Abstract
Rab5 targets to early endosomes and is a master regulator of early endosome fusion and endocytosis in all eukaryotic cells. Like other GTPases, Rab5 functions as a molecular switch by alternating between GTP-bound and GDP-bound forms, with the former being biologically active via interactions with multiple effector proteins. Thus the Rab5-GTP level in the cell reflects Rab5 activity in promoting endosome fusion and endocytosis and is indicative of cellular endocytic activity. In this chapter, we describe a Rab5 activity assay by using GST fusion proteins with the Rab5 effectors such as Rabaptin-5, Rabenosyn-5, and EEA1 that specifically bind to GTP-bound Rab5. We compare the efficiencies of the three GST fusion proteins in the pull-down of mammalian and fungal Rab5 proteins.
Keywords: Rab5, Rabaptin-5, EEA1, Rabenosyn-5, Endocytosis, Endosome
1 Introduction
Rab5 is localized on early endosomes and plasma membrane, and plays an important role in the formation, movement, and fusion of endocytic vesicles and early endosomes during endocytosis [1, 2]. As a member of the Rab GTPase family, Rab5 alternates between inactive GDP-bound and active GTP-bound conformations, and this GTPase cycle is facilitated by guanine nucleotide exchange factors (GEFs), e.g., Rabex-5 [3] and RIN1 [4], and GTPase-activating proteins (GAPs), e.g., RabGAP5 [5]. In the active GTP-bound conformation, Rab5 interacts with multiple effector proteins to promote the aforementioned early events of endocytosis.
Endocytosis is a fundamental function of all eukaryotic cells for uptake of extracellular nutrients and regulation of cell surface proteins such as receptors, channels, and adhesion proteins. Growth factor receptors control cell growth, differentiation, and migration via various signal transduction pathways, which may affect and/or be affected by Rab5 activity and endosomal sorting by recruitment of Rab5 GEFs or GAPs to the membrane. As a result, it’s necessary to determine Rab5 activity, i.e., Rab5-GTP level, in the cell to gauge endocytic activity and physiological changes during signal transduction processes.
The first method for determining Rab5-GTP levels in the cell involves metabolic labeling with [32P]orthophosphate, followed by immunoprecipitation of cell lysates with a Rab5 antibody and then separation of [32P]-labeled GDP and GTP bound to Rab5 by thin-layer chromatography and detection by autoradiography. This approach, however, is time-consuming and involves the usage of radioactivity. More recently, glutathione S-transferase (GST) pull-down assays [6] have been adopted to determine Rab5 activity in the cell (Fig. 1). In this case, GST fusion proteins with a Rab5 effector, which specifically binds to GTP-bound Rab5, is bound to glutathione-coupled Sepharose beads and used to affinity-purify Rab5-GTP in cell lysates. The captured Rab5-GTP is then detected by immunoblot analysis. We previously used the Rab5-binding domain (R5BD) of the Rab5 effector Rabaptin-5 [7, 8] as a bait in the GST fusion protein to determine Rab5-GTP levels in PC12 cells and showed rapid down-regulation of Rab5 activity by nerve growth factor (NGF) signaling to facilitate cell differentiation [9]. In addition to Rabaptin-5, other Rab5 effectors such as Rabenosyn-5 [10] and EEA1 [11] have also been used as GST fusion proteins in determining Rab5 activity in the cell [12, 13]. Here we compare the efficiencies of these three Rab5 effectors in the detection of Rab5-GTP in the cell in GST pull-down assays. In addition, we characterize two Rab5 homologs (MoRab5A and MoRab5B) from Magnaporthe oryzae, a pathogenic fungus that infects plants and causes rice blast disease [14], in the GST pull-down assay and show that they differentially interact with the Rab5 effectors.
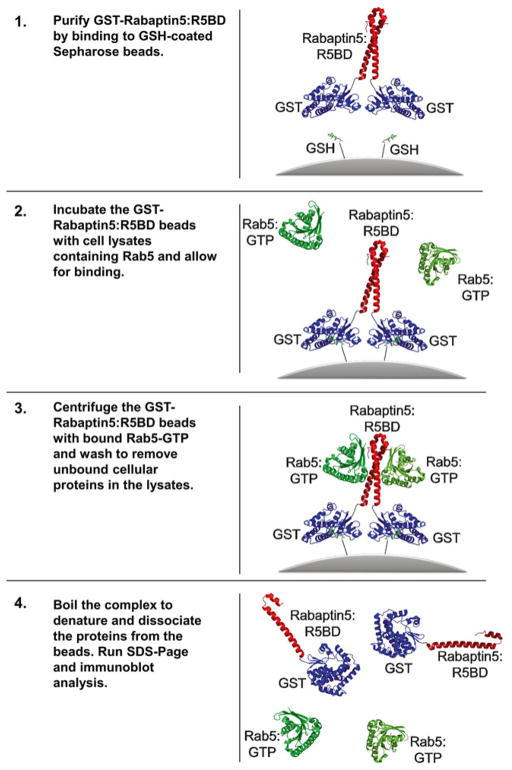
Fig. 1.
Schematic illustration of GST:R5BD pull-down assay. Rab5 (green) and Rabaptin5:R5BD (red) structures are from PDB: 1TU3 [8]. The structure for GST (blue) is from PDB: 2GST [17]. GSH-conjugated Sepharose beads are shown in grey
2 Materials
2.1 Preparation of GST Fusion Proteins
Millipore filtered H2O is used in all experiments.
Plasmids. pGEX-4 T-2/Rabaptin-5:R5BD, pGEX-4 T-2/EEA1:R5BD, and pGEX-2 T/Rabenosyn-5:R5BD. The pGEX vectors are commercially available from GE Healthcare.
Bacterial strains. E. coli DH5α and MC1061.
Growth media. LB liquid and LB Agar as liquid and solid media, respectively, for bacterial growth.
Reduced Glutathione (GSH)-Sepharose 4B resin.
Isopropyl-β-D-thiogalactopyranoside (IPTG): 1,000× stock solution (0.5 M in H2O, aliquoted and stored at −20 °C).
Ampicillin: 1,000× stock solution (50 mg/ml in H2O sterilized by filtration, aliquoted and stored at −20 °C).
Reagents. Phosphate-buffered saline (PBS), Triton X-100.
2.2 Expression of Rab5, MoRab5A, MoRab5B, and Their Mutants in Tissue Cultures and Preparation of Cell Lysates
Plasmids. pBI-Tet-Off, pBI/eGFP, pBI/eGFP/Myc-Rab5, pBI/eGFP/Myc-Rab5:Q79L, pBI/eGFP/Myc-Rab5:S34N, pBI/eGFP/Myc-MoRab5A, pBI/eGFP/Myc-MoRab5A: Q70L, pBI/eGFP/Myc-MoRab5A:S25N, pBI/eGFP/Myc-MoRab5B, pBI/eGFP/Myc-MoRab5B:Q79L, pBI/eGFP/Myc-MoRab5B:S33N.
Tissue cultures. Baby hamster kidney (BHK) cells (BHK-21 cell line from ATCC).
Growth media. α-Minimal Essential Medium (MEM) containing 5 % fetal bovine serum (FBS), glutamine, penicillin/streptomycin.
Lipofectamine 2000 transfection reagent (Invitrogen).
Lysis buffer: 25 mM HEPES-KOH (pH 7.4), 100 mM NaCl, 5 mM MgCl2, 0.1 % NP40, 10 % Glycerol, 1 mM DTT (add before use), 1:250 protease inhibitor cocktail for mammalian tissue cultures (add before use).
Reagents. Phosphate-buffered saline (PBS), Triton X-100.
2.3 SDS-PAGE and Coomassie Blue Staining
3× SDS loading buffer: 150 mM Tris–HCl (pH 6.8), 6 % SDS (W/V), 0.3 % bromophenol blue (W/V), 30 % glycerol (V/V), 300 mM β-mercaptoethanol (add before use).
16 % separation gel for SDS-PAGE.
Electrophoresis buffer: For 1 l of 5× running buffer: 15.2 g Tris base, 72 g Glycine, 5 g SDS, add H2O to 1 l.
Staining buffer: 0.6 % Coomassie Brilliant Blue, 50 % Methanol (v/v), 10 % Glacial acetic acid (v/v), add H2O to 100 %.
Destaining buffer: 15 % methanol (v/v), 10 % acetic acid (v/v).
2.4 Immunoblot Assay with Bio-Rad Semi-dry Transfer Apparatus
Transfer buffer: For 1 l: 2.9 g Glycine, 5.8 g Tris base, 0.37 g SDS, 200 ml methanol, add H2O to 1 l.
Washing buffer: 1× TBS (Tris-buffered Saline) containing 0.04 % Tween-20. For 1 l of 5× TBS: dissolve 40 g NaCl, 1 g KCl, and 15 g Tris base in 800 ml of H2O, adjust pH to 7.4 with HCl, and then add H2O to 1 l.
Blocking buffer: 1× TBS containing 8 % non-fat dry milk.
Antibodies: anti-Myc monoclonal antibody, IRDye 680CW-conjugated goat anti-mouse or anti-rabbit IgG (LI-COR Biosciences).
Immobilon-P PVDF Membrane.
Odyssey Infrared Imaging System (LI-COR Biosciences).
3 Methods
3.1 Expression and Purification of GST-R5BD Fusion Proteins
R5BD here refers to a Rab5-binding domain from Rabaptin-5 (residues 739–862), EEA1 (residues 1–209), or Rabenosyn-5 (residues 1–40). For Rabaptin-5 and EEA1, the R5BD cDNA fragments are generated by PCR using human Rabaptin-5 and EEA1 cDNA as templates and cloned into the BamHI and EcoRI sites of pGEX-4 T-2, resulting in the expression constructs pGEX-4 T-2/Rabaptin-5:R5BD and pGEX-4 T-2/EEA1:R5BD. For Rabenosyn-5, the expression construct pGEX-2 T/Rabenosyn-5:R5BD contains the N-terminal R5BD domain repeated four times in tandem and cloned in-frame in the pGEX vector, as described previously [12], and was kindly provided by Dr. John Colicelli (Department of Biological Chemistry, UCLA).
The GST-R5BD constructs are transformed into bacterial strain DH5α or MC1061, and the transformants are grown on LB/Amp agar plates at 37 °C overnight. Single colonies are picked with toothpicks and grown in 2 ml LB containing 50 μg/ml Amp in a 37 °C incubating shaker at 250 rpm overnight.
Take 1 ml of the overnight culture and dilute into 100 ml of fresh LB/Amp growth media. Continue the incubation in the 37 °C shaker for about 4–5 h until the OD600 reaches 0.5–0.8 (log phase growth).
Add IPTG to a final concentration of 0.5 mM to induce protein expression, and continue the incubation in the 37 °C shaker for 4 h (see Note 1).
Harvest the cells in polypropylene bottles by centrifugation at 8,000×g in a Beckman Aventi J-20 centrifuge for 10 min. Discard the supernatant (see Note 2).
Resuspend the cell pellet in 850 μl of PBS containing 1 % Triton X-100 by pipetting (see Note 3).
Transfer the suspension to a 1.5 ml Eppendorf tube, and then sonicate 6 times (10 s each time), with 10-s interval on ice to avoid overheating. Incubate the bacterial lysates on ice for 5 min.
Centrifuge at 9,300 × g for 30 min in a refrigerated (4 °C) Eppendorf centrifuge and transfer the supernatant to a new tube. Keep it on ice.
Take 250 μl of glutathione (GSH) Sepharose 4B beads and rinse three times in a 1.5 ml Eppendorf tube with PBS containing 1 % Triton X-100, by centrifugation at 2,300 × g for 30 s, aspiration of the supernatant, and resuspension of the beads (see Notes 4 and 5).
After the final wash, aspirate the washing buffer and add 750 μl of the bacterial supernatant prepared above (step 6) to the GSH beads. Mix and incubate at room temperature on a rotating mixer for 30 min.
Centrifuge at 400 × g for 1-min and aspirate the supernatant.
Wash the beads three times with 1 ml of PBS containing 1 % Triton X-100, as described above (step 7).
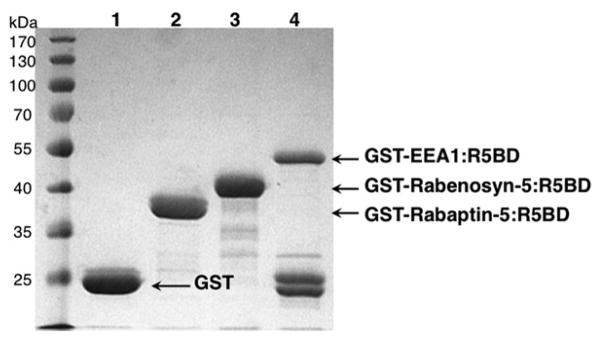
Wash the beads once with PBS, and then resuspend the beads in 150 μl PBS. A fraction of the GST-R5BD fusion protein bound to the beads (2 %) is analyzed by SDS-PAGE and visualized by Coomassie Blue staining (Fig. 2). The remainder of GST-R5BD beads is used for the pull-down assay below (see Note 8).
Fig. 2.
Affinity-purified GST-R5BD fusion proteins on GSH beads analyzed by SDS-PAGE and Coomassie Blue staining. GST and GST-R5BD fusion proteins, as indicated, were dissociated from the GSH beads in SDS sample buffer and subjected to SDS-PAGE (16 % gel), followed by Coomassie Blue staining (1 h) and destaining (2 h). Approximately 20 μg of each protein were loaded in each lane and represented 2 % of the total purified protein. To some extent, the GST-EEA1:R5BD fusion protein undergoes auto-proteolytic cleavage separating GST and EEA1:R5BD (see Note 6), but there is enough fusion protein for the pull-down assay
3.2 Preparation of Mammalian Cell Lysates for the Pull-Down Assay
BHK cell monolayers are grown in individual or 6-well 35 mm tissue culture plates in a 37 °C tissue culture incubator with 5 % CO2, respectively, and transfected with the plasmid constructs listed in Table 1 (3 wells per construct) for expression of human and Magnaporthe oryzae Rab5 and mutant proteins via Lipofectamine 2000 (see Note 9).
At 24 h post-transfection, aspirate the growth medium and wash the cell monolayers once with ice-cold PBS (1 ml per wash).
Add 300 μl of ice-cold lysis buffer to one of the triplicate wells transfected with each construct and incubate on ice for 5 min, then transfer the lysate to the next two wells sequentially and incubate for 5 min on ice each time.
Homogenize the cells by going through a 1 ml syringe with a 25G3/8 needle 10 times, and further incubate the cell lysate on ice for 5 min.
Spin down the nuclei and cell debris by a 3-min centrifugation at 9,300 × g in a refrigerated (4 °C) Eppendorf centrifuge.
An aliquot of the supernatant (10 μl) is directly subjected to immunoblot analysis to gauge the total amount of Rab5 in the cell lysate (Figs. 3 and 4), and the remainder is used for the pull-down assay as described below (Figs. 3 and 4).
Table 1.
List of expression constructs
| Plasmid name | Protein expression |
|---|---|
| pBI/eGFP/MycRab5:WT | Human Rab5 (wild-type), with N-terminal Myc-tag |
| pBI/eGFP/MycRab5:Q79L | Human Rab5 (Q79L mutant), with N-terminal Myc-tag |
| pBI/eGFP/MycRab5:S34N | Human Rab5 (S34N mutant), with N-terminal Myc-tag |
| pBI/eGFP/MycMoRab5A:WT | Magnaporthe oryze Rab5A (wild-type), with N-terminal Myc-tag |
| pBI/eGFP/MycMoRab5A:Q70L | Magnaporthe oryze Rab5A (Q70L mutant), with N-terminal Myc-tag |
| pBI/eGFP/MycMoRab5A:S25N | Magnaporthe oryze Rab5A (S25N mutant), with N-terminal Myc-tag |
| pBI/eGFP/MycMoRab5B:WT | Magnaporthe oryze Rab5B (wild-type), with N-terminal Myc-tag |
| pBI/eGFP/MycMoRab5B:Q79L | Magnaporthe oryze Rab5B (Q79L mutant), with N-terminal Myc-tag |
| pBI/eGFP/MycMoRab5B:S33N | Magnaporthe oryze Rab5B (S33N mutant), with N-terminal Myc-tag |
| pTet-Off | Tet-responsive transcription activator (tTA) |
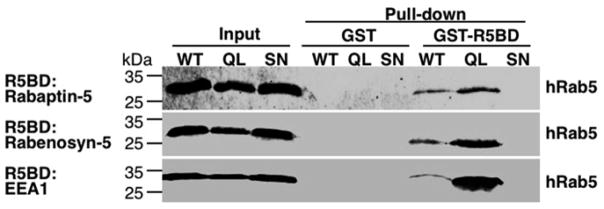
Fig. 3.
GST-R5BD fusion proteins specifically pull-down GTP-bound human Rab5 proteins. Shown are immunoblots with the anti-Myc antibody indicating the levels of human Rab5 proteins (Myc-tagged) expressed in BHK cells, including wild-type (WT) and constitutively active and dominant negative mutants (QL and SN), before and after the pull-down assay. The input represents 4 % of the total cell lysates directly subjected to the immunoblot analysis. The rest of the cell lysates are divided equally for pull-down assays by GST (as the negative control) and GST-R5BD beads, respectively, as indicated. Note that the three types of GST-R5BD beads (Rabaptin-5, Rabenosyn-5, and EEA1) show similar pull-down results with strongest binding for the GTP-bound mutant (QL), followed by WT. No binding was observed for the GDP-bound mutant (SN) (see Note 11)
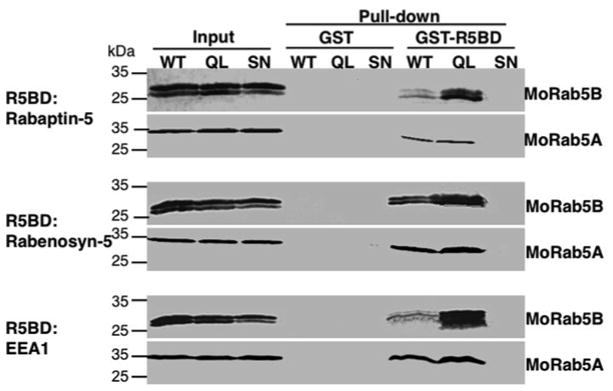
Fig. 4.
Distinct binding properties of MoRab5A and MoRab5B in the GST-R5BD pull-down assay. The pull-down procedure is the same as that for Fig. 3 except the cells expressing MoRab5A and MoRab5B, respectively, as indicated. Note that the pull-down profiles of MoRab5B are similar to those of human Rab5 in Fig. 3, i.e., the QL mutant shows the strongest binding signal while the WT signal is much weaker. In contrast, the WT and the QL mutant of MoRab5A show similar binding strength in the pull-down assay (see Note 12). The MoRab5B doublet is due to post-translational isoprenylation whereas MoRab5A is larger and the two forms are not readily resolved by the SDS-PAGE
3.3 GST-R5BD Pull-Down Assay
Add 150 μl of the pre-cleared cell lysate above to 20 μl of the GSH beads bound with the GST-R5BD fusion protein.
Mix and incubate for 30 min at 4 °C on a rotating mixer.
Spin down the beads by 1-min centrifugation at 400 × g in a refrigerated Eppendorf centrifuge, and aspirate the supernatant to remove unbound cellular proteins.
Wash the beads once by centrifugation and resuspension in 1 ml of ice-cold lysis buffer without detergent.
Spin down the beads and aspirate the supernatant.
Add 10 μl of 1× SDS loading buffer containing freshly added β-Mercaptoethanol to the beads. Boil the samples for 3 min to denature, reduce, and dissociate the proteins from the beads. Spin down the beads for 30 s at 10,000 rpm (9,300 × g) and subject the supernatant for SDS-PAGE and immunoblot analysis with the anti-Myc antibody as described below (Figs. 3 and 4).
3.4 SDS-PAGE and Immunoblot Analysis
Run the 16 % SDS-PAGE at 160 V for about 1 h until the blue dye front reaches the end of the gel.
Disassemble the gel apparatus and carefully pry the plates apart with a spatula, then cut off the stacking gel with a clean razor blade and soak the separating gel in transfer buffer for 10 min.
Cut PVDF membrane and Whatman filter papers to the gel size, wet the PVDF membrane in methanol for 10 s, and then soak it in the transfer buffer with eight pieces of the Whatman papers.
Place four pieces of the wet Whatman papers on the Bio-Rad transfer apparatus, then place the PVDF membrane, the gel, and four Whatman papers in that order (see Note 6).
Connect the electrodes and allow protein transfer from the gel to the PVDF membrane at 25 V for 35 min.
Incubate the membrane in blocking buffer for 1 h at room temperature on a shaker.
Wash the membrane 3 times in washing buffer on the shaker (5 min per wash).
Dilute the primary antibody with washing Buffer (1:10,000 for the anti-Myc antibody) and incubate the membrane in the diluted antibody solution for 1 h at room temperature on the shaker (see Note 10).
Repeat the washing step 7 to remove unbound primary antibody.
Dilute the secondary antibody 1:15,000 with washing buffer and incubate the membrane in the diluted secondary antibody solution in the dark by covering the container with aluminum foil for 1 h at room temperature on the shaker.
Repeat the washing step 7 to remove unbound secondary antibody in the dark.
Visualize and quantify the membrane with a LI-COR Odyssey Infrared Imaging System, according to the manufacturer’s instructions (see Note 11).
Acknowledgments
We thank M. Caleb Marlin for helpful comments and Fig. 1 illustration, and John Colicelli for the generous gift of pGEX-2 T/Rabenosyn-5:R5BD. This work was supported in part by the NIH grant R01 GM074692 (to G.L.) and a scholarship from the China Scholarship Council (to Y.Q.)
Footnotes
The temperature and incubation time are important factors in bacterial protein expression. Lower temperature (e.g., 30 °C or room temperature) and longer incubation time can increase the yield of soluble GST-R5BD fusion proteins.
If desired, the bottles containing the bacterial pellets can be stored at −80 °C for at least a month before use.
Avoid formation of bubbles.
The amount of GSH beads used depends on the GST fusion protein yield and may be adjusted upward if larger volumes of bacterial cultures are grown and higher protein yields are expected.
Use pipette tips with large opening to transfer the GSH beads. Regular tips may be used after cutting the end off with a clean razor blade.
Remove air bubbles by rolling a glass pipette or rod on the membrane.
Human Rab5 expressed in BHK cells binds to all three effectors (Rabaptin-5, Rabenosyn-5, and EEA1) in the pull-down assay (Fig. 3) [16]. The GTP hydrolysis defective Rab5:Q79L mutant shows the strongest binding signal. Wild-type Rab5 shows much weaker signal indicating that less than 10 % of Rab5 in the cell are in GTP-bound state. The GDP-bound Rab5:S34N mutant shows no binding to the GST-R5BD fusion proteins above the background binding to GST alone. GST is used here as a negative control to gauge nonspecific binding in the pull-down assay.
The GST-R5BD beads should be used on the same day to avoid protein degradation. The three GST-R5BD fusion proteins used here exemplify the difference in stability among GST fusion proteins. While GST-Rabaptin-5:R5BD and GST-Rabenosyn-5:R5BD are relatively stable, GST-EEA1:R5BD is less stable and appears to undergo auto-proteolysis separating GST from EEA1:R5BD (Fig. 2). Similar auto-proteolysis was observed in other GST fusion proteins, e.g., the GST-RBD35 in Chapter 18. The mechanism of such auto-proteolysis is unclear at present.
Three 35 mm plates of transfected cell monolayers should provide sufficient amount of Rab5 protein for the pull-down assay. For detection of endogenous Rab5 activity, more cells may be necessary depending on Rab5 levels and activation state in a given cell type [15].
Make sure that the membrane is immersed in the solution rather than floating on the surface.
We use the LI-COR Infra-red Imaging system in immunoblot analysis for quantification and sensitivity of detection, but other detection methods such as chemiluminescence can also be used with similar results.
MoRab5B shows the same binding profile as human Rab5, i.e., wild-type and mutant MoRab5B proteins bind to the effectors in the order of MoRab5B:Q79L>MoRab5B:WT>MoRab5B: S33N (Fig. 4) [16]. In contrast, MoRab5A shows distinct effector binding property, with wild-type and GTP hydrolysis-defective mutant (MoRab5A:Q70L) binding the effectors with identical affinity (Fig. 4) [16], suggesting that the wild-type protein is defective in GTP hydrolysis. As expected, the GDP-bound MoRab5A:S25N does not interact with any of the effectors.
References
- 1.Bucci C, Parton RG, Mather IH, et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 2.Li G. Early endocytosis: Rab5, Rab21 and Rab22. In: Li G, Segev N, editors. Rab GTPases and membrane trafficking. Bentham Science Publishers; Sharjah: 2012. [Google Scholar]
- 3.Horiuchi H, Giner A, Hoflack B, et al. A GDP/GTP exchange-stimulatory activity for the Rab5-RabGDI complex on clathrin-coated vesicles from bovine brain. J Biol Chem. 1995;270:11257–11262. doi: 10.1074/jbc.270.19.11257. [DOI] [PubMed] [Google Scholar]
- 4.Tall GG, Barbieri MA, Stahl PD, et al. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev Cell. 2001;1:73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 5.Haas AK, Fuchs E, Kopajtich R, et al. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat Cell Biol. 2005;7:887–893. doi: 10.1038/ncb1290. [DOI] [PubMed] [Google Scholar]
- 6.Kaelin WG, Jr, Pallas DC, DeCaprio JA, et al. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991;64:521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- 7.Stenmark H, Vitale G, Ullrich O, et al. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhu G, Zhai P, Liu J, et al. Structural basis of Rab5-Rabaptin5 interaction in endocytosis. Nat Struct Mol Biol. 2004;11:975–983. doi: 10.1038/nsmb832. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Lamb D, Chou MM, et al. Nerve growth factor-mediated neurite outgrowth via regulation of Rab5. Mol Biol Cell. 2007;18:1375–1384. doi: 10.1091/mbc.E06-08-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen E, Christoforidis S, Uttenweiler-Joseph S, et al. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol. 2000;151:601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonsen A, Lippe R, Christoforidis S, et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 12.Balaji K, Mooser C, Janson CM, et al. RIN1 orchestrates the activation of RAB5 GTPases and ABL tyrosine kinases to determine the fate of EGFR. J Cell Sci. 2012;125:5887–5896. doi: 10.1242/jcs.113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodhi IJ, Chiang SH, Chang L, et al. Gapex-5, a Rab31 guanine nucleotide exchange factor that regulates Glut4 trafficking in adipocytes. Cell Metab. 2007;5:59–72. doi: 10.1016/j.cmet.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebbole DJ. Magnaporthe as a model for understanding host-pathogen interactions. Annu Rev Phytopathol. 2007;45:437–456. doi: 10.1146/annurev.phyto.45.062806.094346. [DOI] [PubMed] [Google Scholar]
- 15.Dou Z, Pan JA, Dbouk HA, et al. Class IA PI3K p110beta subunit promotes autophagy through Rab5 small GTPase in response to growth factor limitation. Mol Cell. 2013;50:29–42. doi: 10.1016/j.molcel.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi Y, Marlin MC, Liang Z, et al. Distinct biochemical and functional properties of Two Rab5 homologs from the rice blast fungus Magnaporthe oryzae. J Biol Chem. 2014;289:28299–28309. doi: 10.1074/jbc.M114.591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji X, Johnson WW, Sesay MA, et al. Structure and function of the xenobiotic substrate binding site of a glutathione S-transferase as revealed by X-ray crystallographic analysis of product complexes with the diastereomers of 9-(S-glutathionyl)-10-hydroxy-9,10-dihydrophenanthrene. Biochemistry. 1994;33:1043–1052. doi: 10.1021/bi00171a002. [DOI] [PubMed] [Google Scholar]