Abstract
Objective
To investigate whether there are differences in the resting energy expenditure (REE) and body composition of athletes with a spinal cord injury (SCI) compared to active able-bodied controls.
Design
In this cross sectional study, male athletes with a SCI were compared to active able-bodied controls matched for age, stretch stature and body mass. In addition, the accuracy of standard REE prediction equations in estimating REE was assessed.
Participants
Seven male wheelchair athletes with a SCI and six matched active able-bodied controls volunteered to participate.
Outcome Measures
REE was measured using indirect calorimetry and estimated using population-specific prediction equations. Body composition (lean tissue mass, fat mass and bone mineral content) was measured by dual energy X-ray absorptiometry (DXA).
Results
While absolute and adjusted REE in the athletes with SCI was lower than controls, this difference was not significant (P = 0.259). When adjusted for lean tissue mass (LTM), REE was significantly higher (P = 0.038) in the athletes with SCI compared to the controls (146 ± 29kJ/kg LTM vs. 125 ± 8kJ/kg LTM). LTM was significantly lower in the athletes with SCI (44.35 ± 6.98 kg) compared to the able-bodied controls (56.02 ± 4.93 kg; P < 0.01). The differences between predicted and measured REE in the athletes with SCI were not statistically significant (except for the Owen equation), however there was no significant correlation between the measures.
Conclusion
This suggests that existing prediction equations used to estimate energy requirements may require modification for athletes with SCI.
Keywords: Athlete, Body composition, Disability, Resting energy expenditure, Spinal cord injury, Prediction equations
Introduction
The popularity of sport for people with disabilities has increased considerably over the past 10 years, with over 4000 athletes competing at the Rio 2016 Paralympic Games.1 This has generated an increasing interest in the energy requirements for this population and the relationship to body composition.
Energy expended throughout the day or total daily energy expenditure (TDEE) consists of resting energy expenditure (REE) or resting metabolic rate (RMR), thermic effect of food and energy expended throughout physical activity.2 In athletes, while REE is the main determinant of energy requirements, the contribution to TDEE varies widely.3 An inaccurate estimation of REE can lead to the over- or under-prediction of energy requirements, potentially leading to positive or negative energy balance which may impact on sporting performance.
Athletes with spinal cord injury (SCI) have impaired muscle power resulting in loss of function in the lower extremities (paraplegia) or all extremities (tetraplegia).1 Although REE has been examined in the general SCI population, little data is available in active individuals with a SCI.4–8 In a healthy adult SCI population, absolute REE has been reported to be 12 to 27% lower when compared to able-bodied controls, with the degree of difference being related to the level of the spinal injury.9 A strong correlation (r = 0.84) between REE and fat free mass (FFM) has been shown to explain 70–85% of the variation in REE6,10 in individuals with SCI,6 indicating that the larger the depletion of FFM (specifically muscle mass), the greater the reduction in REE.6,10–12 In relation to the level of spinal cord lesion, the higher the injury, the larger the area of FFM affected hence REE tends to be lowest in individuals with tetraplegia compared to paraplegia11 Lower cardiac output13 and reduced sympathetic nervous system (SNS)14 activity may also influence REE in individuals with SCI. As cardiac output is a predictor of the oxygen capacity throughout the body, this may potentially be a factor in the decreased REE of individuals with SCI.15 SNS activity is affected following a SCI, relative to the neurological level of the lesion, resulting in decreased metabolic activity and hence a resulting decreased REE.16 How much these factors influence REE is as yet undetermined, but is likely to be relatively small (2–3% of REE).16
While REE can be determined using direct and indirect calorimetry, these methods are not always suitable for use in a clinical setting. An alternative method is the use of various prediction equations to estimate REE. There are a range of equations that have been developed for specific reference populations varying in age, sex, level of obesity and activity level. Each equation involves substituting one or more variables, including an individual's height, weight, age or lean tissue mass (LTM).3 LTM differs from FFM in that it is based on a three compartment model of body composition that separates bone mineral content and fat mass from lean tissue. However, prediction equations based on reference populations are unlikely to be suitable for estimating REE in individuals with SCI, regardless of level of activity, and there is currently no well validated equation for use in this population.
Currently, there is very little data reported on the actual REE of athletes with a SCI, nor is there a clear understanding of how to estimate best REE in this population. This is important in order to enable dietary manipulations that optimise training capability, body composition and hence performance. The aim of this study was to investigate whether REE differs between male athletes with a SCI compared to able-bodied controls matched for age, stretch stature, body mass and level of activity. In addition, the accuracy of standard REE prediction equations in estimating REE in male athletes with a SCI was assessed.
Methods
Subjects
Two groups of males (18 to 45 years) were recruited for this study. The first group consisted of males with SCI (n = 7) who were classified as wheelchair athletes by the Australian Paralympic Committee.17 To be included in the study, each athlete had to be competing at a State, National and/or International level in a sport of aerobic nature. The second group consisted of physically active able-bodied males (n = 7) who were matched to the athletes with SCI by age (within 1–2 years), stretch stature (< 5%) and body mass (< 5%). The able-bodied males must have been undertaking at least 150 minutes of physical activity per week.
Participants were excluded from the study if they were smokers or taking medications that affect REE, including aspirin, suppressor agents, catecholamines, steroids, sedatives, narcotic analgesics, anesthesia and beta-blockers. Participants who reported that they had exercised or consumed alcohol or caffeine 24 hours preceding the day of REE measurements were also excluded. Participants were asked to report their recent weight history (whether they were weight stable or were attempting to alter their weight). Ethics approval (no. A/05/65) was obtained from the the University of the Sunshine Coast Human Research Ethics Committee.
Body composition
Body mass (BM) of each participant was determined immediately prior to the measurement of REE. All participants were asked to empty their bladders prior to the BM measurement and wore minimal clothing. The able-bodied controls were weighed using an electronic digital scale (Tanita, Illinois, USA) calibrated to within 0.05 g. The BM of the athletes with SCI was obtained using a floor mounted force plate (AMTI 400600NC, JC Measurements Pty. Ltd., Australia) measuring to within 0.001 g.
Stretch stature of able-bodied controls was determined using a wall-mounted stadiometer to a precision of ± 1 mm. A specially designed horizontal length board was constructed for athletes with SCI. The athletes with SCI positioned themselves on the length board with legs outstretched and feet in dorsiflexion pressed against the immovable footboard. In order to obtain stretch stature, an assistant held onto their feet at all times to maintain pressed heels against the footboard and the feet measure (mm) was subtracted from the head measure (mm). Two of the athletes with SCI were unable to stretch their legs on the length board so standard anthropometric landmarks were used on their legs and three measures were taken using an anthropometric steel tape.
Lean tissue mass
Assessment of body composition for all participants was conducted using a Lunar-DPX/NT DXA instrument (Lunar Radiation Corp., Madison, WI, USA). Participants were requested to fast overnight for a minimum of 12 hours prior to measurement. Data obtained from the total body analysis included FM (kg), LTM (kg), bone mineral content (BMC) (g), bone mineral density (BMD) (g.cm−2), and tissue and regional %BF. Total %BF was obtained by calculating TotalFat(g)/TotalTissue(g). Fat free mass (FFM) was determined to be LTM plus BMC.
Indirect calorimetry
The REE of each study participant was measured early in the morning, following a 12-hour fast and abstinence from exercise, caffeine and alcohol for a minimum of 24 hours prior. Participants were requested to undertake minimum movement prior to testing. Each participant lay in a supine position in a dimly lit room, at a thermo-neutral temperature of 21–22°C. Each participant was allowed a 10–20 minute relaxation period prior to commencing the test. Participants were monitored carefully to ensure that they did not fall asleep and any interruptions that occurred were noted.
The volume of oxygen consumed (VO2) and carbon dioxide produced (VCO2) were measured via open-circuit indirect calorimetry using a ventilated hood apparatus. Gas sampling was underneath using a ParvoMedics TrueOne 2400 metabolic cart (ParvoMedics Inc., Sandy, Utah). The instrument was calibrated prior to performing each measurement against a standard mixed reference gas. Prior to a gas calibration, the flow rate was calibrated to between 20 L/min to 150 L/min. If either the gas or flow rate calibrations were >2% different than the previous measurement, calibrations were re-run prior to the commencement of a test. Testing was conducted for 30–60 minutes, with the first 10 minutes considered to be an adaptation period where alterations to the fraction of expired oxygen (FEO2) were made in order to maintain a standard concentration of between 0.65–0.85%. Testing was ceased 10 minutes after steady state was achieved, which was defined as a fluctuation of less than 5% in VO2, VCO2 and respiratory quotient (RQ) together with a metabolic equivalent (MET) value that was stable and less than 1.0 over a 10 minute period as previously described.18–20 If a participant reached steady state more than once, the lowest 10-minute value of REE was selected. For two of the athletes with SCI and two of the able-bodied controls, steady state was not reached over a 10-minute period. In these cases, the time frame in which steady state was determined from was reduced to 5 minutes (n = 3) or 3 minutes (n = 1) in order to determine steady state under the same conditions (i.e. a fluctuation of ≤ 5% in REE, VO2, VCO2 and RQ).
Prediction equations
REE was estimated for each participant by entering the variables of stretch stature, BM, age and LTM (as determined by DXA) into a range of commonly used prediction equations. The REE prediction equations selected for comparison have been presented in Table 1.
Table 1.
REE prediction equations for males.
| Source | No. of subjects | Reference population | Equation for REE |
|---|---|---|---|
| Mifflin, 199021 | 251 | Healthy normal (89-<119% IBW) and obese (> 120% IBW) males aged 19-78y. Excludes underweight (< 80% IBW) and morbidly obese (> 120% IBW) | REE = 10(wt) + 6.25(ht) – 5(age) + 5 |
| Owen, 198722 | 60 | Healthy lean and obese males (60-171 kg); aged 18 to 82y. No athletes included | REE = 290 + 22.3 (LBM) |
| Schofield, 198523 | 3525 | 2879 healthy male volunteers (63 ± 8.7 kg); aged 18 to 30 yrs (22.47 ± 7.48 yrs) 646 healthy male volunteers (64 ± 10.8 kg); aged 30 to 60 yrs (40.09 ± 7.2 yrs) | 0.063(wt) + 2.869 0.048(wt) + 3.653 |
| Cunningham, 198024 | 120 | Male adult subjects from the study of Harris and Benedict. 16 males were excluded for being identified as athletes | REE = 500 + 22 (LBM) |
| Harris and Benedict, 191925 | 136 | Healthy normal weight males aged 27 ± 9. Included trained athletes | REE = 66.47 + 13.75(wt) + 5(ht) – 6.67(age) |
LBM = lean body mass, which is equivalent to LTM.
Data analysis
Descriptive statistics were first completed in order to determine summary statistics, mean, standard deviation (SD), and range for each variable. The Mann-Whitney Rank sum test was used to test for differences between athletes with SCI and able-bodied controls, while differences in variables within a group were tested using Wilcoxon Signed Rank sum test. Spearman's Rank correlation (rs) was conducted to determine the relationship between the variables. For all inferential statistical analysis of distributions, the level of statistical significance was chosen at P ≤ 0.05.
Data analysis was conducted using Microsoft Excel (Microsoft Corporation, USA). SPSS statistical software (Version 13.0, SPSS Inc., Chicago, Illinois) was used for all statistical analyses.
Results
Subject characteristics
Six athletes with SCI presented with complete and incomplete spinal cord lesions at the T3/4 (n = 1), T5 (n = 2), T12 (n = 2) and L3/4/5 (n = 1) levels. The final athlete with SCI presented with bilateral Perthes disease. The duration of injury for the athletes with SCI was between 10 to 15 years. All athletes with SCI competed at national (n = 1) and international (n = 6) level of competition in tennis (n = 2), basketball (n = 1), hand cycling (n = 2) and water skiing (n = 2). No significant difference (P < 0.05) was found in age, stretch stature and BM between the athletes with SCI and the able-bodied controls.
Body composition
No significant difference was found in total body FM and BMD between athletes with SCI and able-bodied controls (Table 2), although the athletes with SCI carried 6.94 ± 4.29 kg more body fat than the able-bodied controls. The %BF of athletes with SCI was on average 10.8 ± 4.0% higher than the able-bodied controls (95% CI: 1.99 to 19.6%BF).
Table 2.
Body composition of athletes with SCI and able-bodied controls (mean ± SD).
| Descriptive | Athletes with SCI | Controls |
|---|---|---|
| Age (years) | 31.3 ± 7.3 | 32.7 ± 7.2 |
| Body mass (kg) | 72.0 ± 15.2 | 76.1 ± 8.5 |
| Stretch Stature (cm) | 173.1 ± 18.5 | 179.4 ± 5.4 |
| FM (kg) LTM (kg)* | 23.4 ± 10.2 33.4 ± 6.9 | 16.5± 5.0 56.0 ± 4.9* |
| LTM (g.kg−1BM)* % BF * BMD (g. cm2) | 628 ± 87 33.0 ± 9.0 1.193 ± 0.056 | 739 ± 51** 22.0 ± 5.0* 1.307 ± 0.112 |
*Mann Whitney U, z = –2.236 P = 0.026; **Mann Whitney U z = –2.364; P = 0.017).
The athletes with SCI had a significantly lower %LTM compared to able-bodied controls (67 ± 9 vs. 78 ± 5% LTM), with athletes with SCI on average having 10.8 ± 4.0% less lean tissue than able-bodied controls (P = 0.026). A similar trend was evident when considering the amount of LTM in grams (g) per kilogram (kg) of BM (Table 2). Total LTM, leg and trunk LTM differed significantly between the athletes with SCI and the able-bodied controls (P ≤ 0.01).
Measured REE
The mean difference in absolute REE between athletes with SCI and able-bodied controls was 527 kJ.d−1 (95% CI: -1329 to 272 kJ.d−1, not significant). REE relative to BM further reduced the difference in REE between the two groups (Table 3). However, a significant difference (P = 0.038) was found when REE relative to LTM was compared between the two groups (Table 3). Athletes with SCI expended, on average, 25 ± 13 kJ more energy per kg of LTM than the able-bodied controls (95% CI: -2.01 to 49.78 kJ.kg LTM−1d−1).
Table 3.
Absolute REE, REE relative to BM, and REE relative to LTM in the athletes with SCI and able-bodied controls (mean ± SD).
| Athletes with SCI | Controls | |
|---|---|---|
| Absolute REE (kJ.d−1) | 6437 ± 769 | 6964 ± 142 |
| REE relative to BM (kJ.kg−1d−1) | 92 ± 4 | 92 ± 4 |
| REE relative to LTM (kJ.kg LTM−1d−1) | 146 ± 29 | 125 ± 8* |
(Mann-Whitney U; z = –2.070; P = 0.038).
Fasting RQ was not significantly different between athletes with SCI (0.84 ± 0.128) and able-bodied controls (0.87 ± 0.035).
Predicted REE
There was no significant difference between the measured REE for the athletes with SCI and the predicted REE from the Mifflin (P = 0.375), Cunningham (P = 0.578), Harris and Benedict (P = 0.156) and Schofield (P = 0.078) equations (Fig. 1a). However, the Owen equation significantly underestimated REE by 17% (P = 0.016). The Cunningham equation best predicted REE with a difference of 268 ± 874 kJ.d−1 between the predicted and measured values (95% CI: –539 to 1074 kJ.d−1). While the differences between the predicted and measured REE were not statistically significant (except for the Owen equation), there was a low to moderate and non-significant correlation between the measures (Table 4). The Mifflin equation had the highest correlation (r = 0.464; P = 0.294) to the measured REE in athletes with SCI (Table 4).
Figure 1.
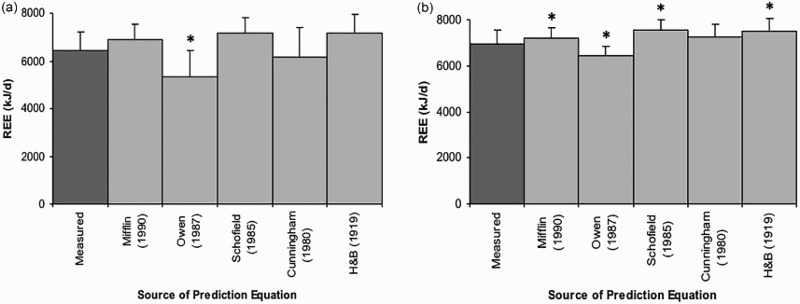
Measured and predicted REE for the athletes with SCI and able-bodied controls (mean ± SD). (a) REE in athletes with SCI. (b) REE in able-bodied controls. Details about the prediction equations are provided in Table 1. H&B = Harris and Benedict. *P ≤ 0.05.
Table 4.
The relationship between measured REE and predicted REE using the Spearman's Rank correlation co-efficient (r) in athletes with SCI and able-bodied controls.
| Prediction Equation | Athletes with SCI | Controls |
|---|---|---|
| Owen (1987) | 0.143 | 0.821* |
| Mifflin (1990) | 0.464 | 0.964* |
| Cunningham (1980) | 0.143 | 0.821* |
| Harris & Benedict (1919) | 0.393 | 1.000* |
| Schofield (1985) | 0.321 | 0.893* |
*significance level at P ≤ 0.05.
Similarly, the REE predicted by the Cunningham equation for the able-bodied controls did not differ significantly from their measured REE. On the other hand, the Owen (P = 0.031), Mifflin (P = 0.047), Harris and Benedict (P = 0.016) and Schofield (P = 0.016) equations over-predicted REE by 3 to 8%, with all measures differing significantly to the measured REE (Figure 1b). In contrast to the results for the athletes with SCI, the predicted REE for all of the equations correlated highly and significantly with the corresponding measured REE (Table 4). Although the predicted REE was significantly different to the measured REE for all equations, the mean differences were smaller than that obtained for the athletes with SCI (543 ± 159 vs. 656 ± 314 kJ.d−1; able-bodied controls vs. athletes with SCI).
Discussion
The primary finding of this study was that the absolute REE of the athletes with SCI was not significantly different to the REE of the able-bodied controls. We also found that when REE was adjusted for BM, the difference between the two groups was further reduced. In contrast, when adjusted for lean tissue mass (LTM), REE was significantly higher in the athletes with SCI compared to the able-bodied controls. These were interesting findings because there were clear differences in the body composition of the two groups, including a greater %BF and lower LTM in the athletes with SCI.
The absolute and relative REE of the athletes with SCI in this study (6437 kJ.d−1 or 146 kJ.kg LTM−1d−1) is similar to previous reports of sedentary male paraplegics (6310-6649 kJ.d−19 and 142 kJ.kg FFM−1.d−1 8). A recent study comparing exercising (150 mins/week) and sedentary individuals with a SCI found a significant difference in REE when reported relative to BM (88 kJ.kg−1d−1 versus 67.kg−1d−1)21, less than the 92 kJ.kg−1d−1reported in this study.21 The same study also found a significant difference between FM and FFM in the sedentary and exercising groups which most likely explained the variance in REE.26
One of the hypotheses for the current study was that there would be a difference in absolute REE between the athletes with SCI and the able-bodied controls. Previous investigations conducted on sedentary individuals with SCI reported that absolute REE was significantly lower when compared to matched able-bodied controls.5,6,8 While absolute REE in the athletes with SCI in the current study was 527 kJ.d−1 lower than the controls, this difference was not statistically significant. REE has also been reported to be significantly lower in individuals with SCI when adjusted for BM as compared to able-bodied controls.6 In contrast, the current study found no difference between the athletes with SCI and controls, which is supported by Bauman et al.5 Buchholz et al.6 suggests that BM is only a crude indicator of body composition and that LTM (or FFM) may provide a better indication of REE per unit of metabolically active tissue. Several researchers have reported that REE did not differ between individuals with SCI and able-bodied controls when adjusted for FFM5,6,27 indicating that the metabolic activity in the fat-free compartment of the body was similar in both groups. This differs to our findings where REE was significantly higher in athletes with SCI than able-bodied controls when adjusted for LTM (146 versus 125 kJ.kg LTM−1d−1). These results were substantially higher than those reported by Sedlock and Laventure7 (112 kJ.kg FFM−1d−1), however their small sample of four subjects were not trained athletes and fat free mass was determined by subtracting fat mass from total body weight and thus may not be as accurate measure of metabolically active tissue. Comparisons with other studies are complicated by the large variation in levels and completeness of SCI, the inclusion of both males and females, the lack of description regarding current activity levels of the subjects and variation in the method to determine body composition.
Our findings that suggest that the athletes with SCI were expending more energy per kg of LTM may appear surprising, however can be explained. The majority of energy expended by LTM during rest is accounted for by the high metabolic activity of the viscera28 and not skeletal muscle which only accounts for 17% of the energy expended.29 Therefore, if the spinal cord lesion was between T1 and T10 (high paraplegia), the affected skeletal muscle areas include the intercostal and thoracic muscle, abdominal muscles and gastrointestinal tract, which may in turn influence the integrity and metabolic activity of the tissues that contribute to the majority of REE. A lesion lower than T10 predominantly affects the LTM of the lower extremities, which contributes a lower proportion to REE.7 Since the majority of our athletes with SCI were classified as low incomplete paraplegia (T10 and below), the majority of the decline in LTM would have involved only the lower extremities, which may explain the similarity between the REE of the athletes with SCI and the able-bodied controls. This proposed mechanism is supported by previous studies that found the REE in sedentary individuals with SCI to be significantly lower than able-bodied controls.5–8 For example, in a study of 13 SCI individuals,5 the six high paraplegia or tetraplegia individuals had a greater decline of LTM, not only from the lower extremities but also from the viscera. Similar findings have been reported in other studies.6,8,30 This suggests that investigation into energy expenditure in those with SCI should consider classifying the level and completeness of the spinal cord lesion as this may impact on the viscera.
In addition to changes in LTM, it has also been proposed that the variability in metabolic rate among able-bodied individuals may result from differences in the activity of the sympathetic nervous system (SNS).31 Muscle sympathetic nerve activity (MSNA) is defined as the sympathetic outflow to skeletal muscle, represents a direct measurement of sympathetic activity and has been shown to play a role in regulating metabolic rate as it was highly correlated to measures of REE.31 Indeed, it has been previously suggested that the activation of spinal sympathetic neurons, which innervate skeletal muscle, is decreased following an injury to the spinal cord.16 This mechanism could contribute to a lower REE in individuals with SCI, albeit most likely to only a small degree (2–3%).6,27 It is possible that the higher level of physical activity of athletes with SCI may result in an increased sympathetic innervation of skeletal muscle in comparison to sedentary individuals with SCI.
Prediction equations used to estimate REE
The study has demonstrated that prediction equations commonly used to estimate REE in able-bodied individuals did not accurately estimate REE in athletes with SCI. In able–bodies controls, prediction equations have been shown to under-report REE by 7 to 15%.32 This study found that only the Owen equation22 under-reported REE in the able-bodied controls, while REE predictions by the Mifflin,21 Schofield,23 and Harris and Benedict25 equations significantly over-reported REE. As per previous results by Thompson and Manore32 in a sample of athletes, our study found the Cunningham equation24 best predicted the able bodied controls’ REE compared to measured.
A comparison of the measured and predicted REE of the athletes with SCI in this investigation found that only REE predicted by the Owen equation differed significantly to the measured REE. As per able-bodied controls, the Cunningham equation best estimated REE in athletes with SCI with a mean difference of 268 kJ.d–1. The Mifflin, Schofield and Harris and Benedict equations all over-predicted REE in the athletes with SCI to a greater extent than in the able-bodied controls. The mean differences in the athletes with SCI were relatively larger than the differences found between measured and predicted REE in their able-bodied counterparts. However, the estimation of REE from a prediction equation is only as accurate as the variables used in the equation.33 Theoretically, the most appropriate REE prediction equation should be the one that best matches the individual to the reference population from which the equation was derived.28 As all equations were validated in healthy able-bodied individuals, the predictive ability of these equations is decreased in athletes with SCI due to alterations in body composition.6 To date, only one prediction equation relevant to sedentary SCI populations has been reported in the literature,6 which was derived from 28 paraplegics of both sexes (17 males and 11 females). However, the small sample size of 17 males, the sedentary level of activity of participants and the use of height which is difficult to accurately measure in many individuals with a SCI, precludes it from comparison in this study. Further investigation is required into the development of prediction equations for the estimation of REE specific to active individuals with SCI.
The findings of this study are influenced by a few limitations. The medication use prior to measurement was not recorded and it is feasible this may have had an impact on the results. One of the athletes with SCI had back pain that persisted throughout the REE measurement, thus testing was only conducted for 20 minutes, resulting in modification of the procedure to determine steady state REE. For this SCI athlete, the time frame in which steady state was achieved was shortened to three minutes as per Reeves et al.20 The same guidelines for obtaining REE, including fluctuation of less than 5% in REE, VO2, VCO2 and RQ were adhered to. As is common with most studies on this population, the sample size was small. However, the results of this study add to limited body of evidence on energy expenditure of athletes with SCI.
Conclusions
We found no difference in absolute REE between athletes with SCI and able-bodied controls matched for age, stretch stature and BM despite the difference in body composition between the two groups. Furthermore, when adjusted for LTM, REE was found to be significantly higher in the athletes with SCI, suggesting that they expend more energy per kg of LTM than their able-bodied counterparts. The physiological mechanisms for this finding require further investigation, and future studies of REE in athletes with SCI should report their findings according to different levels and completeness of SC lesion. In addition, prediction equations developed for healthy able-bodied individuals to estimate REE are not applicable to athletes with SCI, warranting further research into the development of prediction equations specific to this population. It is evident that there is a need for similar studies on larger samples of athletes with SCI using current and reliable measures of body composition.
Funding Statement
This work was supported by The University of the Sunshine Coast [internal seed grant SRG05/10].
Disclaimer statements
Contributors None.
Conflict of interest None.
Ethics approval None.
References
- 1.International Paralympic Committee The Paralympic Movement 2017. [cited 2017 20th January]. Available from: https://www.paralympic.org/rio-2016.
- 2.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C.. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 1986;78(6):1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manore MM, Thompson JL.. Energy requirements of the athlete: assessment and evidence of energy efficiency. In: Burke L, Deakin V, editors. Clinical Sports Nutrition. 5th ed North Ryde: McGraw-Hill Australia Pty Ltd; 2015. p. 114–30. [Google Scholar]
- 4.Price M. Energy expenditure and metabolism during exercise in persons with a spinal cord injury. Sports Med 2010;40(8):681–96. doi: 10.2165/11531960-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 5.Bauman WA, Spungen AM, Wang J, Pierson RN.. The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury. J Rehabil Res Dev 2004;41(1):1–8. doi: 10.1682/JRRD.2004.01.0001 [DOI] [PubMed] [Google Scholar]
- 6.Buchholz AC, McGillivray CF, Pencharz PB.. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr 2003;77(2):371–8. doi: 10.1093/ajcn/77.2.371 [DOI] [PubMed] [Google Scholar]
- 7.Sedlock DA, Laventure SJ.. Body composition and resting energy expenditure in long term spinal cord injury. Paraplegia 1990;28(7):448–54. [DOI] [PubMed] [Google Scholar]
- 8.Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E.. Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr 1998;68(6):1223–7. doi: 10.1093/ajcn/68.6.1223 [DOI] [PubMed] [Google Scholar]
- 9.Mollinger LA, Spurr GB, el Ghatit AZ, Barboriak JJ, Rooney CB, Davidoff DD, et al. Daily energy expenditure and basal metabolic rates of patients with spinal cord injury. Arch Phys Med Rehabil 1985;66(7):420–6. [PubMed] [Google Scholar]
- 10.Nielsen S, Hensrud DD, Romanski S, Levine JA, Burguera B, Jensen MD.. Body composition and resting energy expenditure in humans: role of fat, fat-free mass and extracellular fluid. Int J Obes Relat Metab Disord 2000;24(9):1153–7. doi: 10.1038/sj.ijo.0801317 [DOI] [PubMed] [Google Scholar]
- 11.Sedlock D, Laventure S.. Body composition and resting energy expenditure in long term spinal cord injury. Paraplegia 1990;28(7):448–54. [DOI] [PubMed] [Google Scholar]
- 12.Blissitt PA. Nutrition in acute spinal cord injury. Crit Care Nurs Clin North Am 1990;2(3):375–84. [PubMed] [Google Scholar]
- 13.Hopman MT, Oeseburg B, Binkhorst RA.. Cardiovascular responses in persons with paraplegia to prolonged arm exercise and thermal stress. Med Sci Sports Exerc 1993;25(5):577–83. doi: 10.1249/00005768-199305000-00008 [DOI] [PubMed] [Google Scholar]
- 14.Saad MF, Alger SA, Zurlo F, Young JB, Bogardus C, Ravussin E.. Ethnic differences in sympathetic nervous system-mediated energy expenditure. Am J Physiol 1991;261(6 Pt 1):E789–94. [DOI] [PubMed] [Google Scholar]
- 15.Poehlman ET. Regulation of energy expenditure in aging humans. J Am Geriatr Soc 1993;41(5):552–9. doi: 10.1111/j.1532-5415.1993.tb01895.x [DOI] [PubMed] [Google Scholar]
- 16.Stjernberg L, Blumberg H, Wallin BG.. Sympathetic activity in man after spinal cord injury. Outflow to muscle below the lesion. Brain 1986;109 (Pt 4):695–715. doi: 10.1093/brain/109.4.695 [DOI] [PubMed] [Google Scholar]
- 17.Australian Paralympic Committee. Disability Categories Sydney: Australian Paralympic Committee ; 2007. [2 March 2007]. Available from: http://www.paralympic.org.au/apc_sub.asp?id=258. [Google Scholar]
- 18.Haugen HA, Melanson EL, Tran ZV, Kearney JT, Hill JO.. Variability of measured resting metabolic rate. Am J Clin Nutr 2003;78(6):1141–5. doi: 10.1093/ajcn/78.6.1141 [DOI] [PubMed] [Google Scholar]
- 19.McClave SA, Spain DA, Skolnick JL, Lowen CC, Kjeber MJ, Wickerham PS, et al. Achievement of steady state optimizes results when performing indirect calorimetry. Jpen-Parenter Enter 2003;27(1):16–20. doi: 10.1177/014860710302700116 [DOI] [PubMed] [Google Scholar]
- 20.Reeves MM, Davies PS, CBauer J, Battistutta D.. Reducing the time period of steady state does not affect the accuracy of energy expenditure measurements by indirect calorimetry. J Appl Physiol 2004;97:130–34. doi: 10.1152/japplphysiol.01212.2003 [DOI] [PubMed] [Google Scholar]
- 21.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO.. A new predictive equation for resting energy expentidure in healthy individuals. Am J Clin Nutr 1990;51:241–7. doi: 10.1093/ajcn/51.2.241 [DOI] [PubMed] [Google Scholar]
- 22.Owen OE, Holup JL, D'Alessio DA, Craig ES, Polansky M, Smalley KJ, et al. A reappraisal of the caloric requirements of men. Am J Clin Nutr 1987;46(6):875–85. doi: 10.1093/ajcn/46.6.875 [DOI] [PubMed] [Google Scholar]
- 23.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985;39 Suppl 1:5–41. [PubMed] [Google Scholar]
- 24.Cunningham JJ. A reanalysis of the factors influencing basal metabolic rate in normal adults. Am J Clin Nutr 1980;33(11):2372–4. doi: 10.1093/ajcn/33.11.2372 [DOI] [PubMed] [Google Scholar]
- 25.Harris J, Benedict F.. A biometric study of basal metabolism in man. Philadelphia: FB Lippincott; 1919. [Google Scholar]
- 26.Tanhoffer RA, Tanhoffer AI, Raymond J, Hills AP, Davis GM.. Exercise, energy expenditure, and body composition in people with spinal cord injury. J Phys Act Health 2014;11(7):1393–400. doi: 10.1123/jpah.2012-0149 [DOI] [PubMed] [Google Scholar]
- 27.Jeon JY, Steadward RD, Wheeler GD, Bell G, McCargar L, Harber V.. Intact sympathetic nervous system is required for leptin effects on resting metabolic rate in people with spinal cord injury. J Clin Endocrinol Metab 2003;88(1):402–7. doi: 10.1210/jc.2002-020939 [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Heshka S, Zhang K, Boozer CN, Heymsfield SB.. Resting energy expenditure: systematic organization and critique of prediction methods. Obes Res 2001;9(5):331–6. doi: 10.1038/oby.2001.42 [DOI] [PubMed] [Google Scholar]
- 29.Bray GA, Atkinson RL.. Factors affecting basal metabolic rate. Prog Food Nutr Sci 1977;2(8):395–403. [PubMed] [Google Scholar]
- 30.Schneider DA, Sedlock DA, Gass E, Gass G.. VO2peak and the gas-exchange anaerobic threshold during incremental arm cranking in able-bodied and paraplegic men. Eur J Appl Physiol O 1999;80(4):292–7. doi: 10.1007/s004210050595 [DOI] [PubMed] [Google Scholar]
- 31.Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA.. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J Clin Invest 1993;92(4):1730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson J, Manore MM.. Predicted and measured resting metabolic rate of male and female endurance athletes. J Am Diet Assoc 1996;96(1):30–4. doi: 10.1016/S0002-8223(96)00010-7 [DOI] [PubMed] [Google Scholar]
- 33.Finan K, Larson DE, Goran MI.. Cross-validation of prediction equations for resting energy expenditure in young, healthy children. J Am Diet Assoc 1997;97(2):140–5. doi: 10.1016/S0002-8223(97)00039-4 [DOI] [PubMed] [Google Scholar]


