Abstract
Objective
Central motor conduction time (CMCT) recorded from the abductor pollicis brevis (CMCT-APB) and abductor digiti minimi (CMCT-ADM) muscles may enable the evaluation of patients with C6–7 myelopathy. CMCT is more useful for the evaluation of the function of spinal cord than magnetic resonance imaging (MRI) findings. CMCT may be associated with age and height. However, there are few reports regarding CMCT-APB in normal subjects. This study aimed to investigate the relationships between age, height, and conduction parameters in normal subjects to assess the effectiveness of using CMCT-APB and CMCT-ADM for the evaluation of patients with C6–7 and C7–T1 myelopathy.
Design
Retrospective study.
Methods
Fifteen patients with cervical compressive myelopathy at C6–7 (11 patients) or C7-T1 (4 patients) level were enrolled. The control group consisted of 150 normal subjects (mean age 45.8±17.0 years; mean height 163.6±8.9 cm). Motor evoked potentials induced by transcranial magnetic stimulation and F-waves were used to determine CMCT.
Outcome Measures
CMCT-APB, CMCT-ADM.
Results
The normative values of CMCT-APB and CMCT-ADM were 5.3±0.7 ms and 5.2±0.8 ms, respectively. CMCT-APB was significantly longer than CMCT-ADM for patients with C6–7 myelopathy (P < 0.05). Neither of the CMCTs for those with C7–T1 myelopathy were significantly different from those of controls, but CMCT-APB was more prolonged than CMCT-ADM in patients with C6–C7 myelopathy.
Conclusions
CMCTs improve the accuracy of the diagnosis of myelopathy by pinpointing the lesion in combination with MRI imaging. Selective CMCT-APB prolongation may be seen in patients with C6–7 myelopathy but not C7-T1 myelopathy.
Keywords: Central motor conduction time, Cervical compressive myelopathy, Abductor digiti minimi, Abductor pollicis brevis, Normal subject
Introduction
The central motor conduction time (CMCT) has been used to electrophysiologically evaluate corticospinal tract function and is very useful for diagnosing corticospinal tract disorders.1–4 Measurement of the CMCT is noninvasive and safe for investigating lateral corticospinal tract function. In our previous study, we found a systematic correlation between the CMCT recorded from the abductor digiti minimi (ADM; CMCT-ADM) and the responsible level of cervical compressive myelopathy (CCM).5 However, patients with C6–7 myelopathy could not be diagnosed using the CMCT-ADM.
The myotomal distribution of the ADM muscle is in the C8 and T1 segments, with the main myotome being in C8.6 Anatomical studies on cadavers have shown that the border between the C8 and T1 segments in the spinal cord is at the C6–7 disc level.7 A compressed spinal cord at the C6–7 disc level could lead to involvement of the C8 segment and the long tract caudal to T1.5 The myotomal distribution of the abductor pollicis brevis (APB) muscle is in the C8 and T1 segments, with the main myotome being in T1.8
We hypothesized that the CMCT recorded from the APB (CMCT-APB) innervated by the median nerve and the ADM (CMCT-ADM) innervated by the ulnar nerve could be a reliable method for evaluation of patients with C6–7 myelopathy and for differential diagnosis of early CCM. The CMCT is more useful for the evaluation of the function of the spinal cord than MRI (magnetic resonance imaging) or CT (computed tomography) findings, and it may be associated with the patient's age and height. However, there are few reports in the literature regarding the CMCT-APB in normal subjects. This study aimed to investigate the relationship between age, height, and conduction parameters in normal subjects to assess the importance of the CMCT-APB and CMCT-ADM in patients with C6–7 and C7–T1 myelopathy.
Methods
Patients
A total of 15 patients with CCM (12 males and 3 females; mean age 63.8±10.2 years; range, 40–77) were examined by magnetic resonance imaging (MRI) and computed tomography (CT) and were found to have spinal cord compression at the C6–7 or C7–T1 disc level (Table 1). The responsible level was at C6–7 in 11 patients and at C7–T1 in 4 patients. The CMCT-APB and the CMCT-ADM were measured bilaterally for all patients before surgery, and we evaluated the CMCT-APB and CMCT-ADM on the more symptomatic side. We excluded patients with stroke, radiculomyelopathy, spinal cord injury, brachial plexus injury, motor neuron disease, and peripheral nerve disease using electrophysiological exams. The CMCT-APB, CMCT-ADM, compound muscle action potentials (CMAPs) (deltoid, biceps brachii muscle, triceps brachii muscle, extensor digiti communis, first dorsal interosseous, APB, and ADM), sensory nerve action potentials stimulated by the median and ulnar nerves, and electromyography were recorded bilaterally.
Table 1.
Patient characteristics.
| Level of myelopathy | Case | Age | Sex | Disease |
|---|---|---|---|---|
| C6-7 | 1 | 60 | M | OPLL |
| 2 | 40 | M | CSM | |
| 3 | 73 | F | CSM | |
| 4 | 58 | M | CDH | |
| 5 | 77 | M | OPLL | |
| 6 | 60 | M | OPLL | |
| 7 | 59 | M | CDH | |
| 8 | 71 | F | CSM | |
| 9 | 54 | M | OPLL | |
| 10 | 72 | F | CSM | |
| 11 | 56 | M | OPLL | |
| C7-T1 | 12 | 64 | M | CSM |
| 13 | 62 | M | CSM | |
| 14 | 77 | M | OLF | |
| 15 | 74 | M | OPLL |
M, male; F, female; OPLL, ossification of posterior longitudinal ligament; CSM, cervical spondylotic myelopathy; CDH, cervical disc herniation; OLF, ossification of yellow ligament.
Controls
One hundred fifty normal subjects (81 males and 69 females; mean age 45.8±17.0 years; mean height 163.6±8.9 cm) participated in the study. We interviewed the subjects about their medical histories and performed CMCT-APB, CMCT-ADM, and peripheral nerve conduction studies before conducting this study. The exclusion criteria were subjects with central nervous system disorders, peripheral neuropathies, other neuromuscular diseases, or diabetes mellitus. Informed consent for participation was obtained from all subjects. This study was approved by the Institutional Review Board of Yamaguchi University Hospital.
Measurement of CMCT
Self-adhesive surface recording electrodes were placed on the target muscles by the belly- tendon method. The motor evoked potentials (MEPs) from the APB and the ADM on the left side were recorded during voluntary contraction. Transcranial magnetic stimulation was delivered using a round, 14-cm outer diameter coil (Magstim, Machida City, Tokyo, Japan) positioned flat on the scalp with its center over the Cz (International 10/20 system) for the APB and the ADM. The magnetic stimulus intensity was set at 20% above the threshold of the MEPs during voluntary contraction. We specified that the raw electromyogram (EMG) constantly maintained contractions of approximately 10–20% of the maximum force during slight voluntary contraction. First, we recorded the raw EMG during a maximum voluntary contraction. Then, both the patients with CCM and the normal subjects were informed of the size of the amplitude of their raw EMG, and they practiced maintaining a level that was 10–20% of that recorded during a maximum voluntary contraction. Measurement of the CMCT-APB and CMCT-ADM was then performed on all patients. All stimuli were repeated at least 4 times.
The CMAPs and F-waves were also recorded following supramaximal electrical stimulation (square wave, 0.2 ms) of the median and ulnar nerves of the wrist. Sixteen serial responses were obtained, and the shortest latency of the F-waves was measured. All of the muscle responses were amplified, filtered from 5–5,000 Hz, and recorded using a standard EMG (Viking Select: Nicolet Biomedical Madison, WI, USA). The peripheral motor conduction time (PMCT) was calculated from the latencies of the CMAPs and F-waves, as follows: PMCT= (latency of CMAPs + latency of F-waves -1)/2.9
The conduction time from the motor cortex to the spinal motor neurons, such as the CMCT, was calculated by subtracting the PMCT from the onset latency of the MEPs (Figs. 1, 2, 3, and 4). The latencies of the F-waves and the MEPs, and the CMCT measured to the right and the left were treated as one, because no obvious difference has been found between them.10 Therefore, in this study we only evaluated the left corticospinal tract for normal subjects using the CMCT.
Figure 1.
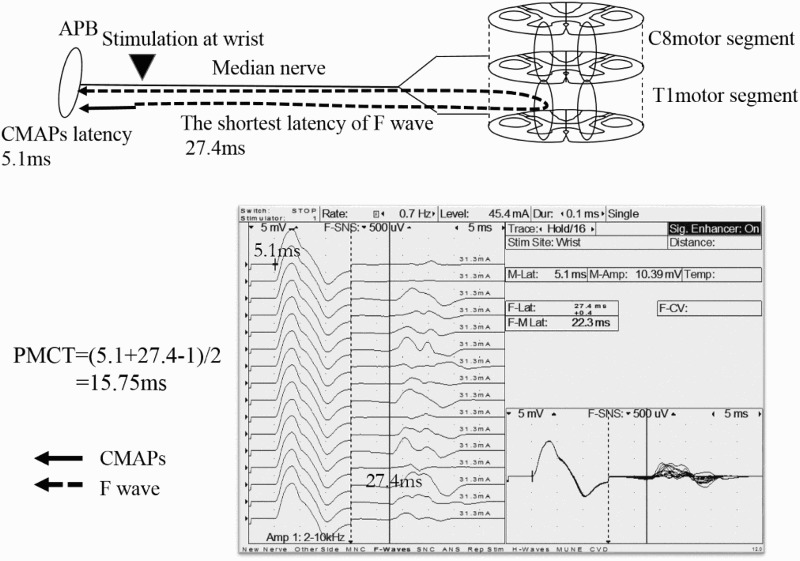
PMCT-APB
The APB was innervated by the median nerves (C8 and T1 nerve roots), with the main myotome being T1. The CMAPs and F-waves were also recorded following supramaximal electrical stimulation of the median nerves of the wrist. The PMCT, excluding the turn-around time at the spinal motor neuron (1 ms), was calculated from the latencies of the CMAPs and F-waves as follows: PMCT=(latency of CMAPs + the shortest latency of F-wave -1)/2. PMCT=(5.1+27.4-1)/2=15.75ms.
PMCT, peripheral motor conduction time; APB, abductor pollicis brevis; CMAPs, compound muscle action potentials.
Figure 2.
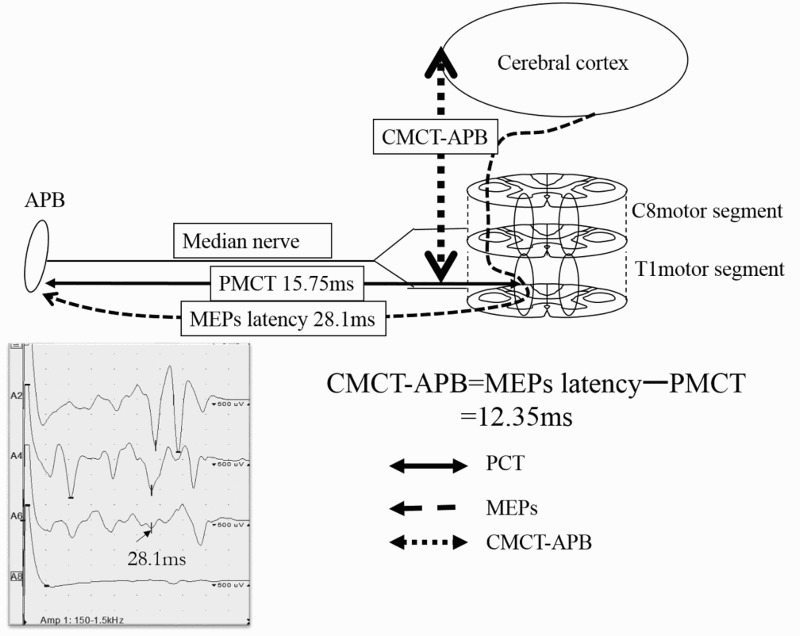
CMCT-APB
The latency of the MEPs was recorded in the APB by transcranial magnetic stimulation. The conduction time from the cerebral cortex mainly to the T1 motor segment was defined as the CMCT-APB; this was derived by subtracting the PMCT from the latency of the MEPs. CMCT-APB=28.1–15.75=12.35ms.
APB, abductor pollicis brevis, MEPs, motor evoked potentials, CMCT, central motor conduction time, PMCT, peripheral motor conduction time.
Figure 3.
PMCT-ADM
The ADM was innervated by the ulnar nerves (C8 and T1 nerve roots), with the main myotome being C8. The CMAPs and F-waves were also recorded following supramaximal electrical stimulation of the ulnar nerves of the wrist. The PMCT, excluding the turn-around time at the spinal motor neuron (1 ms), was calculated from the latencies of the CMAPs and F-waves as follows: PMCT=(latency of CMAPs + the shortest latency of F-wave -1)/2. PMCT=(4.5+29.5-1)/2=16.5ms
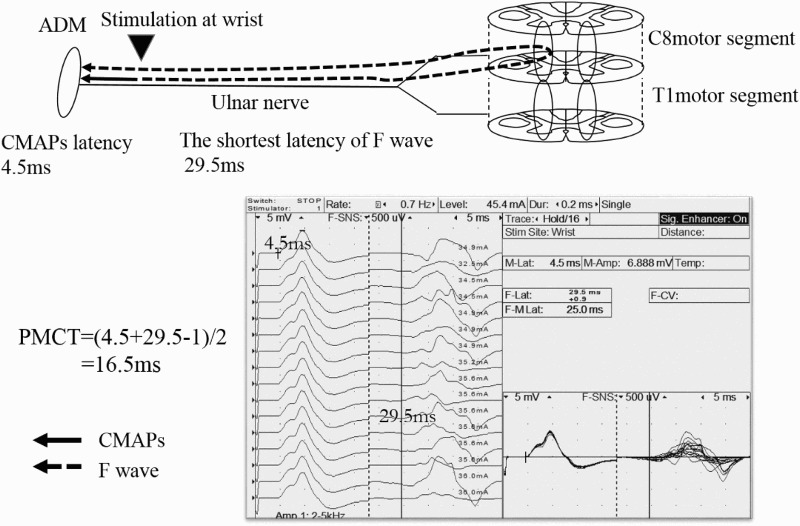
PMCT, peripheral motor conduction time; ADM, abductor digiti minimi; CMAPs, compound muscle action potentials.
Figure 4.
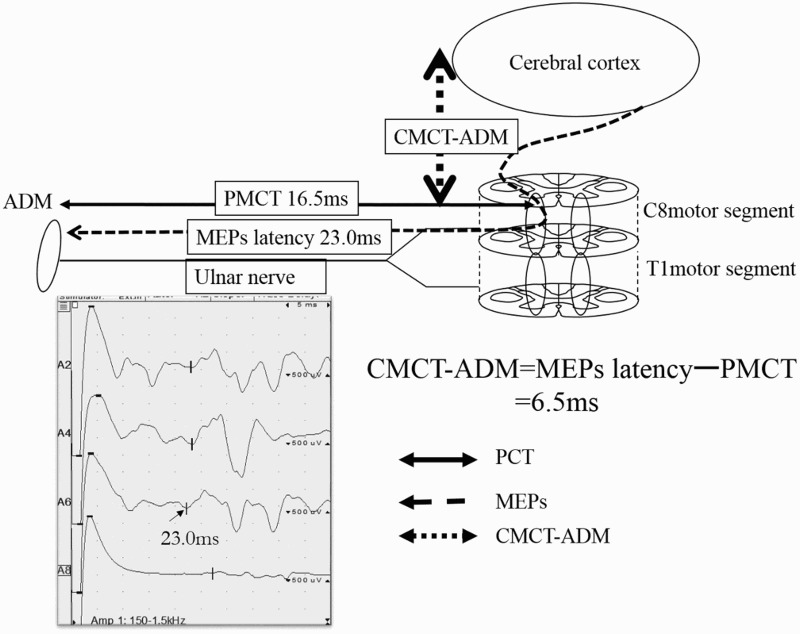
CMCT-ADM
The latency of the MEPs was recorded in the ADM by transcranial magnetic stimulation. The conduction time from the cerebral cortex mainly to the C8 motor segment was defined as CMCT-ADM; this was derived by subtracting the PMCT from the latency of the MEPs. CMCT-ADM=23.0-16.5=6.5ms.
ADM, abductor digiti minimi; MEPs, motor evoked potentials; CMCT, central motor conduction time; PMCT, peripheral motor conduction time.
Statistical analysis
The shortest latency of the F-waves, the latency of the MEPs, and the CMCTs recorded from the APB and the ADM of all subjects were evaluated. Simple linear regression analyses were performed to investigate the relationship between each of the above conduction parameters and the age and body height of the subjects. For the simple linear regression analysis, the coefficient of correlation was expressed as an R value. A P value of < 0.05 was considered to indicate statistical significance.
In addition, the shortest latency of the F-waves, the latency of the MEPs, and the CMCTs recorded from the APB and the ADM of the11patients with C6–7 myelopathy were evaluated. The Wilcoxon signed-rank test was used to compare distributions. Analysis of the clinical findings was performed both for the CMCT-APB and the CMCT-ADM by use of the Mann-Whitney test. A P value of <0.05 was considered to indicate statistical significance.
Results
Patients with myelopathy (Table 2)
Table 2.
The shortest latency of the F-waves, the latency of the MEPs, and the CMCT recorded from the APB and ADM for normal subjects and patients with myelopathy.
| APB |
ADM |
||||||
|---|---|---|---|---|---|---|---|
| F-waves | MEPs | CMCT | F-waves | MEPs | CMCT | ||
| Normal subjects (n=150) |
24.4±1.8 | 18.9±1.2 | 5.3±0.7 | 24.7±2.0 | 18.6±1.3 | 5.2±0.8 | |
| C6–7 myelopathy |
Case1 | 27.4 | 28.1 | 12.4 | 29.5 | 23.0 | 6.5 |
| 2 | 26.5 | 25.0 | 10.9 | 27.2 | 20.0 | 5.7 | |
| 3 | 27.9 | 26.2 | 10.2 | 26.3 | 21.0 | 6.4 | |
| 4 | 24.0 | 23.0 | 9.9 | 26.4 | 20.4 | 6.2 | |
| 5 | 26.0 | 24.4 | 9.9 | 25.8 | 21.4 | 7.4 | |
| 6 | 27.5 | 24.0 | 9.0 | 29.4 | 21.5 | 5.5 | |
| 7 | 25.7 | 22.6 | 8.7 | 26.4 | 19.5 | 5.3 | |
| 8 | 23.0 | 21.6 | 8.5 | 23.1 | 17.8 | 5.2 | |
| 9 | 26.8 | 22.9 | 8.0 | 28.3 | 22.9 | 7.6 | |
| 10 | 23.7 | 21.0 | 7.9 | 23.7 | 19.3 | 5.7 | |
| 11 | 28.8 | 22.7 | 6.5 | 28.6 | 20.6 | 5.2 | |
| Mean values | 26.1±1.9 | 23.8±2.1 | 9.3±1.6 | 26.8±2.1 | 20.7±1.5 | 6.1±0.8 | |
| P vs. normal values | * | * | * | * | * | * | |
| C7-T1 myelopathy | 12 | 27.4 | 19.6 | 4.5 | 26.5 | 19.5 | 5.3 |
| 13 | 26.3 | 20.4 | 5.6 | 28.3 | 20.9 | 5.7 | |
| 14 | 25.2 | 20.3 | 5.8 | 29.3 | 21.1 | 5.1 | |
| 15 | 26.7 | 20.3 | 5.8 | 28.1 | 20.0 | 4.9 | |
| Mean values | 26.4±0.9 | 20.2±0.4 | 5.4±0.6 | 28.1±1.2 | 20.4±0.8 | 5.3±0.3 | |
(ms)
yr, year; APB, abductor pollicis brevis; ADM, abductor digiti minimi; MEPs, motor evoked potentials; CMCT, central motor conduction time.
*P < 0.05.
For the patients with C6–7 myelopathy, the mean values of the F-waves, the MEPs, and the CMCT recorded from the APB were 26.1±1.9, 23.8±2.1, and 9.3±1.6 ms, respectively. The mean values of the F-waves, the MEPs, and the CMCT recorded from the ADM were 26.8±2.1, 20.7±1.5, and 6.1±0.8 ms, respectively. The latencies of the MEPs and the CMCT recorded from the APB were significantly longer than those from the ADM (P < 0.05). There was no significant difference between the F-waves recorded from the APB and those from the ADM. The MEPs and the CMCT recorded from the APB and the ADM were significantly longer than their respective normative values (P < 0.05) (see Normal subjects). For the patients with C7–T1 myelopathy, the mean values of the F-waves, the MEPs, and the CMCT recorded from the APB were 26.4±0.9, 20.2±0.4, and 5.4±0.6 ms, respectively. The mean values of the F-waves, the MEPs, and the CMCT recorded from the ADM were 28.1±1.2, 20.4±0.8, and 5.3±0.3 ms, respectively. The latency of the MEPs and the CMCT recorded from the APB were not significantly longer than those from the ADM. The CMCT-APB and CMCT-ADM were normal (see Normal subjects).
Clinical findings
Details of the clinical findings are shown in Table 3.
Table 3.
Patient's detailed clinical findings.
| Level of myelopathy | Case | Deep tendon reflex |
Manual muscle test |
MRI | ||
|---|---|---|---|---|---|---|
| TTR/PTR | Hoffmann sign | ADM | APB | HIA | ||
| C6–7 | 1 | →/↑ | — | 5 | 5 | + |
| 2 | →/↑ | — | 4 | 5 | + | |
| 3 | →/↑ | — | 5 | 5 | + | |
| 4 | →/↑ | — | 4 | 5 | — | |
| 5 | ↑/↑ | — | 5 | 5 | — | |
| 6 | →/↑ | + | 5 | 5 | — | |
| 7 | →/↑ | — | 4 | 5 | — | |
| 8 | →/↑ | — | 4 | 4 | — | |
| 9 | →/↑ | — | 5 | 5 | — | |
| 10 | →/↑ | — | 4 | 5 | — | |
| 11 | ↑/↑ | — | 4 | 5 | + | |
| C7–T1 | 12 | →/↑ | — | 5 | 5 | — |
| 13 | →/↑ | — | 5 | 5 | + | |
| 14 | →/↑ | — | 5 | 5 | + | |
| 15 | →/↑ | — | 5 | 5 | — | |
TTR, triceps tendon reflex; PTR, patella tendon reflex; →: normal; ↑: exaggerated; —: negative; +: positive; ADM, abductor digiti minimi; APB, abductor pollicis brevis; MRI, magnetic resonance imaging; HIA, high intensity area.
MRI findings
In patients with C6–7 myelopathy, 4 (36.3%) had a high intensity area (HIA) in the spinal cord. Two patients (50.0%) with C7–T1 myelopathy had an HIA in the spinal cord. We did not observe any relationship between the CMCT parameters and the presence of intramedullary high-intensity lesions disclosed by MRI.
Muscle weakness
In the patients with C6–7 myelopathy, 5 (45.4%) had no muscle weakness in the upper limbs, 5 (45.4%) had muscle weakness in the ADM, and 1 (9.2%) had muscle weakness in the ADM and the APB. None of the 4 patients with C7–T1 myelopathy had muscle weakness in their upper limbs.
Deep tendon reflex and Hoffmann sign
In the patients with C6–7 myelopathy, 9 (81.8%) had a normal triceps tendon reflex (TTR), 10 (90.1%) had a negative Hoffmann sign, and all 11 patients (100%) with C6–7 myelopathy had an exaggerated patellar tendon reflex (PTR). All 4 patients (100%) with C7–T1 myelopathy had a normal TTR, a negative Hoffmann sign, and an exaggerated PTR. Because of insufficient numbers in our dataset, we did not detect any statistically significant relationships between the CMCT and the grade of muscle weakness (APB or ADM), or between the CMCT and Hoffmann reflex.
Normal subjects (Table 4)
Table 4.
Relationship between age and each parameter and between body height and each parameter for normal subjects.
| APB |
ADM |
||||||
|---|---|---|---|---|---|---|---|
| F-waves | MEPs | CMCT | F-waves | MEPs | CMCT | ||
| Age (yr) | |||||||
| 20-29 (n=33) | 24.0±2.0 | 18.9±1.4 | 5.6±0.8 | 24.7±2.3 | 18.5±1.6 | 5.2±0.9 | |
| 30–39 (n=26) | 23.9±1.7 | 18.5±1.1 | 5.2±0.6 | 24.3±1.7 | 18.5±1.4 | 5.4±0.9 | |
| 40–49 (n=32) | 24.7±1.3 | 18.9±0.9 | 5.6±0.5 | 24.7±1.9 | 18.5±1.2 | 5.2±0.8 | |
| 50–59 (n=25) | 24.3±1.5 | 18.9±1.1 | 5.4±0.6 | 24.5±2.0 | 18.4±1.1 | 5.1±0.5 | |
| ≥ 60 (n=34) | 24.9±2.0 | 19.3±1.1 | 5.3±0.7 | 25.2±1.8 | 18.9±1.2 | 5.2±0.9 | |
| Body height (cm) | |||||||
| 139≦ <150 (n=10) | 23.0±1.9 | 17.8±1.1 | 4.9±0.7 | 22.8±1.8 | 17.3±0.8 | 5.0±0.7 | |
| 150≦ <160 (n=41) | 23.0±1.8 | 18.2±0.9 | 5.7±0.4 | 23.2±1.2 | 17.7±1.0 | 5.2±0.9 | |
| 160≦ <170 (n=56) | 24.5±1.4 | 19.1±1.0 | 5.4±0.7 | 24.9±1.5 | 18.8±1.1 | 5.3±0.9 | |
| 170≦ <180 (n=37) | 25.8±1.2 | 19.6±0.9 | 5.3±0.7 | 26.2±1.7 | 19.3±1.1 | 5.3±0.7 | |
| 180≦ (n=6) | 26.5±0.9 | 20.1±0.9 | 5.5±0.7 | 27.3±1.1 | 20.0±1.4 | 5.3±0.6 | |
| Total (n=150) | 24.4±1.8 | 18.9±1.2 | 5.3±0.7 | 24.7±2.0 | 18.6±1.3 | 5.2±0.8 | |
yr, year; APB, abductor pollicis brevis; ADM, abductor digiti minimi; MEPs, motor evoked potentials; CMCT, central motor conduction time.
The approximate ages of the subjects were as follows: 33 were in their 20s, 26 were in their 30s, 32 were in their 40s, 25 were in their 50s, and 34 were ≥ 60 years old. The heights of 10, 41, 56, 37, and 6 subjects were <150, 150–160, 160–170, 170–180, and >180 cm, respectively. The findings from the above tests revealed that the CMCT-APB and the CMCT-ADM were normally distributed, and that their normative values were 5.3±0.7 ms and 5.2±0.8 ms, respectively. The ADM control data came from a previously published study.1 No significant correlations were found between the CMCT-APB and the CMCT-ADM (CMCT-APB - CMCT-ADM) = 0.1±1.0 ms (P = 0.44).
Age
No significant correlations were found between the CMCT-APB and the age of the subjects or between the CMCT-ADM and age (CMCT-APB, P = 0.37; CMCT-ADM, P = 0.83).There was a significant difference between the shortest latency of the F-waves recorded from the APB and subject age (P < 0.05), but no significant correlations were found between any other conduction parameter and age.
Body height
No significant correlations were found between the CMCT-APB and body height or between the CMCT-ADM and body height (CMCT-APB, P = 0.37; CMCT-ADM, P = 0.23). However, there were significant differences between body height and the shortest latency of the F-waves and the latency of the MEPs recorded from the APB and the ADM (P < 0.05).
Discussion
In this study, age and height of the patients had no significant impact on the group CMCT results. Our results agree with the findings of Mano et al. and Claus.9, 12 The number of patients with degenerative diseases such as CCM has been increasing in societies with an aging population.13 We diagnosed CCM using neurological findings such as the deep tendon reflex, sensory disturbance, and muscle weakness, as well as radiological findings from plain radiographs, MRI, and CT. However, it is sometimes difficult to diagnose CCM using neurological findings in elderly patients with neurological disorders such as diabetic and entrapment neuropathies Moreover, some elderly subjects have been shown to have asymptomatic spinal cord compression by radiography.14
In general, we found that the CMCT-APB and CMCT-ADM were useful for evaluation of corticospinal tract function, regardless of age or body height. Fujimoto et al. measured the CMCT-ADM in 94 patients with CCM who underwent cervical laminoplasty and reported a mean value of 11.0 ± 3.0 ms (range; 7.4 to 18.7).15 These measurements are considered to be abnormal when they exceed the normal mean value by more than 2.5 standard deviations. Thus, we suggest that patients with CMCT-ADM values ≥7.2 ms and CMCT-APB values ≥7.1 ms may have CCM.1
Myotomal innervation of APB and ADM
Chiba et al. reported T1-dominant innervation of the APB.16 The ADM seemed to be innervated by both the C8 and T1 roots.16 Tsao et al. reported that the APB were T1-innervated by the median intrinsic hand muscles and that the ADM were C8-innervated by the ulnar intrinsic hand muscles.17 Seichi et al. reported that the ADM was the key muscle for diagnosis of C6–7 myelopathy6 and that its main myotome was the C8 motor segment.6 Based on these findings, we considered that the APB might be a mainly T1-innervated muscle and the ADM might be a mainly C8-innervated muscle.
Patients with C6–7 myelopathy
In the patients with myelopathy, muscle weakness was determined by lesions of the anterior horn in this study. The CMCT for the thenar muscle correlated with the long tract motor sign, but not with the segmental motor sign.18 Prolongation of the CMCT could occur with involvement of the corticospinal tract and be poorly correlated with motor segment involvement.18
We classified the patients into 4 groups using measurements of the CMCT. Fig. 5 shows the relationship between the motor segments (APB and ADM) and the C6–7 disc level, using the degree of muscle weakness. Group 1 (cases 5 and 9) had a prolonged CMCT-APB and CMCT-ADM. Group 2 (no patient) had a prolonged CMCT-APB and a non-prolonged CMCT-ADM. Group 3 (cases 1, 2, 3, 4, 6, 7, 8, and 10) had a prolonged CMCT-APB and a non-prolonged CMCT-ADM. Group 4 (case 11) had a non-prolonged CMCT-APB and CMCT-ADM.
Figure 5.
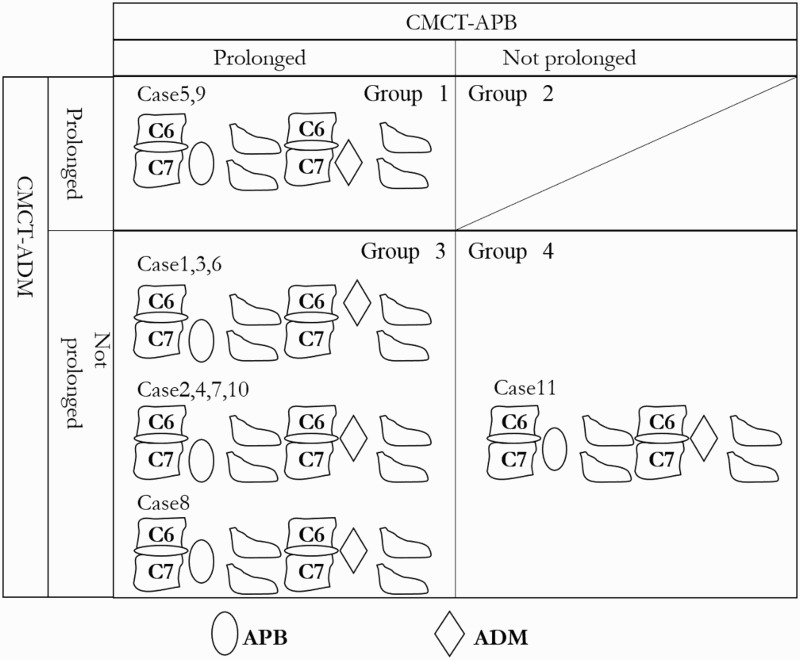
The relationship between the motor segments (APB and ADM) and the C6–7 disc level, using the CMCT and degree of muscle weakness. Group1: a prolonged CMCT-APB and CMCT-ADM. Group 2: a prolonged CMCT-APB and non-prolonged CMCT-ADM. Group 3: a prolonged CMCT-APB and non-prolonged CMCT-ADM. Group 4: a non-prolonged CMCT-APB and CMCT-ADM. Regarding Group 1, cases 5 and 9 had no motor weakness. The motor segment-innervated ADM and APB was caudal to the C6–7 disc level. With regard to Group 3, cases 1, 3, and 6 had no motor weakness. The motor segment-innervated APB was caudal to the C6-7 disc level, and the ADM was rostral to the C6–7 disc level. Cases 2, 4, 7, and 10 had motor weakness of the ADM. The motor segment-innervated APB was caudal to the C6–7 disc level and the ADM was at the C6–7 disc level. Case 8 had motor weakness of the ADM and the APB. The rostral side of the motor segment-innervated APB was at the C6–7 disc level, and the motor segment-innervated ADM was at the C6–7 disc level. With regard to Group 4, case 11 had motor weakness of the ADM and the CMCT-APB was longer than the CMCT-ADM. The rostral side of the motor segment-innervated APB was at the C6–7 disc level and the motor segment-innervated ADM was at the C6–7 disc level.
ADM, abductor digiti minimi; APB, abductor pollicis brevis; CMCT, central motor conduction time.
Regarding Group 1, cases 5 and 9 had no motor weakness. The motor segment-innervated ADM and APB were caudal to the C6–7 disc level. With regard to Group 3, cases 1, 3, and 6 had no motor weakness. The motor segment-innervated APB was caudal to the C6-7 disc level, and the ADM was rostral to the C6–7 disc level. Cases 2, 4, 7, and 10 had motor weakness of the ADM. The motor segment-innervated APB was caudal to the C6–7 disc level, and the ADM was at the C6–7 disc level. Case 8 had motor weakness of the ADM and APB. The rostral side of the motor segment-innervated APB was at the C6–7 disc level, and the motor segment-innervated ADM was at the C6–7 disc level. With regard to Group 4, case 11 had motor weakness of the ADM and the CMCT-APB was longer than the CMCT-ADM. The rostral side of the motor segment-innervated APB was at the C6–7 disc level and the motor segment-innervated ADM was at the C6–7 disc level. Based on these results, we considered that the motor segment-innervated APB was more caudal than the motor segment-innervated ADM.
Patients with C7–T1 myelopathy
Cases 12, 13, 14, and 15 had no motor weakness, and the CMCT-APB and CMCT-ADM were normal. The C8 and T1 motor segments were on the rostral side at the C7–T1 disc level. We could not evaluate patients with C7–T1 myelopathy using the CMCT-APB and CMCT-ADM. According to these results, we found that the motor segment-innervated APB was at the C7 vertebral level in most of these patients. Tuzuki et al. reported the presence of the T1 motor segment cord at the C7 vertebral body in 14 cadaver specimens (Fig. 6),7 and their anatomical findings are consistent with our results.7
Figure 6.
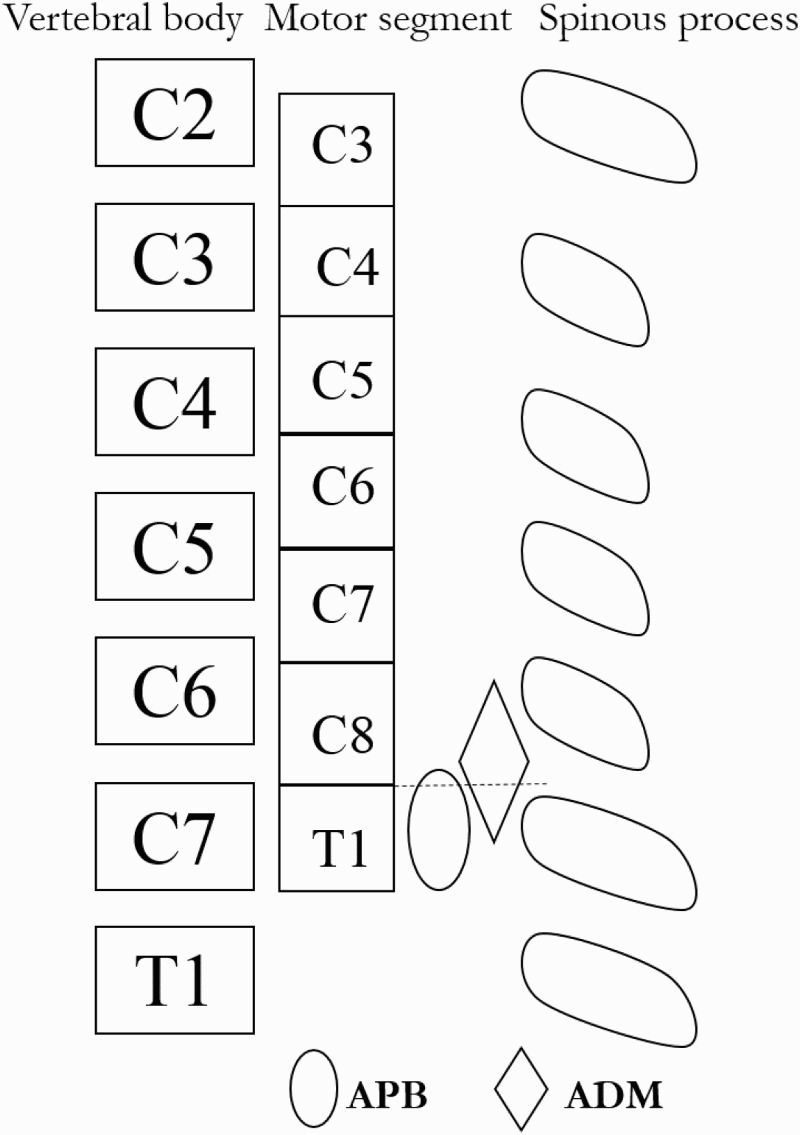
The relationship between vertebral body and the motor segment-innervated APB and ADM.
Tuzuki et al. reported the relationship between the vertebral body and motor segment in 14 cadaver specimens.7 The motor segment-innervated APB was consistent with the presence of the T1 motor segment cord at the C7 vertebral body. The motor segment-innervated APB was more caudal than the motor segment-innervated ADM.
The incidence of CCM at the C6–7 disc level reportedly has ranged from 4.1% to 14.7%.5,19 Occasionally, we can see patients whose spinal cord at C7–-T1 is compressed by ossification of the posterior longitudinal ligament (OPLL) spread over from the cervical and thoracic spines. However, single-level C7–T1 myelopathy is rare. Only a few reports have described this myelopathy as caused by ossification of the ligamentum flavum (OLF) or by degenerative anterolisthesis. There have been no reports of the percentage of C7–T1 myelopathy cases among all patients with cervical myelopathy.20 Aizawa et al. produced a report on 15,714 surgical operations performed between 1988 and 2002 at 30 hospitals in Miyagi Prefecture, Japan. There were 265 patients with thoracic myelopathy including OLF, OPLL, posterior spurs, and disc herniation. Patients with C7–T1 myelopathy accounted for approximately 10 cases (3.8%).21 Therefore, the incidence of patients with C6–7 myelopathy and C7–T1 myelopathy is rare, and the neurological findings in the upper extremities are poor.
Funaba et al. reported that patients with C6–7 myelopathy may not have clinical symptoms in their hands, and the CMCT-ADM tended to be less prolonged.22 In particular, they found it very difficult to diagnose patients with C6–7 myelopathy using neurological findings and the CMCT-ADM since there were no specific findings of deep tendon reflexes in these patients. Funaba et al. also reported that the CMCT-ADM was useful for the diagnosis of CCM rostral to the C5–6 intervertebral level and that diagnosis of patients with C6–7 myelopathy and compressive thoracic myelopathy (CTM) should include assessment of the CMCT-abductor hallucis (AH).5 Measurement of the CMCT-AH is valuable as a noninvasive technique for screening patients with CTM.10 AH is innervated by tibial nerves (S2 and S3).23 Fujimoto et al. reported the presence of the L4 segment of spinal cord from the lower third of the T11 vertebral body to the T11-12 intervertebral disc level using neurological findings for patients with a single ossification of ligamentum fravum.24 According to their results, the S2 and S3 segments of the spinal cord are below the T12 vertebral level. Therefore, the CMCT-AH was useful for the diagnosis of CTM rostral to the T11/12 intervertebral level. When patients have a normal CMCT-ADM and a prolonged CMCT-AH, they may have involvement from the C6-7 to the T11–12 disc level. Most patients with CTM have a normal CMCT-APB and a prolonged CMCT-AH. In this study, all patients with C6-7 and C7-T1 myelopathy had a prolonged CMCT-AH. Although it is difficult to determine surgical levels in patients with C6–7 myelopathy and C7–T1 myelopathy who have coexisting CTM, it is possible for us to diagnose patients with C6-7 myelopathy who have coexisting CTM when we use the CMCT-ADM, CMCT-APB, and CMCT-AH. Therefore, we suggest that CMCT-APB in addition to the CMCT-ADM and CMCT-AH should be considered when evaluating patients with C6–7 myelopathy. Table 5 shows the relationship between CMCTs and the involvement of level. On the other hand, it is difficult to evaluate patients with C7–T1 myelopathy using these CMCTs.
Table 5.
The relationship between the involvement of disc level and CMCTs.
| The involvement of disc level | CMCT-ADM | CMCT-APB | CMCT-AH |
|---|---|---|---|
| Rostral to the C5–6 | × | × | × |
| Rostral to the C6–7 | ○ | × | × |
| From the C7–T1 to the T11–12 | ○ | ○ | × |
APB, abductor pollicis brevis; ADM, abductor digiti minimi; CMCT, central motor conduction time; ×: prolonged; ○: not prolonged.
Limitations
A major limitation of this study was the small number of subjects. Evaluation of F-wave latencies, MEP latencies, and the CMCT may not be applicable in a non-Japanese population, in children, and in those with a height ≥190 cm. Nevertheless, the study findings may be useful to clinicians for correct diagnosis of C6–7 myelopathy.
In conclusion, we found that CMCT-ADM values ≥7.2 ms and CMCT-APB values ≥7.1 ms were proper to diagnose patients with CCM. We found that the motor segment-innervated APB was more caudal than the motor segment-innervated ADM. Patients with C7–T1 myelopathy could not be distinguished using CMCT. However, patients with C6-7 myelopathy were more likely to show selective prolongation of CMCT-APB. Therefore, we believe that it is very important to compare the CMCT-APB with the CMCT-ADM in patients with C6–7 myelopathy in order to facilitate diagnosis.
Disclaimer statements
Contributors None.
Funding None.
References
- 1.Imajo Y, Kanchiku T, Suzuki H, Yoshida Y, Funaba M, Nishida N, et al Effects of differences in age and body height on normal values of central motor conduction time determined by F-waves. J Spinal Cord Med 2017;40(2):181–7. doi: 10.1080/10790268.2015.1117193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaneko K, Taguchi T, Morita H, Yonemura H, Fujimoto H, Kawai S.. Mechanism of prolonged central motor conduction time in compressive cervical myelopathy. Clin Neurophysiol 2001;112(6):1035–40. doi: 10.1016/S1388-2457(01)00533-8 [DOI] [PubMed] [Google Scholar]
- 3.Kaneko K, Kato Y, Kojima T, Imajo Y, Taguchi T.. Epidurally recorded spinal cord evoked potentials in patients with cervical myelopathy and normal central motor conduction time measured transcranial magnetic stimulation. Clin Neurophysiol 2006;117(7):1467–73. doi: 10.1016/j.clinph.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto H, Konoma Y, Shimizu T, Okabe S, Shirota Y, Hanajima R, et al Aging influences central motor conduction less than peripheral motor conduction: a transcranial magnetic stimulation study. Muscle Nerve 2012;46(6):932–6. doi: 10.1002/mus.23430 [DOI] [PubMed] [Google Scholar]
- 5.Funaba M, Kanchiku T, Imajo Y, Suzuki H, Yoshida Y, Nishida N, et al Transcranial magnetic stimulation in the diagnosis of cervical compressive myelopathy. Spine 2015;40(3):E161–7. doi: 10.1097/BRS.0000000000000698 [DOI] [PubMed] [Google Scholar]
- 6.Seichi A, Takeshita K, Kawaguchi H, Matsudaira K, Higashikawa A, Ogata N, et al Neurologic level diagnosis of cervical stenotic myelopathy. Spine 2006;31(12):1338–43. doi: 10.1097/01.brs.0000219475.21126.6b [DOI] [PubMed] [Google Scholar]
- 7.Tsuzuki N, Honda H, Tanaka Y.. Morphological variation of human cervical spine cord segments and roots and their clinical significance. Orthop Surg (in Japanese) 1983;34(2):329–35. [Google Scholar]
- 8.Stoker GE, Kim HJ, Riew KD.. Differentiating C8-T1 radiculopathy from ulnar neuropathy: A survey of 24 spine surgeons. Global Spine J 2014;4(1):1–6. doi: 10.1055/s-0033-1354254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mano Y, Nakamuro T, Ikoma K, Sugata T, Morimoto S, Takayanagi T, et al Central motor conductivity in aged people. Intern Med 1992;31(9):1084–7. doi: 10.2169/internalmedicine.31.1084 [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi K, Tanaka N, Sasaki H, Kamei N, Hamasaki T, Yamada K, et al Assessment of central motor conduction time in the diagnosis of compressive thoracic myelopathy. Spine 2010;35(26):E1593–8. doi: 10.1097/BRS.0b013e3181d9e7a4 [DOI] [PubMed] [Google Scholar]
- 11.Brain L, Walton J.. Brain's Disease of Nervous System, 7th edn. Oxford University press: London, UK: 1969;40–3. [Google Scholar]
- 12.Claus D. Central motor conduction: method and normal results. Muscle Nerve. 1990;13(12):1125–32. doi: 10.1002/mus.880131207 [DOI] [PubMed] [Google Scholar]
- 13.Imajo Y, Taguchi T, Yone K, Okawa A, Otani K, Ogata T, et al Japanese 2011 nationwide survey on complications from spine surgery J Orthop Sci 2015;20(1):38–54. doi: 10.1007/s00776-014-0656-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tani T, Yamamoto H, Kimura J.. Cervical spondylotic myelopathy in elderly people: a high incidence of conduction block at C3–4 or C4–5 J Neurol Neurosurg Psychiatry 1999;66(4):456–64. doi: 10.1136/jnnp.66.4.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto K, Kanchiku T, Imajo Y, Suzuki H, Funaba M, Nishida N, et al Use of central motor conduction time and spinal cord evoked potentials in the electrophysiological assessment of compressive cervical myelopathy. Spine (Phila Pa 1976). 2016 Oct 25. [Epub ahead of print]. [DOI] [PubMed]
- 16.Chiba T, Konoueda F, Higashibara M, Matsudaira K, Oishi C, Hatanaka Y, et al C8 and T1 innervation of forearm muscles. Clin Neurophysiol 2015;126(4):837–42. doi: 10.1016/j.clinph.2014.07.031 [DOI] [PubMed] [Google Scholar]
- 17.Tsao BE, Ferrante MA, Wilbourn AJ, Shields RW.. Electrodiagnostic features of true neurogenic thoracic outlet syndrome. Muscle Nerve 2014;49(5):724–7. doi: 10.1002/mus.24066 [DOI] [PubMed] [Google Scholar]
- 18.Lazzaro VD, Colosimo RC, Tonali P.. The contribution of magnetic stimulation of the motor cortex to the diagnosis of cervical spondylotic myelopathy. Correlation of central motor conduction to distal and proximal upper limb muscles with clinical and MRI findings. Electroencephalogr Clin Neurophysiol 1992;85(5):311–20. doi: 10.1016/0168-5597(92)90107-M [DOI] [PubMed] [Google Scholar]
- 19.Kokubun S. Cervical spondylotic myelopathy at C6/7 disc level; its neurology and spinal factors. Orthop Surg (in Japanese) 1993;28(8):881–5. [Google Scholar]
- 20.Aizawa T, Ozawa H, Hoshikawa T, Kusakabe T, Itoi E.. Severe facet joint arthrosis caused c7/t1 myelopathy: a case report. Case Rep Med 2009;2009:481459 Epub 2009 Jun 16. doi: 10.1155/2009/481459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aizawa T, Sato T, Tanaka Y, Ozawa H, Hoshikawa T, Ishii Y, et al Thoracic myelopathy in Japan: Epidemiological retrospective study in Miyagi prefecture during 15 years. Tohoku J Exp Med 2006;210(3):199–208. doi: 10.1620/tjem.210.199 [DOI] [PubMed] [Google Scholar]
- 22.Funaba M, Kanchiku T, Imajo Y, Suzuki H, Nishida N, Fujimoto K, et al Characteristics of C6-7 myelopathy: assessment of clinical symptoms and electrophysiological findings. Spinal Cord 2016;54(10):798–803. doi: 10.1038/sc.2015.203 [DOI] [PubMed] [Google Scholar]
- 23.Toyokura M, Furukawa T.. F wave duration in mild S1 radiculopathy: comparison between the affected and unaffected sides. Clin Neurophysiol 2002;113(8):1231–5. doi: 10.1016/S1388-2457(02)00148-7 [DOI] [PubMed] [Google Scholar]
- 24.Fujimoto K, Kanchiku T, Imajo Y, Suzuki H, Yoshida Y, Nishida N, et al Neurologic findings caused by ossification of ligamentum flavum at the thoracolumbar junction. J Spinal Cord Med 2015 Dec17:1–5 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]


