Abstract
Background
Pracinostat is a potent histone deacetylase inhibitor with antitumor activity in both solid tumor and acute myeloid leukemia (AML) cell lines. Pracinostat has modest clinical activity in advanced solid tumors. Given the higher preclinical sensitivity of hematologic malignancies to pracinostat, we conducted a phase 1 study to assess the safety, maximum tolerated dose (MTD), recommended phase II dose (RP2D), efficacy, pharmacokinetics and pharmacodynamics of pracinostat in advanced hematological malignancies.
Methods
Pracinostat was administered orally thrice weekly for 3 weeks on a 28-day cycle. Patients were assigned to seven dose levels using a 3+3 dose escalation design.
Results
Forty-four patients were enrolled, 25 had AML and 14 had myelodysplastic syndrome (MDS). The MTD was 120 mg and the RP2D was 60 mg. Two AML patients achieved a response; 1 complete remission (CR) and 1 complete cytogenetic response. Despite dose-dependent increase in the plasma concentration of pracinostat, similar increase in histone acetylation was not observed. As an extension, 10 additional MDS patients were enrolled to assess the safety and efficacy of pracinostat in combination with azacitidine. Six patients achieved CR and 3 achieved CR without platelet recovery with no added toxicity.
Conclusions
Pracinostat is safe with modest single-agent activity in hematological malignancies.
Keywords: acetylation, epigenetic, histone, methylation, pracinostat
INTRODUCTION
Histone deacetylases (HDACs) play a crucial role in regulating gene expression and are considered a potential epigenetic target for cancer therapy.1–3 Acetylation of histone proteins in promoter regions of genes has been found to activate gene transcription and is highly regulated by HDACs and histone acetyltransferases (HATs).4, 5 Overexpression of HDACs induces histone hypoacetylation resulting in chromatin condensation thereby leading to transcriptional repression and epigenetic silencing.1, 6 This aberrant epigenetic suppression of gene expression may result in uncontrolled cell proliferation and arrested differentiation leading to malignancy.7
HDAC inhibitors (HDACIs) can reactivate gene expression and promote cell-cycle arrest, differentiation, and apoptosis through inducing histone acetylation resulting in chromatin relaxation increasing the accessibility of transcription factors to their target genes.1, 5, 8, 9 Pracinostat, 3-[2-butyl-1-(2-diethylaminoethyl)-1H-benzoimidazol-5-yl]-N-hydroxyacrylami-hydrochloride, is a potent pan-HDACI which is about twice the potency of vorinostat.10 In vivo studies have demonstrated favorable pharmacokinetic (PK) properties of pracinostat, with a 4.1-fold increased oral bioavailability and a 3.3-fold increased half-life (t1/2) when compared to vorinostat.10 Furthermore, pracinostat, in contrast to vorinostat, has been found to accumulate in tumor tissue and induce a dose-dependent sustained elevation in the histone H3 acetylation (AcH3) levels in mice models of AML and human colorectal cancer.10, 11
These PK and pharmacodynamic (PD) properties of pracinostat have been confirmed in two studies conducted in patients with advanced solid tumors.12, 13 Despite elevation in target efficacy biomarker, AcH3 in peripheral blood mononuclear cells (PBMCs), antitumor efficacy of pracinostat was not observed, with stable disease (SD) documented as the best response to treatment in 21%13 and 32%12 of the patients, respectively.
Given the higher sensitivity of leukemia and lymphoma to pracinostat,10 as demonstrated in preclinical studies, we conducted a multicenter phase 1 dose escalation study to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of pracinostat in patients with advanced hematological malignancies.
MATERIALS AND METHODS
This multicenter study was developed jointly by the initial pracinostat developer, S*Bio Pte Ltd., and the investigators. The clinical trial registration number is NCT00741234. The study was conducted according to the standards of Good Clinic Practice (GCP) and per institutional research policies and procedures. The study protocol was approved by the institutional review board and ethics committee at each center.
Patient Population
Patients aged ≥ 18 years with an advanced hematological malignancy (relapsed or refractory AML, high-risk MDS [defined by an International Prognostic Scoring System (IPSS) Risk Category Intermediate-1 or greater], multiple myeloma, myelofibrosis, indolent or aggressive Non-Hodgkin’s lymphoma, or Hodgkin’s disease) who failed, relapsed, or were ineligible for conventional therapy or allogeneic stem cell transplantation (SCT) were eligible. Patients had to have an Eastern Cooperative Oncology Group performance status of ≤ 2, adequate renal, hepatic and cardiac function [total bilirubin ≤ 1.5 × upper limit of normal (ULN), aspartate aminotransferase and alanine aminotransferase ≤ 2.5 × ULN, serum creatinine < 1.6 mg/dl, left ventricular ejection fraction ≥ 50%], and no clinically significant co-morbidities.14 Major exclusion criteria were CNS involvement; concomitant treatment with other HDACIs; malabsorption; use of hydroxyurea or any investigational agent within 14 days or five half-lives of study enrollment; major surgery within the past 4 weeks; therapy for malignancy within the past 3 weeks; uncontrolled cardiovascular or cerebrovascular events in the past 6 months; QTcF interval > 450 msec in males and >470 msec in females. All patients provided a written informed consent prior to study enrollment.
Study Design and Treatment
This was a phase 1, multicenter, open-label, single-agent, dose-escalation study designed to assess the safety, tolerability, efficacy, PK, and PD properties of pracinostat in patients with advanced hematologic malignancies. Patients were assigned to 7 dose cohorts (10 to 120 mg) using a 3+3 dose escalation design to determine the maximum tolerated dose (MTD). Pracinostat was administered orally once daily, every other day, 3 days a week, for 3 consecutive weeks in a 28-day cycle. The MTD was defined as the lowest dose level at which ≥ 2 patients out of 6, who completed at least 1 cycle of treatment, experienced a dose-limiting toxicity (DLT). The recommended Phase II dose (RP2D) was defined as the dose level immediately below the MTD. Once RP2D was defined, up to 10 intermediate or high-risk MDS and up to 10 AML patients were added for a total of up to 26 patients treated at the RP2D, to assess the anti-tumor activity and confirm tolerability and PK properties of pracinostat.
Given the known synergy between hypomethylating agents and HDACIs, the study protocol was amended to include a second cohort of patients (Arm 2) to assess the safety, tolerability, and efficacy of pracinostat plus azacitidine (AZA) in patients with intermediate or high-risk MDS not previously treated with AZA.15. AZA was given at a dose of 75 mg/m2 daily for 7 days in a 28-day cycle in combination with pracinostat given at a dose of 60 mg starting on day 1.
Efficacy and Safety
For Arm 1 (Pracinostat), response assessment was performed using the International Working Group Criteria.16–21 For Arm 2 (AZA plus Pracinostat), response was considered when complete remission (CR) or CR with incomplete platelet recovery (CRp) were achieved. CR was defined as bone marrow blasts <5% with complete recovery of peripheral counts (neutrophils >1000/uL and platelets >100,000/uL). CRp was defined using the same criteria except for platelet count <100,000 uL and transfusion independence. Progression-free survival (PFS; from start of therapy to disease progression, death, or last follow-up) and overall survival (OS; from start of therapy to date of death or last follow-up) were assessed.
Adverse effects (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. DLTs were defined as AEs occurring during the first cycle of treatment and fulfilling one of the following criteria: grade ≥3 non-hematologic treatment-related adverse event (TRAE) including grade ≥ 3 nausea and vomiting uncontrolled with optimal therapy; dose delay > 2 weeks or missing ≥ 4 doses within a cycle due to TRAEs or laboratory abnormalities; grade 4 neutropenia lasting ≥ 7 days or grade ≥ 3 neutropenia associated with fever and/or infection (malignant lymphoma only); grade 4 thrombocytopenia or grade 3 thrombocytopenia plus bleeding (malignant lymphoma only). In the event of a DLT, pracinostat was held until resolution of the toxicity after which it could be resumed at a reduced dose.
Pharmacokinetic and Pharmacodynamic Analysis
Blood samples for PK analysis were drawn prior to dosing and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 24 and 30 hours after dosing on Days 1 and 15 of Cycle 1. Pre-dose PK assessment was also performed on Day 8 of Cycle 1 of treatment, excluding additional patients enrolled at the RP2D. Plasma concentrations of pracinostat were determined using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method. Noncompartmental methods were used to estimate the area under the concentration versus time curve from time zero to infinity (AUC0-inf). The t1/2 was estimated using the extrapolation of the terminal segment of the log linear concentration-time curve to infinity including at least the last 3 points. Peak plasma concentration (Cmax) and mean time to peak concentration (Tmax) were estimated from the plasma drug concentration-time curve. PK parameters were calculated using validated WinNonlin software (v. 5.2, Parsight, Mountain View, CA).
For PD evaluation, blood samples were collected for isolation of PBMCs, a surrogate for tumor tissue, to test for changes in the AcH3 levels. Samples were obtained prior to dosing and at 3 and 24 hours after dosing on Days 1 and 15 of Cycle 1. Levels of AcH3 in PBMCs were determined using a validated Western Blot or enzyme-linked immunosorbent assay (ELISA) methods.12 Details regarding blood sample collection, processing, PBMC extraction, and measurement of the pracinostat concentration in plasma and AcH3 levels in PBMCs have been previously reported.12
Statistical Considerations
The efficacy analysis included all subjects who completed ≥ 2 cycles of therapy and had at least one follow-up tumor assessment. The safety population included all subjects who received at least one dose of pracinostat, while the DLT analysis was performed on those who received at least 75% of Cycle 1. Two sided, exact 95% confidence intervals (CIs) were used to estimate the response rates. Median PFS and OS were estimated using Kaplan-Meier method.
RESULTS
Patient Characteristics and Evaluable Populations
Arm 1 (Pracinostat)
From April 2007 to February 2012, 44 patients with advanced hematological malignancies were enrolled. Baseline demographics and disease characteristics are summarized in Table 1. The median age was 70 years (range, 37-84) with male predominance (57%). A total of 25 patients (57%) had AML and 14 (32%) had MDS. Forty-three (98%) patients received systemic chemotherapy prior to enrollment. Patients received a median of 17 doses (range, 2 – 108) of pracinostat.
Table 1.
Patient Characteristics
| Arm 1 Characteristics (N= 44) | Number (%) | Median [range] |
|---|---|---|
| Age (years) | 70 [37-84] | |
|
| ||
| Male Gender | 25 (57) | |
|
| ||
| Hematological Malignancy | ||
| AML | 25 (57) | |
| MDS | ||
| Intermediate-1 | 8 (18) | |
| Intermediate-2 | 3 (7) | |
| High | 3 (7) | |
| Non-Hodgkin’s Lymphoma | 1 (2) | |
| Mantle Cell Lymphoma | 1 (2) | |
| Follicular Lymphoma | 1 (2) | |
| Myelofibrosis | 1 (2) | |
| Myelomonocytic Leukemia | 1 (2) | |
|
| ||
| ECOG Performance Status | ||
| 0 | 13 (30) | |
| 1 | 26 (59) | |
| 2 | 5 (11) | |
|
| ||
| Prior therapy | ||
| Chemotherapy | 43 (98) | 2 [0-9] |
| Radiation therapy | 5 (11) | |
| Surgery | 7 (16) | |
| Stem Cell Transplantation | 7 (16) | |
|
| ||
| Arm 2 Characteristics (N=10) | Number (%) | Median [range] |
|
| ||
| Age (years) | 65 (18-73) | |
|
| ||
| Male gender | 3 (30) | |
|
| ||
| BM blasts (%) | 7 (1-18) | |
|
| ||
| Hemoglobin (g/dL) | 10.1 (8.2-11.1) | |
|
| ||
| Platelet (K/μL) | 42 (13-269) | |
|
| ||
| WBC (K/μL) | 2.4 (0.7-9.3) | |
|
| ||
| ANC (K/μL) | 1.37 (0.2-4.7) | |
|
| ||
| Creatinine (mg/dL) | 0.8 (0.5- 1.0) | |
|
| ||
| Bilirubin (mg/dL) | 0.8 (0.4-1.3) | |
Abbreviations: AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; ECOG: Eastern Cooperative Oncology Group; BM: bone marrow.
Arm 2 (AZA plus Pracinostat)
From May 2011 to February 2012, 10 patients with MDS were enrolled. Median age was 65 years (range, 18-73) with female predominance (70%). Baseline characteristics are demonstrated in Table 1. Eight patients had therapy-related MDS. On cytogenetic analysis; 6 patients had complex karyotype, 3 had monosomy 7, and 1 had translocation (6;9). Four patients received prior MDS therapy (decitabine; N=2, lenalidomide; N=2). Patients received a median of 4 cycles (range, 2- 9) of therapy.
Safety and Toxicity
Arm 1 (Pracinostat)
Thirty-four patients (77%) experienced at least 1 TRAE (Table 2). The most common non-hematological TRAEs were fatigue (41%), nausea (30 %), anorexia (23%), diarrhea (16%), and vomiting (14%). These events were mild to moderate in severity and more frequent at higher dose levels. Nine patients (20%) experienced a grade ≥ 3 TRAE with the most common non-hematological AEs being fatigue (11%), infections (9%), and QTc prolongation (5%). Thrombocytopenia (14 %) was the most common hematological AE and was experienced at grade ≥ 3 with pracinostat doses ≥ 80 mg. AEs leading to a dose-reduction or dose-interruption of pracinostat occurred in 27% and 34% of the patients, respectively. Discontinuation of pracinostat due to an AE was required in 11 patients (25%) of which 3 discontinued therapy due to a TRAE including QTc interval prolongation, fatigue, and elevated troponins.
Table 2.
Treatment-Related Adverse Events occurring in ≥10% Patients
| Adverse Events | Pracinostat Alone | AZA + Pracinostat | ||
|---|---|---|---|---|
| All Grades, % | Grade ≥ 3, % | All Grades, % | Grade ≥3, % | |
| Fatigue | 41 | 11 | 60 | 0 |
|
| ||||
| Nausea | 30 | 0 | 100 | 0 |
|
| ||||
| Anorexia | 23 | 2 | 30 | 0 |
|
| ||||
| Diarrhea | 16 | 2 | 20 | 0 |
|
| ||||
| Thrombocytopenia | 14 | 11 | 60 | 60 |
|
| ||||
| Vomiting | 14 | 0 | 80 | 10 |
|
| ||||
| Infections | 9 | 9 | 10 | 10 |
|
| ||||
| QTc prolongation | 7 | 5 | 10 | 0 |
| Constipation | 5 | 0 | 50 | 0 |
|
| ||||
| Anemia | 5 | 5 | 10 | 10 |
|
| ||||
| Neutropenia | 2 | 2 | 40 | 40 |
|
| ||||
| Elevated ALT | 0 | 0 | 10 | 10 |
Abbreviations: AZA: azacitidine; N: number; ALT alanine aminotransferase.
DLTs were reported in 5 patients (13%) and included grade 3 QTc interval prolongation (N=2, 40 and 100 mg), fatigue (N=2, 100 mg), and febrile neutropenia (N=1, 120 mg). Although the MTD was not reached, multiple dose reductions were required for patients treated at the 120 mg dose level which prompted its selection as the MTD and expansion of the 100 mg dose cohort. Since most patients did not tolerate repeated cycles at doses above 60 mg, this dose level was determined to be the RP2D.
Arm 2 (AZA plus Pracinostat)
All 10 patients reported a TRAE (Table 2). The most common non-hematological TRAEs were nausea (100%), vomiting (80%), fatigue (60%), and constipation (50%). Eight patients experienced a grade ≥ 3 TRAE. Thrombocytopenia (60%) and neutropenia (40%) were the most frequently reported hematological TRAEs. Three subjects required a dose reduction in pracinostat due to an AE.
Pharmacokinetics
Twenty-three patients were assessed for day 1 PK and 18 patients for day 15 PK (Table 3). Due to the small number of subjects, the 10-mg and 20-mg cohorts were excluded from the analysis. Pracinostat showed rapid absorption, Tmax reached in 0.8-1.3 hours, followed by biexponential disposition (Figure 1). The mean t1/2 ranged from 7.0 to 14.1 hours. Mean AUC0-inf and Cmax showed a dose proportional increase between 40 and 120 mg on Day 1. With the exception of the 100-mg dose level, no significant accumulation was seen between Day 1 and Day 15 following repeated dosing of the drug. There was moderate accumulation at the 100-mg dose level; mean AUC0-inf and Cmax on Day 15 were 1985 ng*h/mL and 314 ng/mL, respectively, compared to 1171 ng*h/mL and 187 ng/mL, respectively, on Day 1. Plasma concentrations above the 50% inhibitory concentration (IC50) of pracinostat for HDAC 1, 2, and 3 were reached at all dose levels. PK parameters for pracinostat were not affected by AZA administration.
Table 3.
PK Parameters (Mean±SD) for Pracinostat in Subjects with Hematologic Malignancies
| Dose (mg) | Cmax (ng/mL) | Tmax (h) | t1/2 (h) | AUC0-inf (ng·h/mL) |
|---|---|---|---|---|
| Day 1 | ||||
| 40 (n=6) | 84±34 | 1.1±0.7 | 9.4±3.5 | 434±191 |
| 60 (n=3) | 133±117 | 1.0±0.5 | 12.1±2.3 | 948±1143 |
| 80 (n=3) | 189±182 | 1.3±0.8 | 7.0±1.1 | 1047±803 |
| 100 (n=3) | 187±99 | 1.2±0.3 | 8.4±1.1 | 1171±347 |
| 120 (n=6) | 202±83 | 1.2±0.4 | 10.8±3.0 | 1356±725 |
| Day 15 | ||||
| 40 (n=5)* | 64±19 | 1.3±0.7 | 10.7±2.3 | 477±191 |
| 60 (n=3) | 118±135 | 0.8±0.3 | 11.8±3.4 | 766±746 |
| 80 (n=3) | 164±122 | 1.2±0.3 | 9.3±4.1 | 977±459 |
| 100 (n=3) | 314±143 | 1.0±0.5 | 13.7±5.6 | 1985±682 |
| 120 (n=2) | 96 | 1 | 14.1 | 757 |
Abbreviations: AUC0-inf: area under the concentration versus time curve from time 0 to infinity; Cmax: maximum concentration; PK: pharmacokinetics; SD: standard deviation; Tmax: time to maximum concentration; t1/2: half-life
PK parameters were not estimated from 1 subject as samples were not taken
Figure 1.

Plasma Concentration-time profile of Pracinostat in Day 1 (A) and Day 15 (B)
Pharmacodynamics
PBMCs for PD analysis was obtained from ten subjects. Although AcH3 levels were increased across all dose levels, a dose-dependent increase was not observed. The maximal increase in AcH3 levels were seen at the 3-hour post-dose sampling point, with levels slightly sustained at 24 hours on Day 15 (Figure 2).
Figure 2. Acetylated Histone H3 Levels in Peripheral Blood Mononuclear Cells of Subjects with Hematological Malignancies.

Abbreviations: d: day; h: hour; AML: acute myeloid leukemia; HL: Hodgkin’s lymphoma; nHL: non-Hodgkin’s lymphoma; pre-d: pre-dose
Response Rates
Arm 1 (Pracinostat)
Thirty-two patients were evaluable for response with the remaining 12 patients excluded due to inadequate drug exposure. There were 2 responders (6%), 1 patient achieved CR and 1 achieved complete cytogenetic response (CCyR). Both patients had AML, were enrolled at the 80 and 120 mg dose levels, completed 11 and 8 cycles of pracinostat, and had a PFS of 362 and 197 days, respectively. Among the remaining 30 patients, 20 had SD (63%) and 10 experienced progressive disease (31%). The median duration of follow-up was 2.7 months (range, 2.1-11.8). Median OS was 362 days [95% CI: 114.0- NA]. Thirteen patients (30 %) died; causes of death included disease progression (N=4), infection (N=6), respiratory failure (N=2), and hemorrhagic stroke (N=1).
Arm 2 (AZA plus Pracinostat)
Overall, 6 patients achieved CR and 3 achieved CR without platelet recovery for an overall response rate (ORR) of 90%. Median number of cycles to response was 3 (range, 1 to 6). Five patients achieved cytogenetic response (4 CCyR and 1 major cytogenetic response). Five patients were successfully bridged to SCT. A total of 3 patients relapsed (4, 7, and 10 months, respectively) including 1 relapse occurring after SCT. The 2-year disease-free survival and OS were 57% and 56%, respectively; median OS was not yet reached (Figure 3). Four patients (40%) died; 3 after relapse and 1 patient died in CR after SCT.
Figure 3.
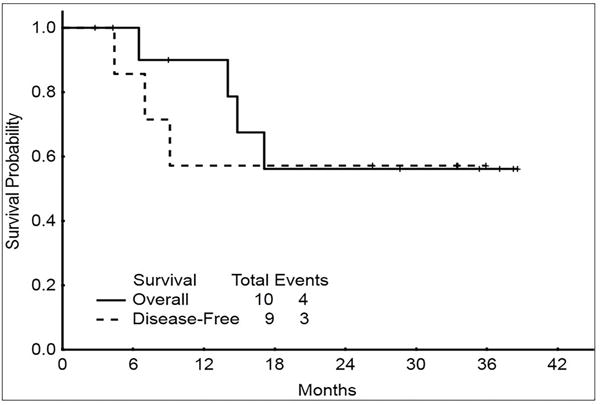
Overall survival and disease-free survival for patients treated with azacitidine plus pracinostat
DISCUSSION
This is the first clinical trial using pracinostat in patients with advanced hematological malignancies. Pracinostat was well tolerated, demonstrated excellent PK properties, and induced histone acetylation in a non-dose dependent fashion. Although the MTD of pracinostat was declared to be 120 mg, the RP2D was determined to be 60 mg due to the multiple dose reductions required with higher doses. Comparable with previous reports, these findings suggests that toxicity assessment during the first cycle of therapy maybe insufficient to assess for dose-limiting toxicities of a drug.22 Pracinostat demonstrated limited but significant anti-tumor activity in patients with advanced hematological malignancies. Although the majority of patients experienced prolonged disease stabilization, 2 AML patients treated at or below the MTD achieved a response.
Consistent with previous pracinostat studies, the most common toxicities reported in our study were gastrointestinal, fatigue and thrombocytopenia.12, 13 Toxicities were mostly mild to moderate in severity and were easily controlled with either standard medical management or dose reduction. High grade thrombocytopenia was observed at doses ≥ 80 mg, as in previous reports.12, 13 DLTs occurred at doses ≥ 40 mg and included QTc interval prolongation, fatigue, and febrile neutropenia. In contrast to our study and that of Yong et al, Razak and colleges used an alternative dosing schedule, continuous 5-day dosing every 2 weeks, and showed the early emergence of grade 3 TRAEs and DLTs at lower doses of pracinostat (20 mg), compared to 40 mg in the above mentioned studies, suggesting better tolerance of the every other day schedule.12, 13 Furthermore, the every other day schedule has been associated with superior therapeutic efficacy as demonstrated in preclinical studies and confirmed by the Yong et al who reported a dose-dependent escalation in AcH3 levels among patients with refractory solid tumors.10, 13 This improved efficacy may be attributed to the continuous pracinostat exposure provided by this regimen. For these reasons, this schedule has been selected for subsequent Phase II studies.
PK analysis of pracinostat showed favorable properties with rapid absorption followed by biexponential disposition, comparable with the solid tumor experience.12, 13 There was a dose dependent increase in the plasma concentration of pracinostat with a prolonged t1/2 reaching 14 hours, which was significantly longer when compared with the 1-2 hour half-lives of vorinostat and belinostat.23, 24 This improved t1/2 allows for the intermittent dosing of pracinostat without jeopardizing its efficacy. Unlike previous pracinostat studies, drug accumulation was seen at the 100 mg dose level which correlated with the intolerance observed with repeated drug administration. As a result, 60 mg was declared as the RP2D, congruent with the results published by Yong et al and Razak and colleagues.12, 13
Despite the sustained elevation of AcH3 levels across all doses, we did not find a dose-dependent increase as reported by Yong and colleagues.13 In vitro, the AcH3 levels varied significantly among the different subtypes of pracinostat-treated PBMCs, with CD20+ B-lymphocytes showing the highest levels and CD16+ monocytes showing the lowest levels of AcH3.11 Therefore, AcH3 levels in pracinostat-treated PBMCs are not comparable in patients with hematological malignancies due to the abnormal variation in the percentages of the different hematopoietic cells.
There is a dynamic intricate interaction between DNA methylation and histone deacetylation which induces gene repression. DNA methyltransferases (DNMTs) catalyze the methylation of CpG islands in the promotor region of tumor suppressor genes which subsequently bind to methyl-CpG-binding proteins recruiting transcriptional corepressors, including HDACs, inducing histone deacetylation and chromatin condensation thereby repressing gene transcription.5, 25, 26 In vitro, HDACs alone could not transcriptionally reactivate densely methylated genes without the prior use of a DNMT inhibitor, signifying the dominance of DNA methylation in epigenetic silencing and the synergistic activity between HDAC and DNMT inhibitors.15, 27
This synergy has been translated clinically with numerous trials showing promising activity of the combination in both AML and MDS.25, 28–31 Response rates increased from 10-20% achieved after a median of 3 to 4 cycles, when using either agent alone, to 30-46% achieved after a median of 2 cycles with the combination. This compares favorably with our results; we observed an ORR of 90% in higher-risk MDS patients treated with AZA plus pracinostat. Based on these results, two phase 2 studies have been conducted to determine the efficacy of this combination in both higher-risk MDS and AML patients.32, 33 Despite the lack of efficacy in higher-risk MDS patients, due to high rate of early discontinuation due to toxicity, there was significant activity in AML patients with an ORR of 52% lasting for a median of 13.2 months. The median OS among AML patients was 19.1 months which compares favorably with the historic AZA data.34
In conclusion, our study confirms the safety and efficacy of pracinostat in patients with advanced hematological malignancies with significant activity in AML and MDS especially in combination with AZA. Given its potency and synergy with DNMT inhibitors, randomized Phase III trials of pracinostat plus AZA for both MDS and AML patients are currently ongoing.
CONDENSED ABSTRACT.
Pracinostat is safe with modest single-agent activity in hematological malignancies. When combined with azacitidine, pracinostat has shown significant anti-tumor activity in MDS patients.
Acknowledgments
This study was supported by research funding from S*Bio.
Footnotes
Authorship Contributions:
Conception and design: Guillermo Garcia-Manero, Toh Han Chong, Charles Chuah, Liang-Piu Koh, Boon-Cher Goh, and Xiao Qin Dong
Provision of study materials or patients: Tapan Kadia, Elias Jabbour, Marina Konopleva, Gautam Borthakur, Alessandra Ferrajoli, Zeev Estrov, William Wierda, Toh Han Chong, Charles Chuah, Liang-Piu Koh, Boon-Cher Goh, Julie E. Chang, Hagop Kantarjian, Xiao Qin Dong, Ana Alfonso, and Guillermo Garcia-Manero
Collection and assembly of data: Yasmin Abaza, Maria Cielo Foudray, and Daniel Durkes
Data analysis and interpretation: Guillermo Garcia-Manero, Toh Han Chong, Charles Chuah, Liang-Piu Koh, Boon-Cher Goh, Yasmin Abaza, and Daniel Durkes
Manuscript writing: Yasmin Abaza, Guillermo Garcia-Manero
Final approval of manuscript: Guillermo Garcia-Manero, Yasmin Abaza, and Daniel Durkes
Disclosure of Conflicts of Interest: Julie E. Chang, M.D. obtains research funding from Genentech and Celgene. Daniel Durkes in an employee of MEI Pharma, the company that in-licensed pracinostat from S*BIO. The remaining authors disclose no relevant conflicts related to this article.
References
- 1.Cai J, Wei H, Hong KH, et al. Discovery, bioactivity and docking simulation of Vorinostat analogues containing 1,2,4-oxadiazole moiety as potent histone deacetylase inhibitors and antitumor agents. Bioorg Med Chem. 2015;23:3457–3471. doi: 10.1016/j.bmc.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Popovic R, Licht JD. Emerging epigenetic targets and therapies in cancer medicine. Cancer Discov. 2012;2:405–413. doi: 10.1158/2159-8290.CD-12-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Manero G. Can we improve outcomes in patients with acute myelogenous leukemia? Incorporating HDAC inhibitors into front-line therapy. Best Pract Res Clin Haematol. 2012;25:427–435. doi: 10.1016/j.beha.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 4.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 6.Bouchain G, Delorme D. Novel hydroxamate and anilide derivatives as potent histone deacetylase inhibitors: synthesis and antiproliferative evaluation. Curr Med Chem. 2003;10:2359–2372. doi: 10.2174/0929867033456585. [DOI] [PubMed] [Google Scholar]
- 7.Sambucetti LC, Fischer DD, Zabludoff S, et al. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J Biol Chem. 1999;274:34940–34947. doi: 10.1074/jbc.274.49.34940. [DOI] [PubMed] [Google Scholar]
- 8.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 9.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 10.Novotny-Diermayr V, Sangthongpitag K, Hu CY, et al. SB939, a novel potent and orally active histone deacetylase inhibitor with high tumor exposure and efficacy in mouse models of colorectal cancer. Mol Cancer Ther. 2010;9:642–652. doi: 10.1158/1535-7163.MCT-09-0689. [DOI] [PubMed] [Google Scholar]
- 11.Novotny-Diermayr V, Sausgruber N, Loh YK, et al. Pharmacodynamic evaluation of the target efficacy of SB939, an oral HDAC inhibitor with selectivity for tumor tissue. Mol Cancer Ther. 2011;10:1207–1217. doi: 10.1158/1535-7163.MCT-11-0044. [DOI] [PubMed] [Google Scholar]
- 12.Razak AR, Hotte SJ, Siu LL, et al. Phase I clinical, pharmacokinetic and pharmacodynamic study of SB939, an oral histone deacetylase (HDAC) inhibitor, in patients with advanced solid tumours. Br J Cancer. 2011;104:756–762. doi: 10.1038/bjc.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yong WP, Goh BC, Soo RA, et al. Phase I and pharmacodynamic study of an orally administered novel inhibitor of histone deacetylases, SB939, in patients with refractory solid malignancies. Ann Oncol. 2011;22:2516–2522. doi: 10.1093/annonc/mdq784. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 15.Yang H, Hoshino K, Sanchez-Gonzalez B, Kantarjian H, Garcia-Manero G. Antileukemia activity of the combination of 5-aza-2′-deoxycytidine with valproic acid. Leuk Res. 2005;29:739–748. doi: 10.1016/j.leukres.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Creutzig U, Kaspers GJ. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2004;22:3432–3433. doi: 10.1200/JCO.2004.99.116. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 19.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 21.Tefferi A, Barosi G, Mesa RA, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT) Blood. 2006;108:1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 22.DiNardo CD, Daver N, Jabbour E, et al. Sequential azacitidine and lenalidomide in patients with high-risk myelodysplastic syndromes and acute myeloid leukaemia: a single-arm, phase 1/2 study. Lancet Haematol. 2015;2:e12–20. doi: 10.1016/S2352-3026(14)00026-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly WK, O’Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steele NL, Plumb JA, Vidal L, et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14:804–810. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 25.Gore SD, Baylin S, Sugar E, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 26.Maslak P, Chanel S, Camacho LH, et al. Pilot study of combination transcriptional modulation therapy with sodium phenylbutyrate and 5-azacytidine in patients with acute myeloid leukemia or myelodysplastic syndrome. Leukemia. 2006;20:212–217. doi: 10.1038/sj.leu.2404050. [DOI] [PubMed] [Google Scholar]
- 27.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 28.Silverman LR, Verma A, Odchimar-Reissig R, et al. A Phase I Trial of the Epigenetic Modulators Vorinostat, in Combination with Azacitidine (azaC) in Patients with the Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML): A Study of the New York Cancer Consortium. Blood. 2008;112:3656–3656. [Google Scholar]
- 29.Kirschbaum M, Gojo I, Goldberg SL, et al. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. Br J Haematol. 2014;167:185–193. doi: 10.1111/bjh.13016. [DOI] [PubMed] [Google Scholar]
- 30.Gore SD, Jiemjit A, Silverman LB, et al. Combined Methyltransferase/Histone Deacetylase Inhibition with 5-Azacitidine and MS-275 in Patients with MDS, CMMoL and AML: Clinical Response, Histone Acetylation and DNA Damage. Blood. 2006;108:517–517. [Google Scholar]
- 31.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia Manero G, Atallah E, Khaled SK, et al. A Phase 2 Study of Pracinostat and Azacitidine in Elderly Patients with Acute Myeloid Leukemia (AML) Not Eligible for Induction Chemotherapy: Response and Long-Term Survival Benefit. Blood. 2016;128:100–100. [Google Scholar]
- 33.Garcia-Manero G, Montalban-Bravo G, Berdeja JG, et al. Phase 2, randomized, double-blind study of pracinostat in combination with azacitidine in patients with untreated, higher-risk myelodysplastic syndromes. Cancer. 2017;123:994–1002. doi: 10.1002/cncr.30533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–299. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]


