Abstract
Since MGUS was first described over 30 years ago, the definition of the entity has evolved. Today, three distinct clinical MGUS subtypes have been defined: non-IgM (IgG or IgA) MGUS, IgM MGUS, and light chain MGUS. Each clinical MGUS subtype is characterized by unique intermediate stages and progression events. Although we now have strong evidence that multiple myeloma is consistently preceded by a precursor state, at the molecular level, there is urgent need to better understand mechanisms which regulate transformation from precursor to full-blown multiple myeloma. In the future, if such knowledge was available, it would allow clinicians to define high-risk and low-risk precursor patients for a more tailored clinical management. Also it would provide insights on the individual patient's disease biology which in turn can be used for targeted and more individualized treatment strategies. Based on current clinical guidelines, patients diagnosed with MGUS and smoldering myeloma should not be treated outside of clinical trials. In the near future, it seems reasonable to believe that high-risk precursor patients will likely become candidates for early treatment strategies. In this review paper we discuss novel insights from recent studies and we propose future directions of relevance for clinical management and research studies.
Keywords: multiple myeloma, monoclonal gammopathy of undetermined significance, MGUS, smoldering myeloma, precursor disease, biomarkers, early treatment
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant plasma cell disorder present in over 3% of the general Caucasian population age 50 and older.(1, 2) On a clinical note, individuals diagnosed with MGUS have a 1% annual risk of progression to multiple myeloma (MM) or related malignancy.(3) Typically, MGUS is found incidentally during the work-up of a variety of symptoms and disorders. It is characterized by abnormal immunoglobulins detectable in the patient's peripheral blood and/or urine, as well as clonal plasma cells present in the bone marrow (Figure 1). Definitions of distinct MGUS subtypes are given in Table 1.
Figure 1.

Bone Marrow Biopsies from A Patient With Non-IgM MGUS (Panel A; H&E and Panel B; CD138) and from A Patient with Multiple Myeloma (Panel C; H&E and Panel D; CD138); Magnification 200X
Table 1. Disease Definitions for the Monoclonal Gammopathies: Monoclonal Gammopathy of Undetermined Significance (MGUS) and Related Disorders.
| Type of Monoclonal Gammopathy | Premalignancy with a low risk of progression (1-2% per year) | Premalignancy with a high risk of progression (10 % per year) | Malignancy |
|---|---|---|---|
| IgG and IgA (Non-IgM) monoclonal gammopathies* |
Non-IgM MGUS All 3 criteria must be met:
|
Smoldering Multiple Myeloma Both criteria must be met:
|
Multiple Myeloma All 3 criteria must be met except as noted:
|
| IgM Monoclonal gammopathies |
IgM MGUS** All 3 criteria must be met:
|
Smoldering Waldenström's Macroglobulinemia Both criteria must be met:
|
Waldenström's Macroglobulinemia All criteria must be met:
All criteria must be met:
|
| Light Chain monoclonal gammopathies |
Light Chain MGUS All criteria must be met:
|
Idiopathic Bence Jones Proteinuria All criteria must be met:
|
Light Chain Multiple Myeloma**
|
Occasionally patients with IgD and IgE monoclonal gammopathies have been described and will be considered to be part of this category as well.
Note that conventionally IgM MGUS is considered a subtype of MGUS, and similarly light chain multiple myeloma is considered as a subtype of multiple myeloma. Unless specifically distinguished, when the terms MGUS and multiple myeloma are used in general, they include IgM MGUS and light chain multiple myeloma respectively.
Furthermore, MGUS has confirmed and reported associations with numerous diseases that are commonly encountered in clinical practice such as osteoporosis and venous thrombosis.(4) Given the fact that MGUS is easily detected in peripheral blood and it can be monitored noninvasively, MGUS represents a readily accessible model to study the conversion of premalignancy to malignancy.(5)
Race and ethnicity play a role in the pathogenesis of MGUS. African Americans, and blacks from Africa, have a 2- to 3-fold higher prevalence of MGUS compared with whites.(6, 7) In contrast, the risk is lower in Asians from Japan,(8)and in Mexicans.(9) Increasing age,(1) male gender, family history of MGUS and/or multiple myeloma,(10) immunosuppression, and exposure to certain pesticides(11) all increase the risk of MGUS. Clearly, future studies are needed to improve our understanding on underlying mechanisms of these associations.
A more advanced premalignant stage of plasma cell proliferation in non-IgM MGUS is termed smoldering myeloma and is characterized by a much higher risk of progression to multiple myeloma (on average, about 10% per year during the first 5 years of follow-up).(12)
During recent years, new concepts and advances have emerged concerning the diagnosis, classification, risk-stratification, and management of myeloma precursor disease (MGUS and smoldering myeloma). In this review paper we discuss novel insights from recent studies and we propose future directions for clinical management and research studies.(13)
Distinct Clinical MGUS Subtypes
Since MGUS was first described in 1978 (14), the definition of the entity has evolved.(15) Today, three distinct clinical MGUS subtypes have been defined: non-IgM (IgG or IgA) MGUS, IgM MGUS, and light chain MGUS (Table 1). Each clinical MGUS subtype is characterized by unique intermediate stages and progression events. For example, as mentioned above, the more advanced premalignant stage of plasma cell proliferation in non-IgM MGUS is smoldering myeloma; it has an average 10% annual risk of progression to multiple myeloma (versus 1% per year collectively for all forms of MGUS).(12) IgM MGUS is associated with a predisposition mainly to Waldenström's macroglobulinemia and rarely to IgM multiple myeloma.(16, 17) Recently, a new disease entity termed “light chain MGUS” was defined. It represents the premalignant precursor of a subtype of multiple myeloma called “light chain multiple myeloma” which accounts for almost 20% of all new multiple myeloma cases.(18) The equivalent of smoldering myeloma and smoldering Waldenström's macroglobulinemia in the spectrum of light chain monoclonal gammopathies is called idiopathic Bence Jones proteinuria (Table 1).(19, 20) At this time, at least one large cohort including patients meeting each of the above listed clinical subtypes has been assembled. These cohorts have allowed clinicians and researchers to study and define the natural history for each MGUS subtype.(3, 12, 16, 18, 19, 21-23) Consequently, we now know how to diagnose each of these entities accurately, and we also know the outcome of patients meeting the specific subtype definition to assist with management and counseling. In Table 1 we have summarized the main features and results from the largest epidemiologic and clinical studies focusing on MGUS subtypes to date.(3, 12, 16, 18, 19, 21-23) More specifically, we have listed and commented on prevalence, risk of progression, and natural history of non-IgM MGUS, IgM MGUS and light chain MGUS.
It should be emphasized that in patients diagnosed with clonal proliferation of plasma cells consistent with a precursor state, the biology and natural history is very different compared with patents diagnosed with multiple myeloma. Importantly, patients with a precursor state should be reassured rather than labeled as having a cancer. For example, patients with less than 10% infiltration of the bone marrow by lymphoplasmacytic cells have an overall survival similar to that of the general population, and should therefore not be labeled as having a lymphoma or Waldenström's macroglobulinemia merely because the bone marrow pathology shows clonal proliferation of lymphoid cells.(22) Furthermore, on a clinical note, increasingly better sensitivity for diagnostic methods will continue to challenge clinical management and the line between malignancy and premalignancy will most likely continue to blur. As our understanding of disease progression improves, it will become more and more important to recognize that well-designed epidemiologic studies and clinicopathologic disease definitions will be required to separate patients who need treatment such as chemotherapy or stem cell transplantation for cancer like myeloma,(24) from those who call for no therapy and need reassurance.(5)
From Precursor to Multiple Myeloma: Current Clinical Risk Models
It is important to keep in mind that the vast majority of MGUS patients will never progress to multiple myeloma. Currently, we do not have access to any reliable markers to predict risk of multiple myeloma progression for individual MGUS patients. At the present time, the risk of progression of MGUS is assessed by a few selected risk factors. Two major models for risk stratification have been proposed: one model by the Mayo Clinic and the other by the Spanish study group (PETHEMA, Programa para el Estudio de la Terapéutica en Hemopatía Maligna)(Figure 2).
Figure 2. Risk Stratification Schemes for MGUS (Left) and Smoldering Myeloma (RIGHT).
Footnote: Two major models for risk stratification have been proposed: one model by the Mayo Clinic and the other by the Spanish study group (PETHEMA). The Mayo Clinic model focuses largely on serum protein abnormalities. For MGUS patients, the following features are considered as adverse risk factors: non-IgG isotype, M-protein concentration ≥ 1.5 g/dL, and an abnormal serum free light chain (FLC)-ratio (normal reference 0.26-1.65) (25). For smoldering myeloma patients, the following features are considered to be adverse risk factors: ≥3 g/dL M-protein, an FLC-ratio outside the reference range of 0.125 to 8, and ≥10% bone marrow plasma cells (12, 26). The Spanish model uses multiparametric flow cytometry of bone marrow aspirates to differentiate aberrant from normal plasma cells (27). Plasma cells characteristically express CD138 and intense (bright) CD38. The features of aberrant plasma cells included decreased CD38 expression, expression of CD56, and the absence of CD19 and/or CD45. In their study, MGUS and smoldering myeloma patients with ≥95% phenotypically aberrant plasma cells (aPC) of total bone marrow plasma cells (BMPC) (i.e. ≥95% aPC/BMPC) at diagnosis had a significantly higher risk of multiple myeloma progression (27). Furthermore, on multivariate analysis, ≥95% aPCs/BMPC, DNA aneuploidy, and immunoparesis were found to be independent predictors of multiple myeloma progression from MGUS/smoldering myeloma. More specifically, for MGUS patients ≥95% aPCs/BMPC and DNA aneuploidy were found to be risk factors for progression. For smoldering myeloma patients the risk factors were ≥95% aPCs/BMPC and immunoparesis (27).
The Mayo Clinic model focuses largely on serum protein abnormalities. For MGUS patients, the following features are considered as adverse risk factors: non-IgG isotype, M-protein concentration ≥1.5 g/dL, and an abnormal serum free light chain (FLC)-ratio (normal reference 0.26-1.65) (Figure 2) (25). In the Mayo Clinic model, at 20 years of follow-up, MGUS patients with all three risk factors, on average, have an absolute risk of multiple myeloma progression of 58%; for MGUS patients with 2, 1, and 0 of these risk factors, the corresponding absolute risk is 37%, 21% and 5%, respectively (25). For smoldering myeloma patients, the following features are considered to be adverse risk factors: ≥3 g/dL M-protein, an FLC-ratio outside the reference range of 0.125 to 8, and ≥10% bone marrow plasma cells (Figure 2) (14, 26). In the Mayo Clinic model, at 5 years of follow-up, smoldering myeloma patients with all three risk factors, on average, have a cumulative risk of multiple myeloma progression of 76% (median time-to-progression (TTP) was 1.9 years); for patients with 2 or 1 risk factors the corresponding risk was 51% (median TTP 5.1 years) and 25% (median TTP 10 years), respectively (12, 26).
The Spanish model uses multiparametric flow cytometry of bone marrow aspirates to differentiate aberrant from normal plasma cells (27). Plasma cells characteristically express CD138 and intense (bright) CD38. The features of aberrant plasma cells included decreased CD38 expression, expression of CD56, and the absence of CD19 and/or CD45. In 93 smoldering myeloma and 407 MGUS patients, the percentage of phenotypically aberrant plasma cells (aPC) of total bone marrow plasma cells (BMPC) at diagnosis allowed risk stratification of MGUS and smoldering myeloma patient's progression to overt multiple myeloma. In their study, MGUS and smoldering myeloma patients with ≥95% aPCs/BMPC at diagnosis had a significantly higher risk of multiple myeloma progression (27). Furthermore, on multivariate analysis, ≥95% aPCs/BMPC, DNA aneuploidy, and immunoparesis were found to be independent predictors of multiple myeloma progression. More specifically, for MGUS patients with 0, 1, or 2 risk factors (≥95% aPCs/BMPC and DNA aneuploidy) the risk of progression at 5 years was 2%, 10%, and 46%, respectively (Figure 2). For smoldering myeloma patients (risk factors: ≥95% aPCs/BMPC and immunoparesis) the corresponding risks at 5 years were 4%, 46%, and 72%, respectively (Figure 2) (27).
Taken together, these studies emphasize the fact that the risk of multiple myeloma progression varies greatly among individuals diagnosed with myeloma precursor disease. As discussed in detail below, we need better markers to define high-risk (versus low-risk) MGUS/smoldering myeloma and to better predict individual risk of multiple myeloma progression.
From Precursor to Multiple Myeloma: Current Knowledge from The Research Laboratory
Two independent studies have shown that multiple myeloma is consistently preceded by MGUS.(28, 29) In the first study, based on a large cancer screening trial including 77,469 volunteers prospectively followed for up to 10 years in a cancer screening trial, 71 individuals were found to develop multiple myeloma. Using stored pre-diagnostic serum samples obtained annually from these subjects, evidence of MGUS was demonstrated prior to multiple myeloma in all participants. (28) In 82% of multiple myeloma cases, evidence of MGUS was present in pre-diagnostic blood collected ≥8 years prior to multiple myeloma diagnosis.(28) The other study was based on a large U.S. Army serum repository. In brief, that study reported that 27 of 30 multiple myeloma cases with available pre-diagnostic serum samples had evidence of a preceding MGUS diagnosis; the other 3 patients either had IgD multiple myeloma or lacked samples ≤8 years prior to multiple myeloma diagnosis.(29)
Of great relevance from a clinical perspective, in approximately half of the 71 multiple myeloma patients from the large cancer screening trial (28), based on the systematically collected pre-diagnostic blood samples, the M-protein concentration increased annually following initial detection; among the remaining patients, serum M-protein was stable until multiple myeloma diagnosis (30). Similar patterns of gradual evolution and sudden increase prior to diagnosis were also observed in the serum free light chain (FLC)-ratio (around 85% had a skewed FLC ratio 2 years prior to multiple myeloma diagnosis).(28) Importantly, these findings emphasize the fact that clinicians must be alert in monitoring patients for myeloma-related end-organ damage regardless of the stability of serum protein markers.
At the molecular level, based in our current knowledge, transformation from precursor to full-blown multiple myeloma does not appear to be a sudden, discontinuous process with specific immunophenotypic markers differentiating plasma cells in patients with MGUS, smoldering myeloma, and multiple myeloma.(31, 32) Instead, several overlapping oncogenic events within plasma cells and the marrow microenvironment accumulate from normal plasma cells through precursor disease to full-blown multiple myeloma. Indeed, early cytogenetic changes are seen amongst almost all patients at the level of MGUS. (31, 32) These potentially overlapping, enduring changes are seen from MGUS onward and include hyperdiploidy and primary immunoglobulin translocations at the 14q32 locus.(33-35) In both states, Cyclin D dysregulation is a very common early event.(36) Importantly, at this time, MGUS from smoldering myeloma cannot be differentiated using conventional cytogenetics or fluorescent in situ hybridization.(37)
Abnormal plasma cells in MGUS, smoldering myeloma as well as multiple myeloma produce a broad range of immunoreceptors which are stimulated by both exogenous molecules and microenvironmental paracrine signals such as interleukin-6, contributing to the clonal proliferation observed in patients' bone marrow biopsies.(38) In contrast, many secondary oncogenic events have been implicated in the transition from MGUS/smoldering myeloma to full-blown multiple myeloma and from newly diagnosed multiple myeloma to advanced/refractory disease. These secondary genetic events may, in part, be dependent on the primary lesion.(39, 40) Furthermore, complex alterations to microenvironmental interactions occur in the transition from MGUS to multiple myeloma.(41) An apparent manifestation in myeloma-genesis is the interaction between abnormal plasma cells, cells in the bone marrow microenvironment as well as the bone (41), which ultimately lead to characteristic lytic lesions in approximately 80% of multiple myeloma patients.(42) While osteoclastic activation and osteoblastic inactivation leading to lytic lesions is a criterion for progression from MGUS/smoldering myeloma to multiple myeloma, studies using quantitative bone biopsy and levels of biomarkers for bone turnover (e.g. RANK ligand), have revealed excess bone resorption in patients with MGUS. (43-45) Future studies are needed to better define the role of activated osteoclasts in the pathogenesis of multiple myeloma.
Treatment Studies for Smoldering Myeloma: Future Challenges and Opportunities
Based on the International Myeloma Working Group 2010 guidelines, patients diagnosed with MGUS and smoldering myeloma should not be treated outside of clinical trials. (2) Overall, treatment trials for MGUS patients are complicated, as these individuals are relatively healthy and the majority has a low life-time risk of progression, especially when other causes of death are taken into account. (3) Therefore, it seems reasonable to propose that an ideal treatment would be effective, non-toxic, and directed towards patients with a high risk of progression. At this time, we do not have access to any such drug.
In contrast to MGUS, early treatment strategies for smoldering myeloma are particularly attractive, as the rate of progression to multiple myeloma is substantially higher. Prior to the advent of novel therapies, a randomized-controlled trial of melphalan-prednisone given initially versus at progression to multiple myeloma found no difference in response rate or overall survival.(46) Furthermore, a single-arm trial using thalidomide and pamidronate in 76 patients with smoldering myeloma failed to show a clear benefit for treatment, with a quite unexpected shorter time to progression among treatment responders versus non-responders.(47) In their report, the authors speculated that their observation perhaps was reflecting greater drug sensitivity of more aggressive disease. (47) Another randomized trial (zoledronic acid versus surveillance during one year) found reduced skeletal complications at progression from smoldering myeloma to multiple myeloma but without impact on the risk of progression.(48) It is unknown whether a more extended bisphosphonate treatment influences the risk of progression. The study based on zoledronic acid versus surveillance was prematurely stopped by the safety committee due to development of osteonecrosis of the jaw in a smoldering myeloma patient in the treatment arm. (48) Currently, a randomized, multicenter phase III trial for patients with high-risk smoldering myeloma is ongoing in Spain (lenalidomide-dexamethasone versus active surveillance). An interim analysis at 19 months of follow-up shows that about 50% of patients in the surveillance arm experienced progression to multiple myeloma while only 2 patients in the treatment arm had progression.(49) A collaborative ECOG/SWOG study based on lenalidomide versus active surveillance just opened in the U.S. Furthermore, at the NCI in Bethesda, MD, novel treatment strategies (designed to facilitate the patient's own immune system, to achieve anti-myeloma effects) are under development and the first treatment study opened in December 2010. Importantly, it is currently unknown whether treating smoldering myeloma patients improves overall survival and/or quality of life, as such data are not yet available. As stated above, in accord with the International Myeloma Working Group 2010 guidelines, smoldering myeloma patients should not be treated outside of clinical trials. (2)
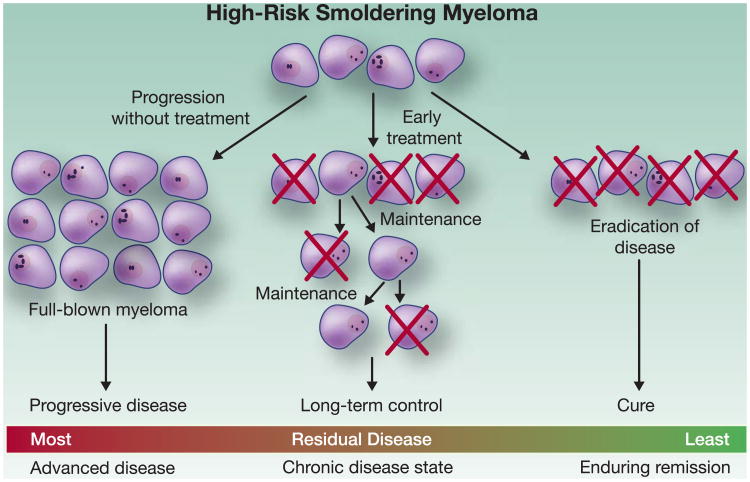
While the above discussed trials underscore the value of ongoing treatment trials for smoldering myeloma patients, one can envision several scenarios resulting from treatment of smoldering myeloma. Aimed at preventing progression, smoldering myeloma could be treated as a chronic disease, with an extended dosing schema used to control the malignant clone (Figure 3). Alternately, in the future, highly active therapy could be used with the goal of cure. However, to responsibly perform any such trial, well-designed correlative studies must be performed to assess for the theoretical possibility of unexpected long-term adverse events or selecting for more aggressive disease (Figure 3).
Figure 3. Treatment Goals in Smoldering Myeloma.
Footnote: Under active surveillance, patients with high-risk SMM have a >84% lifetime risk of progression to full-blown myeloma (52). One can envision several scenarios resulting from treatment of SMM. Aimed at preventing progression, SMM could be treated as a chronic disease, with relatively benign maintenance therapy used to control the malignant clone. Alternately, highly active therapy could be used with the goal of cure, though this may prove challenging in the context of current treatment options. However, to responsibly perform any such trial, well-designed correlative studies should be performed to assess for the theoretical possibility of unexpected long-term adverse events or selecting for more aggressive disease.
Future Directions
In the context of numerous molecular events and heterogeneous risk of progression, developing individualized risk profiles for patients with MGUS and smoldering myeloma represents an ongoing challenge. Clearly, we need future prospective studies based on clinical monitoring and extensive correlative science. The ultimate goal is to develop better molecular markers which will allow (i) clinicians to define high-risk and low-risk precursor patients for a more tailored clinical management, and (ii) to provide insights on the individual patient's disease biology which in turn can be used for targeted and more individualized treatment strategies. In the near future, it seems reasonable to believe that high-risk precursor patients will likely become candidates for early treatment strategies. As discussed in detail in this review paper, future studies need to assess the role of early treatment in relation to overall survival and quality of life (50-52). High response rates among smoldering myeloma patients receiving treatment may not correlate with survival (50, 52). In fact, one may speculate that prolonged “stable disease” may provide key benefit to patients. Currently, the answers to these important questions remain unknown.
Acknowledgments
We thank Irina Maric, M.D., at the National Institutes of Health, Bethesda, Maryland for providing the pictures in Figure 1. We also acknowledge Adam J. Waxman, B.A., at the National Cancer Institute, Bethesda, Maryland for comments and suggestions regarding Figures 2 and 3.
Funding: This work was supported by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health; the grants CA 62242 and CA 107-476-03 from the National Cancer Institute; and the facilities and resources of the Divisions of Hematology, Biostatistics, Clinical Biochemistry and Immunology, and Epidemiology at the Mayo Clinic, Rochester, Minnesota.
Footnotes
Authors' disclosures: None.
References
- 1.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med. 2006;354:1362–9. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Durie BGM, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis of monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–69. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 4.Bida JP, Kyle RA, Therneau TM, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clinic Proceedings. 2009;84:685–93. doi: 10.4065/84.8.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajkumar SV. Prevention of Progression in Monoclonal Gammopathy of Undetermined Significance. Clinical Cancer Research. 2009;15:5606–8. doi: 10.1158/1078-0432.CCR-09-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landgren O, Gridley G, Turesson I, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107:904–6. doi: 10.1182/blood-2005-08-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgren O, Katzmann JA, Hsing AW, et al. Prevalence of Monoclonal Gammopathy of Undetermined Significance Among Men in Ghana. Mayo Clin Proc. 2007;82:1468–73. doi: 10.1016/S0025-6196(11)61089-6. [DOI] [PubMed] [Google Scholar]
- 8.Iwanaga M, Tagawa M, Tsukasaki K, Kamihira S, Tomonaga M. Prevalence of Monoclonal Gammopathy of Undetermined Significance: Study of 52,802 Persons in Nagasaki City, Japan. Mayo Clin Proc. 2007;82:1474–9. doi: 10.1016/S0025-6196(11)61090-2. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Delgado GJ, Ruiz-Arguelles GJ. Genetic Predisposition for Monoclonal Gammopathy of Undetermined Significance. Mayo Clin Proc. 2008;83:601–2. doi: 10.4065/83.5.601-a. [DOI] [PubMed] [Google Scholar]
- 10.Vachon CM, Kyle RA, Therneau TM, et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood. 2009;114:785–90. doi: 10.1182/blood-2008-12-192575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landgren O, Kyle RA, Hoppin JA, et al. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance (MGUS) in the Agricultural Health Study. Blood. 2009;25:6386–91. doi: 10.1182/blood-2009-02-203471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyle RA, Remstein ED, Therneau TM, et al. Clinical Course and Prognosis of Smoldering (Asymptomatic) Multiple Myeloma. N Engl J Med. 2007;356:2582–90. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 13.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a Chronic Disease State in Patients With Smoldering or Indolent Multiple Myeloma by Targeting Interleukin 1Î2-Induced Interleukin 6 Production and the Myeloma Proliferative Component. Mayo Clinic Proceedings. 2009;84:114–22. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyle RA. Monoclonal gammopathy of undetermined significance Natural history in 241 cases. Am J Med. 1978;64:814–26. doi: 10.1016/0002-9343(78)90522-3. [DOI] [PubMed] [Google Scholar]
- 15.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102:3759–64. doi: 10.1182/blood-2003-03-0801. [DOI] [PubMed] [Google Scholar]
- 17.Schuster S, Rajkumar SV, Dispenzieri A, et al. IgM Multiple Myeloma: Disease Definition, Prognosis, and Differentiation from Waldenstrom's Macroglobulinemia. Am J Hematol. 2010 doi: 10.1002/ajh.21845. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–8. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyle R, Therneau T, Dispenzieri A, et al. Idiopathic Bence Jones Proteinuria: Clinical Course and Prognosis. ASH Annual Meeting Abstracts. 2006;108:3493-. [Google Scholar]
- 20.Kyle RA, Maldonado JE, Bayrd ED. Idiopathic Bence Jones proteinuria--a distinct entity? American Journal of Medicine. 1973;55:222–6. doi: 10.1016/0002-9343(73)90172-1. [DOI] [PubMed] [Google Scholar]
- 21.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1,027 patients with newly diagnosed multiple myeloma. Mayo Clinic Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Gobbi PG, Baldini L, Broglia C, et al. Prognostic validation of the international classification of immunoglobulin M gammopathies: a survival advantage for patients with immunoglobulin M monoclonal gammopathy of undetermined significance? Clinical Cancer Research. 2005;11:1786–90. doi: 10.1158/1078-0432.CCR-04-1899. [DOI] [PubMed] [Google Scholar]
- 23.Baldini L, Goldaniga M, Guffanti A, et al. Immunoglobulin M monoclonal gammopathies of undetermined significance and indolent Waldenstrom's macroglobulinemia recognize the same determinants of evolution into symptomatic lymphoid disorders: proposal for a common prognostic scoring system. Journal of Clinical Oncology. 2005;23:4662–8. doi: 10.1200/JCO.2005.06.147. [DOI] [PubMed] [Google Scholar]
- 24.Gertz MA, Ansell SM, Dingli D, et al. Autologous stem cell transplant in 716 patients with multiple myeloma: low treatment-related mortality, feasibility of outpatient transplant, and effect of a multidisciplinary quality initiative. Mayo Clin Proc. 2008;83:1131–8. doi: 10.4065/83.10.1131. [DOI] [PubMed] [Google Scholar]
- 25.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812–7. doi: 10.1182/blood-2005-03-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111:785–9. doi: 10.1182/blood-2007-08-108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Persona E, Vidriales MB, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–92. doi: 10.1182/blood-2007-05-088443. [DOI] [PubMed] [Google Scholar]
- 28.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418–22. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosinol L, Cibeira MT, Montoto S, et al. Monoclonal gammopathy of undetermined significance: predictors of malignant transformation and recognition of an evolving type characterized by a progressive increase in M protein size. Mayo Clin Proc. 2007;82:428–34. doi: 10.4065/82.4.428. [DOI] [PubMed] [Google Scholar]
- 31.Landgren O, Waxman AJ. Multiple myeloma precursor disease. Jama. 304:2397–404. doi: 10.1001/jama.2010.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munshi NC, Avet-Loiseau H. Genomics in multiple myeloma. Clin Cancer Res. 2011;17 doi: 10.1158/1078-0432.CCR-10-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–58. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- 34.Rajkumar SV, Lacy MQ, Kyle RA. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Blood Rev. 2007;21:255–65. doi: 10.1016/j.blre.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Chiecchio L, Protheroe RK, Ibrahim AH, et al. Deletion of chromosome 13 detected by conventional cytogenetics is a critical prognostic factor in myeloma. Leukemia. 2006;20:1610–7. doi: 10.1038/sj.leu.2404304. [DOI] [PubMed] [Google Scholar]
- 36.Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Shaughnessy J., Jr Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106:296–303. doi: 10.1182/blood-2005-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonseca R, Bailey RJ, Ahmann GJ, et al. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 2002;100:1417–24. [PubMed] [Google Scholar]
- 38.Mantovani A, Garlanda C. Inflammation and multiple myeloma: the Toll connection. Leukemia. 2006;20:937–8. doi: 10.1038/sj.leu.2404229. [DOI] [PubMed] [Google Scholar]
- 39.Chng WJ, Glebov O, Bergsagel PL, Kuehl WM. Genetic events in the pathogenesis of multiple myeloma. Best Pract Res Clin Haematol. 2007;20:571–96. doi: 10.1016/j.beha.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Annunziata CM, Davis RE, Demchenko Y, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–30. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balakumaran A, Robey PG, Fedarko N, Landgren O. Bone marrow microenvironment in myelomagenesis: its potential role in early diagnosis. Expert Rev Mol Diagn. 2010;10:465–80. doi: 10.1586/erm.10.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melton LJ, 3rd, Rajkumar SV, Khosla S, Achenbach SJ, Oberg AL, Kyle RA. Fracture risk in monoclonal gammopathy of undetermined significance. J Bone Miner Res. 2004;19:25–30. doi: 10.1359/JBMR.0301212. [DOI] [PubMed] [Google Scholar]
- 43.Bataille R, Chappard D, Basle MF. Quantifiable excess of bone resorption in monoclonal gammopathy is an early symptom of malignancy: a prospective study of 87 bone biopsies. Blood. 1996;87:4762–9. [PubMed] [Google Scholar]
- 44.Politou M, Terpos E, Anagnostopoulos A, et al. Role of receptor activator of nuclear factor-kappa B ligand (RANKL), osteoprotegerin and macrophage protein 1-alpha (MIP-1a) in monoclonal gammopathy of undetermined significance (MGUS) Br J Haematol. 2004;126:686–9. doi: 10.1111/j.1365-2141.2004.05092.x. [DOI] [PubMed] [Google Scholar]
- 45.Raje N, Roodman GD. Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res. 2011;17 doi: 10.1158/1078-0432.CCR-10-1804. [DOI] [PubMed] [Google Scholar]
- 46.Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rodjer S, Westin J. Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I--a randomized study Myeloma Group of Western Sweden. Eur J Haematol. 1993;50:95–102. doi: 10.1111/j.1600-0609.1993.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 47.Barlogie B, van Rhee F, Shaughnessy JD, Jr, et al. Seven-year median time to progression with thalidomide for smoldering myeloma: partial response identifies subset requiring earlier salvage therapy for symptomatic disease. Blood. 2008;112:3122–5. doi: 10.1182/blood-2008-06-164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musto P, Petrucci MT, Bringhen S, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113:1588–95. doi: 10.1002/cncr.23783. [DOI] [PubMed] [Google Scholar]
- 49.Mateos MV, Lopez-Corral L, Hernandez MT, et al. Multicenter, Randomized, Open-Label, Phase III Trial of Lenalidomide-Dexamethasone (Len/dex) Vs Therapeutic Abstention in Smoldering Multiple Myeloma at High Risk of Progression to Symptomatic MM: Results of the First Interim Analysis. Blood (ASH Annual Meeting Abstracts) 2009:114. [Google Scholar]
- 50.Attal M, Palumbo A. Approach to newly diagnosed patients: change in the therapeutic paradigm to include maintenance therapy and new survival data. Clin Cancer Res. 2011;17 doi: 10.1158/1078-0432.CCR-10-1925. [DOI] [PubMed] [Google Scholar]
- 51.Lonial S, Richardson P. Treatment options for relapsed and refractory multiple myeloma. Clin Cancer Res. 2011;17 doi: 10.1158/1078-0432.CCR-10-1805. [DOI] [PubMed] [Google Scholar]
- 52.Waxman AJ, Kuehl WM, Balakumaran A, Weiss B, Landgren O. Smoldering (Asymptomatic) Multiple Myeloma: Revisiting the Clinical Dilemma and Looking into the Future. Clinical Lymphoma, Myeloma & Leukemia. 2010;10:248–57. doi: 10.3816/CLML.2010.n.053. [DOI] [PMC free article] [PubMed] [Google Scholar]