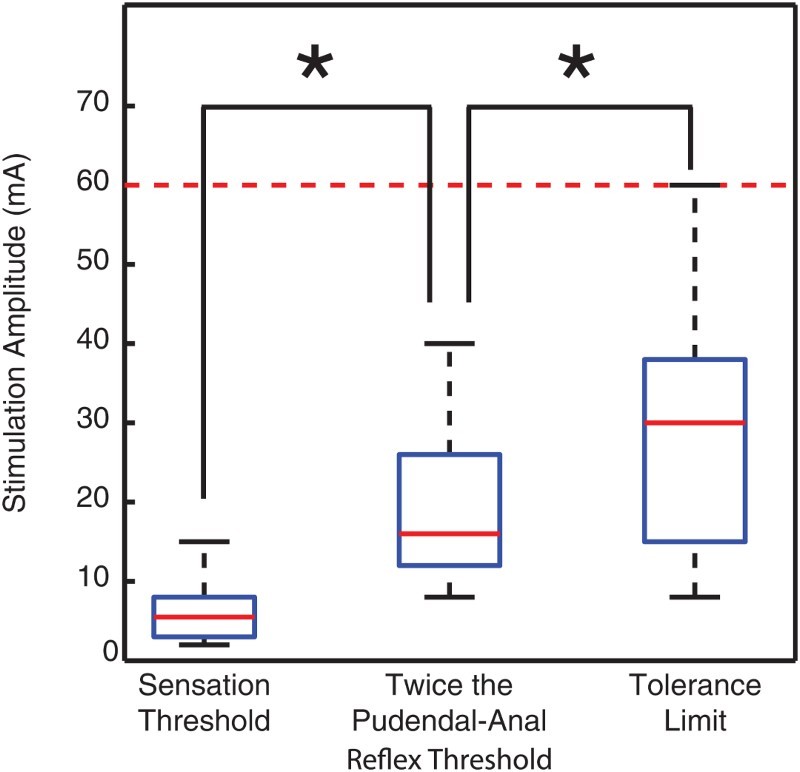
GNS is tolerable. The tolerance threshold amplitude was larger than twice the pudendal-anal reflex threshold, which was larger than the sensation threshold (ANOVA, P < 0.01). Twice the pudendal-anal reflex is the amplitude typically used for GNS. These data demonstrate that individuals with sensation can tolerate stimulation amplitudes typically used for GNS. GNS at tolerable amplitudes either inhibited reflexive bladder contractions or increased bladder capacity in all subjects tested. Boxplots indicate the distributions of amplitudes: the 25th and 75th quantiles and median are horizontal lines within the box and the 10th and 90th quantiles are denoted by the stems above and below the boxes. The dashed line shows the stimulation limit of 60 mA; values above that level were not tested.
The stimulation amplitude for effectiveness testing was set to twice the pudendal-anal reflex if that amplitude was tolerable and the pudendal-anal reflex was observed (n=12). In 7 of those 12 subjects, stimulation amplitudes were set to greater than twice the pudendal-anal reflex. Three subjects demonstrated a tolerance limit greater than 60 mA, but amplitude was not increased above 60 mA. (Table 2) Retesting tolerance amplitude after pudendal-anal reflex testing (and adjusting amplitude, if necessary) was conducted because some subjects became accustomed to the sensation of stimulation after encountering it initially for the first time, and were better able to tolerate it; in subjects 6, 10, 11, 12, and 22, the tolerance limits increased by approximately 5 mA. Subjects 9, 16, and 24 perceived the initial onset of stimulation as intense, and therefore, stimulation was started at half of the amplitude and then immediately increased to the target level.
Although subjects 18, 21 and 23 tolerated GNS for this acute study, at the end of the study they felt that the sensation was too unnatural and uncomfortable and concluded that they would not want to use that level of stimulation chronically. Therefore, the tolerance amplitudes for these 3 subjects are overestimated.
GNS effectiveness
GNS was tested in 23 subjects and significantly increased their bladder capacities by 135±109 mL (Fig. 3, Table 2) across all subjects from 307 mL to 442 mL (ANOVA, P=0.007, n=23). GNS delayed bladder contractions in 19 of those 23 subjects (Table 2); this effect was directly observed under urodynamic monitoring. Bladder capacity significantly increased by 143±118 mL in the 19 subjects that GNS was successful in preventing or arresting bladder contractions (ANOVA, P = 0.01, n=19). The 4 subjects whose bladder contractions were not acutely inhibited were different from the 4 subjects who did not show an increase in bladder capacity. Therefore, all 23 subjects tested had either increased bladder capacity or delayed bladder contractions in response to GNS at tolerable amplitudes.
Figure 3.
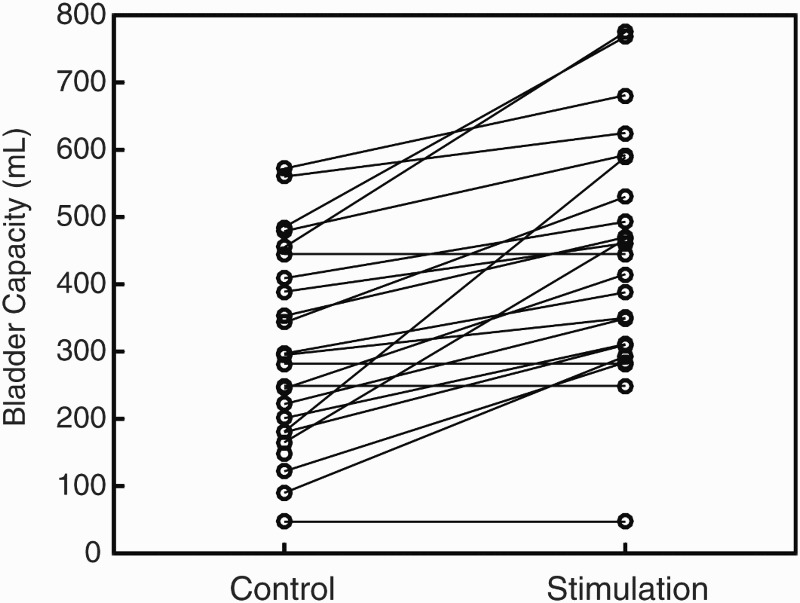
GNS increased bladder capacity and was effective at tolerable stimulation amplitudes. Responses are shown for 23 individual subjects. GNS significantly increased bladder capacities by 135±109 mL from 307 mL (control) to 442 mL (stimulation) (n=23). Bladder capacities increased in 19 of 23 subjects. GNS inhibited acute bladder contractions, but did not increase bladder capacity in four subjects.
GNS was applied with amplitudes of 25±11 mA (Table 2). There was no significant difference between the amplitudes used for bladder inhibition and either the tolerance limit or twice the pudendal-anal reflex threshold.
Discussion
GNS is tolerable in individuals with incomplete SCI and sensation
It was unknown if persons with sensory function can tolerate GNS at effective levels, which is a key factor in the future clinical application of surface GNS in neurogenic bladder populations beyond the subset of sensory-complete SCI. Stimulation amplitudes of twice the threshold required to evoke a pudendal-anal reflex are typically chosen for effective bladder inhibition with surface GNS. Although GNS at these amplitudes has been applied to some individuals with sensation in a number of studies, concerns about the ability of persons with incomplete SCI to tolerate GNS remain. The results of this study demonstrate that GNS is tolerable for individuals with incomplete SCI and pelvic sensation, and that GNS at tolerable levels can inhibit reflexive bladder contractions and increase bladder capacity.
Sensation and tolerance
All subjects could sense stimulation at low levels of amplitude with little variance among subjects. There was much greater variance in the tolerance limits of subjects. Some of this variance may be due to such factors as differences in a subject's individual pain management strategy, variation in level and severity in spinal lesion, or most likely, subjective psychological differences in the perception of sensations, including pain. We believe that the number of subjects included in this study is large enough to reasonably represent the variance in perception of GNS that would occur in the broader population of individuals with incomplete spinal cord injury.
Some subjects initially approached electrical stimulation with caution due to the unusual sensation that it evoked; these subjects were unfamiliar with electrical stimulation and had never experienced it before. Subjects that were naïve to electrical stimulation reported that the sensation was unnatural, but not uncomfortable. Over the course of the testing session, they became accustomed to the sensation of GNS and reported that they were interested in increasing the stimulation amplitude during cystometric fills with GNS.
Subjects 9, 16, and 24 experienced discomfort in the first second or two of stimulation onset. For these subjects, we turned stimulation on at a lower amplitude and then within approximately two seconds increased the stimulation amplitude to effectively inhibit their bladder contractions, which they tolerated. Because the initial onset of stimulation may be perturbing, this approach of increasing the amplitude may be an appropriate strategy.
GNS was not observed to trigger autonomic dysreflexia in any subjects. Subject 4 experienced autonomic dysreflexia in response to bladder filling, however GNS at amplitudes up to 35 mA did not cause symptoms of AD. Bladder distention is one of the most common causes of autonomic dysreflexia after SCI, and GNS presents the potential to decrease AD through inhibiting neurogenic detrusor overactivity and decreasing intravesical pressure8.
GNS is effective at tolerable amplitudes
In agreement with the literature, we found GNS to be acutely effective at inhibiting bladder contractions and increasing bladder capacity. The literature suggests that the amplitude for GNS be set to twice the threshold to evoke the pudendal-anal reflex.5 Nineteen subjects demonstrated a pudendal-anal reflex and, of those, twelve tolerated stimulation at twice this reflex threshold. For the other eleven subjects in our study, we stimulated the genital nerve at amplitudes based on subjects’ tolerance limits and still observed bladder inhibition. There was no significant difference between the amplitudes used for bladder inhibition and either the tolerance limit or twice the pudendal-anal reflex threshold. The effect of stimulation amplitude on GNS effectiveness has not been examined. The results of this study demonstrate that GNS can be effective at stimulation amplitudes between 1-2 times the pudendal-anal reflex.
The GNS amplitudes used for inhibition in this study (25±11 mA) are on the lower end compared to those reported for similar studies. Other reports testing acute GNS have typically used stimulation amplitudes of approximately 50±15 mA, with a range of 20-80 mA.4–7,18,22 The pudendal-anal reflex thresholds were also lower than typically reported, suggesting that either the electrode interface may have had a lower impedance or we were more sensitive in identifying the pudendal-anal reflex.
Thus GNS provides a potential benefit to subjects at lower stimulation amplitudes than expected, a key point for this population of persons with potential for lesser ability to tolerate high stimulus amplitudes. It is encouraging that bladder inhibition is possible at lower amplitudes than previously reported. Percutaneous GNS has been used in women with an intact spinal cord and overactive bladder21, indicating that GNS may be useful in persons with intact sensation. Other sensate populations with detrusor overactivity, such as persons with multiple sclerosis, traumatic brain injury, or stroke, and neurologically intact persons with overactive bladder, may also benefit from GNS.
Limitations
Limitations of this study include neurologic heterogeneity in both source of SCI and neurologic severity of the SCI, including level and degree of completeness. The lack of heterogeneity in the incomplete SCI population was difficult to avoid in the available pool of subjects.
The majority of our subject population was male. The majority of persons with SCI are male nationwide, and the veteran population is similarly largely male, which decreased the available population of female veterans available for study. Future work will evaluate ways to increase the number of women studied.
The tolerance limit was subjective; however, it is a clinically important consideration because it relates to the ability of a subject to actually use the device. The tolerance limit generally correlated with the sensation threshold (6.6±7.5 times) and pudendal-anal reflex thresholds (3.5±2.8 times), suggesting that the variability was reasonable.
Bladder activity and bladder capacity varied across subjects, such that inhibiting one or two bladder contractions might result in an insignificant change in bladder capacity for one person, but a large change in bladder capacity for another. Therefore, we found that while the ability to inhibit a bladder contraction might predict how well GNS can improve urinary continence, it does not necessarily predict how much the bladder capacity will increase. Indeed, even if GNS does not significantly improve an individual's bladder capacity under a particular urodynamics trial, it can inhibit spontaneous bladder contractions. Furthermore, if GNS were to be applied in a chronic setting, this could imply a potential to reduce unwanted urinary incontinence by providing the user with additional time so that they could get to a place where they can empty their bladder at will.
Determining the pudendal-anal reflex is at times challenging due to the varying degree with which it is visible and observable. Using the pudendal-anal reflex to determine appropriate stimulation amplitudes is subject to observer bias. Our research nurse, who is trained and experienced in spinal cord medicine and urodynamics, observed for the pudendal-anal reflex in response to stimulation. We therefore had a skilled observer and had no variance due to inter-observer error. We focused on identifying an accurate threshold for the pudendal-anal reflex, which may account for our comparatively lower GNS amplitudes. Even if we were imprecise in determining the pudendal-anal reflex threshold, it would only result in our GNS amplitudes being underestimated. Surface electromyography of the external anal sphincter could potentially improve pudendal-anal reflex detection, however stimulus artifact limited the utility of EMG in this study.
Conclusions
GNS was acutely tolerable and effectively inhibited neurogenic detrusor overactivity in individuals with pelvic sensation. GNS has been proposed as a solution to neurogenic detrusor overactivity, but concerns have been raised about its clinical feasibility, due to questionable capacity of persons with pelvic sensation to tolerate use of the approach. The majority of persons with SCI have incomplete injuries and pelvic sensation. This report demonstrates the potential to use GNS to inhibit neurogenic detrusor overactivity in persons with incomplete spinal cord injury.
Neurogenic detrusor overactivity after spinal cord injury is an important clinical problem demonstrated to cause urinary incontinence and reduced bladder capacity. Further research is needed to evaluate GNS as a chronic tool for managing neurogenic detrusor overactivity.
Acknowledgements
The authors would like to thank the following individuals for experimental, statistical and data analysis assistance: Melissa Schmitt, RN; Steven Siddik, PhD; Elizabeth Harpster, BA; Hongfei Di, MD; Janel Montfort, BS; Kelsey Aamoth; Michael Jacobson; Katherine Berry; and Heather Schneck. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Funding Statement
This work was supported by Merit Review and Career Development Awards RR&D RX000822, RX000960, and RX001962 and the Cleveland Center for Functional Electrical Stimulation RX-16-002 from the United States (U.S.) Department of Veterans Affairs Rehabilitation Research and Development Service.
Disclaimer statements
Contributors None.
Conflict of interest declaration None.
Ethics approval None.
References
- 1.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 2004;21(10):1371–83. doi: 10.1089/neu.2004.21.1371 [DOI] [PubMed] [Google Scholar]
- 2.Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. Cumulative use of strong anticholinergics and incident dementia. JAMA Intern Med 2015;175(3):401–7. doi: 10.1001/jamainternmed.2014.7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salahudeen MS, Hilmer SN, Nishtala PS.. Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people. J Am Geriatr Soc 2015;63(1):85–90. doi: 10.1111/jgs.13206 [DOI] [PubMed] [Google Scholar]
- 4.Wheeler J, Walter J, Zaszczurynski P.. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol 1992;147(1):100–3. doi: 10.1016/S0022-5347(17)37145-8 [DOI] [PubMed] [Google Scholar]
- 5.Previnaire J, Soler J, Perrigot M, Boileau G, Delahaye H, Schumacker P, et al. Short-term effect of pudendal nerve electrical stimulation on detrusor hyperreflexia in spinal cord injury patients: importance of current strength. Paraplegia 1996;34(2):95–9. [DOI] [PubMed] [Google Scholar]
- 6.Kirkham AP, Shah NC, Knight SL, Shah PJ, Craggs MD.. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord 2001;39(8):420–8. doi: 10.1038/sj.sc.3101177 [DOI] [PubMed] [Google Scholar]
- 7.Dalmose AL, Rijkhoff NJM, Kirkeby HJ, Nohr M, Sinkjaer T, Djurhuus JC.. Conditional stimulation of the dorsal penile/clitoral nerve may increase cystometric capacity in patients with spinal cord injury. Neurourol Urodyn 2003;22(2):130–7. doi: 10.1002/nau.10031 [DOI] [PubMed] [Google Scholar]
- 8.Lee Y-H, Creasey GH, Lim H, Song J, Song K, Kim J.. Detrusor and blood pressure responses to dorsal penile nerve stimulation during hyperreflexic contraction of the bladder in patients with cervical cord injury. Arch Phys Med Rehabil 2003;84(1):136–40. doi: 10.1053/apmr.2003.50075 [DOI] [PubMed] [Google Scholar]
- 9.Hansen J, Media S, Nøhr M, Biering-Sørensen F, Sinkjaer T, Rijkhoff NJ.. Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J Urol 2005;173(6):2035–9. doi: 10.1097/01.ju.0000158160.11083.1b [DOI] [PubMed] [Google Scholar]
- 10.Horvath EE, Yoo PB, Amundsen CL, Webster GD, Grill WM.. Conditional and continuous electrical stimulation increase cystometric capacity in persons with spinal cord injury. Neurourol Urodyn 2010;29(3):401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y, Kim JM, Im HT, Lee K, Kim SH, Hur DM.. Semiconditional electrical stimulation of pudendal nerve afferents stimulation to manage neurogenic detrusor overactivity in patients with spinal cord injury. Ann Rehabil Med 2011;35(5):605–12. doi: 10.5535/arm.2011.35.5.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martens FM, Heesakkers JP, Rijkhoff NJ.. Minimal invasive electrode implantation for conditional stimulation of the dorsal genital nerve in neurogenic detrusor overactivity. Spinal Cord 2011;49(4):566–72. doi: 10.1038/sc.2010.134 [DOI] [PubMed] [Google Scholar]
- 13.Walter J, Sidarous R, Robinson CJ, Wheeler JS, Wurster RD.. Comparison of direct bladder and sacral nerve stimulation in spinal cats. J Rehabil Res 1992;29(2):13–22. doi: 10.1682/JRRD.1992.04.0013 [DOI] [PubMed] [Google Scholar]
- 14.Lindström S, Fall M, Carlsson CA, Erlandson BE.. The neurophysiological basis of bladder inhibition in response to intravaginal electrical stimulation. J Urol 1983;129(2):405–10. doi: 10.1016/S0022-5347(17)52127-8 [DOI] [PubMed] [Google Scholar]
- 15.Vodusek DB, Light JK, Libby JM.. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol Urodyn 1986;5(4):381–9. doi: 10.1002/nau.1930050404 [DOI] [Google Scholar]
- 16.2014 Annual Statistical Report for the Spinal Cord Injury Model Systems Public Version. Birmingham, AL: University of Alabama at Birmingham; 2014. https://www.nscisc.uab.edu/reports.aspx.
- 17.Wheeler JS Jr, Walter JS, Sibley P.. Management of incontinent SCI patients with penile stimulation: preliminary results. J Am Paraplegia Soc 1994;17(2):55–9. doi: 10.1080/01952307.1994.11735917 [DOI] [PubMed] [Google Scholar]
- 18.Opisso E, Borau A, Rodríguez A, Hansen J, Rijkhoff NJ.. Patient controlled versus automatic stimulation of pudendal nerve afferents to treat neurogenic detrusor overactivity. J Urol 2008;180(4):1403–8. doi: 10.1016/j.juro.2008.06.023 [DOI] [PubMed] [Google Scholar]
- 19.Opisso E, Borau A, Rijkhoff NJ.. Subject-Controlled stimulation of dorsal genital nerve to treat neurogenic detrusor overactivity at home. Neurourol Urodyn 2013;32(7):1004–9. doi: 10.1002/nau.22359 [DOI] [PubMed] [Google Scholar]
- 20.Bower WF, Moore KH, Adams RD.. A pilot study of the home application of transcutaneous neuromodulation in children with urgency or urge incontinence. J Urol 2001;166(6):2420–2. doi: 10.1016/S0022-5347(05)65606-6 [DOI] [PubMed] [Google Scholar]
- 21.Goldman HB, Amundsen CL, Mangel J, Grill J, Bennett M, Gustafson KJ, et al. Dorsal genital nerve stimulation for the treatment of overactive bladder symptoms. Neurourol Urodyn 2008;27(6):499–503. doi: 10.1002/nau.20544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fjorback MV, Hansen J, Dalmose AL, Rijkhoff NJ, Sinkjær T.. A portable device for experimental treatment of neurogenic detrusor overactivity. Neuromodulation 2003;6(3):158–65. doi: 10.1046/j.1525-1403.2003.03024.x [DOI] [PubMed] [Google Scholar]


