Abstract
Background
Formal pulmonary function testing with laboratory spirometry (LS) is standard of care for risk stratification before lung resection. LS and handheld office spirometry (OS) are clinically comparable for forced expiratory volume in 1 second (FEV1) and forced vital capacity. We investigated the safety of preoperative risk stratification based solely on OS.
Methods
Patients at low-risk for cardiopulmonary complications were enrolled in a single-center prospective study and underwent preoperative OS. When FEV1% was >60% by OS, formal LS was not performed. Using propensity score matching, patients in the OS group were compared to low-risk institutional database patients (2008–2015) who underwent LS and lung resection. Standardized mean differences determined model covariate balance. McNemar’s test and log-rank test were performed respectively for categorical and continuous paired outcome data.
Results
66 prospectively enrolled patients received OS, and underwent pulmonary resection. 1290 patients received preoperative LS, resulting in 52 propensity score matched pairs (83%). There were no mortalities and two 30-day readmissions per group. Major morbidity risk was similar in each group (7.7%). All analyses of discordant pair morbidity had p >0.56. There was no association between length of stay and exposure to OS vs LS (p=0.31). The estimated annual institutional cost savings from performing OS only and avoiding LS was $38,000.
Conclusions
Low-risk patients undergoing lung resection can be adequately and safely assessed using OS without formal LS, with significant cost savings. With upcoming bundled care reimbursement paradigms, such safe and effective strategies are likely to be more widely employed.
Postoperative pulmonary complications (PPCs) are the leading cause of death after both cardiothoracic and non-cardiothoracic surgeries [1–7]. The most common PPCs are pneumonia and lobar atelectasis requiring bronchoscopy, however PPCs also include postoperative respiratory failure, the need for reintubation, prolonged mechanical ventilation, pleural effusions, pneumothoraces, exacerbations of chronic obstructive pulmonary disease (COPD), and bronchospasm [2]. While the incidence of PPCs after upper abdominal surgery is 12–17%, the incidence after thoracic surgery is 38–59% [2, 8, 9]. In addition to mortality, PPCs are a major driver of postoperative length of stay, rehospitalization, and healthcare utilization after pulmonary resection [2, 10, 11]. Therefore, much emphasis has been placed on appropriate risk stratification prior to pulmonary resection to allow clinicians and patients to make informed decisions regarding treatment options.
Well-established risk factors for PPC after pulmonary resection are COPD, smoking, age, cardiovascular comorbidity, functional status, and abnormal pulmonary function tests (PFTs). Many patients with potentially resectable lung tumors are at greater risk than the general population for both short-term complications and long-term disability owing to tobacco-associated cardiopulmonary disease [2, 3, 12, 13]. Therefore, universal preoperative risk stratification via screening spirometry and electrocardiogram prior to lung surgery has been recommended by the American College of Chest Physicians, the British Thoracic Society, and the European Society of Thoracic Surgeons [14–16]. Specifically, forced expiratory volume in one second (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO) should be measured in order to detect reductions in lung volume and function. Prior to anatomic lung resection, FEV1 or DLCO <30–40% predicted is associated with high risk for postoperative mortality and warrants further physiologic testing, whereas a patient with FEV1 and DLCO >60% predicted is considered to be low-risk and no further testing is indicated [14–16].
Formal laboratory based volume displacement spirometry has conventionally been used because it has high internal and external validity, is non-invasive, and is widely available. It is considered to be the standard of care for risk stratification prior to pulmonary resection [12, 17]. Yet, anecdotally, many clinicians know that on the healthy extreme of patient populations, laboratory spirometry (LS) is predictably normal. In these situations, LS results rarely change clinical management, making it one contributor to the high costs of healthcare.
One potential alternative for preoperative screening of pulmonary function and reserve is handheld office spirometry (OS). For patients undergoing lung surgery, we previously showed that office spirometry FEV1 and FVC are clinically comparable to laboratory spirometry values [24]. Subsequently, we questioned whether LS is necessary prior to surgical resection or whether OS alone is sufficient. In contrast to LS, OS is lower cost, quicker, portable, and easier to use for both providers and patients yet still provides FEV1 and forced vital capacity (FVC) [14, 18]. Several studies have shown good correlation between modern flow-sensing OS and formal LS values across a wide range of manufactured OS devices. OS results are reproducible and have high validity. Many researchers have advocated for more wide-spread use of OS in primary care settings and in epidemiological studies [18–23]. The purpose of this study was to investigate the safety of preoperative risk stratification for lung resection in low-risk patients based solely on OS. We hypothesized that an identifiable proportion of patients exists for which formal LS is not required prior to lung resection.
Patients and Methods
Prospectively, we enrolled patients being evaluated for pulmonary resection who were determined to be at low risk for postoperative cardiopulmonary complications based on easily identifiable markers of pulmonary and functional status (Table 1). Possible participants were identified via convenience sampling at their initial clinic visit to the Washington University Lung Center. Patients who did not meet these criteria were re-categorized as a higher risk population and followed standard of care. The study was approved by the Washington University School of Medicine Human Research Protection Office.
Table 1.
Inclusion and exclusion screening criteria for low-risk patients undergoing pulmonary resection. These indicators can be implemented rapidly and easily in an outpatient clinic setting in order to allow OS to be integrated into the natural clinic flow. ASA, American Society of Anesthesiologists.
| Inclusion criteria |
| Age >18 years |
| Lung lesion requiring lobar, sublobar, or bilobar resection |
| Climb ≥2 flights of stairs without shortness of breath |
| Karnofsky performance status ≥ 80 or Zubrod score ≤1 |
| Exclusion criteria |
| Prior lung resection (i.e. redo) |
| Prior chest radiation or chemotherapy for current lung cancer |
| Recent pneumonia (≤ 30 days) |
| Home oxygen requirement |
| ≥3 months use of inhaled bronchodilators or steroids |
| Known or suspected chest wall invasion |
| Planned pneumonectomy |
| Interstitial fibrosis |
| Emergency or eminent threat to life (ASA class 4–6) |
Participants were enrolled in both pilot and study phases then were combined for final statistical analysis. In order to evaluate and compare OS and LS, pilot phase participants underwent formal LS as well as screening OS. OS was performed using the Micromed CE 0120 spirometer (Micro Direct Inc., Maine, USA) by a dedicated, trained nurse. The equipment was maintained and calibration checks performed per recommendations of the respiratory laboratory. FEV1 and FVC percent predicted values were calculated according to reference standards [24].
Interim analysis confirmed clinical comparability between OS and LS for FEV1 and FEV1% in this low-risk population [25]. Therefore, in the subsequent study phase, the protocol used OS as a universal screening tool. If FEV1% was less than 60%, patients were deemed study ineligible due to higher risk of PPCs and proceeded to formal LS. Participants with FEV1% greater than 60% predicted by OS underwent surgery without LS. The participants received routine workup for surgery including clinical testing as indicated.
The primary end point of the study was discharge from hospital. The secondary end-points were perioperative mortality (death within 30 days of surgery), postoperative respiratory failure (need for postoperative mechanical ventilation or bronchoscopy for atelectasis), hospital readmission within 30 days of surgery, length of stay, and the costs associated with conduction and interpretation of the pulmonary function test (laboratory and office based). Additional major morbidities were also recorded.
Descriptive statistics of continuous variables were reported as mean ± standard deviation. Student t tests were used to analyze normally distributed continuous data. Pearson χ2 tests were used to compare categorical data. Propensity score technique was used to address the influence of selection bias in preoperative testing allocation. Study patients who received OS were matched with similar low-risk patients from our institutional surgical database (2008–2015) who had undergone preoperative LS. Patient, tumor, and treatment variable definitions are in accordance with the Society of Thoracic Surgeons national General Thoracic Surgery Database [26]. The propensity score between the OS and LS groups was based on patient and surgical characteristics related to the outcome and was estimated using a non-parsimonious logistic regression model. Variables with p≤0.05 in univariate analysis or biologic plausibility were included (Supplemental Table 1). Balance within the model was assessed using standardized difference of the means, with an absolute value >0.10 suggestive of a meaningful difference. Propensity score matching was performed in 1:1 fashion without replacement via a caliper of 0.2 standard deviations of the logit of the propensity score. Match pairs were analyzed using McNemar’s test for categorical variables with discordant pairs and log-rank test for continuous variables.
Expenditures were based on the national Medicare Fee Schedule and represent the sum of the technical and interpretive components (in 2015 US $). All statistical analyses were performed in SAS for Windows (Version 9.4. Cary, NC. SAS Institute Inc. 2012).
Results
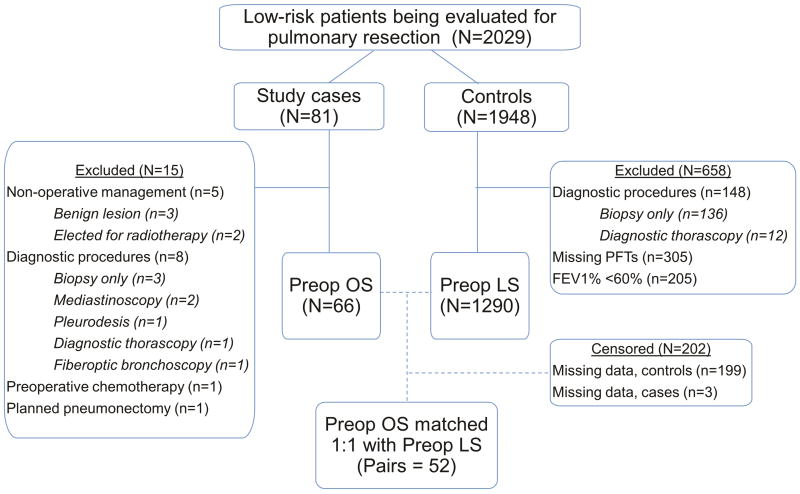
Between May 2008 and February 2012, 81 participants were enrolled in this study. 5 were inevitably non-operative and were excluded from analysis. Additionally, 9 participants underwent only diagnostic procedures while 1 was determined to require a pneumonectomy and therefore required LS. In retrospect, 1 participant was ineligible due to chemotherapy within 6 months of study enrollment (Figure 1). The final cohort was 66 participants (81.5%).
Figure 1.
Inclusion schema and resultant pairs after propensity score matching. FEV1, forced expiratory volume in 1 second; LS, laboratory spirometry; OS, office spirometry; PFT, pulmonary function test.
Within the institutional database (2008–2015), 1948 patients were similar to our study population based on inclusion/exclusion criteria. Of these, 305 patients had missing LS and therefore were excluded. 205 patients were excluded as their FEV1% was less than 60% and were not candidates for matching as our protocol recommended LS for high risk patients. The final control cohort was 1290 low-risk patients, or 63.6% of all low risk patients undergoing surgery.
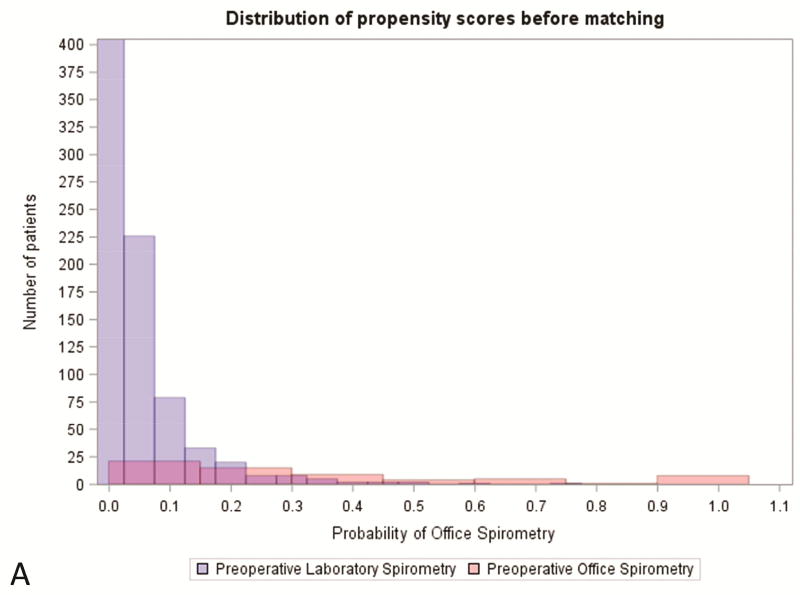
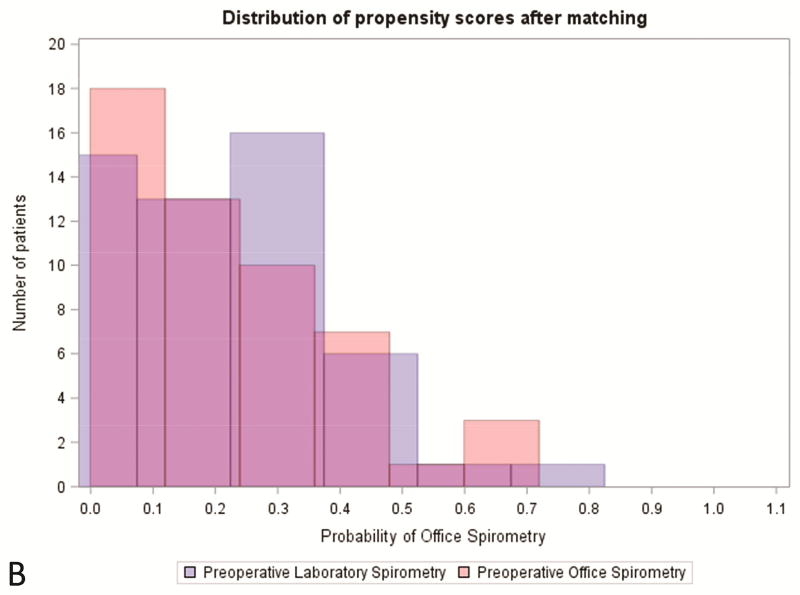
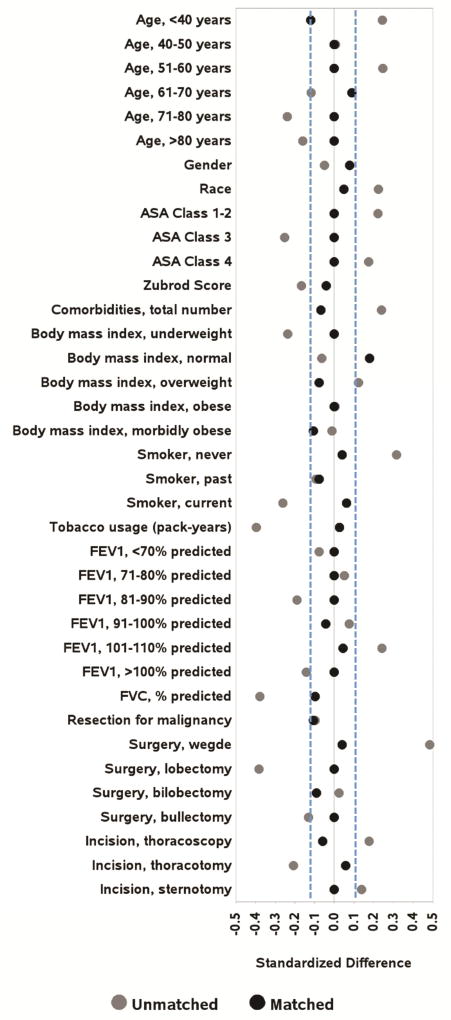
There were statistically significant differences between the study group and the control group pertaining to age, ASA classification, smoking status, FVC%, preoperative comorbidities, pack-year history, surgery type, and incision type (Table 2). Patients were well-matched at baseline for the remainder of characteristics. Using propensity score matching, we identified 52 matched pairs (82.5%, Table 2). ( Covariate balance improved to established thresholds for nearly all variables included in the propensity score (Figures 2–3, Supplemental Table 2).
Table 2.
Univariate analysis comparing office spirometry and laboratory spirometry cohorts both before and after propensity score matching. ASA, American Society of Anesthesiologists; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation.
| Total cohort (N=1356) |
Propensity score matched (N=104) |
||||||
|---|---|---|---|---|---|---|---|
| No. of patients (%) | No. of patients (%) | ||||||
| Characteristics | Office spirometry (N=66) |
Laboratory spirometry (N=1290) |
p value | Office spirometry (N=52) |
Laboratory spirometry (N=52) |
p value | |
| Age, mean (SD), in years | 57.67 (12.54) | 62.51 (12.32) | *0.002 | 57.70 (12.23) | 56.19 (13.42) | 0.553 | |
| Male sex | 30 (45.45) | 554 (42.95) | 0.688 | 27 (51.92) | 25 (48.08) | 0.694 | |
| Race | White | 52 (78.79) | 1124 (87.13) | 0.051 | 42 (80.77) | 43 (82.69) | 0.800 |
| Other | 14 (21.21) | 166 (12.87) | 0.051 | 10 (19.23) | 9 (17.31) | 0.800 | |
| Body mass index, mean (SD) | 28.67 (6.29) | 28.03 (6.39) | 0.425 | 28.77 (6.17) | 29.33 (6.07) | 0.640 | |
| Smoking | Never smoker | 27 (41.54) | 345 (26.76) | *0.009 | 22 (42.31) | 21 (40.38) | 0.842 |
| Former smokera | 28 (43.08) | 614 (47.63) | 0.473 | 24 (46.15) | 26 (50.00) | 0.695 | |
| Current smoker | 10 (15.15) | 330 (25.58) | 0.057 | 6 (11.54) | 5 (9.62) | 0.750 | |
| Smoking exposure, mean (SD), in pack-years | 35.14 (21.54) | 43.26 (30.15) | *0.033 | 18.88 (23.26) | 18.21 (27.62) | 0.893 | |
| FEV1% predicted, mean (SD) | 89.33 (15.89) | 88.15 (16.24) | 0.564 | 90.77 (15.26) | 89.40 (15.27) | 0.648 | |
| FVC% predicted, mean (SD) | 88.61 (22.09) | 95.94 (16.29) | *<0.001 | 89.19 (18.17) | 91.06 (19.95) | 0.618 | |
| Number of preoperative comorbidities, mean (range) | 0.78 (0–3) | 0.98 (0–5) | 0.134 | 0.78 (0–3) | 0.75 (0–4) | 0.901 | |
| ASA classification | 1–2 | 23 (34.85) | 320 (24.81) | 0.067 | 17 (32.69) | 17 (32.69) | 1.000 |
| 3 | 42 (63.64) | 969 (75.12) | *0.034 | 35 (67.31) | 35 (67.31) | 1.000 | |
| 4b | 1 (1.52) | 0 | *<0.001 | 0 | 0 | - | |
| Disease Category | Benign | 12 (18.18) | 187 (14.56) | 0.419 | 9 (17.31) | 7 (13.46) | 0.587 |
| Malignant | 54 (81.82) | 1097 (85.44) | 0.419 | 43 (82.69) | 45 (86.54) | 0.587 | |
| Surgery type | Wedge resection | 39 (59.09) | 459 (35.58) | *<0.001 | 34 (65.38) | 33 (63.46) | 0.838 |
| Segmentectomy | 0 | 56 (4.34) | 0.084 | 0 | 0 | - | |
| Lobectomy | 25 (37.88) | 730 (56.59) | *0.003 | 16 (30.77) | 16 (30.77) | 1.000 | |
| Bilobectomy | 2 (3.03) | 34 (2.64) | 0.846 | 2 (3.85) | 3 (5.77) | 0.647 | |
| Bullectomy | 0 | 11 (0.85) | 0.451 | 0 | 0 | - | |
| Incision typec | Thoracoscopy | 58 (87.88) | 947 (73.41) | *0.009 | 45 (86.54) | 46 (88.46) | 0.767 |
| Thoracotomy | 7 (10.61) | 339 (26.28) | *0.004 | 7 (13.46) | 6 (11.54) | 0.767 | |
| Sternotomy | 1 (1.52) | 3 (0.23) | 0.061 | 0 | 0 | - | |
Quit >1 month prior.
Should have been screen fail.
Intention to treat.
Figure 2.
Distibution of propensity score (A) prior to and (B) after matching office spirometry and laboratory spirometry patients. PFT, pulmonary function test.
Figure 3.
Standardized differences before and after propensity score matching for variables included in the propensity score logistic regression model. Blue dashed vertical lines represent the limits, ±10%, outside of which standardized differences indicate imbalance. ASA, American Society of Anesthesiologists; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
All matched participants met the primary end point of discharge from the hospital. In the propensity matched groups, no patients died within 30 days postoperatively, 1 OS patient and 3 LS patients experienced 30-day readmission, and 1 patient per group had one or more components of postoperative respiratory failure, specifically chest tube airleak or therapeutic bronchoscopy. The rate of major morbidities was similar, at 4 patients per group (7.7%, Table 3). No association between OS and LS was found for 30-day readmission, length of stay, or major morbidities (all p >0.3).
Table 3.
Major postoperative complications seen in the office spirometry (OS) and laboratory spirometry (LS) groups.
| Major complication | Events in total low risk cohort |
Events in OS matched group |
Events in LS matched group |
|
|---|---|---|---|---|
| Additional anesthesia | 25 | 0 | 1 | |
| Chest tube airleak | 44 | 1 | 2 | |
| Delirium Tremens | 6 | 0 | 0 | |
| Deep venous thrombosis | 11 | 0 | 0 | |
| Empyema | 5 | 0 | 0 | |
| Intraoperative transfusion | 37 | 0 | 0 | |
| Myocardial infarction | 3 | 1 | 0 | |
| Other cardiac | 19 | 0 | 1 | |
| Other gastrointestinal | 8 | 0 | 0 | |
| Other miscellaneous | 87 | 0 | 0 | |
| Other neurologic | 20 | 1 | 0 | |
| Other pulmonary | 19 | 0 | 1 | |
| Pneumonia | 38 | 0 | 0 | |
| Pulmonary embolism | 8 | 0 | 0 | |
| Reintubation | 23 | 0 | 0 | |
| Reoperation | 11 | 0 | 0 | |
| Tracheostomy | 17 | 0 | 0 | |
| Urinary tract infection | 34 | 1 | 0 | |
| Ventilation >48 hours | 4 | 0 | 0 | |
| Total events | 402 | 4 | 5 | |
| Total patients | 268 | 4 | 4 | |
Medicare allowable charges for OS are $37 while LS costs $170 (2015 US$). Therefore, the incremental cost savings of OS over LS is $133. During the 8 years included for database analysis, low-risk patients with available pulmonary function data constituted 47% of our surgical population (1356/2889). Using this conservative proportion as a theoretical ideal, performing OS rather than LS in low-risk patients undergoing pulmonary resection would results in an estimated institutional cost savings of approximately $38,000 per year.
Comment
Several national and international evidence-based guidelines recommend preoperative PFTs [14–16] and these are conventionally interpreted to mean laboratory spirometry (LS). However, no such declaration is made and handheld office spirometry (OS) is clinically comparable to LS for FEV1 and FVC. Our findings suggest that screening pulmonary function solely by OS prior to pulmonary resection is sufficient and safe for carefully selected low risk patients.
The purpose of preoperative PFTs is to stratify individuals at perceived risk of postoperative pulmonary complications (PPCs) and to facilitate informed consent about treatment hazards. PPCs are associated with higher rates of mortality and morbidity, as well as higher health care costs [11]. Having used clinical history exclusion criteria to identify patients at high risk for PPCs after thoracic surgery, the incidence of PPCs in our low-risk cohort was well below the described rate of PPCs in the general population of patients undergoing lung resection (38–59%) [2, 8, 9]. Interestingly, the unmatched patient who suffered the greatest morbidity postoperatively was identified as high risk by office spirometry (FEV1% 44%) and not by laboratory spirometry (FEV1% 69%) and was ultimately deemed ASA class 4.. This strengthens our hypothesis that lower risk patients can be readily identified and that our screening criteria were effective in doing so. Furthermore, our findings suggest that formal laboratory based volume displacement spirometry would infrequently result in a change in the clinical management of these low risk patients.
LS and OS are both reproducible, validated, and safe in low risk pulmonary resection. However, OS is more time efficient, user-friendly, lower maintenance, and notably economical [18–23, 25]. The incremental cost savings by forgoing LS in lieu of OS for low risk patients undergoing pulmonary resection was $133 per patient. Based on our surgical volume in 2014 and the fact that 47% of those patients were eligible for OS by our proposed screening criteria, the annual institutional savings would be approximately $38,000. Because Medicare allowable charges are typically lower than commercial payer reimbursement, the true annual avoidable spending is likely substantially greater.
The Choosing Wisely campaign is a well-known example of successful dialogue about unnecessary testing. It has encouraged societies and organizations to identify and question the need for tests and procedures in their fields of practice. In their recent survey of 600 representative physicians, nearly three-quarters felt that unnecessary testing or procedures was a major healthcare problem and almost half indicated that they prescribe unnecessary testing at least weekly. The primary reasons provided for this behavior was for physician reassurances and to assuage malpractice concerns. Only 1 in 5 physicians reported discussing the cost of testing consistently with their patients. Respondents indicated great interest in more evidence-based recommendations regarding unnecessary care [27].
As our understanding of the predictive potential of PFT values grows, several note-worthy changes were made in the 3rd edition of the American College of Chest Physicians evidence-based clinical practice guidelines for physiologic evaluation prior to lung resection. First, the FEV1% threshold for low risk decreased from <80% to <60% based on emerging research in lung-volume reduction surgery. Likewise, the threshold for low risk DLCO decreased from <80% to <60%. FEV1% or DLCO within 30–60% warrant low technology exercise testing, such as a shuttle walk or stair climbing test. If satisfactory performance is met, the patient is considered low risk despite their abnormal PFT values [14, 29, 30]. While FEV1 and DLCO were previously thought to be complementary physiologic tests, the actual correlation is consistently poor. DLCO is associated with both short- and long-term outcomes and may be aberrant despite normal FEV1 [28, 31]. Therefore, the 2013 guidelines recommend DLCO prior to all lung cancer resections [14]. However, this recommendation is not universal among governing societies, especially in otherwise low risk patients [13, 15, 16]. Further evidence of the controversy can be seen in the major thoracic databases in which the Society of Thoracic Surgeons reports only 57% of patients undergoing major pulmonary resection received DLCO, while the European Thoracic Surgery Database reports <25% usage [32].
DLCO measurement is typically a convenient single-breath maneuver that historically was offered only in the laboratory setting. However, portable devices are now being used in office-based settings with results available in less than 20 minutes [33]. Even if laboratory DLCO is combined with OS for preoperative screening, the institutional incremental cost savings over LS is still approximately $24,000 annually. Furthermore, low DLCO values generally reflect interstitial lung disease and correlate well with findings on high-resolution computed tomography [34]. Therefore, as we await uniform consensus of our guidelines, the desire for preoperative DLCO does not preclude the selection of OS. Beyond DLCO, other potential barriers to implementation include force of habit for both surgeons and referring physicians, persistent fears of inferiority of low-technology assessments, and the financial implications for existing PFT laboratories.
It is important to note that this study has limitations. First, the targeted enrollment numbers were based on feasibility and not on power calculations. Second, the residual standard difference of the means for the propensity score implies that minimal residual bias remains in our dataset. This is likely due to variable probability of database patients receiving OS whereas our study population has absolute probability, leading to unmatchable study participants. We chose to sacrifice matching in order to reduce residual confounding, as highlighted by the 83% success rate for matching with the caliper method. However, the final model was well balanced and allowed for a more accurate assessment of the average treatment effect.
In conclusion, low-risk patients undergoing lung resection can be accurately, adequately, and safely assessed using handheld OS without the need for formal LS, with significant cost savings. This study supports the notion that preoperative OS could be the new standard of care for low-risk patients and that LS need not be administered indiscriminately. With upcoming bundled care reimbursement paradigms, such safe and effective strategies are likely to be more widely employed.
Supplementary Material
Abbreviations
- ASA
American Society of Anesthesiologists
- COPD
Chronic obstructive pulmonary disease
- DLCO
Diffusion capacity of the lungs for carbon monoxide
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- LS
Laboratory spirometry
- OS
Office spirometry
- PFT
Pulmonary function test
- PPC
Postoperative pulmonary complication
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amar D, et al. A clinical prediction rule for pulmonary complications after thoracic surgery for primary lung cancer. Anesth Analg. 2010;110(5):1343–8. doi: 10.1213/ANE.0b013e3181bf5c99. [DOI] [PubMed] [Google Scholar]
- 2.Agostini P, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax. 2010;65(9):815–8. doi: 10.1136/thx.2009.123083. [DOI] [PubMed] [Google Scholar]
- 3.Qaseem A, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144(8):575–80. doi: 10.7326/0003-4819-144-8-200604180-00008. [DOI] [PubMed] [Google Scholar]
- 4.McAlister FA, et al. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. Am J Respir Crit Care Med. 2005;171(5):514–7. doi: 10.1164/rccm.200408-1069OC. [DOI] [PubMed] [Google Scholar]
- 5.Arozullah AM, et al. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135(10):847–57. doi: 10.7326/0003-4819-135-10-200111200-00005. [DOI] [PubMed] [Google Scholar]
- 6.Rahmanian PB, Langebartels G KA, et al. Impact of major noncardiac complications on outcome following cardiac surgery procedures: logistic regression analysis in a very recent patient cohort. Interact Cardiovasc Thorac Surg. 2013;2013(17):319–327. doi: 10.1093/icvts/ivt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):581–95. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 8.Canet J, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–50. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Miguel FJ, Serrano-Aguilar PG, Lopez-Bastida J. Preoperative assessment. Lancet. 2003;362(9397):1749–57. doi: 10.1016/s0140-6736(03)14857-x. [DOI] [PubMed] [Google Scholar]
- 10.Stephan F, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest. 2000;118(5):1263–70. doi: 10.1378/chest.118.5.1263. [DOI] [PubMed] [Google Scholar]
- 11.Sabate S, Mazo V, Canet J. Predicting postoperative pulmonary complications: implications for outcomes and costs. Curr Opin Anaesthesiol. 2014;27(2):201–9. doi: 10.1097/ACO.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 12.Tanoue LT. Preoperative evaluation of the high-risk surgical patient for lung cancer resection. Semin Respir Crit Care Med. 2000;21(5):421–32. doi: 10.1055/s-2000-9404. [DOI] [PubMed] [Google Scholar]
- 13.Salati M, Brunelli A. Risk Stratification in Lung Resection. Curr Surg Rep. 2016;4(11):37. doi: 10.1007/s40137-016-0158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunelli A, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3d ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e166S–90S. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- 15.BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56(2):89–108. doi: 10.1136/thorax.56.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunelli A, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34(1):17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein WK. Pulmonary function testing. Curr Opin Anaesthesiol. 2012;25(1):11–6. doi: 10.1097/ACO.0b013e32834e7ad2. [DOI] [PubMed] [Google Scholar]
- 18.Schoh RJ, et al. Performance of a new screening spirometer at a community health fair. Respir Care. 2002;47(10):1150–7. [PubMed] [Google Scholar]
- 19.Gerbase MW, et al. Agreement between spirometers: a challenge in the follow-up of patients and populations? Respiration. 2013;85(6):505–14. doi: 10.1159/000346649. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson GT, et al. Office spirometry for lung health assessment in adults: A consensus statement from the National Lung Health Education Program. Chest. 2000;117(4):1146–61. doi: 10.1378/chest.117.4.1146. [DOI] [PubMed] [Google Scholar]
- 21.Ng TP, Tan WC, Hui KP. Ventilatory function measured with the Micro Spirometer: performance evaluation and reference values. Ann Acad Med Singapore. 1995;24(3):403–10. [PubMed] [Google Scholar]
- 22.Rebuck DA, et al. The accuracy of a handheld portable spirometer. Chest. 1996;109(1):152–7. doi: 10.1378/chest.109.1.152. [DOI] [PubMed] [Google Scholar]
- 23.Liistro G, et al. Technical and functional assessment of 10 office spirometers: A multicenter comparative study. Chest. 2006;130(3):657–65. doi: 10.1378/chest.130.3.657. [DOI] [PubMed] [Google Scholar]
- 24.Knudson RJ, et al. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113(5):587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 25.Puri V, et al. Handheld office-based spirometry versus laboratory spirometry in low-risk patients undergoing lung resection. Innovations (Phila) 2011;6(4):257–61. doi: 10.1097/IMI.0b013e31822a3709. [DOI] [PubMed] [Google Scholar]
- 26.General Thoracic Surgery Database Data Collection. STS National Database Data Managers 2016. 2016 Nov 4; Data Collection Forms and Training Manuals]. Available from: http://www.sts.org/quality-research-patient-safety/nationaldatabase/database-managers/general-thoracic-surgery-databa-1.
- 27.Choosing Wisely. AIBM Foundation. 2016. 2016 Nov 4; Available from: http://http://www.choosingwisely.org/
- 28.Ferguson MK, Siddique J, Karrison T. Modeling major lung resection outcomes using classification trees and multiple imputation techniques. Eur J Cardiothorac Surg. 2008;34(5):1085–9. doi: 10.1016/j.ejcts.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 29.Colice GL, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):161s–77s. doi: 10.1378/chest.07-1359. [DOI] [PubMed] [Google Scholar]
- 30.Awdeh H, et al. The SF-36 and 6-Minute Walk Test are Significant Predictors of Complications After Major Surgery. World J Surg. 2015;39(6):1406–12. doi: 10.1007/s00268-015-2961-4. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg. 2008;85(4):1158–64. doi: 10.1016/j.athoracsur.2007.12.071. discussion 1164-5. [DOI] [PubMed] [Google Scholar]
- 32.Berrisford R, et al. The European Thoracic Surgery Database project: modelling the risk of in-hospital death following lung resection. Eur J Cardiothorac Surg. 2005;28(2):306–11. doi: 10.1016/j.ejcts.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 33.Enright Md P. Office-based DLCO tests help pulmonologists to make important clinical decisions. Respir Investig. 2016;54(5):305–11. doi: 10.1016/j.resinv.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Ungprasert P, et al. Novel Assessment of Interstitial Lung Disease Using the CALIPER Software System in Idiopathic Inflammatory Myopathies. Lung. 2017;195:1–8. doi: 10.1007/s00408-017-0035-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.