Abstract
Purpose
Physical activity (PA) is important in the prevention of type 2 diabetes, yet little is known about the role of specific dimensions of PA, including sedentary time in subgroups at risk of impaired glucose metabolism (IGM). We applied a data driven decision tool to identify dimensions of PA associated with IGM across age, sex and body mass index (BMI) groups.
Methods
This cross-sectional study included 1,501 individuals (mean (SD) age 65.6 (6.8) years) at high risk of type 2 diabetes from the ADDITION-PRO study. PA was measured by an individually calibrated combined accelerometer and heart rate monitor worn for 7 days. PA energy expenditure, time spent in different activity intensities, bout duration and sedentary time were considered determinants of IGM together with age, sex and BMI. Decision tree analysis was applied to identify subgroup-specific dimensions of PA associated with IGM. IGM was based on oral glucose tolerance test results and defined as fasting plasma glucose ≥ 6.1 mmol/L and/or 2-hour plasma glucose ≥ 7.8 mmol/L.
Results
Among overweight (BMI ≥ 25kg/m2) men, accumulating less than 30 minutes/day of moderate-to-vigorous PA was associated with IGM, while in overweight women sedentary time was associated with IGM. Among individuals aged > 53 years with normal weight (BMI < 25kg/m2), time spent in light PA was associated with IGM. None of the dimensions of PA were associated with IGM among individuals aged ≤ 53 years with normal weight.
Conclusion
We identified subgroups in which different activity dimensions were associated with IGM. Methodology and results from this study may suggest a preliminary step towards the goal of tailoring and targeting PA interventions aimed at type 2 diabetes prevention.
Keywords: Decision Tree Analysis, Type 2 Diabetes prevention, Epidemiology, Combined accelerometery, Heart rate monitoring
Introduction
Physical activity (PA) can effectively decrease type 2 diabetes risk in high risk individuals (30). Thus, enhancing PA has long been presented as an effective approach for preventing the transition from a state of impaired glucose metabolism (IGM) to overt type 2 diabetes. Several dimensions related to the type, volume, intensity, duration, frequency and bout-duration of PA have been suggested to be of relevance for reducing risk of type 2 diabetes (1, 29). Yet, which dimension of PA to target and whether this differs in subgroups of individuals remains to be fully explored.
This is in part because epidemiological studies examining the effect of PA and sedentary behavior on health have tended to focus on self-reported measures (3). However, these are prone to bias, have poor levels of validity (34) and do not accurately quantify all dimensions of PA in daily living (37). Although more recent studies employing objective measures of PA and sedentary behavior have been reported, the emphasis have mainly been on assessing main effects applying to the total population of interest while little attention, if any, has been paid to subgroup differences. PA dimensions that only apply to a small subgroup may not show significant effect on a population level and could therefore be missed. None of the commonly used statistical approaches automatically accounts for potential modifying effects of other factors e.g. age, sex and obesity on the associations between dimensions of PA, sedentary behavior and the health outcome being studied. Any complex higher order interactions between risk factors, or potential non-linearity or threshold effect of a risk factor on the outcome of interest need to be pre-specified in conventional regression techniques (including linear- and logistic regression models). As current PA guidelines are developed from conventional regression model results, they are geared toward the typical/average member of the population, without consideration of population subgroups. This is an important limitation as public health recommendations and policies specify that chronic disease prevention strategies should include targeted interventions aimed at the identification and management of high risk individuals (11, 33). Therefore, we need a better understanding of different dimensions of PA and sedentary behavior in individuals with high risk of type 2 diabetes in order to efficiently design PA intervention programs.
Decision Tree Analysis is a data driven methodology with the ability to examine how a set of risk factors jointly influences the risk of an outcome such as IGM. The complex interplay among established risk/protective factors may reveal relationships that would otherwise not have been detected with the use of conventional methods such as comparisons of means and regression models (9, 41). Despite its potential, however, within the PA literature decision tree analysis has to date been infrequently applied. Thus, in a decision tree analysis of the ADDDITION-PRO study we aimed to explore if different dimensions of objectively measured free-living PA, including sedentary time are differently associated with IGM in subgroups of individuals at risk of type 2 diabetes.
Materials and methods
Study sample
We used data from the ADDITION-PRO study, an observational, prospective, population-based cohort study. The ADDITION-PRO participants were recruited from the Danish arm of the ADDITION-Europe study (ADDITION-DK), a stepwise screening program for type 2 diabetes conducted in a defined high-risk group in primary care (28). The ADDITION-Europe study addresses the feasibility of population-based screening for type 2 diabetes, as well as the benefits and costs of screening and intensive multifactorial treatment early in the disease trajectory. The ADDITION-PRO study was the follow-up health examination of individuals without type 2 diabetes at screening in ADDITION-DK but with stratified levels of type 2 diabetes risk defined by the screening procedure in ADDITION-DK. The ADDITION-PRO study aims to quantify type 2 diabetes progression rates and to examine early markers of cardiovascular disease and microvascular diabetic complications as well as the mechanisms that underlie and drive early changes in cardiometabolic physiology. The rationale, methods and definition of diabetes risk groups have been described previously (27).
The present study included cross-sectional data from 2,082 participants who completed the baseline examination between 2009 and 2011. Only participants with a minimum of 48 hours of valid PA data were considered for the present analysis. After excluding participants without PA measurement (n= 238), who had less than 48 hours of combined monitor wear time (n= 16), who had developed type 2 diabetes since screening (n= 336), who did not fast a minimum of 8 hours prior to the oral glucose tolerance test (n= 11) or whose oral glucose tolerance test was unclassified due to missing blood samples (n= 19), a total of 1,501 participants were included in the present analyses.
The ADDITION-PRO study was approved by the ethics committee of the Central Denmark Region (reference no. 20080229) and was conducted in accordance with the Helsinki Declaration. All participants provided oral and written informed consent before participating in the ADDITION-PRO study.
Measurements
General information and body mass index
Information on age and sex was obtained from the unique Danish civil registration number. At the health assessment height was measured without shoes to the nearest millimeter using a stadiometer (Seca, Hamburg, Germany). Participants were weighed in light clothes without shoes using a body composition analyzer (Tanita, Tokyo, Japan). Weight was measured to the nearest 0.1 kg and clothes were estimated to weigh 0.5 kg, which was deducted from the participants' total weight.
Glucose metabolism status
To determine glucose metabolism status, all participants without known diabetes underwent a standard oral glucose tolerance test (75 g glucose dissolved in 250 mL water) after 8 hours of fasting (27). Blood samples were drawn before, 30 minutes and 120 minutes after glucose intake. Plasma glucose levels were assessed using the Hitachi 912 system (Roche Diagnostics, Mannheim, Germany) or the Vitros 5600 integrated system (Ortho Clinical Diagnostic, Illkirch Cedex, France). All ‘Vitros’ values were converted to correspond to ‘Hitachi’ values, using regression equations from validation analyses performed by the study laboratory (See, Table, Supplemental Digital Content 1, Conversion of glucose measures in the ADDITION-PRO study). IGM was defined as fasting plasma glucose ≥ 6.1mmol/l and/or 2 hour plasma glucose ≥ 7.8 mmol/l. Normal glucose metabolism (NGM) was defined as fasting plasma glucose < 6.1mmol/l and 2 hour plasma glucose < 7.8 mmol/l (40).
Physical activity and sedentary time
Free-living PA was measured using a combined accelerometer and heart rate monitor (ActiHeart, CamNTech, Cambridge, UK) worn continuously for seven days (4). The monitor measures uniaxial acceleration and heart rate independently. The monitor was placed horizontally on the participant’s chest with two standard electrocardiogram electrodes (Maxensor, Alton, UK). On the day of the health examination, an 8-minute submaximal step test was performed to account for individual variations in heart rate to PA intensity, as described previously (6). Participants who were physically impaired or had certain cardiovascular conditions, e.g. angina pectoris, were excluded from the step test. Based on 1,046 ADDITION-PRO participants with a valid step test, a “group calibration” based on regression coefficients from the heart rate-to-PA energy expenditure relationship was derived. This group calibration was then used for participants who did not perform the submaximal step test, including information on sex, age, and sleeping heart rate of the individual. A full description of the processing of accelerometer data and heart rate measures from the combined monitor is available elsewhere (5, 7, 35). Briefly, heart rate data was pre-processed using a 2-stage Gaussian Process Robust regression to de-noise the heart rate signal according to the method described by Stegle et al. (35). The procedure works well for dealing with noise when the sensor is worn, and owing to the short-term covariance function, also for brief periods of missing data (e.g., electrode changes). Non-wear periods were identified using the Bayesian uncertainty estimate from the Gaussian Process Robust regression in combination with extended periods of zero-movement (i.e. if also accompanied by non-physiological heart rate data). Such segments are probabilistically marked as non-wear if longer than 90 min, which is taken into account when the time-series are summarized into average daily estimates using adjustment for diurnal bias (i.e. weighting all hours of the day equally in the summation) (8). Physical activity energy expenditure (PAEE) (in kJ/kg/day) was calculated by time-integration of the activity intensity time-series, estimated from heart rate and acceleration in a branched equation modelling framework (5, 6). The time distribution of activity intensity was described by summarizing the intensity time-series in standard metabolic equivalents (METs) within 25 narrowly defined intensity categories. For tabulation purposes these categories were later collapsed into broader intensity categories as sedentary (≤1.5 METs), light intensity PA (LPA) (1.5 to 3 METs), moderate intensity PA (3 to 6 METs), and vigorous intensity PA (VPA) (>6 METs). To indicate the degree of activity accumulation occurring in bouts, time spent in continuous bouts of moderate-to-vigorous PA (MVPA) lasting ≥ 10 minutes was also derived. We used a fixed value of 20.35 J ·ml−1 O2 × 3.5 ml O2·min−1.kg−1 to define 1.0 MET. The broader MET thresholds used have been commonly applied when investigating PA in adults.
Awake sedentary time (SED-time) and sleep are difficult distinguish from one another solely on the basis of heart rate and acceleration data. Therefore, to assist separation of sleep and SED-time, participants were asked to report the times that they went to bed and got up in a sleep diary while wearing the ActiHeart monitor. This self-reported information was fused with the combined heart rate and movement time-series plot to locate a region of interest within which to identify objective markers of sleep onset (considered the beginning of prolonged minimal movement accompanied by a decline in heart rate) and termination (movement initiation together with an abrupt increase in heart rate). All activity plots were inspected by researchers whilst blinded to all other participant characteristics. Every occurrence of time spent ≤ 1.5 METs was given either a sleep or SED-time while awake score depending on whether the activity fell within or outside of a designated sleep phase.
PA level (PAL) was estimated as the ratio between total energy expenditure (PAEE + predicted resting metabolic rate + thermic effect of food) and predicted resting metabolic rate. The predicted resting metabolic rate was estimated using the Oxford 2005 equation (24) and the thermic effect of food accounted for 10% of the total energy expenditure (39).
Statistical analysis
Descriptive characteristics of the study sample by glucose metabolism status were summarized as medians with interquartile ranges (IQR) or as numbers and percentages. Skewed data was logarithmically transformed to fulfill the requirement of normal distribution of the residuals before analysis. Differences between NGM and IGM groups in arithmetic and geometric means for normally distributed and skewed data, respectively were assessed using independent sample t-test while Chi-square test was used to assess differences in categorical data.
Decision-tree modelling of data from the ADDITION-PRO population was performed to explore, describe and visualize the potential impact of different PA dimensions and SED-time as determinants of IGM along with age, sex and BMI. The decision tree model allows for the identification of the strongest dimensions of PA and SED-time as determinants of IGM in different subgroups. Decision tree models were generated by repeated binary partitioning of the population, based on a set of determinants, such that the participants in the subsets called “nodes” are increasingly more homogenous within those nodes further down the tree. For each node, the risk factor and split in this factor which maximizes discrimination is identified. In our study the optimal splits are the ones which give the maximal difference in prevalence of IGM between the two resulting subgroups. Splitting stops when the participants in a node are either entirely, or almost entirely, of the same class, or when further splitting does not improve discrimination between participants. Furthermore, we set an a priori requirement of at least 20 observations in each subset node in order for further splitting to be considered. When a node cannot be split any further, that subgroup is labelled as a terminal ‘leaf’ in the tree. The final set of terminal leaves comprise subgroups in the study population, characterized by a different sequence of classifications by the determinants included in the final decision tree (9, 41).
The following dimensions of PA were considered as determinants. All have in the literature been suggested to be associated with risk of type 2 diabetes: a) overall PA volume expressed as PAEE b) LPA c) MVPA d) MVPA accumulated in bouts ≥ 10minutes and e) VPA. In addition, two variables were computed as categorical variables of meeting the Danish MVPA-guidelines (14): f) 30 minutes of MVPA per day regardless of bout-duration and g) 30 minutes of MVPA per day accumulated in bouts of at least 10 minutes. Finally, h) SED-time was computed. Additional determinants included sex, age as a continuous variable and BMI categorized as underweight/normal weight < 25 kg/m2) or overweight/obese (≥ 25 kg/m2).
The relative risk of IGM was calculated for all subgroups using the subgroup with the lowest prevalence of IGM as reference. In addition, we assessed correlations between the PA dimensions with a pairs plot (see Figure, Supplemental Digital Content 2, Pairs plot showing a clear correlation tendency between all the physical activity dimensions). Secondly, we performed a sensitivity analysis by remodeling the final decision tree alternately without one of the correlated PA dimensions and assessed which other determinants were selected in their place [see Figures, Supplemental Digital Content 3, Decision tree with PAEE left out in the model; Supplemental Digital Content 4, Decision tree with MVPA (30min/day) left out in the model; Supplemental Digital Content 5, Decision tree with LPA left out in the model; and Supplemental Digital Content 6, Decision tree with SED-time left out in the mode)].
All statistical analyses were performed using the statistical software package R version 3.2.0. The Decision tree model was programmed using the package ‘party’ 1.0-25 (26).
Results
Clinical and physical activity characteristics in participants with NGM and IGM
The overall study population consisted of 1,501 participants with a median (IQR) age of 66.2 (61.9, 71.3) years, 53.6% of whom were men. A total of 785 (52.3%) participants had NGM and 716 (47.7%) had IGM (Table 1). Participants with IGM were more often men, smokers, unemployed and had a higher alcohol intake. The IGM participants had an unfavorable metabolic profile and more prevalent use of anti-hypertensive and lipid-lowering drugs compared to participants with NGM.
Table 1.
Descriptive characteristics of the study population according to glucose metabolism status
| NGM n = 785 (52.3%) |
IGM n = 716 (47.7%) |
|
|---|---|---|
| Demographical | 377 (48.0) | 428 (59.8)** |
| Male sex, n (%) | ||
| Age, (years) | 66.0 (61.0, 71.0) | 66.0 (62.0, 71.0)* |
| Employment, n(%)a | 330 (42.0) | 256 (35.8)* |
| Behavioral | 120 (15.4) | 120 (16.9)* |
| Smoking, n(% yes) | ||
| Alcohol consumption, (units/week) | 6.0 (3.0, 12.0) | 8.0 (3.0, 16.0)** |
| Clinical measures | ||
| Weight, (kg) | 75.1 (66.1, 84.5) | 83.2 (73.5, 93.0)** |
| Body mass index, (kg/m2) | 25.7 (23.3, 28.4) | 28.1 (25.5, 31.1)** |
| Waist circumference, (cm) | 91.5 (83.3, 99.7) | 100.5 (91.2, 108.2)** |
| Systolic Blood Pressure, (mmHg) | 130 (118, 141) | 135 (124, 146)** |
| Diastolic Blood Pressure, (mmHg) | 80 (73, 86) | 83 (76, 90)** |
| Total Cholesterol, (mmol/l) | 5.4 (4.8, 6.2) | 5.3 (4.7, 6.0)** |
| High density lipoprotein cholesterol, (mmol/l) | 1.6 (1.3, 1.9) | 1.4 (1.2, 1.7)** |
| Low density lipoprotein cholesterol, (mmol/l) | 3.3 (2.6, 4.0) | 3.2 (2.6, 3.8)* |
| Triacylglycerol, (mmol/l) | 0.96 (0.7, 1.2) | 1.2 (0.9, 1.6)** |
| Fasting plasma glucose, (mmol/l) | 5.5 (5.3, 5.8) | 6.4 (6.2, 6.8)** |
| 2 h plasma glucose, (mmol/l) | 5.5 (4.7, 6.4) | 8.0 (6.5, 9.5)** |
| HbA1c, (mmol/mol) | 38 (36, 40) | 40 (38, 42)** |
| HbA1c, (%) | 5.6 (5.4, 5.8) | 5.8 (5.6, 6.0)** |
| Medication use | ||
| Any antihypertensive drug, n(%) | 266 (34.1) | 356 (49.9)** |
| Any lipid lowering drug, n(%) | 169 (21.6) | 219 (30.7)** |
| Physical activity behavior | ||
| Wear time, (days) | 6.9 (6.3, 7.1) | 6.9 (6.4, 7.1) |
| PAL | 1.53 (1.41, 1.66) | 1.50 (1.39, 1.64)** |
| Physical activity energy expenditure, (kJ/kg/day) | 31.6 (22.9, 42.9) | 28.8 (20.9, 39.5)** |
| Light intensity physical activity, (h/day) | 4.7 (3.7, 6.0) | 4.4 (3.2, 5.7)** |
| MVPA, (min/day) | 48.0 (24.0, 84.0) | 42.0 (18.0, 78.0)* |
| MVPA in bouts >10 min, (min/day) | 8.7 (1.4, 25.3) | 6.4 (0.0, 19.2)** |
| MVPA guidelines ≥ 30 min/day, n(%) | 475 (60.5) | 407 (56.8)* |
| MVPA guidelines ≥ 30 min in bouts >10 min/day, n(%) | 166 (21.2) | 113 (15.9)* |
| Vigorous physical activity, (min/day) | 0.3 (0.0, 2.5) | 0.0 (0.0, 1.0)* |
| Awake sedentary time, (h/day) | 12.1 (10.5, 13.5) | 12.6 (10.8, 14.2)** |
| Sleep, (h/day) | 6.7 (6.2, 7.1) | 6.6 (6.2, 6.9)** |
Data are median (IQR) or n (%).
Employed includes the self-employed. Out of work includes the unemployed, housewives, retired individuals and individuals receiving government provisions.
NGM: normal glucose metabolism; IGM: impaired glucose metabolism; HbA1c: glycated hemoglobin A1c; MVPA: moderate-to-vigorous physical activity; PAL: physical activity level
* for p-value < 0.05 and ** for < 0.01.
The average ActiHeart wear time in the study population equaled to 156 hours, corresponding to 6.4 days. 81% of the study population had 6 or more days of wear time. The average time awake was 17 hours per day. Overall, participants with NGM were more physically active and less sedentary while the lowest levels of PA and highest levels of SED-time were found among the IGM participants (Table 1). A significantly larger proportion of NGM participants reached the MVPA-guideline threshold of at least 30 minutes MVPA/day. Individuals with NGM also accumulated more time in continuous bouts of MVPA lasting ≥ 10 minutes. Both NGM and IGM- groups had a low proportion of individuals who met either criteria of MVPA-guidelines of minimum 30 minutes MVPA (bouted or not).
Decision Tree Analysis
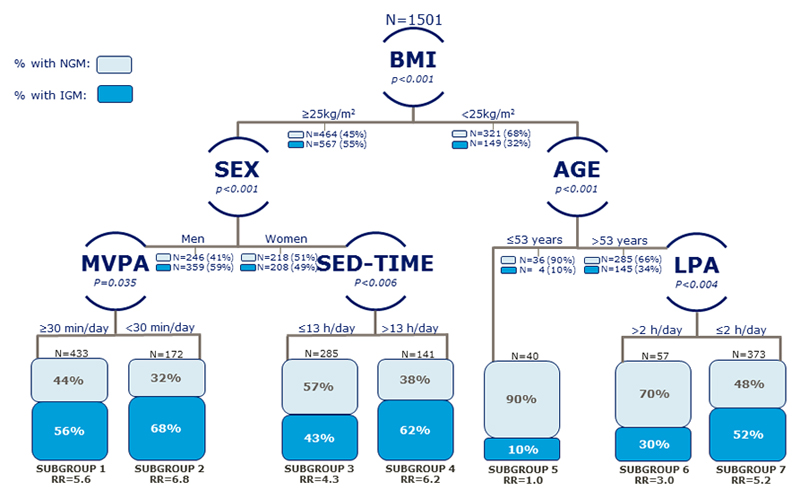
The final decision tree contained seven distinct terminal leaves (i.e. subgroups) (Figure 1). The six determinants that were retained as the most important for discriminating IGM status were in the following order: BMI, sex, age, reaching MVPA-guideline, SED-time and LPA.
Figure 1.
The Decision Tree depicts seven subgroups that emerged from the interplay/combination between retained physical activity determinants and sex, age and BMI. Following every split in each subgroup, the corresponding prevalence is given for normal glucose metabolism (NGM; light turquois) vs. impaired glucose metabolism (IGM; dark turquois). The relative risk of IGM in each subgroup is in reference to the subgroup with the lowest risk of IGM (subgroup 5).
BMI, body mass index; LPA, light intensity physical activity (1.5-3.0METs); MVPA, moderate-to-vigorous physical activity of ≥ or < 30min (≥ 3.0 METs); SED-time, sedentary time while awake (≤ 1.5 MET, subtracting sleep); RR, relative risk.
The strongest determinant was BMI, which formed the first split and divided the study sample into two branches (BMI: ≥ 25 kg/m2, n=1031; < 25 kg/m2, n= 470). Among overweight (BMI: ≥ 25 kg/m2) participants the next split was by sex. In overweight men, accumulating at least 30 minutes of MVPA/day was associated with a lower risk of having IGM. The prevalence of IGM was 56% among overweight men who engaged in ≥ 30 minutes of MVPA daily (subgroup 1, n=433) while the prevalence was 68% in those who engaged in less than 30 minutes of MVPA daily (subgroup 2, n=172). SED-time was associated with IGM among overweight women only and with an optimal split in SED-time at 13 hours. Participants who spent ≤ 13 hours sedentary while awake daily had an IGM prevalence of 43% (subgroup 3, n=285) and those with > 13 hours/day had a prevalence of 62% (subgroup 4, n=285). The differential effect of these two dimensions of PA, indicate significant high order interactions between BMI, sex and PA measures. In the subset branch of participants with normal weight (BMI < 25 kg/m2) LPA was only significantly associated with IGM among participants aged > 53 years, again indicating a significant interaction effect between age and time spent in LPA. The prevalence of IGM was 30% in the subgroup defined by age > 53 years and > 2 hours spent in LPA daily (subgroup 6, n=57), while the prevalence was 52% among those spending < 2 hours in LPA daily (subgroup 7, n=373). None of the dimensions of PA or SED-time were associated with IGM in individuals with normal weight aged ≤ 53 years (Subgroup 5, n=40). In this subgroup the prevalence of IGM was 10%.
The pairs plot showed a clear correlation tendency between all the PA dimensions plot (see, Supplemental Digital Content 2, Figure 1: Pairs plot showing a clear correlation tendency between all the physical activity dimensions). In the sensitivity analysis, the final tree remained unchanged when PAEE was left out in the model. No other dimension was selected among the overweight males when MVPA was left out of the model. However, when SED-time or LPA was alternately left out of the model, PAEE was retained in their place, respectively. This implies a strong correlation between PAEE and LPA and SED-time, respectively (see, Supplemental Digital Content 3, Figure 2: Decision tree with PAEE left out in the model, Supplemental Digital Content 4, Figure 3: Decision tree with MVPA (30min/day) left out in the model, Supplemental Digital Content 5, Figure 4: Decision tree with LPA left out in the model and Supplemental Digital Content 6, Figure 5: Decision tree with SED-time left out in the model)
Discussion
We applied a novel methodology, Decision Tree Analysis, as a way to explore and assess the importance of different dimensions of PA, SED-time and personal characteristics in relation to IGM in a population at high risk of developing type 2 diabetes. Such information may improve the design of tailored PA interventions for the prevention of type 2 diabetes.
Our decision tree analysis suggests that different dimensions of PA are important for glucose metabolism in individuals of different age, sex and obesity level. Our results provide support for a number of PA dimensions and SED-time that have previously been found to be associated with IGM. In contrast to our observations, most studies to date have identified only main effect predictors, implicitly assuming that these apply to the same extent to all individuals. The present study extends upon and broadens these findings by applying a data driven approach to objectively measured PA data in order to understand the potential impact of multiple dimensions of free-living activity most strongly associated with IGM in subgroups with different sex, age and obesity characteristics. Our novel methodological approach makes a direct comparison with other studies challenging. Nevertheless, our findings confirm and extend previous prospective and cross-sectional studies examining the associations of dimensions of PA and SED-time with IGM and hence future type 2 diabetes risk (22).
Physical inactivity and obesity are key modifiable determinants of type 2 diabetes (19). One role of PA in the modulation of type 2 diabetes risk is through the prevention of obesity. However, PA influences type 2 diabetes risk both in the presence and absence of obesity through a direct effect on insulin sensitivity (31). Among the modelled determinants in this study BMI, albeit a crude marker of obesity, was the strongest determinant of IGM as it was the first to form a split in the study population. Among the emerged subset of overweight men, meeting Danish PA guidelines of ≥ 30 minutes/day of MVPA regardless of bout duration was found to be inversely associated with IGM. The American College for Sports Medicine recommends adults who are overweight or obese to perform at least 30 minutes of at least MVPA on 5 or more days a week even if they do not lose weight as a result, because PA can bring other health benefits such as reduced risk of type 2 diabetes (17).
Only a few studies have investigated the potential of LPA as an intervention target in populations at high risk for type 2 diabetes. One study found that objectively measured LPA was inversely associated with 2-hour plasma glucose independently of MVPA in adults (mean age: 53.4 years) (20). This is in contrast to a recent systematic review of 33 intervention studies that examined the effects of LPA on CVD risk factors including markers of glucose metabolism in adults. No consistent evidence that LPA is effective at improving any CVD risk factors was found (2). However, it should be noted that participants studied were primarily young adults (18–39 years old). Also the used doses of LPA were small (ranged from an acute bout of training lasting 5 minutes to chronic training lasting 30 minutes per session, 3 times per week for 9 months). Compared to the dose of LPA typically performed in adults it could be argued that this volume of LPA is not sufficient to promote favorable adaptations in the examined biological markers. The current study found LPA to be the main PA determinant of IGM among individuals with normal weight and aged > 53 years. The decision tree split on LPA with a cut-point of > 2 hours/day which was associated with a 22% lower IGM prevalence compared to the same subset engaging in less daily LPA. Given the amount of time adults, especially the middle aged and the elderly, spend in light intensity activities this finding may have important implications for PA interventions. Such evidence could underpin recommendations regarding light intensity activity in future PA guidelines and inform interventions targeting light intensity activity among the elderly for whom MVPA may present challenges. Therefore, future studies should attempt to elucidate the effects of LPA in elderly populations since activities of light intensity may be more readily attainable, easier to promote and more common than MVPA in middle-aged and elderly populations.
Moreover, studies have been consistent in identifying SED-time as a distinct health risk, even independently of meeting PA guidelines (21). The overall PA level in in our study population was low. Median PAEE was 30 kJ/kg/day. In a healthy Danish population (mean age 58 years), the median ActiHeart-assessed PAEE level was found to be higher (≈ 40 kJ/kg/day) (36). In a PA calibration sub-study the validity of a four-category PA index in 1,941 participants of similar age and sex to those in the ADDITION-PRO study was examined, using combined movement and heart rate-sensing. Results suggest that the average PAEE across categories of PA were: inactive (36 kJ/kg/day); moderately inactive (41 kJ/kg/day); moderately active (46 kJ/kg/day); active (51 kJ/kg/day) (15). In addition, although our study population accumulated a median of 46 min/day of MVPA, they also spent a median of 12.3 hours being sedentary while awake. When excluding time spent sleeping (6.7 hours/day), 70% of the time was spent sedentary while awake (≤1.5 METs), 26% of the time was spent with light intensity activities (>1.5 to 3.0 METs), and only 4% was spent in MVPA (≥3 METs). When summing up the contribution of these fractions of time spent across the PA intensity continuum to PAEE, it becomes apparent that the MVPA contribution is low resulting in an overall low total PAEE of 30 kJ/kg/day. When we calculated the proportion of participants who spent 30 min of MVPA in bouts of ≥10 min per day, as currently recommended by the Danish health authorities (14), more than 80% of the participants did not meet the PA recommendations. Finally, estimated median PAL was 1.51 which classifies the study population’s lifestyle as sedentary (PAL value: 1.40-1.69) (18). These findings mirror reports stating that even adults who meet PA guidelines spend a large proportion of their day engaged in sedentary behavior (21). As such high levels of sedentary behavior may coexist with a high total level of PA and may be associated with an increased risk of a variety of health problems (21). However, a recent meta-analysis concluded that high levels of moderate intensity PA (i.e., about 60–75 minutes per day) seem to eliminate the detrimental effects of SED-time (16). .
We found SED-time to be adversely associated with IGM in a subset of overweight women. A 19% higher IGM prevalence was found among overweight women who were sedentary for more than 13 hours compared to those with less SED-time. These findings could imply that reducing SED-time in this risk group and increasing overall PA, even if it is less than the recommended guidelines could result in important health benefits in terms of IGM risk. This suggestion is in keeping with recent studies that found that replacement of sedentary behavior with either LPA or MVPA showed beneficial associations with risk factors related to adiposity (BMI) and glucose metabolism (plasma insulin, β-cell function and insulin sensitivity) (10). In addition, prolonged sitting interrupted by even brief (≤ 5 minutes) bouts of standing every 20–30 minutes have been shown to improve glycemic control in sedentary overweight/obese populations and in women with IGM (25). Neither of the dimensions of PA that we modelled nor SED-time was associated with IGM in normal weight individuals aged ≤ 53 years. Some reasons for this could be that this subgroup was too small to be classified further by any activity determinant and/or individuals in that subgroup show homogenous PA patterns that do not allow for any activity dimension to discriminate between mutually exclusive subgroups.
Strengths and Limitations
A key strength of our study was the use of a large sample of middle aged and older adults with elevated type 2 diabetes risk, in whom PA and SED-time was measured objectively with a combined accelerometer and heart rate monitor. This has been shown to be a highly accurate method for assessing PA (4). In addition, all measures were either individually or group calibrated. Thus, our estimates of the amounts and intensities of PA and SED-time are more accurate than data based on self-report, solely accelerometry or solely heart rate based measures. Furthermore, the use of a combined accelerometer and heart rate monitor enabled us to examine and quantify associations of several measured PA and SED-time variables with IGM. Median wear time in our study population was 6.9 days with on average 17 waking hours per day, and 81% of our study population provided at least 6 days of data.
Another strength of the study was our innovative decision tree analysis approach. This is the first study of which we are aware to use explorative decision tree modelling of PA data to segment a population by risk of IGM. Only a handful of studies have used decision tree methods to classify and study health-related outcomes (38). Decision tree analysis can identify groups that are similar in outcome and determinants and has the ability to detect interactions without having to make a priori decisions about which interaction terms to include. While much previous research on PA and SED-time has not focused on interaction terms, the present results suggest that interactions may be highly informative. Unlike traditional regression analyses that include only main effects or interaction terms specified a priori by researchers, our analyses allowed for the examination of more complicated interactions that may have not been hypothesized to be important based on the existing research. Our results suggest that classification may be enhanced by systematic evaluation of determinants from multiple dimensions of PA and in combination with one another. Specific PA intervention targets can be identified if we take interactions of factors into account.
This study was not without limitations, including the inherent limitations of the decision tree methodology. In decision tree analysis, variables are selected, based on mathematical conditions, often with different thresholds at different levels of the tree. This can be confusing for the interpretation of findings, as the “split” level of the tree on a certain variable may not be an important, validated or generalizable cut-off. Another constraint is collinearity between the modelled PA dimensions. As with all methodological approaches to prediction and classification, the use of highly correlated variables in such models increases the likelihood that different solutions may be obtained across samples. In a decision tree analysis the variables retained by the model may not be the only significant ones but the most significant ones. However, collinearity is a bigger concern with the use of conventional regression models where correlation makes the estimated regression coefficients more difficult to interpret and increases their variability. One way to test if multicollinearity is a serious concern in a decision tree analysis is to remodel it without one of the correlated variables and see which other variables are selected in their place (see, Supplemental Digital Content 3, Figure 2: Decision tree with PAEE left out in the model, Supplemental Digital Content 4, Figure 3: Decision tree with MVPA (30min/day) left out in the model, Supplemental Digital Content 5, Figure 4: Decision tree with LPA left out in the model and Supplemental Digital Content 6, Figure 5: Decision tree with SED-time left out in the model)
Furthermore, the overall PA level was low to very-low in the ADDITION-PRO population and our findings therefore only apply in similar sedentary high risk populations for type 2 diabetes. However, MVPA levels in our population may be slightly higher than what would have been expected. Some methodological differences may explain discrepancy between our and other studies in observed MVPA levels. We quantified and captured all minutes of MVPA throughout the day for seven days using a combined heart rate and accelerometer. Other studies in high risk individuals for type 2 diabetes have typically used self-report measures which have only low-to-moderate correlation with objective measures and subject to recall bias (32). Alternatively, studies that have used solely accelerometry also show lower MVPA levels in somewhat similar populations to ours (13). However, the limitations of accelerometry are primarily biomechanical. Their validity in assessing moderate intensity of different activities dependent on the type of activity performed. The accelerometry-PA intensity relationship during activities, such as walking on the level and incline, when running, stepping, and cycling, and during load-bearing activities, is highly variable. This may be due to the inability of accelerometers to detect increased energy cost from upper body movement, load carriage, or changes in surface or terrain (23). Because combined heart rate and accelerometry yield more precise measures of MVPA compared with either method used alone we might have captured more complete and precise measures of MVPA (4, 5). Taken together this may explain the higher levels of MVPA measured in our population. Also, we included individual PA recordings ranging from ≥ 48 hours to 7 full days, mostly representing both week and weekend days. Ideally, more days of objective recording would have been preferable to better capture variations in PA during the week and in addition more reliable estimates of habitual PA could have been obtained. This is significant especially for metrics such as MVPA bouts ≥10min that may rarely occur in a population as ours and therefor may not be captured with a cut-point for valid PA data of ≥ 48 hours. However, our large-scale clinical epidemiological setting made objective measurement of PA for more than 7 days unfeasible. Nevertheless, as stated our study population were particularly adherent to the PA measurement protocol as more than 80% provided more than 6 days of PA measures which from a clinical epidemiological perspective is rather satisfactory.
Moreover, considerable study population homogeneity (due to narrow age range and a low proportion of participants with low type 2 diabetes risk) exists in the ADDITION-PRO study. This is reflected by the relatively small albeit significant differences between the NGM and IGM groups in PA dimensions (Table 1). Nevertheless we were able to detect significant associations between different PA dimensions and IGM at subgroup level with a decision tree approach. These may not a have been found with the use of conventional statistical methods such as comparisons of means/medians and regression models because higher order interactions are cumbersome to model and interpret in regression models. Secondly, as our study is exploratory and cross-sectional, the findings need to be replicated in other data sets and tested in prospective cohorts or intervention trials. Nevertheless, empirical findings of exploratory analyses can stimulate the generation of new hypotheses and inform subsequent theory. As such, the findings from this study may have important methodological and public health implications. Finally, measurement of glucose in plasma of fasting subjects is widely accepted as a diagnostic criterion for IGM (40). However due to day-to-day variations in the 2h plasma glucose following an oral glucose tolerance test, there might be a risk of misclassification in relation to our outcome measure.
A position statement of the American Diabetes Association on PA and type 2 diabetes specify PA recommendations for individuals at high risk or with IGM (12). The statement stresses that because PA recommendations may vary depending on individual characteristics and health status, they should be tailored. At the present time, Danish PA recommendations for individuals at high risk or with type 2 diabetes are not different from those for the general population. This study provides novel objective evidence that, in specific subgroups of individuals at risk of type 2 diabetes, different dimensions of PA and SED-time may have stronger associations with IGM than e.g. MVPA. Diabetes prevention programs that concentrate solely on MVPA may overlook and limit the potential benefit of other dimensions of PA (e.g. reducing SED-time and promoting LPA) that are of importance to type 2 diabetes prevention.
This study found implications for reducing time spent in sedentary behaviors and increasing time spent in both LPA and MVPA in terms of a beneficial impact on glucose metabolism status. Importantly, these dimensions of activity were specific to sex, age and obesity characteristics. Given the limitations of the cross sectional design, this study should not be used to confirm a causal link between PA, SED-time and IGM, but should inform further research in high risk populations in order to refine future PA and sedentary behavior interventions aimed at type 2 diabetes prevention.
Supplementary Material
Acknowledgements
The ADDITION-Denmark study was supported by the National Health Services in the counties of Copenhagen, Aarhus, Ringkøbing, Ribe, and Southern Jutland in Denmark; the Danish Council for Strategic Research; the Danish Research Foundation for General Practice; Novo Nordisk Foundation; the Danish Centre for Evaluation and Health Technology Assessment; the Diabetes Fund of the National Board of Health; the Danish Medical Research Council; and the Aarhus University Research Foundation. Additionally, the ADDITION-PRO study was funded by an unrestricted grant from the European Foundation for the Study of Diabetes/Pfizer for Research into Cardiovascular Disease Risk Reduction in Patients with Diabetes (74550801), the Danish Council for Strategic Research, and internal research and equipment funds from Steno Diabetes Center.
Footnotes
Published ahead of Print contains articles in unedited manuscript form that have been peer reviewed and accepted for publication. This manuscript will undergo copyediting, page composition, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered that could affect the content.
Conflict of Interest and Source of Funding
AB., N.J., M.J., T.L., D.V and D.W. own shares in Novo Nordisk A/S. No other potential conflicts of interest relevant to this article were reported for the remaining authors. H.A. has received scholarship funding from the Danish Ministry of Higher Education and Science, the Danish Heart Foundation and Aarhus University. D.W. is supported by the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation. K.F. is supported by the Novo Nordisk Foundation. The work of Soren Brage is supported by UK Medical Research Council [MC_UU_12015/3].
Contribution statement
H.A. K.F. and D.V. contributed to study design. H.A. performed the analysis and wrote the manuscript. D.V. helped with analyzing data and contributed to revising the article. M.J., A.S., N.J., A.B, S.B., D.W., K.F contributed with interpretation of data and revising the content of the manuscript. H.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by ACSM.
References
- 1.Assah FK, Brage S, Ekelund U, Wareham NJ. The association of intensity and overall level of physical activity energy expenditure with a marker of insulin resistance. Diabetologia. 2008;51(8):1399–407. doi: 10.1007/s00125-008-1033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batacan RB, Jr, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of Light Intensity Activity on CVD Risk Factors: A Systematic Review of Intervention Studies. BioMed research international. 2015;2015:596367. doi: 10.1155/2015/596367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besson H, Brage S, Jakes RW, Ekelund U, Wareham NJ. Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self-report in adults. The American journal of clinical nutrition. 2010;91(1):106–14. doi: 10.3945/ajcn.2009.28432. [DOI] [PubMed] [Google Scholar]
- 4.Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. European journal of clinical nutrition. 2005;59(4):561–70. doi: 10.1038/sj.ejcn.1602118. [DOI] [PubMed] [Google Scholar]
- 5.Brage S, Brage N, Franks PW, et al. Branched equation modeling of simultaneous accelerometry and heart rate monitoring improves estimate of directly measured physical activity energy expenditure. Journal of applied physiology (Bethesda, Md. : 1985) 2004;96(1):343–51. doi: 10.1152/japplphysiol.00703.2003. [DOI] [PubMed] [Google Scholar]
- 6.Brage S, Ekelund U, Brage N, et al. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. Journal of applied physiology (Bethesda, Md. : 1985) 2007;103(2):682–92. doi: 10.1152/japplphysiol.00092.2006. [DOI] [PubMed] [Google Scholar]
- 7.Brage S, Westgate K, Franks PW, et al. Estimation of Free-Living Energy Expenditure by Heart Rate and Movement Sensing: A Doubly-Labelled Water Study. PLoS One. 2015;10(9):e0137206. doi: 10.1371/journal.pone.0137206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brage SWK, Wijndaele K, Godinho J, Griffin S, Wareham N. Evaluation of a method for minimising diurnal information bias in objective sensor data. ICAMPAM (Amherst) Conference Proceeding; 2013. [Google Scholar]
- 9.Breiman L. Classification and Regression Trees. New York, NY: Chapman & Hall; 1984. p. 368. [Google Scholar]
- 10.Buman MP, Winkler EA, Kurka JM, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005-2006. American journal of epidemiology. 2014;179(3):323–34. doi: 10.1093/aje/kwt292. [DOI] [PubMed] [Google Scholar]
- 11.Chatterton H, Younger T, Fischer A, Khunti K. Risk identification and interventions to prevent type 2 diabetes in adults at high risk: summary of NICE guidance. BMJ (Clinical research ed.) 2012;345:e4624. doi: 10.1136/bmj.e4624. [DOI] [PubMed] [Google Scholar]
- 12.Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes care. 2016;39(11):2065–79. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper AR, Sebire S, Montgomery AA, et al. Sedentary time, breaks in sedentary time and metabolic variables in people with newly diagnosed type 2 diabetes. Diabetologia. 2012;55(3):589–99. doi: 10.1007/s00125-011-2408-x. [DOI] [PubMed] [Google Scholar]
- 14.Danish Health and Medicines Authority. [cited 2017 04 May]; [Internet]. Available from: https://www.sst.dk/en/health-and-lifestyle/physical-activity/recommendations/recommendations-for-adults.
- 15.Ekelund U, Palla L, Brage S, et al. Physical activity reduces the risk of incident type 2 diabetes in general and in abdominally lean and obese men and women: the EPIC-InterAct Study. Diabetologia. 2012;55(7):1944–52. doi: 10.1007/s00125-012-2532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekelund U, Steene-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. The Lancet. 388(10051):1302–10. doi: 10.1016/S0140-6736(16)30370-1. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson B. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. The Journal of the Canadian Chiropractic Association. 2014;58(3):328. [Google Scholar]
- 18.Food and Agriculture Organization of the United Nations. [Internet]. Available from: ftp://ftp.fao.org/docrep/fao/007/y5686e/y5686e00.pdf.
- 19.Gillett M, Royle P, Snaith A, et al. Non-pharmacological interventions to reduce the risk of diabetes in people with impaired glucose regulation: a systematic review and economic evaluation. Health technology assessment (Winchester, England) 2012;16(33):1–236. doi: 10.3310/hta16330. iii-iv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Healy GN, Dunstan DW, Salmon J, et al. Objectively Measured Light-Intensity Physical Activity Is Independently Associated With 2-h Plasma Glucose. Diabetes care. 2007;30(6):1384–9. doi: 10.2337/dc07-0114. [DOI] [PubMed] [Google Scholar]
- 21.Healy GN, Dunstan DW, Salmon J, Shaw JE, Zimmet PZ, Owen N. Television time and continuous metabolic risk in physically active adults. Medicine and science in sports and exercise. 2008;40(4):639–45. doi: 10.1249/MSS.0b013e3181607421. [DOI] [PubMed] [Google Scholar]
- 22.Helmerhorst HJ, Wijndaele K, Brage S, Wareham NJ, Ekelund U. Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes. 2009;58(8):1776–9. doi: 10.2337/db08-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendelman D, Miller K, Baggett C, Debold E, Freedson P. Validity of accelerometry for the assessment of moderate intensity physical activity in the field. Medicine and science in sports and exercise. 2000;32(9 Suppl):S442–9. doi: 10.1097/00005768-200009001-00002. [DOI] [PubMed] [Google Scholar]
- 24.Henry CJ. Basal metabolic rate studies in humans: measurement and development of new equations. Public health nutrition. 2005;8(7a):1133–52. doi: 10.1079/phn2005801. [DOI] [PubMed] [Google Scholar]
- 25.Henson J, Davies MJ, Bodicoat DH, et al. Breaking Up Prolonged Sitting With Standing or Walking Attenuates the Postprandial Metabolic Response in Postmenopausal Women: A Randomized Acute Study. Diabetes care. 2016;39(1):130–8. doi: 10.2337/dc15-1240. [DOI] [PubMed] [Google Scholar]
- 26.Hothorn T. R Foundation for Statistical Computing Web site. 2015. Package ‘party’ A Laboratory for Recursive Partytioning. [Google Scholar]
- 27.Johansen NB, Hansen AL, Jensen TM, et al. Protocol for ADDITION-PRO: a longitudinal cohort study of the cardiovascular experience of individuals at high risk for diabetes recruited from Danish primary care. BMC public health. 2012;12:1078. doi: 10.1186/1471-2458-12-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G. The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24(Suppl 3):S6–11. doi: 10.1038/sj.ijo.0801420. [DOI] [PubMed] [Google Scholar]
- 29.Loprinzi PD, Cardinal BJ. Association between biologic outcomes and objectively measured physical activity accumulated in >/= 10-minute bouts and <10-minute bouts. American journal of health promotion : AJHP. 2013;27(3):143–51. doi: 10.4278/ajhp.110916-QUAN-348. [DOI] [PubMed] [Google Scholar]
- 30.Laaksonen DE, Lindstrom J, Lakka TA, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes. 2005;54(1):158–65. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 31.Mayer-Davis EJ, D'Agostino R, Jr, Karter AJ, et al. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Jama. 1998;279(9):669–74. doi: 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- 32.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes care. 2007;30(2):203–9. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 33.Paulweber B, Valensi P, Lindstrom J, et al. A European evidence-based guideline for the prevention of type 2 diabetes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2010;42(Suppl 1):S3–36. doi: 10.1055/s-0029-1240928. [DOI] [PubMed] [Google Scholar]
- 34.Rikli RE. Reliability, validity, and methodological issues in assessing physical activity in older adults. Research quarterly for exercise and sport. 2000;71(2 Suppl):S89–96. [PubMed] [Google Scholar]
- 35.Stegle O, Fallert SV, MacKay DJ, Brage S. Gaussian process robust regression for noisy heart rate data. IEEE transactions on bio-medical engineering. 2008;55(9):2143–51. doi: 10.1109/TBME.2008.923118. [DOI] [PubMed] [Google Scholar]
- 36.The InterAct C. Validity of a short questionnaire to assess physical activity in 10 European countries. European Journal of Epidemiology. 2012;27(1):15–25. doi: 10.1007/s10654-011-9625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tudor-Locke CE, Myers AM. Challenges and Opportunities for Measuring Physical Activity in Sedentary Adults. Sports Medicine. 2001;31(2):91–100. doi: 10.2165/00007256-200131020-00002. [DOI] [PubMed] [Google Scholar]
- 38.Vistisen D, Andersen GS, Hansen CS, et al. Prediction of First Cardiovascular Disease Event in Type 1 Diabetes Mellitus: The Steno Type 1 Risk Engine. Circulation. 2016;133(11):1058–66. doi: 10.1161/CIRCULATIONAHA.115.018844. [DOI] [PubMed] [Google Scholar]
- 39.Westerterp KR. Diet induced thermogenesis. Nutrition & metabolism. 2004;1(1):5. doi: 10.1186/1743-7075-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. Geneva: [Internet]. Available from: http://whqlibdoc.who.int/publications/2006/9241594934_eng.pdf. [Google Scholar]
- 41.Zhang HSB. Recursive Partitioning and Applications. 2nd ed. New York, NY: Springer; 2010. p. 262. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.