SUMMARY
SETTING
Current passive case finding strategies are not effective at identifying tuberculosis (TB) patients in rural China.
OBJECTIVE
To evaluate a community-based, active case finding (ACF) scheme in identifying symptomatic individuals with TB.
DESIGN
We conducted door-to-door household visits of all residents aged ⩾15 years at two rural sites to screen for TB symptoms. Individuals with symptoms were enrolled and asked to provide three sputum samples. All participants underwent chest X-ray, and microbiologic detection of Mycobacterium tuberculosis from sputum samples using microscopy, solid culture and Xpert® MTB/RIF was performed.
RESULTS
Among the 19 334 residents screened for TB symptoms, 865 (4.5%) reported having ⩾1 symptom. A total of 52 TB cases were detected, 11 of whom had microbiologic confirmation. Xpert identified all five M. tuberculosis culture-positive cases and yielded an additional three diagnoses. Prevalence of newly detected TB at the two sites through ACF was respectively 475 and 196 per 100 000 population. These estimates are respectively four and eight times, on average, higher than those identified through passive surveillance during the previous 5-year period for the two sites.
CONCLUSION
Community-based symptom screening followed by laboratory tests was found to be feasible and effective in increasing TB case finding in rural China.
Keywords: epidemiology, population-based study, Xpert, rural settings
TUBERCULOSIS (TB) is a significant public health concern in China, as evidenced by the approximately 930 000 new cases and 38 000 deaths in 2014.1 Despite the progress China has made in tackling the TB epidemic,2 the prevalence of pulmonary TB (PTB) nationwide declined only nominally from 466 per 100 000 population in 2000 to 459/100 000 in 2010, with rural areas and western provinces experiencing prevalence increases.3 In China’s 2010 National TB Survey, the prevalence of PTB in rural areas remained as high as 569/100 000—more than 1.8 times that of urban areas—and displayed an escalating trend from east to west.3
China’s current case finding strategy is a passive scheme that relies principally on symptomatic individuals voluntarily seeking medical attention. Evidence shows that more than half of individuals with TB symptoms in China fail to seek care in a timely manner, and those living in rural areas are 1.8 times more likely to delay such behavior.4 A more proactive strategy, such as community-based screening, can identify large numbers of undiagnosed prevalent TB cases, as demonstrated by epidemiologic studies in China and other high-burden countries.2,5–7 One epidemiologic study conducted at eight sites in China compared passive vs. active case finding (ACF), and found TB prevalence obtained through active screening was significantly higher than TB registry data of other townships in the same region over the same period.8 Based on a review of available evidence, the World Health Organization (WHO) issued active case screening recommendations in 2013.9 While encouraging screening in some high-risk groups, these guidelines called for more research to evaluate new screening approaches and the impact of such screening modalities.
TB detection depends on the availability of efficient and accurate diagnostic tools. In addition to the traditional assays, the Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) could potentially contribute to the detection of TB cases. Further to the WHO’s 2011 recommendation that Xpert be used as the initial assay in individuals with suspected PTB,10 several studies have evaluated Xpert in conjunction with ACF in a variety of settings and populations, with a focus on human immunodeficiency virus infected persons and household contacts of TB cases;11–15 however, incorporating Xpert into community-based screening in the general population has been explored by relatively few studies,16,17 and has not been documented in China.
The purpose of the present study was to explore community-based ACF for TB in rural areas of western China using sputum smear, culture and Xpert as diagnostic tools. We also aimed to yield robust TB prevalence estimates to facilitate the planning and conduct of future TB studies in China.
METHODS
Study population
This was a cross-sectional TB ACF study of residents at two rural sites in western China. Site A comprised 14 villages with an estimated total population of 20 361, including 17 711 (87.0%) adults aged ⩾15 years. Site B covered seven villages with an estimated total population of 29 586 and an adult (aged ⩾15 years) population of 24 899 (84.2%). Household screening was conducted at Site A from November 2013 to January 2014 and at Site B from March to May 2014.
Ethics approval was granted by the Institutional Review Board of Fudan University, Shanghai, China. Written informed consent was obtained from each study participant and/or legal guardian.
Screening and enrollment
At each site, door-to-door household visits of all residents were performed by three 3-person teams consisting of one TB physician, one village doctor and one village official. Current resident status (residing at home, residing elsewhere) was queried for all registered residents aged ⩾15 years. Registered residents who had resided at the study site for at least 6 months in the past year, and unregistered/temporary residents who had resided continuously at the study site for ⩾6 months were eligible for the study. We excluded registered residents if they had moved or visited the home only occasionally during the previous year. Sociodemographic information, such as age, sex, and occupation, was verified or collected at the time of household screening.
All eligible residents were screened for TB symptoms (unremitting cough ⩾2 weeks, unremitting expectoration ⩾2 weeks, blood with cough, fever, night sweats, weight loss or chest pain) as described in the national guidelines.18 The study teams also collected data on whether the resident was currently on anti-tuberculosis treatment, which was crosschecked with the National TB Information Management System. Where a resident’s self-report of antituberculosis treatment was not in accordance with the data obtained from the national system, the resident remained in the study pool. For residents who resided in the area but were not at home at first visit, village doctors and officials conducted follow-up visits or calls to conduct symptom screening.
Individuals with TB symptoms were provided with two sputum containers for the collection of one overnight and one early morning specimen. Symptomatic individuals were then referred to county Centers for Disease Control and Prevention (CDC) clinics for study enrollment. Once at the clinic, a third (spot) sputum sample was collected, and chest X-ray (CXR; posteroanterior) was performed to identify lung abnormalities consistent with TB.
Laboratory procedures
Sputum specimens were processed at the biosafety level-2 TB laboratory at each county CDC clinic. Each of the three sputum samples was prepared and stained with Ziehl-Neelsen for detection of acid-fast bacilli (AFB) using direct smear light microscopy. ‘AFB-negative’ was defined as no AFB detected after examination of 300 fields at 100× magnification. One of the two most mucoid sputum samples was randomly selected for culture on conventional solid medium (Löwenstein-Jensen). Xpert was performed according to the manufacturer’s instructions using the other sample or using the same specimen as culture if the volume was sufficient.
A clinical diagnosis of active TB was made by the physicians based on clinical history, TB symptoms, and CXR abnormalities. Patients with microbiological confirmation were those with at least one positive result on smear, culture, or Xpert. Participants diagnosed with active TB were registered in the National TB Information Management System and initiated on standard anti-tuberculosis treatment according to national guidelines.
Statistical analyses
Prevalence of newly detected PTB was calculated and compared with the case notification rate over the same period for the previous 5 years at both sites. Historical case notification rates were estimated by dividing the number of reported TB cases by the number of residents residing at the study site. On investigation, we learned of no major changes to migration patterns over the previous 5 years. As such, for the denominator we used the number of actual residents obtained from our household screening. Participant characteristics between the two sites were compared using χ2 tests for categorical measures and t-tests for continuous measures. Trend of symptomatic rate and active TB rate was assessed using the Cochran-Armitage trend test. A two-sided P value <0.05 was considered statistically significant. Stata 13 (StataCorp, College Station, TX, USA) was used to perform statistical analyses.
RESULTS
A total of 36 859 registered residents ⩾15 years of age at the two sites (Site A 17 383; Site B 19 476) were queried about residence. Approximately half (19 334; 52.5%) were identified as residing in the study areas, and were screened for TB symptoms. Of these, 865 (4.5%) individuals reported having any symptoms at the time of screening, including 17 individuals who were on anti-tuberculosis treatment and 44 who had had a recent diagnosis with signs/symptoms compatible with TB but for whom TB was ruled out on their most recent medical visit. An additional 10 individuals on anti-tuberculosis treatment reported no symptoms. As a result, 804 (4.2%) symptomatic individuals were identified through the efforts of the study. Of the 804 residents with symptoms, 734 (91.3%) were evaluated for TB disease through the collection of sputum and CXR. Fifty-two (7.1%) participants were diagnosed as having TB, of whom 11 (21.2%) had bacteriological confirmation (Figure 1).
Figure 1.

Household screening flowchart. CXR = chest X-ray.
Demographic characteristics of the study participants
Half of the residents screened were female (49.8%), and the majority were farmers (89.0%) (Table 1). The difference between the two sites in sex, age and occupation distribution was statistically significant (P < 0.001). At Site A, the median age was 59 years (interquartile range [IQR] 45–68), with a large proportion of residents (82.1%) aged ⩾40 years. At Site B, age was more normally distributed, with a median age of 42 years (IQR 29–57). Despite these demographic differences across the sites, individuals with TB symptoms showed consistent statistically significant demographic characteristics: most residents with symptoms were aged ⩾50 years (88.7%) and 57.8% were male. An increasing trend in the prevalence of TB symptoms and rate of active TB was observed with increasing age for both sites and sexes (P for trend <0.001; Figure 2, Appendix Figure A.1).* A higher proportion of residents at Site A presented with TB symptoms than at Site B in each age group (Figure 2).
Table 1.
Demographic characteristics of residents screened for TB symptoms and symptomatic residents to be evaluated for TB
| Residents screened for TB symptoms
|
Symptomatic residents to be evaluated for TB*
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Site A n (%) |
Site B n (%) |
Total n (%) |
P(χ2) | Site A n (%) |
Site B n (%) |
Total n (%) |
P(χ2) | |
| Sex | <0.001 | 0.587 | ||||||
| Male | 2376 (46.9) | 7 334 (51.4) | 9 710 (50.2) | 307 (57.2) | 158 (59.2) | 465 (57.8) | ||
| Female | 2 686 (53.1) | 6 938 (48.6) | 9 624 (49.8) | 230 (42.8) | 109 (40.8) | 339 (42.2) | ||
| Age, years median [IQR] | 59 [45–68] | 42 [29–57] | 46 [31–61] | <0.001† | 64 [59–71] | 64 [58–72] | 64 [58.25–71] | 0.250† |
| Age, years | <0.001 | 0.145 | ||||||
| 15–19 | 228 (4.5) | 1 025 (7.2) | 1 253 (6.5) | 1 (0.2) | 0 | 1 (0.1) | ||
| 20–29 | 351 (6.9) | 2 597 (18.2) | 2 948 (15.3) | 6 (1.1) | 4 (1.5) | 10 (1.2) | ||
| 30–39 | 329 (6.5) | 2 857 (20.0) | 3 186 (16.5) | 10 (1.9) | 11 (4.1) | 21 (2.6) | ||
| 40–49 | 774 (15.3) | 2 905 (20.4) | 3 679 (19.0) | 36 (6.7) | 23 (8.6) | 59 (7.3) | ||
| 50–59 | 928 (18.3) | 1 852 (13.0) | 2 780 (14.4) | 96 (17.9) | 47 (17.6) | 143 (17.8) | ||
| 60–69 | 1 429 (28.2) | 1 668 (11.7) | 3 097 (16.0) | 225 (41.9) | 89 (33.3) | 314 (39.1) | ||
| ⩾70 | 1 023 (20.2) | 1 368 (9.6) | 2 391 (12.4) | 163 (30.4) | 93 (34.8) | 256 (31.8) | ||
| Occupation | <0.001 | 0.218 | ||||||
| Farmers | 4 914 (97.1) | 12 296 (86.2) | 17 210 (89.0) | 535 (99.6) | 263 (98.5) | 798 (99.3) | ||
| Students | 141 (2.8) | 787 (5.5) | 928 (4.8) | 1 (0.2) | 2 (0.8) | 3 (0.4) | ||
| Other | 7 (0.1) | 1 189 (8.3) | 1 196 (6.2) | 1 (0.2) | 2 (0.8) | 3 (0.4) | ||
| Symptoms | <0.001 | |||||||
| Yes | 570 | 295 | 865 | |||||
| No | 4 492 | 13 977 | 18 469 | |||||
| Total | 5 062 | 14 272 | 19 334 | 537 | 267 | 804 | ||
61 individuals who were on anti-tuberculosis treatment or had a recent disease diagnosis with signs/symptoms compatible with TB were excluded.
t-test.
TB = tuberculosis; IQR = interquartile range.
Figure 2.
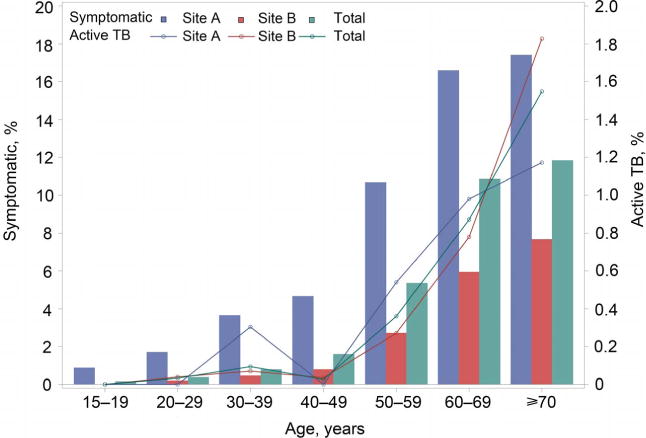
Symptoms and active TB by age and site. TB = tuberculosis. This image can be viewed online in colour at http://www.ingentaconnect.com/content/iuatld/ijtld/2017/00000021/00000011/art00008
Radiographic and microbiologic results
CXR results were available for 728 individuals (728/734, 99.2%), 49 (6.7%) of whom were diagnosed with active TB (Appendix Table A). Eight of these 49 clinical cases (16.3%) had microbiological confirmation. An additional three bacteriologically confirmed cases did not present with clinical abnormalities on CXR. A total of 733 individuals with symptoms (733/734, 99.9%) provided at least one sputum sample. Six of the 11 cases with microbiological confirmation (54.5%) would have been detected by culture alone. Xpert results were positive for five cases (except for one M. bovis). Furthermore, Xpert and smear detected respectively three and two cases not picked up by culture (Table 2). No rifampicin-resistant cases were identified, except for one sample that had an indeterminate result.
Table 2.
Diagnostic test results for newly detected TB cases by study site
| Site A |
Site B |
Total | |
|---|---|---|---|
| TB cases with microbiologic confirmation | |||
| Smear-positive, culture-positive, Xpert-positive | 2 | 1 | 3 |
| Smear-positive, culture-negative, Xpert-negative | 1 | 1 | 2 |
| Smear-negative, culture-negative, Xpert-positive | 2 | 1 | 3 |
| Smear-negative, culture-positive, Xpert-positive | 1 | 1 | 2 |
| Smear-negative, culture-positive, Xpert-negative | 1* | 0 | 1 |
| TB cases with radiographic diagnosis only | 17 | 24 | 41 |
| Total | 24 | 28 | 52 |
M. bovis.
TB = tuberculosis.
Active vs. passive case finding
Considering all individuals screened, the prevalence of newly detected TB cases was estimated to be 475/100 000 (24/5054) at Site A and 196/100 000 (28/14 253) at Site B. These estimates were on average 4 and 8 times higher than those identified by the passive routine strategy that relied on sputum smear, culture and CXR in the same period of the previous 5 years for Site A (3–6 times) and Site B (5–14 times) (Appendix Figure A.2). If we include the 27 cases found to be on treatment at screening, the prevalence rates of PTB (existing + newly identified) at Sites A and B were respectively 632 (32/5062) and 329/100 000 (47/14 272).
DISCUSSION
To our knowledge, this is the first community-based study to explore ACF for TB using sputum smear, culture and Xpert in rural China. Of 19 334 residents screened for TB symptoms, 52 new TB diagnoses were made. The prevalence at Site A obtained in this study (632/100 000) is comparable to the prevalence from the 2010 national survey using CXR, smear and solid culture, in which western areas of China were found to have the highest prevalence (695/100 000) among three geographical areas.3 The lower prevalence of TB at Site B (329/100 000) is believed to reflect the higher-than-average proportion of young adults at the site.
Our study revealed that a significant proportion of TB morbidity at these sites is found in individuals aged ⩾50 years, which, together with the finding that the rate of symptoms increased with age, indicates that the elderly are a high-risk group in rural western China. This echoes the findings from the 2010 national survey,3 as well as a latent tuberculous infection study in China, which found that the infection rate increased with age.19 These findings may direct us to focus efforts on the detection and prevention of TB in elderly populations in China. Considering that a large proportion of rural residents are older adults, active case finding in the elderly population may effectively contribute to TB control in rural China. Nevertheless, in view of the human and financial resources required, incorporating active screening of high-risk groups (e.g., elderly) into the existing case finding system merits further exploration. In areas where population density is high and residents are more likely to delay seeking medical care (e.g., some rural areas of China),4 community-based screening might yield more timely diagnosis and thus aid in improving TB prevention in the entire area.
The distinctive migratory patterns at the two sites serve to partially explain the differences in demographic distribution and TB prevalence. Nearly 70% of registered residents at Site A were found not to be residing in their registered homes. This is a phenomenon common to many rural areas of China, where the young adult population moves to the cities for employment opportunities, resulting in a shift towards over-representation of the elderly in rural areas.20 Site B is near an industrial park, where many residents of working age are employed while continuing to reside in their registered homes. It is demographics and characteristics like these—in addition to the more commonly considered criteria such as epidemiology, infrastructure, and capacity— that require careful consideration when selecting suitable sites for TB research.
Consistent with previous studies,16,17 our study found that Xpert performed well in community-based ACF, especially when compared with culture. Xpert detected all the culture M. tuberculosis-positive cases and yielded three additional diagnoses in a much shorter turnaround time. In Cambodia, 48% of additional diagnoses were confirmed using Xpert among those at risk of multidrug-resistant and initially smear-negative TB.16 Similarly, a study in Nigeria reported the detection of an additional 189 M. tuberculosis-positive cases by a follow-up Xpert test among 1685 individuals with at least two smear-negative results.17 Xpert is a diagnostic test that can be run outside centralized laboratories by individuals with minimal technical expertise.21 In our setting, Xpert proficiency was rapidly acquired by technicians at both sites with the help of a short training course. A major obstacle to the sustained use of Xpert for these resource-constrained settings will be the relatively high cost of the system. In addition to Xpert, our study demonstrated the utility of CXR in identifying TB cases without microbiologic confirmation in community-based case finding.
Several study limitations should be noted. First, due to logistical and financial constraints, we did not perform a CXR or sputum analysis on every resident as a screening tool, but only those deemed symptomatic for TB. This may have resulted in the under-diagnosis of non-symptomatic TB cases. Second, less than a quarter of the newly detected TB cases were bacteriologically confirmed; many TB cases were diagnosed clinically based on symptoms and CXR abnormalities. Given the non-specific nature of cough and CXR abnormalities, it is possible that this led to an over-estimation of the TB burden. Third, we did not conduct a thorough workup to rule out the possibility of TB for the 44 individuals who had a recent disease diagnosis with signs/symptoms compatible with TB. Finally, there is an aluminum smelting factory near Site B, where young workers might have some indirect exposure to bauxite dust; however, as they were not working directly at the mine area, we expect the impact of this exposure on the development of TB to be limited.
This study demonstrates the feasibility and effectiveness of ACF with the use of Xpert as an additional diagnostic tool in the setting of a well-functioning TB program in rural China. Application of these techniques revealed that TB prevalence was substantially higher than had been identified using routine passive surveillance in the communities targeted. Active community-based screening of this nature can add significant value to TB case detection and should be considered as a complementary strategy in high TB burden areas.
Acknowledgments
The authors thank the National Center for Tuberculosis Control and Prevention, Chinese Centers for Disease Control and Prevention (CDC) for their support and guidance; the team of village doctors, county CDC TB physicians, laboratory technicians, and other staff at both sites for their outstanding work in detecting and caring for TB patients; and K S Goh for his support in installing the Xpert machines and training the laboratory technicians.
Funding for this study was provided by Aeras. CGY, XG, QG and site staff were also supported by the Key Project of Chinese National Programs, China [2013ZX10003004-001]. CGY was additionally supported by National Institutes for Health Research Training Grant [R25 TW009343] funded by the Fogarty International Center, Bethesda, MD, and the University of California Global Health Institute, San Francisco, CA, USA.
APPENDIX
Figure A.1.
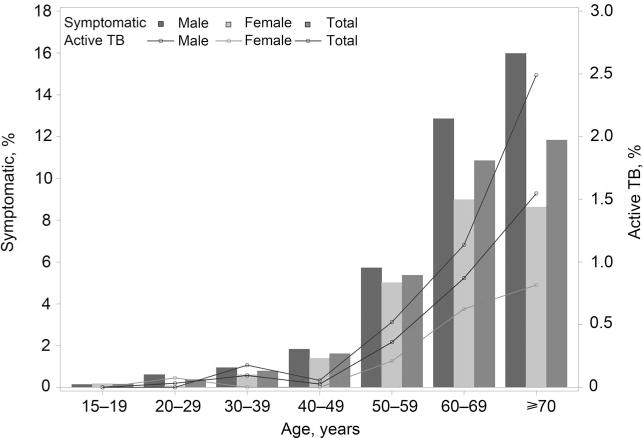
Symptoms and active TB by age and sex. TB = tuberculosis.
Figure A.2.
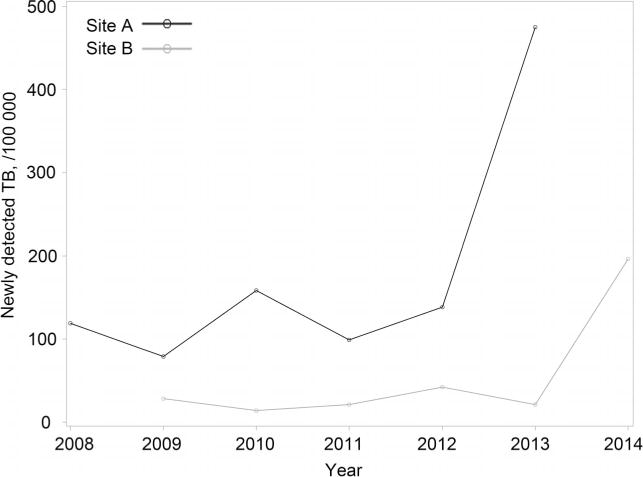
Newly detected TB by site, 2008–2014. TB = tuberculosis.
Table A.
Laboratory results for residents with symptoms suggestive of TB
| Site A | Site B | Total | |
|---|---|---|---|
| Chest X-ray | |||
| Active TB | 21 | 28 | 49 |
| Possible TB suspects | 6 | 21 | 27 |
| Old TB based on scar | 38 | 10 | 48 |
| Lung cancer | 3 | 0 | 3 |
| Pneumonia | 16 | 21 | 37 |
| Heart disease | 29 | 1 | 30 |
| Pneumoconiosis | 2 | 0 | 2 |
| Chronic bronchitis and emphysema | 158 | 3 | 161 |
| Other abnormalities | 13 | 8 | 21 |
| Normal | 218 | 132 | 350 |
| Smear* | |||
| Positive | 5† | 2 | 7 |
| 1–8 AFB/300 fields | 3 | 2 | 5 |
| 1+ | 0 | 0 | 0 |
| 2+ | 1 | 0 | 1 |
| 3+ | 1 | 0 | 1 |
| Negative | 499 | 226 | 725 |
| Culture | |||
| M. tuberculosis-positive | 3 | 2 | 5 |
| M. tuberculosis-negative | 495 | 215 | 710 |
| Contaminated | 6 | 6 | 12 |
| Unknown | 0 | 5 | 5 |
| Xpert | |||
| Positive | 5 | 3 | 8 |
| Very low | 3 | 2 | 5 |
| Low | 0 | 1 | 1 |
| Medium | 2 | 0 | 2 |
| Negative | 500 | 225 | 725 |
For smear results: 1+=3–9 AFB/100 fields; 2+=1–9 AFB/10 fields; 3+=1–9 AFB/field.
Including two false-positive isolates.
TB = tuberculosis; AFB = acid-fast bacilli; Xpert = Xpert® MTB/RIF.
Footnotes
Conflict of interest statement: CC and VC were employees of Aeras during the study period. All other authors declare no conflict of interest.
The appendix is available in the online version of this article, at http://www.ingentaconnect.com/content/iuatld/ijtld/2017/00000021/00000011/art00008
References
- 1.World Health Organization. Global tuberculosis report, 2. WHO/HTM/TB/2015.22.2015. [Google Scholar]
- 2.Wang L, Zhang H, Ruan Y, et al. Tuberculosis prevalence in China, 1990–2010: a longitudinal analysis of national survey data. Lancet. 2014;383:2057–2064. doi: 10.1016/S0140-6736(13)62639-2. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Cheng S, Chen M. [The fifth national tuberculosis epidemiological survey in 2010] Chinese J Antituberculosis. 2012;34:485–508. [Chinese] [Google Scholar]
- 4.Li Y, Ehiri J, Tang S, et al. Factors associated with patient, and diagnostic delays in Chinese TB patients: a systematic review and meta-analysis. BMC Med. 2013;11:156. doi: 10.1186/1741-7015-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kebede AH, Alebachew Z, Tsegaye F, et al. The first population-based national tuberculosis prevalence survey in Ethiopia, 2010–2011. Int J Tuberc Lung Dis. 2014;18:635–639. doi: 10.5588/ijtld.13.0417. [DOI] [PubMed] [Google Scholar]
- 6.Hoa NB, Sy DN, Nhung NV, Tiemersma EW, Borgdorff MW, Cobelens FG. National survey of tuberculosis prevalence in Viet Nam. Bull World Health Organ. 2010;88:273–280. doi: 10.2471/BLT.09.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F, Wang W, Wang X, Xu H, Zhu J, Fan J, et al. [Evaluation of community-based active case screening for tuberculosis] Zhejiang J Prev Med. 2006;18:52–52. [Chinese] [Google Scholar]
- 8.Liu E, Zhou L, Cheng J, et al. [The comparative study of health check and passive identification in tuberculosis case detection] Chinese J Antituberculosis. 2014;36:327–330. [Chinese] [Google Scholar]
- 9.World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.04. [PubMed] [Google Scholar]
- 10.World Health Organization. Policy statement: automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.4. [PubMed] [Google Scholar]
- 11.Dorman S, Chihota VN, Lewis JJ, et al. Performance characteristics of the Cepheid Xpert MTB/RIF test in a tuberculosis prevalence survey. PLOS ONE. 2012;7:e43307. doi: 10.1371/journal.pone.0043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abed Al-Darraji HA, Abd Razak H, Ng KP, Altice FL, Kamarulzaman A. The diagnostic performance of a single GeneXpert MTB/RIF assay in an intensified tuberculosis case finding survey among HIV-infected prisoners in Malaysia. PLOS ONE. 2013;8:e73717. doi: 10.1371/journal.pone.0073717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balcha TT, Sturegård E, Winqvist N, et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PLOS ONE. 2014;9:e85478. doi: 10.1371/journal.pone.0085478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adelman M, Tsegaye M, Kempker R, et al. Intensified tuberculosis case finding among HIV-infected persons using a WHO symptom screen and Xpert® MTB/RIF. Int J Tuberc Lung Dis. 2015;19:1197–1203. doi: 10.5588/ijtld.15.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ntinginya E, Squire S, Millington K, et al. Performance of the Xpert® MTB/RIF assay in an active case-finding strategy: a pilot study from Tanzania. Int J Tuberc Lung Dis. 2012;16:1468–1470. doi: 10.5588/ijtld.12.0127. [DOI] [PubMed] [Google Scholar]
- 16.Lorent N, Choun K, Thai S, et al. Community-based active tuberculosis case finding in poor urban settlements of Phnom Penh, Cambodia: a feasible and effective strategy. PLOS ONE. 2014;9:e92754. doi: 10.1371/journal.pone.0092754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John S, Gidado M, Dahiru T, Fanning A, Codlin A, Creswell J. Tuberculosis among nomads in Adamawa, Nigeria: outcomes from two years of active case finding. Int J Tuberc Lung Dis. 2015;19:463–468. doi: 10.5588/ijtld.14.0679. [DOI] [PubMed] [Google Scholar]
- 18.Disease Control Bureau of the Ministry of Health, Department of Medical Administration of the Ministry of Health, Chinese Center for Disease Control and Prevention. Guidelines for implementing the National Tuberculosis Control Program in China 2008. Beijing, China: Peking Union Medical College Press; 2009. [Chinese] [Google Scholar]
- 19.Gao L, Lu W, Bai L, et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis. 2015;15:310–319. doi: 10.1016/S1473-3099(14)71085-0. [DOI] [PubMed] [Google Scholar]
- 20.Gong P, Liang S, Carlton EJ, et al. Urbanisation and health in China. Lancet. 2012;379:843–852. doi: 10.1016/S0140-6736(11)61878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6:1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]


