Abstract
Our earlier studies demonstrated that the ozone-sensitive hybrid poplar clone NE-388 displays an attenuated level of ozone-, wound-, and phytopathogen-induced defense gene expression. To determine if this reduced gene activation involves signal transduction pathways dependent on salicylic acid (SA) and/or jasmonic acid (JA), we compared the responses of NE-388 and an ozone-tolerant clone, NE-245, to these signal molecules. JA levels increased in both clones in response to ozone, but only minimal increases in SA levels were measured for either clone. Treatment with SA and methyl jasmonate induced defense gene expression only in NE-245, indicating that NE-388 is insensitive to these signal molecules. DNA fragmentation, an indicator of programmed cell death (PCD), was detected in NE-245 treated with either ozone or an avirulent phytopathogen, but was not detected in NE-388. We conclude that these clones undergo two distinct mechanisms of ozone-induced lesion formation. In NE-388, lesions appear to be due to toxic cell death resulting from a limited ability to perceive and subsequently activate SA- and/or JA-mediated antioxidant defense responses. In NE-245, SA-dependent PCD precedes lesion formation via a process related to the PCD pathway activated by phytopathogenic bacteria. These results support the hypothesis that ozone triggers a hypersensitive response.
Plants must efficiently adapt to changing conditions to ensure their continued productivity. To adapt to environmental change, plants use several distinct regulatory mechanisms to alter the patterns of gene expression that ultimately cause the biochemical and physiological changes most favorable for survival. The ability to rapidly respond to environmental change is especially important in woody plant species, which must be able to adapt through changing seasons over a lifespan that may range up to hundreds of years. Among the conditions to which trees must adapt, ozone is one of the major anthropogenic stresses contributing to forest decline (Johnson and Taylor, 1989; Schmeiden and Wild, 1995).
Recently, molecular tools have been applied to discern the molecular basis of ozone-induced responses in herbaceous plants (for review, see Kangasjarvi et al., 1994; Sharma and Davis, 1997; Sandermann et al., 1998). These studies have shown that there is an overlap in the signaling pathways and defense-related genes that are induced by ozone and other stresses such as pathogen infection (Sharma et al., 1996), wounding (Örvar et al., 1997; Koch et al., 1998), UV (Rao et al., 1996), cold, drought, and heavy metal toxicity (Kangasjarvi et al., 1994; Sharma and Davis, 1997; Sandermann et al., 1998) in various plant species, including hybrid poplar (Koch et al., 1998).
Several signal molecules, including salicylic acid (SA), jasmonic acid (JA), ethylene, and active oxygen species (AOS) such as superoxide and hydrogen peroxide, have been implicated in the modulation of plant responses to ozone and other stresses. The connection between these stresses most likely involves AOS in primary signaling events that activate multiple signal transduction pathways. When ozone enters the plant via the stomata, it interacts with cellular constituents and water in the mesophyll, leading to the rapid generation of AOS (Kanofsky and Sima, 1995). Furthermore, recent results in tobacco (Schraudner et al., 1998) and Arabidopsis (Sharma et al., 1996; Rao and Davis, 1999) indicate that ozone induces an oxidative burst, generating AOS and activating signal transduction pathways that overlap with those triggered during pathogen infection. This ozone-induced production of AOS is potentiated by SA, which is required for induction of antioxidant defense pathways and in some cases leads to activation of a programmed cell death (PCD) pathway (Rao and Davis, 1999; I. Aguilar and K.R. Davis, unpublished results).
Although it is clear that SA is an important factor in regulating ozone responses in plants, the available data also support the notion that ozone activates a second, SA-independent pathway that is likely to require JA and/or ethylene (Kangasjarvi et al., 1994; Sharma et al., 1996; Rao and Davis, 1999). The induction of PAL and cytAPX gene expression by ozone has been shown to be SA independent, suggesting the involvement of a second signal transduction pathway (Sharma and Davis, 1996; Rao and Davis, 1999). Örvar et al. (1997) demonstrated that mechanical wounding or the direct application of JA prior to ozone exposure resulted in a decrease in the amount of ozone injury in tobacco plants. Our laboratory demonstrated in hybrid poplar that ozone induced accumulation of transcripts encoding WIN 3.7, a gene that is both wound and JA inducible (Koch et al., 1998). These results clearly implicate JA as an important signal in ozone-induced responses.
Although the physiological responses of forest trees to ozone have been well characterized, very little is known about their defense responses to ozone and other stresses at the molecular level. Of the defense-related signal molecules that have been identified in herbaceous plants, only ethylene has been studied in some detail in trees (Telewski 1990, 1992; Kargiolaki et al., 1991). Given that the roles of signal molecules previously characterized in herbaceous plants have been found to vary somewhat from one plant species to another (Coquoz et al., 1995; Silverman et al., 1995; Chen et al., 1997), and that tree species have unique characteristics that distinguish them from herbaceous plant species, e.g. longevity and the ability to undergo the process of wood formation, it is possible that trees may have defense signaling systems that are distinct from those found in herbaceous plants.
To further our understanding of ozone-induced signal transduction pathways in tree species, our previous studies used WIN 3.7 and PR-1 as marker genes for JA- and SA-mediated defense response pathways and characterized the ozone induction of these genes in ozone-sensitive (NE-388) and ozone-tolerant (NE-245) nonisogenic hybrid poplar clones (Koch et al., 1998). Our findings showed that when treated with 300 μL L−1 ozone, clone NE-388 developed large necrotic regions on up to 90% of all leaves and had greatly attenuated levels of both JA- and SA-dependent defense gene expression compared with NE-245. Lesion development on NE-245 was observed on less than 20% of all leaves, and the lesions themselves resembled small, HR-like lesions. The attenuated level of induced gene expression was also observed in clone NE-388 in response to either wounding or infiltration with an avirulent Pseudomonas syringae strain, indicating a lack of responsiveness of signaling pathway(s) shared by all three stresses. This non-responsiveness could be attributed to either the inability of NE-388 to produce signal molecules such as SA and/or JA or to its inability to perceive these signal molecules.
In our current report, we show that the attenuated ozone response of clone NE-388 is attributable to insensitivity to both SA and JA. In addition, we demonstrate that the SA-/JA-insensitive clone NE-388 undergoes a mechanism of lesion formation that is distinct from the ozone-tolerant clone NE-245. The characterization of this hybrid poplar clone as being insensitive to both SA and JA establishes an experimental system based on a woody plant species that can be used to study signal transduction pathways that regulate defense gene expression. Furthermore, this system may provide a unique opportunity to define novel signal transduction pathways that are largely SA and JA independent.
RESULTS
NE-388 Is Insensitive to Exogenous SA and Methyl Jasmonate (MeJA)
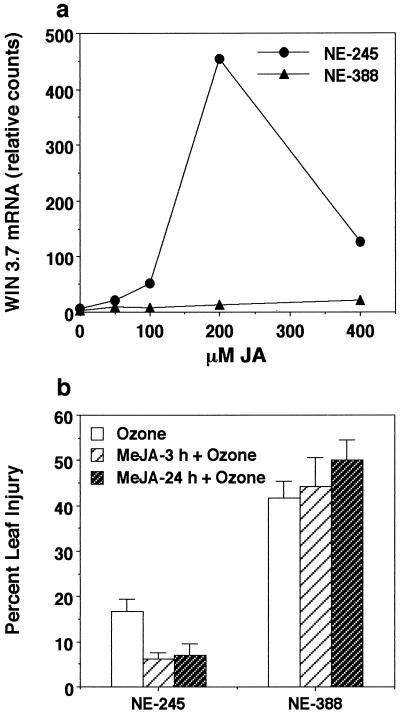
Previous results demonstrating that ozone, pathogen, and wound induction of both SA- and JA-regulated gene expression is attenuated in clone NE-388 indicate that a deficiency exists in a signal transduction pathway(s) that is activated by all three stresses. This deficiency may be due to the inability to synthesize SA and JA or to the inability to perceive these signal molecules. To distinguish between these two possibilities, experiments were performed using exogenously applied SA and MeJA (Fig. 1). Application of MeJA caused induction of WIN 3.7 transcripts in NE-245 (Fig. 1a). At 100 μm MeJA, a modest induction of WIN3.7 transcripts was detected, and at 200 μm MeJA, a 10-fold increase in WIN 3.7 transcripts was observed. No visible lesions formed, even at concentrations as high as 400 μm MeJA. In contrast, WIN 3.7 transcripts were barely detectable in NE-388, even at 400 μm MeJA. Again, there were no visible signs of lesion formation.
Figure 1.
Effects of pretreatment with exogenous MeJA on ozone-induced leaf damage and defense gene induction. a, Individual leaves were sprayed with different concentrations of JA in 0.1% (w/v) Triton X-100 until runoff occurred. The fourth fully expanded leaf from three trees per concentration was harvested 24 h after treatment, and pooled for RNA isolation and subsequent northern-blot analysis using WIN 3.7 as a probe. b, Plants were treated either 3 or 24 h prior to ozone fumigation with a solution of 200 μm MeJA in 0.1% (w/v) Triton X-100, and 24 h after the start of the ozone treatment, plants were assessed for the percent leaf injury. The data for each test condition represent the mean ± se of two to six individual plants. Data shown are representative of one of three independently performed experiments.
It has been reported previously that in tobacco, pretreatment with wounding or JA resulted in a reduction of ozone-induced lesion formation (Örvar et al., 1997). To further explore the role of JA in ozone tolerance and to confirm that NE-388 is non-responsive to JA, experiments in which plants were treated with MeJA prior to ozone exposure were performed. A concentration of 200 μm MeJA was chosen for use in these experiments because it produced the greatest extent of WIN 3.7 induction 24 h after application (Fig. 1a). MeJA pretreatments were performed both 3 and 24 h prior to ozone exposure. A statistically significant reduction in percent leaf injury (from 19%–6%, P = 0.01) was observed in the tolerant clone NE-245 when treated with 200 μm MeJA both 3 and 24 h prior to ozone treatment (Fig. 1b). A slight increase in percent leaf injury was recorded for MeJA-pretreated NE-388 plants; however, this increase was not statistically significant (P = 0.20). These results further confirm that NE-388 does not respond to JA.
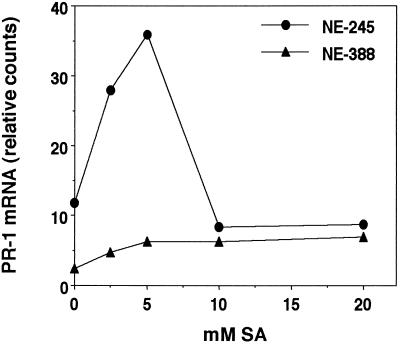
Experiments with exogenously applied SA revealed that NE-388 is also not able to respond to SA. A 3-fold induction of PR-1 transcripts was observed at 24 h in the ozone-tolerant clone NE-245 when the plants were treated with 2.5 mm SA (Fig. 2). Maximum levels of PR-1 transcript accumulation were observed at 5 mm SA, while higher concentrations did not cause significant PR-1 induction. The lack of induction at the higher concentrations correlated with the appearance of SA-induced lesions. Conversely, in the ozone-sensitive clone NE-388 there was no detectable induction of PR-1 transcripts by treatment with SA concentrations as high as 20 mm. Interestingly, as was observed with NE-245, SA-induced lesion formation in NE-388 also occurred at 10 and 20 mm SA.
Figure 2.
Defense gene induction by SA. Individual leaves were sprayed with different concentrations of SA in 0.1% (w/v) Triton X-100 until runoff occurred. The fourth fully expanded leaves from three trees per concentration were harvested 24 h after treatment and pooled for RNA isolation and subsequent northern-blot analysis using PR-1 as a probe. Data shown are representative of one of three independently performed experiments.
Measurement of Endogenous Levels of SA and JA
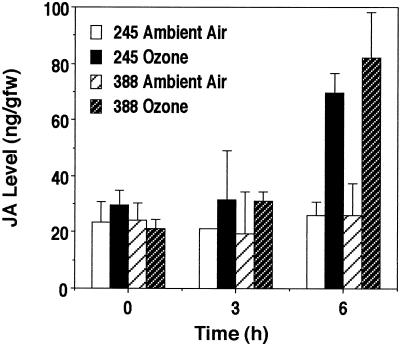
To determine if both clones accumulate SA and JA in response to ozone exposure, the levels of these two molecules were measured in both clones that had been kept in ambient air or exposed to 300 μL L−1 ozone. Ozone exposure of both NE-388 and NE-245 increased JA levels by 3.2- and 2.7-fold, respectively, compared with control plants (Fig. 3). Although an increase in JA was detected within 3 h of ozone exposure, the induction of JA in ozone-treated plants compared with control plants was not statistically significant until 6 h.
Figure 3.
Ozone-induced JA accumulation. Data shown represent the mean ± se of three individual trees from a representative experiment. Similar results were obtained in an independently performed duplicate experiment.
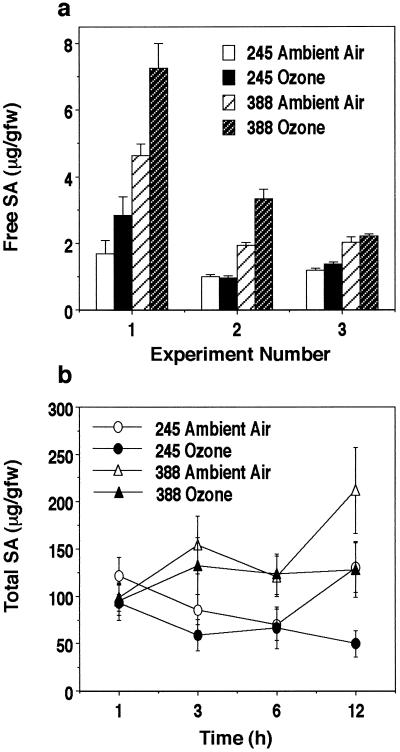
Endogenous levels of SA were also determined, and both clones were found to have high constitutive levels of free SA compared with herbaceous plant species such as tobacco and Arabidopsis. The basal levels of free and total SA were 2.4- and 1.5-fold higher, respectively, in NE-388 compared with NE-245 (Fig. 4). Time course experiments revealed that 6 h after the start of ozone treatment, a modest increase in free SA levels of 36% for NE-388 and 31% for NE-245 was detected (Fig. 4a), but these increases were not found to be statistically significant (P = 0.09 for NE-388 and P = 0.22 for NE-245). Levels of free SA were measured in three independent experiments, and in each experiment modest increases were observed at 6 h, ranging from 8% to 56% in clone NE-388 and from 16% to 68% in clone NE-245. In only one experiment was this increase found to be statistically significant for both clones. Total SA was also measured and no statistically significant increase due to ozone exposure was observed at 1, 3, 6, or 12 h (Fig. 4b). Although no significant induction of either free or total SA was found in ozone-treated trees, SA was determined to be present in both clones, and, in fact, the basal levels of both free (P ≤ 0.0001) and total (P = 0.0002) SA were significantly higher in NE-388 (1.5- to 2.7-fold). Thus, the lack of response of NE-388 to ozone treatment is not related to a lack of SA production.
Figure 4.
Accumulation of SA in ozone-treated plants 6 h after onset of treatment. a, Free SA accumulation. Data shown represent the mean ± se of six to eight trees from three independent experiments. The average values for free SA levels in untreated plants for the three experiments were 3.12 ± 0.33 for NE-388 and 1.31 ± 0.18 μg/g fresh weight for NE-245. ANOVA resulted in the following P values: P ≤ 0.0001 for interaction due to clone, P = 0.2177 for interaction due to treatment in clone NE-245, and P = 0.0891 for interaction due to treatment in clone NE-388. b, Total SA accumulation. Data represent the mean ± se for both the free and conjugated SA measured in two independent experiments. The average values for total SA in untreated plants in the two experiments were 146 ± 17 for NE-388 and 99 ± 10 μg/g fresh weight for NE-245. ANOVA resulted in the following P values: P = 0.0002 for interaction due to clone, P = 0.1523 for interaction due to treatment in clone NE-245, and P = 0.1163 for interaction due to treatment in clone NE-388.
TUNEL Analysis of Ozone- and Pathogen-Treated Plants
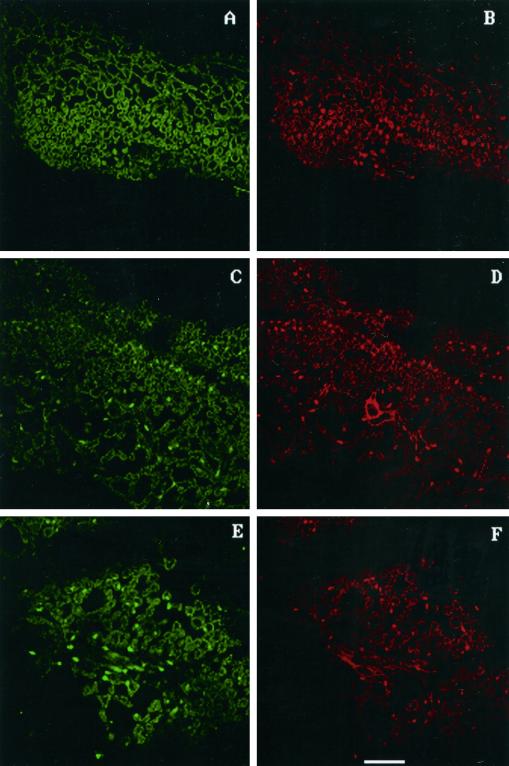
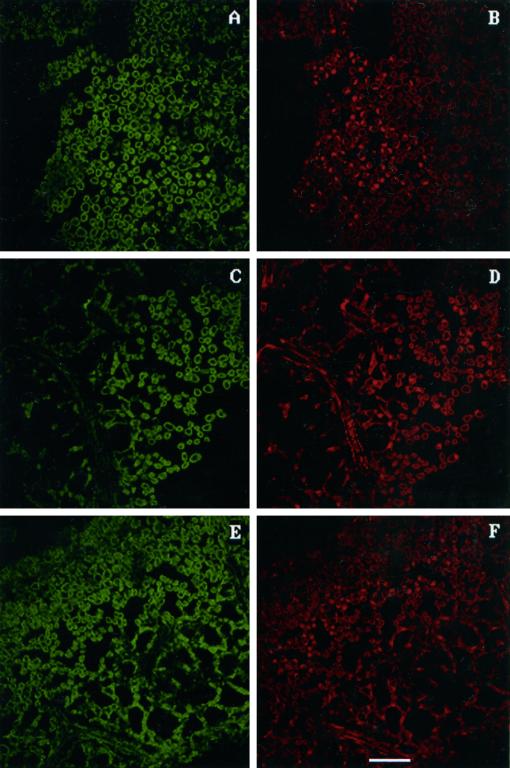
Our previous experiments in both hybrid poplar (Koch et al., 1998) and Arabidopsis (Rao and Davis, 1999; I. Aguilar and K.R. Davis, unpublished results) suggest that ozone-induced lesion formation can occur by two different mechanisms: by the activation of PCD or by necrosis. To test this further, we examined DNA fragmentation as an indicator of PCD using terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) analysis. PCD is characteristic of the SA-dependent activation of a hypersensitive response (HR) by pathogens or ozone (Dangl et al., 1996; Greenberg, 1997; Sharma and Davis, 1997). DNA fragmentation was detected in ozone-treated leaf tissue from the tolerant clone NE-245 at both 3 h (Fig. 5C) and 6 h (data not shown) after treatment, as indicated by the incorporation of fluoroscein-labeled dUTP. Sections were counterstained with propidium iodide to allow nuclei to be visualized (Fig. 5D). By comparing the pattern of fluoroscein incorporation (Fig. 5C) with the pattern of propidium-iodide-stained nuclei, it is clear that the fluoroscein is localized to a subset of nuclei. There was no evidence of fluoroscein incorporation (Fig. 5A) in untreated tissue samples, even though numerous nuclei are visible in the propidium-iodide-stained sample (Fig. 5B). No evidence for DNA fragmentation was found in ozone-treated tissue of clone NE-388, even though numerous propidium-iodide-staining nuclei are visible (Fig. 6D). Sections were taken from both clones from the second and fourth fully expanded leaves from regions near the petiole, which typically develop lesions first.
Figure 5.
TUNEL assay to detect DNA fragmentation in clone NE-245. C shows incorporation of fluoroscein-labeled dUTP in the ozone-treated NE-245 tissue visualized as bright green spots within the cells. D is the same section as seen in C except propidium iodide was used to stain all nuclei present, which appear as bright red spots. When C and D are compared, it is clear that most nuclei in this particular section have undergone DNA fragmentation. E shows fluoroscein incorporation in pathogen-treated tissue, and F is the propidium iodide staining of tissue shown in E. No evidence of fluoroscein incorporation was seen in untreated tissue (A), even though numerous nuclei were visible in the propidium-iodide-stained control tissue (B). The white line at the bottom of F represents 50 μm.
Figure 6.
TUNEL assay to detect DNA fragmentation in clone NE-388. C is a section of ozone-treated tissue from clone NE-388 that has been treated with terminal transferase. No bright green nuclei were visible, although numerous stained nuclei were clearly evident after staining with propidium iodide. Similar results were obtained with pathogen-treated tissue (E and F) and untreated tissue (A and B). The white line at the bottom of F represents 50 μm.
Given the clear differences in lesion formation in clones NE-388 and NE-245 in response to ozone, we investigated whether pathogen-induced lesion formation was also different in these two clones. Pathogen infiltration resulted in the appearance of DNA fragmentation in clone NE-245 (Fig. 5E), but no DNA fragmentation was detectable in NE-388 (Fig. 6E). Similar to ozone-treated tissue, DNA fragmentation was detected at both 3 h (Fig. 5E) and 6 h (data not shown) after infiltration in the tolerant clone, and occurred prior to visible lesion formation.
DISCUSSION
The attenuated response to ozone, phytopathogen infection, and wounding of both SA- and JA-inducible defense gene expression in clone NE-388 indicates a deficiency in a component of a signaling pathway that is common for all three stresses (Koch et al., 1998). Our current results demonstrate that exogenous application of SA and MeJA fails to cause induction of PR-1 and WIN 3.7, respectively, in the ozone-sensitive clone NE-388, even at levels that far exceed endogenous levels, indicating that this clone is deficient in perceiving SA and JA. This conclusion is supported by quantitative analyses demonstrating that clone NE-388 synthesizes at least as much SA and JA as the ozone-tolerant clone NE-245. The levels of JA were not significantly different between the two clones, while the levels of SA were significantly higher in NE-388 compared with NE-245. Therefore, because NE-388 produces just as much JA as NE-245, and even more SA than NE-245, the attenuated response of the ozone-sensitive clone NE-388 cannot be attributed to a deficiency in the biosynthesis or increased metabolism of these signal molecules. These data strongly suggest that the ozone-sensitive clone NE-388 lacks the ability to efficiently perceive both SA and JA.
When levels of JA were measured in hybrid poplar plants that had been treated with ozone, approximately a 2.5-fold increase at 6 h after the start of treatment was found in both the sensitive and tolerant clones. To our knowledge, this is the first report of ozone induction of endogenous levels of JA, and, in conjunction with our previous work in hybrid poplar (Koch et al., 1998) and tobacco (Örvar et al., 1997), provides direct evidence that JA is an important ozone-induced signal molecule. Our finding that treatment with MeJA prior to ozone exposure results in a reduction in visible leaf injury in the tolerant clone NE-245 supports similar findings in tobacco (Örvar et al., 1997) and Arabidopsis (M.V. Rao and K.R. Davis, unpublished results). However, JA pretreatment does not reduce levels of leaf injury in the JA-insensitive clone NE-388. These data provide additional evidence that JA is an important signal molecule involved in modulating ozone responses in a tree system as well.
SA, also known to be an important signal in ozone-induced responses, has been shown to increase 4- to 5-fold after 3 to 6 h of ozone exposure in Arabidopsis (Sharma et al., 1996), with a concomitant increase in conjugated forms of SA over a 24-h period. Our current results with ozone-treated hybrid poplar clearly differ from these findings. We measured only a slight increase in free SA levels (31%–36%), which was not statistically significant, and no increase at all in total SA levels for both clones. However, both free and total SA levels were approximately 2-fold higher in NE-388. This may be due to natural variation between these two clones or may indicate that NE-388 has aberrant SA metabolism. The average free SA level found for NE-245 was 1.3 ± 0.2 and 3.1 ± 0.3 μg/g fresh weight for NE-388 compared with <50 ng/g fresh weight in tobacco and Arabidopsis (Malamy et al., 1990; Enyedi et al., 1992; Vernooij et al., 1994; Sharma et al., 1996).
When comparing basal levels of SA between herbaceous plant species, a great degree of variability also exists. For example, in tobacco, a 1.2- to 4-fold increase in the low basal levels of SA is associated with the induction of PR gene expression (Malamy et al., 1990; Ohshima et al., 1990). However, the basal levels of tomato, potato, and soybean have been reported to exceed even the elevated levels measured in tobacco that are associated with systemic acquired resistance (Raskin et al., 1990), as do the levels we found in hybrid poplar. The levels of both free and total SA in both of the hybrid poplar clones were found to be constitutively higher than the levels measured in tobacco and Arabidopsis that are associated with systemic acquired resistance. It appears that although hybrid poplar shares certain characteristics of SA and JA signaling with some herbaceous plant systems, it does not clearly fit all of the patterns established in any of the well-studied herbaceous plant systems.
Our results distinctly show that DNA fragmentation, an indicator of SA-dependent PCD, occurs in response to ozone in the ozone-tolerant, SA-responsive hybrid poplar clone (NE-245) but not in the ozone-sensitive SA-insensitive clone (NE-388). Identical results were obtained using tissue infiltrated with an avirulent strain of P. syringae. In regions of the leaf that were know to have been infiltrated with bacteria, NE-388 did not display DNA fragmentation yet it was readily detectable in NE-245. These data provide evidence that SA perception is required for the activation of a hypersensitive cell death pathway and that ozone can in fact induce lesion formation via the activation of PCD. In addition, the inability of NE-388 to undergo PCD in response to ozone or pathogen infection, while DNA fragmentation is readily detectable in NE-245, indicates that the mechanism for lesion formation in NE-245 in response to either stress not only requires SA, but is also essentially the same. Thus, ozone-induced lesion formation occurs via two distinct mechanisms in the two hybrid poplar clones. Cell death in the tolerant clone is caused by the induction of a PCD pathway associated with a HR, while cell death in the sensitive clone is likely to be necrosis caused by the lack of induction of antioxidant defenses to a level sufficient to prevent the formation of toxic ozone-induced AOS intermediates (Koch et al., 1998).
The hypothesis that two distinct mechanisms of lesion formation occur in hybrid poplar in response to ozone is supported by recent work in Arabidopsis (Rao and Davis, 1999). Using transgenic plants expressing salicylate hydroxylase (NahG), which converts SA into biologically inactive catechol (Gaffney et al., 1993; Delaney et al., 1994), it was determined that NahG plants undergo ozone-induced toxic cell death due to an inability to maintain the cellular redox state. This mechanism of lesion formation is distinct from Cvi, an ecotype that hyperaccumulates SA and has been shown to undergo ozone-induced nDNA fragmentation (I. Aguilar and K.R. Davis, unpublished results). Furthermore, pretreatment of Cvi with MeJA results in a reduction of ozone-induced leaf injury (M.V. Rao and K.R. Davis, unpublished results) similar to what we observed for the ozone-tolerant hybrid poplar clone. These results indicate that JA may modulate SA-mediated responses, including PCD. Although both Cvi and NE-245 undergo PCD, the higher induced levels of SA in Cvi compared with more tolerant ecotypes appear to stimulate a runaway hypersensitive response that leads to high levels of lesion formation. These results suggest that ozone sensitivity is determined by a delicate balance of several distinct, interacting signaling pathways. For ozone tolerance, optimal concentrations (and perception) of SA and JA are required to achieve maximal induction of defense responses with minimal induction of the PCD pathway.
Although SA appears to function similarly in ozone induction of a PCD pathway leading to lesion formation in both hybrid poplar clone NE-245 and Arabidopsis ecotype Cvi, these plants appear to differ in how the SA signal is transmitted. In Cvi, ozone induces SA levels at least 10-fold, while no ozone-induced elevation in SA levels was detected in the hybrid poplar ozone-tolerant clone, even though both undergo SA-dependent DNA fragmentation in response to ozone. These results demonstrate that direct extrapolation from model herbaceous plants to trees is not always possible; in fact, if reliable data are to be obtained on signal transduction pathways in a woody plant species, a woody plant system must be used.
In conclusion, we have shown that not only is the ozone-sensitive hybrid poplar clone (NE-388) insensitive to both SA and JA, but it also undergoes a mechanism of lesion formation via necrosis that is distinct from the SA-mediated activation of PCD that precedes lesion formation in the ozone-tolerant clone (NE-245). The SA-mediated activation of PCD appears to be inhibited by JA, and supports the growing body of literature indicating significant interaction between these two pathways (Sano et al., 1996; Seo et al., 1997; Romeis et al., 1999; Shah et al., 1999). This naturally occurring variant will be useful in further studies aimed at dissecting these interacting signal transduction pathways involved in defense responses in hybrid poplar, and may prove extremely useful in identifying novel SA/JA-independent signaling pathways.
MATERIALS AND METHODS
Growth and Treatment of the Plants
Greenwood cuttings of hybrid poplar (Populus maximowizii × Populus trichocarpa) were rooted under mist and transplanted as described previously (Koch et al., 1998). Six weeks following transplantation, cuttings were transferred to growth chambers modified for ozone fumigation and acclimated for 2 to 3 d. Ozone treatments were carried out as previously described (Koch et al., 1998) using an ozone generator (model 03V10—0 Orec, Ozone Research and Equipment, Phoenix).
SA and MeJA Treatments
SA was diluted in distilled water containing 0.1% (v/v) Triton X-100 as a surfactant to concentrations of 2.5, 5.0, 10.0, and 20 mm. A 100 mm stock solution of MeJA was made in dimethylformamide. Dilutions of 50, 100, 200, and 400 μm were subsequently made in distilled water containing 0.1% (w/v) Triton X-100. Control plants were treated with either 0.1% (v/v) Triton X-100 or dimethylformamide plus 0.1% (v/v) Triton X-100. Three plants per concentration per experiment were sprayed on both the abaxial and adaxial surfaces until runoff occurred. Twenty-four hours after treatment, the fourth fully expanded leaf from each plant was harvested and three leaves from each concentration were pooled and frozen in liquid nitrogen for subsequent RNA extraction. The data shown are from a representative experiment of three independently replicated experiments.
RNA Extraction and Analysis
Total RNA was extracted as described by Parsons et al. (1989). RNA was fractionated, blotted, and hybridized as previously described (Koch et al., 1998). WIN 3.7 (Bradshaw et al., 1989), a kind gift from M. Gordon (University of Washington, Seattle), was used as a marker gene for JA-mediated gene expression. A poplar partial cDNA for PR-1 (Koch et al., 1998) was used as a marker gene for SA-mediated gene expression. Hybridized filters were exposed to phosphor imager screens (Molecular Dynamics, Sunnyvale, CA) and the signal intensity was quantified using ImageQuant software (Molecular Dynamics). The data shown have been corrected for loading differences by measuring counts obtained by rehybridizing with a 28S ribosomal gene from pea (Wanner and Gruissem, 1991). All experiments were performed at least twice, with at least two replicates per test condition. The data shown are from representative experiments.
JA Measurements
Due to the large amount of tissue required to measure JA, careful consideration was given to experimental design. Based on previous observations that middle-aged leaves (fully expanded leaves 2–10) in both clones respond similarly to ozone with respect to defense gene induction, reduction of stomatal conductance, and photosynthetic rate, and the timing of lesion formation (Koch et al., 1998; J. Koch and K.R. Davis, unpublished results), we pooled leaves 2 through 8 from individual trees for JA analysis. Tissue was collected from plants treated with either ambient air or 300 ppb ozone, major veins were removed, and tissue was frozen in liquid nitrogen and ground to a homogeneous mixture. JA analysis was performed by suspending frozen aliquots of 4 g of pulverized leaf tissue in 60:40 (v:v) acetone:methanol, and homogenizing using a polytron. Leaf tissue was removed by filtration, 2 to 5 mL of water was added, and a known amount of [13C]-labeled JA was added as an internal standard. Acetone and methanol were removed by rotary evaporation, 50 mL of 0.1 m sodium phosphate and 5% (w/v) NaCI (pH 8.5) was added, and the sample was extracted twice with dichloromethane. The aqueous phase was then acidified to pH 2.0 by the addition of 6 n HCI, and extracted once with hexane and once with dichloromethane.
JA was partitioned under acidic conditions into dichloromethane, which was collected and evaporated to near dryness. Following the addition of a small volume of 5 mm HCl, the remaining dichloromethane was evaporated. One to 2 mL of 5 mm HCl was added, and the sample was sonicated, filtered, and loaded onto a C18 cartridge (Sep-Pak, Waters, Milford, MA) previously equilibrated with 5 mm HCl. The cartridge was rinsed with 5 mm HCl followed by water, and centrifuged to remove residual water. The sample was eluted from the cartridge using methanol, and then dried, methylated using etherial diazomethane, and analyzed by gas chromatography/mass spectrometry/ selected ion monitoring according to the method of Creelman and Mullet (1995). Data shown are representative of a single experiment in which three individual trees were assayed per time point. The entire experiment was duplicated with similar results.
SA Measurements
Tissue was collected for SA analysis as described above for JA quantification. SA was extracted as described previously (Enyedi et al., 1992), with the following modifications. Leaves were ground in liquid nitrogen with a mortar and pestle, and 3-hydroxybenzoic acid in methanol was added at a level of 50μg/g fresh weight weight as a recovery measurement spike. Total SA (free plus glucosyl SA) was determined from methanol extracts digested with β-glucosidase (Sigma, St. Louis) as described by Seskar et al. (1998). Dried extraction samples were resuspended in an HPLC mobile phase of 75% (w/v) 40 mm sodium acetate (pH 3.5):25% (w/v) methanol, and filtered through 0.2-μm nylon filters (Costar Spin-X, Corning, Corning, NY). Fifty-microliter samples were injected onto a Nova-Pak C18 60Å 4-μm Guard-Pak insert column (Waters) linked to a Nova-Pak C18 60Å 4-μm column (3.9 × 300 mm; Waters) maintained at 40°C. A linear segment gradient of methanol to 40 mm sodium acetate, pH 3.5, was applied at a constant flow rate of 1 mL/min as follows: 25% to 45% (w/v) methanol over 12 min, 45% to 100% (w/v) methanol over 6 min, and 100% to 25% (w/v) methanol in 5 min to re-equilibrate the column. A 490 absorbance detector (Waters) was used for 3-hydroxybenzoic acid quantitation at 236 nm. In tandem, a 474 scanning fluorescence detector (Waters) was used for SA quantitation with the gain set to 10, excitation wavelength at 295 nm, and emission wavelength at 405 nm; SA (eluting at 7.4 min) and 3-hydroxybenzoic acid (eluting at 6.7 min) were resolved baseline-to-baseline as monitored by A236, and identified using authentic standards.
Data Analyses
StatView software (Abacus Concepts Inc., Berkeley, CA) was used for all statistical analyses, and the data presented include means plus se of at least two independent experiments. All data were subjected to ANOVA, with the exception of the percent injury after MeJA treatment data, which were subjected to the non-parametric Kolmogorov-Smirnov test.
TUNEL Analysis
Tissue samples were taken from the second through fifth fully expanded leaves 1, 3, and 6 h after the start of ozone treatment. Tissue was vacuum infiltrated for 5 min with 4% (w/v) paraformaldehyde in PBS, then incubated overnight at 4°C prior to sectioning. TUNEL analysis was performed on frozen sections using an in situ cell death detection kit using fluorescein (Boehringer Mannheim, Indianapolis), and propidium iodide (R&D Systems, Minneapolis) was used as a counterstain. Sections were viewed using a confocal microscope (LSM, Zeiss, Thornwood, NY). Fluoroscein incorporation was visualized using fluorescein isothiocyanate filter sets, and nuclear staining was visualized using P1 filter sets. Two independent experiments were performed, and for each sampling time at least five slides containing at least five separate sections were screened per clone.
ACKNOWLEDGMENTS
The authors thank Ilya Raskin (Rutgers University) for providing assistance in the SA determinations, Amy Scherzer (U.S. Department of Agriculture Forest Service, Northeastern Research Station) for her advice on statistical analyses of data, MaryAnn Tate (U.S. Department of Agriculture Forest Service, Northeastern Research Station) for her technical expertise and assistance in performing the ozone treatments, Mary Macone (Children's Hospital) for tissue sectioning, Cindy McAllister (Children's Hospital, Columbus, OH) for her technical assistance in performing TUNEL assays and confocal microscopy, and Stacey Putney (Ohio State University, Columbus) for maintaining the plants.
Footnotes
This work was supported in part by a cooperative agreement with the Northeastern Research Station of the Forest Service, by the U.S. Department of Agriculture (K.R.D.), by a National Science Foundation grant (no. MCB–9514034 to J.E.M.), and by a U.S. Department of Agriculture National Research Initiative Competitive Grants Program grant (no. 95–37304–2440 to J.E.M.).
LITERATURE CITED
- Bradshaw HD, Hollick JB, Clarke HRG, Gordon MP. Systemically wound-responsive genes in poplar trees encode proteins similar to sweet potato sporamins and legume Kunitz trypsin inhibitors. Plant Mol Biol. 1989;14:51–59. doi: 10.1007/BF00015654. [DOI] [PubMed] [Google Scholar]
- Chen Z, Iyer S, Caplan A, Klessig D, Fan B. Differential accumulation of salicylic acid and salicylic acid-sensitive catalase in different rice tissues. Plant Physiol. 1997;114:193–201. doi: 10.1104/pp.114.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquoz J-L, Buchala AJ, Meuwly PH, Metraux J-P. Arachidonic acid induces local but not systemic synthesis of salicylic acid and confers resistance in potato plants to Phytopobthora infestans and Altemaria solani. Phytopathlogy. 1995;85:1219–1224. [Google Scholar]
- Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 1995;92:4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy: cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Enyedi AJ, Yalpani N, Silverman P, Raskin I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci USA. 1992;89:2480–2848. doi: 10.1073/pnas.89.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernocij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Greenberg JT. Programmed cell death in plant-pathogen interactions. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:525–545. doi: 10.1146/annurev.arplant.48.1.525. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Taylor GE., Jr Role of air pollution in forest decline in eastern North America. Water Air Soil Pollut. 1989;48:21–43. [Google Scholar]
- Kangasjarvi J, Talvinen J, Utriainen M, Karjalainen R. Plant defense systems induced by ozone. Plant Cell Environ. 1994;17:783–794. [Google Scholar]
- Kanofsky JR, Sima PS. Singlet oxygen generation from the reaction of ozone with plant leaves. J Biol Chem. 1995;270:7850–7852. doi: 10.1074/jbc.270.14.7850. [DOI] [PubMed] [Google Scholar]
- Kargiolaki H, Osborne DJ, Thompson FB. Leaf abscission and stem lesions (intumescences) on poplar clones after SO2 and O3 fumigation: a link with ethylene release? J Exp Bot. 1991;42:1189–1198. [Google Scholar]
- Koch JR, Scherzer AJ, Eshita SM, Davis KR. Ozone sensitivity in hybrid poplar is correlated with a lack of defense-gene activation. Plant Physiol. 1998;118:1243–1252. doi: 10.1104/pp.118.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Ohshima M, Itoh H, Matuoka M, Murakami T, Ohashi Y. Analysis of stress-induced or salicylic acid-induced expression of pathogenesis-related 1A-protein gene in transgenic tobacco. Plant Cell. 1990;2:95–106. doi: 10.1105/tpc.2.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Örvar BL, McPherson J, Ellis BE. Pre-activating wounding response in tobacco prior to high-level ozone exposure prevents necrotic injury. Plant J. 1997;11:203–212. doi: 10.1046/j.1365-313x.1997.11020203.x. [DOI] [PubMed] [Google Scholar]
- Parsons TJ, Bradshaw HD, Gordon MP. Systemic accumulation of specific mRNAs in response to wounding in poplar trees. Proc Natl Acad Sci USA. 1989;86:7895–7899. doi: 10.1073/pnas.86.20.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Rao MV, Pallyath G, Ormrod DP. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996;110:125–136. doi: 10.1104/pp.110.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Skabatz H, Tang W, Meeuse BJD. Salicylic acid levels in thermogenic and non-thermogenic plants. Ann Bot. 1990;66:369–373. [Google Scholar]
- Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JDG. Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell. 1999;11:273–287. doi: 10.1105/tpc.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H, Jr, Ernst D, Heller W, Langebartels C. Ozone: an abiotic elicitor of plant defense reactions. Trends Plant Sci. 1998;3:47–50. [Google Scholar]
- Sano H, Seo S, Koizumi N, Niki T, Iwamura H, Ohashi Y. Regulation by cytokinins of endogenous levels of jasmonic acid and salicylic acid in mechanically wounded tobacco plants. Plant Cell Physiol. 1996;37:762–769. [Google Scholar]
- Schmeiden U, Wild A. The contribution of ozone to forest decline. Physiol Plant. 1995;94:371–378. [Google Scholar]
- Schraudner M, Moeder W, Wiese C, Van Camp W, Inze D, Langebartels C, Sandermann H., Jr Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. 1998;16:235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Seo S, Sano H, Ohashi Y. Jasmonic acid in wound signal transduction pathways. Physiol Plant. 1997;101:740–745. [Google Scholar]
- Seskar M, Shulaev V, Raskin I. Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol. 1998;116:387–392. [Google Scholar]
- Shah J, Kachroo P, Klessig DF. The Arabidopsis ssi1 mutation restores pathogenesis-related gene expression in npr1 plants and renders defensin gene expression salicylic acid dependent. Plant Cell. 1999;11:191–206. doi: 10.1105/tpc.11.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma YK, Davis KR. The effects of ozone on antioxidant responses in plants. Free Radic Biol Med. 1997;23:480–488. doi: 10.1016/s0891-5849(97)00108-1. [DOI] [PubMed] [Google Scholar]
- Sharma YK, Leon J, Raskin I, Davis KR. Ozone-induced responses in Arabidopsis thaliana: the role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc Natl Acad Sci USA. 1996;93:5099–5104. doi: 10.1073/pnas.93.10.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman P, Seskar M, Kanter D, Schweizer P, Metraux J-P, Raskin I. Salicylic acid in rice: biosynthesis, conjugation, and possible role. Plant Physiol. 1995;108:633–639. doi: 10.1104/pp.108.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telewski FW. Growth, wood density, and ethylene production in response to mechanical perturbation in Pinus taeda. Can J For Res. 1990;20:1277–1282. [Google Scholar]
- Telewski FW. Ethylene production by different age class ponderosa and Jeffery pine needles as related to ozone exposure and visible injury. Trees. 1992;6:195–198. [Google Scholar]
- Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessman H, Ryals J. Salicylic acid is not the translocated signal responsible for inducing systemic required resistance but is required in signal transduction. Plant Cell. 1994;6:959–965. doi: 10.1105/tpc.6.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner LA, Gruissem W. Expression dynamics of the tomato rbcS gene family during development. Plant Cell. 1991;3:1289–1303. doi: 10.1105/tpc.3.12.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]