Abstract
Background
Although children <5 years old in sub-Saharan Africa are vulnerable to both malaria and influenza, little is known about coinfection.
Methods
This retrospective, cross-sectional study in rural western Kenya examined outpatient visits and hospitalizations associated with febrile acute respiratory illness (ARI) during a 2-year period (July 2009–June 2011) in children <5 years old.
Results
Across sites, 45% (149/331) of influenza-positive patients were coinfected with malaria, whereas only 6% (149/2408) of malaria-positive patients were coinfected with influenza. Depending on age, coinfection was present in 4%–8% of outpatient visits and 1%–3% of inpatient admissions for febrile ARI. Children with influenza were less likely than those without to have malaria (risk ratio [RR], 0.57–0.76 across sites and ages), and children with malaria were less likely than those without to have influenza (RR, 0.36–0.63). Among coinfected children aged 24–59 months, hospital length of stay was 2.7 and 2.8 days longer than influenza-only-infected children at the 2 sites, and 1.3 and 3.1 days longer than those with malaria only (all P < .01).
Conclusions
Coinfection with malaria and influenza was uncommon but associated with longer hospitalization than single infections among children 24–59 months of age.
Children aged <5 years in sub-Saharan Africa are among the most vulnerable to morbidity and mortality due to malaria [1–3] and influenza [4]. The 2 diseases share many clinical features [5], can be difficult to differentiate on clinical grounds [6], and are in circulation year-round in many tropical areas [3, 7]. Yet, little is known about the prevalence, clinical presentation, or public health relevance of influenza and malaria coinfection [6, 7].
As part of ongoing health facility–based surveillance for febrile and respiratory illnesses in rural western Kenya, 2009–2011, children aged <5 years who presented to 2 hospitals were tested for both influenza and malaria. This gave us the opportunity to examine 3 questions: (1) How common is coinfection in this population? (2) How does the clinical presentation of coinfection differ from either infection alone? and (3) Does coinfection result in worse clinical outcomes? To examine the latter 2 questions, we distinguished between manifestations of respiratory disease (expected to be more common in children with influenza) and hematologic disease (expected to be more common in children with malaria). Because the risk of these factors varies by age [2, 4, 8], we stratified analyses into 4 subcategories defined by age group (0–24 vs 24–59 months) and health facility.
METHODS
Study Design and Population
The study was conducted at 2 hospitals, Lwak Mission Hospital (LMH) and Siaya District Hospital (SDH), in rural western Kenya (Nyanza Province), bordering Lake Victoria, where surveillance for acute respiratory illness (ARI) is conducted through a collaboration between the Kenya Medical Research Institute (KEMRI) and the Centers for Disease Control and Prevention–Kenya (CDC-Kenya). The area is one of the poorest in Kenya, has a high child mortality rate, and is malaria endemic, with a community-based parasite prevalence of approximately 40% among children aged <5 years [8–11]. This retrospective, cross-sectional study examined inpatient and outpatients who had febrile ARI and were dual tested for influenza and malaria during a 2-year period (1 July 2009–31 June 2011). At both hospitals, surveillance was conducted for severe ARI (SARI) (cough or difficulty breathing with an elevated respiratory rate or a respiratory danger sign) in hospitalized patients. At LMH only, surveillance was also conducted for influenza-like illness (ILI) (temperature ≥ 38.0°C and cough or sore throat) in outpatients. For the purposes of this analysis, we defined ARI as any patient with SARI or ILI.
Trained surveillance officers, primarily nurses, collected nasopharyngeal (NP) and oropharyngeal (OP) specimens from SARI and ILI patients and blood for malaria testing from patients with a history of fever with the illness. In addition, they collected demographic and clinical information from patients using structured questionnaires. Vital signs, including axillary temperature, were recorded upon enrollment. Oxygen saturation levels were measured using fingertip pulse oximetry. Parental reports of cough, convulsion, diarrhea, or vomiting were recorded. The admitting clinician indicated whether respiratory distress (difficulty breathing, wheezing, chest indrawing, stridor, or crackles) was present, and clinical diagnoses (anemia, pneumonia) were recorded from the physical exam. Clinicians had access to malaria test results, but not influenza test results. Duration of hospitalization (in days) and ordering of chest radiographs were recorded. Blood transfusions, hemoglobin concentration, and human immunodeficiency virus (HIV) test results were recorded at SDH only.
Laboratory Methods
NP and OP swabs were collected from SARI and ILI patients at enrollment and combined in the same cryovial with viral transport medium and transported to the KEMRI/CDC laboratory in Nairobi, where they were tested for influenza A and B using real-time reverse-transcription polymerase chain reaction (RTPCR) assay. The specimen management and testing protocol, which used primers, probes, and reagents from the CDC, has been described in detail elsewhere [12]. Malaria testing included thick and thin blood smears stained with 10% Giemsa read independently by 2 microscopists trained at a regional center of excellence. Asexual parasites ≥ 1 on examination of 100 high-power oil immersion fields indicated a positive result.
Statistical Analysis
Only patients who had a history of fever within 7 days of enrollment were included in the analysis. The unit of observation was a medical visit or admission, and >1 illness per child could contribute to the data set.
To test the null hypothesis of no differences between children with malaria and influenza coinfection, either infection alone, or neither infection, proportions were compared by χ2 or Fisher exact test, and differences between group means were analyzed using 1-way analysis of variance. Risk ratios (RRs) were calculated to compare the presence of respiratory or hematologic indicators in the infection groups; consistent findings across sites and age groups are highlighted. Logistic and linear regression was applied in univariate and multivariate analyses of dichotomous and continuous outcomes, respectively. Final multivariate models predicting hospital admission or duration of hospitalization were estimated including 3 dichotomous variables to represent infection categories (coinfection, influenza-only, and malaria-only) and included covariates starting with significant (P < .05) univariate associations and then removed nonsignificant factors (P > .05) in a stepwise backward fashion [13, 14]. To aid in interpretation, the difference in the adjusted mean duration of hospitalization for the coinfected children compared with those with a single infection was calculated by subtracting the unstandardized β value associated with the single infection from the β value associated with coinfection. All analyses were conducted using PASW Statistics version 18.0.
Human Subjects Review
This study was approved by the institutional review board of the US CDC and the ethical review committee of KEMRI. Written informed consent was obtained from all participants/ parents.
Role of Funding Sources
The CDC funded this study. In collaboration with KEMRI, CDC participated in the design and conduct of the study and the collection, management, and analysis of the data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Prevalence of Influenza and Malaria Coinfection
From 1 July 2009 through 31 June 2011 at LMH, 1992 outpatients were enrolled, and 76 (3.8%) who did not have a history of fever within 7 days were excluded; of the remaining 1917 subjects, 891(46.5%) were children aged 0–23 months and 1026 (53.5%) were aged 24–59 months. At SDH, during the same period, 2266 children were enrolled at hospital admission, and 130 (5.7%) without fever within 7 days were excluded; of the remaining 2133 subjects, 1630 (76.4%) were aged 0–23 months and 503 (23.6%) were aged 25–59 months.
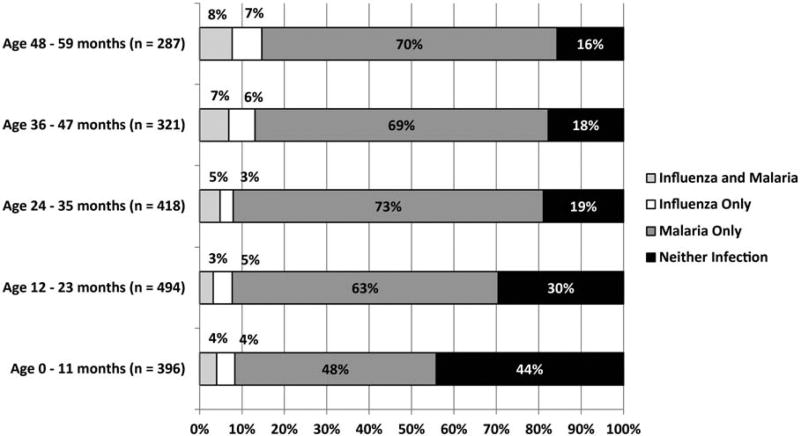
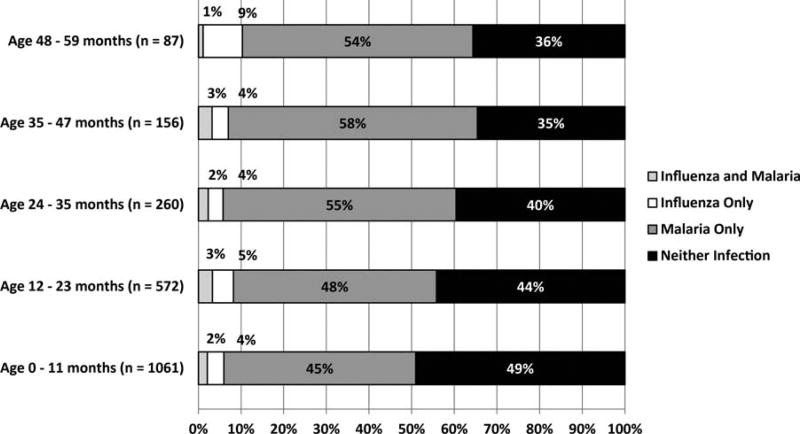
Across sites and age groups, 331 of 4052 (8.2%) children had influenza, including 253 (76.4%) with influenza A and 78 (23.6%) with influenza B; 2408 of 4052 (59.4%) had malaria, including 2303 (95.6%) with Plasmodium falciparum and 105 (4.4%) with other species. Forty-five percent (149/331) of influenza-positive patients were coinfected with malaria, whereas only 6% (149/2408) of malaria-positive patients were coinfected with influenza. Coinfection was noted in 96 of 1917 (5%) of outpatient visits to LMH (range, 4%–8% depending on age) and 53 of 2133 (2.5%) of admissions to SDH (range, 1%–3%) (Figures 1 and 2). Coinfections did not differ significantly from single infections in influenza type or malaria species (data not shown). Positive influenza and malaria cases were identified during every month of the study period.
Figure 1.
Percentage of outpatient visits or admissions for acute febrile respiratory illness among children aged <5 years by influenza and malaria test results and by years of age at Lwak Mission Hospital, 1 July 2009–31 June 2011.
Figure 2.
Percentage of inpatient admissions for acute febrile respiratory illness among children aged <5 years by influenza and malaria test results and years of age at Siaya District Hospital, 1 July 2009–31 June 2011.
At both sites and for both age groups, being positive for one infection was associated with a reduced likelihood of being positive for the other. Children with influenza were less likely than those without influenza to have malaria (aged 0– 23 months at LMH [RR, 0.74; 95% confidence interval {CI}, .57–.96] and SDH [RR, 0.76; 95% CI, .59–.97]; aged 24–59 months at LMH [RR, 0.68; 95% CI, .58–.81] and SDH [RR, 0.57; 95% CI, .46–.91]). Similarly, children with malaria were less likely than those without malaria to have influenza (aged 0–23 months at LMH [RR, 0.56; 95% CI, .36–.87] and SDH [RR, 0.63; 95% CI, .44–.92]; aged 24–59 months at LMH [RR, 0.36; 95% CI, .26–.50] and SDH [RR, 0.38; 95% CI, .19–.74]).
Clinical Presentation by Infection Category, Age, and Site
Patient visit characteristics by 4 infection groups and 2 age groups are presented for outpatient visits to LMH in Table 1 and inpatient admissions to SDH in Table 2. Comparisons of influenza only, malaria only, and coinfection are presented in Table 3, highlighting significant risk ratios by 4 subgroups stratified by age and study site. Respiratory indicators were more common among children with influenza only than in children with malaria only across most subgroups (Table 3), but there was no consistent indication that respiratory disease was worse with coinfection vs influenza only. Similarly, hematologic disease was less common for children with influenza only vs malaria only for some subgroups, but no consistent differences between coinfection and malaria only on hematologic indicators were noted. Finally, no consistent differences in nonspecific indicators were noted.
Table 1.
Outpatient Clinical Presentation and Hospital Admissions by Influenza and Malaria Test Results Among Children Aged 0–23 Months and Aged 24–59 Months With Acute Febrile Respiratory Illness at Lwak Mission Hospital, Rural Western Kenya, 2009–2011
| Characteristic | Aged 0–23 mo | Aged 24–59 mo | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Influenza and Malaria |
Influenza Only |
Malaria Only |
Neither | P Value* | Influenza and Malaria |
Influenza Only |
Malaria Only |
Neither | P Value* | |
| Total No. (%) | 32 (3.6) | 39 (4.4) | 498 (56.0) | 321 (36.1) | 64 (6.2) | 53 (5.2) | 728 (71.0) | 181 (17.6) | ||
| Female | 12 (37.5) | 14 (35.9) | 195 (39.2) | 149 (46.4) | .157 | 34 (53.1) | 26 (49.1) | 357 (49.0) | 84 (46.4) | .823 |
| Age, mo, mean (SD) | 13.5 (5.9) | 12.1 (5.2) | 13.9 (6.0) | 11.9 (6.2) | <.001 | 42.1 (9.9) | 43.4 (10) | 39.9 (10.6) | 39.4 (11) | .035 |
| Days from lever onset to visit, mean (SD) | 2.2 (1.5) | 2.7 (1.6) | 2.0 (1.1) | 2.4 (1.5) | <.001 | 2.2 (1.4) | 2.2 (1.4) | 2.1 (1.2) | 2.2 (1.4) | .690 |
| Physical exam vitals | ||||||||||
| Temperature, °C, mean (SD) | 40.0 (0.7) | 38.6 (0.9) | 39.0 (0.8) | 38.5 (0.9) | <.001 | 39.0 (0.7) | 38.5 (0.9) | 40.0 (0.8) | 38.6 (0.8) | <.001 |
| Fever (>38°C) | 28 (87.5) | 29 (74.4) | 459 (92.2) | 245 (76.3) | <.001 | 58 (90.6) | 39 (73.6) | 667 (91.6) | 143 (79.0) | <.001 |
| Respiration, mean (SD) | 44.7 (12.6) | 47.0 (10.8) | 45.0 (11.3) | 43.1 (11.7) | .061 | 36.1 (9.9) | 32.6 (7.8) | 39.0 (9.9) | 36.9 (8.6) | <.001 |
| Tachypneaa | 17 (53.1) | 25 (64.1) | 299 (60.0) | 173 (53.9) | .286 | 17 (26.6) | 6 (11.3) | 260 (35.7) | 51 (28.2) | .001 |
| Oxygen saturation, mean (SD) | 96.8 (1.9) | 94.9 (4.4) | 96.5 (3.2) | 96.1 (3.9) | .029 | 97.8 (1.9) | 96.5 (3.2) | 96.8 (3.5) | 96.3 (2.9) | .023 |
| Low oxygen saturationb | 0 (0.0) | 5 (12.8) | 5 (1.0) | 8 (2.5) | <.001 | 0 (0.0) | 2 (3.8) | 7 (1.0) | 4 (2.2) | .120 |
| Signs and symptoms | ||||||||||
| Cough | 31 (96.9) | 36 (92.3) | 482 (96.8) | 309 (96.3) | .537 | 61 (95.3) | 52 (98.1) | 712 (97.8) | 179 (98.9) | .402 |
| Respiratory distress | 5 (15.6) | 9 (23.1) | 39 (7.83) | 72 (22.4) | <.001 | 4 (6.3) | 8 (15.1) | 46 (6.32) | 32 (17.7) | <.001 |
| Convulsion | 3 (9.4) | 5 (12.8) | 42 (8.4) | 11 (3.4) | .016 | 7 (10.9) | 1 (1.9) | 35 (4.8) | 11 (6.1) | .117 |
| Diarrhea | 1 (3.1) | 8 (20.5) | 72 (14.5) | 57 (17.8) | .105 | 0 (0.0) | 0 (0.0) | 35 (4.8) | 10 (5.5) | .101 |
| Vomiting | 12 (37.5) | 12 (30.8) | 157 (31.5) | 97 (30) | .858 | 8 (12.5) | 6 (11) | 237 (32.6) | 37 (20.4) | <.001 |
| Clinical diagnoses | ||||||||||
| Anemia | 4 (12.5) | 1 (2.6) | 49 (9.8) | 9 (2.8) | .001 | 6 (9.4) | 0 (0.0) | 45 (6.2) | 13 (7.2) | .181 |
| Pneumonia | 6 (18.8) | 10 (25.6) | 30 (6.0) | 77 (24.0) | <.001 | 3 (4.7) | 9 (17.0) | 22 (3.0) | 33 (18.2) | <.001 |
| Disposition | ||||||||||
| Admitted to hospital | 9 (28.1) | 11 (28.2) | 68 (13.7) | 67 (20.9) | .004 | 8 (12.5) | 5 (9.4) | 74 (10.2) | 24 (13.3) | .630 |
| Days in hospital, mean (SD) | 2.0 (0.7) | 3.7 (1.8) | 2.5 (1.4) | 2.9 (1.5) | .013 | 3.5 (1.6) | 1.6 (0.5) | 2.4 (1.1) | 2.7 (1.3) | .022 |
| Chest radiograph orderedc | 2 (22.2) | 3 (27.3) | 1 (1.5) | 7 (10.4) | .009 | 2 (25.0) | 4 (80.0) | 2 (2.7) | 3 (12.5) | <.001 |
Data are presented as no. (%) unless otherwise specified.
Tachypnea (>60 breaths/min for age <2 months; >50 breaths/min for age <12 months; >40 breaths/min for age ≥12 months).
Low oxygen saturation (<90%).
Denominator is the number hospitalized in the infection group.
P value reflects χ2 or Fisher exact test (if expected cell value <5), testing the null hypothesis of no heterogeneity between groups.
Table 2.
Clinical Presentation and Hospitalization Characteristics by Influenza and Malaria Test Results Among Children Aged 0–23 Months and 24–59 Months With Acute Febrile Respiratory Illness at Siaya Hospital, Rural Western Kenya, 2009–2011
| Characteristic | Aged 0–23 months | Aged 24–59 Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Influenza and Malaria |
Influenza Only | Malaria Only | Neither | P Value* | Influenza and Malaria |
Influenza Only | Malaria Only | Neither | P Value* | |
| Total no. (%) | 41 (2.5) | 69 (4.2) | 748 (45.8) | 772 (47.3) | 12 (2.4) | 23 (4.6) | 280 (55.7) | 188 (37.4) | ||
| Female | 18 (43.9) | 33 (47.8) | 363 (48.5) | 339 (43.9) | .368 | 6 (50.0) | 9 (39.1) | 122 (43.6) | 85 (45.2) | .902 |
| Age, mo, mean (SD) | 11.5 (6.7) | 10.6 (6.1) | 10.6 (6.0) | 9.8 (6.1) | .039 | 36 (8.9) | 39.5 (12) | 37.6 (9.3) | 36.6 (9.7) | .423 |
| Days from lever onset to visit, mean (SD) | 3.1 (1.7) | 3.9 (2.9) | 3.4 (2.0) | 3.9 (2.7) | .002 | 3.4 (1.0) | 2.9 (0.6) | 2.1 (0.1) | 2.9 (0.2) | .032 |
| Physical exam vitals | ||||||||||
| Temperature, °C, mean (SD) | 37.4 (1.1) | 37.8 (1.2) | 37.5 (1.2) | 37.3 (1.1) | .025 | 37.3 (0.8) | 37.5 (1.1) | 37.5 (1.2) | 37.4 (1.2) | .702 |
| Fever (>38°C) | 10 (24.4) | 26 (37.7) | 233 (31.1) | 202 (26.2) | .053 | 2 (16.7) | 9 (39.1) | 96 (34.3) | 50 (26.6) | .175 |
| Respiration, mean (SD) | 52.9 (14.1) | 55.5 (14.0) | 54.0 (13.8) | 51.4 (12.9) | .001 | 46.5 (10.9) | 47.4 (11.4) | 46.4 (11.2) | 47.5 (11.5) | .766 |
| Tachypneaa | 34 (82.9) | 59 (85.5) | 627 (83.8) | 612 (79.3) | .111 | 8 (66.7) | 18 (78.3) | 191 (68.2) | 139 (73.9) | .472 |
| Oxygen saturation, mean (SD) | 96.0 (3.8) | 91.3 (10.9) | 93.8 (6.2) | 92.9 (7.5) | .001 | 91.0 (9.3) | 94.5 (5.2) | 95.4 (5.3) | 94.1 (5.6) | .005 |
| Low oxygen saturationb | 1 (2.4) | 17 (24.6) | 107 (14.3) | 159 (20.6) | <.001 | 4 (33.3) | 1 (4.3) | 19 (6.8) | 27 (14.4) | .002 |
| Hemoglobin g/dL, mean (SD) | 6.9 (2.6) | 9.1 (2.5) | 7.2 (2.4) | 8.8 (2.4) | <.001 | 7.0 (3.0) | 8.9 (2.6) | 7.9 (2.4) | 7.8 (2.8) | .134 |
| Critical anemiac | 8 (19.5) | 5 (7.2) | 148 (19.8) | 48 (6.2) | <.001 | 3 (25.0) | 3 (13.0) | 29 (10.4) | 35 (18.6) | .050 |
| Signs and symptoms | ||||||||||
| Cough | 38 (92.7) | 67 (97.1) | 660 (88.2) | 719 (93.1) | .002 | 12 (100) | 23 (100) | 243 (86.8) | 176 (93.6) | .023 |
| Respiratory distress | 27 (65.9) | 51 (73.9) | 450 (60.2) | 519 (67.2) | .010 | 4 (33.3) | 14 (60.9) | 142 (50.7) | 116 (61.7) | |
| Convulsion | 8 (19.5) | 8 (11.6) | 180 (24.1) | 123 (15.9) | <.001 | 3 (25.0) | 1 (4.3) | 124 (44.3) | 52 (27.7) | <.001 |
| Diarrhea | 12 (29.3) | 28 (40.6) | 321 (42.9) | 327 (42.4) | .383 | 6 (50.0) | 9 (39.1) | 107 (38.2) | 71 (37.8) | .869 |
| Vomiting | 5 (12.2) | 13 (18.8) | 181 (24.2) | 182 (23.6) | .267 | 5 (41.7) | 3 (13.0) | 66 (23.6) | 42 (22.3) | .291 |
| Clinical diagnoses at admission | ||||||||||
| Anemia | 16 (39.0) | 8 (11.6) | 305 (40.8) | 125 (16.2) | <.001 | 7 (58.3) | 3 (13.0) | 88 (31.4) | 56 (29.8) | .050 |
| Pneumonia | 14 (34.1) | 40 (58.0) | 286 (38.2) | 390 (50.5) | <.001 | 3 (25.0) | 8 (34.8) | 52 (18.6) | 70 (37.2) | <.001 |
| HIV positived | 0/22 (0.0) | 3/34 (8.8) | 11/309 (3.6) | 36/303 (11.9) | .002 | 0/9 (0.0) | 0/9 (0.0) | 13/131 (9.9) | 16/97 (16.5) | .179 |
| Hospital treatment | ||||||||||
| Days in hospital, mean (SD) | 5.1 (2.2) | 5.2 (2.8) | 4.7 (2.8) | 5.3 (3.4) | .001 | 6.3 (4.3) | 3.9 (2.6) | 3.4 (2.1) | 3.7 (2.3) | <.001 |
| Chest radiograph ordered | 8 (19.5) | 18 (26.1) | 60 (8.0) | 115 (14.9) | <.001 | 0 (0) | 1 (4.3) | 8 (2.9) | 7 (3.7) | .854 |
| Given blood transfusion | 14 (34.1) | 11 (15.9) | 215 (28.7) | 80 (10.4) | <.001 | 7 (58.3) | 2 (8.7) | 57 (20.4) | 40 (21.3) | .007 |
| Died in hospital | 1 (2.4) | 0 (0.0) | 10 (1.3) | 26 (3.4) | .033 | 0(0.0) | 1 (4.3) | 3 (1.1) | 6 (3.2) | .320 |
Data are presented as no. (%) unless otherwise specified.
Tachypnea (>60 breaths/min for age <2 months; >50 breaths/min for age <12 months; >40 breaths/min for age >12 months).
Low oxygen saturation (<90%).
Critical anemia (hemoglobin <5 g/dL).
Human immunodeficiency virus (HIV) test results with positives (numerator) of all tested (denominator).
P value reflects χ2 or Fisher exact test (if expected cell value <5), testing the null hypothesis of no heterogeneity between groups.
Table 3.
Risk Ratios Comparing Influenza Only, Malaria Only, and Coinfection at Lwak Mission Hospital and Siaya District Hospital, Rural Western Kenya, 2009–2011
| Influenza Only vs Malaria Only | Coinfection vs Influenza Only | Coinfection vs Malaria Only | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMH | SDH | LMH | SDH | LMH | SDH | |||||||
| 0–23 mo | 24–59 mo | 0–23 mo | 24–59 mo | 0–23 mo | 24–59 mo | 0–23 mo | 24–59 mo | 0–23 mo | 24–59 mo | 0–23 mo | 24–59 mo | |
| Respiratory indicatorsa | ||||||||||||
| Chest radiograph orderedb | 18.55 | 30.0 | 3.21 | ns | ns | ns | ns | ns | 13.6 | 9.38 | 2.40 | ns |
| Cough | ns | ns | 1.1 | 1.15 | ns | ns | ns | ns | ns | ns | ns | ns |
| Low oxygen saturation | 12.72 | ns | 1.72 | ns | nc | nc | 0.10 | 7.67 | ns | ns | ns | 4.91 |
| Pneumonia clinical diagnosis | 4.26 | 5.62 | 1.51 | 1.87 | ns | 0.28 | 0.59 | ns | 3.11 | ns | ns | ns |
| Respiratory distress | 2.95 | ns | 1.23 | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Tachypnea | ns | 0.32 | ns | ns | ns | 2.35 | ns | ns | ns | ns | ns | ns |
| Hematologic indicators | ||||||||||||
| Anemia clinical diagnosis | nsc | nsc | 0.29c | nsc | ns | nc | 3.32 | 4.47 | ns | ns | ns | 1.86 |
| Blood transfusion receivedb | … | … | 0.55 | ns | … | … | 2.40 | 6.71 | … | … | ns | 2.86 |
| Critical anemia | … | … | 0.37 | ns | … | … | ns | ns | … | … | ns | ns |
| Nonspecific indicators | ||||||||||||
| Admission to hospitalb | 2.07 | ns | … | … | ns | ns | … | … | ns | ns | … | … |
| Convulsion | ns | ns | 0.48 | 0.10 | ns | ns | ns | ns | ns | 2.28 | ns | ns |
| Diarrhea | ns | nc | ns | ns | ns | nc | ns | ns | ns | nc | ns | ns |
| Fever (>38°C) | 0.81 | 0.80 | ns | ns | ns | 1.23 | ns | ns | ns | ns | ns | ns |
| HIV positive | … | … | ns | ns | … | … | ns | ns | … | … | ns | ns |
| Vomiting | ns | 0.35 | ns | ns | ns | ns | ns | ns | ns | 0.38 | ns | ns |
Values represent statistically significant risk ratios for the indicated comparison.
Abbreviations: LMH, Lwak Mission Hospital; HIV, human immunodeficiency virus; ns, not statistically significant (P > .05); nc, not calculated because denominator was zero; SDH, Siaya District Hospital.
Tachypnea defined as >60 breaths/min for age <2 months, >50 breaths/min for age <12 months, >40 breaths/min for age >12 months; low oxygen saturation defined as <90; critical anemia (hemoglobin <5 gm/dL) was only available at SDH; human immunodeficiency virus test results were only available at SDH.
These indicators are also considered to be outcomes, others are considered clinical presentation.
Overall odds ratio for all 4 strata is 0.20 (95% CI, .11–.37) adjusting for age group and site.
Predictors of Admission to LMH
Seventeen percent (155/889) of children aged <24 months and 11% (111/1026) of children aged 24–59 months with febrile ARI were admitted to LMH from the outpatient clinic (Table 1). The likelihood of hospital admission was not higher for coinfected children in either age category. For both age groups, convulsions and clinical diagnoses of anemia or pneumonia increased the likelihood of admission (Supplementary Table A). After adjusting for these factors, the likelihood of hospitalization among children aged <24 months with malaria only was 38% lower than children with neither infection (adjusted odds ratio, 0.62; 95% CI, .42–.99).
Predictors of Duration of Hospitalization Among Children Aged 0–23 Months
Among children aged <24 months, the average duration of hospitalization was 2.7 days (SD, 1.5) among 155 patients at LWH and 5.0 days (SD, 3.1) among 1633 patients at SDH. In univariate analyses, 3 factors were associated with longer stays at both hospitals: younger age, signs of respiratory distress, and a clinical diagnosis of pneumonia (Table 4). Coinfection was not associated with duration of hospitalization in this age group. In multivariate models, compared to children with neither infection, influenza-only infection was associated with approximately 1 additional day in hospital (β = .90; 95% CI, .01–1.72) at LMH (Table 4) and malaria only was associated with a half-day shorter stay (β = −.50; 95% CI, −.81 to −.18) at SDH (Table 4).
Table 4.
Days in Lwak Mission Hospital and Siaya District Hospital Predicted by Infection Type and Covariates in Univariate and Multivariate Linear Regression Models for Children Aged 0–23 Months and Aged 24–59 Months, Rural Western Kenya, 2009–2011
| Days in Lwak Mission Hospital | Days in Siaya District Hospital | |||
|---|---|---|---|---|
| Univariatea β (95% CI) |
Multivariateb β (95% CI) |
Univariatea β (95% CI) |
Multivariateb β (95% CI) |
|
| Aged 0–23 mo | ||||
| Infection | ||||
| Influenza and malaria | −0.76 (−1.74, .23) | −0.59 (−1.59, .43) | 0.04 (−.93, .99) | 0.13 (−1.05, .88) |
| Influenza only | 1.09* (.20, 1.98) | 0.90* (.01, 1.72) | 0.18 (−.57, .93) | −0.12 (−.88, .64) |
| Malaria only | −0.40 (−.86, .06) | −0.10 (−.50, −.06) | −0.60*** (−.90, −.30) | −0.50*** (−.81, −.18) |
| Neither infection (referent) | 0.28 (−.18, .74) | 0.56*** (.26, .87) | ||
| Covariates | ||||
| Age in mo | −0.04* (−.08, −.00) | −0.03* (−.06, −.01) | ||
| Days since illness onset | 0.09 (−.06, .25) | 0.15*** (.08, .21) | 0.13*** (.07, .20) | |
| Other clinical predictors | ||||
| Temperature (°C) | −0.33*** (−.52, −.13) | −0.30** (−.05, −.07) | 0.10 (−.04, .23) | |
| Respiration (per min) | 0.02 (−.01, .04) | 0.01 (−.00, .02) | ||
| Oxygen saturation | −0.01 (−.05, .02) | −0.05** (−.04, −.00) | ||
| Cough | −0.74* (−1.49, .17) | 0.36 (−.17, .89) | ||
| Respiratory distress | 0.59* (.10, 1.02) | 0.32* (−.01, .62) | ||
| Convulsion | −0.29 (−.81, .23) | −1.18** (−1.92, −.43) | −0.93** (−1.67, −.19) | |
| Anemia clinical diagnosis | −0.12 (−.74, .50) | −0.11 (−.45, .23) | ||
| Pneumonia clinical diagnosis | 0.58** (.14, 1.05) | 0.48*** (.18, .78) | 0.38** (.08, .68) | |
| HIVc | ||||
| Tested and positive | … | 0.99* (.03, 1.94) | ||
| Not tested | … | −0.19 (−.49, .11) | ||
| Aged 24–59 mo | ||||
| Infection | ||||
| Influenza and malaria | 1.09** (.23, 1.85) | 1.15** (.34, 1.96) | 2.76*** (1.45, 4.07) | 2.98*** (1.51, 4.45) |
| Influenza only | −0.99* (−2.02, −.16) | −1.58** (−2.62, −.43) | 0.24 (−.73, 1.22) | 0.20 (−1.24, 1.63) |
| Malaria only | −0.32 (−.81, .16) | −0.11 (−.66, .43) | −0.45* (−.86, −.04) | −0.09 (−.65, .46) |
| Neither infection (referent) | 0.23 (−.33, .79) | 0.15 (−.27, .57) | ||
| Covariates | ||||
| Age in mo | −0.00 (−.02. .02) | −0.01 (−.03, .02) | ||
| Days since illness onset | 0.07 (−.09, .23) | 0.05 (−.03, .13) | ||
| Other clinical predictors | ||||
| Temperature | 0.04 (−.16, .25) | 0.05 (−.12, .23) | ||
| Respiration | 0.00 (−.013, .03) | 0.00 (−.01, .02) | ||
| Oxygen saturation | −0.07** (−.13, −.02) | −0.08 (−.13, −.02) | −0.07*** (−.10, −.03) | −0.07*** (−.12, −.02) |
| Cough | −0.15 (−1.04, −.74) | −0.40 (−1.09, .30) | ||
| Respiratory distress | 0.69* (.98, 1.10) | 0.30 (−.11, .70) | ||
| Convulsion | 0.02 (−.46, .50) | −0.17 (−.93, .58) | ||
| Anemia clinical diagnosis | 0.02 (−.58, .61) | 0.19 (−.25, .64) | ||
| Pneumonia clinical diagnosis | 0.52* (−.01, 1.05) | 0.63* (.09, 1.18) | 0.27 (−.19, .73) | |
| HIVc | ||||
| Tested and positive | … | 1.28** (.43, 2.14) | 1.26** (.43, 2.09) | |
| Not tested | … | −0.11 (−.52, .30) | ||
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
Days in hospital (unstandardized) regressed on each infection category (rows) as dummy variables and covariates separately with linear regression.
Days in hospital (unstandardized) regressed on infection category dummy variables and covariates, simultaneously, with stepwise backward elimination of nonsignificant covariate effects.
HIV test results were available for a subset of Siaya inpatients only; the 2 dichotomous variables represent 3 potential categories: (1) tested and positive, (2) tested and negative, and (3) not tested.
P < .05.
P < .01.
P < .001.
Predictors of Duration of Hospitalization Among Children Aged 24–59 Months
The duration of hospitalization among children aged 24–59 months was shorter among 111 patients at LMH (mean, 2.5 [SD, 1.2]) than among 503 patients at SDH (mean, 3.6 days [SD, 2.3]). For this age group, similar predictors of duration of hospitalization were observed at both hospitals (Table 4). In the multivariate models, compared to children with neither infection, coinfection was associated with approximately 1 additional hospitalization day (β = 1.15; 95% CI, .34–1.96) at LMH and 3 days (β = 2.98; 95% CI, 1.51–4.45) at SDH. At LDH, influenza only was associated with shorter stays for this age group. Using these estimates, the adjusted mean duration of hospitalization for coinfected children aged 24–59 months was significantly longer than children with single infections at both sites. At LDH, coinfected children spent 2.73 days (95% CI, 1.33–3.98) longer in hospital than children with influenza only (β 1.15 minus −1.58) and 1.26 days (95% CI, .35–2.0) longer than children with malaria only (β 1.15 minus −0.11). At SDH, coinfected children spent 2.78 days (95% CI, .83– 4.74 days) (β 2.98 minus 0.20) and 3.07 (95% CI, 1.61–4.53) (β 2.98 minus −0.09) days longer in hospital than children with influenza only or malaria only, respectively.
Chest Radiographs and Blood Transfusions During Hospitalization
Clinicians were no more likely to order chest radiographs for coinfected children than children with influenza only in any of the 4 age by site subgroups (Table 3). Instead, chest radiographs were ordered more often for children with coinfection and influenza only than those with malaria only in 3 of 4 subgroups. At SDH where records on blood transfusions were available (Table 2), coinfected children were more likely to receive blood transfusions than children with malaria only in the 24- to 59-month-old subgroup. In a multivariate model, adjusting for clinical features associated with transfusions (critical anemia, oxygen saturation, tachypnea, and signs of respiratory distress), coinfected children aged 24–59 months were 6.3-fold (95% CI, 1.7–24.1) more likely to receive transfusions than those with malaria only.
DISCUSSION
During 2009–2011, a period when malaria and influenza were co-circulating in rural western Kenya, among children <5 years of age who were tested for both infections, coinfection accounted for about 5% of outpatient visits and 3% of hospital admissions for febrile ARI. In contrast to studies showing that HIV infection increases risk for malaria infection [15], as well as other studies showing syndemic or synergetic associations between diseases [16–18], we did not find that testing positive for influenza increased the likelihood of testing positive for malaria. These findings are not unexpected; although HIV infection impairs T-cell immunity, making it more difficult for the body to protect against malaria infection [19], influenza infection has no such effect. In fact, we found that having one infection was associated with a reduced likelihood of detecting the other. This finding is consistent with a prior study that found lower rates of malaria among older children with RTPCR–confirmed influenza [6]. Although mechanisms whereby infection with 1 pathogen may suppress expression of another have been proposed [20], our findings may simply reflect the fact that either infection alone can cause illness and be sufficient to prompt a medical visit.
Although we found significant differences in clinical presentation between influenza-only vs malaria-only, we observed no consistent differences in clinical presentation between coinfected children and those with single infections with regards to respiratory, hematologic, and other nonspecific clinical indicators. Therefore, in addition to being uncommon, coinfection is difficult to distinguish clinically from either infection alone using the variables that we evaluated.
Nonetheless, several of our findings suggest that coinfection can be associated with more severe illness than single infections. At both hospitals, coinfected children aged 24–59 months had longer hospitalizations than children with either infection alone or neither infection. Having a coinfection increased hospital stays by about 1–3 days in settings where the typical stay was 3–5 days. Among patients aged 24–59 months, coinfected children were also more likely to receive blood transfusions than children with malaria only. Chest radiographs were also ordered more often for coinfected vs malaria-only-infected children. Clinicians had malaria microscopy and hemoglobin results to guide care, but did not have influenza test results, and thus were not aware that the children were coinfected. It may be that coinfected children presented with a more ill appearance, with signs that we did not capture in our study but that clinicians recognized, prompting more aggressive and prolonged care.
However, there were also indications that malaria and influenza coinfection is not consistently more severe than single infection in patients with febrile ARI. Coinfection did not increase the likelihood of admission to LMH, and, among children under age 24 months, was not associated with longer hospital stays.
Our study included 2 full years of data and utilized highly sensitive and specific testing for influenza. We were also able to compare trends among outpatients and inpatients in 2 hospitals with differing clinical practices. These study attributes increase our confidence in trends we noted across sites and age groups.
The study also had several limitations. First, given that the risks and severity of malaria vary considerably by age and community [2, 8, 21, 22] and that influenza strains can change each year and circulate in unique ways in Africa [4], the extent to which our findings regarding coinfection generalize beyond this setting, age group, and time period are unknown. Second, since the unit of observation was medical encounters rather than specific children, we do not know the extent to which chronically ill children may have contributed more than once to the study denominator, though repeat visits or admissions within a year represent a small proportion of total encounters in this region [8, 23]. Third, our participants were enrolled as part of ILI and SARI surveillance, and therefore, children with malaria only may not be representative of those with malaria but not respiratory illness [24]. Fourth, the low number of coinfections we observed limited our statistical power to detect differences in clinical presentation. Fifth, our study examined only 2 pathogens in a population that is exposed to many others [6, 18, 25, 26]. Although our multivariate models adjusted for clinical pneumonia and HIV test results when available, we do not know the extent to which clinical presentation and outcome may have been influenced by unmeasured factors such as coinfections with bacteria and other viruses [26].
Sixth, our study did not examine the role care seeking prior to the facility visit may have played in shaping clinical presentation and outcomes. Since informal care-seeking is common in this region [27], especially for antimalarials [28], the clinical presentation of malaria may have been altered or attenuated by earlier treatment. This may have led us to underestimate the prevalence of coinfection, since some children originally coinfected may have taken antimalarials and then been misclassified as having influenza only when assessed at the health facility. This is a limitation of any facility-based surveillance program [29] and highlights the need to conduct community-based cohort surveillance to gain a complete picture of disease burden, including coinfection.
Seventh, the potential role of asymptomatic infection for either of our pathogens in the results is unclear. Both malaria parasitemia [5, 30] and influenza virus infection [4] can be asymptomatic or incidental to the presenting illness. The lack of malaria parasite counts prevented us from considering alternative classifications by parasite density. Nonetheless, our focus on infants and young children with limited opportunity to develop malaria immunity [5, 22], and the fact that we tested for both infections during acute illness (≤7 days of onset) increases our confidence that most infections were clinically relevant to coinfected children.
Further research is needed to understand the relevance and implications of malaria-influenza coinfection to the epidemiology and clinical course of both diseases. The potential relevance of these coinfections to influenza prevention and control efforts in malaria-endemic parts of Africa [4, 7] is especially clear, given that half of all influenza patients in this study were coinfected with malaria. Fewer malaria patients were coinfected with influenza (about 1 in 20 in our study), but the impact of influenza coinfection should also be considered by malaria researchers, clinicians, and policy makers. Influenza is only one of many viruses that may complicate malaria infection [7, 18, 25, 26], but it is unique in being a vaccine-preventable disease with an effective antiviral treatment. Although our data are specific to western Kenya, if further research confirms that influenza and malaria coinfection indeed results in longer hospital stays and more blood transfusions in some age groups, it would bolster arguments for the value of influenza vaccination and antiviral treatment for young children in tropical regions where malaria is endemic.
Finally, the similar clinical presentation of malaria and influenza coinfections and the heightened or additive severity of coinfection that we observed are consistent with arguments that both influenza and malaria are fundamentally systemic inflammatory diseases [31, 32] and can produce clinical syndromes similar to a variety of infectious agents [16, 33]. Further research on malaria and influenza coinfection may help advance both fields by clarifying how these 2 pathogens interact and potentially share pathological consequences.
Supplementary Material
Acknowledgments
We thank Marc-Alain Widdowson, Josh Mott, and Timothy Uyeki for their input on preliminary analyses, and Jerome Tokars, Sue Reynolds, Alicia Fry, Michael Shaw, David Swerdlow, and 2 anonymous reviewers for their feedback on final analyses and drafts of the article. We thank the patients and parents who took part in the study and the many staff at Lwak Mission Hospital and Siaya District Hospital who contributed to the study, including clinical research staff and laboratory staff. This paper is published with the permission of the Director of KEMRI. This work was submitted under KEMRI Protocol (SSC #1801, CDC #3308): health, demographic, socioeconomic, and health facility surveillance in western Kenya.
Financial support. This work was funded by a cooperative agreement (5U19CI000323–05) from the US CDC to KEMRI and with support from CDC-Kenya.
Footnotes
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US CDC, KEMRI, or the CDC-Kenya.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snow RW, Omumbo JA, Lowe B, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–4. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 3.Crawley J, Chu C, Mtove G, Nosten F. Malaria in children. Lancet. 2010;375:1468–81. doi: 10.1016/S0140-6736(10)60447-3. [DOI] [PubMed] [Google Scholar]
- 4.Gessner BD, Shindo N, Briand S. Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2011;11:223–35. doi: 10.1016/S1473-3099(11)70008-1. [DOI] [PubMed] [Google Scholar]
- 5.Rafael ME, Taylor T, Magill A, Lim YW, Girosi F, Allan R. Reducing the burden of childhood malaria in Africa: the role of improved. Nature. 2006;444(suppl 1):39–48. doi: 10.1038/nature05445. [DOI] [PubMed] [Google Scholar]
- 6.Waitumbi JN, Kuypers J, Anyona SB, et al. Outpatient upper respiratory tract viral infections in children with malaria symptoms in western Kenya. Am J Trop Med Hyg. 2010;83:1010–3. doi: 10.4269/ajtmh.2010.10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yazdanbakhsh M, Kremsner PG. Influenza in Africa. PLoS Med. 2009;6:e1000182. doi: 10.1371/journal.pmed.1000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Hemelrijck MJ, Lindblade KA, Kubaje A, et al. Trends observed during a decade of paediatric sick visits to peripheral health facilities in rural western Kenya, 1997–2006. Trop Med Int Health. 2009;14:62–9. doi: 10.1111/j.1365-3156.2008.02184.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamel MJ, Adazu K, Obor D, et al. A reversal in reductions of child mortality in Western Kenya, 2003–2009. Am J Trop Med Hyg. 2011;85:597–605. doi: 10.4269/ajtmh.2011.10-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adazu K, Lindblade KA, Rosen DH, et al. Health and demographic surveillance in rural western Kenya: a platform for evaluating interventions to reduce morbidity and mortality from infectious diseases. Am J Trop Med Hyg. 2005;73:1151–8. [PubMed] [Google Scholar]
- 11.Central Bureau of Statistics MoPaND, editor. Statistics CBo. Kenya demographic and health survey 2003. Nairobi, Kenya: Kenya National Bureau of Statistics; 2004. pp. 13–21. [Google Scholar]
- 12.Kim C, Ahmed JA, Eidex RB, et al. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PLoS One. 2011;6: e21610. doi: 10.1371/journal.pone.0021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Routledge Academic NY: Routledge, Taylor & Francis Group; 2002. [Google Scholar]
- 15.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–6. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 16.Graham AL, Cattadori IM, Lloyd-Smith JO, Ferrari MJ, Bjornstad ON. Transmission consequences of coinfection: cytokines writ large? Trends Parasitol. 2007;23:284–91. doi: 10.1016/j.pt.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24:351–76. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott JA, Berkley JA, Mwangi I, et al. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011;378:1316–23. doi: 10.1016/S0140-6736(11)60888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezeamama AE, Spiegelman D, Hertzmark E, et al. HIV infection and the incidence of malaria among HIV-exposed children from Tanzania. J Infect Dis. 2012;205:1486–94. doi: 10.1093/infdis/jis234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rooth I, Bjorkman A. Fever episodes in a holoendemic malaria area of Tanzania: parasitological and clinical findings and diagnostic aspects related to malaria. Trans R Soc Trop Med Hyg. 1992;86:479–82. doi: 10.1016/0035-9203(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 21.Reyburn H, Mbatia R, Drakeley C, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–32. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 23.Idro R, Ndiritu M, Ogutu B, et al. Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA. 2007;297:2232–40. doi: 10.1001/jama.297.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh K, Snow RW. Host-parasite interaction and morbidity in malaria endemic areas. Philos Trans R Soc Lond B Biol Sci. 1997;352:1385–94. doi: 10.1098/rstb.1997.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazlett DT, Bell TM, Tukei PM, et al. Viral etiology and epidemiology of acute respiratory infections in children in Nairobi, Kenya. Am J Trop Med Hyg. 1988;39:632–40. doi: 10.4269/ajtmh.1988.39.632. [DOI] [PubMed] [Google Scholar]
- 26.Berkley JA, Munywoki P, Ngama M, et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303:2051–7. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton DC, Flannery B, Onyango B, et al. Healthcare-seeking behaviour for common infectious disease-related illnesses in rural Kenya: a community-based house-to-house survey. J Health Popul Nutr. 2011;29:61–70. doi: 10.3329/jhpn.v29i1.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuma J, Abuya T, Memusi D, et al. Reviewing the literature on access to prompt and effective malaria treatment in Kenya: implications for meeting the Abuja targets. Malar J. 2009;8:243. doi: 10.1186/1475-2875-8-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breiman RF, Olack B, Shultz A, et al. Healthcare-use for major infectious disease syndromes in an informal settlement in Nairobi, Kenya. J Health Popul Nutr. 2011;29:123–33. doi: 10.3329/jhpn.v29i2.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olotu A, Fegan G, Williams TN, et al. Defining clinical malaria: the specificity and incidence of endpoints from active and passive surveillance of children in rural Kenya. PLoS One. 2010;5:e15569. doi: 10.1371/journal.pone.0015569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark IA, Alleva LM, Budd AC, Cowden WB. Understanding the role of inflammatory cytokines in malaria and related diseases. Travel Med Infect Dis. 2008;6:67–81. doi: 10.1016/j.tmaid.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Clark IA, Alleva LM, Mills AC, Cowden WB. Pathogenesis of malaria and clinically similar conditions. Clin Microbiol Rev. 2004;17:509–39. doi: 10.1128/CMR.17.3.509-539.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark IA, Budd AC, Alleva LM. Sickness behaviour pushed too far— the basis of the syndrome seen in severe protozoal, bacterial and viral diseases and post-trauma. Malar J. 2008;7:208. doi: 10.1186/1475-2875-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.