Abstract
Polyphenols are a widely used class of compounds in dermatology. While phenol itself, the most basic member of the phenol family, is chemically synthesized, most polyphenolic compounds are found in plants and form part of their defense mechanism against decomposition. Polyphenolic compounds, which include phenolic acids, flavonoids, stilbenes, and lignans, play an integral role in preventing the attack on plants by bacteria and fungi, as well as serving as cross-links in plant polymers. There is also mounting evidence that polyphenolic compounds play an important role in human health as well. One of the most important benefits, which puts them in the spotlight of current studies, is their antitumor profile. Some of these polyphenolic compounds have already presented promising results in either in vitro or in vivo studies for non-melanoma skin cancer and melanoma. These compounds act on several biomolecular pathways including cell division cycle arrest, autophagy, and apoptosis. Indeed, such natural compounds may be of potential for both preventive and therapeutic fields of cancer. This review evaluates the existing scientific literature in order to provide support for new research opportunities using polyphenolic compounds in oncodermatology.
1. Introduction
Polyphenolic compounds are found in numerous plants and vegetables and are part of the human diet [1]. Both plants and fungi (mushrooms) are considered among the most important sources of polyphenolic compounds [2]. Polyphenolic compounds are rapidly metabolized in the human body [3–5], circulating as their conjugated forms such as glucuronide, methylated, and sulphated derivatives [1]. Several polyphenolic compounds have a very well known anti-angiogenic profile [6–8], mainly because of their anti-inflammatory, antimicrobial, antitumor, and antioxidant properties [1, 9–11]. Rapid metabolism and poor absorption have limited the therapeutic potential of polyphenolic compounds up to now.
Phenol, a mono-phenyl ring bonded to a hydroxyl group, is the most important representative of the phenolic compounds. It is considered a kerato-coagulant agent at a concentration of 88 % [12, 13], with similar effects to a 70 % trichloroacetic acid (TCA) peel [12], or CO2 non-fractional laser [13]. Nonetheless, its skin penetration can be decreased by alcohol; for example, applying poly-ethylene glycol 400, or 70 % isopropanol diluted with water [14]. Skin treated with topical application of phenol presents with immediate vascular coagulation, keratin epidermal coagulation, and injury to the upper reticular dermis, which is why it is commonly used for deep-tissue peels in cosmetic dermatology [12]. In addition, phenol decreases melanogenesis [15], as a peroxidase inhibitor, reducing the polymerization of melanogenic intermediates [16, 17]. Furthermore, it decreases melanocyte proliferation [17].
Although phenol has not been used specifically against skin cancer, many other polyphenolic compounds show potential in skin cancer therapy. We started our review of the literature at 1973, exploring PubMed manuscripts that had as describers the name of the polyphenolic compound in ‘Title’, and the words ‘skin cancer’, ‘skin’, ‘cancer’, ‘dermatology’, ‘cutaneous’, ‘neoplasia’, and ‘tumor’ in ‘Title/Abstract’. Moreover, we also used some iconic books and international standards as additional references. Based on the results obtained from this search, we explored polyphenolic compounds in detail, evaluating their merits as possible agents in dermatologic oncology.
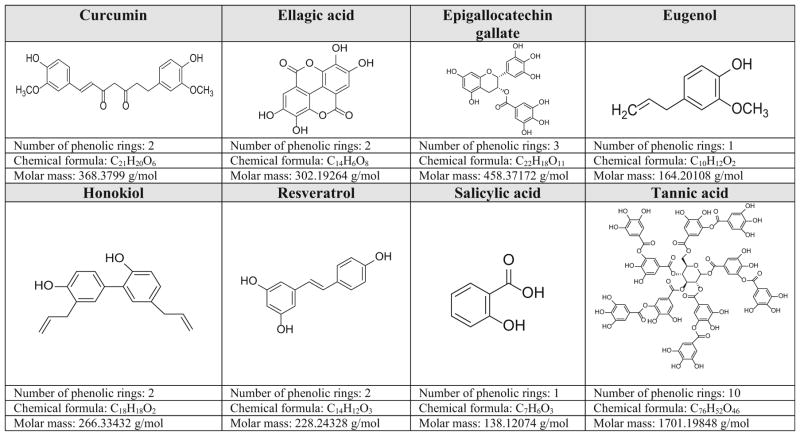
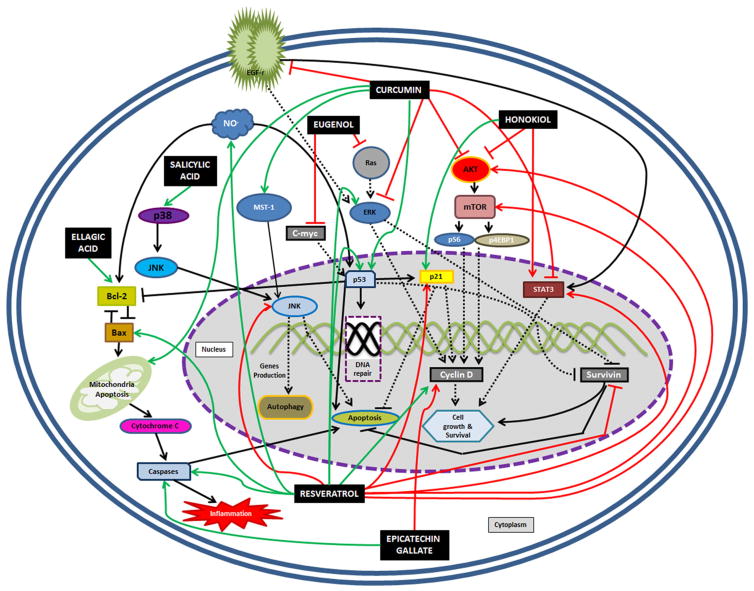
All polyphenolic compounds studied in this manuscript are described in alphabetical order and their chemical structures and characteristics are summarized in Fig. 1 [18–25]. A summary of mechanisms of action of these polyphenolic compounds can be found either in Table 1 or Fig. 2, which are complementary to each other.
Fig. 1.
Chemical overview of the phenolic compounds explored in this manuscript [18–25]
Table 1.
Chemically dependent biomolecular pathways changed by some polyphenolic compounds, as a complement to Fig. 2
| Name of the polyphenolic compound | Anti-oncogenic mechanisms of action in skin cancer |
|---|---|
| Curcumin | Inhibition of p-4EBP1 [48, 49] Decreases 12-O-tetradecanoylphorbol-13-acetate-induced protein kinase C translocation and inhibits IGF-1- carcinogenic signaling [48–53] Decreasing of UVB-induced thymine dimer-positive cells, expression of PCNA, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling, and apoptotic sunburn cells [54] Inhibition of PGE-2, NO, and COX-2 NF-κB [54–56] Inhibition of ROS production and toxic membrane UVA-induced damage [56] Stimulates cytotoxic T-lymphocyte response downregulating myeloid-derived suppressor cells, regulatory T cells, and IL-6 and chemokine ligand-2 [65] Increases TNF-α, IFN-γ, and CD8+ T-cell population [65, 66] |
| Ellagic acid | Promotion of G1 cell cycle arrest, increasing of apoptosis and decreasing of IL-1β, IL-6, IL-8, IL-10, and NF-κβ [94, 100, 101] Reduction of MCP-1, and TNF-α [101] Expression of ICAM-1-UVB-dependent [100] Expression of superoxide dismutase and heme-oxygenase 1 [105] Inhibition of apoptosis: prevention of DNA fragmentation and decreasing of both mitrochondria impairment and endoplasmatic reticulum stress [105] |
| Epicatechin gallate | Suppression of UVA-mediated release of labile iron [117] Suppression of cyclin D1 [121] Induction of caspase-3 activity [121, 122] Inhibition of ribonuclease-A by copper complexes [123] |
| Honokiol | Induces cycle G0/G1 cell arrest [157] Inhibition of ROS-dependent NF-κB [158] Decreasing of ATP pool [153] Decreasing of Notch signaling [154, 156] Inhibition on the UVB-induced expression of COX-2, PGE2, proliferating cell nuclear antigen, and some pro-inflammatory cytokines [137] Inhibition of Ki-67 expression and an increase in TUNEL-positive cells [160] |
| Resveratrol | Stimulates Nrf2 [181–184] Produces glutathione and superoxide dismutase, decreases maloaldehyde content [182–184] Stimulates AMPK-FOXO3 [185] Downregulates Rictor [190] Stimulation of TLR-4 pathway [192] Blocking of IκB kinase activity [193, 194] Activation of MAPKs and AP-1 pathway [194, 195] Increases Apaf-1 [196, 197] Decreases the expression of extracellular signal-regulated kinase 1/2 and p38 [198] Inhibition of accumulation of hypoxia-inducible factor-1α and VEGF expression [204] Blockage of NF-κB pathway [216] Cell survival prolongation: reduces ROS formation, leads to PARP cleavage, and induces autophagy [217] Accumulation of ceramide and autophagy [232] Inhibition of APE/Ref-1, with decreasing of Ap-1/JunD, MMP-1, and iNOS, α-MSH [227, 230, 231] Reduction of AMP-activated protein kinase, COX-2, vasodilator-stimulated phosphoprotein, and VEGF [211, 233] Increasing the expression of thrombospondin-1 with decreasing of the VEGF-sensitive hypoxia inducible factor-1α [42] Inhibition of lipopolysaccharide-induced epithelian-mesenchymal transition of IL-8 [235] Inhibition of NO production in VEGF-stimulated endothelial cells and of COX-2 induction [236] |
| Salicylic acid | Intracellular depletion of gluthatione [237] |
| Tannic acid | Downregulation of UVB-induced IL-18 [261] |
AMP adenosine monophosphate, AMPK-FOXO3 5′AMP-activated protein kinase/forkhead box protein O3, AP-1 activator protein-1, AP-1/JunD activator protein-1/JunD protein, Apaf-1 apoptotic protease-activating factor-1, APE/Ref-1 apurinic/apyrimidinic endonuclease/redox effector factor-1; ATP adenosine triphosphate, CD8+ T-cell cytotoxic T cell CD8 positive, COX-2 cyclooxygenase-2, COX-2 NF-κB cyclooxygenase-2 expression through nuclear factor-kappa B, DNA deoxyribonucleic acid, dUTP 2′-deoxyuridine 5′-triphosphate, G1 Gap 1, ICAM-1 intercellular adhesion molecule-1, IFN-γ interferon gamma, IGF-1 insulin growth factor-1, IL-1β interleukin-1 beta, IL-6 interleukin-6, IL-8 interleukin-8, IL-18 interleukin-18, iNOS inducible nitric oxide synthase, Ki-67 protein Ki-67, MAPK mitogen-activated protein kinase, MCP-1 monocyte chemoattractant protein-1, MMP-1 metalloproteinase-1, α-MSH alpha-melanocyte-stimulating hormone, NF-κβ nuclear factor–kappa B, NO nitric oxide, Nrf2 nuclear factor-like 2, p-4EBP1 phosphorylation of eukaryotic translation initiation factor 4E binding protein-1, p38 protein p38, PARP poly ADP (adenosine diphosphate) ribose polymerase, PCNA proliferation cell nuclear antigen, PGE-2 prostaglandin E2, ROS reactive oxygen species, TLR-4 toll-like receptor-4, TNF-α tumor necrosis factor alpha, TUNEL terminal dUTP nick end labeling, UVA ultraviolet A, UVB ultraviolet B, VEGF vascular endothelial growth factor
Fig. 2.
Protein-dependent biomolecular pathways changed by some polyphenolic compounds, as a complement to the information in Table 1. Solid black arrow indicates physiological stimulation, solid black line with an end block indicates physiological blockage, black dotted arrow indicates physiological stimulation with intermediate pathways that weren’t studied in this manuscript, black dotted line with an end block indicates pathway blocked by the polyphenolic compound, solid green arrow indicates pathway stimulated by the polyphenolic compound, solid red arrow indicates pathway downregulated by the polyphenolic compound, solid red line with an end block indicates pathway blocked by the polyphenolic compound, red dotted line with an end block indicates physiological block with intermediate pathways that weren’t studied in this manuscript. AKT protein kinase B (also named PKB), Bax Bcl-2-associated X protein, Bcl-2 B-cell lymphoma 2, C-myc v-myc avian myelocytomatosis viral oncogene homolog, EGF-r epidermal growth factor receptor, ERK extracellular signal-regulated kinase, iNO inducible nitric oxide, JNK Jun amino-terminal kinases, MST-1 macrophage stimulating 1 protein, mTOR mechanistic target of rapamycin, NO nitric oxide, p-4EBP1 phosphorylation of eukaryotic translation initiation factor 4E binding protein-1, p21 protein p21, p38 protein p38, p53 protein p53, pS6 protein S6, Ras ‘rat sarcoma’ protein family, STAT-3 signal transducer and activator of transcription 3
2. Curcumin
Curcumin is a natural plant polyphenol pigment found in Curcuma longa [26]. Obtained from the turmeric rhizome, curcumin is widely used in traditional Chinese medicine [27] as an anti-inflammatory, antioxidant, anticancer, antimicrobial, cosmetic depigmenting agent and wound healing agent [28–31], and for the treatment of psoriasis [32–34].
However, curcumin has poor systemic bioavailability when administered orally [35]; it is poorly insoluble in water [36], and suffers first-pass metabolism in the liver [37–39]. This has stimulated its use via transdermal application [40], since topical use of curcumin is considered as effective as systemic use [40, 41], including when dissolved into modern topical vehicles [41, 42]. Modern topical vehicles can protect curcumin against ultraviolet B (UVB) degradation; these novel topical formulations include ethyl cellulose and/or methyl cellulose nanoparticles [43, 44], β-cyclodextrin-curcumin-nanoparticle complexes [41], curcumin-loaded soybean phospholipid liposomes [42], curcumin-encapsulated chitosan-bioglass [45], lipid vesicle-propylene glycol liposomes [46], and curcumin-loaded chitin nanogels [47].
Curcumin and some of its derivatives, such a 1,3-bis-(2-substituted-phenyl)-propane-1,3-dione [48], are promising for therapy against non-melanoma skin cancer. In animal models, topical and/or systemic curcumin reduced the size of human squamous cell carcinoma SRB12-p9 by inhibiting phosphorylated protein kinase B (p-AKT), protein S6 (pS6), phosphorylation of eukaryotic translation initiation factor 4E binding protein-1 (p-4EBP1), phosphorylated-signal transducer and activator of transcription 3 (p-STAT-3), and phosphorylated-extracellular signal-regulated kinase 1/2 (p-ERK1/2) in vitro, as well as p-STAT-3 and p-ERK1/2 activation of p-ERK and pS6 in vivo [48, 49]. These actions can be enhanced because curcumin is a selective and non-competitive phosphory-lase-kinase agent, helping to decrease 12-O-tetrade-canoylphorbol-13-acetate-induced protein kinase C translocation [50–52] and because curcumin inhibits the insulin growth factor-1 (IGF-1)-carcinogenic signaling as well [53]. The effect of curcumin on signal transducer and activator of transcription 3 (STAT-3) seems to be important for preventing growth of A341 melanoma cells as well [26] (Table 1; Fig. 2).
In an animal study, curcumin (10 mmol/200 mL acetone) was topically applied 30 minutes prior to UVB (180 mJ/cm2) irradiation [54]: curcumin decreased UVB-induced thymine dimer-positive cells, expression of proliferation cell nuclear antigen (PCNA), terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate (dUTP) nick end labeling, apoptotic sunburn cells, and increased protein p53 (p53) and cyclin-dependent kinase inhibitor 1A (Cip/p21)-positive cells. Such findings were associated with inhibition of prostaglandin E2 (PGE-2) and nitric oxide (NO) [54], as well as the cyclooxygenase-2 (COX-2) expression through nuclear factor-kappa B (NF-κB) [54, 55]. By blocking NF-κB, curcumin inhibits T-cell response, and both epidermal and fibroblast proliferation [50] (Table 1).
Under ultraviolet A (UVA) or visible light exposure, curcumin inhibits keratinocyte proliferation, enhances the release of cytochrome C, and inhibits NF-κB [56]. Furthermore, under UVA radiation, curcumin inhibits induction of reactive oxygen species (ROS) and toxic membrane UVA-induced damage, preventing increased production of apoptotic bodies and activated caspases, while inhibiting protein kinase B (AKT), ERK1/2, and epidermal growth factor receptor (EGF-r) [56] (Table 1).
In melanoma cell studies, curcumin and analogs [57, 58] reduce proliferation, migration, and invasion of melanoma and induce apoptosis [59–62]. Curcumin’s lipophilic profile has encouraged its use in transdermal devices [63]; moreover, when curcumin is used topically, visible light enhances the anti-melanoma effects of curcumin, implying a potential photodynamic effect [64].
When curcumin is administered intravenously concurrently with an anti-melanoma vaccine, it boosts in vivo cytotoxic T-lymphocyte response, downregulates levels of some immunosuppressive factors, such as myeloid-derived suppressor cells, regulatory T cells, interleukin-6 (IL-6) and chemokine ligand-2, and increases levels of some proinflammatory cytokines [65], including tumor necrosis factor-α (TNF-α) [65, 66] and interferon gamma (IFN-γ) [65], as well as resulting in an elevation of the cytotoxic T cell CD8-positive (CD8+ T-cell) population [65, 66] (Table 1).
Curcumin leads to apoptosis of melanoma in a mitochondrial-caspase-dependent pathway [67, 68], since curcumin is also associated with such organelle permeability transition pore opening [69]. Such an apoptotic scenario involves the activation of macrophage stimulating 1 protein (MST-1), which activates Jun amino-terminal kinases (JNK) and forkhead box protein O3 (FOXO3) nuclear translocation [70], as well as downregulation of NF-κβ [71–73]. Furthermore, decreased pulmonary tumor formation has been seen in a murine model of experimental metastatic melanoma using orally administered curcumin, which was associated with significantly decreased expression of metalloproteinases [74, 75]; this mechanism could be one of the reasons why curcumin sensitizes melanoma cells to tamoxifen [76] (Table 1; Fig. 2).
3. Ellagic Acid
Ellagic acid is found in different fruits such as berries, pomegranates, and nuts, and is a popular dietary supplement [77]. Experiments have already shown that ellagic acid has antifibrotic, antiproliferative, antibiofilm, and antitumorigenic properties [77–84]. Ellagic acid is a potent polyphenolic agent, despite its poor solubility and permeability [85].
Ellagic acid is metabolized by gut microbiota to yield urolithins [86–88]. There are three human phenotypes for urolithin production: phenotype A, in 25–85 % of the population that produces only urolithin A conjugates; phenotype B, in 10–50 % of the population that produces isourolithin A and/or urolithin B in addition to urolithin A and is associated with gut microbial imbalance (i.e., dysbiosis in which there is also an increased risk of chronic illness such as metabolic syndrome) or colorectal cancer; and phenotype 0, in 5–25 % of the population in which no urolithins are observed [89]. The systemic anti-inflammatory role of ellagic acid [90–92] seems to stem mainly from its metabolite, urolithin-A; it inhibits activation of NF-κB and mitogen-activated protein kinase (MAPK), downregulates COX-2 and membrane-associated PGE synthase 1 (mPGES-1) expression, reducing PGE-2 production [92].
Potential dermatologic uses of ellagic acid are suggested by its in vitro anti-proliferative effect against melanoma cells [93, 94]. Ellagic acid promotes gap 1 (G1) cell cycle arrest, increases apoptosis, and decreases synthesis of IL-1β, IL-8, and NF-κβ [94]. Topical [95] and systemic use of ellagic acid in animals [96, 97] inhibits epidermal tumor generation caused by some environmental tumorigenesis chemicals such as 12-O-tetradecanoyl-phorbol-13-acetate (TPA) [95], 3-methylcholantrene [96, 97], or benzo[a]-pyrene and (±)-7β, 8α-dihydroxy-9 α,10 α-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene [98]. Ellagic acid also acts on oral carcinoma cells [99] (Table 1).
Ellagic acid reduces in vitro UVB-induced expression of IL-1β [100], IL-6 [100, 101], IL-8, monocyte chemoattractant protein-1 (MCP-1), and TNF-α [101], increases anti-UVB protective IL-10 expression [101], and inhibits intercellular adhesion molecule-1 (ICAM-1)-UVB-dependent expression [100] in keratinocytes. Ellagic acid also helps UVB-exposed fibroblast to block metalloproteinase (MMP) secretion and collagen degradation, leading to less wrinkles and thickness [100]. A 4-week oral supplementation with 100–200 mg/day of ellagic acid produced a slight increase in the minimum erythema dose value caused by UV exposure [102], since it is shown to inhibit tyrosinase activity [103], which means it may also be useful as an anti-melasma agent [104] (Table 1).
Finally, ellagic acid-treated HaCaT cells show increased viability and decreased ROS generation after UVA exposure, with a higher expression of superoxide dismutase, and hemeoxygenase 1. These actions may prevent deoxyribonucleic acid (DNA) fragmentation, decrease mitochondrial impairment, activate caspase-3, prevent endoplasmatic reticulum stress, and deregulate B-cell lymphoma 2(BCL2)/BCL2-associated X protein (Bcl/Bax) pathway [105] (Table 1; Fig. 2).
4. Epicatechin Gallate
Epicatechin gallate, or (2)2-epigallocatechin-3-gallate, is a catechin derived from Camellia sinensis [106] and has anti-inflammatory, antioxidative, antimutagenic, anticarcinogenic, apoptotic [106–110], and wound-healing properties [111–113], among others. Nonetheless, paradoxically, at high doses, epicatechin gallate may stimulate hypoxia-inducible factor 1-alpha (HIF1-α) and tumor cell survival [114].
Green tea is one of the most important sources of epicatechin gallate, as well as for other catechins, such as (2)2-epicatechin gallate (ECG), (2)2-epicatechin (EC), and (2)2-epigallocatechin [106]. In fact, epicatechin gallate is the most abundant polyphenolic compound found in green tea [107, 108], albeit final biological green tea effects likely result from a combination of catechins [115].
Epicatechin gallate protects HaCaT keratinocytes against UVB- and UVA-induced oxidative damage [116–119]. A potential mechanism is that epicatechin gallate suppresses UVA-mediated release of labile iron, since it contributes to lysosomal protection, avoids protease release, and blocks ferritin degradation [117]. This prevents iron-mediated fibroblast and keratinocyte apoptosis [120]. In head and neck squamous cell carcinoma, epicatechin gallate leads to suppression of cyclin D1 [121], induces caspase-3 activity [121, 122], and inhibits ribonuclease-A by copper complexes [123]. However, further studies need to be done with epicatechin gallate and melanoma (Table 1; Fig. 2).
5. Eugenol
Eugenol, also named 4-allyl-2-methoxyphenol, a component of cloves or Syzygium aromaticum [124], is a polyphenolic compound also obtained from other aromatic plants, such as basil, cinnamon, and bay leaves [125, 126]. This polyphenolic compound has anti-fungal activity [127]. Eugenol can be used in perfumes and fragrance-based creams [128, 129]; however, it is considered an important sensitizer [127–130].
Some studies have shown that eugenol has anti-neoplastic activity, since eugenol suppresses mutagenicity caused by furylfuramide, 4-nitroquinoline N-oxide, and aflatoxin B in Salmonella typhimurium [131–135]; additionally, it modulates enzymatic detoxification by inhibiting 7,12-dimethyl-benz[a]anthracene (DMBA)-induced DNA damage [133, 134]. Furthermore, when mice receive oral supplementation with eugenol, it restricts croton oil-induced skin carcinogenesis through attenuation of v-myc avian myelocy-tomatosis viral oncogene homolog (c-Myc), human-‘rat sarcoma’ protein family (H-Ras), and modification of some p53-associated gene expression [136] (Table 1; Fig. 2).
6. Honokiol
Honokiol is a bioactive, biphenolic constituent found in the leaves and bark of Magnolia officinalis and other plants of the genus Magnolia [137–139]. Honokiol use comes from the traditional Japanese medicine ‘Saiboku-to’, which has been used for a long time as an anxiolytic, antithrombotic, antidepressant, and antibacterial compound [140]. Moreover, honokiol has also demonstrated antibacterial, anti-inflammatory, antifungicidal, antioxidative, anticarcinogenic [139–147], and antiviral activities [148–151], besides ameliorating pre-existing cardiac hypertrophy [152].
At a glance, as an anticarcinogenic agent in melanoma, honokiol inhibits proliferation, viability, and clonogenicity, induces autophagy, inhibits melanosphere formation, and controls cell size in a dose-dependent manner [153, 154]. However, several other additional mechanisms of action of honokiol have been described.
First, honokiol increases cytosolic cytochrome C in cytoplasm, resulting in increased caspase activity, and increased mitochondrial depolarization [137, 138, 153, 155–157]. Second, honokiol directly inhibits ROS-dependent NF-κB via scavenging superoxide and peroxy radicals [158]. Third, honokiol antagonizes AKT via increased phosphorylation/activation of adenosine monophosphate kinase (AMPK). Fourth, honokiol decreases the cellular adenosine triphosphate (ATP) pool in a dose- and time-dependent manner [153]. Fifth, honokiol targets Notch signaling [154, 156], a kind of single transmembrane receptor family, by suppressing the expression of astrocyte and endothelial cell expression of TNF-α converting enzyme (TACE or ADAM 17) [154], and γ-secretase complex proteins in cells [154, 156], reducing stem cell markers such as cytotoxic T cell CD271-positive (CD271), cytotoxic T cell CD166-positive (CD166), Jumonji/ARID Domain-Containing Protein 1B (Jarid1b), and ATP-binding cassette sub-family B member 5 (ABCB5), thus reducing the cleaved Notch-2 expression, which is involved in stem cell self-renewal [154] (Table 1; Fig. 2).
In vivo experiments also support the in vitro activity of honokiol against both melanoma and non-melanoma skin cancer [137, 138, 157, 158]. Honokiol demonstrates a dose-dependent effect with topical usage, reducing volume and tumor growth, either before or after UVB radiation [138, 157], possibly through inhibition of COX-2, PGE-2, PCNA, and pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), further inhibiting cyclins D1, D2, and E, and phosphatidylinositol 3-kinase (which decreases phosphorylation of AKT at Ser473) and of associated cyclin-dependent kinases (CDK) 2, CDK4, and CDK6, and consequently upregulating Cip/p21 and CDK inhibitor 1B (Kip/p27) expression [137] (Table 1).
In non-melanoma skin cancer, honokiol downregulates the expression of cyclin D1, D2, CDK2, CDK4, and CDK6, upregulating the expression of the CDK inhibitor proteins p21 and p27 in human epidermoid squamous carcinoma, inducing apoptosis and DNA fragmentation, gap 0/gap1 (G0/G1) cell cycle arrest, and decreasing the percentage of cells in the synthesis (S) and gap2/mitosis (G2/M) phases [157]. The activity of honokiol against skin cancer can be increased when associated with α-santolol—an active component of sandalwood oil—and magnolol [159] (Table 1).
Honokiol also inhibits protein Ki-67 expression and increases terminal deoxyuridine triphosphate nick end labeling (TUNEL)-positive cells, by inducing mitochondria-dependent and death receptor-mediated apoptosis, secondary to inhibition of EGF-r/STAT-3 signaling and downregulation of STAT-3 target genes, helping paclitaxel to improve its chemotherapy capability [160]. Additionally, honokiol inhibits other EGF-r signaling, as a downstream inhibition of MAPK, AKT, and expression of STAT-3 target genes, such as B-cell lymphoma-extra large (Bcl-XL) and cyclin D1 [161]. These two main actions synergize with paclitaxel and erlotinib chemotherapy [160, 161] (Table 1; Fig. 2).
Finally, honokiol induces endoplasmatic reticulum stress and unfolded protein response in neuroectodermal tumor cells lines, such as melanoma, glioblastoma, neuroblastoma, and angiosarcoma, after it binds and activates glucose regulated protein 78 (grp78) [159].
7. Resveratrol
Resveratrol is a polyphenolic compound found in grapes, nuts, and berries [162] that increases mitochondrial biogenesis, acting as an anti-inflammatory, anti-diabetic, and anti-cancer compound [163], skin-depigmenting agent [162, 164, 165], anti-psoriatic [166], and wound-healing agent [167], among other properties.
In normal keratinocytes, resveratrol inhibits their proliferation [164] by downregulating aquaporin 3 via a sirtuin-1/aryl hydrocarbon receptor nuclear translocator/extracellular signal-regulated kinase (SIRT1/ARN/ERK)-dependent pathway [165], being cytotoxic at high concentrations [164]. Moreover, it has anti-inflammatory effects on macrophage, adipocyte, cultured adipocytes, and adipose tissue [162], as well as down-regulating tyrosinase [168] on melanocytes [166–171].
Using Franz vertical diffusion with porcine ear skin as a biological membrane, resveratrol was shown to improve sunscreen safety when associated with beta-carotene, by reducing by 63 % the delivery of octocrylene, octyl methoxycinnamate, avobenzone, and bemotrizinole into the stratum corneum and viable epidermis [172]. New delivery systems can enhance its epidermal penetration [173–178].
Resveratrol acts as a potent antioxidant. In a cigarette-based in vitro keratinocyte model, resveratrol shows an important antioxidative action [179, 180], stimulating the nuclear factor-like 2 (Nrf2) pathway by decreasing the Nrf2 repressor Kelch-like ECH-associated protein 1 (Keap1) protein [181], allowing translocation of Nrf2 to the nucleus, producing glutathione [182–184] and super-oxide dismutase [182], which protects the dermal–epidermal junction in full-thickness reconstructed human skin [183, 184]. In addition, resveratrol stimulates the 5′ adenosine monophosphate (AMP)-activated protein kinase/forkhead box protein O3 (AMPK-FOXO3) cascade [185] and dose-dependently reduces intracellular hydrogen peroxide in human fibroblasts [186]. By decreasing malonaldehyde content in HaCaT cells under UVA irradiation [182], resveratrol may be useful as an alternative approach for non-melanoma skin cancers [187, 188] (Table 1).
In skin squamous cell carcinoma, resveratrol increases apoptosis by upregulating expression of p53 and ERK and downregulating expression of survivin (SVV), the strongest inhibitor of apoptosis protein (IAP) family members [189]. By downregulating the complex known as the regulatory associated protein of mTOR (RPTOR)-independent companion of mechanistic target of rapamycin (MTOR), or simply RICTOR, which is an mTOR complex 2 (mTORC2) component, resveratrol promotes premature senescence [190]; and by inhibiting autophagy, it enhances apoptosis [191] (Table 1; Fig. 2).
Resveratrol also interacts with the toll-like receptor 4 (TLR-4)-dependent anti-tumor pathway. In this case, resveratrol acts as an antiangiogenic factor, as well as enhancing IFN-γ and IL-12 cell-mediated immune response [192]. Furthermore, resveratrol inhibits phorbol ester-induced expression of COX-2, since resveratrol either blocks IκB kinase (IKKα) activity (and activation of NF-κB) [193, 194], or activates MAPK and the activator protein-1 (AP-1) pathway [194, 195] (Table 1).
With regards to using chemical agents as tumor enhancers, topical use of resveratrol before 7,12-dimethyl benz(a)anthracene topical application reduces tumorigenesis in mice, since it increases p53 and Bax expression [196, 197], decreases Bcl-2 and SVV expression [198], releases cytochrome C [191, 196], activates caspases-3 and -9 [196, 197], increases apoptotic protease-activating factor-1 (Apaf-1) [196, 197], and regulates phosphatidylinositol-3-kinase (PI3K)/AKT proteins [196]. All those findings show that resveratrol uses pro-apoptotic mitochondrial pathways [196, 197, 199]. Finally, if chemically induced tumor cells are infected with Epstein-Barr virus, resveratrol acts as an anti-oxidant and pro-cytotoxic chemopreventive agent [200] (Table 1; Fig. 2).
As with topical use [172], a systemic combination of resveratrol with other compounds may be useful in chemo-preventive and therapeutic strategies. When combined with black tea polyphenol, resveratrol decreases the expression of phosphorylated MAPK family proteins, such as ERK1/2, JNK1/2, and protein p38 (p38), increases the total amount of p53 and phospho-p53 (Ser 15) in skin tissue and tumor, and decreases expression of PCNA [198]. These activities enhance the combination with ursolic acid to enhance its anti-cancer effect in skin carcinoma cells [201] (Fig. 2).
Since resveratrol can cause DNA damage to squamous cell carcinoma in the head and neck area [202], causing p53-dependent apoptosis [203], and inhibiting accumulation of hypoxia-inducible factor-1α and vascular endothelial growth factor (VEGF) expression [204], resveratrol can be used as either a chemopreventive agent [205, 206] or a therapeutic one, improving cytotoxic irradiation-based therapy, by decreasing cyclin B, cyclin D, CDK2, CDK4, and the anti-apoptotic molecule Fas-associated protein with death domain-like IL-1β-converting enzyme-inhibitory protein (FLIP), Bcl-2, and SVV [178, 207–209]. Resveratrol may also improve the cytotoxicity of 5-fluorouracil [210, 211] (Table 1; Fig. 2).
Resveratrol is also photoprotective against either UVB-or UVA-induced responses in keratinocytes [212]. In a UVB-induced skin cancer mouse model, SVV seems to be decreased in either pre- or post-topical treatment with resveratrol [213, 214]; further, if resveratrol is applied before, it modulates the expression and function of cell cycle regulatory proteins cyclin D1 and D2, as well as CDK2, CDK4 and CDK6, and WAF1/p21, perhaps associated with inhibition of the MAPK pathway [215]. Resveratrol also blocks, in a dose-dependent manner, the UVB-mediated NF-κB pathway, through phosphorylation and degradation of IκBα and activation of IKKα [216]. In association with these findings, resveratrol prolongs cell survival, once it reduces ROS formation, inhibits caspase-8, leads to poly ADP (adenosine diphosphate) ribose polymerase (PARP) cleavage, and induces autophagy [217]. For this reason, it could be useful for preventing UVB-mediated damage [187], and enhancing the response to radiation therapy against inflammatory and premalignant conditions [218]. Furthermore, Resveratrol leads UVA-damaged keratinocytes to apoptosis by producing oxidative stress in mitochondria, which increases the organelle permeability transition pores [219] (Table 1; Fig. 2).
Finally, resveratrol has been shown to be a pro-apoptotic compound against melanoma [219], inducing apoptosis once it induces G1/S cell cycle arrest [27, 220–223], through expression of dihydronicotinamide riboside quinone reductase 2 and p53 [221], upregulation of cyclins A, E, and B1 [223], suppression of STAT-3/β-catenin-pathway [224], and production of NO, which stimulates p53 and Bax-Bcl2 mitochondrial apoptosis [225, 226]. Subsequently, cytochrome C, and caspase-9 and -3 are released [227, 228]. Furthermore, apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1), a protein enrolled in tumorigenesis, is inhibited [226, 229], resulting in a coordinated decrease in AP-1/JunD protein, MMP-1 and inducible NO (iNO) protein levels [226]. It reduces tumor invasiveness by inhibition of α-melanocyte-stimulating hormone (α-MSH) expression [230]. Furthermore, autophagy is also observed through ceramide accumulation and AKT/mTOR inhibition [231] (Table 1; Fig. 2).
As an oral supplementation, resveratrol downregulates and inactivates AKT, an anti-apoptotic protein, reducing primary tumor volume, AKT expression, and the propensity for metastasis in animals [232]. Resveratrol also decreases AMP-activated protein kinase, COX-2, vasodilator-stimulated phosphoprotein [232], VEGF [211, 232] and its VEGF-sensitive HIF1-α, and increases expression of anti-angiogenic factors thrombospondin-1 and p53 [42]. Those anti-angiogenic metastatic pathways inhibit liver melanoma metastasis [233] by inhibiting lipopolysaccharide-induced epithelian-mesenchymal transition of IL-8 [234], VEGF-stimulated NO production, and basic fibroblast growth factor (bFGF)-stimulated COX-2 induction in fibroblasts [235] (Table 1; Fig. 2).
8. Salicylic Acid
Salicylic acid, or ortho-hydroxybenzoic acid, is a beta-hydroxy acid [236]. It is found in the bark of the willow tree, Salix alba, and has been used for skin disorders for over 2000 years [237]. Salicylic acid is also described as having keratolytic, bacteriostatic, fungicidal, and photo-protective properties [238].
Under topical use, salicylic acid reduces skin sensitivity in solar-simulated radiation, reducing erythema, DNA damage, and sunburn cell formation [236], increasing cutaneous minimum phototoxic dose and minimum erythema dose prior to UVA [239] and UVB [240] exposures. This explains why salicylic acid interferes with skin cancer development in animal models [241] and treats actinic keratosis, alone and in combination with 0.5 % 5-fluorouracil [242–245].
Salicylic acid is metabolized by tyrosinase [246, 247]. This process seems to be the cause for suppressing the proliferation of B16 and SK-28 human melanoma cells at a plasma-attainable and nontoxic level, probably because it induces the activation of p38 and JNK MAPKs, being synergistic with 1,3-bis(2-chloroethyl)-1-nitrosourea [248] (Fig. 2).
Finally, salicylic acid significantly depletes intracellular glutathione in melanoma cell lines, as well as ROS formation, which suggests that quinone species, intracellular glutathione depletion, and mitochondrial toxicity significantly contribute toward s the selective toxicity of salicylic acid in melanocytic melanoma cells, which is not observed in amelanotic ones [246] (Table 1).
9. Tannic Acid
Penta-m-digalloyl-glucose, or simply tannic acid, is a polyphenolic compound found in the bark and fruits of many plants [249], and is formed in animals’ bodies as the secondary metabolism of those plants [249–251]. Tannic acid is the main source of the pro-apoptotic agent gallic acid [251], but is considered more potent than ellagic acid [252]. Tannic acid is an antioxidant, anti-inflammatory, and anti-proliferative agent [253–255].
Tannic acid’s antioxidant effect [253–255] occurs because it reduces lipid peroxidation, COX-2, inducible nitric oxide synthase (i-NOS), and PCNA expression, increases reduced glutathione, decreases pro-inflammatory cytokines such as IL-6 and NF-κB, and depletes both hydrogen peroxide and xanthine oxidase [253]. Indeed, it is highly likely that these mechanisms are also important in providing tannic acid’s anti-carcinogenic profile.
Tannic acid’s important anti-carcinogenic mechanism of action is its capability of strongly inhibiting EGF-r tyrosine kinase [256]. Furthermore, tannic acid acts against topical skin cancer inducers [81, 95, 252, 257–259] such as DMBA plus croton oil-induced skin cancer [249, 258] and TPA [259]. More recent studies have shown that tannic acid prevents UVB-induced cutaneous carcinogenesis [260, 261], because it downregulates IL-18 [260]. The effect of tannic acid on the prevention and/or treatment of melanoma has not been studied yet (Table 1).
10. Conclusion
Polyphenolic compounds are already being used in different areas of dermatology and this manuscript provides a comprehensive review focused on the activity of these compounds in oncodermatology. Phenol itself acts as a physical destructive agent, but the addition of hydroxyl groups and rings results in additional biological properties, including disruption of hydrogen bonding, enhancement of mitochondrial metabolism, blockage of NF-κB and other effects. On the other hand, natural polyphenolic compounds can be delivered in relatively high concentrations to the skin using optimized formulations, allowing the proposed beneficial effects on cellular signaling to occur, as opposed to systemic delivery, in which these compounds are poorly absorbed and rapidly metabolized.
Given that knowledge of the polyphenolic compounds may be considered essential for practicing dermatologists, we aim to help dermatologists understand the variety of mechanisms of actions associated with these compounds. In fact, the diverse and distinct mechanisms of actions available provide new opportunities for researchers to explore polyphenolic compounds not only in dermatology but also in oncology. Curcumin, ellagic acid, epicatechin gallate, eugenol, honokiol, resveratrol, salicylic acid and tannic acid act as anti-cancer agents in important pro-neoplastic cell aspects, such as inflammation, autophagy, apoptosis, cell growth, and cell survival. Without doubt, this diversity of actions represents a unique advantage.
Polyphenolic compounds are already used in over-the-counter preparations, prescription drugs, sunscreens, and oral supplements. Increased frequency of the use of polyphenolic compounds in commercial products is expected. We believe that sunscreens are most likely to see an increase in use of polyphenolic compounds. However, it is likely that many other compounds will enter the dermatologic marketplace within the next decade. For instance, currently, there is one FDA-approved product of topical catechins, based on sinecatechins ointment 10 % (Veregen®; PharmaDerm®, a division of Fougera Pharmaceuticals Inc., Princeton, NJ, USA), which is formulated from green tea (Camellia sinensis) extracts, for the treatment of external genital warts and perianal warts in patients 18 years and older [262].
Because of the scientific evidence and benefits related to polyphenolic compounds as described in this review, it is considered essential for the practicing dermatologist to understand the variety of mechanisms of action related to these ubiquitous compounds.
Key Points.
Polyphenolic compounds have both current and future potential impact in dermatologic oncology.
We strongly believe that, in addition to non-melanoma skin cancer, melanoma can be targeted by polyphenolic compounds.
These compounds are already in use in both prescription and over-the-counter formulations, but the impact on dermatologic oncology is not yet fully understood.
Acknowledgments
Funding This manuscript was supported by two National Institute of Health fundings: RO1 AR47901 and P30 AR42687.
Footnotes
Compliance with Ethical Standards
Conflict of interest Adilson Costa, M.D., M.Sc., Ph.D, Michael Yi Bonner and Jack Arbiser, M.D., Ph.D. have no conflicts of interest related to this manuscript.
References
- 1.Sun Q, Heilmann J, Konig B. Natural phenolic metabolites with anti-angiogenic properties—a review from the chemical point of view. Beilstein J Org Chem. 2015;11:249–64. doi: 10.3762/bjoc.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heleno SA, Martins A, Queiroz MJ, Ferreira IC. Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chem. 2015;15(173):501–13. doi: 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 3.Nardini M, Forte M, Vrhovsek U, Mattivi F, Viola R, Scaccini C. White wine phenolics are absorbed and extensively metabolized in humans. J Agric Food Chem. 2009;57(7):2711–8. doi: 10.1021/jf8034463. [DOI] [PubMed] [Google Scholar]
- 4.Rechner AR, Kuhnle G, Bremner P, Hubbard GP, Moore KP, Rice-Evans CA. The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med. 2002;33(2):220–35. doi: 10.1016/s0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- 5.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130(8S Suppl):2073S–85S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 6.Ahn KS, Sethi G, Shishodia S, Sung B, Arbiser JL, Aggarwal BB. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-kappaB activation pathway. Mol Cancer Res. 2006;4(9):621–33. doi: 10.1158/1541-7786.MCR-06-0076. [DOI] [PubMed] [Google Scholar]
- 7.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 1. Curr Oncol. 2006;13(1):14–26. [PMC free article] [PubMed] [Google Scholar]
- 8.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 2. Curr Oncol. 2006;13(3):99–107. [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad S, Phromnoi K, Yadav VR, Chaturvedi MM, Aggarwal BB. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010;76(11):1044–63. doi: 10.1055/s-0030-1250111. [DOI] [PubMed] [Google Scholar]
- 10.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72(11):1439–52. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63(7):1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 12.Mradula PR, Sacchidanand S. A split-face comparative study of 70 % trichloroacetic acid and 80 % phenol spot peel in the treatment of freckles. J Cutan Aesthet Surg. 2012;5(4):261–5. doi: 10.4103/0974-2077.104914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langsdon PR, Milburn M, Yarber R. Comparison of the laser and phenol chemical peel in facial skin resurfacing. Arch Otolaryngol Head Neck Surg. 2000;126(10):1195–9. doi: 10.1001/archotol.126.10.1195. [DOI] [PubMed] [Google Scholar]
- 14.Monteiro-Riviere NA, Inman AO, Jackson H, Dunn B, Dimond S. Efficacy of topical phenol decontamination strategies on severity of acute phenol chemical burns and dermal absorption: in vitro and in vivo studies in pig skin. Toxicol Ind Health. 2001;17(4):95–104. doi: 10.1191/0748233701th095oa. [DOI] [PubMed] [Google Scholar]
- 15.Kligman AM, Baker TJ, Gordon HL. Long-term histologic follow-up of phenol face peels. Plast Reconstr Surg. 1985;75(5):652–9. doi: 10.1097/00006534-198505000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Yanez J, Vicente V, Alcaraz M, Castillo J, Benavente-Garcia O, Canteras M, et al. Cytotoxicity and antiproliferative activities of several phenolic compounds against three melanocytes cell lines: relationship between structure and activity. Nutr Cancer. 2004;49(2):191–9. doi: 10.1207/s15327914nc4902_11. [DOI] [PubMed] [Google Scholar]
- 17.Kasraee B. Peroxidase-mediated mechanisms are involved in the melanocytotoxic and melanogenesis-inhibiting effects of chemical agents. Dermatology. 2002;205(4):329–39. doi: 10.1159/000066439. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed 12 Apr 2016]; https://pubchem.ncbi.nlm.nih.gov/compound/16129778.
- 19. [Accessed 12 Apr 2016]; https://pubchem.ncbi.nlm.nih.gov/compound/969516.
- 20. [Accessed 12 Apr 2016]; https://pubchem.ncbi.nlm.nih.gov/compound/5281855.
- 21. [Accessed 12 Apr 2016]; https://pubchem.ncbi.nlm.nih.gov/compound/65064.
- 22. [Accessed 12 Apr 2016]; https://pubchem.ncbi.nlm.nih.gov/compound/3314.
- 23. [Accessed 12 Apr 2016]; https://www.ncbi.nlm.nih.gov/pccompound?term=honokiol.
- 24. [Accessed 12 Apr 2016]; https://pubchem.ncbi.nlm.nih.gov/compound/445154.
- 25. [Accessed 12 Apr 2016]; https://pubchem.ncbi.nlm.nih.gov/compound/338.
- 26.Wu J, Lu WY, Cui LL. Inhibitory effect of curcumin on invasion of skin squamous cell carcinoma A431 cells. Asian Pac J Cancer Prev. 2015;16(7):2813–8. doi: 10.7314/apjcp.2015.16.7.2813. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z, Liu B, Liu J, Zhang Q, Liu J, et al. Resveratrol inhibits the proliferation of human melanoma cells by inducing G1/S cell cycle arrest and apoptosis. Mol Med Rep. 2015;11(1):400–4. doi: 10.3892/mmr.2014.2716. [DOI] [PubMed] [Google Scholar]
- 28.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–7. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 29.De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, et al. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53(4):1592–7. doi: 10.1128/AAC.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen TA, Friedman AJ. Curcumin: a novel treatment for skin-related disorders. J Drugs Dermatol. 2013;12(10):1131–7. [PubMed] [Google Scholar]
- 31.Lopez-Jornet P, Camacho-Alonso F, Jimenez-Torres MJ, Orduna-Domingo A, Gomez-Garcia F. Topical curcumin for the healing of carbon dioxide laser skin wounds in mice. Photomed Laser Surg. 2011;29(12):809–14. doi: 10.1089/pho.2011.3004. [DOI] [PubMed] [Google Scholar]
- 32.Antiga E, Bonciolini V, Volpi W, Del Bianco E, Caproni M. Oral Curcumin (Meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with pso-riasis vulgaris. Biomed Res Int. 2015;2015:283634. doi: 10.1155/2015/283634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Zhao Y, Hu J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS One. 2013;8(6):e67078. doi: 10.1371/journal.pone.0067078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurd SK, Smith N, VanVoorhees A, Troxel AB, Badmaev V, Seykora JT, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58(4):625–31. doi: 10.1016/j.jaad.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 36.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and mother nature. Biochem Pharmacol. 2008;76(11):1590–611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Demirovic D, Rattan SI. Curcumin induces stress response and hormetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitro. Biogerontology. 2011;12(5):437–44. doi: 10.1007/s10522-011-9326-7. [DOI] [PubMed] [Google Scholar]
- 38.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27(4):486–94. [PubMed] [Google Scholar]
- 39.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90(5):1011–5. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rachmawati H, Edityaningrum CA, Mauludin R. Molecular inclusion complex of curcumin-beta-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech. 2013;14(4):1303–12. doi: 10.1208/s12249-013-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73(1):29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 42.Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC, Lee CH. Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun. 2012;18(5):685–93. doi: 10.1177/1753425912436589. [DOI] [PubMed] [Google Scholar]
- 43.Suwannateep N, Wanichwecharungruang S, Haag SF, Deva-hastin S, Groth N, Fluhr JW, et al. Encapsulated curcumin results in prolonged curcumin activity in vitro and radical scavenging activity ex vivo on skin after UVB-irradiation. Eur J Pharm Biopharm. 2012;82(3):485–90. doi: 10.1016/j.ejpb.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Suwannateep N, Wanichwecharungruang S, Fluhr J, Patzelt A, Lademann J, Meinke MC. Comparison of two encapsulated curcumin particular systems contained in different formulations with regard to in vitro skin penetration. Skin Res Technol. 2013;19(1):1–9. doi: 10.1111/j.1600-0846.2011.00600.x. [DOI] [PubMed] [Google Scholar]
- 45.Jebahi S, Saoudi M, Farhat L, Oudadesse H, Rebai T, Kabir A, et al. Effect of novel curcumin-encapsulated chitosan-bioglass drug on bone and skin repair after gamma radiation: experimental study on a Wistar rat model. Cell Biochem Funct. 2015;33(3):150–9. doi: 10.1002/cbf.3098. [DOI] [PubMed] [Google Scholar]
- 46.Zhao YZ, Lu CT, Zhang Y, Xiao J, Zhao YP, Tian JL, et al. Selection of high efficient transdermal lipid vesicle for curcumin skin delivery. Int J Pharm. 2013;454(1):302–9. doi: 10.1016/j.ijpharm.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 47.Mangalathillam S, Rejinold NS, Nair A, Lakshmanan VK, Nair SV, Jayakumar R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale. 2012;4(1):239–50. doi: 10.1039/c1nr11271f. [DOI] [PubMed] [Google Scholar]
- 48.Lin CC, Liu Y, Ho CT, Huang MT. Inhibitory effects of 1,3-bis-(2-substituted-phenyl)-propane-1,3-dione, beta-diketone structural analogues of curcumin, on chemical-induced tumor promotion and inflammation in mouse skin. Food Funct. 2011;2(1):78–83. doi: 10.1039/c0fo00098a. [DOI] [PubMed] [Google Scholar]
- 49.Sonavane K, Phillips J, Ekshyyan O, Moore-Medlin T, Roberts Gill J, Rong X, et al. Topical curcumin-based cream is equivalent to dietary curcumin in a skin cancer model. J Skin Cancer. 2012;2012:147863. doi: 10.1155/2012/147863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heng MC. Signaling pathways targeted by curcumin in acute and chronic injury: burns and photo-damaged skin. Int J Dermatol. 2013;52(5):531–43. doi: 10.1111/j.1365-4632.2012.05703.x. [DOI] [PubMed] [Google Scholar]
- 51.Garg R, Ramchandani AG, Maru GB. Curcumin decreases 12-O-tetradecanoylphorbol-13-acetate-induced protein kinase C translocation to modulate downstream targets in mouse skin. Carcinogenesis. 2008;29(6):1249–57. doi: 10.1093/carcin/bgn114. [DOI] [PubMed] [Google Scholar]
- 52.Huang MT, Smart RC, Wong CQ, Conney AH. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48(21):5941–6. [PubMed] [Google Scholar]
- 53.Kim H, Park J, Tak KH, Bu SY, Kim E. Chemopreventive effects of curcumin on chemically induced mouse skin carcinogenesis in BK5.insulin-like growth factor-1 transgenic mice. In vitro cellular & developmental biology. Animal. 2014;50(9):883–92. doi: 10.1007/s11626-014-9791-9. [DOI] [PubMed] [Google Scholar]
- 54.Tsai KD, Lin JC, Yang SM, Tseng MJ, Hsu JD, Lee YJ, et al. Curcumin protects against UVB-induced skin cancers in SKH-1 hairless mouse: analysis of early molecular markers in carcinogenesis. Evid Based Complement Alternat Med. 2012;2012:593952. doi: 10.1155/2012/593952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extra-cellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. 2003;24(9):1515–24. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 56.Dujic J, Kippenberger S, Hoffmann S, Ramirez-Bosca A, Miquel J, Diaz-Alperi J, et al. Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. J Invest Dermatol. 2007;127(8):1992–2000. doi: 10.1038/sj.jid.5700801. [DOI] [PubMed] [Google Scholar]
- 57.Yu T, Chen C, Sun Y, Sun H, Li TH, Meng J, et al. ABT-737 sensitizes curcumin-induced anti-melanoma cell activity through facilitating mPTP death pathway. Biochem Biophys Res Commun. 2015;464(1):286–91. doi: 10.1016/j.bbrc.2015.06.144. [DOI] [PubMed] [Google Scholar]
- 58.Faiao-Flores F, Quincoces Suarez JA, Fruet AC, Maria-Engler SS, Pardi PC, Maria DA. Curcumin analog DM-1 in monotherapy or combinatory treatment with dacarbazine as a strategy to inhibit in vivo melanoma progression. PLoS One. 2015;10(3):e0118702. doi: 10.1371/journal.pone.0118702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang P, Bai H, Liu G, Wang H, Chen F, Zhang B, et al. MicroRNA-33b, upregulated by EF24, a curcumin analog, suppresses the epithelial-to-mesenchymal transition (EMT) and migratory potential of melanoma cells by targeting HMGA2. Toxicol Lett. 2015;234(3):151–61. doi: 10.1016/j.toxlet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 60.Rozzo C, Fanciulli M, Fraumene C, Corrias A, Cubeddu T, Sassu I, et al. Molecular changes induced by the curcumin analogue D6 in human melanoma cells. Mol Cancer. 2013;12:37. doi: 10.1186/1476-4598-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bill MA, Nicholas C, Mace TA, Etter JP, Li C, Schwartz EB, et al. Structurally modified curcumin analogs inhibit STAT3 phosphorylation and promote apoptosis of human renal cell carcinoma and melanoma cell lines. PLoS One. 2012;7(8):e40724. doi: 10.1371/journal.pone.0040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang YP, Li YQ, Lv YT, Wang JM. Effect of curcumin on the proliferation, apoptosis, migration, and invasion of human melanoma A375 cells. Genet Mol Res. 2015;14(1):1056–67. doi: 10.4238/2015.February.6.9. [DOI] [PubMed] [Google Scholar]
- 63.Sun Y, Du L, Liu Y, Li X, Li M, Jin Y, et al. Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-beta-cyclodextrin for melanoma treatment. Int J Pharm. 2014;469(1):31–9. doi: 10.1016/j.ijpharm.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 64.Buss S, Dobra J, Goerg K, Hoffmann S, Kippenberger S, Kaufmann R, et al. Visible light is a better co-inducer of apoptosis for curcumin-treated human melanoma cells than UVA. PLoS One. 2013;8(11):e79748. doi: 10.1371/journal.pone.0079748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu Y, Miao L, Wang Y, Xu Z, Zhao Y, Shen Y, et al. Curcumin micelles remodel tumor microenvironment and enhance vaccine activity in an advanced melanoma model. Mol Ther. 2016;24(2):364–74. doi: 10.1038/mt.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang GM, Xie WY, Wang HS, Du J, Wu BP, Xu W, et al. Curcumin combined with FAPalphac vaccine elicits effective antitumor response by targeting indolamine-2,3-dioxygenase and inhibiting EMT induced by TNF-alpha in melanoma. Oncotarget. 2015;6(28):25932–42. doi: 10.18632/oncotarget.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang AJ, Jiang G, Li LT, Zheng JN. Curcumin induces apop-tosis through mitochondrial pathway and caspases activation in human melanoma cells. Mol Biol Rep. 2015;42(1):267–75. doi: 10.1007/s11033-014-3769-2. [DOI] [PubMed] [Google Scholar]
- 68.Lu C, Song E, Hu DN, Chen M, Xue C, Rosen R, et al. Curcumin induces cell death in human uveal melanoma cells through mitochondrial pathway. Curr Eye Res. 2010;35(4):352–60. doi: 10.3109/02713680903521944. [DOI] [PubMed] [Google Scholar]
- 69.Qiu Y, Yu T, Wang W, Pan K, Shi D, Sun H. Curcumin-induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. Biochem Biophys Res Commun. 2014;448(1):15–21. doi: 10.1016/j.bbrc.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 70.Yu T, Ji J, Guo YL. MST1 activation by curcumin mediates JNK activation, Foxo3a nuclear translocation and apoptosis in melanoma cells. Biochem Biophys Res Commun. 2013;441(1):53–8. doi: 10.1016/j.bbrc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 71.Marin YE, Wall BA, Wang S, Namkoong J, Martino JJ, Suh J, et al. Curcumin downregulates the constitutive activity of NF-kappaB and induces apoptosis in novel mouse melanoma cells. Melanoma Res. 2007;17(5):274–83. doi: 10.1097/CMR.0b013e3282ed3d0e. [DOI] [PubMed] [Google Scholar]
- 72.Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104(4):879–90. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- 73.Zheng M, Ekmekcioglu S, Walch ET, Tang CH, Grimm EA. Inhibition of nuclear factor-kappaB and nitric oxide by curcumin induces G2/M cell cycle arrest and apoptosis in human melanoma cells. Melanoma Res. 2004;14(3):165–71. doi: 10.1097/01.cmr.0000129374.76399.19. [DOI] [PubMed] [Google Scholar]
- 74.Loch-Neckel G, Santos-Bubniak L, Mazzarino L, Jacques AV, Moccelin B, Santos-Silva MC, et al. Orally administered chitosan-coated polycaprolactone nanoparticles containing curcumin attenuate metastatic melanoma in the lungs. J Pharm Sci. 2015;104(10):3524–34. doi: 10.1002/jps.24548. [DOI] [PubMed] [Google Scholar]
- 75.Banerji A, Chakrabarti J, Mitra A, Chatterjee A. Effect of curcumin on gelatinase A (MMP-2) activity in B16F10 melanoma cells. Cancer Lett. 2004;211(2):235–42. doi: 10.1016/j.canlet.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Chatterjee SJ, Pandey S. Chemo-resistant melanoma sensitized by tamoxifen to low dose curcumin treatment through induction of apoptosis and autophagy. Cancer Biol Ther. 2011;11(2):216–28. doi: 10.4161/cbt.11.2.13798. [DOI] [PubMed] [Google Scholar]
- 77.Thresiamma KC, Kuttan R. Inhibition of liver fibrosis by ellagic acid. Indian J Physiol Pharmacol. 1996;40(4):363–6. [PubMed] [Google Scholar]
- 78.Devipriya N, Sudheer AR, Srinivasan M, Menon VP. Effect of ellagic acid, a plant polyphenol, on fibrotic markers (MMPs and TIMPs) during alcohol-induced hepatotoxicity. Toxicol Mech Methods. 2007;17(6):349–56. doi: 10.1080/15376510601077003. [DOI] [PubMed] [Google Scholar]
- 79.Losso JN, Bansode RR, Trappey A, 2nd, Bawadi HA, Truax R. In vitro anti-proliferative activities of ellagic acid. J Nutr Biochem. 2004;15(11):672–8. doi: 10.1016/j.jnutbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 80.Narayanan BA, Geoffroy O, Willingham MC, Re GG, Nixon DW. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999;136(2):215–21. doi: 10.1016/s0304-3835(98)00323-1. [DOI] [PubMed] [Google Scholar]
- 81.Mukhtar H, Das M, Khan WA, Wang ZY, Bik DP, Bickers DR. Exceptional activity of tannic acid among naturally occurring plant phenols in protecting against 7,12-dimethylbenz(a)anthracene-, benzo(a)pyrene-, 3-methylcholanthrene-, and N-methyl-N-nitrosourea-induced skin tumorigenesis in mice. Cancer Res. 1988;48(9):2361–5. [PubMed] [Google Scholar]
- 82.Kowalczyk MC, Kowalczyk P, Tolstykh O, Hanausek M, Walaszek Z, Slaga TJ. Synergistic effects of combined phyto-chemicals and skin cancer prevention in SENCAR mice. Cancer Prev Res (Phila) 2010;3(2):170–8. doi: 10.1158/1940-6207.CAPR-09-0196. [DOI] [PubMed] [Google Scholar]
- 83.Quave CL, Estevez-Carmona M, Compadre CM, Hobby G, Hendrickson H, Beenken KE, et al. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS One. 2012;7(1):e28737. doi: 10.1371/journal.pone.0028737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hancock V, Dahl M, Vejborg RM, Klemm P. Dietary plant components ellagic acid and tannic acid inhibit Escherichia coli biofilm formation. J Med Microbiol. 2010;59(Pt 4):496–8. doi: 10.1099/jmm.0.013680-0. [DOI] [PubMed] [Google Scholar]
- 85.Junyaprasert VB, Singhsa P, Suksiriworapong J, Chantasart D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int J Pharm. 2012;423(2):303–11. doi: 10.1016/j.ijpharm.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 86.Cerda B, Ceron JJ, Tomas-Barberan FA, Espin JC. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J Agric Food Chem. 2003;51(11):3493–501. doi: 10.1021/jf020842c. [DOI] [PubMed] [Google Scholar]
- 87.Cerda B, Espin JC, Parra S, Martinez P, Tomas-Barberan FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004;43(4):205–20. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- 88.Cerda B, Periago P, Espin JC, Tomas-Barberan FA. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem. 2005;53(14):5571–6. doi: 10.1021/jf050384i. [DOI] [PubMed] [Google Scholar]
- 89.Tomas-Barberan FA, Garcia-Villalba R, Gonzalez-Sarrias A, Selma MV, Espin JC. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem. 2014;62(28):6535–8. doi: 10.1021/jf5024615. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez-Sarrias A, Azorin-Ortuno M, Yanez-Gascon MJ, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Dissimilar in vitro and in vivo effects of ellagic acid and its microbiota-derived metabolites, urolithins, on the cytochrome P450 1A1. J Agric Food Chem. 2009;57(12):5623–32. doi: 10.1021/jf900725e. [DOI] [PubMed] [Google Scholar]
- 91.Larrosa M, Gonzalez-Sarrias A, Yanez-Gascon MJ, Selma MV, Azorin-Ortuno M, Toti S, et al. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21(8):717–25. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez-Sarrias A, Larrosa M, Tomas-Barberan FA, Dolara P, Espin JC. NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br J Nutr. 2010;104(4):503–12. doi: 10.1017/S0007114510000826. [DOI] [PubMed] [Google Scholar]
- 93.Kim S, Liu Y, Gaber MW, Bumgardner JD, Haggard WO, Yang Y. Development of chitosan-ellagic acid films as a local drug delivery system to induce apoptotic death of human melanoma cells. J Biomed Mater Res B Appl Biomater. 2009;90(1):145–55. doi: 10.1002/jbm.b.31266. [DOI] [PubMed] [Google Scholar]
- 94.Jensen JD, Dunn JH, Luo Y, Liu W, Fujita M, Dellavalle RP. Ellagic acid inhibits melanoma growth in vitro. Dermatol Rep. 2011;3(3):e36. doi: 10.4081/dr.2011.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perchellet JP, Gali HU, Perchellet EM, Klish DS, Armbrust AD. Antitumor-promoting activities of tannic acid, ellagic acid, and several gallic acid derivatives in mouse skin. Basic Life Sci. 1992;59:783–801. doi: 10.1007/978-1-4615-3476-1_47. [DOI] [PubMed] [Google Scholar]
- 96.Mukhtar H, Das M, Bickers DR. Inhibition of 3-methylcholan-threne-induced skin tumorigenicity in BALB/c mice by chronic oral feeding of trace amounts of ellagic acid in drinking water. Cancer Res. 1986;46(5):2262–5. [PubMed] [Google Scholar]
- 97.Mukhtar H, Das M, Del Tito BJ, Jr, Bickers DR. Protection against 3-methylcholanthrene-induced skin tumorigenesis in Balb/C mice by ellagic acid. Biochem Biophys Res Commun. 1984;119(2):751–7. doi: 10.1016/s0006-291x(84)80314-9. [DOI] [PubMed] [Google Scholar]
- 98.Chang RL, Huang MT, Wood AW, Wong CQ, Newmark HL, Yagi H, et al. Effect of ellagic acid and hydroxylated flavonoids on the tumorigenicity of benzo[a]pyrene and (+/−)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahy-drobenzo[a]pyrene on mouse skin and in the newborn mouse. Carcinogenesis. 1985;6(8):1127–33. doi: 10.1093/carcin/6.8.1127. [DOI] [PubMed] [Google Scholar]
- 99.Weisburg JH, Schuck AG, Reiss SE, Wolf BJ, Fertel SR, Zuckerbraun HL, et al. Ellagic acid, a dietary polyphenol, selectively cytotoxic to HSC-2 oral carcinoma cells. Anticancer Res. 2013;33(5):1829–36. [PubMed] [Google Scholar]
- 100.Bae JY, Choi JS, Kang SW, Lee YJ, Park J, Kang YH. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp Dermatol. 2010;19(8):e182–90. doi: 10.1111/j.1600-0625.2009.01044.x. [DOI] [PubMed] [Google Scholar]
- 101.Lembo S, Balato A, Di Caprio R, Cirillo T, Giannini V, Gasparri F, et al. The modulatory effect of ellagic acid and rosmarinic acid on ultraviolet-B-induced cytokine/chemokine gene expression in skin keratinocyte (HaCaT) cells. Biomed Res Int. 2014;2014:346793. doi: 10.1155/2014/346793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kasai K, Yoshimura M, Koga T, Arii M, Kawasaki S. Effects of oral administration of ellagic acid-rich pomegranate extract on ultraviolet-induced pigmentation in the human skin. J Nutr Sci Vitaminol (Tokyo) 2006;52(5):383–8. doi: 10.3177/jnsv.52.383. [DOI] [PubMed] [Google Scholar]
- 103.Yoshioka S, Terashita T, Yoshizumi H, Shirasaka N. Inhibitory effects of whisky polyphenols on melanogenesis in mouse B16 melanoma cells. Biosci Biotechnol Biochem. 2011;75(12):2278–82. doi: 10.1271/bbb.100514. [DOI] [PubMed] [Google Scholar]
- 104.Ertam I, Mutlu B, Unal I, Alper S, Kivcak B, Ozer O. Efficiency of ellagic acid and arbutin in melasma: a randomized, prospective, open-label study. J Dermatol. 2008;35(9):570–4. doi: 10.1111/j.1346-8138.2008.00522.x. [DOI] [PubMed] [Google Scholar]
- 105.Hseu YC, Chou CW, Senthil Kumar KJ, Fu KT, Wang HM, Hsu LS, et al. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol. 2012;50(5):1245–55. doi: 10.1016/j.fct.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 106.Kim HJ, Ryu JH, Kim CH, Lim JW, Moon UY, Lee GH, et al. Epicatechin gallate suppresses oxidative stress-induced MUC5AC overexpression by interaction with epidermal growth factor receptor. Am J Respir Cell Mol Biol. 2010;43(3):349–57. doi: 10.1165/rcmb.2009-0205OC. [DOI] [PubMed] [Google Scholar]
- 107.Kuroda Y, Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res. 1999;436(1):69–97. doi: 10.1016/s1383-5742(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 108.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89(24):1881–6. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 109.Kondo K, Kurihara M, Miyata N, Suzuki T, Toyoda M. Scavenging mechanisms of (−)-epigallocatechin gallate and (−)-epicatechin gallate on peroxyl radicals and formation of super-oxide during the inhibitory action. Free Radic Biol Med. 1999;27(7–8):855–63. doi: 10.1016/s0891-5849(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 110.Terao J, Piskula M, Yao Q. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch Biochem Biophys. 1994;308(1):278–84. doi: 10.1006/abbi.1994.1039. [DOI] [PubMed] [Google Scholar]
- 111.McKelvey KJ, Appleton I. Epicatechin gallate improves healing and reduces scar formation of incisional wounds in type 2 diabetes mellitus rat model. Wounds. 2012;24(3):55–7. [PubMed] [Google Scholar]
- 112.Demeule M, Brossard M, Page M, Gingras D, Beliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta. 2000;1478(1):51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 113.Kapoor M, Howard R, Hall I, Appleton I. Effects of epicatechin gallate on wound healing and scar formation in a full thickness incisional wound healing model in rats. Am J Pathol. 2004;165(1):299–307. doi: 10.1016/S0002-9440(10)63297-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou YD, Kim YP, Li XC, Baerson SR, Agarwal AK, Hodges TW, et al. Hypoxia-inducible factor-1 activation by (−)-epicatechin gallate: potential adverse effects of cancer chemoprevention with high-dose green tea extracts. J Nat Prod. 2004;67(12):2063–9. doi: 10.1021/np040140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morre DJ, Morre DM, Sun H, Cooper R, Chang J, Janle EM. Tea catechin synergies in inhibition of cancer cell proliferation and of a cancer specific cell surface oxidase (ECTO-NOX) Pharmacol Toxicol. 2003;92(5):234–41. doi: 10.1034/j.1600-0773.2003.920506.x. [DOI] [PubMed] [Google Scholar]
- 116.Huang CC, Wu WB, Fang JY, Chiang HS, Chen SK, Chen BH, et al. (−)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes. Molecules. 2007;12(8):1845–58. doi: 10.3390/12081845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Basu-Modak S, Ali D, Gordon M, Polte T, Yiakouvaki A, Pourzand C, et al. Suppression of UVA-mediated release of labile iron by epicatechin–a link to lysosomal protection. Free Radic Biol Med. 2006;41(8):1197–204. doi: 10.1016/j.freeradbiomed.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 118.Huang CC, Fang JY, Wu WB, Chiang HS, Wei YJ, Hung CF. Protective effects of (−)-epicatechin-3-gallate on UVA-induced damage in HaCaT keratinocytes. Arch Dermatol Res. 2005;296(10):473–81. doi: 10.1007/s00403-005-0540-5. [DOI] [PubMed] [Google Scholar]
- 119.Basu-Modak S, Gordon MJ, Dobson LH, Spencer JP, Rice-Evans C, Tyrrell RM. Epicatechin and its methylated metabolite attenuate UVA-induced oxidative damage to human skin fibroblasts. Free Radic Biol Med. 2003;35(8):910–21. doi: 10.1016/s0891-5849(03)00436-2. [DOI] [PubMed] [Google Scholar]
- 120.Zhong JL, Yiakouvaki A, Holley P, Tyrrell RM, Pourzand C. Susceptibility of skin cells to UVA-induced necrotic cell death reflects the intracellular level of labile iron. J Invest Dermatol. 2004;123(4):771–80. doi: 10.1111/j.0022-202X.2004.23419.x. [DOI] [PubMed] [Google Scholar]
- 121.Lim YC, Lee SH, Song MH, Yamaguchi K, Yoon JH, Choi EC, et al. Growth inhibition and apoptosis by (−)-epicatechin gallate are mediated by cyclin D1 suppression in head and neck squamous carcinoma cells. Eur J Cancer. 2006;42(18):3260–6. doi: 10.1016/j.ejca.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Babich H, Krupka ME, Nissim HA, Zuckerbraun HL. Differential in vitro cytotoxicity of (−)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicol In Vitro. 2005;19(2):231–42. doi: 10.1016/j.tiv.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 123.Ghosh KS, Maiti TK, Mandal A, Dasgupta S. Copper complexes of (−)-epicatechin gallate and (−)-epigallocatechin gallate act as inhibitors of Ribonuclease A. FEBS Lett. 2006;580(19):4703–8. doi: 10.1016/j.febslet.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 124.Pisano M, Pagnan G, Loi M, Mura ME, Tilocca MG, Palmieri G, et al. Antiproliferative and proapoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol Cancer. 2007;6:8. doi: 10.1186/1476-4598-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim SS, Oh OJ, Min HY, Park EJ, Kim Y, Park HJ, et al. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003;73(3):337–48. doi: 10.1016/s0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- 126.Ito M, Murakami K, Yoshino M. Antioxidant action of eugenol compounds: role of metal ion in the inhibition of lipid peroxidation. Food Chem Toxicol. 2005;43(3):461–6. doi: 10.1016/j.fct.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 127.Bertrand F, Basketter DA, Roberts DW, Lepoittevin JP. Skin sensitization to eugenol and isoeugenol in mice: possible metabolic pathways involving orthoquinone and quinone methide intermediates. Chem Res Toxicol. 1997;10(3):335–43. doi: 10.1021/tx960087v. [DOI] [PubMed] [Google Scholar]
- 128.Barratt MD, Basketter DA. Possible origin of the skin sensitization potential of isoeugenol and related compounds. (I). Preliminary studies of potential reaction mechanisms. Contact Dermatitis. 1992;27(2):98–104. doi: 10.1111/j.1600-0536.1992.tb05217.x. [DOI] [PubMed] [Google Scholar]
- 129.Johansen JD, Menne T. The fragrance mix and its constituents: a 14-year material. Contact Dermatitis. 1995;32(1):18–23. doi: 10.1111/j.1600-0536.1995.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 130.Marzulli FN, Maibach HI. Contact allergy: predictive testing of fragrance ingredients in humans by Draize and Maximization methods. J Environ Pathol Toxicol. 1980;3(5–6):235–45. [PubMed] [Google Scholar]
- 131.Miyazawa M, Hisama M. Suppression of chemical mutagen-induced SOS response by alkylphenols from clove (Syzygium aromaticum) in the Salmonella typhimurium TA1535/pSK1002 umu test. J Agric Food Chem. 2001;49(8):4019–25. doi: 10.1021/jf0103469. [DOI] [PubMed] [Google Scholar]
- 132.Rompelberg CJ, Evertz SJ, Bruijntjes-Rozier GC, van den Heuvel PD, Verhagen H. Effect of eugenol on the genotoxicity of established mutagens in the liver. Food Chem Toxicol. 1996;34(1):33–42. doi: 10.1016/0278-6915(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 133.Rompelberg CJ, Verhagen H, van Bladeren PJ. Effects of the naturally occurring alkenylbenzenes eugenol and trans-anethole on drug-metabolizing enzymes in the rat liver. Food Chem Toxicol. 1993;31(9):637–45. doi: 10.1016/0278-6915(93)90046-2. [DOI] [PubMed] [Google Scholar]
- 134.Han EH, Hwang YP, Jeong TC, Lee SS, Shin JG, Jeong HG. Eugenol inhibit 7,12-dimethylbenz[a]anthracene-induced genotoxicity in MCF-7 cells: bifunctional effects on CYP1 and NAD(P)H:quinone oxidoreductase. FEBS Lett. 2007;581(4):749–56. doi: 10.1016/j.febslet.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 135.Abraham SK. Anti-genotoxicity of transanethole and eugenol in mice. Food Chem Toxicol. 2001;39(5):493–8. doi: 10.1016/s0278-6915(00)00156-3. [DOI] [PubMed] [Google Scholar]
- 136.Pal D, Banerjee S, Mukherjee S, Roy A, Panda CK, Das S. Eugenol restricts DMBA croton oil induced skin carcinogenesis in mice: downregulation of c-Myc and H-ras, and activation of p53 dependent apoptotic pathway. J Dermatol Sci. 2010;59(1):31–9. doi: 10.1016/j.jdermsci.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 137.Vaid M, Sharma SD, Katiyar SK. Honokiol, a phytochemical from the Magnolia plant, inhibits photocarcinogenesis by targeting UVB-induced inflammatory mediators and cell cycle regulators: development of topical formulation. Carcinogenesis. 2010;31(11):2004–11. doi: 10.1093/carcin/bgq186. [DOI] [PubMed] [Google Scholar]
- 138.Guillermo RF, Chilampalli C, Zhang X, Zeman D, Fahmy H, Dwivedi C. Time and dose-response effects of honokiol on UVB-induced skin cancer development. Drug Discov Ther. 2012;6(3):140–6. [PubMed] [Google Scholar]
- 139.Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal. 2009;11(5):1139–48. doi: 10.1089/ars.2009.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem Photobiol Sci. 2006;5(2):243–53. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 141.Li TSC. Chinese and related North American herbs: phytopharmacology and therapeutic values. Boca Raton: CRC Press; 2002. [Google Scholar]
- 142.Hahm ER, Arlotti JA, Marynowski SW, Singh SV. Honokiol, a constituent of oriental medicinal herb magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin Cancer Res. 2008;14(4):1248–57. doi: 10.1158/1078-0432.CCR-07-1926. [DOI] [PubMed] [Google Scholar]
- 143.Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278(37):35501–7. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 144.Munroe ME, Arbiser JL, Bishop GA. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J Immunol. 2007;179(2):753–63. doi: 10.4049/jimmunol.179.2.753. [DOI] [PubMed] [Google Scholar]
- 145.Pyo MK, Lee Y, Yun-Choi HS. Anti-platelet effect of the constituents isolated from the barks and fruits of Magnolia obovata. Arch Pharm Res. 2002;25(3):325–8. doi: 10.1007/BF02976634. [DOI] [PubMed] [Google Scholar]
- 146.Clark AM, El-Feraly FS, Li WS. Antimicrobial activity of phenolic constituents of Magnolia grandiflora L. J Pharm Sci. 1981;70(8):951–2. doi: 10.1002/jps.2600700833. [DOI] [PubMed] [Google Scholar]
- 147.Park J, Lee J, Jung E, Park Y, Kim K, Park B, et al. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur J Pharmacol. 2004;496(1–3):189–95. doi: 10.1016/j.ejphar.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 148.Amblard F, Delinsky D, Arbiser JL, Schinazi RF. Facile purification of honokiol and its antiviral and cytotoxic properties. J Med Chem. 2006;49(11):3426–7. doi: 10.1021/jm060268m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Amblard F, Govindarajan B, Lefkove B, Rapp KL, Detorio M, Arbiser JL, et al. Synthesis, cytotoxicity, and antiviral activities of new neolignans related to honokiol and magnolol. Bioorg Med Chem Lett. 2007;17(16):4428–31. doi: 10.1016/j.bmcl.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lan KH, Wang YW, Lee WP, Lan KL, Tseng SH, Hung LR, et al. Multiple effects of Honokiol on the life cycle of hepatitis C virus. Liver Int. 2012;32(6):989–97. doi: 10.1111/j.1478-3231.2011.02621.x. [DOI] [PubMed] [Google Scholar]
- 151.Ishikawa C, Arbiser JL, Mori N. Honokiol induces cell cycle arrest and apoptosis via inhibition of survival signals in adult T-cell leukemia. Biochim Biophys Acta. 2012;1820(7):879–87. doi: 10.1016/j.bbagen.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 152.Pillai VB, Samant S, Sundaresan NR, Raghuraman H, Kim G, Bonner MY, et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun. 2015;6:6656. doi: 10.1038/ncomms7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kaushik G, Kwatra D, Subramaniam D, Jensen RA, Anant S, Mammen JM. Honokiol affects melanoma cell growth by targeting the AMP-activated protein kinase signaling pathway. Am J Surg. 2014;208(6):995–1002. doi: 10.1016/j.amjsurg.2014.09.014. (discussion 1–2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kaushik G, Venugopal A, Ramamoorthy P, Standing D, Subramaniam D, Umar S, et al. Honokiol inhibits melanoma stem cells by targeting notch signaling. Mol Carcinog. 2015;54(12):1710–21. doi: 10.1002/mc.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mannal PW, Schneider J, Tangada A, McDonald D, McFadden DW. Honokiol produces anti-neoplastic effects on melanoma cells in vitro. J Surg Oncol. 2011;104(3):260–4. doi: 10.1002/jso.21936. [DOI] [PubMed] [Google Scholar]
- 156.Kaushik G, Ramalingam S, Subramaniam D, Rangarajan P, Protti P, Rammamoorthy P, et al. Honokiol induces cytotoxic and cytostatic effects in malignant melanoma cancer cells. Am J Surg. 2012;204(6):868–73. doi: 10.1016/j.amjsurg.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chilampalli S, Zhang X, Fahmy H, Kaushik RS, Zeman D, Hildreth MB, et al. Chemopreventive effects of honokiol on UVB-induced skin cancer development. Anticancer Res. 2010;30(3):777–83. [PubMed] [Google Scholar]
- 158.Dikalov S, Losik T, Arbiser JL. Honokiol is a potent scavenger of superoxide and peroxyl radicals. Biochem Pharmacol. 2008;76(5):589–96. doi: 10.1016/j.bcp.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chilampalli C, Zhang X, Kaushik RS, Young A, Zeman D, Hildreth MB, et al. Chemopreventive effects of combination of honokiol and magnolol with alpha-santalol on skin cancer developments. Drug Discov Ther. 2013;7(3):109–15. [PubMed] [Google Scholar]
- 160.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21(24):8247–54. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Budd RC, Yeh WC, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol. 2006;6(3):196–204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]
- 162.Kjaer TN, Thorsen K, Jessen N, Stenderup K, Pedersen SB. Resveratrol ameliorates imiquimod-induced psoriasis-like skin inflammation in mice. PLoS One. 2015;10(5):e0126599. doi: 10.1371/journal.pone.0126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Farris P, Yatskayer M, Chen N, Krol Y, Oresajo C. Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin. J Drugs Dermatol. 2014;13(12):1467–72. [PubMed] [Google Scholar]
- 164.Holian O, Walter RJ. Resveratrol inhibits the proliferation of normal human keratinocytes in vitro. J Cell Biochem Suppl. 2001;(Suppl 36):55–62. doi: 10.1002/jcb.1085. [DOI] [PubMed] [Google Scholar]
- 165.Wu Z, Uchi H, Morino-Koga S, Shi W, Furue M. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J Dermatol Sci. 2014;75(1):16–23. doi: 10.1016/j.jdermsci.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 166.Bae SJ, Ha YM, Kim JA, Park JY, Ha TK, Park D, et al. A novel synthesized tyrosinase inhibitor: (E)-2-((2,4-dihydroxyphenyl)-diazenyl)phenyl 4-methylbenzenesulfonate as an azo-resveratrol analog. Biosci Biotechnol Biochem. 2013;77(1):65–72. doi: 10.1271/bbb.120547. [DOI] [PubMed] [Google Scholar]
- 167.Ohguchi K, Tanaka T, Ito T, Iinuma M, Matsumoto K, Akao Y, et al. Inhibitory effects of resveratrol derivatives from diptero-carpaceae plants on tyrosinase activity. Biosci Biotechnol Biochem. 2003;67(7):1587–9. doi: 10.1271/bbb.67.1587. [DOI] [PubMed] [Google Scholar]
- 168.Park J, Park JH, Suh HJ, Lee IC, Koh J, Boo YC. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch Dermatol Res. 2014;306(5):475–87. doi: 10.1007/s00403-014-1440-3. [DOI] [PubMed] [Google Scholar]
- 169.Park J, Boo YC. Isolation of resveratrol from vitis viniferae caulis and its potent inhibition of human tyrosinase. Evid Based Complement Alternat Med. 2013;2013:645257. doi: 10.1155/2013/645257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Song YM, Ha YM, Kim JA, Chung KW, Uehara Y, Lee KJ, et al. Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg Med Chem Lett. 2012;22(24):7451–5. doi: 10.1016/j.bmcl.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 171.Lee TH, Seo JO, Baek SH, Kim SY. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in Guinea pig skin. Biomol Ther (Seoul) 2014;22(1):35–40. doi: 10.4062/biomolther.2013.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Freitas JV, Praca FS, Bentley MV, Gaspar LR. Trans-resveratrol and beta-carotene from sunscreens penetrate viable skin layers and reduce cutaneous penetration of UV-filters. Int J Pharm. 2015;484(1–2):131–7. doi: 10.1016/j.ijpharm.2015.02.062. [DOI] [PubMed] [Google Scholar]
- 173.Caddeo C, Manconi M, Cardia MC, Diez-Sales O, Fadda AM, Sinico C. Investigating the interactions of resveratrol with phospholipid vesicle bilayer and the skin: NMR studies and confocal imaging. Int J Pharm. 2015;484(1–2):138–45. doi: 10.1016/j.ijpharm.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 174.de Almeida PA, Alves MC, Polonini HC, Calixto SL, Braga Gomes TB, Barros Lataliza AA, et al. Studies with emulsion containing trans-resveratrol: in vitro release profile and ex vivo human skin permeation. Curr Drug Deliv. 2015;12(2):157–65. doi: 10.2174/1567201812666150130161736. [DOI] [PubMed] [Google Scholar]
- 175.Yutani R, Kikuchi T, Teraoka R, Kitagawa S. Efficient delivery and distribution in skin of chlorogenic acid and resveratrol induced by microemulsion using sucrose laurate. Chem Pharm Bull (Tokyo) 2014;62(3):274–80. doi: 10.1248/cpb.c13-00820. [DOI] [PubMed] [Google Scholar]
- 176.Detoni CB, Souto GD, da Silva AL, Pohlmann AR, Guterres SS. Photostability and skin penetration of different E-resveratrol-loaded supramolecular structures. Photochem Photobiol. 2012;88(4):913–21. doi: 10.1111/j.1751-1097.2012.01147.x. [DOI] [PubMed] [Google Scholar]
- 177.Hung CF, Lin YK, Huang ZR, Fang JY. Delivery of resveratrol, a red wine polyphenol, from solutions and hydrogels via the skin. Biol Pharm Bull. 2008;31(5):955–62. doi: 10.1248/bpb.31.955. [DOI] [PubMed] [Google Scholar]
- 178.Zhang G, Flach CR, Mendelsohn R. Tracking the dephosphorylation of resveratrol triphosphate in skin by confocal Raman microscopy. J Control Release. 2007;123(2):141–7. doi: 10.1016/j.jconrel.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sticozzi C, Belmonte G, Cervellati F, Muresan XM, Pessina F, Lim Y, et al. Resveratrol protects SR-B1 levels in keratinocytes exposed to cigarette smoke. Free Radic Biol Med. 2014;69:50–7. doi: 10.1016/j.freeradbiomed.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sticozzi C, Cervellati F, Muresan XM, Cervellati C, Valacchi G. Resveratrol prevents cigarette smoke-induced keratinocytes damage. Food Funct. 2014;5(9):2348–56. doi: 10.1039/c4fo00407h. [DOI] [PubMed] [Google Scholar]
- 181.Liu Y, Chan F, Sun H, Yan J, Fan D, Zhao D, et al. Resveratrol protects human keratinocytes HaCaT cells from UVA-induced oxidative stress damage by downregulating Keap1 expression. Eur J Pharmacol. 2011;650(1):130–7. doi: 10.1016/j.ejphar.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 182.Chen ML, Li J, Xiao WR, Sun L, Tang H, Wang L, et al. Protective effect of resveratrol against oxidative damage of UVA irradiated HaCaT cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31(5):635–9. [PubMed] [Google Scholar]
- 183.Soeur J, Eilstein J, Lereaux G, Jones C, Marrot L. Skin resistance to oxidative stress induced by resveratrol: from Nrf2 activation to GSH biosynthesis. Free Radic Biol Med. 2015;78:213–23. doi: 10.1016/j.freeradbiomed.2014.10.510. [DOI] [PubMed] [Google Scholar]
- 184.Krajka-Kuzniak V, Szaefer H, Stefanski T, Sobiak S, Cichocki M, Baer-Dubowska W. The effect of resveratrol and its methylthio-derivatives on the Nrf2-ARE pathway in mouse epidermis and HaCaT keratinocytes. Cell Mol Biol Lett. 2014;19(3):500–16. doi: 10.2478/s11658-014-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ido Y, Duranton A, Lan F, Weikel KA, Breton L, Ruderman NB. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS One. 2015;10(2):e0115341. doi: 10.1371/journal.pone.0115341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jagdeo J, Adams L, Lev-Tov H, Sieminska J, Michl J, Brody N. Dose-dependent antioxidant function of resveratrol demonstrated via modulation of reactive oxygen species in normal human skin fibroblasts in vitro. J Drugs Dermatol. 2010;9(12):1523–6. [PubMed] [Google Scholar]
- 187.Park K, Lee JH. Protective effects of resveratrol on UVB-irradiated HaCaT cells through attenuation of the caspase pathway. Oncol Rep. 2008;19(2):413–7. [PubMed] [Google Scholar]
- 188.Bastianetto S, Dumont Y, Duranton A, Vercauteren F, Breton L, Quirion R. Protective action of resveratrol in human skin: possible involvement of specific receptor binding sites. PLoS One. 2010;5(9):e12935. doi: 10.1371/journal.pone.0012935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hao Y, Huang W, Liao M, Zhu Y, Liu H, Hao C, et al. The inhibition of resveratrol to human skin squamous cell carcinoma A431 xenografts in nude mice. Fitoterapia. 2013;86:84–91. doi: 10.1016/j.fitote.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 190.Back JH, Zhu Y, Calabro A, Queenan C, Kim AS, Arbesman J, et al. Resveratrol-mediated downregulation of Rictor attenuates autophagic process and suppresses UV-induced skin carcinogenesis. Photochem Photobiol. 2012;88(5):1165–72. doi: 10.1111/j.1751-1097.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tang Q, Li G, Wei X, Zhang J, Chiu JF, Hasenmayer D, et al. Resveratrol-induced apoptosis is enhanced by inhibition of autophagy in esophageal squamous cell carcinoma. Cancer Lett. 2013;336(2):325–37. doi: 10.1016/j.canlet.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 192.Yusuf N, Nasti TH, Meleth S, Elmets CA. Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis. Mol Carcinog. 2009;48(8):713–23. doi: 10.1002/mc.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27(7):1465–74. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- 194.Szaefer H, Cichocki M, Krajka-Kuzniak V, Stefanski T, Sobiak S, Licznerska B, et al. The effect of resveratrol and its methylthio-derivatives on NF-kappaB and AP-1 signaling pathways in HaCaT keratinocytes. Pharmacol Rep. 2014;66(5):732–40. doi: 10.1016/j.pharep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 195.Kundu JK, Chun KS, Kim SO, Surh YJ. Resveratrol inhibits phorbol ester-induced cyclooxygenase-2 expression in mouse skin: MAPKs and AP-1 as potential molecular targets. BioFactors. 2004;21(1–4):33–9. doi: 10.1002/biof.552210108. [DOI] [PubMed] [Google Scholar]
- 196.Roy P, Kalra N, Prasad S, George J, Shukla Y. Chemopreventive potential of resveratrol in mouse skin tumors through regulation of mitochondrial and PI3K/AKT signaling pathways. Pharm Res. 2009;26(1):211–7. doi: 10.1007/s11095-008-9723-z. [DOI] [PubMed] [Google Scholar]
- 197.Kalra N, Roy P, Prasad S, Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 2008;82(7–8):348–58. doi: 10.1016/j.lfs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 198.George J, Singh M, Srivastava AK, Bhui K, Roy P, Chaturvedi PK, et al. Resveratrol and black tea polyphenol combination synergistically suppress mouse skin tumors growth by inhibition of activated MAPKs and p53. PLoS One. 2011;6(8):e23395. doi: 10.1371/journal.pone.0023395. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 199.Jang M, Pezzuto JM. Effects of resveratrol on 12-O-tetrade-canoylphorbol-13-acetate-induced oxidative events and gene expression in mouse skin. Cancer Lett. 1998;134(1):81–9. doi: 10.1016/s0304-3835(98)00250-x. [DOI] [PubMed] [Google Scholar]
- 200.Kapadia GJ, Azuine MA, Tokuda H, Takasaki M, Mukainaka T, Konoshima T, et al. Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein-Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis. Pharmacol Res. 2002;45(6):499–505. doi: 10.1006/phrs.2002.0992. [DOI] [PubMed] [Google Scholar]
- 201.Junco JJ, Mancha A, Malik G, Wei SJ, Kim DJ, Liang H, et al. Resveratrol and P-glycoprotein inhibitors enhance the anti-skin cancer effects of ursolic acid. Mol Cancer Res. 2013;11(12):1521–9. doi: 10.1158/1541-7786.MCR-13-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tyagi A, Gu M, Takahata T, Frederick B, Agarwal C, Siriwardana S, et al. Resveratrol selectively induces DNA Damage, independent of Smad4 expression, in its efficacy against human head and neck squamous cell carcinoma. Clin Cancer Res. 2011;17(16):5402–11. doi: 10.1158/1078-0432.CCR-11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lin HY, Sun M, Tang HY, Simone TM, Wu YH, Grandis JR, et al. Resveratrol causes COX-2- and p53-dependent apoptosis in head and neck squamous cell cancer cells. J Cell Biochem. 2008;104(6):2131–42. doi: 10.1002/jcb.21772. [DOI] [PubMed] [Google Scholar]
- 204.Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J, Le AD. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol Cancer Ther. 2005;4(10):1465–74. doi: 10.1158/1535-7163.MCT-05-0198. [DOI] [PubMed] [Google Scholar]
- 205.Shrotriya S, Agarwal R, Sclafani RA. A perspective on chemoprevention by resveratrol in head and neck squamous cell carcinoma. Adv Exp Med Biol. 2015;815:333–48. doi: 10.1007/978-3-319-09614-8_19. [DOI] [PubMed] [Google Scholar]
- 206.Shan Z, Yang G, Xiang W, Pei-jun W, Bin Z. Effects of resveratrol on oral squamous cell carcinoma (OSCC) cells in vitro. J Cancer Res Clin Oncol. 2014;140(3):371–4. doi: 10.1007/s00432-013-1575-1. [DOI] [PubMed] [Google Scholar]
- 207.Osmond GW, Augustine CK, Zipfel PA, Padussis J, Tyler DS. Enhancing melanoma treatment with resveratrol. J Surg Res. 2012;172(1):109–15. doi: 10.1016/j.jss.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 208.Kma L. Synergistic effect of resveratrol and radiotherapy in control of cancers. Asian Pac J Cancer Prev. 2013;14(11):6197–208. doi: 10.7314/apjcp.2013.14.11.6197. [DOI] [PubMed] [Google Scholar]
- 209.Guan H, Singh NP, Singh UP, Nagarkatti PS, Nagarkatti M. Resveratrol prevents endothelial cells injury in high-dose interleukin-2 therapy against melanoma. PLoS One. 2012;7(4):e35650. doi: 10.1371/journal.pone.0035650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mohan A, Narayanan S, Sethuraman S, Krishnan UM. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. Biomed Res Int. 2014;2014:424239. doi: 10.1155/2014/424239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Trapp V, Parmakhtiar B, Papazian V, Willmott L, Fruehauf JP. Anti-angiogenic effects of resveratrol mediated by decreased VEGF and increased TSP1 expression in melanoma-endothelial cell co-culture. Angiogenesis. 2010;13(4):305–15. doi: 10.1007/s10456-010-9187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pastore S, Lulli D, Pascarella A, Maurelli R, Dellambra E, Potapovich A, et al. Resveratrol enhances solar UV-induced responses in normal human epidermal keratinocytes. Photochem Photobiol. 2012;88(6):1522–30. doi: 10.1111/j.1751-1097.2012.01195.x. [DOI] [PubMed] [Google Scholar]
- 213.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19(9):1193–5. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 214.Aziz MH, Afaq F, Ahmad N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in survivin. Photochem Photobiol. 2005;81(1):25–31. doi: 10.1562/2004-08-13-RA-274. [DOI] [PubMed] [Google Scholar]
- 215.Reagan-Shaw S, Afaq F, Aziz MH, Ahmad N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene. 2004;23(30):5151–60. doi: 10.1038/sj.onc.1207666. [DOI] [PubMed] [Google Scholar]
- 216.Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5(1):74–82. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vitale N, Kisslinger A, Paladino S, Procaccini C, Matarese G, Pierantoni GM, et al. Resveratrol couples apoptosis with autophagy in UVB-irradiated HaCaT cells. PLoS One. 2013;8(11):e80728. doi: 10.1371/journal.pone.0080728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Reagan-Shaw S, Mukhtar H, Ahmad N. Resveratrol imparts photoprotection of normal cells and enhances the efficacy of radiation therapy in cancer cells. Photochem Photobiol. 2008;84(2):415–21. doi: 10.1111/j.1751-1097.2007.00279.x. [DOI] [PubMed] [Google Scholar]
- 219.Niles RM, McFarland M, Weimer MB, Redkar A, Fu YM, Meadows GG. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett. 2003;190(2):157–63. doi: 10.1016/s0304-3835(02)00676-6. [DOI] [PubMed] [Google Scholar]
- 220.Gatouillat G, Balasse E, Joseph-Pietras D, Morjani H, Madoulet C. Resveratrol induces cell-cycle disruption and apoptosis in chemoresistant B16 melanoma. J Cell Biochem. 2010;110(4):893–902. doi: 10.1002/jcb.22601. [DOI] [PubMed] [Google Scholar]
- 221.Hsieh TC, Wang Z, Hamby CV, Wu JM. Inhibition of melanoma cell proliferation by resveratrol is correlated with upregulation of quinone reductase 2 and p53. Biochem Biophys Res Commun. 2005;334(1):223–30. doi: 10.1016/j.bbrc.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 222.Fuggetta MP, D’Atri S, Lanzilli G, Tricarico M, Cannavo E, Zambruno G, et al. In vitro antitumour activity of resveratrol in human melanoma cells sensitive or resistant to temozolomide. Melanoma Res. 2004;14(3):189–96. doi: 10.1097/01.cmr.0000130007.54508.b2. [DOI] [PubMed] [Google Scholar]
- 223.Larrosa M, Tomas-Barberan FA, Espin JC. Grape polyphenol resveratrol and the related molecule 4-hydroxystilbene induce growth inhibition, apoptosis, S-phase arrest, and upregulation of cyclins A, E, and B1 in human SK-Mel-28 melanoma cells. J Agric Food Chem. 2003;51(16):4576–84. doi: 10.1021/jf030073c. [DOI] [PubMed] [Google Scholar]
- 224.Habibie H, Yokoyama S, Abdelhamed S, Awale S, Sakurai H, Hayakawa Y, et al. Survivin suppression through STAT3/beta-catenin is essential for resveratrol-induced melanoma apoptosis. Int J Oncol. 2014;45(2):895–901. doi: 10.3892/ijo.2014.2480. [DOI] [PubMed] [Google Scholar]
- 225.Kang NE, Ha AW, Kim JY, Kim WK. Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3T3-L1 preadipocytes. Nutr Res Pract. 2012;6(6):499–504. doi: 10.4162/nrp.2012.6.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yang Z, Yang S, Misner BJ, Chiu R, Liu F, Meyskens FL., Jr Nitric oxide initiates progression of human melanoma via a feedback loop mediated by apurinic/apyrimidinic endonuclease-1/redox factor-1, which is inhibited by resveratrol. Mol Cancer Ther. 2008;7(12):3751–60. doi: 10.1158/1535-7163.MCT-08-0562. [DOI] [PubMed] [Google Scholar]
- 227.Kim MY. Nitric oxide triggers apoptosis in A375 human melanoma cells treated with capsaicin and resveratrol. Mol Med Rep. 2012;5(2):585–91. doi: 10.3892/mmr.2011.688. [DOI] [PubMed] [Google Scholar]
- 228.van Ginkel PR, Darjatmoko SR, Sareen D, Subramanian L, Bhattacharya S, Lindstrom MJ, et al. Resveratrol inhibits uveal melanoma tumor growth via early mitochondrial dysfunction. Invest Ophthalmol Vis Sci. 2008;49(4):1299–306. doi: 10.1167/iovs.07-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen YJ, Chen YY, Lin YF, Hu HY, Liao HF. Resveratrol inhibits alpha-melanocyte-stimulating hormone signaling, viability, and invasiveness in melanoma cells. Evid Based Complement Alternat Med. 2013;2013:632121. doi: 10.1155/2013/632121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bhattacharya S, Darjatmoko SR, Polans AS. Resveratrol modulates the malignant properties of cutaneous melanoma through changes in the activation and attenuation of the antiapoptotic protooncogenic protein Akt/PKB. Melanoma Res. 2011;21(3):180–7. doi: 10.1097/CMR.0b013e3283456dfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang M, Yu T, Zhu C, Sun H, Qiu Y, Zhu X, et al. Resveratrol triggers protective autophagy through the ceramide/Akt/mTOR pathway in melanoma B16 cells. Nutr Cancer. 2014;66(3):435–40. doi: 10.1080/01635581.2013.878738. [DOI] [PubMed] [Google Scholar]
- 232.Lee SH, Koo BS, Park SY, Kim YM. Anti-angiogenic effects of resveratrol in combination with 5-fluorouracil on B16 murine melanoma cells. Mol Med Rep. 2015;12(2):2777–83. doi: 10.3892/mmr.2015.3675. [DOI] [PubMed] [Google Scholar]
- 233.Junco JJ, Mancha-Ramirez A, Malik G, Wei SJ, Kim DJ, Liang H, et al. Ursolic acid and resveratrol synergize with chloroquine to reduce melanoma cell viability. Melanoma Res. 2015;25(2):103–12. doi: 10.1097/CMR.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 234.Salado C, Olaso E, Gallot N, Valcarcel M, Egilegor E, Mendoza L, et al. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. J Transl Med. 2011;9:59. doi: 10.1186/1479-5876-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Uchiyama T, Toda K, Takahashi S. Resveratrol inhibits angiogenic response of cultured endothelial F-2 cells to vascular endothelial growth factor, but not to basic fibroblast growth factor. Biol Pharm Bull. 2010;33(7):1095–100. doi: 10.1248/bpb.33.1095. [DOI] [PubMed] [Google Scholar]
- 236.Kornhauser A, Wei RR, Yamaguchi Y, Coelho SG, Kaidbey K, Barton C, et al. The effects of topically applied glycolic acid and salicylic acid on ultraviolet radiation-induced erythema, DNA damage and sunburn cell formation in human skin. J Dermatol Sci. 2009;55(1):10–7. doi: 10.1016/j.jdermsci.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lin AN, Nakatsui T. Salicylic acid revisited. Int J Dermatol. 1998;37(5):335–42. doi: 10.1046/j.1365-4362.1998.00452.x. [DOI] [PubMed] [Google Scholar]
- 238.Madan RK, Levitt J. A review of toxicity from topical salicylic acid preparations. J Am Acad Dermatol. 2014;70(4):788–92. doi: 10.1016/j.jaad.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 239.Birgin B, Fetil E, Ilknur T, Tahsin Gunes A, Ozkan S. Effects of topical petrolatum and salicylic acid upon skin photoreaction to UVA. Eur J Dermatol. 2005;15(3):156–8. [PubMed] [Google Scholar]
- 240.Fetil E, Ozka S, Soyal MC, Ilknur T, Erdem Y, Gunes AT. Effects of topical petrolatum and salicylic acid on the erythemogenicity of UVB. Eur J Dermatol. 2002;12(2):154–6. [PubMed] [Google Scholar]
- 241.Dainichi T, Ueda S, Isoda M, Koga T, Kinukawa N, Nose Y, et al. Chemical peeling with salicylic acid in polyethylene glycol vehicle suppresses skin tumour development in hairless mice. Br J Dermatol. 2003;148(5):906–12. doi: 10.1046/j.1365-2133.2003.05282.x. [DOI] [PubMed] [Google Scholar]
- 242.Stockfleth E, Kerl H, Zwingers T, Willers C. Low-dose 5-fluo-rouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165(5):1101–8. doi: 10.1111/j.1365-2133.2011.10387.x. [DOI] [PubMed] [Google Scholar]
- 243.Goncalves JC. Treatment of solar keratoses with a 5-fluorouracil and salicylic acid varnish. Br J Dermatol. 1975;92(1):85–8. doi: 10.1111/j.1365-2133.1975.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 244.Simon JC, Dominicus R, Karl L, Rodriguez R, Willers C, Dirschka T. A prospective randomized exploratory study comparing the efficacy of once-daily topical 0.5 % 5-fluorouracil in combination with 10.0 % salicylic acid (5-FU/SA) vs. cryosurgery for the treatment of hyperkeratotic actinic keratosis. J Eur Acad Dermatol Venereol. 2015;29(5):881–9. doi: 10.1111/jdv.12702. [DOI] [PubMed] [Google Scholar]
- 245.Ulrich M, Alarcon I, Malvehy J, Puig S. In vivo reflectance confocal microscopy characterization of field-directed 5-fluo-rouracil 0.5 %/salicylic acid 10 % in actinic keratosis. Dermatology. 2015;230(3):193–8. doi: 10.1159/000370148. [DOI] [PubMed] [Google Scholar]
- 246.Vad NM, Yount G, Moridani MY. Biochemical mechanism of acetylsalicylic acid (Aspirin) selective toxicity toward melanoma cell lines. Melanoma Res. 2008;18(6):386–99. doi: 10.1097/CMR.0b013e3283107df7. [DOI] [PubMed] [Google Scholar]
- 247.Tamura S, Nitoda T, Kubo I. Effects of salicylic acid on mushroom tyrosinase and B16 melanoma cells. Z Naturforsch C. 2007;62(3–4):227–33. doi: 10.1515/znc-2007-3-412. [DOI] [PubMed] [Google Scholar]
- 248.Ordan O, Rotem R, Jaspers I, Flescher E. Stress-responsive JNK mitogen-activated protein kinase mediates aspirin-induced suppression of B16 melanoma cellular proliferation. Br J Pharmacol. 2003;138(6):1156–62. doi: 10.1038/sj.bjp.0705163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hait DSaM. An ontological design: two stage mouse skin carcinogenesis induced By DMBA and promoted by croton oil. Asian J Res Pharm Sci. 2012;2(1):1–3. [Google Scholar]
- 250.Andrade RG, Jr, Dalvi LT, Silva JM, Jr, Lopes GK, Alonso A, Hermes-Lima M. The antioxidant effect of tannic acid on the in vitro copper-mediated formation of free radicals. Arch Biochem Biophys. 2005;437(1):1–9. doi: 10.1016/j.abb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 251.Srivastava RC, Husain MM, Hasan SK, Athar M. Green tea polyphenols and tannic acid act as potent inhibitors of phorbol ester-induced nitric oxide generation in rat hepatocytes independent of their antioxidant properties. Cancer Lett. 2000;153(1–2):1–5. doi: 10.1016/s0304-3835(99)00400-0. [DOI] [PubMed] [Google Scholar]
- 252.Athar M, Khan WA, Mukhtar H. Effect of dietary tannic acid on epidermal, lung, and forestomach polycyclic aromatic hydrocarbon metabolism and tumorigenicity in Sencar mice. Cancer Res. 1989;49(21):5784–8. [PubMed] [Google Scholar]
- 253.Heng MC, Allen SG, Kim A. Tannic-acid staining material on high endothelial venules and lymphocytes in skin and peripheral lymph nodes in Staphylococcus aureus-associated erythroderma. Clin Exp Dermatol. 1990;15(6):415–21. doi: 10.1111/j.1365-2230.1990.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 254.Chou WW, Wang YS, Chen KC, Wu JM, Liang CL, Juo SH. Tannic acid suppresses ultraviolet B-induced inflammatory signaling and complement factor B on human retinal pigment epithelial cells. Cell Immunol. 2012;273(1):79–84. doi: 10.1016/j.cellimm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 255.Feliciani C, Ruocco E, Zampetti A, Toto P, Amerio P, Tulli A, et al. Tannic acid induces in vitro acantholysis of keratinocytes via IL-1alpha and TNF-alpha. Int J Immunopathol Pharmacol. 2007;20(2):289–99. doi: 10.1177/039463200702000209. [DOI] [PubMed] [Google Scholar]
- 256.Yang EB, Wei L, Zhang K, Chen YZ, Chen WN. Tannic acid, a potent inhibitor of epidermal growth factor receptor tyrosine kinase. J Biochem. 2006;139(3):495–502. doi: 10.1093/jb/mvj050. [DOI] [PubMed] [Google Scholar]
- 257.Das M, Bickers DR, Mukhtar H. Protection against chemically induced skin tumorigenesis in SENCAR mice by tannic acid. Int J Cancer. 1989;43(3):468–70. doi: 10.1002/ijc.2910430321. [DOI] [PubMed] [Google Scholar]
- 258.Majed F, Rashid S, Khan AQ, Nafees S, Ali N, Ali R, et al. Tannic acid mitigates the DMBA/croton oil-induced skin cancer progression in mice. Mol Cell Biochem. 2015;399(1–2):217–28. doi: 10.1007/s11010-014-2248-3. [DOI] [PubMed] [Google Scholar]
- 259.Khan WA, Wang ZY, Athar M, Bickers DR, Mukhtar H. Inhibition of the skin tumorigenicity of (+/−)-7 beta,8 alpha-dihy-droxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene by tannic acid, green tea polyphenols and quercetin in Sencar mice. Cancer Lett. 1988;42(1–2):7–12. doi: 10.1016/0304-3835(88)90232-7. [DOI] [PubMed] [Google Scholar]
- 260.Park HJ, Kim HJ, Kwon HJ, Lee JY, Cho BK, Lee WJ, et al. UVB-induced interleukin-18 production is downregulated by tannic acids in human HaCaT keratinocytes. Exp Dermatol. 2006;15(8):589–95. doi: 10.1111/j.1600-0625.2006.00449.x. [DOI] [PubMed] [Google Scholar]
- 261.Gensler HL, Gerrish KE, Williams T, Rao G, Kittelson J. Prevention of photocarcinogenesis and UV-induced immuno-suppression in mice by topical tannic acid. Nutr Cancer. 1994;22(2):121–30. doi: 10.1080/01635589409514337. [DOI] [PubMed] [Google Scholar]
- 262. [Accessed 12 Apr 2016]; http://www.drugs.com/pro/veregen.html.