Abstract
Background
Treatment of urinary tract infections (UTI) in the spinal cord injury (SCI) population is often difficult due to the lack of symptoms, increased resistance, and increased morbidity and mortality associated with UTIs.
Objective
To develop an algorithm-based order set for the treatment of UTIs for patients with SCI based on SCI-specific antibiogram data in order to assess and improve current antimicrobial prescribing practices at the Clement J. Zablocki Veterans Affairs Medical Center (ZVAMC).
Methods
This study is a retrospective, pre- and post-implementation analysis of an order set based on SCI antibiogram data. Descriptive statistics were used to compare baseline data and characteristics and chi squared tests were used to evaluate the primary outcome and all secondary outcomes. To achieve a power of 80% with an effect size of 0.3, the goal was to assess 45 antimicrobial treatment courses in the pre-implementation group and 45 antimicrobial treatment courses in the post-implementation group.
Results
The percentage of appropriate antimicrobial treatment courses increased from 47.9% in the pre-intervention group (n = 73) to 71.8% in the post-intervention group (n = 39), which was statistically significant (P = 0.015).
Conclusions
Patients with SCI treated for UTIs within the ZVAMC had a significantly higher percentage of appropriate treatment courses following the implementation of a unit-specific antibiogram, electronic order set, and educational in-service for providers. An order set and unit-specific antibiogram with related education may be beneficial in improving antimicrobial therapy from a stewardship perspective.
Keywords: Antibiogram, Unit-specific antibiogram, Urinary tract infections, Antibiotics, Spinal cord injury
Introduction
Catheter-associated (CA) bacteriuria, which is a general term for both catheter-associated asymptomatic bacteriuria (CA-ASB) and catheter-associated urinary tract infection (CA-UTI), is currently the most common healthcare-associated infection throughout the world.1 Most CA bacteriuria consists of CA-ASB, and there are significant concerns regarding the over-treatment of CA-ASB. Treatment of CA-ASB can lead to increased resistance rates of the organisms that typically cause urinary tract infections (UTIs) and make recurring infections more difficult to treat. Compared to the general able-bodied population, patients with a diagnosis of spinal cord injury (SCI) have been shown to have a higher incidence of UTIs, higher rates of resistance, and higher mortality associated with UTIs. Those with SCI have a 22% chance to develop a UTI within the first 50 days after the injury and develop a UTI 83 times more frequently than the general population. Spinal cord injury, along with the common sequelae of partial paralysis and neurogenic bladder, may hide or diminish the classic signs and symptoms of UTI including the sensation of painful or burning urination, increased urge, increased incontinence, or flank pain or costovertebral angle tenderness. In addition to having higher incidence of UTIs, patients with SCI have been shown to have a higher incidence of colonization, which can make the diagnosis of symptomatic bacteriuria and decision to treat less clear.
Although there are significant data regarding the epidemiology of SCI, the data and guidance for the treatment and outcomes linked with CA-UTIs in this patient population are limited.2 The most recent Infectious Diseases Society of America (IDSA) guideline on CA-UTI and CA-ASB was published in 2010 and most recommendations are based on expert opinion. However, of the available literature, these guidelines provide the most consistency in terms of definitions and recommendations and can be used as a reference for appropriate treatment evaluation.
The implementation of a hospital-wide local antibiogram is highly recommended by both the IDSA and the Clinical Laboratory Standards Institute (CLSI) and has become a common practice throughout most tertiary care hospitals in the United States. Since the use of hospital-wide local antibiograms have become more accepted, newer studies have started to evaluate the benefit of department-specific antibiograms.3,4 A 2006 study showed significantly higher rates of antibiotic resistance from Staphylococcus aureus, Enterococcus spp., Escherichia coli, and Pseudomonas aeruginosa isolates in the ICU when compared to hospital-wide isolates.5 Based on current literature research, there are limited studies evaluating SCI unit-specific antibiograms or microbiological data, and no studies evaluating appropriate treatment or outcomes based on implemented unit-specific antibiograms.
The Clement J. Zablocki VA Medical Center (ZVAMC) utilizes electronic algorithm-based order sets to treat various conditions, including infections, for inpatient and outpatient practice areas. Currently, there is an algorithm-based order set for the treatment for UTIs for inpatient care, but none for outpatient care or for the SCI population. The purpose of this study was to retrospectively assess the current antimicrobial prescribing practices for outpatients with SCI at the ZVAMC and develop an algorithm-based order set for the treatment of UTIs for this patient population. The order set was based on SCI-specific antibiogram data in order to improve appropriate treatment and outcomes in this population.
Materials and methods
Study design and setting
This study was a retrospective analysis that measured the appropriate use of antibiotic therapy for outpatients pre- and post-implementation of an algorithm-based order set. There were three phases including the pre-implementation data collection (December 31, 2014 – August 31, 2015), the implementation of the SCI unit antibiogram, development and implementation of the UTI treatment electronic order set, and a provider in-service (January 6, 2016), and the post-implementation data collection (January 7, 2016 – April 7, 2016). The study took place at the Clement J. Zablocki VA Medical Center (ZVAMC), which has 185 acute care beds and includes one of the 24 VA Regional Spinal Cord Injury Centers in the United States. The Spinal Cord Injury Center contains both inpatient and outpatient care and serves about 450 patients annually.
Inclusion and exclusion criteria
Antibiotic courses were included for analysis if they were prescribed from a ZVAMC SCI provider for the treatment of UTI in the outpatient setting. Antibiotic courses had to be prescribed for a patient with the diagnosis of SCI. Treatment courses were excluded from the data analysis if they were for urologic procedures. They were also excluded if the urine culture was obtained at an outside hospital or clinic with antibiotic recommendations provided from the outside hospital or clinic provider. This was excluded because, although the SCI provider from the ZVAMC would still ultimately be responsible for the appropriateness of the prescription order, they were not responsible for the initial work-up.
Antibiogram, order set, and provider education
Organisms isolated from any site within the SCI Center were included in the unit-specific antibiogram data. Culture data was collected over three years from August 18, 2012 to August 17, 2015. This is in contrast to the standard one year of data that is collected for the hospital-wide antibiogram in order to create a comparable number of isolates between the two antibiograms. Per the most recent CLSI recommendations, susceptibility percentages were reported using a patient-based algorithm. In this approach, for one patient with multiple isolates recovered of a single species, only the first isolate of that species during the time period is analyzed.
The main purpose of the order set was to help guide appropriate empiric treatment of UTIs in the outpatient setting, which was the focus for this study. Links to the patient's allergies, to the SCI antibiogram, and to a renal function calculator were included throughout the order set for the providers’ reference. The infectious disease team was consulted during the development of this process in order to identify which antibiotics should be recommended for UTIs in patients with SCI and in what preference or order they should be listed within the order set. Questions were created that ask the ordering provider about previous patient allergies, adverse drug reactions, and renal function in order to determine what empiric antibiotic should be started if any.
Provider education was presented as an in-service to all physicians and nurse practitioners actively practicing in the ZVAMC SCI Center. The in-service took place on January 6, 2016 and included education on the study's purpose, specific data from the SCI-antibiogram, and recommendations from the microbiology department and infectious disease team. The session ended with an open discussion for questions and comments, and provider feedback was then incorporated into the order set prior to its implementation.
Outcomes
The primary outcome was the rate of appropriate antimicrobial treatment courses in which the definition of appropriate treatment was based on the criteria from the 2010 IDSA CA-UTI guideline.2 Appropriate treatment in this study was defined as treatment that was initiated for patients with symptomatic bacteriuria (i.e. CA-UTI) and not catheter-associated asymptomatic bacteriuria (CA-ASB). Symptomatic bacteriuria was further defined as patients with evidence of either fever, rigors, altered mental status, malaise, or increased fatigue with no other identifiable cause, flank pain, costovertebral angle tenderness, acute hematuria, or pelvic or suprapubic tenderness plus the presence of at least 103 colony forming units (cfu)/mL of at least one bacterial species from one urine sample. Per the IDSA guidelines, odorous or cloudy urine by itself should not be reason to obtain a urinalysis, and pyuria by itself should not be a reason to treat with antibiotics. In addition, treatment was only considered appropriate if the empiric antibiotics prescribed covered the most common organisms in SCI-related UTIs and organisms identified through individual patients’ past urine cultures if relevant. The most common organisms for UTIs were based on population studies (e.g. E. coli). Appropriate treatment in this study was further defined based on dosing, timing, and duration of therapy. This included antibiotic courses that were initiated after the urinalysis or urine culture were obtained and then changed to cover susceptible organisms or discontinued based on urine culture results if applicable. Duration was considered appropriate for this study if antibiotics were prescribed for seven to fourteen days depending on how quickly the patients’ symptoms resolved.
The secondary outcomes included assessing the association between number of antibiotics prescribed and catheter type (e.g. indwelling versus intermittent), the association between incidence of UTI and prophylactic antibiotic use, and the inpatient admission rate following treatment of UTIs.
Statistical analyses
Descriptive statistics were used to gather and compare baseline data and characteristics. The chi squared test was used to evaluate the primary outcome as well as the secondary outcomes comparing one independent variable. One-way analysis of variance (ANOVA) was performed in order to evaluate the secondary outcomes with greater than two independent variables. The goal was to achieve a power of 80% with an effect size of 0.3. In order to detect a difference of 30 percent, our goal was to assess 45 patients pre-implementation and 45 patients post-implementation.
Results
A total of 74 pre-implementation antibiotic courses and 40 post-implementation antibiotic courses were initially reviewed. One antibiotic course was excluded from the pre-implementation group because the agent was used as prophylaxis for a urologic procedure. Another antibiotic course was excluded from the post-implementation group because the urine culture was obtained from an outside hospital, and the empiric antibiotic recommendations were provided from the outside hospital provider. Therefore, the results include 73 pre-implementation and 39 post-implementation antibiotic courses. There were no statistically significant differences between baseline characteristics between the pre- and post-intervention groups (Table 1).
Table 1.
Baseline characteristics.
| Pre-Implementation | Post-Implementation | P-value | |
|---|---|---|---|
| Sex – n (%) | |||
| Male | 72 (98.6) | 38 (97.4) | 0.65 |
| Catheter – n (%) | |||
| Indwelling | 46 (63) | 26 (66.7) | 0.70 |
| Intermittent | 16 (21.9) | 7 (17.9) | 0.62 |
| Suprapubic | 4 (5.5) | 4 (10.2) | 0.35 |
| Condom | 4 (5.5) | 1 (2.6) | 0.48 |
| Other | 3 (4.1) | 1 (2.6) | 0.67 |
| UTI Frequency – n (%) | |||
| ≥3 UTIs per year | 18 (24.7) | 13 (33.3) | 0.33 |
| Prophylactic antibiotic use – n (%) | |||
| Yes | 4 (5.5) | 5 (12.8) | 0.17 |
The SCI-specific antibiogram demonstrated different resistance profiles compared to the hospital-wide antibiogram (Figs. 1A and B). Gram negative and gram positive bacterial isolates identified within the SCI Center were noted to be generally more resistant to fluoroquinolones compared to the hospital-wide resistance profile. Some of the most common causative pathogens of UTI retained high susceptibilities with nitrofurantoin including E. coli (93%), Klebsiella oxytoca (80%), and methicillin-resistant Staphylococcus aureus (98%). These pathogens also retained relatively high susceptibilities with sulfamethoxazole/trimethoprim including E. coli (72%), Klebsiella pneumoniae (85%), K. oxytoca (90%), methicillin-resistant S. aureus (100%), and with a third generation cephalosporin including E. coli (95%), K. pneumoniae (99%), K. oxytoca (97%), Proteus mirabilis (99%), and methicillin-susceptible S. aureus (100%).
Figure 1.
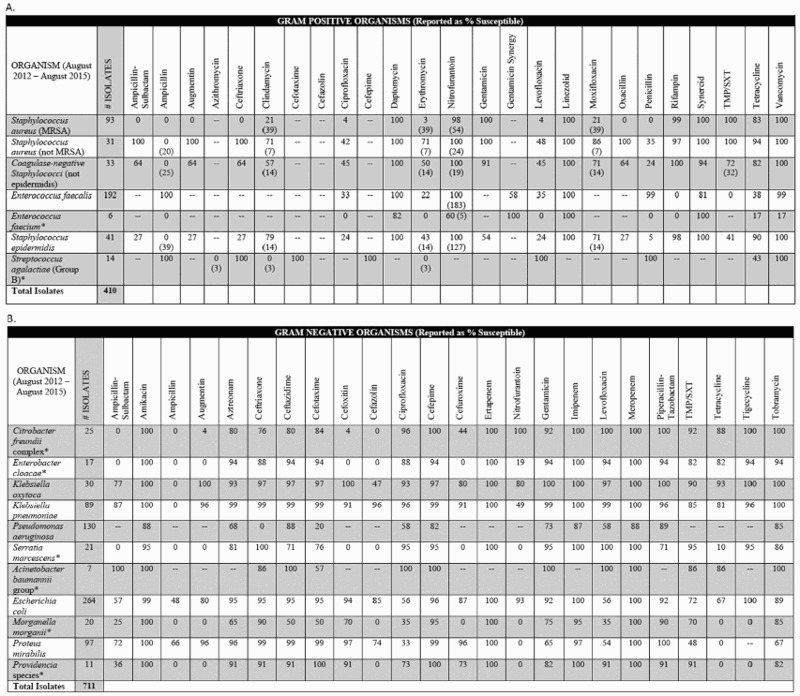
VA Medical Center Spinal Cord Injury Antibiogram Comments included within the antibiogram: 1) Spaces filled with (-) represent organism/antibiotic combinations not tested. 2) Clinicians should use caution when choosing empiric antibiotic regimens based on estimates of susceptibility derived from <30 isolates, as noted by the asterisk above (*), due to a low statistical validity of the estimates of % susceptibility. 3) Percent susceptible for each organism/antibiotic combination was generated by including only the first isolate of that organism recovered from a given patient during the time period analyzed. 4) For some results, there is a number in parenthesis below the % susceptibility; this number is the actual number of isolates tested for this bug/drug combination. Fewer isolates were tested for these combinations based on the specimen type (i.e. Nitrofurantoin is only reported on urine isolates, not isolates cultured from other specimen types). 5) Daptomycin restricted to criteria for use AND requires an ID consult to be placed. 6) Meropenem and linezolid restricted to criteria for use and must be evaluated by Pharmacy. 7) Per CLSI M100-S24 (January 2015), “Organisms that are susceptible to tetracycline are also considered susceptible to doxycycline and minocycline. However, some organisms that are intermediate or resistant to tetracycline may be susceptible to doxycycline, minocycline, or both.” 8) Tigecycline restricted to criteria for use AND requires an ID consult to be placed. 9) Providencia stuartii should be considered resistant to gentamicin and tobramycin, but not intrinsically resistant to amikacin.
A. Gram positive organism unit-specific antibiogram data from the Zablocki VA Spinal Cord Injury Center.
B. Gram negative organism unit-specific antibiogram data from the Zablocki VA Spinal Cord Injury Center.
TMP-SXT = trimethoprim-sulfamethoxazole.
For the primary outcome, there was a significantly higher rate of appropriate antimicrobial prescribing following the provider in-service and electronic order set implementation (Table 2). In the post-implementation group, 71.79% (n=39) of antibiotic courses were considered appropriate compared to 47.95% (n=73) in the pre-implementation group (P = 0.015). The most common cause of inappropriate antimicrobial prescribing was due to either a lack of symptoms or lack of documentation regarding UTI symptoms in both the pre- and post-implementation group (Table 3). Patients with indwelling catheters had the highest number of antibiotic courses with 2.73 courses per patient per year while patients with condom catheters had the lowest number of antibiotic courses with 1.36 courses per patient per year. Those patients included in the data analysis on prophylactic antibiotics received four antibiotic treatment courses per patient per year on average, and those without prophylactic antibiotics received 2.2 antibiotic treatment courses per patient per year on average. Finally, patients treated with appropriate antibiotic courses tended to have less hospitalizations following treatment compared to those treated inappropriately (0.56 hospitalizations/patient/year vs. 0.86 hospitalizations/patient/year, respectively). There were no statistically significant differences in any of the secondary outcomes (Table 4).
Table 2.
Primary outcome.
| Pre-Implementation | Post-Implementation | P-value | |
|---|---|---|---|
| Appropriate treatment – n (%) | 35 (47.95) | 28 (71.79) | 0.015 |
Table 3.
Reasons for inappropriate antimicrobial prescribing.
| Reasons for Inappropriateness | Pre-Implementation – n (%) | Post-Implementation – n (%) |
|---|---|---|
| Asymptomatic | 17 (36.2) | 5 (38.5) |
| Timing of urine culture | 6 (12.8) | 3 (23.1) |
| Empiric antibiotic choice | 9 (19.1) | 1 (7.7) |
| Therapy change based on urine culture | 15 (31.9) | 4 (30.8) |
| Dose or duration | 0 (0) | 0 (0) |
Table 4.
Secondary outcomes.
| Secondary Outcome | Dependent Variable | Independent Variable | P-value |
|---|---|---|---|
| Association between catheter type and antibiotic courses prescribed | Catheter type | Antibiotic courses per patient per year | 0.42 |
| Indwelling | 2.73 | ||
| Intermittent | 2.51 | ||
| Suprapubic | 1.53 | ||
| Condom | 1.36 | ||
| Association between prophylactic antibiotic use and UTI incidence | Prophylactic antibiotic use | Antibiotic courses per patient per year | 0.14 |
| Yes | 4.0 | ||
| No | 2.3 | ||
| Inpatient admissions following UTI treatment | Appropriate treatment | Inpatient admissions following treatment | 0.58 |
| Yes | 0.42 | ||
| No | 0.52 |
Discussion
To our knowledge, this was the first study conducted that evaluated antimicrobial prescribing practices in a SCI Center following the implementation of a SCI unit-specific antibiogram or order set. Following the intervention of the order set and related education, there was a 23.8% improvement in appropriate antimicrobial therapy for the treatment of UTIs in outpatients with SCI within the ZVAMC (71.79% vs. 47.95%, respectively; P = 0.015).
The antibiogram data was consistent with previous studies in that there was an overall higher resistance profile in the SCI Center than in the whole hospital. The most notable trend was with fluoroquinolones including ciprofloxacin and levofloxacin, which demonstrated higher resistance in the SCI Center for every organism tested except K. pneumoniae. Per the IDSA guidelines,2 ciprofloxacin and levofloxacin are considered first line empiric treatment choices for CA-UTIs. However, this particular patient population is known to have increased incidence of UTIs and increased exposure to antibiotics, which likely contribute to the higher rates of resistance. In addition, prescriber-specific and institution-specific prescribing patterns may influence rates of resistance to these particular drugs. The increased rate of fluoroquinolone resistance shown by our SCI antibiogram is an important finding since it is somewhat contradictory of the IDSA CA-UTI guidelines. Our data suggest that even if there are relatively high susceptibility rates for fluoroquinolones throughout the hospital, this class may not be an appropriate empiric option for patients with spinal cord injury depending on the institution's SCI resistance rates.
The Food and Drug Administration (FDA) recently released a safety warning in May of 2016 about the use of fluoroquinolones that is pertinent to the results of this study.6 In their report, they recommend against the use of fluoroquinolones in patients with certain infectious diseases including uncomplicated UTIs on the basis that the risk of serious toxicity outweighs the benefit of treatment. They note that there has been a strong trend of increasing resistance with fluoroquinolones in the general population related to the overuse and inappropriate prescribing of these medications. Although the FDA does not specifically recommend against the use of fluoroquinolones in complicated UTIs or CA-UTIs, the data from our institution's SCI-specific antibiogram supports the idea of increased caution with the use of these agents, especially within the ZVAMC SCI Center where higher fluoroquinolone resistance rates have been shown.
This study contained several strengths. First, this study used real-world antimicrobial stewardship strategies to increase the appropriateness of antibiotic therapy for UTIs. These strategies included use of an electronic order set, unit-specific antibiogram data to improve empiric therapy, and related provider education. The study also evaluated a patient population that is associated with higher incidence of UTIs, higher rates of resistance, and higher morbidity and mortality related to UTIs. In improving antimicrobial therapy for patients with SCI, there may be a more significant improvement in patient outcomes compared to able-bodied patients. In terms of the intervention, education alone has generally been shown not to be time-sustainable. The actual intervention in this study included implementation of an electronic order set in addition to provider education with the goal being to improve the sustainability of our results over time. Because the workflow, prescribing patterns, and resistance patterns can vary substantially between institutions, we believe the most impact can be achieved from implementing unit-specific antibiograms for patient populations suspected to have different resistance profiles from the general population and incorporating antibiograms as part of the institution's normal antimicrobial stewardship activities.
This study also has limitations including that it may not be as generalizable to other patient populations and possibly other institutions that do not contain a SCI Center or have a significant proportion of patients with SCI. However, the idea of unit-specific antibiograms influencing empiric antibiotics for patient populations that are known or suspected to have higher resistance rates can be applied to most institutions.
The study design itself was retrospective in nature, which limits the strength of our conclusions compared to those from prospective studies. There was also a relatively small sample size with 112 total antibiotic courses assessed and included in the results. Although the goal number of total antibiotic courses to assess was 90, we did not technically meet power because the number of antibiotic courses in the post-implementation group was less than 45. This may limit the strength of conclusions strictly due to the number of antibiotic courses assessed; however, the primary outcome was still considered statistically significant. Because of this, there was no concern with type II error for the primary outcome. Due to the retrospective nature of this study and the particular electronic medical record within the institution, it was not practical to assess and differentiate between patients that had UTIs associated with kidney stones or those without. Similarly, it was not assessed whether patients had local irrigation performed either with or without antibiotics. The presence of kidney stones and the use of local irrigation may not have been evenly distributed throughout the pre- and post-implementation groups and may have had some influence on the secondary outcomes.
There are a few limitations related to the unit-specific antibiogram. Due to relatively smaller number of patients within the SCI Center, there were significantly less culture isolates to include in the antibiogram data. The CLSI recommends that antibiograms contain at least 30 isolates per species, or that antibiograms include a comment explaining to use caution when interpreting susceptibility data for organisms with less than 30 isolates. One suggestion they provide to institutions with a lower number of annual culture isolates is to lengthen the amount of time that culture data is collected in order to increase the amount of isolates over 30. Like the hospital-wide ZVAMC antibiogram, the unit-specific antibiogram included culture data from patients with any disease state and included data from outpatients and inpatients. For the purposes of this study, it was not practical to separate outpatient urine cultures from all other culture isolates. Implementing an antibiogram that incorporates any culture specimen as well as data from inpatients or outpatient with SCI increases the utility and generalizability. However, this has to be taken into account when making empiric antibiotic choices based on antibiograms. Certain isolates may be more or less resistant in different types of infections, and the number and proportion of isolates identified are not representative of the proportion found with UTIs specifically. Outpatients are generally considered less at risk for multi-drug resistant organisms, and the inpatient data included in the SCI-antibiogram likely influences the overall resistance rates. Therefore, our antibiogram may show higher resistance profiles than is necessarily true for the outpatient population. Based on the susceptibilities from the antibiogram and recommendations from the infectious disease team, cefpodoxime was incorporated into the electronic order set as a potential empiric treatment option. Within the ZVAMC, not all antibiotics are tested for every culture and every organism, and oral third generation cephalosporins are often not included. Because of this, some of the recommendations for cefpodoxime were based off of data from ceftriaxone, another third generation cephalosporin. Although the spectrum of activity for antibiotics within the 3rd generation cephalosporin class are fairly similar, there may be slight differences from one to another that should be taken into account when making empiric antibiotic recommendations.
This study was fairly general in that the overarching purpose was to improve any part of the antimicrobial treatment course. Further studies may be warranted in order to identify specific processes of antimicrobial treatment and focus on improving those processes. Since there was still a significant percent of inappropriate antimicrobial therapy post-implementation of the unit-specific antibiogram and order set, recognizing and solving specific issues and changing specific processes in antimicrobial prescribing and management may have a more substantial effect on improving appropriate therapy. For example, if the most common cause of inappropriate therapy was that culture results were not being followed up on, adding an alert to the ordering provider when a culture results as either positive or negative may increase the chances that the antibiotics are changed appropriately. Other potential future directions include assessing appropriate treatment of UTIs for inpatients with SCI, direct and indirect costs associated with improvement in antibiotic use (antibiotic days, hospital admissions, recurrent infections), and improvement in patient-specific outcomes such as morbidity and mortality related to antibiotic treatment. A future direction for the ZVAMC is to continue to update the SCI-specific antibiogram and electronic order set if applicable similar to how the hospital-wide antibiogram and related infectious disease order sets are updated on a regular basis.
Conclusion
Based on the above results, our antibiogram-driven order set and provider education was beneficial and improved appropriate treatment of UTIs within the ZVAMC Spinal Cord Injury Center.
Disclaimer statements
Contributors All authors were responsible for the study design, data collection and analysis, and preparation of the article.
Funding The authors received no funding or financial support for any part of this study.
Conflicts of interest No authors had any actual or potential conflicts of interest related to the research, authorship, or publication of this article.
Ethics approval Not applicable. This study is considered a quality assurance analysis.
ORCID
Clayton Patros http://orcid.org/0000-0002-8251-4827
References
- 1.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004. Am J Infect Control 2004;32(8):470–85. doi: 10.1016/j.ajic.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 2.Hooton T, Bradley S, Cardenas D, Colgan R, Geerlings S, Rice J, et al Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50(1):625–63. doi: 10.1086/650482 [DOI] [PubMed] [Google Scholar]
- 3.Dellit T, Owens R, McGowan J, Gerding D, Weinstein R, Burke J, et al Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44(2):159–77. doi: 10.1086/510393 [DOI] [PubMed] [Google Scholar]
- 4.Hindler J, Stelling J.. Analysis and presentation of cumulative antibiograms: a new consensus guideline from the Clinical and Laboratory Standards Institute. Clin Infect Dis 2007;44(6):867–73. doi: 10.1086/511864 [DOI] [PubMed] [Google Scholar]
- 5.Binkleya S, Fishmana N, LaRosa L, Marr A, Nachamkin I, Wordell D, et al.. Comparison of unit-specific and hospital-wide antibiograms potential implications for selection of empirical antimicrobial therapy. Infect Control 2006;27(7):682–7. [DOI] [PubMed] [Google Scholar]
- 6.The Food and Drug Administration Fluoroquinolone antibacterial drugs: drug safety communication – FDA advises restricting use for certain uncomplicated infections. U.S. Food and Drug Administration website; http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm500665.htm. Updated May 25, 2016. Accessed June 1, 2016. [Google Scholar]


