Abstract
Objectives
Gait training interventions that target paretic propulsion induce improvements in walking speed and function in individuals post-stroke. Previously, we demonstrated that able-bodied individuals increase propulsion unilaterally when provided real-time biofeedback targeting anterior ground reaction forces (AGRF). The purpose of this study was to, for the first time, investigate short-term effects of real-time AGRF gait biofeedback training on post-stroke gait.
Methods
Nine individuals with post-stroke hemiparesis (6 females, age = 54 ± 12.4 years 39.2 ± 24.4 months post-stroke) completed three 6-minute training bouts on an instrumented treadmill. During training, visual and auditory biofeedback were provided to increase paretic AGRF during terminal stance. Gait biomechanics were evaluated before training, and during retention tests conducted 2, 15, and 30 minutes post-training. Primary dependent variables were paretic and non-paretic peak AGRF; secondary variables included paretic and non-paretic peak trailing limb angle, plantarflexor moment, and step length. In addition to evaluating the effects of biofeedback training on these dependent variables, we compared effects of a 6-minute biofeedback training bout to a non-biofeedback control condition.
Results
Compared to pre-training, significantly greater paretic peak AGRFs were generated during the 2, 15, and 30-minute retention tests conducted after the 18-minute biofeedback training session. Biofeedback training induced no significant effects on the non-paretic leg. Comparison of a 6-minute biofeedback training bout with a speed-matched control bout without biofeedback demonstrated a main effect for training type, with greater peak AGRF generation during biofeedback.
Discussion
Our results suggest that AGRF biofeedback may be a feasible and promising gait training strategy to target propulsive deficits in individuals post-stroke.
Keywords: Feedback, locomotor training, motor learning, walking, push-off, gait biomechanics, hemiparesis
Introduction
Decreased propulsive force generation from the paretic leg during terminal stance, measured as anterior ground reaction force (AGRF), is an important post-stroke gait deficit that has received attention in biomechanics and rehabilitation literature.1–3 Generation of propulsive force by the ankle plantarlexor muscles is considered crucial for a smooth stance-to-swing transition and for accelerating the center of mass and limb forward during the swing phase.4 In individuals with post-stroke hemiparesis, reduced paretic AGRF has been correlated to hemiparetic severity, gait speed, and gait asymmetry.1,2,4–11 Due to the importance of paretic propulsion as a post-stroke gait impairment, recent gait training research has focused on development and testing of interventions that target paretic propulsion.12–16 Paretic AGRF can be modified with gait retraining interventions, such as treadmill training and functional electrical stimulation.12–14 Changes in paretic AGRF induced by 12-week locomotor training intervention correlated with training-induced changes in self-selected and fast walking speeds,12,17 demonstrating the importance of targeting paretic propulsion during stroke gait retraining. Baseline AGRF and the capacity to modulate AGRF were major predictors of clinical response to locomotor training combining fast walking and functional electrical stimulation.11,18 Given the importance of paretic propulsion as a post-stroke locomotor deficit, further research is needed to develop novel and efficacious gait training interventions that target paretic propulsion.
Biofeedback training involves inducing a change in behavior by providing the user with quantitative information regarding a targeted performance variable.19–24 Real-time biofeedback is a promising strategy to enhance an individual’s awareness of the impairment targeted during gait training, encouraging self-correction of aberrant gait patterns.19–25 Several studies have evaluated the use of real-time biofeedback for post-stroke gait training.23,24,26,27 Previously, Wolf and Binder-Macleod evaluated electromyography biofeedback for post-stroke gait rehabilitation.28 Two studies found that gait training with real-time biofeedback was effective at modulating step length asymmetry in individuals post-stroke.29,30 In a case-series, 2 post-stroke individuals improved step length asymmetry following 10-weeks of gait training comprising step length biofeedback.29 Druzbicki et al. demonstrated that real-time biofeedback to increase step length induced greater improvements in stride duration and other temporal parameters compared to treadmill training without biofeedback.30 Jonsdottir and colleagues showed that biofeedback regarding triceps surae electromyographic activity during walking resulted in increased peak ankle power, gait velocity, and stride length in individuals with post-stroke hemiparesis.26,27 Biofeedback can also promote motor learning and enhance neuroplasticity during stroke rehabilitation.31 These previous studies showcase the potential for biofeedback as a post-stroke gait training strategy.
Franz et al. demonstrated that neurologically unimpaired older adults have the ability to increase AGRF and gait speed within a single session of AGRF biofeedback gait training.32 Recently, Schenck and Kesar25 demonstrated that young able-bodied individuals can increase propulsion unilaterally with real-time AGRF gait biofeedback. Surprisingly, the use of AGRF biofeedback has not yet been evaluated in individuals post-stroke. Therefore, based on recent findings in neurologically intact individuals,25,32 the objective of this study was to investigate whether real-time AGRF biofeedback training can improve paretic propulsion in individuals with post-stroke hemiparesis. We hypothesized that a single session of gait training with real-time biofeedback about paretic AGRF would lead to increased paretic leg AGRF and improved inter-limb propulsive symmetry. Additionally, we hypothesized that a training bout comprising AGRF biofeedback would induce greater improvements in paretic AGRF compared to a control, speed-matched training bout without biofeedback.
Methods
Nine post-stroke individuals (6 females, age = 54 ± 12.4 years, Table 1) participated in one session. All participants provided informed consent, approved by the institutional Human Subject Review Board. The study procedures complied with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. The study was conducted in the setting of a research laboratory (motion analysis laboratory). The study recruitment, data-collection, and manuscript preparation spanned a period of 2 years. Inclusion criteria included:>6 months post-stroke, ability to walk on a treadmill continuously for 6-minutes, and ability to communicate with investigators. Exclusion criteria included neurologic diagnosis other than stroke, hemi-neglect, orthopedic conditions limiting walking, and cerebellar dysfunction. Eleven participants were screened and included in the study. Data from 2 participants were excluded from analysis due to not meeting study inclusion criteria (hemi-neglect, demonstrated inability to follow instructions during the gait session).
Table 1.
Participant demographics and clinical characteristics.
| Participant | Gender | Age (years) | Time post stroke (months) |
Side of Lesion | Fugl–Meyer score | Berg balance score | Self-selected speed (m/s) |
|---|---|---|---|---|---|---|---|
| 1 | Female | 63 | 42 | Left | 28 | 48 | 0.7 |
| 2 | Male | 72 | 82 | Left | 32 | 50 | 0.4 |
| 3 | Male | 54 | 47 | Right | 30 | 55 | 0.85 |
| 4 | Female | 65 | 42 | Right | 25 | 48 | 0.25 |
| 5 | Female | 56 | 40 | Right | 21 | 34 | 0.3 |
| 6 | Female | 47 | 64 | Right | – | 50 | 0.55 |
| 7 | Male | 56 | 11 | Right | – | – | 0.6 |
| 8 | Female | 43 | 10 | Right | 26 | 47 | 0.4 |
| 9 | Female | 31 | 15 | Left | 27 | 55 | 0.4 |
| Average | 54.1 | 39.2 | 27 | 48.4 | 0.5 | ||
| Std dev | 12.4 | 24.4 | 3.6 | 6.6 | 0.2 |
Setup and determination of gait speed for training
Reflective markers were attached to pelvis, and bilateral thigh, shank, and foot segments.33 Marker data were recorded using a 7-camera motion capture system (Vicon Inc., Colorado, USA). Participants walked on a dual-belt treadmill instrumented with force platforms (Bertec Corporation, Ohio, USA), with one foot on each treadmill belt to enable collection of GRF data from each leg. After a 2-minute warm up, the self-selected treadmill speed was determined for each participant. All subsequent gait tests and training were performed at this self-selected speed.
Control bout
To determine whether a 6-minute bout without biofeedback induced changes in gait biomechanics, participants completed a six-minute bout of walking at their self-selected speed. Marker position and GRF data were collected during 30-second walking trials immediately before (Pre) and after (Post) the bout (Figure 1(A)). The 6-minute bout duration was determined based on previous stroke gait training studies.13,34
Figure 1.
(A) Flowchart summarizing the experimental protocol. The arrows indicate time points when gait data were collected during 30-second treadmill walking trials at self-selected speed. Experimental time points when participants took rest breaks (seated or standing) are also indicated. During the training bouts, intermittent (alternating 1-minute epochs) real-time AGRF biofeedback was provided to study participants. (B) Schematic showing biofeedback paradigm (adapted from Schenck and Kesar, 2016). The symbol x indicates current antero-posterior GRF, and the bars indicate targeted AGRF. An auditory tone indicating successful achievement of ARGF every step cycle (B).
Methodology for biofeedback
Visual and auditory biofeedback was provided using a screen placed in front of the treadmill and a speaker (Figure 1(B)). The visual display comprised a horizontal line with a cursor (X) that represented the current paretic leg antero-posterior ground reaction force25 (MotionMonitor, Illinois, USA). The targeted AGRF range was represented by a green line with a 6-Newton error-tolerance range centered at the AGRF target (Figure 1(B)).25 Audio feedback comprised an audible tone produced when the AGRF entered target range during each gait cycle, indicating success. The participants were told that the cursor on the screen represented a measurement of how hard they were pushing the ground backward with their paretic foot, and their goal was to push-off harder with the paretic leg to achieve their target. Participants were not given any other instructions regarding strategies to increase AGRF.
Determination of AGRF target for biofeedback
Five AGRF target values were generated using paretic and non-paretic peak AGRFs measured during the baseline trial (See Equation (1)) to set a challenging, individualized target AGRF for each participant.
Following calculation of AGRF targets, the participant completed five 30-second trials with AGRF biofeedback at each target value. We opted to use brief 30-second trials for determination of target AGRF to minimize fatigue, and to obtain sufficient gait cycles of data for measuring the immediate effects of biofeedback at each target. Before beginning the biofeedback, participants were provided verbal instruction regarding the targeted gait parameter (paretic AGRF) and the biofeedback interface. The AGRF target selected for training corresponded to the 30-second trial where the participant achieved the target for ≥ 50% gait cycles as an index for appropriate challenge level. The 50% success rate criterion was selected based on our preliminary experiments, as well as to ensure that an adequate level of challenge was achieved during AGRF biofeedback. Observation (whether the participant visibly used compensatory gait deviations such as lateral or forward trunk lean) and verbal feedback from participant (whether the participants perceived the selected AGRF target to be sufficiently challenging) were also used to confirm appropriate target AGRF. The selected target AGRF was used throughout training.
Biofeedback gait training
Gait training comprised three 6-minute bouts of treadmill walking, with 5-minute seated breaks between bouts (Figure 1(A)). Ground reaction force and kinematic data were collected during 30-second walking trials before (Pre) and after (Post) the first training bout; biofeedback was not provided while data were collected. Data from these Pre and Post trials were used to compare effects of treadmill walking bouts with feedback and without biofeedback (control bout). The Pre trials were compared to retention trials to evaluate the effects of biofeedback training (Figure 1(A)). During training, biofeedback was provided intermittently with an alternating 1-minute-on, 1-minute-off protocol. We implemented intermittent instead of continuous biofeedback dosage to reduce dependence on biofeedback and enhance motor learning.19 During periods with biofeedback, participants were instructed to try and bring the cursor within the target AGRF on the biofeedback screen (Figure 1(B)). During periods without biofeedback, participants were instructed to maintain the gait they used to achieve the targeted AGRF during biofeedback. The participants completed 3 training bouts (total 18 minutes of biofeedback training) (Figure 1(A)).
Retention tests following training
After the final training bout, participants were provided a 2-minute standing break (Figure 1(A)). Following the break, a retention test was completed to evaluate short-term recall of the trained gait pattern. Retention tests were conducted without biofeedback, during a 30-second trial at the self-selected speed. Participants were instructed to walk such that their walking matched the pattern employed during biofeedback training. Participants then took another seated break. Similar retention tests were repeated following seated breaks at 15- and 30-minutes post-training (Figure 1(A)).
Data analysis and dependent variables
The primary dependent variables were peak AGRF for paretic and non-paretic legs. Secondary variables included peak trailing limb angle (angle between the laboratory’s vertical axis and vector joining the 5th metatarsal foot marker and greater trochanter marker), peak ankle plantarflexor moment, and step length for paretic and non-paretic legs. Step length for the leading leg was calculated as the distance between heel markers of the leading and trailing leg at initial contact35. Additionally, for each variable, the difference between paretic and non-paretic value was calculated as the “deficit” (peak AGRF, trailing limb angle, and plantarflexor moment deficit). A positive deficit indicates a greater non-paretic value, and a decrease in deficit with training indicates reduction in inter-limb asymmetry. For step length, deficit or step asymmetry was calculated as paretic step length divided by the sum of non-paretic and paretic step lengths35.
Statistical analyses
To evaluate effects of biofeedback training on gait biomechanics, for each variable, one-way repeated measures analysis of variance was performed to compare 4 time points – Pre (conducted before training bout 1), and 2-, 15-, and 30-minute retention tests. Planned post-hoc Bonferroni-corrected paired t-tests were conducted to compare pre-training data to each of the 3 retention tests. To compare changes in gait biomechanics induced by the control walking bout without biofeedback and the first exposure to biofeedback (training bout 1), a two-way repeated measures analysis of variance was performed for each dependent variable with time (Pre, Post) and type of training bout (biofeedback, control) as independent variables. Significance level was set at α ≤ 0.05 for all tests.
Results
Complete gait biomechanics data were collected on all 9 participants (Table 1). None of the participants reported any discomfort or adverse effects during biofeedback training.
Effects of AGRF biofeedback training on stroke gait biomechanics
Primary gait variables (paretic and non-paretic AGRF)
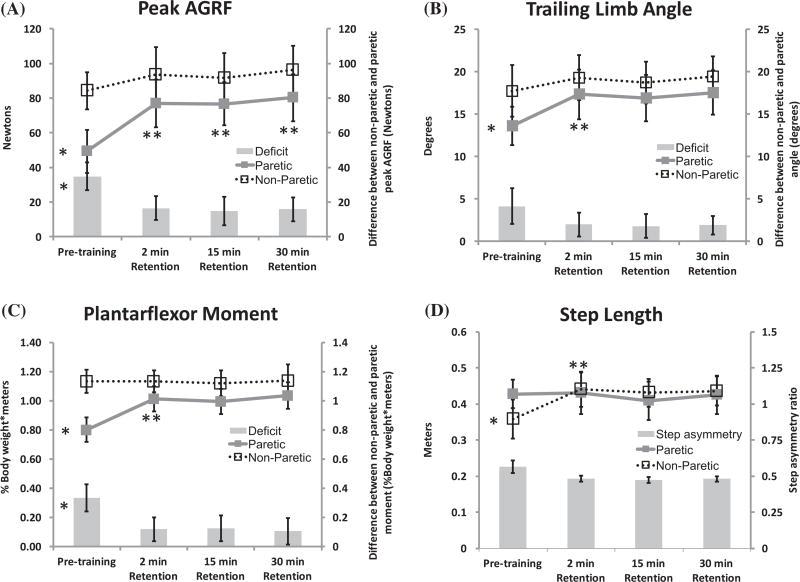
The one-way ANOVA for paretic peak AGRF showed a significant main effect of time (F = 39.24, p < .001) (Figure 2(A)). Planned post-hoc, pairwise, Bonferroni-adjusted comparisons revealed a significant difference in paretic peak AGRF between pre-training and 2-minute retention (p < 0.001), 15-minute retention (p < 0.001), and 30-minute retention (p < 0.001) (Figure 2(A)). The non-paretic peak AGRF demonstrated no main effect of time (all p’s > 0.097) (Figure 2(A)). The AGRF deficit showed a main effect of time (p = 0.003) (Figure 2(A)). There was no significant difference found on post-hoc pairwise testing of AGRF deficit.
Figure 2.
Average peak AGRF (A), peak trailing limb angle (B), peak ankle plantarflexor moment (C), and step length (D) for the study participants (N = 9). Each graph shows the values for the paretic leg (line graphs with filled symbols and bold lines), non-paretic leg (line graphs with open symbols and dashed lines), and the deficit or asymmetry between the non-paretic and paretic leg value (depicted in the bar plots). Error bars denote standard errors. For each plot, the symbol *to the left of a metric indicate a significant main effect of time detected by the 1-way repeated measures ANOVA. The repeated measures one-way ANOVA showed a significant main effect of time for each of the 4 secondary variables (peak AGRF, trailing limb angle, plantarflexor momen for the paretic leg, and step length of non-paretic leg) indicating an increase in these variables during the retention tests compared to pre-training. The symbol **indicates a significant difference at that time point compared to Pre (detected by pairwise post-hoc comparisons). No significant effect of time was observed for the non-paretic leg peak AGRF, trailing limb angle, plantarflexor moment, and paretic step length.
Secondary gait variables
The one-way ANOVA for paretic peak trailing limb angle showed a main effect of time (F = 6.785, p = 0.002) (Figure 2(B)). Post-hoc pairwise Bonferroni-adjusted comparisons showed a significant difference in paretic trailing limb angle between pre-training and 2-minute retention (p = 0.021) but not the other retention test time-points (all p’s > 0.17). No main effect of time was found for non-paretic trailing limb angle or trailing limb angle deficit (all p’s > 0.07).
The ANOVA revealed a significant main effect of time on paretic peak plantarflexor moment (F = 6.31, p = 0.003) (Figure 2(C)). Pairwise comparisons revealed a significant difference in paretic peak plantarflexor moment at pre-training vs. 2-minute retention (p = 0.044) but not compared to the other retention tests (all p’s > 0.16). There was no main effect of time for non-paretic peak plantarflexor moment (p = 0.93), but a main effect of time was shown for plantarflexor moment deficit (p = 0.014); planned pairwise comparisons revealed no significant differences for plantarflexor moment deficit (all p’s > 0.28).
The one-way ANOVA for non-paretic step length showed a main effect of time (F = 8.297, p = 0.001) (Figure 2(D)). The non-paretic step length measures the distance between the non-paretic and paretic leg at non-paretic initial contact, reflecting an improved trailing limb position for the paretic leg at terminal stance. Pairwise comparisons for non-paretic step length showed a significant difference between pre-training vs. 2-minute retention (p = 0.03), but not between pre-training and the other 2 retention tests (p’s > 0.11). A main effect of time was not detected for paretic step length or step asymmetry (p’s > 0.07).
Comparison of the effects of training bouts with and without biofeedback
The two-way ANOVA with type of training (AGRF feedback, control) and time (Pre, Post) as independent variables for paretic peak AGRF during a 6-minute training bout showed a significant main effect of training type (F = 13.51, p = 0.006), but no effect of time (p = 0.06) and no interaction effects (p = 0.10) (Figure 3(A)). The two-way ANOVA showed a significant effect of type of training on paretic peak trailing limb angle (F = 9.80, p = 0.01) (Figure 3(B)). No significant effect of time (p = 0.051) or interaction (p = 0.23) was found for paretic peak trailing limb angle. The two-way ANOVA showed a significant effect of training type on paretic peak plantarflexor moment (F = 6.72, p = 0.03). No significant effect of time (p = 0.78) or interaction (p = 0.41) was found for plantarflexor moment. No effects were detected for the non-paretic leg parameters.
Figure 3.
Average (N = 9) peak AGRF (A) and peak trailing limb angle (B) immediately before (Pre) and after (Post) a 6-minute training bout with biofeedback and a control training bout without biofeedback (speed- and duration-matched). The symbol *indicates a significant main effect of training type detected by the two-way repeated measures ANOVA. The two-way repeated measures ANOVA for paretic peak AGRF revealed a main effect of training type (F = 13.51, p = 0.006). A significant effect of type of training was also observed for paretic peak trailing limb angle (F = 0.80, p = 0.01) and plantarflexor moment (F = 6.72, p = 0.03).
Discussion
During retention gait tests conducted following a single 18-minute session of real-time AGRF biofeedback gait training, individuals post-stroke demonstrated increased peak AGRF production from their paretic leg. There was no concomitant increase in the non-paretic leg AGRF during the post-training retention tests. Additionally, real-time AGRF biofeedback training induced improvements in trailing limb angle, and ankle plantarflexor moment, with no changes in the non-paretic leg for these gait variables. Consistent with our hypothesis, based on calculation of step length with respect to the leading leg (at initial contact), biofeedback training resulted in a significant increase in the non-paretic step length, indicating that after biofeedback training, the paretic leg was positioned farther posteriorly with respect to the non-paretic leg during the paretic terminal stance phase of gait. Our findings of significantly improved gait biomechanics parameters specific to the paretic leg suggest that in response to biofeedback, participants acquired a modified gait pattern by changing their paretic leg parameters, rather than compensating with the non-paretic leg. Moreover, stroke participants demonstrated sustained improvements in paretic AGRF during retention gait tests conducted without biofeedback at 2-, 15-, and 30-minutes following training, demonstrating recall of the gait patterns learned during biofeedback training. Finally, our comparison of the effects of 6-minute bout of gait training with vs. without biofeedback demonstrated significantly greater AGRF during the training bout with biofeedback, suggesting that improvements in AGRF were induced by biofeedback rather than repetitive treadmill walking. Our study, for the first time, used technological tools to provide accurate and immediate feedback regarding paretic AGRF, an important post-stroke gait deficit. Our study demonstrates that real-time AGRF biofeedback has potential as a feasible and promising strategy for post-stroke gait retraining.
Decreased propulsion is a major contributor to gait dysfunction.1,2,4–10 To ameliorate deficits in gait parameters such as paretic propulsion, there is a paucity of training strategies that provide preferential or focused practice for the paretic leg, while minimizing compensations from the non-paretic leg. Recent studies have developed and tested new stroke gait training treatments targeting paretic propulsion, such as fast treadmill training, functional electrical stimulation, and powered exoskeletons.13,14,17,36,37 During post-stroke gait training, improvements in gait speed or endurance can be attained by increasing the contribution of the non-paretic leg toward total propulsion without increasing paretic leg propulsion, likely worsening inter-leg asymmetry. Our results show that following a single session of biofeedback training, improvements were induced only in paretic leg propulsion and not both legs or the non-paretic leg. Therefore, a unique advantage of biofeedback, in addition to the provision of accurate, real-time knowledge of performance, is that biofeedback directs practice of correct gait patterns toward the paretic leg. Because gait is a bilateral movement, few gait training treatments provide a similar advantage of specific practice to the paretic leg. Additionally, biofeedback empowers each participant to explore and develop their own individualized biomechanical solutions for achieving greater paretic leg propulsion.
Following biofeedback training, study participants demonstrated a significant increase in paretic trailing limb angle and paretic ankle plantarflexor moment. Ankle plantarflexor moment and the position of the trailing foot relative to the body’s center of mass (trailing limb angle) are major biomechanical parameters contributing to peak AGRF.9,38 Paretic ankle plantarflexor moment has a stronger correlation to walking speed compared to moment generation by any other lower extremity muscle group post-stroke,39 and has been correlated to propulsive force in stroke survivors.1 Trailing limb angle affects the position and orientation of the ground reaction force vector with respect to the center of mass, and therefore determines the proportion of GRF distributed anteriorly.9 In the present study, the participants were not given specific cues or verbal instructions regarding biomechanical strategies they could use to increase paretic push-off. Through biofeedback, we wanted to allow study participants to experiment with different movement strategies to increase paretic AGRF. Our results suggest that study participants used appropriate biomechanical gait parameters to influence paretic propulsion, but require more rigorous investigation in a larger sample size.
Hsiao and colleagues showed that after 12-weeks of fast treadmill training combined with functional electrical stimulation to ankle muscles, stroke participants increased paretic AGRF by 23% and non-paretic AGRF by 17% from pre-training to post training.40 In this study, following a single session of AGRF biofeedback training, we found an average 115.16 ± 121.48% increase in paretic peak AGRF and 1.59 ± 24.57% increase in non-paretic peak AGRF. There was considerable inter-individual variability in the training-induced increase of paretic AGRF (33.78% to 293.02%), inviting more systematic investigation of patient characteristics influencing response to biofeedback training. Potentially, AGRF biofeedback may have a more targeted effect on paretic propulsion compared to other locomotor training strategies. Here, we demonstrated the feasibility and short-term effects of AGRF biofeedback as a gait training interventional strategy in the laboratory. The use of wearable sensors41,42 can enable translation of gait biofeedback outside the gait laboratory to clinical and community settings. Additionally, future studies are needed to compare AGRF biofeedback with other gait training interventions that target paretic propulsion.
Our study has several limitations. Although our study was limited by a small sample size (N = 9), we detected statistically significant effects of biofeedback training on gait biomechanics. Due to the preliminary nature of this single-session study, we compared the effects of a 6-minute training bout with and without biofeedback (control bout), but did not compare the effect of 18-minutes of biofeedback training with a control training conducted in a separate session. Additionally, for each participant, the same target paretic AGRF was used throughout biofeedback training. Ongoing studies are exploring the incorporation of behavioral shaping strategies by modifying the target AGRF according to success rate and gait performance during training. Our future endeavors will also focus on development of algorithms to enable progression of gait speed based on ongoing performance during AGRF biofeedback training. The target AGRF given to participants in this study was selected based on success rates during a brief 30-second exposure to biofeedback. The selection of 50% success rate as a determinant for target AGRF was based on our preliminary testing as well as observed and perceived challenge during exposure to biofeedback. Future studies are needed to develop protocols for identifying an optimal target AGRF. Here, we provided alternating, intermittent, 1-minute periods with and without biofeedback, without incorporating a faded feedback schedule. The within-session time course of change in gait performance (immediate effects of biofeedback) or retention following a longer (e.g. 24-hour) interval were not explored in this preliminary study. The pursuit of optimal dosage, feedback frequency, and biofeedback training schedule, carryover to over ground gait, as well as long-term effects of biofeedback gait training need further investigation and, based upon the present findings, appear justified. The effects of utilizing alternate gait parameters such as trailing limb angle or step length as biofeedback target variables for increasing paretic propulsion merit systematic study. Future studies should also consider comparing specific verbal feedback provided by a clinician to quantitative, computerized biofeedback to increase paretic propulsion.
In summary, here, for the first time, we demonstrated that post stroke individuals can increase paretic leg AGRF within one session of real-time AGRF biofeedback gait training. Training-induced improvements in paretic peak AGRF were sustained during retention tests performed 15- and 30-minutes following training, demonstrating short-term recall of gait patterns learned during biofeedback training. Lack of significant changes in the non-paretic biomechanical parameters suggest that gait biofeedback provided preferential practice of targeted gait patterns to the paretic leg, without encouraging concomitant modifications to the non-paretic leg post-stroke. Real-time AGRF biofeedback may be a feasible strategy to improve paretic push-off post-stroke that merits additional investigation.
Acknowledgments
Funding
This work was supported by National Institute of Child Health and Human Development [grant number K01 HD079584]; and National Institutes of Health [grant numbers U10NS086607, U01 NS091951, R03 HD083727].
References
- 1.Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37(3):872–876. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88(1):43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Awad LN, Binder-Macleod SA, Pohlig RT, Reisman DS. Paretic propulsion and trailing limb angle are key determinants of long-distance walking function after stroke. Neurorehabil Neural Repair. 2015;29(6):499–508. doi: 10.1177/1545968314554625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34(11):1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22(1):51–56. doi: 10.1016/j.gaitpost.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech (Bristol, Avon) 1999;14(2):125–135. doi: 10.1016/s0268-0033(98)00062-x. [DOI] [PubMed] [Google Scholar]
- 7.Mulroy SJ, Klassen T, Gronley JK, et al. Gait parameters associated with responsiveness to treadmill training with body-weight support after stroke: an exploratory study. Phys Ther. 2010;90(2):209–223. doi: 10.2522/ptj.20090141. [DOI] [PubMed] [Google Scholar]
- 8.Hall AL, Peterson CL, Kautz SA, Neptune RR. Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis. Clin Biomech (Bristol, Avon) 2011;26(5):509–515. doi: 10.1016/j.clinbiomech.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson CL, Cheng J, Kautz SA, Neptune RR. Leg extension is an important predictor of paretic leg propulsion in hemiparetic walking. Gait Posture. 2010;32(4):451–456. doi: 10.1016/j.gaitpost.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson CL, Hall AL, Kautz SA, Neptune RR. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J Biomech. 2010;43(12):2348–2355. doi: 10.1016/j.jbiomech.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA. Identifying candidates for targeted gait rehabilitation after stroke: better prediction through biomechanics-informed characterization. J Neuroeng Rehabil. 2016;13(1):e46. doi: 10.1186/s12984-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowden MG, Behrman AL, Neptune RR, Gregory CM, Kautz SA. Locomotor rehabilitation of individuals with chronic stroke: difference between responders and nonresponders. Arch Phys Med Rehabil. 2013;94(5):856–862. doi: 10.1016/j.apmr.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA. Targeting paretic propulsion to improve poststroke walking function: a preliminary study. Arch Phys Med Rehabil. 2014;95(5):840–848. doi: 10.1016/j.apmr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA. Reducing the cost of transport and increasing walking distance after stroke: a randomized controlled trial on fast locomotor training combined with functional electrical stimulation. Neurorehabil Neural Repair. 2015;30(7):661–670. doi: 10.1177/1545968315619696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowden MG, Embry AE, Gregory CM. Physical therapy adjuvants to promote optimization of walking recovery after stroke. Stroke Res Treat. 2011;2011:601416. doi: 10.4061/2011/601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charalambous CC, Bonilha HS, Kautz SA, Gregory CM, Bowden MG. Rehabilitating walking speed poststroke with treadmill-based interventions: a systematic review of randomized controlled trials. Neurorehabil Neural Repair. 2013;27(8):709–721. doi: 10.1177/1545968313491005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsiao H, Higginson JS, Binder-Macleod SA. Baseline predictors of treatment gains in peak propulsive force in individuals poststroke. J Neuroeng Rehabil. 2016;13(1):e28. doi: 10.1186/s12984-016-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsiao H, Awad LN, Palmer JA, Higginson JS, Binder-Macleod SA. Contribution of paretic and nonparetic limb peak propulsive forces to changes in walking speed in individuals poststroke. Neurorehabil Neural Repair. 2015;30(8):743–752. doi: 10.1177/1545968315624780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giggins OM, Persson UM, Caulfield B. Biofeedback in rehabilitation. J Neuroeng Rehabil. 2013;10:60. doi: 10.1186/1743-0003-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf SL, Binder-Macleod SA. Use of the Krusen limb load monitor to quantify temporal and loading measurements of gait. Phys Ther. 1982;62(7):976–982. doi: 10.1093/ptj/62.7.976. [DOI] [PubMed] [Google Scholar]
- 21.Wolf SL, Binder-Macleod SA. Electromyographic biofeedback applications to the hemiplegic patient. Changes in upper extremity neuromuscular and functional status. Phys Ther. 1983;63(9):1393–1403. doi: 10.1093/ptj/63.9.1393. [DOI] [PubMed] [Google Scholar]
- 22.Wolf SL, Hudson JE. Feedback signal based upon force and time delay: modification of the Krusen Limb Load Monitor. Phys Ther. 1980;60(10):1289–1290. doi: 10.1093/ptj/60.10.1289. [DOI] [PubMed] [Google Scholar]
- 23.Stanton R, Ada L, Dean CM, Preston E. Biofeedback improves activities of the lower limb after stroke: a systematic review. J Physiother. 2011;57(3):145–155. doi: 10.1016/S1836-9553(11)70035-2. [DOI] [PubMed] [Google Scholar]
- 24.Stanton R, Ada L, Dean CM, Preston E. Biofeedback improves performance in lower limb activities more than usual therapy in people following stroke: a systematic review. J Physiother. 2017;63(1):11–16. doi: 10.1016/j.jphys.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Schenck C, Kesar TM. Effects of unilateral real-time biofeedback on propulsive forces during gait. J Neuroeng Rehabil. 2017;14(1):52. doi: 10.1186/s12984-017-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsdottir J, Cattaneo D, Recalcati M, et al. Task-oriented biofeedback to improve gait in individuals with chronic stroke: motor learning approach. Neurorehabil Neural Repair. 2010;24(5):478–485. doi: 10.1177/1545968309355986. [DOI] [PubMed] [Google Scholar]
- 27.Jonsdottir J, Cattaneo D, Regola A, et al. Concepts of motor learning applied to a rehabilitation protocol using biofeedback to improve gait in a chronic stroke patient: an A–B system study with multiple gait analyses. Neurorehabil Neural Repair. 2007;21(2):190–194. doi: 10.1177/1545968306290823. [DOI] [PubMed] [Google Scholar]
- 28.Wolf SL, Binder-Macleod SA. Electromyographic biofeedback applications to the hemiplegic patient. Changes in lower extremity neuromuscular and functional status. Phys Ther. 1983;63(9):1404–1413. doi: 10.1093/ptj/63.9.1404. [DOI] [PubMed] [Google Scholar]
- 29.Lewek MD, Feasel J, Wentz E, Brooks FP, Jr, Whitton MC. Use of visual and proprioceptive feedback to improve gait speed and spatiotemporal symmetry following chronic stroke: a case series. Phys Ther. 2012;92(5):748–756. doi: 10.2522/ptj.20110206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Druzbicki M, Guzik A, Przysada G, Kwolek A, Brzozowska-Magon A. Efficacy of gait training using a treadmill with and without visual biofeedback in patients after stroke: A randomized study. J Rehabil Med. 2015;47(5):419–425. doi: 10.2340/16501977-1949. [DOI] [PubMed] [Google Scholar]
- 31.Del Din S, Bertoldo A, Sawacha Z, et al. Assessment of biofeedback rehabilitation in post-stroke patients combining fMRI and gait analysis: a case study. J Neuroeng Rehabil. 2014;11:53. doi: 10.1186/1743-0003-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franz JR, Maletis M, Kram R. Real-time feedback enhances forward propulsion during walking in old adults. Clin Biomech (Bristol, Avon) 2014;29(1):68–74. doi: 10.1016/j.clinbiomech.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Kesar TM, Binder-Macleod SA, Hicks GE, Reisman DS. Minimal detectable change for gait variables collected during treadmill walking in individuals post-stroke. Gait Posture. 2011;33(2):314–317. doi: 10.1016/j.gaitpost.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reisman D, Kesar T, Perumal R, et al. Time course of functional and biomechanical improvements during a gait training intervention in persons with chronic stroke. J Neurol Phys Ther. 2013;37(4):159–165. doi: 10.1097/NPT.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabil Neural Repair. 2015;29(5):416–423. doi: 10.1177/1545968314552528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsiao H, Knarr BA, Higginson JS, Binder-Macleod SA. Mechanisms to increase propulsive force for individuals poststroke. J Neuroeng Rehabil. 2015;12:40. doi: 10.1186/s12984-015-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi KZ, Lewek MD, Sawicki GS. A neuromechanics-based powered ankle exoskeleton to assist walking post-stroke: a feasibility study. J Neuroeng Rehabil. 2015;12:23. doi: 10.1186/s12984-015-0015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsiao H, Knarr BA, Higginson JS, Binder-Macleod SA. The relative contribution of ankle moment and trailing limb angle to propulsive force during gait. Hum Mov Sci. 2015;39:212–221. doi: 10.1016/j.humov.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003;83(1):49–57. [PubMed] [Google Scholar]
- 40.Hsiao H, Knarr BA, Pohlig RT, Higginson JS, Binder-Macleod SA. Mechanisms used to increase peak propulsive force following 12-weeks of gait training in individuals poststroke. J Biomech. 2016;49(3):388–395. doi: 10.1016/j.jbiomech.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs DA, Ferris DP. Estimation of ground reaction forces and ankle moment with multiple, low-cost sensors. J Neuroeng Rehabil. 2015;12:90. doi: 10.1186/s12984-015-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Washabaugh EP, Kalyanaraman T, Adamczyk PG, Claflin ES, Krishnan C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture. 2017;55:87–93. doi: 10.1016/j.gaitpost.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]