Abstract
Episodic memory relies on memory for the relations among multiple elements of an event and the ability to discriminate among similar elements of episodes. The latter phenomenon, termed pattern separation, has been studied mainly in young and older adults with relatively little research on children. Building on prior work with young children, we created an engaging computer-administered relational memory task assessing what-where relations. We also modified the Mnemonic Similarity Task used to assess pattern discrimination in young and older adults for use with preschool children. Results showed that 4 year-olds performed significantly worse than 6 year-olds and adults on both tasks, whereas 6 year-olds and adults performed comparably, even though there were no ceiling effects. However, performance on the two tasks did not correlate, suggesting that two distinct mnemonic processes with different developmental trajectories may contribute to age-related changes in episodic memory.
Keywords: hippocampus, relational memory, pattern separation, episodic memory development
Episodic memory is memory for events that occur within a specific spatiotemporal context (Tulving, 2002). For example, I can remember that the last time I visited my favorite restaurant with a friend, we sat by the window and I had salmon and she ordered steak, but that the time before, we sat in a back booth and both ordered steak. Adults are better able to retrieve episodic memories like these than children younger than the age of 6 (Rubin, 2000), and some children fail to distinguish between real events and imagined events (Sluzenski, Newcombe, & Ottinger, 2004).
Remembering and discriminating between similar events presents at least two distinct challenges. First, I need to relate the various aspects of each event to form a cohesive episode (e.g., window seat with ate salmon). That is, I need to bind different elements, such as what, where, and when the event occurred, to form a memory of a complex event– i.e., relational memory (Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001; Schacter & Tulving, 1994). Second, I need to discriminate between similar events and elements within them (e.g., type of steak at time 1 was rib-eye, type of steak at time 2 was sirloin). That is, I need to distinguish similar memories from one another – pattern separation (Complementary Learning Systems: Norman & O’Reilly, 2003; Norman, 2010). These two component processes of episodic memory are the focus of this paper.
Relational Memory
Episodic memory requires the formation of relational structures that bind information to the specific context. Thus, the ability to form, retain, and retrieve relational information has been thought to be a crucial component of episodic memory (Eichenbaum & Cohen, 2001). Relational memory is typically tested using paired-associates tasks, which assess memory for the co-occurrence of multiple items that are not semantically related to one another (e.g., a tiger and a pool). Relational memory undergoes protracted development, whereas memory for single items develops relatively early and improves gradually (e.g., Lloyd, Doydum, & Newcombe, 2009; Sluzenski, Newcombe, & Kovacs, 2006; Sluzenski et al., 2004; Riggins, 2014). When asked to remember either single items (e.g., a tiger, a library) or unique item-place pairs (e.g., a tiger and a library), 6 year-olds outperformed 4 year-olds in relational memory test trials, but their item memory performances were comparable (e.g., Lloyd et al., 2009; Sluzenski et al., 2006).
More recent studies have employed episodic memory tasks that tap complex relational representations as opposed to unique pairings of items. For example, Newcombe and colleagues (2015) examined children’s ability to remember two arbitrary associations, each occurring in a specific context. Children were shown two different rooms (e.g., a rainbow and a cloud room) by two different experimenters. Both rooms contained an identical set of four containers but their arrangements differed between the two rooms. Children witnessed a different toy being hidden inside a different container in each room, and were later asked to retrieve a given toy. This task required the use of contextual memory (e.g., the rainbow room) to retrieve the correct object pair association (the bubbles hidden in a basket). Children as young as 15-20-months olds performed better than chance, with a significant increase in performance in 21-and 26-month-olds, 34- and 40-month-olds, and 64-and 72-month-olds.
In a similar paradigm, Richmond and Pan (2013) studied 3- to 5-year-old children who learned two series of animal-place pairs (e.g., the duck likes the train station), each presented in a storybook. Every place was associated with two animals, each learned from one of the two storybooks. This relational structure is referred to as ABAC format: A is paired with B in context 1 and paired with C in context 2. At test, children were asked to choose the correct place paired with an animal in a two-alternative force-choice test. Age significantly correlated with performance on this task: older children required fewer blocks to reach 70% accuracy, and were overall more accurate on this task, compared to younger children. Similar results were found by Yim, Dennis, and Sloutsky (2013). In this study, 4-year-olds, 7-year-olds, and young adults first learned two lists of object pairs, each list contained 6 pairs. Each pair consisted of an overlapping item – an object that occurred in both lists, and one unique item – an object that occurred only in one list (e.g., the bike was paired with a spoon in list 1 and paired with a mug in list 2). Participants first learned all object pairs in the first list until they reached 100% accuracy before learning the object pairs in the second list. Adults outperformed 7-year-old children, who outperformed 4-year-olds (Yim et al., 2013). Interestingly, increasing the saliency of the contextual cue improved 4-year-old children’s performance, suggesting that poor attention to the context information at encoding may account for the age-related differences in learning relational information.
Together, these findings suggest important developmental changes in children’s abilities to form and retrieve relational representations during early childhood. The marked improvement in relational memory between the ages of 3 to 7 coincides with the age window in which gains in episodic memory are robust (Peterson, Warren, & Short, 2011), consistent with the idea that the developmental changes in relational memory may contribute to the overall maturation of episodic memory in early childhood (reviewed in Olson & Newcombe, 2014). Given these findings, many researchers suggest that the age-related improvement in relational memory may be at the core of the development of episodic memory (Richmond & Pan, 2013; Yim et al., 2013).
Pattern Separation
Relational memory has been used as a proxy for episodic memory across all age groups. While relational memory is crucial in the formation of episodic memory, it is equally important to consider how multiple episodic memories are discerned from one another. Accurate episodic memory also relies on the ability to form distinct memory representations that share overlapping elements. Returning to the restaurant example, the two visits to my favorite restaurant were highly similar: both took place in similar environments with the same company, but with subtle variations between the two episodes (e.g., which dish I ordered and even more subtly, what type of steak my friend ordered). Memories for common day-to-day events share a considerable amount of feature overlap, which creates memory interference (Gomez & Edgin, 2015). A proposed process by which the overlap among similar memories is reduced to minimize memory interference is pattern separation (Complementary Learning Systems theory: O’Reilly & McClelland, 1994; Norman & O’Reilly, 2003; Norman, 2010). The hippocampus is thought to perform pattern separation, rapidly assigns distinct representations to specific events by transforming similar memories into highly dissimilar and non-overlapping patterns of activation. Specifically, the dentate gyrus – a hippocampal subfield – is thought to play a crucial role in pattern separation by assigning non-overlapping representations even for highly similar inputs. The ability to remember similar, but not identical, memories as distinct from one another – i.e., lure discrimination – is thought to represent the behavioral outcome of pattern separation (reviewed in Yassa & Stark, 2011).
Grounded in the idea that fine discrimination between similar memories depends on pattern separation, previous studies have used the Mnemonic Similarity Task (MST) – a recognition task that uses lure items, which are perceptually similar exemplars of some items in the study list (e.g., Bakker, Kirwan, Miller, & Stark, 2008; Bennett, Huffman, & Stark, 2014; Kirwan & Stark, 2007; Lacy, Yassa, Stark, Muftuler, & Stark, 2011; Stark et al., 2013; Stark et al., 2015; Toner, Pirogovsky, Kirwan, & Gilbert, 2009). In one version of the MST, participants perform an incidental outdoor/indoor task on a series of object images while scanned. Within this series, some objects are identical to previous objects within the series (repeat), some objects are similar exemplars of the previous objects (lure), and some are dissimilar to the rest of the objects within the series (novel). Separation-like activation profiles are only found in the hippocampal subregions CA3/dentate gyus, but not in other hippocampal subregions (Bakker et al., 2008; Lacy et al., 2011), or other MTL areas (Kirwan & Stark, 2007). These results are congruent with the idea that the dentate gyrus is responsive to small changes in input and may more readily assign similar inputs using non-overlapping representations despite only subtle differences. Together, there is evidence to suggest that the MST taxes hippocampal pattern separation and that this task can provide a behavioral index of pattern separation.
Importantly, pattern separation has been proposed as one key aspect of episodic memory that is sensitive to typical and atypical aging. In a MST overt recognition task variant, in which participants make either “old”, “similar”, or “new” judgments to each test item, correctly identifying lures as “similar” items is thought to rely on pattern separation. Lure discrimination is worse in old age (Bennett et al., 2014; Stark, Stevenson, Wu, Rutledge, & Stark, 2015; Stark, Yassa, Lacy, Stark, 2013; Toner et al., 2009), correlates with standardized episodic memory performance (Bennett et al., 2014; Stark, Yassa, Lacy, & Stark, 2013), and underlies memory dysfunction associated with amnestic mild cognitive impairment (Bakker et al., 2012; Yassa et al., 2010b). Previous studies found that an age-related decrease in lure discrimination is accompanied by higher activation in CA3/dentate gyrus subfields (Yassa et al., 2010b), and a decrease in white matter pathways within hippocampal circuitry (Yassa, Muftuler, & Stark, 2010a; Yassa et al., 2011a). Thus, the Complementary Learning Systems theory introduced a key mechanism by which episodic memory may change with age. Surprisingly, to our knowledge, there have been no assessments of pattern separation abilities in young children.
Present Study
Both relational memory and pattern separation are critical component processes of episodic memory. Whereas relational memory has been positioned front and center within the episodic memory development literature, pattern separation has been largely overlooked. The goal of the current study was to identify developmental changes in both components – relational memory and pattern separation – that underlie the development of episodic memory during early childhood. Given that the ages of 4 and 6 are an age window in which gains in episodic memory are most robust (Peterson et al., 2011), we tested 4- and 6-year-olds, as well as young adults, on two tasks. Our relational memory task was inspired by Newcombe et al. (2015)’s and Yim et al. (2013)’s studies. Engaging, narrated animations were created that contained item-item associations tied to a specific context using an AB-AC paradigm (A is paired with B in one context, but paired with C in another context). Our pattern separation task was a variant of the MST, which requires participants to incidentally encode pictures of objects. The ability to mnemonically discriminate between two similar items at test was used as a behavioral index of pattern separation.
Developing these two tasks allowed us to test whether performances on the relational memory and pattern separation tasks correlate with one another within each age group. Although both relational memory and pattern separation have been ascribed to the hippocampal functions, they have been hypothesized to rely on distinct neural substrates, each of which follows a different maturation profile. Relational memory may rely on CA3 as well frontal regions (e.g., Norman & O’Reilly, 2003; reviewed in Ghetti & Bunge, 2012), whereas pattern separation may rely on the dentate gyrus (Norman & O’Reilly, 2003). Previous work in non-human primates have shown that the dentate gyrus is a late-developing subfield relative to other hippocampal subfields such as CA1, CA2, and CA3 (Lavenex & Banta Lavenex, 2013; Serres, 2001). Given that the developmental trajectories of these regions are dissociable, it could be the case that relational memory and pattern separation abilities have an uneven development in young children.
To preview, we found a significant improvement in relational memory between the ages of 4 and 6, but 6-year-olds and young adults performed comparably. We also found a significant improvement in pattern separation, indexed by the MST lure discrimination, between the ages of 4 and 6, but a non-significant difference between 6-year-olds and young adults. Performances on the relational memory task and MST did not correlate for any age group.
Methods
Participants
A total of 39 4-year-old (23 females; Mmonth = 54.17 ± 3.87; range = 48.39 - 59.47) children and 30 6-year-old (15 females; Mmonth = 75.93 ± 3.19; range = 72.11 - 83.35) children recruited from the Philadelphia suburban areas participated in the study at the Temple Ambler Infant and Child Laboratory. All children were free of neurological damage and had no history of developmental disorders as reported by a parent. Of these children, 9 4-year-olds did not pass the training for the MST thus they were excluded from the MST analyses. All children completed the Relational Memory task. Informed consent was obtained from parents. All children received a small toy for their participation. The adult sample consisted of 52 undergraduate students from Temple University who participated for partial course credit. Two participants did not complete the experiment due to experimenter error. The remaining 50 young adults (25 females; Mage = 22.22 ± 4.79; range =18 - 49) were included in the analyses. All participants gave informed consent and reported to have normal or corrected-to-normal vision.
Relational Memory Task
Materials
We created a novel relational memory task that was engaging while also controlling for important psychologically important variables. Two animation sequences were created using images created in Microsoft PowerPoint or obtained from the internet and manipulated in Adobe Photoshop CS6. Each animation consisted of a tour to two locations (e.g., a red and a blue house), each containing four associations, totaling to eight associations per animation (Cronbach’s α of 16 associations = .82). Every association was made up of one common item (e.g., Pooh bear) – an item that appeared in both locations, and a unique item (e.g., book) – an item that only appears in one location (see Figure 1A). The entire tour was narrated by a female voice recording. A total of 16 animations were created to counterbalance the unique items and the order in which the locations were visited. Each animation lasted approximately 2.5 minutes. Example animations can be viewed at http://www.olson-lab.com/memory-test/.
Figure 1.

A schematic timeline of the associations encountered in one example of the house animation (A). Note that the animations are dynamic; thus Figure 1A illustrates an example of how the common and unique items were paired across two locations (ABAC format), not the actual images from the animations. An example of a test trial is shown in (B).
Procedure
Young children
All children watched two videos: a house video and a park video. For each video, they followed an encoding-delay-test procedure. At the beginning of each video, the voice recording stated “We’re going on an adventure today. We’re going to see two different houses/parks. We will have to remember the things we see in each place, so let’s watch very carefully, okay?” The house video toured two different color houses: a red house and a blue house. Four associations were presented in each house. Each association contained one common element and one unique element, i.e., ABAC format (see Figure 1A). For each association, the common element was first introduced “Look, there is Pooh bear. What does Pooh bear like to do in the red house?” The unique item then appeared on the screen (“A book. Pooh bear likes to read a book the in red house”). Each association was presented statically for 5 s with 12 transition frames (100ms/frame) before the next association appeared.
After the encoding phase of each video, there was 5-minute delay, during which the experimenter took the children into another room to play. The test phase consisted of 8, three-alternative forced choice trials for each animation. In each test trial, children were presented with a static screen shot of the common item in a location (e.g., Pooh bear in the red house), with three options shown below: target, across-context lure, and foil. Targets were the correct unique items in the particular location (e.g., book). Across-context lures were the unique items seen in the other location (e.g., paint). Foils were novel items that were not seen. Children were asked to choose one item that they saw with a given scene either by pointing or by the verbal response (see Figure 1B). The experimenter recorded children’s responses on paper. The order of the test trials was randomized. The locations of the targets, across-context lures, and foils were counterbalanced across test trials. All unique items were counterbalanced such that they were assigned as targets, across-context lures, and foils an equal number of times across participants. The order of the two locations and the order of the animations were counterbalanced across participants.
Mnemonic Similarity Task (MST)
Materials
A total of 230 digital images of common objects (115 pairs of similar exemplars) were obtained from the Internet, and 46 pairs of object exemplars were sampled from Craig Stark’s laboratory MST stimuli database (http://faculty.sites.uci.edu/starklab/mnemonic-similarity-task-mst/). We selected objects based on their appeal to children (e.g., toys, animals) and the likelihood that children would have had some familiarity with these objects (e.g., hat, bicycle). Each object pair was matched for size and orientation. The sizes of the objects were comparable across all objects (300 × 300 pixels). An independent sample of 31 young adults rated the levels of similarities for 161 pairs of objects on a 7-point Likert scale, ranging from completely different (1) to identical (7) (see Figure 2A), on Qualtrics (http://www.qualtrics.com/). The rating survey was interspersed with 42 catch trials (21 pairs of identical objects and 21 pairs of completely different objects) in order to ensure that participants did not respond randomly. All participants accurately rated the 21 pairs of identical objects as “identical”; therefore we included all participants’ rating results. The similarity ratings for 161 pairs of objects ranged from 4.66 - 6.00. They were binned into three levels of similarities based on the ratings: 20 level-1 items (4.66 - 4.99), 72 level-2 items (5.00 - 5.49) and 69 level-3 items (5.50 - 6.00). Of these, 88 pairs of objects were randomly selected (20 level-1, 34 level-2, and 34 level-3 items) in order to create a study and test set sizes of 66 items (see Figure 2B). The study and test set sizes were determined based on pilot work in order to ensure that 4-year-old children would be able to complete the task. To fully counterbalance all of the items, 88 object pairs were divided into 4 sets of 22 items, approximately matched on the level of similarities. Each set contained approximately equal number of stimuli from each semantic (e.g., animals, vegetables/fruit, toys). Eight versions of the study and test lists were created such that each set of objects were assigned as the target, lure, foil, and not-tested items an equal number of time across participants (mean Cronbach’s α of all test versions = .89, range = .84 – 94). The experiment was programmed in Eprime 2.0 (Psychology Software Tools, Pittsburgh, PA).
Figure 2.
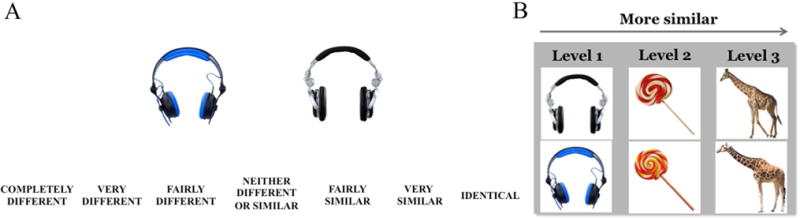
An example of the rating survey used to gauge the similarity between two object exemplars (A). Examples of the level-1, level-2, and level-3 object pairs, based on similarity rating results (B).
Procedure
Young children
The first part of the study consisted of an incidental encoding task. Children were told that they would play a game about pictures of objects. The task was to look at pictures of objects presented one at a time on a computer screen and to make an indoor/outdoor judgment for each object (see Figure 3A). Responses were entered by depressing one of two large buttons on a wood box which when pressed played the recorded word “inside” or “outside” (see Figure 3B). The experimenter instructed “I will show you a lot of pictures on this computer screen; whenever you see a picture of a an object that usually belong inside, you should press the white button, which says “inside”, and whenever you see a picture of an object that usually belongs outside, you should press the yellow button, which says “outside”.” Preceding the encoding phase were 2 self-paced practice trials (a bird and a spoon) to acquaint the children with the general rule of the game. The experimenter informed the children that the pictures would appear and disappear quickly so they would have to press the buttons quickly. Sixty-six objects were presented sequentially in a randomized order for 3 s each followed by a .5 s ISI (see Figure 3A). The incidental encoding task lasted approximately 5 minutes.
Figure 3.
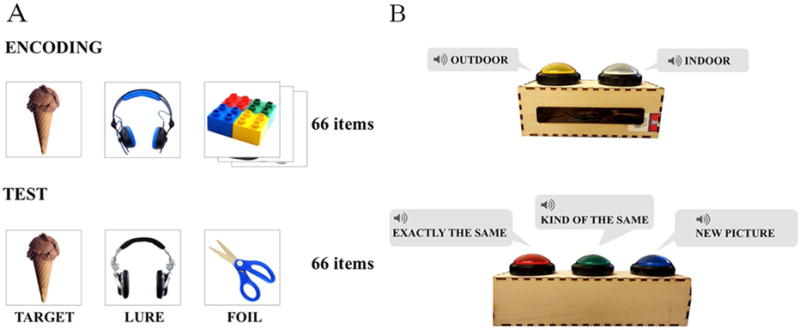
The encoding phase consisted of 66 object images shown sequentially (A top), in which young children decided whether the object belonged indoors or outdoors by pressing a two-button toy box with audio recordings (B top). At test, children were tested on 66 object images (22 targets, 22 lures, and 22 foils) (A bottom). Children responded by pressing a three-button toy box with audio recordings analogous to “old”, “similar”, and “new” for each test trial (B bottom).
The training phase immediately followed the incidental encoding task. The experimenter introduced a new game with a new toy box consisting of three buttons: a red, a green, and a blue button which when pressed played the audio phrases “exactly the same”, “kind of the same”, and “new picture,” respectively. Children were told to push each button by themselves and hear what each one said. The experimenter then explained the new game to the children: “Remember when we play the inside/outside game? Now we are going to play a different game with this toy box but with the pictures that we saw before, okay?” Items assigned as “not-tested” at encoding (e.g., the Lego in Figure 3A) were used for the training session to help children understand the task. During training, children were first shown one object that they previously saw presented on the left side of the screen, and triad of objects (one identical, one similar, and one completely different) shown on the right side of the screen (see Figure 4A). The experimenter then explained to the child while pointing that if the object on the right looked exactly the same as the one on the left, they should press the red button because the red button. If the object on the right looked kind of the same, but not exactly the same as the one on the left (e.g., they are both Legos, but not exactly the same Lego) they should press the green button. If the object on the right was completely different from the one on the left, they should press the blue button. After the first practice trial, children were encouraged to press the buttons by themselves for the following three trials with immediate feedback from the experimenter (see Figure 4B). After a total of four distinct examples, children then completed two “screening” practice trials in which the object was shown by itself (see Figure 4C). If the child chose the correct button for all three objects for both practice trials (total of six trials), they continued onto the test phase. If the child was not 100% correct, they repeated the training phase with distinct objects. Only when the child provided 100% correct responses for the two “screening” practice trials could they enter the test phase. If not, the experiment ended the MST and continued with the Relational Memory task. All lure items included in the “screening” practice trials were level-2 items, ensuring that these lures would not be too easy or too difficult. Among 69 children, nine 4-year-olds did not pass the training. Of these children, two children pressed the same button throughout the training session; two children pressed all three buttons a systematic order (e.g., red, green, blue), the remaining 5 children made errors on a combination of test trials (targets, lures, and foils). The training session took approximately 3-6 minutes.
Figure 4.
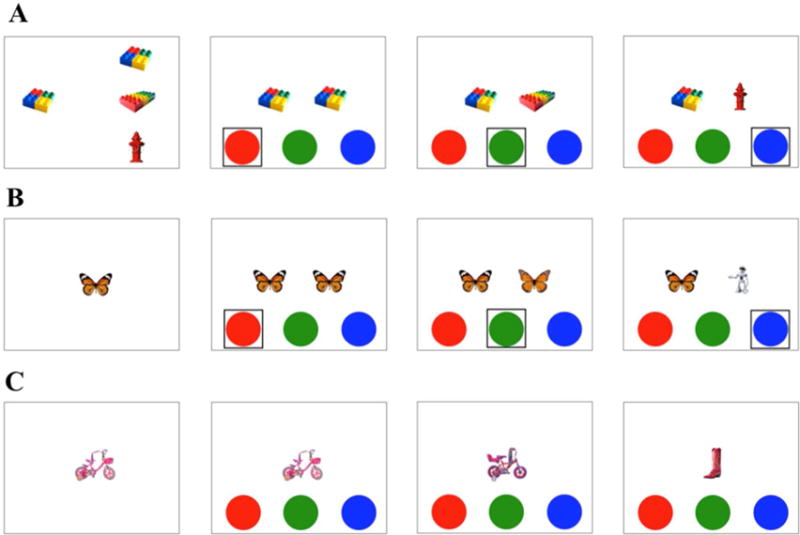
Examples from the training session. (A) First, children were shown an item studied at encoding and a triad of objects, each corresponding to one of the buttons (target: red, lure: green, and foil: blue). Children were explained on when to use each button to respond to different type of items. This repeats three more times with different items and immediate feedbacks were provided (B). Children were given two “screening” practice trials in which they asked to use the buttons to respond by themselves (C).
Immediately after the training session, children were given a self-paced test on 66 items: 22 targets, 22 lures, and 22 foils. Targets were identical items to those studied at encoding. Lures were similar exemplars of the studied items. Foils were novel items that were dissimilar from other objects in the stimuli set. For every trial, the experimenter asked, “Is this exactly the same, kind of the same, or completely new from the ones you saw before?”. Once the children pressed a button on the toy box, the experimenter recorded their responses by pressing keys “o”, “s”, or “n” for “old”, “similar”, and “new” on the keyboard. The test image remained on the screen until the experimenter inputted the children’s response. The order of the test items was randomized for each participant. The test phase took approximately 5-6 minutes.
Young adults
The stimuli were identical to those used in children. The procedure for young adults was similar to that used in children. The main difference was that responses were entered by computer keyboard and the instructions were modified to be appropriate for adult listeners. Adult participants completed an incidental indoor/outdoor encoding task, similar to that described earlier, which included 66 items presented serially (2 s each with the 0.5 s ISI.). After the encoding phase, participants were given a self-paced memory test on 66 items (22 targets, 22 lures, and 22 foils). The test instruction was as follow: “if the objects were identical to the ones they saw, press key “o” for old; if the objects were similar, but not identical to the ones they saw, press key “s” for similar, if the objects were completely different from the ones they saw, press key “n” for new.”
Overall procedure
All participants performed two tasks: the MST, and the relational memory task. Both tasks were presented on a 29.5-inch Dell desktop. For 4- and 6-year-olds, the MST was always administered first, followed by the relational memory task. Based on our pilot work, the MST appeared to be more taxing than the relational memory task and thus was always administered first for young children to avoid low performance due to fatigue. Adults were first administered the relational memory task encoding phase, followed by the MST, followed by the relational memory task’s test phase. Our pilot work also showed that adults reached ceiling performance on the relational memory task if administered in the encoding-delay-test procedure for each video, thus the MST was flanked by the relational memory task encoding and the test phases to serve as a delay. All parts of the experiment took place in the same room. Children were only taken to the playroom during the delay period of the Relational Memory Task.
Results
Relational Memory Task
These analyses included all children: 39 4-year-olds, 30 6-year-olds, and 50 young adults. First, we tested whether there was an age effect in overall memory accuracy, defined as the proportion of correct trials. A one-way ANOVA showed a significant age effect, F(2,116) = 9.55, p<.001. Tukey post-hoc tests showed that 4-year-olds (M = .69, SE= .04) performed significantly worse than 6-year-olds (M = .86, SE = .03), p = .002, and young adults (M = .86, SE = .03), p< .001. There was no difference in accuracy between 6-year-olds and young adults, p = .99 (see Figure 5).
Figure 5.
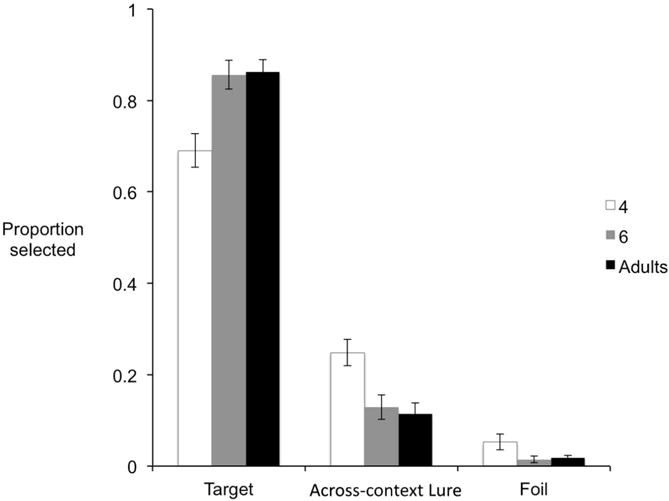
Mean proportions of targets, across-context lures, and foils selected in the relational memory task in three age groups.
Next, we calculated a corrected hit rate for each participant by subtracting the proportion of lure-chosen trials from the proportion of correct trials. The corrected hit rate reflects participants’ accuracy corrected for their lure error rate. There was a significant age effect, F(2,116)= 9.39,p < .001. Four-year-olds (M = .44, SE = .06) performed significantly worse than 6-year-olds (M = .73, SE = .06), p = .004, and young adults (M = .75, SE = .06), p < .001. There was no difference between 6-year-olds and young adults, p = .98.
It is worth noting that overall performance did not differ between the house and the park animations, or between the first and the second animations, all p’s > .05, suggesting that there were no unintended differences in difficulty between the two animations, and that participants did not improve or get worse from fatigue between the first and the second animations.
Mnemonic Similarity Task
Lure discrimination and item memory
The proportions of memory responses (old, similar, and new) for each item type (target, lure, and foil) were calculated for each participant (see Figure 6). Similar to Toner and colleagues (2009), lure discrimination index was calculated for each participant by subtracting the proportion of old responses to lures from the proportion of similar responses to lures. This value ranges from −1 to 1, and indexes the extent to which participants accurately identified lures corrected for the extent to which they falsely endorsed lures as targets. Positive values denote successful discrimination between targets and lures, whereas negative values denotes higher tendency to overgeneralize between two similar items. A lure discrimination index of zero denotes chance-level discrimination. A one-way ANOVA test revealed a significant age effect in the lure discrimination index, F(2, 109) = 22.82, p < .001, MSE = 2.53, ηp2= .30. Tukey post-hoc tests showed that 4-year-olds (M = −.29, SE = .06) performed significantly worse than 6-year-olds (M = .07, SE = .06), p = .002, and young adults (M = .23, SE = .05), all p’s < .001. There was no significant difference between the 6-year-olds and young adults, p = .13. One-sample t-tests (contrasting chance level at 0) revealed that 4-year-olds called lures “old” significantly more often than they would at chance, t(29) = −5.11, p < .001, 6-year-olds did not differ from chance, p = .29, whereas young adults correctly identified lures above chance, t(49) = 4.76, p < .001.
Figure 6.
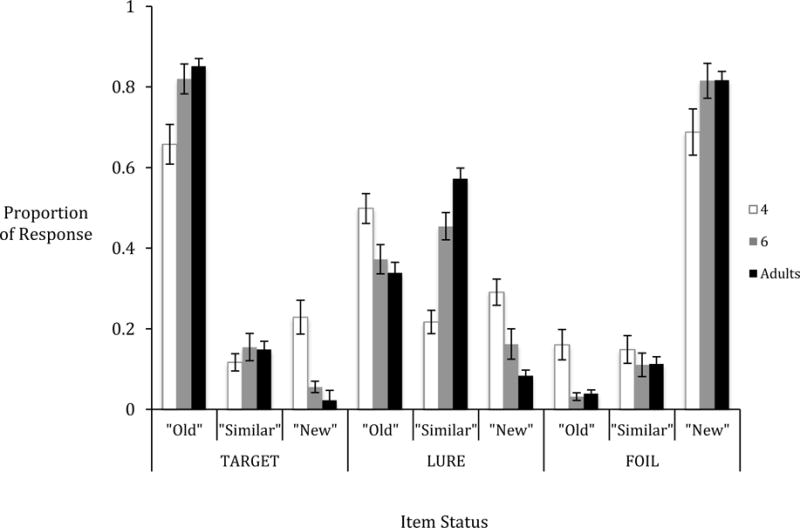
Mean proportions of responses to each type of test item status in the MST across three age groups.
Next, we examined item memory performance by assessing how well participants discriminated between dissimilar items (targets versus foils). Item memory was calculated by subtracting the proportion of proportion of old responses to foils from the proportion of old responses to targets (Bennett et al., 2014; Toner et al., 2009; Lacy et al., 2011; Stark et al., 2013, 2015). We found an age effect in item memory, F(2, 109) = 15.28, p < .001, MSE = 1.03, ηp2 = .22. Four-year-olds (M = .50, SE = .07) performed significantly worse than 6-year-olds (M = .79, SE = .04), p < .001, and adults (M = .81, SE = .02), p < .001, whereas 6-year-olds and adults performed comparably,p = .92. All age groups performed significantly above chance level (0), all p’s < .001. Given the significant age effect in item memory, we tested whether the age differences in lure discrimination were accounted for by age differences in item memory. We conducted a one-way ANCOVA, with item memory performance as covariate. The corrected model was significant, F(3,106) = 16.57, p < .001, MSE = 1.80. Age was the only factor that was significant, F(1,106) = 22.89, p < .001, whereas item memory was not significant, F(1,106) = 2.90, p = .09 (see Figure 7). These results show a significant improvement in lure discrimination between the ages of 4 and 6, but no increase was found between 6-year-olds and young adults.
Figure 7.
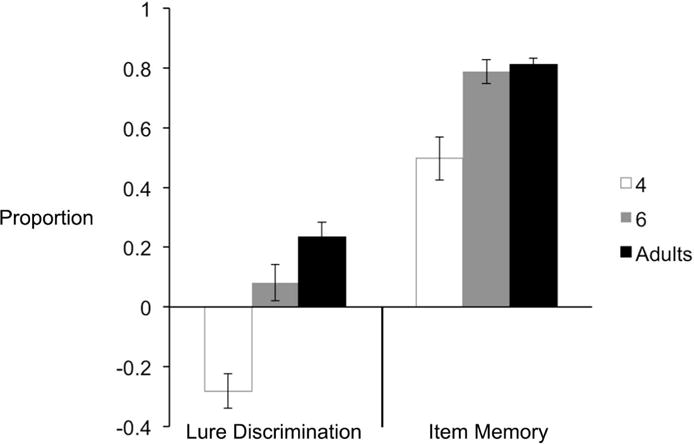
Lure discrimination and item memory performance in the MST across three age groups.
Levels of similarity
As the exemplar pairs were more similar to each other, we expected lure discrimination to decrease. We tested whether lure discrimination differed across three levels of similarity as a function of age. We conducted a 3 (levels of similarity: 1, 2, 3) × 3 (age: 4, 6, adults) mixed factorial analysis. We found a main effect of levels of similarity, F(2, 106) = 106.00, p < .001, a main effect of age, F(2, 107) = 25.16, p < .001, and a significant interaction, F(4, 105) = 5.53, p < .001. To unpack the significant interaction, pairwise comparisons for the levels of similarities were conducted for each age group separately. In 4-year-olds, lure discrimination did not significantly differ between level-1 (M = −.17, SE = .08) and level-2 (M = −.27, SE = .07), p = .21, or between level-2 and level-3 (M = −.36, SE = .06), p = .16, but the difference between level-1 and -3 was significant p = .05. In 6-year-olds, lure discrimination was significantly higher for level-1 items (M = .51, SE = .07) compared to level-2 (M = .06, SE = .06) and level-3 items (M = −.09, SE = .10), all p’s < .001. The difference between level-2 and level-3 was not significant, p = .06. In young adults, lure discrimination was significantly highest for level-1 items (M = .61, SE = .06), followed by level-2 items (M = .21, SE = .06), followed by level-3 items (M = −.03, SE = .06), all p’s < .001 (see Figure 8). Overall, these findings are consistent with the similarity ratings results such that the more similar the exemplars were to each other, the more likely the participants were to misremember them as the same.
Figure 8.
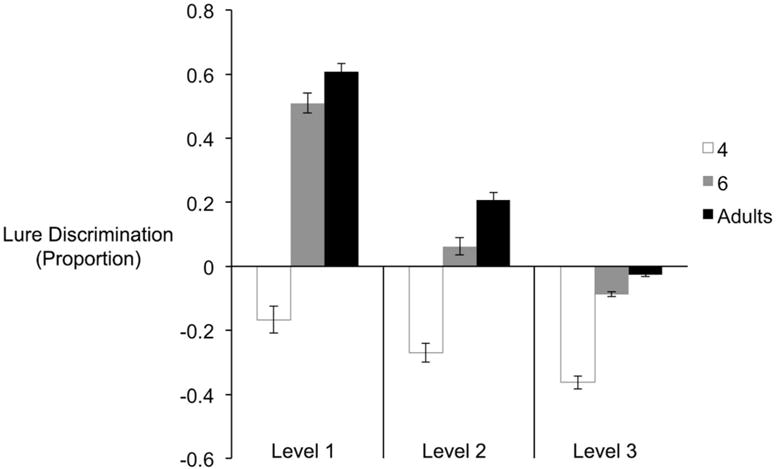
Lure discrimination broken down into three levels of similarities in the MST for each age group.
Across all three levels of similarities, 4-year-olds’ lure discrimination indices were significantly lower than 0, all p’s < .05, suggesting that they were more likely to falsely endorse lures as targets than to correctly identify lures. Six-year-olds’ lure discrimination was only significantly above chance for level-1 items, whereas young adults’ lure discrimination was significantly above chance for level-1 and -2 items, all p’s < .001. Together, these findings show that unlike 6-year-olds and young adults, 4-year-olds have a tendency to over-generalize, even for exemplar pairs in the lowest level of similarity. However, the fact that they were more likely to call a lure “old” as the items were more similar (level 3 < level 1) suggests that they understood the task procedure.
Correlation between Pattern Separation and Relational Memory
To examine whether relational memory is related to pattern separation, we correlated the corrected hit rate in the relational memory task with lure discrimination in the MST for each age group. Our normality tests showed that neither the MST lure discrimination nor the corrected hit (target – lure) of the relational memory task was normally distributed (Shapiro-Wilk < .001), thus violating one of the assumptions of the Pearson correlation. Therefore, we conducted the Spearman rank correlation. There was no significant correlation between MST lure discrimination and the relational memory corrected hit rate in either the 4-year-olds, r(28) = −.04, p = .83, the 6-year-olds, r(28) = .12, p = .52, or adults, r(48) = .10, p = .49. Across all participants, MST lure discrimination and Relational Memory corrected hit rate significantly correlates, r(108) = .23, p = .02. However, when age is entered as a covariate, the partial correlation is non-significant, r(107) = .15, p = .13.
Discussion
Episodic memory is a complex and multifaceted construct. Thus, characterizing the development of episodic memory development requires an examination of its component processes. In a previous study, Cheke and Clayton (2013) aimed to delineate a single common underlying construct of episodic memory by administering various “episodic memory” tasks in the same children including free and cued recall, what-where-when, and source memory tasks, an assessment of pattern separation was not included. Our goal is to fill this gap in the memory development literature. The current study examined the development of two crucial aspects of episodic memory: relational memory and pattern separation in early childhood. In our relational memory task, 4-year-olds performed significantly worse than 6-year-olds and young adults, whereas 6-year-olds and young adults performed comparably. In addition to choosing a lower proportion of targets, 4-year-olds showed higher false alarm rates for across-context lures – i.e., the correct paired objects presented in the other context. Our results echo the findings of previous studies examining relational memory using different paradigms and stimuli (e.g., Lloyd et al., 2009; Sluzenski et al., 2006) in suggesting significant improvement in children’s ability to remember the relations among multiple items between the ages of 4 and 6. It is important to note that although 6-year-olds and young adults performed very similarly in our task, it is likely that relational memory continues to develop well into adolescence, although in a less dramatic fashion (Ghetti & Lee, 2011; reviewed in Ghetti & Bunge, 2012).
Compared to 4-year-olds’ performance in a previous study using face-scene relational memory task (Koski, Newcombe, & Olson, 2013), in which they chose targets 44% of the time in a three-alternative forced-choice test, 4-year-old children performed well in our relational memory task (69%). The use of engaging and dynamic presentation of the associations via animations in the current study may be more appropriate for children in this age range. In contrast to Yim and colleagues (2013) who found that children, but not adults, showed proactive interference such that learned associations from List 1 interfered with learning the associations from List 2, we did not find any difference in memory performance for the first location versus the second location for any age group. An important methodological difference between our relational memory task and those used Yim and colleagues (2013) and Richmond and Pan (2013) should be noted. In those studies, participants learned the associations in the first list until they reached a learning criterion before learning the associations on the second list. In our task, participants were only shown each association once, because episodic memory is often a single-trial learning phenomenon.
To assess pattern separation, we tested 4-year-olds, 6-year-olds, and young adults using the MST, a lure discrimination task, in which similar developmental effects were observed. We found a significant improvement in lure discrimination between 4- and 6 year-olds, and a non-significant difference between 6 year-olds and young adults. Unlike 6-year-olds and young adults, 4-year-olds frequently misidentified lures as “old” more often than chance – an indicator of pattern separation failure. These results suggest that ages of 4 and 6 may be an important age window in which pattern separation increases significantly. Although there was no significant difference in lure discrimination between 6-year-olds and young adults, 6-year-olds did not correctly identify lures above chance, whereas adults did. These results suggest that pattern separation likely undergoes improvement well beyond the age of 6. The age effect in lure discrimination held when controlling for item memory performance, suggesting that 4-year-olds’ poor performance on lure discrimination cannot be accounted for by lower general item memory alone. Four year-olds did not discriminate between targets and lures for any levels of the similarity, even for lures that were least similar to targets (level-1 items). Six year-olds, on the other hand, were successful at discriminating between targets and lures for level-1, but showed no discrimination when the items were more similar (level-2 and -3 items). Young adults were successfully at lure discrimination except for when the lures were very similar to targets (level-3 items). Importantly, in all age groups, the tendency to misidentify lures as “old” items systematically increased as the similarity of the lure items increased suggesting that young children’s poor performance is unlikely due to task procedural difficulty.
Although we did not find any differences between 6-year-olds and young adults in either the MST or the relational memory task, there are methodological differences between the tasks administered to children and adults that may account for the lack of finding. With regards to the relational memory task, children were shown the first animation, followed by a 5-minute delay, then tested. This procedure repeated for the second animation. In contrast, adults were shown both animations sequentially then were tested on both. With regards to the MST, the encoding time and the delay differed for children versus adults. Children saw each item for 3 s while adults saw each item for 2 s. After encoding, children were given training, which lasts on average 3-4 minutes, whereas adults performed the relational memory task in between MST encoding and test phases, which lasts approximately 10 minutes. These methodological differences were intended to level the playing field for children and young adults, because these tasks were developed specifically for children. Thus it is likely that there are developmental changes that occur in both relational memory and pattern separation after the age 6 and well into adulthood, which were not detected in our paradigms.
In our study, although 6-year-olds performed better than 4-year-olds in lure discrimination, they did not correctly identify lures at above chance levels. On the other hand, 6-year-olds outperformed 4-year-olds at relational memory, and both age groups performed significantly above chance (target selected = .33). These findings suggest that pattern separation and relational memory may have dissociable developmental trajectories. Relational memory and pattern separation have been hypothesized to rely on distinct neural substrates, each of which follows a different maturation profile. Relational memory may rely on CA3 (Norman & O’Reilly, 2003) as well as portions of the frontal lobe (DeMaster & Ghetti, 2013; reviewed in Ghetti & Bunge, 2012), whereas pattern separation may rely critically on the dentate gyrus (Norman & O’Reilly, 2003). Findings in non-human primates show that the dentate gyrus is a late developing subfield relative to other hippocampal subfields such as CA1, CA2, and CA3 (Lavenex & Banta Lavenex, 2013; Serres, 2001). Consistent with this, we found that pattern separation and relational memory performance did not correlate with each other for any age group. One possible explanation for our findings is that relational memory and pattern separation tap different aspects of episodic memory. Relational memory may be important for forming an episodic memory (Cohen & Eichebaum, 1993), whereas pattern separation allows for similar memories to be represented separately with reduced overlap in order to minimize interference at retrieval (O’Reilly & Norman, 2003).
It is important to note that the MST demands mnemonic distinction between similar items with similarity confined along the dimension of conceptual (object identity/category) and perceptual properties (e.g., color, shape). However, this is not the only way in which two items can share similarities. Perhaps what would be more critical for episodic memory, are ways in which objects are similar on the “episodic” dimension. Returning to our restaurant example, one defining difference between the two events is that I ordered salmon in one episode, steak in the other. In this example, salmon and steak, which possess numerous perceptual differences, are similar due to the overlap in the contexts in which they were encountered. It remains unclear whether similar items and similar episodes are handled differently by hippocampal pattern separation. This question has important theoretical implications and merits empirical investigation. A pivotal future direction for this line of research is to examine the development of pattern separation for contexts as it pertains to the development of episodic memory.
Another point worth noting is that our relational memory task, as well as others used in previous work, may require some degree of pattern separation. In tasks that employ the AB-AC paradigm, both associations can be said to be similar such that they share an overlapping component. In addition, these associations may also share other overlapping episodic features depending on the similarities between the contexts in which they were learned. In our task, although the contexts had distinct background colors (e.g., red versus blue house), as well as unique decorative details in each location, the structures of the house/park tours were made similar (e.g., the living room was first visited, followed by the kitchen in both houses). Similarly, tasks by Richmond and Pan (2013), Yim and colleagues (2013), and Newcombe and colleagues (2015) also employed variants of AB-AC relational structure, in which the to-be-remembered associations shared overlapping elements. This type of relational memory is not process-pure. That is, in addition to relational memory, they may place some demand on pattern separation. This can be contrasted to relational memory tasks that have an AB-CD relational structure, in which the to-be-remembered associations are always made up of unique components, and thus have fewer overlaps. However, given that the similarity of the associations and their encoding contexts was not manipulated in these studies, the development of pattern separation for associations or contextual memory in early childhood remains unknown.
Relational memory has been used as a proxy for episodic memory across all age groups. While relational memory is crucial in the formation of episodic memory, it is equally important to consider new memories are formed against a backdrop of existing memories, and in many cases, these memories share overlapping elements with one another. However, the development of pattern separation has been overlooked in the memory development literature. The current study, to our knowledge, was the first to investigate the development of pattern separation, indexed by MST lure discrimination, in young children. We found that 4-year-olds exhibit a tendency to overgeneralize, whereas 6-year-olds do not. Newcombe and colleagues (2007) previously argued that it may be more important to build semantic knowledge than to remember specific episodes in early years of life. Aligned with this argument, generalizing across similar memories to learn about the regularities of the environment may be prioritized over fine-grained discrimination among them. That is, younger children may need to learn that objects such as chairs, regardless of subtle variations in colors and shapes, serve the same function. In parallel, we examined the development of relational memory - another key characteristic of episodic memory - in the same children. The current study provides important implications on the mechanisms of memory development. Our findings suggest that pattern separation undergoes a significant improvement in early childhood, and that it is crucial to consider the developmental changes of both relational memory and pattern separation to account for episodic memory development.
Research Highlights.
Pattern separation, indexed by a new measure of mnemonic lure discrimination suitable for children, undergoes changes between ages of 4 and 6, and continues to develop into adulthood.
Relational memory also improves significantly between the ages of 4 and 6, with a non-significant difference between 6-year-olds and young adults.
Pattern separation and relational memory may be separable cognitive components of episodic memory, with developmental changes in each likely contributing to episodic memory gains in early childhood.
References
- Bakker A, Kirwan CB, Miller MI, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Huffman DK, Stark CE. Limbic tract integrity contributes to pattern separation performance across the lifespan. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheke LG, Clayton NS. The six blind men and the elephant: Are episodic memory tasks tests of different things of different tests of the same thing? Journal of Experimental Child Psychology. 2015;137:164–171. doi: 10.1016/j.jecp.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- DeMaster DM, Ghetti S. Developmental differences in hippocampal and cortical contributions to episodic retrieval. Cortex. 2013;49(6):1482–1493. doi: 10.1016/j.cortex.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: memory systems of the brain. New York: Oxford University Press; 2001. [Google Scholar]
- Ghetti S, Bunge SA. Neural changes underlying the development of episodic memory during middle childhood. Developmental Cognitive Neuroscience. 2012;2(4):381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, Lee JK. Children’s episodic memory. Wiley Interdisciplinary reviews: Cognitive Science. 2011;2(4):365–373. doi: 10.1002/wcs.114. [DOI] [PubMed] [Google Scholar]
- Gomez R, Edgin J. The extended trajectory of hippocampal development: Implications for early memory development and disorder. Developmental Cognitive Neuroscience. 2015 doi: 10.1016/j.dcn.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski JE, Newcombe NS, Olson IR. Tracking the eyes to see what children remember. Memory. 2013;21:396–407. doi: 10.1080/09658211.2012.735241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Banta-Lavenex P. Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behavioral Brain Research. 2013;254(2013):8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA1 and CA3/DG revealed using high-resolution fMRI and mnemonic similarity. Learning and Memory. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd ME, Doydum AO, Newcombe NS. Memory binding in early childhood: evidence for a retrieval deficit. Child Development. 2009;80(5):1321–8. doi: 10.1111/j.1467-8624.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- Newcombe NS, Balcomb F, Ferrara K, Hansen M, Koski J. Two rooms, two representations. Episodic-like memory in toddlers and preschoolers. Developmental Science. 2015;17(5):743–756. doi: 10.1111/desc.12162. [DOI] [PubMed] [Google Scholar]
- Newcombe NS, Lloyd ME, Ratliff KR. Development of episodic and autobiographical memory: A cognitive neuroscience perspective. Advances in Child Development and Behavior. 2007;35:37–85. doi: 10.1016/b978-0-12-009735-7.50007-4. [DOI] [PubMed] [Google Scholar]
- Norman KA. How hippocampus and cortex contribute to recognition memory: revisiting the complementary learning systems model. Hippocampus. 2010;20:1217–1227. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling Hippocampal and Neocortical Contributions to Recognition Memory: A Complementary-Learning-Systems Approach. Psychological Review. 2003;110(4):611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Olson IR, Newcombe NS. Binding together the elements of episodes: Relational memory and the developmental trajectory of the hippocampus. In: Bauer Patricia J, Fivush Robyn., editors. The Wiley handbook and the development of children’s memory, Volume I/II. 2014. [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4(6):661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Peterson C, Warren KL, Short MM. Infantile amnesia across the years: A 2 year follow-up of children’s earliest memories. Child Development. 2011;82:1092–1105. doi: 10.1111/j.1467-8624.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- Richmond JL, Pan R. Thinking about the future early in life: The role of relational memory. Journal of Experimental Child Psychology. 2013;114:510–521. doi: 10.1016/j.jecp.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Riggins T. Longitudinal investigation of source memory reveals qualitative differences between item memory and binding. Developmental Psychology. 2014;50(2):449–459. doi: 10.1037/a0033622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC. The distribution of early childhood memories. Memory. 2000;8:265–269. doi: 10.1080/096582100406810. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Tulving E. Memory Systems. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- Serres L. Morphological changes of the human hippocampal formation from midgestation to early childhood. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2001. pp. 45–58. [Google Scholar]
- Sluzenski J, Newcombe NS, Kovacs SL. Binding, relational memory, and recall of naturalistic events: A developmental perspective. Journal of Experimental Psychology. 2006;32:89–100. doi: 10.1037/0278-7393.32.1.89. [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, Ottinger W. Changes in reality monitoring and episodic memory in early childhood. Developmental Science. 2004;7:225–245. doi: 10.1111/j.1467-7687.2004.00341.x. [DOI] [PubMed] [Google Scholar]
- Stark SM, Stevenson R, Wu C, Rutledge S, Stark CE. Stability of age-related deficits in the mnemonic similarity task across task variations. Behavioral Neuroscience. 2015;129(3):257–268. doi: 10.1037/bne0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CE. A task to assess behavioral pattern separation in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan B, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning & Memory. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resoultion micro-structural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. PNAS. 2010a;107:12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage. 2010b;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences USA. 2011a;108(21):8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Wu J, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011b;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends in Neuroscience. 2011;24(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim H, Dennis SJ, Sloutsky VM. The development of episodic memory: Items, contexts, and relations. Psychological Science. 2013;24:2153–2172. doi: 10.1177/0956797613487385. [DOI] [PMC free article] [PubMed] [Google Scholar]


