Abstract
Background
The anaesthetic dose causing neurotoxicity in animals has been evaluated, but the relationship between duration of volatile anaesthetic (VA) exposure and neurodevelopment in children remains unclear.
Methods
Data were obtained from the Western Australian Pregnancy Cohort (Raine) Study, with language (Clinical Evaluation of Language Fundamentals: Receptive [CELF-R] and Expressive [CELF-E] and Total [CELF-T]) and cognition (Coloured Progressive Matrices [CPM]) assessed at age 10 yr. Medical records were reviewed, and children divided into quartiles based on total VA exposure duration before age three yr. The association between test score and exposure duration quartile was evaluated using linear regression, adjusting for patient characteristics and comorbidity.
Results
Of 1622 children with available test scores, 148 had documented VA exposure and were split into the following quartiles: ≤25, >25 to ≤35, >35 to ≤60 and >60 min. Compared with unexposed children, CELF-T scores for children in the first and second quartiles did not differ, but those in the third and fourth quartiles had significantly lower scores ([3rd quartile – Unexposed] -5.3; 95% confidence interval [CI], (-10.2 – -0.4), [4th quartile – Unexposed] -6.2; 95% CI, (-11.6 – -0.9). CELF-E showed similar findings, but significant differences were not found in CELF-R or CPM for any quartile.
Conclusions
Children with VA exposures ≤35 min did not differ from unexposed children, but those with exposures >35 min had lower total and expressive language scores. It remains unclear if this is a dose-response relationship, or if children requiring longer exposures for longer surgeries have other clinical reasons for lower scores.
Keywords: anaesthetic neurotoxicity, neurodevelopment, paediatric anaesthesia
Editor's key points.
-
•
Preclinical data support anaesthetic neurotoxicity, but the clinical implications and possible dose-response relationship are unclear.
-
•
A retrospective analysis probed medical records for children exposed to general anaesthesia before age 3 yr who were also tested for language and cognition at age 10 yr.
-
•
Children with anaesthetic exposures >35 min had lower expressive language scores but no difference in cognition.
-
•
This could indicate dose-dependent toxicity or an effect of comorbidities on neurodevelopment after anaesthesia.
Exposure of developing brains to N-methyl-D-aspartate antagonists (such as nitrous oxide and ketamine) and γ-aminobutyric acid agonists (such as benzodiazepines, propofol, and volatile anaesthetics), lead to dose-dependent neuroapoptosis and neurodegenerative changes in animal models, with long-term cognitive deficits observed in adulthood.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Studies in rodents have used various combinations of i.v. and volatile anaesthetic (VA) agents at differing doses.11 Despite this heterogeneity, the preclinical data overall support a dose-response relationship, with higher doses, longer durations, increased number of different agents, and multiple discrete anaesthetic exposures associated with higher levels of histological damage and worse functional deficits.
Compared with hundreds of published preclinical studies, there are relatively few clinical studies of neurotoxicity. Of the published clinical studies, several have found an increased risk of neurodevelopmental deficit associated with exposure to surgery and anaesthesia, but few have specifically assessed dose-response.12, 13, 14, 15, 16, 17 The effect of specific durations of anaesthetic exposure has been explored in two studies assessing learning disability and academic achievement, with both reporting an association between longer durations of anaesthetic exposure and increased risk of neurodevelopmental deficits.18 19 Neither study however used neuropsychological testing, which might be more sensitive than other measures of neurodevelopmental function.
We previously found an association between surgery and anaesthesia and long-term neuropsychological deficits in language and cognition.20 Using the same patient cohort, the present study builds upon this by evaluating further the association between total duration of VA exposure before age 3 yr and neuropsychological test scores at age 10 yr.
Methods
This study was approved by the Columbia University Institutional Review Board, with approval for data collection and storage at each age by Ethics Committees at King Edward Memorial Hospital, Princess Margaret Hospital, and the University of Western Australia, and medical record review by the University of Western Australia.
Data source
Raine cohort data
Data was obtained from the Raine Study, an established birth cohort in Perth, Western Australia, consisting of 2,868 children born from 1989 to 1992. As part of the study, detailed patient characteristics and medical data were collected prenatally and at birth from medical records and parental self-report. After birth, all children were assessed at one, two, three, five, eight, 10, 13, 17, 20, and 22 yrs of age. Comprehensive neuropsychological testing was performed at age 10. During follow-up visits, parents completed questionnaires describing illnesses and medical problems, which were coded by research staff into International Classification of Diseases, 9th Revision (ICD-9) codes as described.21
Medical and anaesthetic record review
All children in the Raine cohort with parentally reported history of any procedure or admission to the hospital before age five yr were identified. Medical and anaesthetic records for these children were requested from the Princess Margaret Hospital for Children and surrounding hospitals and outpatient surgical centres in Perth, Australia. Data extracted from the records were stored on a database maintained by the Columbia University Data Coordinating Center.
Children with procedures requiring anaesthetic exposure before age three yr based on either anaesthetic records or parental report were classified as being “exposed”. All surgical and anaesthetic exposures occurred between 1989 and 1995. Children with no parental report of a procedure and no available anaesthetic records before age three yr were classified as “unexposed”. While an exact age of vulnerability to anaesthesia in children is unknown, exposure before three yr old was used to define exposure status as it is the time of peak synaptogenesis in various regions of the brain including the prefrontal cortex.22 23 For exposed children with available anaesthetic records, VA exposure in min from each procedure was quantified. Children with more than one anaesthetic exposure, had the durations of VA from separate procedures summed together to calculate a total duration of exposure before age three. Children with parental report of anaesthetic exposure where anaesthetic records could not be obtained, were classified as “exposed with no anaesthetic record”.
Neuropsychological tests
Outcomes included in this study were the neuropsychological tests previously found to be different between exposed and unexposed children.20 All assessments were performed at age 10 by trained research staff directly administering the tests. Language was evaluated with the Clinical Evaluation of Language Fundamentals (CELF), which is composed of individual scores for Receptive (CELF-R), or language comprehension ability, Expressive (CELF-E), or speaking language ability, and a Total (CELF-T) language.24 25 Cognition was assessed by Coloured Progressive Matrices (CPM) which is a test of nonverbal intelligence, with a specific focus on abstract reasoning.26
Comorbid illness
Comorbid illness was quantified using the Johns Hopkins ACG Case-Mix System, a method for predicting past and future healthcare utilization and costs.27 ICD-9 codes from all follow-up visits up to and including age 10 were used to evaluate medical resource use by each child and calculate Resource Utilization Band (RUB) scores. ICD-9 codes for mental, behavioural, and neurodevelopmental disorders were excluded from the calculation of RUB scores, as they were directly related to the outcomes of interest. For each child, RUB was coded as 0) No diagnoses, 1) Healthy, 2) Low, 3) Moderate, 4) High, and 5) Very High. Children with no diagnoses and presented for follow-up, and the healthy category, were collapsed in the Low resource utilization category, and children in the High and Very High utilization groups were also combined. Coding of the RUB score was performed with the Johns Hopkins ACG version 10.0.1.
Statistical analysis
Children with available anaesthetic records and neuropsychological outcomes were split into quartiles based on the total duration of exposure to VA for each child. Coding of the duration variable as categorical as opposed to continuous, allowed assessment of a non-linear relationship between anaesthesia duration and outcome measures.
A multilevel categorical exposure group variable was created, that included levels for each quartile, in addition to the unexposed group and the exposed with no anaesthetic records group. Linear regression was used to calculate test score differences and 95% confidence intervals between the unexposed group and each individual exposure group. Multivariable linear regression was subsequently used to adjust for patient characteristics and clinical covariates (sex, race, birth weight (<2500g), maternal school level and income) in addition to comorbidity as measured by the Johns Hopkins ACG system.
Results
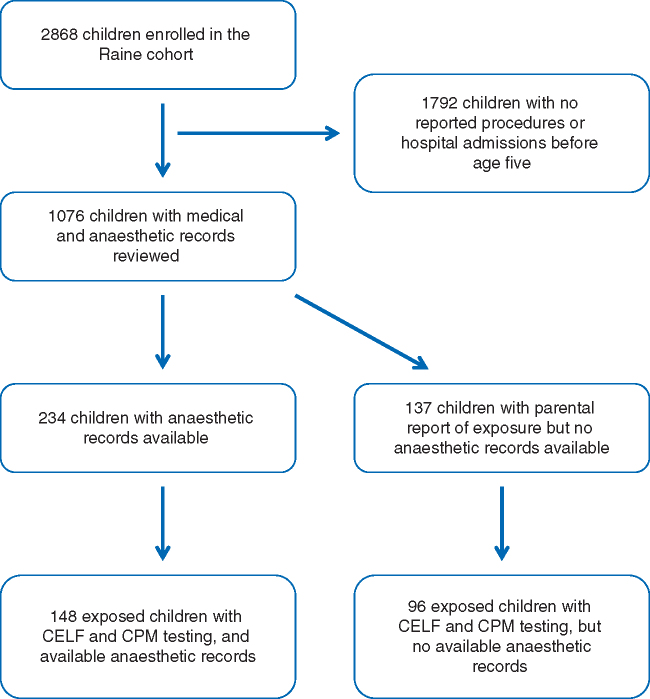
The Raine cohort consists of 2,868 children, with 1076 children having a history of a procedure or admission to a hospital before age five yr. Available medical and anaesthetic records for these children were evaluated, and data for procedures requiring anaesthesia before the age of three yr were entered into our database. (Fig. 1) Records for 348 procedures with anaesthetics were identified, of which 337 were performed at PMH and 11 were performed at other institutions. These procedures pertained to 234 children. Of these children, 184 had a prior parental report attesting to exposure before age three based on the Raine database, while the remaining 50 had an anaesthetic record, but no associated parental report.
Fig 1.
Children classified as exposed to anaesthesia in Raine cohort.
In addition to children with available anaesthetic records, another 137 children were identified as being exposed to surgery and anaesthesia before age three based on parental report, but the associated records could not be obtained. These children were therefore classified as “exposed with no anaesthetic record”.
Duration of VA exposure and neuropsychological test score
A total of 148 children had anaesthetic record information and CELF and CPM scores available. These children were split into quartiles based on total duration of VA exposure: 1st quartile: ≤25 min (n = 36), 2nd quartile: >25 to ≤35 min (n = 43), 3rd quartile: >35 to ≤60 min (n = 38), and 4th quartile: >60 min (n = 31). The numbers of patients in each quartile were unequal because some children at the quartile cut points had the same duration of exposure. The mean durations of exposure in each quartile were 19, 32, 50 and 160 min respectively. A total of 96 children were “exposed with no anaesthetic record” and had available test scores.
Compared with unexposed children, those who were exposed were found to have a higher percentage of boys. (Table 1) Children in the 4th quartile also had a higher percentage of low birth weight children compared with the other exposure groups, and higher resource utilization was also found in the 3rd and 4th exposure quartiles. All children in the 1st quartile had a single exposure, while three children (7%) in the 2nd quartile had two exposures. In the 3rd quartile, two children (5.3%) had two exposures, while 16 children (51.6%) in the 4th quartile had two or more exposures, with the number ranging from two to 23 exposures.
Table 1.
Characteristics of children in each exposure group with CELF and CPM Scores. *Because of rounding, percentages may not sum to 100. AUD: Australian Dollar
| Unexposed (n = 1378), n (%) | First quartile (n = 36), n (%) | Second quartile (n = 43), n(%) | Third quartile (n = 38), n(%) | Fourth quartile (n = 31), n(%) | No record (n = 96), n(%) | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Girls | 687(49.9) | 15(41.7) | 13(30.2) | 13(34.2) | 12(38.7) | 35(36.5) |
| Boys | 691(50.1) | 21(58.3) | 30(69.8) | 25(65.8) | 19(61.3) | 61(63.5) |
| Birth Weight | ||||||
| < 2500g | 109(7.9) | 3(8.3) | 4(9.3) | 3(7.9) | 6(19.4) | 12(12.5) |
| ≥ 2500g | 1268(92) | 33(91.7) | 39(90.7) | 35(92.1) | 25(80.6) | 84(87.5) |
| Missing | 1(0.1) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Race | ||||||
| Caucasian | 1213(88) | 33(91.7) | 40(93) | 37(97.4) | 28(90.3) | 83(87.4) |
| Non-Caucasian | 139(10.1) | 3(8.3) | 2(4.7) | 0(0) | 3(9.7) | 7(7.4) |
| Missing | 26(1.9) | 0(0) | 1(2.3) | 1(2.6) | 0(0) | 5(5.3) |
| Household Income (AUD) | ||||||
| Less than $7000 | 81(5.9) | 1(2.8) | 3(7) | 0(0) | 2(6.5) | 7(7.3) |
| $7000-$23999 | 380(27.6) | 11(30.6) | 15(34.9) | 11(28.9) | 13(41.9) | 28(29.2) |
| $24000 - $35999 | 352(25.5) | 8(22.2) | 12(27.9) | 8(21.1) | 7(22.6) | 15(15.6) |
| $36,000 | 473(34.3) | 15(41.7) | 12(27.9) | 18(47.4) | 9(29) | 39(40.6) |
| Missing | 92(6.7) | 1(2.8) | 1(2.3) | 1(2.6) | 0(0) | 7(7.3) |
| Maternal Education Beyond High School | ||||||
| None | 640(46.4) | 13(36.1) | 20(46.5) | 18(47.4) | 17(54.8) | 46(47.9) |
| Trade certificate, Professional registration or other | 311(22.6) | 13(36.1) | 8(18.6) | 12(31.6) | 6(19.4) | 18(18.8) |
| College or University degree | 401(29.1) | 10(27.8) | 14(32.6) | 7(18.4) | 8(25.8) | 27(28.1) |
| Missing | 26(1.9) | 0(0) | 1(2.3) | 1(2.6) | 0(0) | 5(5.2) |
| Resource Utilization Band | ||||||
| Healthy | 62(4.5) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Low | 353(25.6) | 6(16.7) | 6(14) | 0(0) | 3(9.7) | 12(12.5) |
| Moderate | 906(65.7) | 27(75) | 32(74.4) | 24(63.2) | 13(41.9) | 72(75) |
| High or Very High | 57(4.1) | 3(8.3) | 5(11.6) | 14(36.8) | 15(48.4) | 12(12.5) |
| Total Number of Exposures | ||||||
| 1 | N/A | 36(100) | 40(93) | 36(94.7) | 15(48.4) | N/A |
| 2 | N/A | 0(0) | 3(7) | 2(5.3) | 9(29) | N/A |
| 3 or greater | N/A | 0(0) | 0(0) | 0(0) | 7(22.6) | N/A |
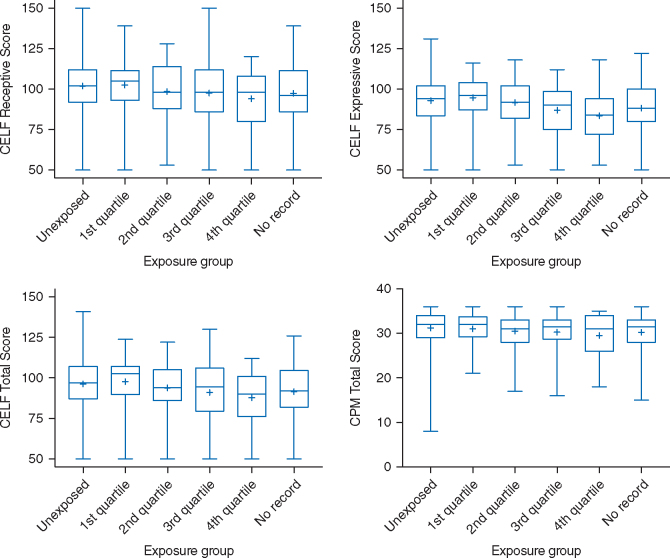
The neuropsychological test scores for each quartile were initially evaluated, with lower mean and median scores seen in the quartiles with longer exposure duration. (Fig. 2) Linear regression was subsequently used to calculate the difference in test scores between unexposed children and those in each exposure group. After adjusting for patient characteristics and comorbidity, children in the 1st and 2nd quartiles were found to have CELF-T scores that were similar to unexposed children [CELF-T: (1st quartile – Unexposed), -0.7; 95% confidence interval (CI), (-4.2 – 5.6) and (2nd quartile – Unexposed), -1.9; 95% CI, (-6.3 – 2.6)]. (Table 2) Children in the 3rd and 4th quartiles of exposure however had mean total language scores approximately six points lower than unexposed children [CELF-T: (3rd quartile – Unexposed), -5.3; 95% CI, (-10.2 – -0.4) and (4th quartile – Unexposed), -6.2; 95% CI, (-11.6 – -0.9)]. Similar findings were seen with the CELF-E expressive language scores, with no differences between unexposed and the 1st and 2nd quartiles [CELF-E: (1st quartile – Unexposed), 1; 95% CI, (-3.9 – 5.9) and (2nd quartile – Unexposed), and -1; 95% CI (-5.4 – 3.5)], but a significant difference in the 3rd and 4th quartiles [CELF-E: (3rd quartile – Unexposed), -6.1; 95% CI (-11 – -1.2) and (4th quartile – Unexposed), and (-6.3; 95% CI (-11.6 – -0.9)]. After adjusting for covariates, significantly lower scores were not found with CELF-R or CPM scores in any exposure quartile.
Fig 2.
Box plots of neuropsychological outcomes and exposure groups. The + represents the mean score of each exposure group.
Table 2.
Crude and adjusted differences in language and cognitive test scores between unexposed children and children in each exposure group. *Adjusted for sex, race, birthweight (<2500g), maternal school level and income. **Adjusted for the above patient characteristics and comorbidity measured by resource utilization band (RUB)
| Crude and adjusted differences in neuropsychological test scores in children in each exposure duration quartile compared with unexposed children | ||||
|---|---|---|---|---|
| n | Crude, difference (95% CI) | Adjusted for patient characteristics | Adjusted for patient characteristics and RUB | |
| Adjusted difference* (95% CI) | Adjusted difference** (95% CI) | |||
| CELF Receptive | ||||
| Unexposed | 1378 | Reference | Reference | Reference |
| First Quartile (≤25 min) | 36 | 0.7 (-4.7 – 6) | 0 (-5.2 – 5.1) | 0.1 (-5.1 – 5.2) |
| Second Quartile (>25 to ≤ 35 min) | 43 | −3.4 (-8.3 – 1.5) | −2.8 (-7.5 – 1.8) | −2.7 (-7.4 – 2) |
| Third Quartile (>35 to ≤ 60 min) | 38 | −4.3 (-9.5 – 0.9) | −4.8 (-9.9 – 0.2) | −4.1 (-9.3 – 1.1) |
| Fourth Quartile (>60 min) | 31 | −7.8 (-13.6 – -2.1) | −6.6 (-12.1 – -1.1) | −5.6 (-11.3 – 0.05) |
| No Record | 96 | −4.5 (-7.8 – -1.1) | −3.9 (-7.2 – -0.6) | −3.7 (-7 – -0.4) |
| CELF Expressive | ||||
| Unexposed | 1378 | Reference | Reference | Reference |
| First Quartile (≤25 min) | 36 | 1.7 (-3.3 – 6.8) | 1 (-3.9 – 5.9) | 1 (-3.9 – 5.9) |
| Second Quartile (>25 to ≤ 35 min) | 43 | −1.3 (-5.9 – 3.4) | −1 (-5.4 – 3.4) | −1 (-5.4 – 3.5) |
| Third Quartile (>35 to ≤ 60 min) | 38 | −5.9 (-10.8 – -1) | −6.6 (-11.4 – -1.8) | −6.1 (-11 – -1.2) |
| Fourth Quartile (>60 min) | 31 | −8.3 (-13.7 – -2.9) | −7.1 (-12.3 – -1.9) | −6.3 (-11.6 – -0.9) |
| No Record | 96 | −4.8 (-7.9 – -1.6) | −4.3 (-7.5 – -1.2) | −4.3 (-7.5 – -1.2) |
| CELF Total | ||||
| Unexposed | 1378 | Reference | Reference | Reference |
| First Quartile (≤25 min) | 36 | 1.4 (-3.7 – 6.5) | 0.7 (-4.2 – 5.6) | 0.7 (-4.2 – 5.6) |
| Second Quartile (>25 to ≤ 35 min) | 43 | −2.4 (-7.1 – 2.2) | −2 (-6.4 – 2.5) | −1.9 (-6.3 – 2.6) |
| Third Quartile (>35 to ≤ 60 min) | 38 | −5.3 (-10.3 – -0.4) | −5.9 (-10.7 – -1.2) | −5.3 (-10.2 – -0.4) |
| Fourth Quartile (>60 min) | 31 | −8.5 (-13.9 – -3) | −7.2 (-12.4 – -2) | −6.2 (-11.6 – -0.9) |
| No Record | 96 | −4.8 (-8 – -1.6) | −4.3 (-7.4 – -1.1) | −4.2 (-7.3 – -1) |
| CPM Total | ||||
| Unexposed | 1378 | Reference | Reference | Reference |
| First Quartile (≤25 min) | 36 | −0.2 (-1.4 – 1) | −0.4 (-1.6 – 0.8) | −0.4 (-1.6 – 0.8) |
| Second Quartile (>25 to ≤ 35 min) | 43 | −0.7 (-1.8 – 0.4) | −0.8 (-1.9 – 0.3) | −0.7 (-1.8 – 0.4) |
| Third Quartile (>35 to ≤ 60 min) | 38 | −0.9 (-2.1 – 0.3) | −1.1 (-2.3 – 0.1) | −0.9 (-2.1 – 0.3) |
| Fourth Quartile (>60 min) | 31 | −1.7 (-3 – -0.4) | −1.5 (-2.7 – -0.2) | −1.3 (-2.6 – 0.1) |
| No Record | 96 | −1 (-1.7 – -0.2) | −0.9 (-1.7 – -0.2) | −0.9 (-1.7 – -0.1) |
Children who were exposed with no anaesthetic record available, after adjusting for covariates, had significantly lower scores compared with unexposed children in Receptive [CELF-R: (No Record – Unexposed)], -3.7; 95% CI, (-7 – -0.4), Expressive [CELF-E: (No Record – Unexposed)], -4.3; 95% CI, (-7.5 – -1.2), and Total language scores [CELF-T: (No Record – Unexposed)], -4.2; 95% CI, (-7.3 – -1), and CPM scores [CPM: (No Record – Unexposed)], -0.9; 95% CI (-1.7 – -0.1).
Types of anaesthetics used
In the 148 children exposed to VA, 99.3% had been exposed to nitrous oxide, 91.2% to halothane, 15.5% to isoflurane, and 12.8% to enflurane. While nearly all children received nitrous oxide and halothane, more children received isoflurane and enflurane in the 3rd and 4th quartiles of exposure compared with those in the 1st and 2nd quartiles. Aside from VA, they received other anaesthetic agents, with thiopental used in 35.8% of children, propofol in 9.5%, benzodiazepines in 15.5%, pethidine in 53.4%, morphine in 11.5%, and fentanyl in 6.8%.
Types of procedures
Most procedures performed in children in the 1st and 2nd quartiles were otolaryngological and other minor procedures, including myringotomies and tonsillectomies. Those children were found to be similar to unexposed children with regard to language and cognitive scores. Children with durations of exposures in the 3rd and 4th quartiles had more varied types of surgical and diagnostic procedures. (Appendix Tables 1 to 4) The parentally reported procedures in children exposed with no anaesthetic record include a variety of procedures from minor procedures to more complex procedures. (Appendix Table 5)
Discussion
Compared with unexposed children in this cohort, children exposed to longer durations of VA before age three had mean CELF-R and CELF-T language scores at age 10 that were approximately six points, or 0.4 standard deviations, lower than unexposed children, even after adjusting for underlying patient characteristics and clinical differences. These results suggest that children in this cohort who had surgery requiring >35 min of VA had significantly decreased language test scores during middle childhood. Wilder and colleagues19 also identified duration for a potentially toxic anaesthetic exposure at >120 min, but our results show that shorter exposures might also be associated with lower neuropsychological test scores. Possibilities for this discrepancy include differences in the patient populations or in the neurodevelopmental test sensitivity, as direct neuropsychological testing might be more sensitive than other tests. While the IQ test differs from the CELF test, as a reference point, lead levels of 10 μg dL − 1 are associated with decreases in IQ of 5.8 points, or 0.39 standard deviations, but the US Center for Disease Control recommends that public health actions are initiated at blood lead levels of 5 μg dL − 1.28 29
It is however important to note that this is only an association, and could be as a result of patient comorbidity. Children requiring longer and more complex surgical procedures and having other comorbid conditions could have higher baseline risk for neurodevelopmental deficits. Unfortunately, teasing out whether this is as a result of the anaesthetic dose or comorbid disease is difficult using observational studies. While we adjusted for comorbidity using the Johns Hopkins ACG Case-Mix System, this comorbidity score is designed to measure resource utilization, not specifically the increased risk of long-term neurodevelopmental deficit. As a result, while it will likely adjust for some of the difference in comorbidity between the exposed and unexposed children, there might be residual confounding because of medical comorbidity.
In order to determine the causal effect of anaesthetic exposure on neurodevelopmental deficit, clinical trials are essential. However, designing a clinical trial to evaluate different doses and durations of anaesthesia present ethical and logistical challenges, as it is difficult to randomize a child to get more or less anaesthesia for a particular procedure. Currently the General Anaesthesia compared with Spinal anaesthesia (GAS) study is the only ongoing randomized trial evaluating anaesthetic neurotoxicity, and assesses neuropsychological differences in children exposed to either sevoflurane or a pure regional technique at under six months of age. The interim results of the GAS study recently found that a mean exposure duration of 54 min of sevoflurane, did not result in any measureable neuropsychological difference at age two yr, with the final results of the study expected after the children are tested at age five yr.30 It should be recognized that the duration of exposure is not the only difference between the GAS study and other observational studies. Children in the GAS study were exposed to anaesthesia between the yr 2007 and 2013, while in the study by Wilder and colleagues children were exposed from 1976 and 1986, and in the present Raine cohort children were exposed from 1989 and 1995. Pulse oximetry and standard anaesthetic monitoring was available for children in the Raine cohort, but the medications from both observational studies included halothane, enflurane, and isoflurane in conjunction with nitrous oxide and other i.v. agents, while the GAS study used sevoflurane only.
The GAS study can isolate the effect of a short sevoflurane exposure, but it is possible that a single short exposure to a single anaesthetic agent is not sufficient to cause a neurotoxic injury. Preclinical evidence has found neurotoxic effects in rodents after at least a one-h exposure, and in non-human primates after a five-h exposure, so the impact of longer or multiple anaesthetic exposures in children, if any, is still unknown.31 32 Combinations of anaesthetic agents, such as VA used in conjunction with i.v. agents and nitrous oxide could cause more neuronal damage than sole agents, as has been described in rodent and non-human primate models.2 7 33 The neurotoxic potency of different volatile anaesthetics is also unclear with some animal studies showing that desflurane and isoflurane have greater toxicity than sevoflurane, while others have found all three anesthetics to be equally toxic, with no studies comparing them in humans.34, 35, 36
Based on the preclinical data and data from other observational studies, the total number of discrete exposures might also play a role.17 19 Our results show that while the test scores for children in the 2nd and 3rd quartiles differed, the number of children with multiple exposures in those quartiles was similar. This study was not designed to evaluate multiple exposures, but this suggests that there might be other factors such as total duration of exposure or comorbidity associated with needing multiple procedures that contribute to lower scores in multiple exposure patients, independent of the number of discrete exposures.
In evaluating children who were exposed but had no records available, we found an increased risk for all neuropsychological outcomes. While the differences were significant for all tests, the estimated test score differences were approximately the average of those seen in the four quartiles. The reason for significance was therefore likely not as a result of an increased effect in the children exposed with no records, but instead because of a larger sample size compared with the number of children in each individual quartile.
It is difficult to establish a dosage threshold beyond which neurotoxic injury might occur based on our results, but our findings add to the general understanding of clinical anaesthetic neurotoxicity. Importantly, they provide some additional reassurance to parents and clinicians that short anaesthetic exposures (≤35 min) do to not appear to have detrimental long-term effects on sensitive neuropsychological tests of language. This lack of an effect with a short single exposure to anaesthesia is consistent with the PANDA study results, which also suggest that a short exposure for hernia repair does not result in a significant long-term deficit.30 37 As the GAS and PANDA studies both only involve hernia patients, the results from the present study suggest this lack of an effect might also be applicable to patients undergoing a variety of other minor surgical procedures, including myringotomies.
There are several limitations to our study, including the retrospective extraction of anaesthetic exposure data, patient characteristics and clinical differences between exposed and unexposed children, and attrition of the cohort over time. Neuropsychological testing however was performed prospectively, and independent of the hypothesis currently being tested. As anaesthetic data were extracted from paper records from over 20 yr ago, significant fluctuations in haemodynamics or other intraoperative vital signs were difficult to determine. Quantification of cumulative volatile anaesthetic dose (i.e. MAC-h), even though important in evaluating a dose-response effect, would have been relatively inaccurate as the documentation was on paper charts, and not electronic records.38 In addition, some anaesthetic records might have been missed, resulting in exposed children misclassified as unexposed, which could bias toward a null result because of potentially lower scores in unexposed children.
Conclusion
Based on our results, it remains unclear if this is truly a dose-response relationship, or if children who require longer anaesthetic exposures for longer surgeries have other associated medical reasons for lower scores in language assessments. While we could not separate the effect of long anaesthetic exposure from the contribution of different clinical conditions, we were able to further confirm the safety of a short anaesthetic exposure (≤35 min) using a cocktail of anaesthetic medications. Our results also suggest that in future studies of anaesthetic neurotoxicity, children with longer exposures to anaesthesia should be evaluated.
Authors' contributions
Study design/planning: C.I., M.K.H., C.J.D., G.L., L.S.S., B.S.vU-S. Study conduct: M.K.H., J.W.P., A.J.O.W., H.A., B.S.vU-S. Data analysis: C.I., M.S., G.L., L.S.S. Writing paper: C.I., M.K.H., J.W.P., A.J.O.W., C.J.D., M.S., H.A., G.L., L.S.S., B.S.vU-S. Revising paper: all authors
Acknowledgments
We acknowledge the Raine study investigators and staff responsible for the collection of the data presented in this manuscript. Sincere thanks are extended to all study families, as this research could not have been conducted without their participation. We are also grateful to Professor Peter D. Sly, MBBS, FRACP, MD, DSc, Deputy Director, Queensland Children's Medical Research Institute (Brisbane, Australia) and Jenny Mountain, MClinEpi, Study Manager, Raine Study Team, University of Western Australia (Perth, Australia) for their help in acquiring the data for the manuscript. We acknowledge the Columbia University Data Coordinating Center and the Irving Institute for their support of the DCC. We also acknowledge Troy Wooding, MBBS, Angel Dong, MBBS, and Eli Lawson, MBBS for their help in reviewing medical and anaesthetic records.
Declaration of interest
None declared.
Funding
This work was supported in part by a grant from SmartTots (San Francisco, CA, USA) and a grant of the Australian and New Zealand College of Anaesthetists (Melbourne, Australia). The Western Australian Pregnancy Cohort (Raine) Study is funded by project and program grants from the National Health and Medical Research Council of Australia (Canberra, Australia). Core management funding is provided by the Raine Medical Research Foundation, the Telethon Kids Institute, the University of Western Australia (UWA), the UWA Faculty of Medicine, Dentistry and Health Sciences, the Women and Infants Research Foundation, Curtin University, and Edith Cowan University. All institutions providing core management funding reside in Perth, Australia. C.I. is supported by the Agency for Healthcare Research and Quality (AHRQ) award number K08HS022941. The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ. A.W. is supported by a Senior Research Fellowship from the National Health and Medical Research Council (APP1077966). B.vU.-S. is supported by the Princess Margaret Hospital Foundation (Australia), the Callahan Estate and the Stan Perron Fellowship (Australia). This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), Grant Number UL1 TR000040. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Handling editor: Hugh C. Hemmings Jr
References
- 1.Cattano D, Young C, Straiko MM, Olney JW. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg. 2008;106:1712–1714. doi: 10.1213/ane.0b013e318172ba0a. [DOI] [PubMed] [Google Scholar]
- 2.Jevtovic-Todorovic V, Hartman RE, Izumi Y. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–1707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 4.Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5:515–521. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- 5.Slikker W, Jr, Zou X, Hotchkiss CE. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 6.Young C, Jevtovic-Todorovic V, Qin YQ. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou X, Liu F, Zhang X. Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicol Teratol. 2011;33:592–597. doi: 10.1016/j.ntt.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Bercker S, Bert B, Bittigau P. Neurodegeneration in newborn rats following propofol and sevoflurane anesthesia. Neurotox Res. 2009;16:140–147. doi: 10.1007/s12640-009-9063-8. [DOI] [PubMed] [Google Scholar]
- 9.Paule MG, Li M, Allen RR. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satomoto M, Satoh Y, Terui K. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 11.Disma N, Mondardini MC, Terrando N, Absalom AR, Bilotta F. A systematic review of methodology applied during preclinical anesthetic neurotoxicity studies: important issues and lessons relevant to the design of future clinical research. Paediatr Anaesth. 2016;26:6–36. doi: 10.1111/pan.12786. [DOI] [PubMed] [Google Scholar]
- 12.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–291. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen TG, Pedersen JK, Henneberg SW. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 14.O'Leary JD, Janus M, Duku E. A population-based study evaluating the association between surgery in early life and child development at primary school entry. Anesthesiology. 2016;125:272–279. doi: 10.1097/ALN.0000000000001200. [DOI] [PubMed] [Google Scholar]
- 15.Stratmann G, Lee J, Sall JW. Effect of general anesthesia in infancy on long-term recognition memory in humans and rats. Neuropsychopharmacology. 2014;39:2275–2287. doi: 10.1038/npp.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backeljauw B, Holland SK, Altaye M, Loepke AW. Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics. 2015;136:e1–12. doi: 10.1542/peds.2014-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flick RP, Katusic SK, Colligan RC. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–e1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117:494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 19.Wilder RT, Flick RP, Sprung J. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ing C, Dimaggio C, Whitehouse A. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–e485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 21.Ing CH, DiMaggio CJ, Malacova E. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120:1319–1332. doi: 10.1097/ALN.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 22.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 24.Dunn L, Dunn L, Williams K, Wang J. Peabody Picture Vocabulary Test III. American Guidance Services Inc; Circle Pines, MN: 1997. [Google Scholar]
- 25.Semel E, Wiig E, Secord W. Clinical Evaluation of Language Fundamentals. 3rd Edn. Psychological Corporation Harcourt Brace Co; San Antonio, TX: 1995. [Google Scholar]
- 26.Raven J, Court J, Raven J. Manual for Raven's Progressive Matricies and Vocabulary Scales-section 2: Coloured progressive matrices. Oxford Psychologists Press; Oxford: 1990. [Google Scholar]
- 27.PC (DOS/WIN/NT) and Unix Version 10.0 - December 2011. Johns Hopkins Bloomberg School of Public Health; Baltimore, MD: 2010. The Johns Hopkins ACG Case Mix System: Version 10.0 Release Notes. [Google Scholar]
- 28.Bellinger DC, Stiles KM, Needleman HL. Low-level lead exposure, intelligence and academic achievement: a long-term follow-up study. Pediatrics. 1992;90:855–861. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention: Lead 2016. Available from http://www.cdc.gov/nceh/lead/
- 30.Davidson AJ, Disma N, de Graaff JC. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brambrink AM, Evers AS, Avidan MS. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valentim AM, Di Giminiani P, Ribeiro PO, Rodrigues P, Olsson IA, Antunes LM. Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology. 2010;113:1099–1108. doi: 10.1097/ALN.0b013e3181f79c7c. [DOI] [PubMed] [Google Scholar]
- 33.Lee BH, Hazarika OD, Quitoriano GR. Effect of combining anesthetics in neonates on long-term cognitive function. Int J Dev Neurosci. 2014;37:87–93. doi: 10.1016/j.ijdevneu.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kodama M, Satoh Y, Otsubo Y. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115:979–991. doi: 10.1097/ALN.0b013e318234228b. [DOI] [PubMed] [Google Scholar]
- 35.Liang G, Ward C, Peng J, Zhao Y, Huang B, Wei H. Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology. 2010;112:1325–1334. doi: 10.1097/ALN.0b013e3181d94da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Istaphanous GK, Howard J, Nan X. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–587. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 37.Sun LS, Li G, Miller TL. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson SA, Young C, Olney JW. Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol. 2008;20:21–28. doi: 10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]