Abstract
Pemphigus is a group of IgG-mediated autoimmune diseases of stratified squamous epithelia, such as the skin and oral mucosa, in which acantholysis (the loss of cell adhesion) causes blisters and erosions. Pemphigus has three major subtypes: pemphigus vulgaris, pemphigus foliaceus and paraneoplastic pemphigus. IgG autoantibodies are characteristically raised against desmoglein 1 and desmoglein 3, which are cell–cell adhesion molecules found in desmosomes. The sites of blister formation can be physiologically explained by the anti-desmoglein autoantibody profile and tissue-specific expression pattern of desmoglein isoforms. The pathophysiological roles of T cells and B cells have been characterized in mouse models of pemphigus and patients, revealing insights into the mechanisms of autoimmunity. Diagnosis is based on clinical manifestations and confirmed with histological and immunochemical testing. The current first-line treatment is systemic corticosteroids and adjuvant therapies, including immunosuppressive agents, intravenous immunoglobulin and plasmapheresis. Rituximab, a monoclonal antibody against CD20+ B cells, is a promising therapeutic option that may soon become first-line therapy. Pemphigus is one of the best-characterized human autoimmune diseases and provides an ideal paradigm for both basic and clinical research, especially towards the development of antigen-specific immune suppression treatments for autoimmune diseases.
The term pemphigus stems from the Greek ‘pemphix’, which means blister or bubble, and it describes a group of chronic blistering epithelial diseases in which the production of IgG autoantibodies against extracellular domains of cell membrane proteins of keratinocytes results in acantholysis (the loss of cell–cell adhesion between keratinocytes)1 (FIG. 1). In pemphigus, IgG autoantibodies are characteristically directed against desmogleins (desmoglein 1 and desmoglein 3), which are part of the cadherin family of cell–cell adhesion molecules that are found in desmosomes, which are the structures primarily responsible for maintaining intercellular adhesion in stratified squamous epithelia, such as the skin and oral mucosa2–4. Pemphigus can be classified into three major forms: pemphigus vulgaris, pemphigus foliaceus and paraneoplastic pemphigus (BOX 1). Pemphigus vulgaris and pemphigus foliaceus are the originally characterized, classic forms of pemphigus. Histological analysis shows that pemphigus vulgaris blisters develop deep in the epidermis or oral epithelium above the basal layer, whereas pemphigus foliaceus blisters occur in the superficial layers of the epidermis, mostly in the granular layer. Paraneoplastic pemphigus is distinguished by the presence of a known-associated or occult-associated neoplasm, usually of lymphoid tissue5. The phenotype of paraneoplastic pemphigus is typically characterized by blisters in the deep epithelial layers, suprabasal blisters in the deep epithelial layers and interface dermatitis (BOX 1) and is caused by the combination of humoral and cellular autoimmune reactions.
Figure 1. Clinical features of pemphigus.
Pemphigus vulgaris can present with erosions on the buccal mucosa (part a) and cutaneous flaccid blisters and haemorrhagic erosions (part b). Scaly, crusted erosions in pemphigus foliaceus (part c). Intractable stomatitis in paraneoplastic pemphigus, with erosions and ulcerations that characteristically extend onto the vermilion (the edge between the lip and the adjacent skin; partd).
Box 1. Classification of pemphigus subtypes.
Pemphigus vulgaris
Caused by humoral autoimmune response; the three subtypes are:
Mucosal-dominant type (limited cutaneous involvement): blisters in the deep layers of the oral mucosa owing to anti-desmoglein 3 IgG autoantibodies
Mucocutaneous type (both mucosal and cutaneous involvement): blisters in the deep layers of the oral mucosa and epidermis, owing to anti-desmoglein 3 and anti-desmoglein 1 IgG autoantibodies, respectively
Cutaneous type (cutaneous involvement alone): blisters in the deep layers of the epidermis owing to anti-desmoglein 1 and pathogenically weak anti-desmoglein 3 autoantibodies
Pemphigus foliaceus
Caused by humoral autoimmune response; no apparent mucosal involvement, blisters in the superficial layers of the epidermis owing to anti-desmoglein 1 IgG autoantibodies
Paraneoplastic pemphigus
Caused by both humoral and cellular autoimmune responses; mucosal and cutaneous blisters owing to anti-desmoglein 3 and/or anti-desmoglein 1 IgG autoantibodies in combination with interface dermatitis (vacuolization of basal cells, apoptosis of keratinocytes, dyskeratotic cells (cells with abnormal keratinization) and inflammation at the dermal–epidermal junction) or severe oral lichenoid reaction (chronic inflammation of the oral mucosa) owing to self-reacting T cells. Patients with paraneoplastic pemphigus can also develop IgG autoantibodies against α2-macroglobulin-like protein 1 (a protease inhibitor) and several cytoplasmic proteins of the plakin family: epiplakin, plectin, desmoplakin I, desmoplakin II, BPAG1 (also known as dystonin), envoplakin and periplakin
Pemphigus variants
Pemphigus vegetans: a variant of pemphigus vulgaris with fungoid vegetations (eroded areas do not heal as usual but form papillomatous growth of epidermis) characterized by anti-desmoglein 3 IgG autoantibodies
Pemphigus erythematosus: a variant of pemphigus foliaceus with localized involvement, mainly on the face and upper part of the chest and back, mediated by anti-desmoglein 1 IgG autoantibodies
Fogo selvagem: an endemic form of pemphigus foliaceus found in rural areas in Brazil that is characterized by anti-desmoglein 1 IgG autoantibodies
Herpetiform pemphigus: a subtype characterized by small vesicles and pustules and mainly anti-desmoglein 1 IgG autoantibodies
Drug-induced pemphigus
In this Primer, we focus on the major forms of pemphigus: pemphigus vulgaris, pemphigus foliaceus and paraneoplastic pemphigus.
Epidemiology
Incidence estimates of pemphigus substantially vary around the world6. Pemphigus vulgaris is the most common subtype of pemphigus in Europe, the United States and Japan; it preferentially affects women, and most of the patients are 50–60 years of age at disease onset6,7. Pemphigus foliaceus is the most common type observed in South America and North Africa owing to the endemic form6, with sex predisposition differing among the regions and a preferential occurrence in young adults. These differences in disease onset might be related to genetic, hormonal and environmental factors, as discussed below. The incidence of paraneoplastic pemphigus is not known, but it is much less common than pemphigus vulgaris and pemphigus foliaceus. Around 300 cases have been reported in the literature5,8–10. Paraneoplastic pemphigus mostly occurs in patients 45–70 years of age, with equal prevalence in both sexes8.
In pemphigus vulgaris, epidemiological studies evaluating different European regions suggest that the incidence of pemphigus tends to be lower at higher latitudes than at lower latitudes11, with incidences ranging from 0.5 cases per million population in Germany to 8 cases per million population in Greece12,13. Reported incidences are 1.6, 16.1 and 50 cases per million population in Saudi Arabia, Israel and Iran, respectively14–16. Of note, the reported pemphigus incidence is 32 cases per million population in a Jewish population in the United States17.
The increased number of pemphigus foliaceus cases in South America (Brazil and neighbouring countries) and North Africa (Tunisia and neighbouring countries) is the result of an endemic form of the disease in these areas, where it affects mostly young adults, with a peak incidence between the second and third decade of life18,19. In Brazil, pemphigus foliaceus is approximately 20-times more frequent than pemphigus vulgaris and has a prevalence of around 3–5% in the Limao Verde Amerindian reservation in the state of Mato Grosso do Sul18. In Tunisia, the overall incidence of pemphigus (both vulgaris and foliaceus) is up to 8.6 cases per million population, although the incidence of pemphigus foliaceus can increase to up to 20 cases per million individuals in young adult women living in rural areas of central and southern Tunisia19,20. These epidemiological associations suggest that an environmental agent might trigger a low-level autoantibody response against desmoglein 1 that in some genetically susceptible individuals becomes pathogenic. In fogo selvagem, the endemic form of pemphigus foliaceus in Brazil, an initial immune response against the sand fly salivary antigen LJM11 (a non-infectious protein from the sand fly Lutzomyia longipalpis, the vector of leishmaniasis21) is speculated to cause a cross-reaction with desmoglein 1 (REFS 22,23), which may trigger the disease.
Mechanisms/pathophysiology
Genetic factors
A large body of evidence supports the role of genetic factors in pemphigus24. Pemphigus is a polygenic disease and, although sporadic forms only rarely affect more than one family member, an increased prevalence of low titres of disease-associated autoantibodies in healthy first-degree relatives of patients with pemphigus has been reported25. Several HLA alleles have been identified as risk factors, but the correlation between a certain HLA genetic profile and the patient’s clinical profile is still unclear24. A strong association with pemphigus vulgaris has been observed for HLA-DRB1*0402 (which is predominant in Ashkenazi Jews), HLA-DRB1*1401, HLA-DRB1*1404 and HLA-DQB1*0503 (which are both prevalent in non-Jewish patients of European and Asian descent)26. However, in a North American cohort of Ashkenazi Jews, HLA-DRB1*1401 does not directly confer risk, but is overrepresented in patients because of a linkage disequilibrium with HLA-DQB1*0503 (REF. 27). Three other alleles (HLA-DRB1*03, HLA-DRB1*07 and HLA-DRB1*15) had a lower prevalence in patients with pemphigus vulgaris26, whereas HLA-E*0103, a non-classical class IB allele, and a non-HLA marker encoding suppression of tumorigenicity 18 protein (ST18; a molecule that regulates apoptosis and inflammation) have both been associated with pemphigus vulgaris28,29. However, the association between ST18 and pemphigus vulgaris found in Jewish and Egyptian patients was not confirmed in German patients, suggesting that ST18-associated variants may predispose to pemphigus vulgaris in a population-specific manner29. Moreover, a pemphigus vulgaris-associated functional risk variant residing within the ST18 promoter region may be linked to the secretion of key inflammatory molecules by keratinocytes after autoantibody binding (tumour necrosis factor (TNF), IL-1α and IL-6)30. In addition to regulating autoantibody expression, the HLA status is also an important driver of oxidative stress in pemphigus vulgaris31,32.
The HLA class II loci HLA-DRB1*04 and HLA-DRB1*14 were linked to non-endemic pemphigus foliaceus, and a strong correlation has been found in fogo selvagem with HLA-DRB1*1402 and HLA-DR*0404 (REFS 24,33).
The HLA-DRB1*03 allele confers strong susceptibility to paraneoplastic pemphigus in French Caucasian patients34. In Chinese patients with paraneoplastic pemphigus, the HLA-Cw14 allele was identified more frequently than in healthy controls regardless of the underlying tumour, whereas HLA-DRB1*03 was not detected, indicating that different ethnic backgrounds confer different susceptibilities to this pemphigus subtype35.
Environmental factors
Genetic factors alone are not sufficient for pemphigus onset, and triggering environmental determinants seem to have a role. Certain drugs, especially those containing a thiol group such as penicillamine (a metal-chelating agent) and captopril (an angiotensin-converting enzyme inhibitor), might interfere with the biochemistry of the keratinocyte membrane or the immune balance and, therefore, promote acantholysis in pemphigus36; however, there is no case–control study that corroborates this potential link. Other environmental factors under investigation include, among others, viruses (such as herpes simplex virus), dietary factors and physiological and psychological stressors36.
Desmogleins as autoimmune targets
The intraepithelial expression patterns of desmoglein 1 and desmoglein 3 are different in the oral mucosa and in the skin4,37. In the skin, desmoglein 1 is expressed throughout the epidermis but more abundantly in the superficial layers, whereas desmoglein 3 is almost exclusively expressed in the basal and parabasal cell layers (FIG. 2). In mucous membranes, both desmoglein 1 and desmoglein 3 are expressed throughout the squamous layer, but desmoglein 1 levels are much lower than those of desmoglein 3. The profile of autoantibodies against desmoglein 1 and desmoglein 3 largely corresponds to specific clinical features4,37 (FIG. 2). Patients with pemphigus foliaceus have essentially only anti-desmoglein 1 autoantibodies. Patients with the mucosal-dominant type of pemphigus vulgaris have mostly, if not only, anti-desmoglein 3 autoantibodies, whereas those with the mucocutaneous type of pemphigus vulgaris have both anti-desmoglein 3 and anti-desmoglein 1 autoantibodies. Anti-desmoglein 1 and anti-desmoglein 3 IgG antibodies can be found circulating in serum and bound to the surface of skin and mucosal keratinocytes in patients with pemphigus, even in epithelia that appear normal. However, application of lateral pressure on this normal-appearing, perilesional skin can induce blisters and epidermal splitting (known as positive Nikolsky sign), thereby revealing the compromised cell adhesion.
Figure 2. Pathogenesis of pemphigus.
a | The distribution and expression levels of desmoglein 1 (DSG1; blue coloured box in the first column) and DSG3 (yellow coloured box in the first column) vary in the skin and mucous membranes. The compensation theory states that the development of blisters in the skin or mucosa or both depends on the DSG type being targeted by the autoantibodies. In the mucosal-dominant type of pemphigus vulgaris, anti-DSG3 IgG antibodies induce erosions in the oral mucosa, where DSG3 is the most abundant DSG type, but fail to induce cutaneous blisters, as there is compensation from DSG1. Similarly, the anti-DSG1 IgG antibodies in pemphigus foliaceus induce superficial blisters in the skin but not in the oral mucosa. In the mucocutaneous type of pemphigus vulgaris, both anti-DSG3 and anti-DSG1 IgG antibodies are present, resulting in extensive blisters and erosions of the skin and mucous membranes235. In paraneoplastic pemphigus, in addition to acantholysis (loss of cell adhesion) induced by humoral autoimmune reactions, a cellular autoimmune response to the epidermis is also observed, which results in keratinocyte apoptosis with T cell infiltration within the epidermis, obscuring of the boundary of epidermis and dermis, and a band-like dense T cell infiltration in the upper dermis indicating interface dermatitis. b | Haematoxylin and eosin staining demonstrates a suprabasilar and subcorneal blister with acantholysis in skin biopsies from patients with pemphigus vulgaris and pemphigus foliaceus, respectively, and interface dermatitis in addition to blister formation in a sample from a patient with paraneoplastic pemphigus.
The correlation between autoantibody profile and clinical phenotype can be physiologically explained by the desmoglein compensation theory, which is based on the findings that desmoglein 1 and desmoglein 3 can compensate for each other when they are co-expressed in the same cell and the adhesive function is impaired in one of them4,38,39 (FIG. 2). The desmoglein compensation theory provides a logical base to understand how pathogenic autoantibodies induce the phenotype of typical cases of pemphigus vulgaris and pemphigus foliaceus. Consistent with this compensation theory in pemphigus, exfoliative toxins produced by Staphylococcus aureus, which specifically cleave desmoglein 1 (REF. 40), cause superficial blisters in the skin, but not in the oral mucosa, as the mucosa expresses high levels of desmoglein 3.
Anti-desmoglein autoantibodies are necessary and sufficient to generate the specific pathology of pemphigus. However, in very rare cases of pemphigus vulgaris, IgG autoantibodies against desmocollin 3 (another desmosomal cadherin) can cause the disease phenotype without coexisting anti-desmoglein autoantibodies41,42. In addition to desmoglein and desmocollin, other autoimmune targets have also been found with various approaches31,43–45. Although their potential role in the pathogenesis of pemphigus is still under investigation, these non-desmoglein autoantibodies might have some supporting roles and contribute the complex phenotype of pemphigus or might have pathogenic roles on their own that are sufficient for blister formation in rare cases.
Patients with paraneoplastic pemphigus develop characteristic IgG autoantibodies against multiple antigens, including desmoglein 3, desmoglein 1 or both46, as well as various proteins of the plakin family5,47 (BOX 1). Anti-desmoglein antibodies have a pathogenic role in inducing blister formation, whereas the pathophysiological relevance of the anti-plakin autoantibodies is unclear, because plakin proteins are cytosolic and, therefore, cannot be directly attacked by IgG antibodies in vivo. In addition to humoral immunity, cell-mediated cytotoxicity is involved in the pathogenesis of paraneoplastic pemphigus and induces more-severe and refractory oral lichenoid stomatitis or mucositis, as well as polymorphic lichenoid skin eruptions, which are caused by autoimmune T cells reacting to epithelial and epidermal cells, respectively48,49.
Blister formation and acantholysis
Studies on experimental pemphigus models in mice, human keratinocyte cultures and skin biopsies from patients with pemphigus suggest that autoantibody binding to desmoglein antigens can cause epithelial acantholysis through several synergistic mechanisms. Collectively, the studies in the field support a model in which autoantibody-mediated steric hindrance of desmosomal adhesion and/or interference with desmosome assembly cause the pathognomonic pemphigus pathology, whereas cell signalling pathways augment the pathological autoimmune response, and all these mechanisms contribute to acantholysis (FIG. 3).
Figure 3. Mechanisms of acantholysis.
Anti-desmoglein autoantibodies can cause acantholysis through steric hindrance of desmoglein-mediated trans- (between molecules on opposing cells) and cis- (between molecules on the same cells) adhesion, inhibition of desmosome assembly and promotion of desmosome disassembly (by clustering and/or endocytosis of desmogleins) and stimulation of signalling pathways that can synergize with these mechanisms to modulate keratinocyte cell adhesion. In pemphigus vulgaris, IgG-induced desmoglein 3 endocytosis is regulated by the p38 mitogen-activated protein kinase (MAPK) pathway and its downstream effector MAPK-activated protein kinase 2 (MK2)68–71. Inhibition of either p38 or MK2 prevents pemphigus vulgaris IgG-induced acantholysis and monoclonal antibody-induced spontaneous blistering in passive transfer mouse models71,236. p38 similarly regulates pemphigus foliaceus IgG-induced acantholysis237. The trans-adhesive and cis-adhesive interactions can be homophilic (that is, between desmogleins) or heterophilic (between desmoglein and desmocollin). Dashed arrows indicate that the signal transduction mechanism can affect both steric hindrance and desmoglein depletion. DAG, diacylglycerol; DP, desmoplakin; HSP27, heat shock protein 27; PG, plakoglobin; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PKP, plakophilin; PLC, phospholipase C.
The direct pathogenic role of autoantibodies in pemphigus has been clearly established. Blister formation in pemphigus, as opposed to other autoimmune skin diseases (for example, bullous pemphigoid and epidermolysis bullosa acquisita), does not require complement activation50, and monovalent antibody fragments are sufficient to cause skin blisters51,52. Consistent with these observations, pemphigus autoantibodies are predominantly of the IgG4 subclass53–55, which does not activate complement, poorly activates immune effector cells via its Fc region and does not effectively crosslink antigen56. Thus, autoantibody binding can directly compromise desmosomal function.
Desmogleins have four cadherin repeats in their extracellular domains, (EC1 to EC4), plus a membrane-proximal extracellular anchor domain. The amino-terminal EC1 and EC2 domains contain residues important for adhesive interactions57–59, and these domains are most often targeted by pemphigus autoanti-bodies. Pathogenic anti-desmoglein 3 monoclonal antibodies cloned from patients with pemphigus vulgaris and model mice directly bind to residues that mediate trans-adhesion59 and cis-adhesion60; in addition, atomic force microscopy experiments indicate that polyclonal serum IgG antibodies from patients with pemphigus vulgaris can directly inhibit homophilic desmoglein 3 trans-interactions61. Together, these data support steric hindrance as a mechanism for autoantibody-induced acantholysis in pemphigus (FIG. 3).
Additional events triggered by anti-desmoglein autoantibody binding also contribute to, and might be required for, pemphigus acantholysis. Serum IgG antibodies from patients with pemphigus vulgaris, as well as recombinant monovalent human anti-desmoglein 3 antibodies, interfere with desmosome assembly by causing internalization and degradation of desmoglein 3 proteins that are not yet integrated in desmosomes, leading to desmoglein 3-depleted desmosomes62–64. Interference with desmosome assembly by pathogenic pemphigus autoantibodies is supported by both immunofluorescence and electron microscopy findings in skin from patients with pemphigus foliaceus or pemphigus vulgaris, which show interdesmosomal intercellular widening in areas of desmoglein clustering65,66.
Supporting a role for cellular signalling in pemphigus pathogenesis, polyclonal serum IgG antibodies from patients with pemphigus foliaceus can cause dissociation of desmoglein 1 junctions without detectable interference with homophilic desmoglein 1 trans-interactions67. Numerous signalling molecules and metabolic pathways have been implicated in pemphigus acantholysis, involving, for example, p38 mitogen-activated protein kinase (MAPK) and its downstream effector MAPK-activated protein kinase 2 (MK2)68–71, epidermal growth factor receptor72,73, RHO GTPases74, MYC75, caspases76,77 and mitochondria78. However, no signalling event is sufficient to induce the specific histology of pemphigus vulgaris or pemphigus foliaceus epithelial blistering. Furthermore, blisters induced by monoclonal antibodies isolated from patients with pemphigus vulgaris are typically not sensitive to p38 or MK2 inhibition, suggesting that this skin fragility phenotype predominantly reflects steric hindrance mechanisms69–71.
B cell repertoire profiling
Cloning of anti-desmoglein antibodies from patients and model mice has elucidated the composition of the autoreactive B cell repertoire59,60. Both pathogenic and non-pathogenic human monoclonal antibodies that bind to desmoglein 1, desmoglein 3 or both have been identified; pathogenicity is defined as the ability to cause acantholysis in cultured keratinocytes, human skin organ culture or animal models79,80. The presence of non-pathogenic autoantibodies might in part explain the persistently high titres of anti-desmoglein antibodies observed in some patients despite disease remission, as well as IgG binding to uninvolved epithelia81. Of note, a polyclonal mixture of supposedly non-pathogenic antibodies could cause blisters through antigen crosslinking or other signalling-mediated mechanisms69,82. Thus, all anti-desmoglein antibodies have pathogenic potential.
In pemphigus vulgaris and paraneoplastic pemphigus, VH1-46 antibody heavy chain gene usage by desmoglein 3-reactive B cells is commonly observed83,84. Surprisingly, anti-desmoglein 3-secreting B cells that use VH1-46 have relatively few somatic mutations and require few to none of these mutations to bind to desmoglein 3, in contrast to B cells using other VH genes, which are highly mutated and require somatic mutations to maintain desmoglein 3 autoreactivity60,83. These data indicate that naive VH1-46 B cells might be prone to desmoglein 3 autoreactivity. Interestingly, AK23, a pathogenic mouse monoclonal anti-desmoglein 3 antibody cloned from pemphigus vulgaris model mice, was transcribed from a gene that was highly homologous to the human VH1-46 gene and had few somatic mutations83, suggesting similar selection mechanisms in response to desmoglein 3 in humans and mice.
Both healthy individuals and patients with pemphigus foliaceus had antibodies against intracellular desmoglein 1 precursor proteins, but only the patients had antibodies against the mature, cell surface-anchored desmoglein 1 protein85. Similar to anti-desmoglein 3 autoantibodies, anti-desmoglein 1 monoclonal antibodies cloned from patients with fogo selvagem showed enriched VH3-23 usage and similar patterns of somatic mutation before and after disease onset, suggesting similar antigen-driven binding and selection mechanisms86.
The anti-desmoglein B cell repertoire can crossreact with foreign antigens. VH1-46 usage has been described in B cells responding to the rotavirus capsid protein VP6 (REFS 83,87). However, crossreactivity of VH1-46 B cells to both desmoglein 3 and VP6 in patients with pemphigus vulgaris is rare owing to the different nature of the somatic mutations that favour reactivity against desmoglein 3 versus VP6. Nevertheless, monoclonal antibodies were identified that could both inhibit rotavirus infectivity and induce keratinocyte acantholysis88. In endemic pemphigus foliaceus, anti-desmoglein 1 IgG4 monoclonal antibodies can crossreact with the sand fly antigen LJM11 (REF. 21). Collectively, these findings underscore the potentially double-edged sword of immunity, which can lead to autoimmunity in pemphigus.
Longitudinal analysis of B cell repertoires of patients with pemphigus vulgaris and pemphigus foliaceus before and after therapy showed that patients who relapsed after a complete clinical (but not serological) remission of the disease had the same anti-desmoglein B cell clones that were identified during a previous bout of active disease89. The persistence of identical anti-desmoglein B cell clones over years is consistent with serum antibody epitope mapping studies that showed a lack of epitope-spreading throughout the disease course in patients with pemphigus vulgaris and pemphigus foliaceus90,91. Thus, the development of pemphigus seems to be associated with the rare stochastic occurrence of a fixed set of autoreactive B cell clones that do not show a substantial dynamic evolution over time. Hence, the elimination of this finite set of autoimmune B cells could lead to long-lasting disease remission, as has been observed in some patients treated with rituximab92.
Pathogenic role of T cells
T cells are mainly classified as CD4+ or CD8+ T cells. CD4+ T cells regulate antibody production by interacting with B cells and directly infiltrate the tissue expressing the target antigen, where they modulate inflammation through cytokines and surface molecules. Evidence supports that autoreactive CD4+ T cells are involved in anti-desmoglein 3 antibody production (FIG. 4). Some HLA class II haplotypes that have been associated with pemphigus vulgaris in various populations93,94 seem to encode MHC that are particularly suitable to present specific desmoglein 3 peptides to CD4+ T cells. The finding that IgG4 is the predominant subclass of anti-desmoglein 3 antibodies suggests T cell-dependent immunoglobulin class switching in pemphigus54,95. In addition, somatic hypermutations were observed mainly in complementarity determining regions of anti-desmoglein 3 antibodies, although some pathogenic antibodies were barely mutated60,83. This mutation leads to affinity maturation, an event that, in turn, occurs in addition to class switching after T cells and B cells interact in germinal centres.
Figure 4. B cell and T cell response in pemphigus.
Dendritic cells presenting desmoglein (DSG) antigens can activate CD4+ and CD8+ T cells. These autoreactive T cells can be identified even in healthy individuals and mainly produce IL-10 and interferon-γ (IFNγ). IFNγ has a potential to suppress DSG-specific T helper 2 (TH2) cell development. IL-10 is a key mediator of the peripheral tolerance mechanism that suppresses the activity of pathogenic TH2 cells. However, IL-10 has complex effects, as it can promote immunoglobulin class switching to IgG4, the predominant subclass of anti-DSG antibodies, and has opposite effects in different phases of disease pathogenesis. IL-4-producing TH cells can be isolated from patients with pemphigus (but not healthy individuals) and presumably drive anti-DSG antibody production from B cells. Acantholysis seen in pemphigus vulgaris and pemphigus foliaceus is induced by a humoral autoimmune response. DSG-reactive (or reactive to other epidermal autoantigens) CD4+ and CD8+ T cells with cytotoxic activity can rarely be generated and mediate a cellular autoimmune response that causes interface dermatitis, which can be observed in paraneoplastic pemphigus. TCR, T cell receptor; Tr1, T regulatory type 1.
Furthermore, desmoglein 3-reactive CD4+ T cells have been isolated from peripheral blood mononuclear cells from both patients with pemphigus vulgaris and healthy controls, after in vitro stimulation with recombinant desmoglein 3 or phytohaemagglutinin96–98. Desmoglein 3-reactive, interferon-γ (IFNγ)-producing CD4+ T cells were detected in patients with pemphigus vulgaris as well as healthy controls with either HLA-DRB1*0402 or HLA-DQB1*0503, but they were not detected in controls with pemphigus vulgaris-unrelated HLA alleles98. By contrast, IL-4-producing CD4+ T cells (which presumably drive the production of anti-desmoglein antibodies by B cells) are detected only in patients with pemphigus vulgaris but not unaffected individuals, and IL-4 blockade suppressed anti-desmoglein 3 IgG production in a mouse model99. The frequency of IL-4-producing CD4+ T cells did not change between disease phases, but IFNγ-producing CD4+ T cells were more frequently detected in the chronic active phase than at disease onset or during disease remission98. Desmoglein 3-reactive CD4+ T cells detected in healthy controls produced IL-10, an immunoregulatory cytokine, more frequently than the cells detected in patients with pemphigus vulgaris100. The IL-10-producing clones could suppress immune reactions against desmoglein 3 by autoreactive T cells, at least in vitro. Thus, the balance among clones of autoreactive CD4+ T cells that produce different cytokines is important for pemphigus pathogenesis (FIG. 4).
In paraneoplastic pemphigus, autoreactive T cells also have cytotoxic activity, as interface dermatitis is mediated by direct infiltration of autoimmune CD4+ and CD8+ T cells9,10,48,49 (FIG. 4). Notably, bronchiolitis obliterans (an inflammation and fibrotic change that cause the obstruction of bronchioles), which shows pulmonary T cell infiltration, occurs as a complication of paraneoplastic pemphigus but not in pemphigus vulgaris or pemphigus foliaceus101,102. Desmoglein 3-specific CD4+ T cells could induce pulmonary inflammation probably due to ectopic expression of desmoglein 3 by squamous metaplasia in the lung, as has been observed in patients with paraneoplastic pemphigus101,103. Ectopic expression of epidermal autoantigens might help to explain the multiple organ involvement in paraneoplastic pemphigus.
Thus, autoreactive T cells have roles in pemphigus pathogenesis and can be targeted to regulate the autoimmune responses.
Animal models
Several animal models are available for pemphigus and are useful for preclinical studies of newly developed therapeutic strategies, although no model completely mimics the entire sequence of events found in patients (FIG. 5).
Figure 5. Mouse models for pemphigus.
a | Passive transfer model. IgG fractions prepared from patients’ sera, or other engineered anti-desmoglein 3 (DSG3) antibodies, are injected intraperitoneally or subcutaneously into neonatal mice. The model develops blisters that show the typical histology observed in patients, with IgG deposition on keratinocyte cell surfaces and an acantholytic blister (asterisk).b | Active disease model. DSG3-deficient (Dsg3−/−) mice do not establish tolerance against DSG3 because DSG3 is never exposed to immune cells106. After adoptive transfer of lymphocytes from Dsg3−/− mice, immunodeficient mice, such as Rag2−/− mice, stably produce anti-DSG3 IgG antibodies and show the typical gross and histological phenotype of the mucosal-dominant type of pemphigus vulgaris106. This model mice also develop telogen hair loss (a feature that is not observed in patients to the same extent) because, in mice, intercellular adhesion of follicular epidermis is mainly mediated by DSG3 during the telogen (rest) phase of hair growth106,238. Another active disease model is generated by adoptive transfer of DSG3-specific T cells prepared from transgenic mice expressing DSG3-specific T cell receptor (TCR) to immunodeficient mice. Transferred T cells directly infiltrate into the skin and attack DSG3-expressing keratinocytes, causing experimental autoimmune dermatitis (EAD). This model is useful to analyse T cell- mediated cellular autoimmune mechanisms in T cell-mediated skin inflammation. c | Humanized model. In this model, MHC class II (MHC II)-deficient mice are engineered to express transgenic HLA-DRB1*0402. After immunization with human recombinant DSG3, the mice produce anti-human DSG3 antibodies that induce blister formation in human skin specimens. Scale bar indicates 50 μm. Histology image in part c courtesy of M. Hertl and R. Eming, Philipps University of Marburg, Germany.
A passive transfer model using neonatal mice can be used to study the pathogenicity of IgG autoantibodies and evaluate drugs that target antibody–antigen interaction and the following molecular events in keratinocytes104 (FIG. 5a). When IgG fractions prepared from patients’ sera, monoclonal human or mouse antibodies or single chain variable fragments from phage display are injected intraperitoneally or subcutaneously into neonatal mice, the mice develop blisters in the skin spontaneously 12–18 h after the injection46,59,79,80. To study the pathogenicity of anti-desmoglein 3 IgG antibodies in this model, a minimal dose of exfoliative toxin A or anti-desmoglein 1 monoclonal antibody needs to be co-injected to eliminate the compensatory adhesion by desmoglein 1. A modified version of the passive transfer model can be generated by inoculation of hybridoma cells producing monoclonal anti-desmoglein 3 antibodies to adult mice59,82,105.
Active disease models are valuable to evaluate therapeutic strategies that target desmoglein 3-reactive T cells and B cells. To overcome immunological tolerance to self-antigens, Dsg3 (which encodes desmoglein 3) knockout mice were used to generate an immune response against desmoglein 3 and produce anti-desmoglein 3 IgG antibodies106. Adoptive transfer of peripheral lymphocytes (that it, splenocytes) from Dsg3−/− mice to immune-deficient but desmoglein 3-expressing recipient mice generates an artificial autoimmune state in the recipient mice106 (FIG. 5b). This active disease model of pemphigus vulgaris is useful to isolate and characterize anti-desmoglein 3 monoclonal antibodies59 and desmoglein 3-reactive CD4+ T cell clones99, and to evaluate the efficacy of pharmacological reagents as well as future cell therapies to block antibody production107. Furthermore, transgenic mice expressing desmoglein 3-reactive T cell receptors can be used to understand some aspects of tolerance mechanisms108 and the role of T cells in antibody production and cellular immunity48 (FIG. 5b). Another approach is a mouse model with a MHC class II-null background that expresses the pemphigus-associated HLA-DRB1*0402 allele109 (FIG. 5c). This model provides a useful tool to analyse the effects of the interaction between human desmoglein 3 peptides and pemphigus vulgaris-associated HLA molecules on the mouse immune response.
Diagnosis, screening and prevention
Clinical manifestations
Pemphigus vulgaris can manifest as a mucosal-dominant, mucocutaneous or, less frequently, solely cutaneous type1,110 (FIG. 1a,b). Lesions classically start in the oral mucosa and might then extend to the skin. Oral mucosa involvement consists of flaccid blisters that easily rupture, leaving painful erosions, especially in the buccal area (the inside of the cheeks), which might potentially lead to weight loss and malnutrition. In addition to the oropharyngeal mucosa, other mucous membranes, such as those lining the larynx, oesophagus, conjunctiva, nose, genitalia and anus, might be less frequently affected. Lesions of the skin, which frequently affect the head, upper trunk and groin, are characterized by flaccid blisters and partly crusted erosions on healthy-appearing or erythematous skin. Erosions of the intertriginous areas (where skin folds) might develop into vegetative lesions (abnormal growth of keratinocytes with granular papillomatous appearance) in the pemphigus vegetans variant. In pemphigus vulgaris and pemphigus foliaceus, Nikolsky sign might be positive in the active phase of the disease. A substantial number of patients with pemphigus vulgaris can relapse with a pemphigus foliaceus clinical and histological phenotype, especially when the relapses occur after a long remission111,112.
Unlike pemphigus vulgaris, pemphigus foliaceus typically does not affect mucosal sites; both non-endemic pemphigus foliaceus and fogo selvagem share the same clinical, histological and immunological findings (BOX 1; FIG. 1c). Patients usually present with multiple pruritic, scaly and crusted erosions with flaky circumscribed patches in mostly seborrhoeic areas that can extend, merge and progress to exfoliative erythroderma (erythema and scaling that covers >90% of the body surface area); blisters are rarely seen owing to their superficial localization and consecutive rupture.
The clinical hallmark of paraneoplastic pemphigus is a painful, persistent and therapy-refractory haemorrhagic stomatitis (inflammation of the mouth and lips), especially involving the vermilion of the lips (the edge between the lip and the adjacent skin) and lateral borders of the tongue (FIG. 1d). Cutaneous lesions, which predominantly affect the upper body and usually develop after the onset of mucous membrane lesions, show heterogeneous phenotypes, including polymorphic bullae, erosions, papulae (small superficial solid elevations of the skin), target lesions, lichenoid lesions, scales or pustulae (circumscribed elevation of the skin containing purulent fluid). These clinical features might be associated with a positive Nikolsky sign. Compared with pemphigus vulgaris, paraneoplastic pemphigus typically shows a more widespread involvement of the oral mucosa, more frequent involvement of other mucosal sites, such as conjunctivae, and involvement of palms and soles but not the scalp. Because paraneoplastic pemphigus is associated with and might precede a clinically manifest or occult malignancy and can also be accompanied by bronchiolitis obliterans, a comprehensive diagnostic examination, including a CT scan of the chest, abdomen and pelvis, is mandatory8.
Pregnant women with pemphigus can passively transfer pathogenic autoantibodies to their child, leading to transient blisters and erosions of the skin of the neonates and, in about one-quarter of cases, of mucous membranes; 34 cases of neonatal pemphigus (31 of pemphigus vulgaris and 3 of pemphigus foliaceus) have been reported113. The low prevalence of neonatal pemphigus foliaceus might be explained by the overexpression of desmoglein 3 in the neonatal epidermis114,115.
Confirmation of diagnosis
Histopathology
Lesional biopsy specimens from all types of pemphigus present acantholysis, which can progress to the formation of intraepithelial blisters (FIG. 2). In pemphigus vulgaris, a ‘tombstone effect’, which is the presence of residual basal keratinocytes at the basement membrane zone, might be seen at the site of the floor of the blister. Although the epidermal blisters are classically non-inflammatory, in both pemphigus vulgaris and pemphigus foliaceus, a slight eosinophilic or neutrophilic infiltration can be occasionally seen in the upper dermis and epidermis. The features of paraneoplastic pemphigus might vary depending on the morphology of the clinical lesion.
Immunofluorescence
Direct immunofluorescence microscopy of perilesional biopsy specimens is the most reliable and sensitive diagnostic test for all forms of pemphigus; direct immunofluorescence of lesional samples might yield false-negative results owing to internalization of immune reactants on the cell surface by acantholytic keratinocytes (FIG. 6a,b). In both pemphigus vulgaris and pemphigus foliaceus, IgG antibodies, and occasionally complement C3 protein, deposit on the cell surface and are visible as a honeycomb-like pattern. In some cases of paraneoplastic pemphigus, an additional deposition of immunoreactants (IgG or C3) can be seen along the dermal–epidermal junction in a band-like pattern. Of note, false-negative results might be possible in paraneoplastic pemphigus owing to necrotic tissue or dense inflammatory infiltrates.
Figure 6. Immunopathological characteristics of pemphigus vulgaris, pemphigus foliaceus and paraneoplastic pemphigus.
Direct immunofluorescence microscopy of skin biopsies of perilesions from patients with pemphigus vulgaris (parta) and pemphigus foliaceus (part b) reveals an intercellular staining of IgG antibodies. Indirect immunofluorescence microscopy of a monkey oesophagus exposed to the serum of a patient with pemphigus vulgaris shows an epithelial intercellular surface staining of IgG (part c). Indirect immunofluorescence microscopy of a rat bladder exposed to the serum of a patient with paraneoplastic pemphigus presents both epithelial intercellular and cytoplasmic staining (part d).
Indirect immunofluorescence microscopy using monkey or guinea pig oesophagus or human skin as substrates enables a semiquantitative detection of serum IgG autoantibodies that bind to the epithelium in an intercellular pattern (FIG. 6c). In paraneoplastic pemphigus, circulating autoantibodies might also stain the basement membrane zone and bind to the plakin-rich transitional epithelium of the rat bladder (FIG. 6d).
Serological tests
The molecular specificity of circulating pemphigus autoantibodies can be analysed using highly sensitive and specific enzyme-linked immunosorbent assays (ELISAs) with recombinant autoantigens108,116–118. The desmoglein ELISA has 96–100% sensitivity and specificity for disease diagnosis, indicating that nearly all patients with pemphigus have anti-desmoglein antibodies and that anti-desmoglein antibodies largely do not occur in healthy individuals108,117. In addition, biochips using tissue sections of monkey oesophagus and cells transfected with recombinant auto-antigens are available to detect anti-desmoglein 1 and anti-desmoglein 3 autoantibodies semiquantitatively by indirect immunofluorescence microscopy119. Autoantibody levels often correlate with disease activity and are, therefore, an appropriate biomarker for clinical follow-up monitoring. Anti-desmoglein 1 antibody titres tend to show a closer correlation with the course of the disease activity compared with anti-desmoglein 3 antibody titres120,121. However, active lesions might occur, albeit rarely, even in the absence of detectable anti-desmoglein 3 and desmoglein 1 IgG autoantibodies122, whereas 20–40% of patients in clinical remission still have detectable levels of circulating autoantibodies123,124, and low levels of anti-desmoglein IgG autoantibodies are also occasionally detected among healthy individuals25,31,125,126.
Western blotting or immunoprecipitation of epithelial and keratinocytes extracts are not practical in the diagnosis of pemphigus vulgaris and pemphigus foliaceus, but they can be used to identify several other non-desmoglein target proteins in patients with paraneoplastic pemphigus. A multivariant ELISA and a biochip assay are also available to detect anti-envoplakin autoantibodies118,127. Anti-epiplakin autoantibodies might be associated with bronchiolitis obliterans in Japanese patients with paraneoplastic pemphigus128.
Differential diagnoses
Several diseases broadly classified based on their autoimmune, inflammatory, infective, genetic or metabolic aetiology can also cause skin and mucosal blisters (BOX 2). In particular, different disorders of the pemphigoid group cause subepidermal blistering and are characterized by the presence of autoantibodies against distinct structural components of the dermal–epidermal junction. There are many differential considerations that may particularly apply to paraneoplastic pemphigus, in which eruptions might have a diverse morphology and the clinical phenotype might depend on the predominance of antibody-mediated versus cell-mediated cytotoxicity8.
Box 2. Differential diagnoses of pemphigus.
Autoimmune diseases: bullous pemphigoid, mucous membrane pemphigoid, lichen planus pemphigoides, anti-p200 pemphigoid, epidermolysis bullosa acquisita, linear IgA bullous dermatosis and bullous lupus erythematosus
Infectious diseases: staphylococcal scalded skin syndrome, bullous impetigo and acute herpetic stomatitis
Genetic diseases: Hailey–Hailey disease
Others: aphthous stomatitis, erythema multiforme, Stevens–Johnson syndrome, toxic epidermal necrolysis, severe drug eruption, lichen planus, graft-versus-host disease, Grover disease (transient acantholytic dermatosis), seborrheic dermatitis and subcorneal pustular dermatosis
Management
Most therapies aim to improve symptoms through the reduction of serum autoantibodies, either directly or through generalized immune suppression. Published guidelines for pemphigus therapy mostly rely on expert consensus129–131, given the paucity of randomized clinical trials with large sample sizes and rigorous randomization methods132.
The goal for the initial phase of therapy is disease control, which means preventing the formation of new blisters and starting the healing process of the existing ones. The initial phase ends when no new blisters appear for 2 weeks and most existing lesions have healed (disease control). This moment usually marks a change in the therapeutic regimen and the end of the consolidation phase of therapy.
Generally, patients with pemphigus vulgaris and pemphigus foliaceus respond to the same types of therapy as outlined below. However, patients with paraneoplastic pemphigus are notoriously refractory to therapy. Therapy for paraneoplastic pemphigus aims to ameliorate both T cell-mediated and B cell-mediated attack on epithelial tissues, although combined T cell and B cell immunosuppression is associated with a high risk of infections. Anecdotal evidence supports the use of corticosteroids133, cyclophosphamide134–136, plasmapheresis137,138, rituximab139,140, cyclosporine141,142, rituximab plus daclizumab49, and alemtuzumab143,144.
Systemic corticosteroids
The first-line treatment during the initial phase of therapy is corticosteroids, owing to their rapid effect (within days). Split-dose regimens (two to three times daily) have not been directly compared with once-daily regimens in pemphigus trials, but are anecdotally associated with better therapeutic effect in refractory cases, at the potential expense of greater adrenal suppression. Japanese guidelines recommend short-term high-dose intravenous methylprednisolone in refractory cases, an approach that is supported by retrospective and open-label studies145,146. Notably, corticosteroids can improve disease within days despite the same titre of circulating autoantibodies. The rapid therapeutic effect of corticosteroids is attributed to increased transcription of desmogleins and other cell adhesion molecules, which counteracts the autoantibody-induced interference with desmoglein adhesive function147. Accordingly, topical and intralesional corticosteroids can be used as adjunctive therapy or even monotherapy in localized mild disease.
Osteoporosis counselling should be provided if corticosteroid treatment is anticipated to last >3 months148. Pneumocystis prophylaxis and tuberculosis screening should be considered for patients who will receive high doses of corticosteroids together with another immunosuppressive drug for >1 month149,150. Coordination among all care providers is advised for monitoring of other complications of corticosteroid therapy.
At the end of the consolidation phase of therapy, most clinicians begin to taper steroids. Approximately half of patients will relapse during steroid taper, whereas half will achieve complete remission off therapy after a mean treatment duration of 3 years151. Steroid-sparing immunosuppressive drugs are prescribed if patients relapse during this phase, and they can also be used at the initial phase of therapy in patients with severe disease who will probably require adjunctive treatments or who have increased risk of corticosteroid adverse effects.
Mycophenolate mofetil and azathioprine
The steroid-sparing agents mycophenolate mofetil and azathioprine are considered to be first-line adjunctive immunosuppressive therapies in pemphigus and demonstrate approximately comparable safety and efficacy152,153. Mycophenolate mofetil has shown faster and more-durable treatment responses than placebo when added to prednisone regimens154; however, these results were not reproduced in another randomized trial155. The steroid-sparing effect of azathioprine was independently confirmed156.
Azathioprine can cause potentially life-threatening bone marrow suppression. Sensitivity of the patient to azathioprine can be estimated by measuring the enzymatic activity of thiopurine methyltransferase, the protein that inactivates purine analogues such as azathioprine (BOX 3).
Box 3. Genetic susceptibility to azathioprine toxicity.
The levels of thiopurine methyltransferase (TPMT) are intermediate in 10–11% of individuals of Caucasian ethnicity, and 0.3–0.6% of individuals have TPMT deficiency217. Studies have shown a 9-fold reduction of haematological adverse events in the 10% of individuals with low-activity TPMT who receive reduced doses of azathioprine; thus, the US FDA and European pemphigus guidelines recommend TPMT testing before azathioprine therapy218,219. However, genetic susceptibility to azathioprine toxicity differs in Asian individuals; mutations in the gene encoding nucleotide triphosphate diphosphatase NUDT15 occurred in 89% of Korean patients treated with azathioprine who developed early-onset leukopenia, whereas only 1% of patients demonstrated TPMT variants220. NUDT15 mutations are also associated with azathioprine sensitivity in Guatemalan and white populations, albeit at lower frequencies220. Furthermore, azathioprine toxicity from ITPA (which encodes inosine triphosphate pyrophosphatase) polymorphisms can occur in Caucasian populations. Ultimately, pharmacogenetic risk profiling may screen for all known genetic determinants of azathioprine toxicity, although commercial testing is currently only available for TPMT.
Mycophenolate mofetil is not recommended during pregnancy owing to the risk of teratogenicity. Azathioprine has been associated with increased risk of lymphoma157,158. Azathioprine is generally preferred for patients with renal failure, because the active moiety of mycophenolate mofetil, mycophenolic acid, is not cleared by haemodialysis and leads to drug intolerance159. Weight-based dosing of mycophenolate mofetil should be considered, particularly for patients at the extremes of the weight range160.
Rituximab
Rituximab is a monoclonal anti-CD20 antibody that targets CD20+ B cells. A multicentre, prospective, randomized trial of rituximab as a first-line therapy for pemphigus was recently published161, resulting in designation of rituximab as a US FDA Breakthrough Therapy. Previously, prospective, open-label trials of rituximab (with or without intravenous immunoglobulin)92,162–164 and meta-analyses165,166 have supported the remarkable efficacy of rituximab in pemphigus, with 59–100% of patients achieving complete clinical remission after treatment, a mean time to clinical remission of 3–6 months and a median remission duration of 15–19 months. Rituximab therapy early in the disease course might be associated with better clinical response92,167. Relapse rates after rituximab therapy range between 40% and 81%92,166 and generally increase with the length of the follow-up. During long-term follow-up, 35–45% of patients with pemphigus treated with rituximab remain in complete clinical disease remission when off systemic therapy92,165,166, with rates as high as 100% in a small study of rituximab and intravenous immunoglobulin as adjunctive treatments164.
Dosing regimens used to treat lymphoma and rheumatoid arthritis have shown comparable rates of complete clinical remission, with the lymphoma protocol showing slightly higher rates of complete remission, shorter time to disease control and longer duration of remission166. High-dose rituximab was associated with a longer duration of complete clinical remission than low-dose rituximab (17 and 9 months, respectively)166. Longitudinal analysis in patients with pemphigus who received rituximab has shown that relapse is associated with the same anti-desmoglein B cells observed during active disease, indicating that relapse is probably caused by incomplete depletion of the autoreactive clones, whereas anti-desmoglein B cells were undetectable in patients in long-term remission after rituximab89,92. Relapse might also occur due to the production of pathogenic antibodies by long-lived CD20− B cells that are not targeted by rituximab. Regularly scheduled low-dose infusions have been suggested as maintenance therapy to prevent disease relapse92,161, although the relative safety and efficacy of such regimens have not yet been tested in clinical trials. The overall data indicate that a rituximab regimen that aims for complete B cell depletion offers the highest chance for long-term clinical disease remission.
Resistance to rituximab efficacy
Resistance to rituximab therapy can occur owing to either genetic polymorphisms (BOX 4) or the development of human anti-chimeric antibodies against the murine fragment of rituximab that prevent the drug from binding to B cells. In pemphigus, human anti-chimeric antibodies were associated with adverse reactions to rituximab infusions and poor treatment response168. Human anti-chimeric antibodies rarely occur in patients with cancer who were treated with the rituximab dosing regimen used for lymphoma, but were observed in 11% of patients with rheumatoid arthritis who received the corresponding dosing regimen169. As there are no commercially available tests for polymorphisms in FCGR3A (which encodes Fc fragment of IgG receptor IIIa) or human anti-chimeric antibodies, it follows that the dosing regimen used for lymphoma — by increasing the serum levels of rituximab and reducing the risk of human anti-chimeric antibodies — should optimize B cell depletion in most individuals.
Box 4. Genetic susceptibility to rituximab.
The 158FF variant of the Fc fragment of IgG receptor IIIa gene (FCGR3A), a relatively common low-affinity variant found in 44% of patients with systemic lupus erythematosus and 26% of healthy control individuals221, causes decreased antibody-dependent cellular cytotoxicity leading to inefficient rituximab-mediated B cell killing222. Patients with systemic lupus erythematosus who are homozygous for the low-affinity F allele require 10-fold higher serum rituximab levels to achieve the same level of B cell depletion as patients with the high-affinity 158VV genotype223. Similar findings have been reported in patients with lymphoma treated with rituximab224, although the presence of FCGR3A polymorphisms might not always affect rituximab efficacy225,226.
Adverse effects
Rituximab therapy carries the risk of developing serious adverse events. In a meta-analysis of 153 patients with pemphigus who received rituximab, infections occurred in 11 (7.2%) patients and 2 (1.3%) cases were fatal; hypogammaglobulinaemia was observed in 3 (2%) patients165. Similar results were observed in a German registry of 370 patients with an autoimmune disease who were treated with rituximab, which documented a 1.9% rate of fatal infection170,171. Administration of intravenous immunoglobulin in addition to rituximab might prevent infectious adverse events163, although comparative studies would be necessary to determine the safety and efficacy of this combination therapy compared with rituximab alone. Patients should be screened for hepatitis B virus infection; those who test positive should be considered for prophylactic tenofovir therapy and regularly screened again for 6–12 months after rituximab therapy.
Intravenous immunoglobulin
The advantage of intravenous immunoglobulin as an adjunctive therapy for pemphigus is that it is not immunosuppressive. Intravenous immunoglobulin is obtained by pooling human plasma and typically contains ≥95% IgG antibodies and only trace amounts of IgA or IgM. Intravenous immunoglobulin has several proposed mechanisms of action in autoantibody-mediated diseases: it can saturate IgG receptor FcRn large subunit p51 (FcRn, also known as neonatal Fc receptor) leading to the catabolism of serum autoantibodies172 and inhibition of antigen presentation173,174, and can also upregulate inhibitory immunoglobulin gamma Fc receptors via a subset of Fc-sialylated IgG, which in turn mitigates autoantibody-induced inflammation175. The efficacy of intravenous immunoglobulin in pemphigus was evaluated in a randomized, double-blind, placebo-controlled trial, in which time to escape from protocol (that is, the duration of the treatment before another additional treatment is required) was significantly prolonged in the patients who received intravenous immunoglobulin compared with those who received placebo176. Baseline creatinine and IgA levels should be determined before starting intravenous immunoglobulin therapy; IgA-depleted formulations can be safely administered in cases of IgA deficiency177.
Immunoglobulin therapy is not without risks. Thrombotic complications178 and aseptic meningitis can occur, the latter manifesting as severe headache 2–7 days after the last immunoglobulin infusion, sometimes accompanied by fever, vomiting and photophobia. Studies of intravenous immunoglobulin in patients with immunodeficiency have reported no increased risk of thrombotic complications179, although these patients received a lower dose than the high-dose regimens used in pemphigus. The risk of complications should be discussed, particularly with patients with underlying risk factors, and can be reduced through slow infusion rates, hydration and lower doses or increased time between cycles.
Plasmapheresis and immunoadsorption
Plasmapheresis, involving plasma exchange with albumin or fresh frozen plasma, or extracorporeal immunoadsorption with protein A (a staphylococcal cell wall component that binds IgG) to selectively remove serum IgG antibodies can be effective in severe or refractory pemphigus. IgG has a 3-week serum half-life, a property that contributes to the 3-month delay to treatment response in most pemphigus therapies. Although one randomized controlled study found plasma exchange ineffective180, small case series of plasmapheresis or immunoadsorption have shown a rapid reduction in the serum levels of pemphigus autoantibodies and short time to disease control181–183. Thus, a rational therapy for severe pemphigus would be to combine plasmapheresis or immunoadsorption (for immediate reduction in the levels of pathogenic autoantibodies) with a subsequent rituximab regimen (for long-term disease control), although this approach has not yet been tested in randomized clinical trials. Additional treatment options are listed in BOX 5.
Box 5. Other adjunctive therapies.
Dapsone (an antibiotic with anti-inflammatory effects) has been used as a steroid-sparing agent227 and offers the additional benefit of pneumocystis prophylaxis. Glucose-6-phosphate 1-dehydrogenase (G6PD) activity screening should be performed before dapsone therapy to avoid haemolytic adverse events, particularly in individuals of African and Mediterranean descent, in whom the frequencies of mutations in G6PD can be as high as 11–20%228. Although G6PD is X-linked, women who are heterozygous for G6PD mutations can demonstrate wide phenotypic variability owing to random X-inactivation in erythrocytes228. Methotrexate (a chemotherapeutic drug) is another steroid-sparing agent, although only small case series support its efficacy in pemphigus229. Small case series support the efficacy of cyclosporine (a T cell inhibitor) in pemphigus230,231, although larger clinical trials have suggested inferior to no benefit and a higher number of adverse effects in the treatment group232,233. Both oral and intravenous cyclophosphamide are effective in pemphigus153,234; however, given its high toxicity, including risk of bladder cancer and infertility, cases that might benefit from cyclophosphamide need to be carefully considered.
Quality of life
Prognosis and comorbidities
Pemphigus vulgaris and pemphigus foliaceus are associated with considerable morbidity and mortality. Mortality has considerably decreased to 1.6–12% since the regular use of corticosteroids and adjuvant immunosuppressants was introduced, but is approximately 2–3-fold higher in patients with pemphigus than in the general population184–186. The most frequently reported causes of death include respiratory tract infections, septicaemia, cardiovascular disease and peptic ulcer disease. Besides corticosteroid-related comorbidities (for example, infections, Cushing syndrome (hypercortisolism), adrenal insufficiency and osteoporosis)186, pemphigus has been correlated to other autoimmune diseases, such as autoimmune thyroid diseases (such as Graves disease and Hashimoto thyroiditis), rheumatoid arthritis and type 1 diabetes mellitus187,188.
The prognosis of paraneoplastic pemphigus is poor: mortality is as high as 90%, with 1-year, 2-year and 5-year overall survival rates of 49, 41 and 38%, respectively189. The disease is often fatal owing to the presence of underlying neoplasms, such as non-Hodgkin lymphoma, chronic lymphocytic leukaemia (both of which account for about two-thirds of paraneoplastic pemphigus-associated neoplasms in Europe, Japan and the United States), Castleman disease (the most common paraneoplastic pemphigus-associated neoplasia in Chinese and paediatric patients), thymoma, epithelial carcinoma or other malignancies8, but mortality might also result from associated infections or bronchiolitis obliterans.
Disease measures
Pemphigus burden can result in substantially detrimental effects on quality of life, owing to its severity with an unpredictable, variable and potentially life-threatening course. A systematic review of outcome measures in pemphigus highlighted the heterogeneity of the outcome measures used across different studies190, an observation that led to the development of standardized scoring systems that are pivotal to accurately monitor changes in the severity of the disease and the associated effects on the quality of life of the patient191.
Two indices for pemphigus disease activity have gained widespread international acceptance191: the Pemphigus Disease Area Index (PDAI) and the Autoimmune Bullous Skin Disorder Intensity Score (ABSIS)192,193. The PDAI is preferable as a severity measure, with good reliability, validity, responsiveness to change and accuracy192,193. The PDAI has a scoring system ranging from 0 to 263, with 250 points measuring disease activity (the number and maximum size of the lesions on the skin, scalp and mucosa) and the remaining 13 points measuring post-inflammatory lesions. The main advantage of the PDAI is its sensitivity to low numbers of lesions, which improves the consistency of score values assessed by different operators192. Unlike the PDAI, the ABSIS sets body surface area and lesion type as scoring variables, an approach that can amplify small differences between scores measured by different users, and also includes patients’ subjective symptoms concerning discomfort experienced while eating and drinking193. Cut-off values for both indices have been defined to identify different pemphigus disease severity subgroups194,195.
Measures of quality of life
The WHO defines the assessment of quality of life as the “individuals’ perception of their position in life in the context of the culture and values systems in which they live and in relation to their goals, expectations, standards, and concerns” (REF. 196). Integrating measures of quality of life into the clinical evaluation of patients with pemphigus facilitates the assessment of disease severity and can capture treatment outcomes that are relevant to the patient191. A systematic review and meta-analysis of 16 studies from 8 different countries reported a severely impaired health-related quality of life in patients with pemphigus that was determined by 41 possible factors. Higher disease severity, anxiety and depression were clearly associated with reduced quality of life, whereas there was no clear effect of age, sex, pemphigus type, disease duration, mucocutaneous involvement, clinical activity, itching, skin burning or treatment with adjuvant drugs197. Quality of life can be affected even by quiescent disease, as over 20% of patients with quiescent disease believed that they “often/always suffered from pemphigus” (REF. 198).
The great heterogeneity in the generic or dermatology-specific quality-of-life scores used in the 16 studies that were analysed highlighted the need for standardized assessment of quality of life in pemphigus197. Two disease-specific questionnaires, each containing 17 quality-of-life-related parameters, have been developed: Autoimmune Bullous Disease Quality of Life (ABQOL) and Treatment of Autoimmune Bullous Disease Quality of Life (TABQOL). Both indices are reliable and valid tools to measure quality of life for patients with autoimmune bullous diseases including pemphigus, but further validation studies in different populations and cultures are ongoing199–201.
Outlook
Current research in pemphigus addresses a broad range of topics in both the basic science of autoimmunity and cell adhesion and the translational and clinical research.
Basic research
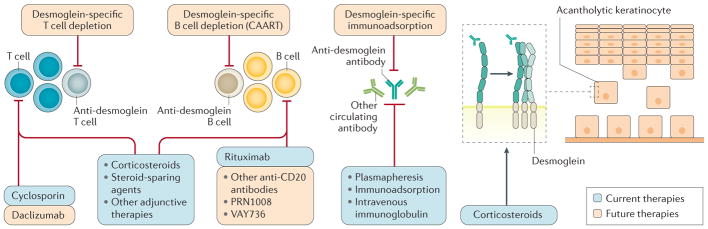
Pemphigus is one of the best-characterized human autoimmune diseases, particularly regarding the pathogenic contributions of B cell and T cell populations. Active areas of research focus on detailed characterization of desmoglein-reactive B cell lineages and their crossreactivity and specificity23,88, genetic susceptibility and gene expression profiles30,202, and immunological mechanisms in novel animal models48,203,204, among other topics. These studies have created translational research opportunities to develop novel strategies for autoimmune disease therapy (FIG. 7). Several approaches for inducing antigen-specific tolerance could be evaluated in pemphigus, including autoantigen vaccination205 and antigen-coupled cell tolerance206 to induce desmoglein-specific tolerance, the generation of antigen-specific T regulatory cells207 to suppress anti-desmoglein autoimmunity, and chimeric autoantibody receptor T cells (CAARTs)105 to eliminate desmoglein-specific B cells.
Figure 7. Current and future therapeutic strategies for pemphigus.
Current and future therapeutic strategies for pemphigus are outlined based on their mechanisms of action. Whereas conventional treatments such as systemic steroid and immunosuppressive agents affect broad ranges of cells and tissues, antigen-specific treatments targeted against desmoglein-reactive cells or IgG antibodies provide more-tailored and potentially safer strategies. One of these approaches uses T cells that are engineered to express a chimeric immunoreceptor consisting of the desmoglein 3 extracellular domain fused to the T cell receptor cytoplasmic signalling and co-stimulatory domains105. These desmoglein 3 chimeric autoantibody receptor T cells (CAARTs) specifically bind to and kill anti-desmoglein 3 B cells, leading to disease remission in a pemphigus mouse model. Owing to the potential for generation of long-term memory CAARTs, this technology offers the potential for long-term disease remission without the immunosuppressive effects from global B cell depletion.
Samples from patients with pemphigus provide a unique biological tool to investigate the mechanisms of epidermal cell adhesion, which in turn can lead to novel strategies for adjunctive therapy through inhibition of end-organ pathology. Advances in elucidating the crystal structure of desmocollins and desmogleins58 and in high-resolution imaging of skin from patients with pemphigus66,208 can further our understanding of the pathophysiological regulation of keratinocyte cell adhesion.
Clinical research
Several novel B cell-depleting agents are under investigation as pemphigus therapy. Veltuzumab, a humanized anti-CD20 monoclonal antibody, has shown promise in refractory pemphigus and has received FDA orphan drug status for this indication. A single cycle of subcutaneous veltuzumab in a patient previously refractory to prednisone, azathioprine, mycophenolate mofetil and rituximab, resulted in complete clinical remission of disease while off therapy209. Rituximab (NCT02383589)210, as well as next-generation CD20-targeted agents211, including fully human or glycoengineered antibodies with greater affinity for immunoglobulin gamma Fc receptors, are currently in trials for patients with B cell cancers and hold promise as future options for pemphigus therapy212. PRN1008, an inhibitor of tyrosine-protein kinase BTK (which is required for B cell maturation), is under evaluation in a phase II trial in pemphigus (NCT02704429)213. Covalent binding of PRN1008 to its target kinase selectively concentrates the drug in B cells; this pharmacodynamic profile has the advantage of achieving effective B cell depletion with minimal plasma trough concentrations214. VAY736 is a fully human IgG1 monoclonal antibody that targets the TNF receptor superfamily member 13C (also known as B cell-activating factor receptor). This receptor is expressed on most B cell subsets and promotes the survival of naive B cells and plasmablasts (which are short-lived antibody-secreting cells)215, suggesting that VAY736 could have a broader range of effects on B cell depletion and plasmablast survival than CD20-targeted therapies. Results from a phase II study of VAY736 in pemphigus are pending (NCT01930175)216.
Clinical research has also actively focused on developing instruments for measurement of disease activity and quality of life192–195,199. Studies are ongoing to determine the minimal change in disease activity that constitutes a clinically meaningful difference, which could ultimately be an end point in pemphigus clinical trials.
Acknowledgments
The authors thank R. Eming and M. Hertl (Philipps University of Marburg, Germany) for kindly providing the histology image in Figure 5c.
Footnotes
Author contributions
Introduction (M.A.); Epidemiology (M.K. and D.Z.); Mechanisms/pathophysiology (M.A., C.T.E., H.T., J.Y. and A.S.P.); Diagnosis, screening and prevention (M.K. and D.Z.); Management (C.T.E. and A.S.P.); Quality of life (M.K. and D.Z.); Outlook (C.T.E. and A.S.P.); Overview of the Primer (M.A.). M.K., C.T.E. and H.T. contributed equally to this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Competing interests
M.A. receives speaker honoraria and grants from Nihon Pharmaceutical, research support from Medical & Biological Laboratories, Health Sciences Research Grants for Research on Rare and Intractable Diseases from Ministry of Health, Labour, and Welfare, and grants from Japan Society for the Promotion of Science and Agency for Medical Research and Development. H.T. receives grants from Japan Society for the Promotion of Science. A.S.P. is a consultant for Syntimmune and TG Therapeutics and receives grants or research support from the US NIH (R01-AR057001, R56-AR064220 and R01-068288), the Dermatology Foundation and Sanofi. She is co-inventor on a patent related to chimeric immunoreceptor therapy of pemphigus. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US NIH. C.T.E. receives grants or research support from Deutsche Forschungsgemeinschaft (EL711/1-1) and is co-inventor on a patent related to chimeric immunoreceptor therapy of pemphigus. D.Z. is an advisory board member for Roche Pharma, and a consultant for Euroimmun, Almirall, UCB, Fresenius and arGEN-X. He received speakers honoraria and/or travel/accommodations/meeting compensation from Biotest, Fresenius, Miltenyi, Roche Pharma, Biogen Idec, AbbVie, UCB, Janssen and grants or research support from Euroimmun, Miltenyi, Fresenius, Biotest, Dompe, Almirall, Biogen and Roche. He holds the patent Euroimmun: DE 10 2006 059 574 A1. M.K. and J.Y. declare no competing interests.
References
- 1.Amagai M. In: Dermatology. Bolognia JL, Jorizzo JL, Schaffer JV, editors. Vol. 1. Mosby; 2012. pp. 461–474. Ch. 29. [Google Scholar]
- 2.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-b. This study reports the cDNA cloning of a pemphigus vulgaris antigen, which turned out to be a desmosomal cadherin called desmoglein 3. [DOI] [PubMed] [Google Scholar]
- 3.Koch PJ, et al. Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol. 1990;53:1–12. [PubMed] [Google Scholar]
- 4.Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med. 2006;355:1800–1810. doi: 10.1056/NEJMra061111. [DOI] [PubMed] [Google Scholar]
- 5.Anhalt GJ, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323:1729–1735. doi: 10.1056/NEJM199012203232503. [DOI] [PubMed] [Google Scholar]
- 6.Alpsoy E, Akman-Karakas A, Uzun S. Geographic variations in epidemiology of two autoimmune bullous diseases: pemphigus and bullous pemphigoid. Arch Dermatol Res. 2015;307:291–298. doi: 10.1007/s00403-014-1531-1. [DOI] [PubMed] [Google Scholar]
- 7.Shah AA, et al. Development of a disease registry for autoimmune bullous diseases: initial analysis of the pemphigus vulgaris subset. Acta Derm Venereol. 2015;95:86–90. doi: 10.2340/00015555-1854. [DOI] [PubMed] [Google Scholar]
- 8.Yong AA, Tey HL. Paraneoplastic pemphigus. Australas J Dermatol. 2013;54:241–250. doi: 10.1111/j.1440-0960.2012.00921.x. [DOI] [PubMed] [Google Scholar]
- 9.Anhalt GJ. Paraneoplastic pemphigus. Adv Dermatol. 1997;12:77–96. [PubMed] [Google Scholar]
- 10.Anhalt GJ. Paraneoplastic pemphigus. J Investig Dermatol Symp Proc. 2004;9:29–33. doi: 10.1111/j.1087-0024.2004.00832.x. [DOI] [PubMed] [Google Scholar]
- 11.Meyer N, Misery L. Geoepidemiologic considerations of auto-immune pemphigus. Autoimmun Rev. 2010;9:A379–A382. doi: 10.1016/j.autrev.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Bertram F, Brocker EB, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J Dtsch Dermatol Ges. 2009;7:434–440. doi: 10.1111/j.1610-0387.2008.06976.x. This study reports the lowest known incidence of patients with pemphigus. [DOI] [PubMed] [Google Scholar]
- 13.Michailidou EZ, et al. Epidemiologic survey of pemphigus vulgaris with oral manifestations in northern Greece: retrospective study of 129 patients. Int J Dermatol. 2007;46:356–361. doi: 10.1111/j.1365-4632.2006.03044.x. [DOI] [PubMed] [Google Scholar]
- 14.Tallab T, et al. The incidence of pemphigus in the southern region of Saudi Arabia. Int J Dermatol. 2001;40:570–572. doi: 10.1046/j.1365-4362.2001.01247.x. [DOI] [PubMed] [Google Scholar]
- 15.Pisanti S, Sharav Y, Kaufman E, Posner LN. Pemphigus vulgaris: incidence in Jews of different ethnic groups, according to age, sex, and initial lesion. Oral Surg Oral Med Oral Pathol. 1974;38:382–387. doi: 10.1016/0030-4220(74)90365-x. [DOI] [PubMed] [Google Scholar]
- 16.Asilian A, Yoosefi A, Faghini G. Pemphigus vulgaris in Iran: epidemiology and clinical profile. Skimmed. 2006;5:69–71. doi: 10.1111/j.1540-9740.2006.03756.x. [DOI] [PubMed] [Google Scholar]
- 17.Simon DG, Krutchkoff D, Kaslow RA, Zarbo R. Pemphigus in Hartford County, Connecticut, from 1972 to 1977. Arch Dermatol. 1980;116:1035–1037. [PubMed] [Google Scholar]
- 18.Culton DA, et al. Advances in pemphigus and its endemic pemphigus foliaceus (Fogo Selvagem) phenotype: a paradigm of human autoimmunity. J Autoimmun. 2008;31:311–324. doi: 10.1016/j.jaut.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaraa I, et al. Spectrum of autoimmune blistering dermatoses in Tunisia: an 11-year study and a review of the literature. Int J Dermatol. 2011;50:939–944. doi: 10.1111/j.1365-4632.2010.04801.x. [DOI] [PubMed] [Google Scholar]
- 20.Bastuji-Garin S, et al. Comparative epidemiology of pemphigus in Tunisia and France. Incidence of foliaceus pemphigus in young Tunisian women. Ann Dermatol Venereol. 1996;123:337–342. (in French) [PubMed] [Google Scholar]
- 21.Qian Y, et al. Cutting edge: Brazilian pemphigus foliaceus anti-desmoglein 1 autoantibodies cross-react with sand fly salivary LJM11 antigen. J Immunol. 2012;189:1535–1539. doi: 10.4049/jimmunol.1200842. This paper shows that antibodies to sand fly salivary antigens crossreact with human desmoglein 1 and, therefore, may be a trigger for endemic pemphigus foliaceus, fogo selvagem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian Y, et al. IgE anti-LJM11 sand fly salivary antigen may herald the onset of fogo selvagem in endemic Brazilian regions. J Invest Dermatol. 2015;135:913–915. doi: 10.1038/jid.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian Y, et al. Overlapping IgG4 responses to self- and environmental antigens in endemic pemphigus foliaceus. J Immunol. 2016;196:2041–2050. doi: 10.4049/jimmunol.1502233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha AA. The genetics of pemphigus. Dermatol Clin. 2011;29:381–391. doi: 10.1016/j.det.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Torzecka JD, et al. Circulating pemphigus autoantibodies in healthy relatives of pemphigus patients: coincidental phenomenon with a risk of disease development? Arch Dermatol Res. 2007;299:239–243. doi: 10.1007/s00403-007-0760-y. [DOI] [PubMed] [Google Scholar]
- 26.Yan L, Wang JM, Zeng K. Association between HLA-DRB1 polymorphisms and pemphigus vulgaris: a meta-analysis. Br J Dermatol. 2012;167:768–777. doi: 10.1111/j.1365-2133.2012.11040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee E, et al. Disease relevant HLA class II alleles isolated by genotypic, haplotypic, and sequence analysis in North American Caucasians with pemphigus vulgaris. Hum Immunol. 2006;67:125–139. doi: 10.1016/j.humimm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Bhanusali DG, et al. HLA-E*0103X is associated with susceptibility to pemphigus vulgaris. Exp Dermatol. 2013;22:108–112. doi: 10.1111/exd.12077. [DOI] [PubMed] [Google Scholar]
- 29.Sarig O, et al. Population-specific association between a polymorphic variant in ST18, encoding a pro-apoptotic molecule, and pemphigus vulgaris. J Invest Dermatol. 2012;132:1798–1805. doi: 10.1038/jid.2012.46. This is the first genome-wide association study in pemphigus vulgaris, which implicates the HLA region as well as ST18 as genomic susceptibility loci. [DOI] [PubMed] [Google Scholar]
- 30.Vodo D, et al. Identification of a functional risk variant for pemphigus vulgaris in the ST18 gene. PLoS Genet. 2016;12:e1006008. doi: 10.1371/journal.pgen.1006008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sajda T, et al. Multiplexed autoantigen microarrays identify HLA as a key driver of anti-desmoglein and -non-desmoglein reactivities in pemphigus. Proc Natl Acad Sci USA. 2016;113:1859–1864. doi: 10.1073/pnas.1525448113. References 30 and 31 provide novel important insights into the complex relationship between genetics and disease development in pemphigus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah AA, Dey-Rao R, Seiffert-Sinha K, Sinha AA. Increased oxidative stress in pemphigus vulgaris is related to disease activity and HLA-association. Autoimmunity. 2016;49:248–257. doi: 10.3109/08916934.2016.1145675. [DOI] [PubMed] [Google Scholar]
- 33.Moraes ME, et al. An epitope in the third hypervariable region of the DRB1 gene is involved in the susceptibility to endemic pemphigus foliaceus (fogo selvagem) in three different Brazilian populations. Tissue Antigens. 1997;49:35–40. doi: 10.1111/j.1399-0039.1997.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 34.Martel P, et al. Paraneoplastic pemphigus is associated with the DRB1*03 allele. J Autoimmun. 2003;20:91–95. doi: 10.1016/s0896-8411(02)00092-6. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Bu DF, Li D, Zhu XJ. Genotyping of HLA-I and HLA-II alleles in Chinese patients with paraneoplastic pemphigus. Br J Dermatol. 2008;158:587–591. doi: 10.1111/j.1365-2133.2007.08361.x. [DOI] [PubMed] [Google Scholar]
- 36.Ruocco V, et al. Pemphigus: etiology, pathogenesis, and inducing or triggering factors: facts and controversies. Clin Dermatol. 2013;31:374–381. doi: 10.1016/j.clindermatol.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Amagai M. Desmoglein as a target in autoimmunity and infection. J Am Acad Dermatol. 2003;48:244–252. doi: 10.1067/mjd.2003.7. [DOI] [PubMed] [Google Scholar]
- 38.Amagai M. Autoimmunity against desmosomal cadherins in pemphigus. J Dermatol Sci. 1999;20:92–102. doi: 10.1016/s0923-1811(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 39.Mahoney MG, et al. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103:461–468. doi: 10.1172/JCI5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amagai M, Matsuyoshi N, Wang ZH, Andl C, Stanley JR. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat Med. 2000;6:1275–1277. doi: 10.1038/81385. This study identifies the target molecule of the exfoliative toxin produced by S. aureus as desmoglein 1, the autoantigen of pemphigus foliaceus. [DOI] [PubMed] [Google Scholar]
- 41.Mao X, et al. Autoimmunity to desmocollin 3 in pemphigus vulgaris. Am J Pathol. 2010;177:2724–2730. doi: 10.2353/ajpath.2010.100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafei D, et al. IgG autoantibodies against desmocollin 3 in pemphigus sera induce loss of keratinocyte adhesion. Am J Pathol. 2011;178:718–723. doi: 10.1016/j.ajpath.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen VT, Ndoye A, Shultz LD, Pittelkow MR, Grando SA. Antibodies against keratinocyte antigens other than desmoglein 1 and 3 can induce pemphigus vulgaris-like lesions. J Clin Invest. 2000;106:1467–1479. doi: 10.1172/JCI10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalantari-Dehaghi M, et al. Pemphigus vulgaris autoantibody profiling by proteomic technique. PLoS ONE. 2013;8:e57587. doi: 10.1371/journal.pone.0057587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed AR, et al. Monopathogenic versus multipathogenic explanations of pemphigus pathophysiology. Exp Dermatol. 2016;25:839–846. doi: 10.1111/exd.13106. [DOI] [PubMed] [Google Scholar]
- 46.Amagai M, Nishikawa T, Nousari HC, Anhalt GJ, Hashimoto T. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J Clin Invest. 1998;102:775–782. doi: 10.1172/JCI3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schepens I, et al. The protease inhibitor alpha-2-macroglobulin-like-1 is the p170 antigen recognized by paraneoplastic pemphigus autoantibodies in human. PLoS ONE. 2010;5:e12250. doi: 10.1371/journal.pone.0012250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi H, et al. Desmoglein 3-specific CD4+ T cells induce pemphigus vulgaris and interface dermatitis in mice. J Clin Invest. 2011;121:3677–3688. doi: 10.1172/JCI57379. This study reports that desmoglein 3-specific CD4+ T cells not only help B cells to produce pathogenic autoantibodies but also induce interface dermatitis by cellular autoimmunity, as found in paraneoplastic pemphigus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cummins DL, et al. Lichenoid paraneoplastic pemphigus in the absence of detectable antibodies. J Am Acad Dermatol. 2007;56:153–159. doi: 10.1016/j.jaad.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Anhalt GJ, et al. Defining the role of complement in experimental pemphigus vulgaris in mice. J Immunol. 1986;137:2835–2840. [PubMed] [Google Scholar]
- 51.Mascaro JM, Jr, et al. Mechanisms of acantholysis in pemphigus vulgaris: role of IgG valence. Clin Immunol Immunopathol. 1997;85:90–96. doi: 10.1006/clin.1997.4408. [DOI] [PubMed] [Google Scholar]
- 52.Rock B, Labib RS, Diaz LA. Monovalent Fab’ immunoglobulin fragments from endemic pemphigus foliaceus autoantibodies reproduce the human disease in neonatal Balb/c mice. J Clin Invest. 1990;85:296–299. doi: 10.1172/JCI114426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rock B, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–1469. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 54.Futei Y, et al. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. J Dermatol Sci. 2001;26:55–61. doi: 10.1016/s0923-1811(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 55.Funakoshi T, et al. Enrichment of total serum IgG4 in patients with pemphigus. Br J Dermatol. 2012;167:1245–1253. doi: 10.1111/j.1365-2133.2012.11144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2002;105:9–19. doi: 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boggon TJ, et al. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 58.Harrison OJ, et al. Structural basis of adhesive binding by desmocollins and desmogleins. Proc Natl Acad Sci USA. 2016;113:7160–7165. doi: 10.1073/pnas.1606272113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsunoda K, et al. Induction of pemphigus phenotype by a mouse monoclonal antibody against the amino-terminal adhesive interface of desmoglein 3. J Immunol. 2003;170:2170–2178. doi: 10.4049/jimmunol.170.4.2170. [DOI] [PubMed] [Google Scholar]
- 60.Di Zenzo G, et al. Pemphigus autoantibodies generated through somatic mutations target the desmoglein-3 cis-interface. J Clin Invest. 2012;122:3781–3790. doi: 10.1172/JCI64413. This paper demonstrates that human anti-desmoglein 3 antibodies binding to the cis-adhesive desmoglein 3 interface can induce acantholysis, providing support for the steric hindrance model of pemphigus pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heupel WM, Zillikens D, Drenckhahn D, Waschke J. Pemphigus vulgaris IgG directly inhibit desmoglein 3-mediated transinteraction. J Immunol. 2008;181:1825–1834. doi: 10.4049/jimmunol.181.3.1825. [DOI] [PubMed] [Google Scholar]
- 62.Sato M, Aoyama Y, Kitajima Y. Assembly pathway of desmoglein 3 to desmosomes and its perturbation by pemphigus vulgaris-IgG in cultured keratinocytes, as revealed by time-lapsed labeling immunoelectron microscopy. Lab Invest. 2000;80:1583–1592. doi: 10.1038/labinvest.3780168. [DOI] [PubMed] [Google Scholar]
- 63.Calkins CC, et al. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. J Biol Chem. 2006;281:7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- 64.Mao X, Choi EJ, Payne AS. Disruption of desmosome assembly by monovalent human pemphigus vulgaris monoclonal antibodies. J Invest Dermatol. 2009;129:908–918. doi: 10.1038/jid.2008.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oktarina DA, van der Wier G, Diercks GF, Jonkman MF, Pas HH. IgG-induced clustering of desmogleins 1 and 3 in skin of patients with pemphigus fits with the desmoglein nonassembly depletion hypothesis. Br J Dermatol. 2011;165:552–562. doi: 10.1111/j.1365-2133.2011.10463.x. [DOI] [PubMed] [Google Scholar]
- 66.Sokol E, et al. Large-scale electron microscopy maps of patient skin and mucosa provide insight into pathogenesis of blistering diseases. J Invest Dermatol. 2015;135:1763–1770. doi: 10.1038/jid.2015.109. [DOI] [PubMed] [Google Scholar]
- 67.Waschke J, Bruggeman P, Baumgartner W, Zillikens D, Drenckhahn D. Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction. J Clin Invest. 2005;115:3157–3165. doi: 10.1172/JCI23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jolly PS, et al. p38MAPK signaling and desmoglein-3 internalization are linked events in pemphigus acantholysis. J Biol Chem. 2010;285:8936–8941. doi: 10.1074/jbc.M109.087999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saito M, et al. Signaling dependent and independent mechanisms in pemphigus vulgaris blister formation. PLoS ONE. 2012;7:e50696. doi: 10.1371/journal.pone.0050696. This paper provides evidence that monoclonal and polyclonal antibodies contribute to acantholysis in non-redundant and complementary ways, with polyclonal serum IgG inducing signalling-dependent desmoglein clustering not observed with monoclonal antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mao X, Sano Y, Park JM, Payne AS. p38 MAPK activation is downstream of the loss of intercellular adhesion in pemphigus vulgaris. J Biol Chem. 2011;286:1283–1291. doi: 10.1074/jbc.M110.172874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mao X, et al. MAPKAP kinase 2 (MK2)-dependent and -independent models of blister formation in pemphigus vulgaris. J Invest Dermatol. 2014;134:68–76. doi: 10.1038/jid.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bektas M, Jolly PS, Berkowitz P, Amagai M, Rubenstein DS. A pathophysiologic role for epidermal growth factor receptor in pemphigus acantholysis. J Biol Chem. 2013;288:9447–9456. doi: 10.1074/jbc.M112.438010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sayar BS, et al. EGFR inhibitors erlotinib and lapatinib ameliorate epidermal blistering in pemphigus vulgaris in a non-linear, V-shaped relationship. Exp Dermatol. 2014;23:33–38. doi: 10.1111/exd.12290. [DOI] [PubMed] [Google Scholar]
- 74.Waschke J, et al. Inhibition of Rho A activity causes pemphigus skin blistering. J Cell Biol. 2006;175:721–727. doi: 10.1083/jcb.200605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williamson L, et al. Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. EMBO J. 2006;25:3298–3309. doi: 10.1038/sj.emboj.7601224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li N, Zhao M, Wang J, Liu Z, Diaz LA. Involvement of the apoptotic mechanism in pemphigus foliaceus autoimmune injury of the skin. J Immunol. 2009;182:711–717. doi: 10.4049/jimmunol.182.1.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luyet C, et al. Preclinical studies identify non-apoptotic low-level caspase-3 as therapeutic target in pemphigus vulgaris. PLoS ONE. 2015;10:e0119809. doi: 10.1371/journal.pone.0119809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, Chernyavsky A, Webber RJ, Grando SA, Wang PH. Critical role of the neonatal Fc receptor (FcRn) in the pathogenic action of antimitochondrial autoantibodies synergizing with anti-desmoglein autoantibodies in pemphigus vulgaris. J Biol Chem. 2015;290:23826–23837. doi: 10.1074/jbc.M115.668061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Payne AS, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–899. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamagami J, et al. Homologous regions of autoantibody heavy chain complementarity-determining region 3 (H-CDR3) in patients with pemphigus cause pathogenicity. J Clin Invest. 2010;120:4111–4117. doi: 10.1172/JCI44425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamiya K, et al. A higher correlation of the antibody activities against the calcium-dependent epitopes of desmoglein 3 quantified by ethylenediaminetetraacetic acid-treated enzyme-linked immunosorbent assay with clinical disease activities of pemphigus vulgaris. J Dermatol Sci. 2013;70:190–195. doi: 10.1016/j.jdermsci.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 82.Kawasaki H, et al. Synergistic pathogenic effects of combined mouse monoclonal anti-desmoglein 3 IgG antibodies on pemphigus vulgaris blister formation. J Invest Dermatol. 2006;126:2621–2630. doi: 10.1038/sj.jid.5700450. [DOI] [PubMed] [Google Scholar]
- 83.Cho MJ, et al. Shared VH1-46 gene usage by pemphigus vulgaris autoantibodies indicates common humoral immune responses among patients. Nat Commun. 2014;5:4167. doi: 10.1038/ncomms5167. This paper demonstrates that VH1-46 B cells require no or few somatic mutations to bind desmoglein 3 and may, therefore, be prone to desmoglein 3 autoreactivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saleh MA, et al. Pathogenic anti-desmoglein 3 mAbs cloned from a paraneoplastic pemphigus patient by phage display. J Invest Dermatol. 2012;132:1141–1148. doi: 10.1038/jid.2011.449. [DOI] [PubMed] [Google Scholar]
- 85.Yamagami J, et al. Antibodies to the desmoglein 1 precursor proprotein but not to the mature cell surface protein cloned from individuals without pemphigus. J Immunol. 2009;183:5615–5621. doi: 10.4049/jimmunol.0901691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qian Y, et al. Antigen selection of anti-DSG1 autoantibodies during and before the onset of endemic pemphigus foliaceus. J Invest Dermatol. 2009;129:2823–2834. doi: 10.1038/jid.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian C, et al. Immunodominance of the VH1-46 antibody gene segment in the primary repertoire of human rotavirus-specific B cells is reduced in the memory compartment through somatic mutation of nondominant clones. J Immunol. 2008;180:3279–3288. doi: 10.4049/jimmunol.180.5.3279. [DOI] [PubMed] [Google Scholar]
- 88.Cho MJ, et al. Determinants of VH1-46 cross-reactivity to pemphigus vulgaris autoantigen desmoglein 3 and rotavirus antigen VP6. J Immunol. 2016;197:1065–1073. doi: 10.4049/jimmunol.1600567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hammers CM, et al. Persistence of anti-desmoglein 3 IgG+ B-cell clones in pemphigus patients over years. J Invest Dermatol. 2015;135:742–749. doi: 10.1038/jid.2014.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan PT, et al. Immune response towards the amino-terminus of desmoglein 1 prevails across different activity stages in nonendemic pemphigus foliaceus. Br J Dermatol. 2010;162:1242–1250. doi: 10.1111/j.1365-2133.2010.09696.x. [DOI] [PubMed] [Google Scholar]
- 91.Ohyama B, et al. Epitope spreading is rarely found in pemphigus vulgaris by large-scale longitudinal study using desmoglein 2-based swapped molecules. J Invest Dermatol. 2012;132:1158–1168. doi: 10.1038/jid.2011.448. [DOI] [PubMed] [Google Scholar]
- 92.Colliou N, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30. doi: 10.1126/scitranslmed.3005166. [DOI] [PubMed] [Google Scholar]
- 93.Ahmed AR, et al. Major histocompatibility complex haplotype studies in Ashkenazi Jewish patients with pemphigus vulgaris. Proc Natl Acad Sci USA. 1990;87:7658–7662. doi: 10.1073/pnas.87.19.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyagawa S, et al. HLA-DRB1*04 and DRB1*14 alleles are associated with susceptibility to pemphigus among Japanese. J Invest Dermatol. 1997;109:615–618. doi: 10.1111/1523-1747.ep12337585. [DOI] [PubMed] [Google Scholar]
- 95.Jones CC, Hamilton RG, Jordon RE. Subclass distribution of human IgG autoantibodies in pemphigus. J Clin Immunol. 1988;8:43–49. doi: 10.1007/BF00915155. [DOI] [PubMed] [Google Scholar]
- 96.Lin MS, et al. Development and characterization of desmoglein-3 specific T cells from patients with pemphigus vulgaris. J Clin Invest. 1997;99:31–40. doi: 10.1172/JCI119130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hertl M, et al. Recognition of desmoglein 3 by autoreactive T cells in pemphigus vulgaris patients and normals. J Invest Dermatol. 1998;110:62–66. doi: 10.1046/j.1523-1747.1998.00086.x. [DOI] [PubMed] [Google Scholar]
- 98.Veldman C, et al. Dichotomy of autoreactive Th1 and Th2 cell responses to desmoglein 3 in patients with pemphigus vulgaris (PV) and healthy carriers of PV-associated HLA class II alleles. J Immunol. 2003;170:635–642. doi: 10.4049/jimmunol.170.1.635. [DOI] [PubMed] [Google Scholar]
- 99.Takahashi H, et al. Novel system evaluating in vivo pathogenicity of desmoglein 3-reactive T cell clones using murine pemphigus vulgaris. J Immunol. 2008;181:1526–1535. doi: 10.4049/jimmunol.181.2.1526. [DOI] [PubMed] [Google Scholar]
- 100.Veldman C, Hohne A, Dieckmann D, Schuler G, Hertl M. Type I regulatory T cells specific for desmoglein 3 are more frequently detected in healthy individuals than in patients with pemphigus vulgaris. J Immunol. 2004;172:6468–6475. doi: 10.4049/jimmunol.172.10.6468. [DOI] [PubMed] [Google Scholar]
- 101.Nousari HC, et al. The mechanism of respiratory failure in paraneoplastic pemphigus. N Engl J Med. 1999;340:1406–1410. doi: 10.1056/NEJM199905063401805. [DOI] [PubMed] [Google Scholar]
- 102.Hoffman MA, Qiao X, Anhalt GJ. CD8+ T lymphocytes in bronchiolitis obliterans, paraneoplastic pemphigus, and solitary Castleman’s disease. N Engl J Med. 2003;349:407–408. doi: 10.1056/NEJM200307243490421. [DOI] [PubMed] [Google Scholar]
- 103.Hata T, et al. Ectopic expression of epidermal antigens renders the lung a target organ in paraneoplastic pemphigus. J Immunol. 2013;191:83–90. doi: 10.4049/jimmunol.1203536. This study shows that ectopic expression of epidermal antigens in the lungs as a form of squamous metaplasia could be a reason why fatal pulmonary involvement occurs in patients with paraneoplastic pemphigus. [DOI] [PubMed] [Google Scholar]
- 104.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- 105.Ellebrecht CT, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. This paper describes a novel therapeutic strategy for antigen-specific B cell depletion in pemphigus that could avoid generalized immune suppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amagai M, et al. Use of autoantigen-knockout mice in developing an active autoimmune disease model for pemphigus. J Clin Invest. 2000;105:625–631. doi: 10.1172/JCI8748. This study describes the active disease mouse model for pemphigus vulgaris developed with a novel versatile method using adoptive transfer of lymphocytes from autoantigen-knockout mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takae Y, Nishikawa T, Amagai M. Pemphigus mouse model as a tool to evaluate various immunosuppressive therapies. Exp Dermatol. 2009;18:252–260. doi: 10.1111/j.1600-0625.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 108.Amagai M, et al. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol. 1999;140:351–357. doi: 10.1046/j.1365-2133.1999.02752.x. [DOI] [PubMed] [Google Scholar]
- 109.Eming R, et al. Pathogenic IgG antibodies against desmoglein 3 in pemphigus vulgaris are regulated by HLA-DRB1*04:02-restricted T cells. J Immunol. 2014;193:4391–4399. doi: 10.4049/jimmunol.1401081. [DOI] [PubMed] [Google Scholar]
- 110.Yoshida K, et al. Cutaneous type pemphigus vulgaris: a rare clinical phenotype of pemphigus. J Am Acad Dermatol. 2005;52:839–845. doi: 10.1016/j.jaad.2005.01.106. [DOI] [PubMed] [Google Scholar]
- 111.Espana A, et al. Antibodies to the amino-terminal domain of desmoglein 1 are retained during transition from pemphigus vulgaris to pemphigus foliaceus. Eur J Dermatol. 2014;24:174–179. doi: 10.1684/ejd.2014.2277. [DOI] [PubMed] [Google Scholar]
- 112.Kimoto M, Ohyama M, Hata Y, Amagai M, Nishikawa TA. Case of pemphigus foliaceus which occurred after five years of remission from pemphigus vulgaris. Dermatology. 2001;203:174–176. doi: 10.1159/000051737. [DOI] [PubMed] [Google Scholar]
- 113.Zhao CY, Chiang YZ, Murrell DF. Neonatal autoimmune blistering disease: a systematic review. Pediatr Dermatol. 2016;33:367–374. doi: 10.1111/pde.12859. [DOI] [PubMed] [Google Scholar]
- 114.Rocha-Alvarez R, et al. Pregnant women with endemic pemphigus foliaceus (fogo selvagem) give birth to disease-free babies. J Invest Dermatol. 1992;99:78–82. doi: 10.1111/1523-1747.ep12611868. [DOI] [PubMed] [Google Scholar]
- 115.Wu H, et al. Protection of neonates against pemphigus foliaceus by desmoglein 3. N Engl J Med. 2000;343:31–35. doi: 10.1056/NEJM200007063430105. [DOI] [PubMed] [Google Scholar]
- 116.Ishii K, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–2017. [PubMed] [Google Scholar]
- 117.Schmidt E, et al. Novel ELISA systems for antibodies to desmoglein 1 and 3: correlation of disease activity with serum autoantibody levels in individual pemphigus patients. Exp Dermatol. 2010;19:458–463. doi: 10.1111/j.1600-0625.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- 118.van Beek N, et al. Prospective studies on the routine use of a novel multivariant enzyme-linked immunosorbent assay for the diagnosis of autoimmune bullous diseases. J Am Acad Dermatol. 2017;76:889–894.e5. doi: 10.1016/j.jaad.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 119.van Beek N, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49. doi: 10.1186/1750-1172-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Belloni-Fortina A, et al. Detection of autoantibodies against recombinant desmoglein 1 and 3 molecules in patients with pemphigus vulgaris: correlation with disease extent at the time of diagnosis and during follow-up. Clin Dev Immunol. 2009;2009:187864. doi: 10.1155/2009/187864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abasq C, et al. ELISA testing of anti-desmoglein 1 and 3 antibodies in the management of pemphigus. Arch Dermatol. 2009;145:529–535. doi: 10.1001/archdermatol.2009.9. [DOI] [PubMed] [Google Scholar]
- 122.Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84–89. doi: 10.1016/j.autrev.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 123.Kwon EJ, Yamagami J, Nishikawa T, Amagai M. Anti-desmoglein IgG autoantibodies in patients with pemphigus in remission. J Eur Acad Dermatol Venereol. 2008;22:1070–1075. doi: 10.1111/j.1468-3083.2008.02715.x. [DOI] [PubMed] [Google Scholar]
- 124.Naseer SY, Seiffert-Sinha K, Sinha AA. Detailed profiling of anti-desmoglein autoantibodies identifies anti-Dsg1 reactivity as a key driver of disease activity and clinical expression in pemphigus vulgaris. Autoimmunity. 2015;48:231–241. doi: 10.3109/08916934.2014.976629. [DOI] [PubMed] [Google Scholar]
- 125.Anzai H, Stanley JR, Amagai M. Production of low titers of anti-desmoglein 1 IgG autoantibodies in some patients with staphylococcal scalded skin syndrome. J Invest Dermatol. 2006;126:2139–2141. doi: 10.1038/sj.jid.5700341. [DOI] [PubMed] [Google Scholar]
- 126.Prussmann W, et al. Prevalence of pemphigus and pemphigoid autoantibodies in the general population. Orphanet J Rare Dis. 2015;10:63. doi: 10.1186/s13023-015-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Probst C, et al. Development of ELISA for the specific determination of autoantibodies against envoplakin and periplakin in paraneoplastic pemphigus. Clin Chim Acta. 2009;410:13–18. doi: 10.1016/j.cca.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 128.Tsuchisaka A, et al. Epiplakin is a paraneoplastic pemphigus autoantigen and related to bronchiolitis obliterans in Japanese patients. J Invest Dermatol. 2016;136:399–408. doi: 10.1038/JID.2015.408. [DOI] [PubMed] [Google Scholar]
- 129.Murrell DF, et al. Consensus statement on definitions of disease endpoints and therapeutic response for pemphigus. J Am Acad Dermatol. 2008;58:1043–1046. doi: 10.1016/j.jaad.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Amagai M, et al. Japanese guidelines for the management of pemphigus. J Dermatol. 2014;41:471–486. doi: 10.1111/1346-8138.12486. This report describes current pemphigus treatment guidelines in Japan, which are based on published evidence as well as confirmatory studies in Japanese patients. [DOI] [PubMed] [Google Scholar]
- 131.Hertl M, et al. Pemphigus. S2 guideline for diagnosis and treatment — guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV) J Eur Acad Dermatol Venereol. 2015;29:405–414. doi: 10.1111/jdv.12772. This report describes evidence-based consensus on pemphigus treatment guidelines in Europe. [DOI] [PubMed] [Google Scholar]
- 132.Atzmony L, Hodak E, Gdalevich M, Rosenbaum O, Mimouni D. Treatment of pemphigus vulgaris and pemphigus foliaceus: a systematic review and meta-analysis. Am J Clin Dermatol. 2014;15:503–515. doi: 10.1007/s40257-014-0101-9. [DOI] [PubMed] [Google Scholar]
- 133.Martinez De Pablo MI, et al. Paraneoplastic pemphigus associated with non-Hodgkin B-cell lymphoma and good response to prednisone. Acta Derm Venereol. 2005;85:233–235. doi: 10.1080/00015550510025542. [DOI] [PubMed] [Google Scholar]
- 134.Hertzberg MS, Schifter M, Sullivan J, Stapleton K. Paraneoplastic pemphigus in two patients with B-cell non-Hodgkin’s lymphoma: significant responses to cyclophosphamide and prednisolone. Am J Hematol. 2000;63:105–106. doi: 10.1002/(sici)1096-8652(200002)63:2<105::aid-ajh14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 135.Nousari HC, Brodsky RA, Jones RJ, Grever MR, Anhalt GJ. Immunoablative high-dose cyclophosphamide without stem cell rescue in paraneoplastic pemphigus: report of a case and review of this new therapy for severe autoimmune disease. J Am Acad Dermatol. 1999;40:750–754. doi: 10.1016/s0190-9622(99)70157-x. [DOI] [PubMed] [Google Scholar]
- 136.Becker LR, et al. Paraneoplastic pemphigus treated with dexamethasone/cyclophosphamide pulse therapy. Eur J Dermatol. 1998;8:551–553. [PubMed] [Google Scholar]
- 137.Izaki S, et al. Paraneoplastic pemphigus: potential therapeutic effect of plasmapheresis. Br J Dermatol. 1996;134:987–989. doi: 10.1111/j.1365-2133.1996.tb06349.x. [DOI] [PubMed] [Google Scholar]
- 138.Jing L, Shan Z, Yongchu H, Xixue C, Xuejun Z. Successful treatment of a paraneoplastic pemphigus in a teenager using plasmapheresis, corticosteroids and tumour resection. Clin Exp Dermatol. 2011;36:752–754. doi: 10.1111/j.1365-2230.2011.04081.x. [DOI] [PubMed] [Google Scholar]
- 139.Heizmann M, Itin P, Wernli M, Borradori L, Bargetzi MJ. Successful treatment of paraneoplastic pemphigus in follicular NHL with rituximab: report of a case and review of treatment for paraneoplastic pemphigus in NHL and CLL. Am J Hematol. 2001;66:142–144. doi: 10.1002/1096-8652(200102)66:2<142::AID-AJH1032>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 140.Vezzoli P, Berti E, Marzano AV. Rationale and efficacy for the use of rituximab in paraneoplastic pemphigus. Expert Rev Clin Immunol. 2008;4:351–363. doi: 10.1586/1744666X.4.3.351. [DOI] [PubMed] [Google Scholar]
- 141.Gergely L, Varoczy L, Vadasz G, Remenyik E, Illes A. Successful treatment of B cell chronic lymphocytic leukemia-associated severe paraneoplastic pemphigus with cyclosporin A. Acta Haematol. 2003;109:202–205. doi: 10.1159/000070972. [DOI] [PubMed] [Google Scholar]
- 142.Hwang YY, Chan JC, Trendell-Smith NJ, Kwong YL. Recalcitrant paraneoplastic pemphigus associated with follicular dendritic cell sarcoma: response to prolonged rituximab and ciclosporin therapy. Intern Med J. 2014;44:1145–1146. doi: 10.1111/imj.12576. [DOI] [PubMed] [Google Scholar]
- 143.Ekback M, Uggla B. Paraneoplastic pemphigus associated with chronic lymphocytic leukaemia: treatment with alemtuzumab. Leuk Res. 2012;36:e190–e191. doi: 10.1016/j.leukres.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 144.Bech R, et al. Alemtuzumab is effective against severe chronic lymphocytic leukaemia-associated paraneoplastic pemphigus. Br J Dermatol. 2013;169:469–472. doi: 10.1111/bjd.12324. [DOI] [PubMed] [Google Scholar]
- 145.Werth VP. Treatment of pemphigus vulgaris with brief, high-dose intravenous glucocorticoids. Arch Dermatol. 1996;132:1435–1439. [PubMed] [Google Scholar]
- 146.Mignogna MD, et al. High-dose intravenous ‘pulse’ methylprednisone in the treatment of severe oropharyngeal pemphigus: a pilot study. J Oral Pathol Med. 2002;31:339–344. doi: 10.1034/j.1600-0714.2002.00085.x. [DOI] [PubMed] [Google Scholar]
- 147.Nguyen VT, et al. Pemphigus vulgaris IgG and methylprednisolone exhibit reciprocal effects on keratinocytes. J Biol Chem. 2004;279:2135–2146. doi: 10.1074/jbc.M309000200. [DOI] [PubMed] [Google Scholar]
- 148.Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1–9. doi: 10.1016/j.jaad.2016.01.062. [DOI] [PubMed] [Google Scholar]
- 149.Thomas CF, Jr, Limper AH. Treatment and prevention of Pneumocystis pneumonia in HIV-uninfected patients. UpToDate. 2016 https://www.uptodate.com/contents/treatment-and-prevention-of-pneumocystis-pneumonia-in-hiv-uninfected-patients.
- 150.Centers for Disease Control and Prevention. Targeted tuberculosis testing and interpreting tuberculin skin test results: introduction. CDC; 2016. https://www.cdc.gov/tb/publications/factsheets/testing/skintestresults.htm. [Google Scholar]
- 151.Almugairen N, et al. Assessment of the rate of long-term complete remission off therapy in patients with pemphigus treated with different regimens including medium- and high-dose corticosteroids. J Am Acad Dermatol. 2013;69:583–588. doi: 10.1016/j.jaad.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 152.Beissert S, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of pemphigus. Arch Dermatol. 2006;142:1447–1454. doi: 10.1001/archderm.142.11.1447. [DOI] [PubMed] [Google Scholar]
- 153.Chams-Davatchi C, et al. Randomized controlled open-label trial of four treatment regimens for pemphigus vulgaris. J Am Acad Dermatol. 2007;57:622–628. doi: 10.1016/j.jaad.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 154.Beissert S, et al. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: a multicenter, randomized, placebo-controlled trial. J Invest Dermatol. 2010;130:2041–2048. doi: 10.1038/jid.2010.91. [DOI] [PubMed] [Google Scholar]
- 155.Ioannides D, Apalla Z, Lazaridou E, Rigopoulos D. Evaluation of mycophenolate mofetil as a steroid-sparing agent in pemphigus: a randomized, prospective study. J Eur Acad Dermatol Venereol. 2012;26:855–860. doi: 10.1111/j.1468-3083.2011.04170.x. [DOI] [PubMed] [Google Scholar]
- 156.Chams-Davatchi C, et al. Randomized double blind trial of prednisolone and azathioprine, versus prednisolone and placebo, in the treatment of pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2013;27:1285–1292. doi: 10.1111/j.1468-3083.2012.04717.x. [DOI] [PubMed] [Google Scholar]
- 157.Kotlyar DS, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol. 2015;13:847–858.e4. doi: 10.1016/j.cgh.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 158.Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005;5:2954–2960. doi: 10.1111/j.1600-6143.2005.01125.x. [DOI] [PubMed] [Google Scholar]
- 159.MacPhee IA, et al. Pharmacokinetics of mycophenolate mofetil in patients with end-stage renal failure. Kidney Int. 2000;57:1164–1168. doi: 10.1046/j.1523-1755.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 160.Kaplan B, Gaston RS, Meier-Kriesche HU, Bloom RD, Shaw LM. Mycophenolic acid exposure in high- and low-weight renal transplant patients after dosing with mycophenolate mofetil in the Opticept trial. Ther Drug Monit. 2010;32:224–227. doi: 10.1097/FTD.0b013e3181d18baa. [DOI] [PubMed] [Google Scholar]
- 161.Joly P, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017 doi: 10.1016/S0140-6736(17)30070-3. http://dx.doi.org/10.1016/S0140-6736(17)30070-3. This study demonstrates the remarkable efficacy of rituximab as a first-line therapy for pemphigus. [DOI] [PubMed]
- 162.Joly P, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357:545–552. doi: 10.1056/NEJMoa067752. [DOI] [PubMed] [Google Scholar]
- 163.Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355:1772–1779. doi: 10.1056/NEJMoa062930. [DOI] [PubMed] [Google Scholar]
- 164.Ahmed AR, Kaveri S, Spigelman Z. Long-term remissions in recalcitrant pemphigus vulgaris. N Engl J Med. 2015;373:2693–2694. doi: 10.1056/NEJMc1508234. [DOI] [PubMed] [Google Scholar]
- 165.Feldman RJ, Ahmed AR. Relevance of rituximab therapy in pemphigus vulgaris: analysis of current data and the immunologic basis for its observed responses. Expert Rev Clin Immunol. 2011;7:529–541. doi: 10.1586/eci.11.22. [DOI] [PubMed] [Google Scholar]
- 166.Wang HH, Liu CW, Li YC, Huang YC. Efficacy of rituximab for pemphigus: a systematic review and meta-analysis of different regimens. Acta Derm Venereol. 2015;95:928–932. doi: 10.2340/00015555-2116. [DOI] [PubMed] [Google Scholar]
- 167.Lunardon L, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031–1036. doi: 10.1001/archdermatol.2012.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lunardon L, Payne AS. Inhibitory human antichimeric antibodies to rituximab in a patient with pemphigus. J Allergy Clin Immunol. 2012;130:800–803. doi: 10.1016/j.jaci.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Buch MH, et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:909–920. doi: 10.1136/ard.2010.144998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tony HP, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID) Arthritis Res Ther. 2011;13:R75. doi: 10.1186/ar3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kasperkiewicz M, et al. Efficacy and safety of rituximab in pemphigus: experience of the German Registry of Autoimmune Diseases. J Dtsch Dermatol Ges. 2012;10:727–732. doi: 10.1111/j.1610-0387.2012.07931.x. [DOI] [PubMed] [Google Scholar]
- 172.Li N, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. 2005;115:3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Paquin Proulx D, Aubin E, Lemieux R, Bazin R. Inhibition of B cell-mediated antigen presentation by intravenous immunoglobulins (IVIg) Clin Immunol. 2010;135:422–429. doi: 10.1016/j.clim.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 174.Baker K, Rath T, Pyzik M, Blumberg RS. The role of FcRn in antigen presentation. Front Immunol. 2014;5:408. doi: 10.3389/fimmu.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. J Exp Med. 2007;204:11–15. doi: 10.1084/jem.20061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Amagai M, et al. A randomized double-blind trial of intravenous immunoglobulin for pemphigus. J Am Acad Dermatol. 2009;60:595–603. doi: 10.1016/j.jaad.2008.09.052. This study reports the first placebo-controlled, randomized, multicentre clinical trial for pemphigus and demonstrates the efficacy of intravenous immunoglobulin. [DOI] [PubMed] [Google Scholar]
- 177.Cunningham-Rundles C, Zhou Z, Mankarious S, Courter S. Long-term use of IgA-depleted intravenous immunoglobulin in immunodeficient subjects with anti-IgA antibodies. J Clin Immunol. 1993;13:272–278. doi: 10.1007/BF00919386. [DOI] [PubMed] [Google Scholar]
- 178.Caress JB, Cartwright MS, Donofrio PD, Peacock JE., Jr The clinical features of 16 cases of stroke associated with administration of IVIg. Neurology. 2003;60:1822–1824. doi: 10.1212/01.wnl.0000068335.01620.9d. [DOI] [PubMed] [Google Scholar]
- 179.Basta M. Intravenous immunoglobulin-related thromboembolic events — an accusation that proves the opposite. Clin Exp Immunol. 2014;178(Suppl 1):153–155. doi: 10.1111/cei.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guillaume JC, et al. Controlled study of plasma exchange in pemphigus. Arch Dermatol. 1988;124:1659–1663. [PubMed] [Google Scholar]
- 181.Turner MS, Sutton D, Sauder DN. The use of plasmapheresis and immuosupression in the treatment of pemphigus vulgaris. J Am Acad Dermatol. 2000;43:1058–1064. doi: 10.1067/mjd.2000.109297. [DOI] [PubMed] [Google Scholar]
- 182.Behzad M, et al. Combined treatment with immunoadsorption and rituximab leads to fast and prolonged clinical remission in difficult-to-treat pemphigus vulgaris. Br J Dermatol. 2012;166:844–852. doi: 10.1111/j.1365-2133.2011.10732.x. [DOI] [PubMed] [Google Scholar]
- 183.Kasperkiewicz M, et al. Treatment of severe pemphigus with a combination of immunoadsorption, rituximab, pulsed dexamethasone and azathioprine/mycophenolate mofetil: a pilot study of 23 patients. Br J Dermatol. 2012;166:154–160. doi: 10.1111/j.1365-2133.2011.10585.x. [DOI] [PubMed] [Google Scholar]
- 184.Langan SM, et al. Bullous pemphigoid and pemphigus vulgaris — incidence and mortality in the UK: population based cohort study. BMJ. 2008;337:a180. doi: 10.1136/bmj.a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huang YH, Kuo CF, Chen YH, Yang YW. Incidence, mortality, and causes of death of patients with pemphigus in Taiwan: a nationwide population-based study. J Invest Dermatol. 2012;132:92–97. doi: 10.1038/jid.2011.249. [DOI] [PubMed] [Google Scholar]
- 186.Hsu DY, Brieva J, Sinha AA, Langan SM, Silverberg JI. Comorbidities and inpatient mortality for pemphigus in the USA. Br J Dermatol. 2016;174:1290–1298. doi: 10.1111/bjd.14463. [DOI] [PubMed] [Google Scholar]
- 187.Leshem YA, Katzenelson V, Yosipovitch G, David M, Mimouni D. Autoimmune diseases in patients with pemphigus and their first-degree relatives. Int J Dermatol. 2011;50:827–831. doi: 10.1111/j.1365-4632.2010.04818.x. [DOI] [PubMed] [Google Scholar]
- 188.Parameswaran A, Attwood K, Sato R, Seiffert-Sinha K, Sinha AA. Identification of a new disease cluster of pemphigus vulgaris with autoimmune thyroid disease, rheumatoid arthritis and type I diabetes. Br J Dermatol. 2015;172:729–738. doi: 10.1111/bjd.13433. [DOI] [PubMed] [Google Scholar]
- 189.Leger S, et al. Prognostic factors of paraneoplastic pemphigus. Arch Dermatol. 2012;148:1165–1172. doi: 10.1001/archdermatol.2012.1830. [DOI] [PubMed] [Google Scholar]
- 190.Martin L, Murrell DF. Measuring the immeasurable: a systematic review of outcome measures in pemphigus. Australas J Dermatol. 2006;47:A32–A33. [Google Scholar]
- 191.Zhao CY, Murrell DF. Outcome measures for autoimmune blistering diseases. J Dermatol. 2015;42:31–36. doi: 10.1111/1346-8138.12711. This paper reviews all the standardized and validated outcome measures in pemphigus. [DOI] [PubMed] [Google Scholar]
- 192.Rosenbach M, et al. Reliability and convergent validity of two outcome instruments for pemphigus. J Invest Dermatol. 2008;129:2404–2410. doi: 10.1038/jid.2009.72. The PDAI is widely considered as the most reliable and valid pemphigus disease activity index for use in clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pfütze M, Niedermeier A, Hertl M, Eming R. Introducing a novel autoimmune bullous skin disorder intensity score (ABSIS) in pemphigus. Eur J Dermatol. 2007;17:4–11. doi: 10.1684/ejd.2007.0090. The ABSIS is a pemphigus disease activity scoring system that is commonly used in clinical and translational research studies in pemphigus. [DOI] [PubMed] [Google Scholar]
- 194.Shimizu T, et al. Grading criteria for disease severity by pemphigus disease area index. J Dermatol. 2014;41:969–973. doi: 10.1111/1346-8138.12649. [DOI] [PubMed] [Google Scholar]
- 195.Boulard C, et al. Calculation of cutoff values based on the ABSIS and PDAI pemphigus scoring systems for defining moderate, significant, and extensive types of pemphigus. Br J Dermatol. 2016;175:142–149. doi: 10.1111/bjd.14405. [DOI] [PubMed] [Google Scholar]
- 196.The WHOQOL Group. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41:1403–1409. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 197.Rencz F, et al. Health-related quality of life and its determinants in pemphigus: a systematic review and meta-analysis. Br J Dermatol. 2015;173:1076–1080. doi: 10.1111/bjd.13848. This paper provides an overview of studies on quality of life in pemphigus. [DOI] [PubMed] [Google Scholar]
- 198.Tabolli S, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087–1091. doi: 10.1111/bjd.12836. [DOI] [PubMed] [Google Scholar]
- 199.Sebaratnam DF, et al. Development of a quality-of-life instrument for autoimmune bullous disease: the Autoimmune Bullous Disease Quality of Life questionnaire. JAMA Dermatol. 2013;149:1186–1191. doi: 10.1001/jamadermatol.2013.4972. The ABQOL is the major quality-of-life index in use for patients with an autoimmune blistering disease. [DOI] [PubMed] [Google Scholar]
- 200.Tjokrowidjaja A, et al. The development and validation of the treatment of autoimmune bullous disease quality of life questionnaire, a tool to measure the quality of life impacts of treatments used in patients with autoimmune blistering disease. Br J Dermatol. 2013;169:1000–1006. doi: 10.1111/bjd.12623. [DOI] [PubMed] [Google Scholar]
- 201.Sebaratnam DF, Okawa J, Payne A, Murrell DF, Werth VP. Reliability of the autoimmune bullous disease quality of life (ABQOL) questionnaire in the USA. Qual Life Res. 2015;24:2257–2260. doi: 10.1007/s11136-015-0965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dey-Rao R, Seiffert-Sinha K, Sinha AA. Genome-wide expression analysis suggests unique disease-promoting and disease-preventing signatures in pemphigus vulgaris. Genes Immun. 2013;14:487–499. doi: 10.1038/gene.2013.44. [DOI] [PubMed] [Google Scholar]
- 203.Schmidt T, et al. Induction of T regulatory cells by the superagonistic anti-CD28 antibody D665 leads to decreased pathogenic IgG autoantibodies against desmoglein 3 in a HLA-transgenic mouse model of pemphigus vulgaris. Exp Dermatol. 2016;25:293–298. doi: 10.1111/exd.12919. [DOI] [PubMed] [Google Scholar]
- 204.Hata T, et al. Transgenic rescue of desmoglein 3 null mice with desmoglein 1 to develop a syngeneic mouse model for pemphigus vulgaris. J Dermatol Sci. 2011;63:33–39. doi: 10.1016/j.jdermsci.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 205.Luo J, Lindstrom J. AChR-specific immunosuppressive therapy of myasthenia gravis. Biochem Pharmacol. 2015;97:609–619. doi: 10.1016/j.bcp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 206.Lutterotti A, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5:188ra75. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.MacDonald KG, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Stahley SN, et al. Super-resolution microscopy reveals altered desmosomal protein organization in tissue from patients with pemphigus vulgaris. J Invest Dermatol. 2016;136:59–66. doi: 10.1038/JID.2015.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ellebrecht CT, et al. Subcutaneous veltuzumab, a humanized anti-CD20 antibody, for treatment of refractory pemphigus vulgaris. JAMA Dermatol. 2014;150:1331–1335. doi: 10.1001/jamadermatol.2014.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.US National Library of Medicine. ClinicalTrialsgov. 2017 https://clinicaltrials.gov/ct2/show/NCT02383589.
- 211.US National Library of Medicine. ClinicalTrialsgov. 2016 https://clinicaltrials.gov/ct2/show/NCT01939899.
- 212.Huang A, Madan RK, Levitt J. Future therapies for pemphigus vulgaris: rituximab and beyond. J Am Acad Dermatol. 2016;74:746–753. doi: 10.1016/j.jaad.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 213.US National Library of Medicine. ClinicalTrialsgov. 2016 https://clinicaltrials.gov/ct2/show/NCT02704429.
- 214.Hill RJS, et al. Discovery of PRN1008, a novel, reversible covalent BTK inhibitor in clinical development for rheumatoid arthritis [abstract] Arthritis Rheumatol. 2015;67(Suppl 10):1671. [Google Scholar]
- 215.Avery DT, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.US National Library of Medicine. ClinicalTrialsgov. 2017 https://clinicaltrials.gov/ct2/show/NCT01930175.
- 217.Schaeffeler E, et al. Comprehensive analysis of thiopurine S-methyltransferase phenotype–genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants. Pharmacogenetics. 2004;14:407–417. doi: 10.1097/01.fpc.0000114745.08559.db. [DOI] [PubMed] [Google Scholar]
- 218.Coenen MJ, et al. Identification of patients with variants in TPMT and dose reduction reduces hematologic events during thiopurine treatment of inflammatory bowel disease. Gastroenterology. 2015;149:907–917.e7. doi: 10.1053/j.gastro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 219.Sanderson JD. TPMT testing before starting azathioprine or mercaptopurine: surely just do it? Gastroenterology. 2015;149:850–853. doi: 10.1053/j.gastro.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 220.Yang SK, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017–1020. doi: 10.1038/ng.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu J, et al. A novel polymorphism of FcγRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–1070. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dall’Ozzo S, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64:4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 223.Anolik JH, et al. The relationship of FcγRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 2003;48:455–459. doi: 10.1002/art.10764. [DOI] [PubMed] [Google Scholar]
- 224.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 225.Farag SS, et al. FcγRIIIa and FcγRIIa polymorphisms do not predict response to rituximab in B-cell chronic lymphocytic leukemia. Blood. 2004;103:1472–1474. doi: 10.1182/blood-2003-07-2548. [DOI] [PubMed] [Google Scholar]
- 226.Weng WK, Weng WK, Levy R. Immunoglobulin G Fc receptor polymorphisms do not correlate with response to chemotherapy or clinical course in patients with follicular lymphoma. Leuk Lymphoma. 2009;50:1494–1500. doi: 10.1080/10428190903128660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Werth VP, et al. Multicenter randomized, double-blind, placebo-controlled, clinical trial of dapsone as a glucocorticoid-sparing agent in maintenance-phase pemphigus vulgaris. Arch Dermatol. 2008;144:25–32. doi: 10.1001/archderm.144.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Peters AL, Van Noorden CJ. Glucose-6-phosphate dehydrogenase deficiency and malaria: cytochemical detection of heterozygous G6PD deficiency in women. J Histochem Cytochem. 2009;57:1003–1011. doi: 10.1369/jhc.2009.953828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tran KD, Wolverton JE, Soter NA. Methotrexate in the treatment of pemphigus vulgaris: experience in 23 patients. Br J Dermatol. 2013;169:916–921. doi: 10.1111/bjd.12474. [DOI] [PubMed] [Google Scholar]
- 230.Barthelemy H, et al. Treatment of nine cases of pemphigus vulgaris with cyclosporine. J Am Acad Dermatol. 1988;18:1262–1266. doi: 10.1016/s0190-9622(88)70132-2. [DOI] [PubMed] [Google Scholar]
- 231.Campolmi P, et al. The role of cyclosporine A in the treatment of pemphigus erythematosus. Int J Dermatol. 1991;30:890–892. doi: 10.1111/j.1365-4362.1991.tb04361.x. [DOI] [PubMed] [Google Scholar]
- 232.Olszewska M, et al. Efficacy and safety of cyclophosphamide, azathioprine, and cyclosporine (ciclosporin) as adjuvant drugs in pemphigus vulgaris. Am J Clin Dermatol. 2007;8:85–92. doi: 10.2165/00128071-200708020-00004. [DOI] [PubMed] [Google Scholar]
- 233.Ioannides D, Chrysomallis F, Bystryn JC. Ineffectiveness of cyclosporine as an adjuvant to corticosteroids in the treatment of pemphigus. Arch Dermatol. 2000;136:868–872. doi: 10.1001/archderm.136.7.868. [DOI] [PubMed] [Google Scholar]
- 234.Sharma VK, Khandpur S. Evaluation of cyclophosphamide pulse therapy as an adjuvant to oral corticosteroid in the management of pemphigus vulgaris. Clin Exp Dermatol. 2013;38:659–664. doi: 10.1111/ced.12073. [DOI] [PubMed] [Google Scholar]
- 235.Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40:167–170. doi: 10.1016/s0190-9622(99)70183-0. [DOI] [PubMed] [Google Scholar]
- 236.Berkowitz P, et al. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc Natl Acad Sci USA. 2006;103:12855–12860. doi: 10.1073/pnas.0602973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Berkowitz P, Chua M, Liu Z, Diaz LA, Rubenstein DS. Autoantibodies in the autoimmune disease pemphigus foliaceus induce blistering via p38 mitogen-activated protein kinase-dependent signaling in the skin. Am J Pathol. 2008;173:1628–1636. doi: 10.2353/ajpath.2008.080391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Koch PJ, et al. Desmoglein 3 anchors telogen hair in the follicle. J Cell Sci. 1998;111:2529–2537. doi: 10.1242/jcs.111.17.2529. [DOI] [PubMed] [Google Scholar]