INTRODUCTION
Patients with American Joint Commision on Cancer (AJCC) stage I and II pancreatic adenocarcinoma who are treated with multimodality therapy including surgery have a potential for cure and a median survival that may exceed two years.1 However, surgery is not an option for the 80 to 90% of patients who initially present with locally advanced (AJCC stage III) or metastatic (AJCC stage IV) disease. Indeed, for these patients median survival is usually less than 12 months despite the use of chemotherapy and/or chemoradiation (for patients with stage III disease).2 If the primary tumor can not be surgically removed, long-term survival is uncommon and it is generally felt that cure is not possible because currently available non-surgical therapies rarely result in a complete histologic response.
Over the past several years, a distinct subset of patients has been described, patients with “borderline resectable” tumors. Patients with borderline resectable disease comprise a subset that clarifies the imprecise continuum between radiologically and technically resectable and unresectable disease. The National Comprehensive Cancer Network (NCCN) has recognized borderline resectable pancreatic adenocarcinoma as a unique sub-stage of pancreatic cancer.3 However, thorough understanding of this group of patients has been elusive due to inconsistencies and imprecision in both the definitions and treatment philosophies that have been adopted in different centers. In an attempt to clarify these issues, we recently proposed an objectively defined, CT-based classification for tumors of the pancreatic head, neck and proximal body, consistent with the current AJCC staging system, which clearly distinguishes borderline resectable, from both resectable, and locally advanced primary tumors.4 Borderline tumors are defined as those that abut the superior mesenteric artery (SMA), abut or encase the common hepatic artery over a short segment, or occlude the superior mesenteric vein (SMV) - portal vein (PV) confluence with suitable vein above and below such that venous reconstruction is possible. In addition to this established definition, our evolving experience with the use of multimodality therapy for patients with pancreatic cancer has brought to light two other subsets of patients that may also be considered “borderline resectable” based on additional clinical criteria: 1) patients with indeterminate or questionable metastatic disease at presentation, and 2) patients with a suboptimal performance status or extensive medical comorbidities requiring prolonged evaluation that preclude immediate major abdominal surgery.
From a therapeutic standpoint, no standardized treatment strategy exists for any of these three subsets of patients with borderline resectable disease because there has been no consensus regarding the optimal management of these patients. Patients with borderline resectable disease based on radiologic criteria may be at higher than usual risk for perioperative complications due to the additional complexity of surgery, may be at high risk for early systemic failure due to the advanced nature of the primary tumor, and are at high risk for a margin-positive resection with surgery alone. Therefore, we have advocated the use of multidisciplinary therapy for these patients that utilize neoadjuvant systemic chemotherapy and/or chemoradiation in an attempt to select patients for surgery who are most likely to undergo complete resection and experience long-term survival.4 Likewise, we have approached the other 2 groups of patients with borderline resectable disease with an initial program of non-operative therapy; those patients with an acceptable performance status, fully evaluated comorbidities, and the absence of evolving metastatic disease on post-treatment (preoperative) restaging are considered for pancreatic resection.
The objectives of this report were to present our classification system for patients with borderline disease and to evaluate the outcome of patients prospectively defined as meeting one or more of these M. D. Anderson Cancer Center (MDACC) borderline categories.
PATIENTS AND METHODS
Clinical data on all patients who were evaluated for pancreatic adenocarcinoma between October 1999 and August 2006 were retrieved from an institutional pancreatic tumor database prospectively maintained in the Department of Surgical Oncology. All patients had a pre-treatment cytologic or histologic diagnosis of adenocarcinoma of the pancreas which was obtained or confirmed at our institution; patients with invasive intraductal papillary mucinous neoplasms, mucinous cystadenocarcinomas and other non-pancreatic adenocarcinomas of the periampullary region were excluded.
Baseline evaluation of all patients consisted of a detailed medical history and physical examination, complete blood count and blood chemistries, chest radiograph, and multi-detector contrast-enhanced computed tomography (CT) of the abdomen. Performance status was recorded using the Zubrod/ECOG scale.5 Serum CA 19-9 levels were recorded at the time of referral to our institution; they were not obtained in all patients, particularly during the early years of this report. Serum levels of CA19-9 which were not measurable (indicative of individuals with the Lewis a-b- blood group antigen who do not synthesize CA 19-9) and those associated with an elevated serum bilirubin (> 1.5 mg/dL) were excluded from analysis. Patient age was recorded at the date of the first evaluation.
After initial assessment, patients were reviewed by our multidisciplinary pancreatic tumor study group to determine their stage of disease. All patients who were prospectively characterized as being borderline resectable were included in this analysis. The MDACC borderline resectable categories included three patient subsets as defined by the following clinical and radiographic characteristics: Type A: patients with borderline resectable tumor anatomy as defined on CT images to include the following findings: 1) tumor abutment (180° or less of the circumference of the vessel) of the SMA or celiac axis; 2) tumor abutment or encasement (> 180° of the circumference of the vessel) of a short segment of the hepatic artery typically at the origin of the gastroduodenal artery, or 3) short-segment occlusion of the SMV, PV, or SMV-PV confluence which was amenable to vascular resection and reconstruction because of a patent SMV and PV below and above the area of tumor -related occlusion4 MDACC Type B: patients with borderline resectable disease due to the concern for possible extrapancreatic metastatic disease. This subgroup of borderline resectable patients included those with CT findings suspicious but not diagnostic of metastatic disease, and those with known N1 disease from either pre-referral laparotomy or endoscopic ultrasound-guided fine-needle aspiration. Type B patients may have had a technically resectable6 or a borderline resectable primary tumor as defined on CT images. MDACC Type C patients were those with borderline resectable disease due to a marginal performance status (ECOG 3), or those with a better performance status and significant pre-existing medical co-morbidity thought to require protracted evaluation thereby precluding immediate surgery. Type C patients may also have had a radiologically resectable or a borderline resectable primary tumor. For purposes of analysis, all patients were assigned just one MDACC type; if a patient’s radiographic and clinical findings included more than one borderline subgroup, they were classified in priority of C > B > A (for example, a patient with both MDACC type B and C features would be classified as type C).
Treatment Schema
All patients received an initial treatment program of chemotherapy and/or chemoradiotherapy. Treatment was generally administered off-protocol, but some patients were treated on protocols designed for patients with locally advanced disease. Therapy was administered either at our institution or under the care of the patient’s referring oncologist. External-beam radiation therapy was delivered using either 50.4 Gy in 28 fractions or 30 Gy in 10 fractions. Concomitant chemotherapy included 5-fluorouracil, paclitaxel, gemcitabine or capecitabine in radiosensitizing doses. When systemic therapy was administered, it consisted of gemcitabine alone or in combination; some patients, particularly those treated most recently, received targeted agents. The most common treatment sequence involved 2 to 4 months of systemic therapy followed by chemoradiation with restaging evaluations every 2 months. Approximately 4 to 6 weeks after the completion of all therapy, patients underwent restaging evaluation to include CT and complete physiologic assessment to determine suitability for surgery. Patients who were found on restaging evaluation to have no evidence of progressive disease and who were, in the opinion of the operating surgeon and the multidisciplinary treatment group, suitable for major abdominal operation, were brought to the operating room for planned resection of the primary tumor. The complete treatment algorithm is illustrated in FIGURE 1.
FIGURE 1.
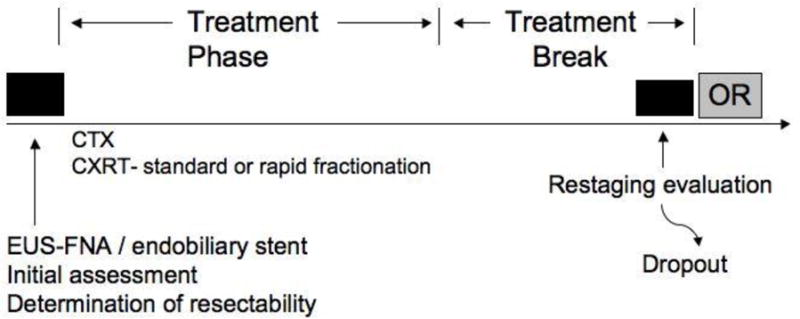
Treatment approach for patients with borderline resectable pancreatic cancer. All patients were initially evaluated with a comprehensive staging evaluation and an assessment of performance status (see text). Patients staged as borderline resectable were treated with induction chemotherapy and/or chemoradiation. After preoperative therapy was complete, restaging was performed to select those patients most likely to benefit from surgery. CTX, chemotherapy; CXRT, chemoradiation.
Pancreaticoduodenectomy and distal pancreatectomy were performed in a standard fashion, as previously described.7,8 Tangential or segmental resection of the SMV, PV, or SMV-PV confluence was performed when the operating surgeon could not separate the pancreatic head and/or the uncinate process from these vessels without leaving gross tumor on the vessel or risking uncontrolled venotomy.9 When limited involvement of the common hepatic artery was identified, segmental resection of this vessel was performed with primary anastomosis or interposition grafting. Patients who were found to be unresectable at surgery, largely due to the presence of radiographically occult extrapancreatic disease, underwent surgical bypass as clinically indicated. Operative time (incision to application of all wound dressings) and blood loss (in mL) were recorded from the anesthesia record. Major postoperative complications were defined as previously described.10 Hospital stay was calculated by considering the day of surgery as day number one; the day of discharge was not counted as a hospital day.
Histopathologic Evaluation and Assessment of Treatment Response
Standardized pathologic evaluation of the surgical specimen was performed as previously described.1,11 The SMA margin was defined as the soft tissue margin directly adjacent to the proximal 3 to 4 cm of the SMA. In all cases, the SMA margin was evaluated according to the AJCC Cancer Staging Manual (6th edition) guidelines.12 This margin was identified and inked by the surgeon and pathologist immediately upon specimen removal, and evaluated by permanent-section microscopic examination of the margin; when tumor extended to the inked margin, the margin was considered positive. The technique for assessment of the SMA margin was the same regardless of whether or not vascular resection was performed. The pancreatic transection margin and the common bile/hepatic duct transection margins were evaluated by examining a complete en face section of each margin. At the discretion of the surgeon, these two margins were usually evaluated using frozen-section analysis, and if positive, additional bile duct or pancreatic parenchyma was usually resected. An operation was designated “R0” if all final margins were negative (no tumor cells were identified at any of the three resection margins) and “R1” if any of the final margins were positive (tumor cells were present at one or more of the margins).
Tumor size was calculated by the pathologist by measuring the maximum gross transverse diameter of the tumor after resection. This measurement was difficult to determine in some patients after preoperative therapy because gross tumor was often hard to distinguish from uninvolved adjacent pancreatic parenchyma. The grade of neoadjuvant treatment effect was assessed on permanent sections by a faculty gastrointestinal pathologist and scored using a previously published grading system.13 A minimal pathologic response was defined as a treatment effect score of either grade I (90% or more viable tumor cells remaining after induction therapy) or IIa (50-89% remaining viable tumor cells). A partial pathologic response was defined as a treatment effect score of IIb (10-49% remaining viable tumor cells) or III (less than 10% remaining viable tumor cells). A treatment effect score of IV, indicative of no remaining viable tumor cells, was used to designate a complete pathologic response.
Follow-up and Statistical Analysis
After completion of all treatment, patients were evaluated every three to four months by physical examination, chest radiography, and abdominal CT. In patients without evidence of disease after two years of follow-up, evaluations were reduced to six month intervals. The development of a new low-density mass in the region of the resected pancreas or root of mesentery was considered evidence of local recurrence even in the absence of symptoms. Similarly, radiographic evidence of a new low-density mass in the liver or lungs was considered evidence of distant recurrence; biopsy was rarely performed for radiographic findings consistent with recurrent cancer. Peritoneal recurrence was defined as the finding of new ascites on physical examination or CT. Only the first site(s) of recurrent disease were documented.
Overall survival was calculated from the date of cytologic or histologic diagnosis until the date of death or last contact; progression free survival was calculated, in resected patients only, from the date of cytologic or histologic diagnosis until the date of recurrence or the last date at which the patient was known to be free of disease. The Kaplan-Meier method was used to generate survival curves by clinical characteristics. The log-rank test was used to compare differences between survival curves. Median follow-up time from time of diagnosis was calculated for all patients as well as for only patients who were censored. All statistical tests were two-tailed with a significance level of p < 0.05. SPSS version 15.0 (Chicago, IL) was used for all statistical analyses.
RESULTS
Patient Demographics and Clinical Variables
Between October 1999 and August 2006, 2,454 patients were evaluated at our institution for the treatment of pancreatic adenocarcinoma. Of these, 160 patients (7%) were prospectively characterized as borderline resectable based on the criteria outlined above: 84 (52%) type A, 44 (28%) type B, and 32 (20%) type C (TABLE 1). The median age of these patients was 63 years (range 36 – 90 years) and 84 (52%) patients were male. Patients classified as type C were significantly older (median 73 years) than type A or B patients (median 60 and 61 years, p < 0.001). Tumors were located in the head or uncinate process in 142 (89%), and the body or tail in 18 (11%) of the 160 patients. Pre-referral laparotomy with an unsuccessful attempt at tumor resection had been performed in 38 (24%) of the 160 patients, with half of these patients classified as type B (p < 0.001 when comparing the frequency of pre-referral laparotomy between groups). Chemotherapy or chemoradiation had been utilized prior to referral in 12 (8%) of 160 patients. Pretreatment serum levels of CA19-9 were evaluable in 116 (73%) of 160 patients; these patients had a median CA 19-9 level of 203 U/mL (range 2.3 – 11482 U/mL). There was no difference in the initial pre-treatment serum level of CA 19-9 between type A, B and C patients.
TABLE 1.
Clinical and demographic characteristics of 160 borderline resectable patients
| All Patients (%) |
Borderline Type
|
p | |||
|---|---|---|---|---|---|
| A | B | C | |||
|
| |||||
| Total No. of Patients | 160 | 84 | 44 | 32 | |
|
| |||||
| Age (Years) | |||||
| Median (Mean) | 63 (63) | 60 (61) | 61 (61) | 73 (71) | 0.001 |
| Range | 36 – 90 | 37 – 81 | 36 – 77 | 50 – 90 | |
|
| |||||
| Gender | NS | ||||
| Male | 84 (52) | 37 (44) | 26 (59) | 21 (66) | |
| Female | 76 (48) | 47 (56) | 18 (41) | 11 (34) | |
|
| |||||
| Tumor Location | NS | ||||
| Head/Uncinate | 142 (89) | 73 (87) | 40 (91) | 29 (91) | |
| Body/Tail | 18 (11) | 11 (13) | 4 (9) | 3 (9) | |
|
| |||||
| Pre-referral Laparotomy | 0.001 | ||||
| Bypass | 31 (19) | 12 (14) | 16 (36) | 3 (9) | 0.003 |
| Exploration only | 7 (4) | 4 (5) | 3 (7) | 0 (0) | NS |
|
| |||||
| Pre-referral Therapy | 12 (8) | 7 (8) | 5 (11) | 0 (0) | NS |
| Systemic Chemotherapy | 6 (4) | 3 (4) | 3 (7) | 0 (0) | NS |
| External-beam Radiation | 7 (4) | 5 (6) | 2 (5) | 0 (0) | NS |
|
| |||||
| Pre-treatment CA19-9 (U/ml) | |||||
| All patients | |||||
| Median (Mean) | 203 (778) | 171 (728) | 266 (871) | 324 (767) | NS |
| Range | 2.3 – 11482 | 2.3 – 11482 | 3 – 7194 | 13 – 3787 | |
|
| |||||
| Patients who underwent pancreatectomy | |||||
| Median (Mean) | 207 (877) | 82 (974) | 21 (755) | 324 (830) | NS |
| Range | 9 – 11482 | 19 – 11482 | 9 – 7194 | 32 – 2797 | |
|
| |||||
| Patients who did not undergo pancreatectomy | |||||
| Median (Mean) | 203 (699) | 187 (551) | 323 (993) | 268 (730) | NS |
| Range | 2 – 6725 | 2 – 6725 | 3 – 3996 | 13 – 3787 | |
|
| |||||
| Underwent Pancreatectomy | |||||
| Yes | 66 (41) | 32 (38) | 22 (50) | 12 (38) | NS |
| No | 94 (59) | 52 (62) | 22 (50) | 20 (62) | |
Treatment
The flow of all patients through the treatment schema is illustrated in FIGURE 2. Post-treatment preoperative restaging evaluation was not completed in 35 (22%) of the 160 patients. After initial evaluation, 9 (26%) of these 35 patients were lost to follow-up. These were patients that were sent back to their local oncologists after initial staging and multidiscilplinary assessment with therapeutic recommendations and a plan to return to MDACC for restaging. We believe that these patients did not return as a result of disease progression and/or a decline in performance status. Prior to the completion of all planned neoadjuvant therapy or scheduled preoperative restaging, 14 (40%) of 35 patients manifested distant (8) or local-regional (6) disease progression. The remaining 12 patients (34%) tolerated the therapy poorly and/or suffered what was felt to be an irreversible decline in performance status, of whom 6 patients died prior to restaging. Of these 6 deaths, 4 occurred (after the completion of all neoadjuvant therapy) due to disease progression (1), complications associated with profound dehydration and acute renal failure (1), cholangitis secondary to an occluded endobiliary stent (1) and complications of small bowel obstruction (1). The cause of death of two patients who died during initial treatment is unknown.
FIGURE 2.
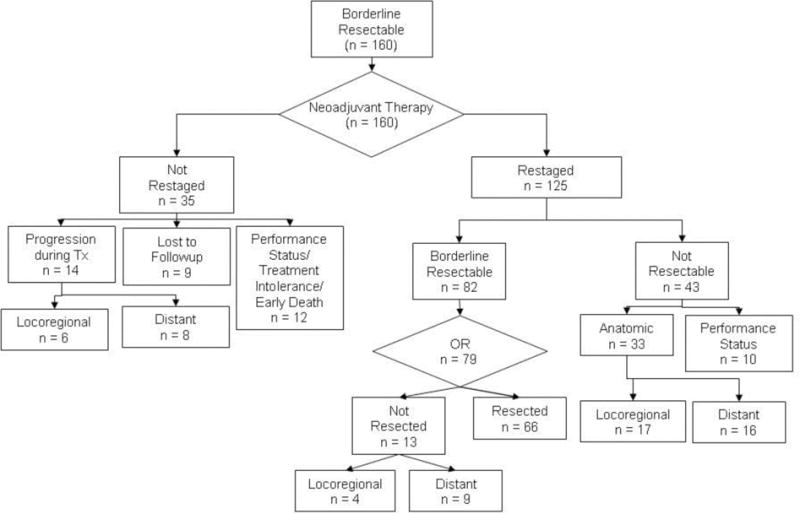
Treatment of 160 patients with borderline resectable pancreatic cancer. All patients were received induction therapy; 125 (78%) patients completed therapy and returned for preoperative restaging. Surgery was recommended in 82 patients, of whom 79 underwent operation; 66 (41% of 160 total patients) were resected. Reasons for failure to receive all therapy included the development of disease progression and/or an irreversible decline in performance status.
In total, 125 (78%) of the 160 patients underwent a complete restaging evaluation after a median of 15 weeks (range 10 days to 40 weeks) of induction therapy. The duration of neoadjuvant therapy of type C patients was significantly shorter (median 4 weeks, range 10 days–34 weeks) than either type A (median 16, range 2-40 weeks) or B patients (median 15, range 2-37 weeks, p = 0.002). During preoperative therapy, 82 (66%) of the 125 patients received systemic chemotherapy and 117 (94%) received chemoradiation. When pre-referral therapy was included, 83 (66%) of 125 patients had received systemic chemotherapy and 122 (98%) received chemoradiation prior to preoperative restaging.
At the time of restaging evaluation, 43 (34%) of the 125 patients were determined to be ineligible for surgery. These 43 patients included 10 (23%) that were felt to have a performance status insufficient for major abdominal operation and 33 (77%) with CT evidence of distant disease progression (16) or unresectable local-regional disease (17). The remaining 82 patients (66%) were without evidence of metastatic disease and were determined to have tumor anatomy and a performance status suitable for surgery. Of these 82 patients, 3 refused surgery; the remaining 79 patients (65% of the 125 who were restaged) were brought to the operating room for planned pancreatectomy. The median time from completion of neoadjuvant therapy to surgery in these 79 patients was 7 weeks (range 2 – 51 weeks). Of the 79 patients brought to surgery, 13 (16%) were found to have unresectable disease due to the presence of radiographically occult distant metastases (9) or locally advanced disease (4), and 66 underwent a complete gross resection of the primary tumor.
In total, 66 (41%) of 160 borderline resectable patients underwent pancreaticoduodenectomy (57) or distal subtotal pancreatectomy (9) (TABLE 2). There was no statistically significant difference in the resectability rate between borderline types. The 66 resected patients underwent surgery a median of 22 weeks (range 7 – 65 weeks) after initiation of therapy, with no significant difference in the length of time from start of therapy to surgery between borderline types. Venous resection was performed in 18 (27%) of 66 patients; two of these patients also required short-segment resection of the common hepatic artery. There was no difference in the number of patients who required vascular resection and reconstruction among the three borderline types. For the 66 patients who underwent pancreatectomy, median operative blood loss was 700 mL (range 75 – 3300 mL), median operative time was 6.8 hours (range 2.9 – 12.9 hours) and median length of hospital stay was 10 days (range 6 – 42 days). There was no difference in median blood loss or median operative time between the three borderline types, but type C patients had a longer length of hospital stay than either type A or B patients (p = 0.05). Additional postoperative adjuvant therapy was delivered to 13 (20%) of the 66 patients; chemoradiation was administered to both patients who had not received it preoperatively, and 11 additional patients received systemic chemotherapy.
TABLE 2.
Preoperative, operative, and histopathologic data of 66 patients who received all therapy (underwent pancreatectomy)
| All Patients (%) |
Borderline Type
|
p | |||
|---|---|---|---|---|---|
| A | B | C | |||
|
| |||||
| Total No. of Patients | 66 | 32 | 22 | 12 | |
|
| |||||
| Preoperative Factors | |||||
|
| |||||
| Laparotomy prior to referral | |||||
| Bypass | 14 (21) | 4 (13) | 7 (32) | 3 (25) | NS |
| Exploration Only | 4 (6) | 2 (6) | 2 (9) | 0 (0) | |
|
| |||||
| Any Neoadjuvant Therapy* | 63 (100) | 32 (100) | 20 (100) | 11 (100) | |
| Systemic Chemotherapy | 49 (74) | 24 (75) | 19 (86) | 6 (50) | NS |
| Chemoradiation | 63 (95) | 32 (100) | 19 (86) | 12 (100) | |
|
| |||||
| Duration of Neoadjuvant Therapy (Weeks) | |||||
| Beginning to end of therapy | |||||
| Median (Mean) | 15 (15) | 16 (16) | 17 (16) | 11 (11) | NS |
| Range | 1.1 – 40 | 2.4 – 40 | 2.3 – 37 | 1.1 - 34 | |
| End of therapy to surgery | |||||
| Median (Mean) | 6 (8) | 6 (7) | 7 (7) | 8 (12) | NS |
| Range | 2 – 51 | 2 – 18 | 3 – 15 | 5 - 51 | |
|
| |||||
| Restaging (preoperative) CA19-9 (U/mL) | |||||
| Median (Mean) | 39 (138) | 28 (75) | 41 (234) | 46 (140) | NS |
| Range | 3 – 2908 | 3 – 566 | 8 – 2908 | 27 – 960 | |
|
| |||||
| Operative Factors | |||||
|
| |||||
| Surgical Procedure | |||||
| Pancreaticoduodenectomy | 57 (86) | 28 (87) | 19 (86) | 10 (83) | NS |
| Distal subtotal Pancreatectomy | 9 (14) | 4 (13) | 3 (14) | 2 (17) | |
|
| |||||
| Operative Time (Minutes) | |||||
| Median (Mean) | 408 (425) | 451 (444) | 374 (399) | 371 (419) | NS |
| Range | 174 – 774 | 227 – 772 | 184 - 623 | 174 – 774 | |
|
| |||||
| Estimated Blood Loss (mL) | |||||
| Median (Mean) | 700 (850) | 738 (977) | 650 (785) | 600 (633) | NS |
| Range | 75 – 3300 | 90 – 3300 | 125 – 2500 | 75 – 1500 | |
|
| |||||
| Hospital Stay (Days) | |||||
| Median (Mean) | 10 (11) | 9 (11) | 9 (12) | 13 (12) | .03 |
| Range | 6 – 42 | 6 - 21 | 6 – 42 | 7 – 20 | |
|
| |||||
| Vascular Resection | |||||
| Hepatic Artery | 2 (3) | 2 (3) | 0 | 0 | NS |
| SMV/PV | 18 (27) | 12 (38) | 3 (14) | 3 (25) | |
|
| |||||
| Histopathologic Factors | |||||
|
| |||||
| Treatment Effect Score** | |||||
| I | 0 | 0 | 0 | 0 | |
| IIa | 26 (40) | 11 (34) | 8 (36) | 7 (58) | |
| IIb | 25 (38) | 12 (38) | 9 (41) | 4 (33) | NS |
| III | 8 (12) | 4 (13) | 4 (18) | 0 | |
| IV | 4 (6) | 3 (9) | 0 | 1 (8) | |
|
| |||||
| Tumor Size (cm) | |||||
| Median (Mean) | 2.5 (2.6) | 2.5 (2.5) | 2.9 (2.7) | 2.5 (2.5) | NS |
| Range | 0.2 – 6 | 0.2 – 6 | 0.6 – 4.3 | 0.8 – 3.5 | |
|
| |||||
| R status | |||||
| R0 | 62 (94) | 31 (97) | 21 (95) | 10 (83) | NS |
| R1 | 4 (6) | 1 (3) | 1 (5) | 2 (17) | |
|
| |||||
| Lymph Node Status | |||||
| No. Patients With Positive Nodes (%) | 26 (39) | 12 (38) | 8 (40) | 6 (50) | NS |
|
| |||||
| No. Lymph Nodes Reported† | |||||
| Median (Mean) | 20 (21) | 21 (21) | 20 (20) | 17 (19) | NS |
| Range | 2 – 50 | 5 – 50 | 2 – 43 | 10 – 40 | |
|
| |||||
| No. of Lymph Nodes Positive‡ | |||||
| Median (Mean) | 3 (3) | 2 (3) | 4 (6) | 2 (2) | NS |
| Range | 1 – 21 | 1 – 7 | 1 – 21 | 1 - 4 | |
Includes pre-referral and post-referral treatment
Treatment effect score was not available for 3 patients
Pancreaticoduodenectomy was associated with a higher number of lymph nodes retrieved (median 21,
range 10-50) than distal subtotal pancreatectomy (median 10, range 2-21)
In patients with at least one positive lymph node
Toxicity and Complications
Of 125 patients who underwent a complete restaging evaluation, endobiliary stent exchange was necessary during induction treatment or in the interval between induction treatment and restaging in 19 (15%) due to stent occlusion or cholangitis. In total, 25 (20%) of the 125 patients required hospitalization prior to restaging. The primary indications for the 27 hospitalizations in these 25 patients were dehydration (8), hematologic toxicity (2), gastrointestinal toxicity (3), low-grade sepsis (3) and endobiliary stent occlusion (11). It should be noted that toxicities recorded prior to restaging may have been incompletely reported, because not all patients received therapy at our institution.
Major postoperative complications occurred in 13 (20%) of 66 patients who underwent pancreatectomy. Perioperative death occurred in two patients. The first of these was a 77-year old, type C patient who underwent an uncomplicated pancreaticoduodenectomy but required an emergent return to the operating room on postoperative day four for intra-abdominal hemorrhage at which time no discrete source of surgical bleeding was identified. Two days later she suffered bilateral cerebral cortical infarcts leading to a progressive neurologic decline and death on postoperative day 13. The other death occurred in a 54-year old type C patient who underwent pancreaticoduodenectomy with PV resection and reconstruction. After an uncomplicated postoperative course, she required readmission to the hospital 30 days after initial discharge for upper gastrointestinal hemorrhage secondary to an inferior pancreaticoduodenal artery pseudoaneurysm which was successfully treated by exclusion with an SMA stent. She subsequently developed recurrent upper gastrointestinal hemorrhage localized to a second pseudoaneurysm identified from the right hepatic artery. Despite successful embolization, she never fully recovered satisfactory end organ function and ultimately expired five months after her initial pancreatectomy. Other major complications included pulmonary embolus treated by anticoagulation (1), abdominal wall dehiscence requiring re-operation (1), upper gastrointestinal hemorrhage managed conservatively (1), chylous drainage from an abdominal drain site which resolved (1), clostridium difficile colitis (2), intra-abdominal fluid collection requiring percutaneous drainage (1), transient acute pulmonary failure (2) and cardiac tachyarrythmia (2).
Histopathology and Treatment Effect
All 66 patients who underwent surgical resection were confirmed to have pancreatic adenocarcinoma on final pathologic analysis (TABLE 2). Median tumor size was 2.5 cm (range 0.2 – 6 cm) with no difference between borderline types. Surgical margins were grossly negative in all patients; 4 (6%) of 66 patients, all of whom underwent pancreaticoduodenectomy, were found to have a microscopically positive margin on permanent histologic sections, including the SMA (2), pancreatic (1) and bile/hepatic duct (1) margins. The patient with a positive pancreatic margin had atypia on intraoperative frozen-section, underwent re-resection of the margin (x2) and the permanent section analysis of the margin was positive for invasive adenocarcinoma. A median of 20 lymph nodes per specimen were examined (range 2 – 50), with a higher number of nodes retrieved and examined in patients who underwent pancreaticoduodenectomy (median 21, range 10-50) compared to patients who underwent distal subtotal pancreatectomy (median 10, range 2-21, p = 0.001). Lymph node metastases were identified in 26 (39%) of the 66 patients who underwent pancreatectomy, including 12 (38%) type A patients, 8 (40%) type B, and 6 (50%) type C patients. The relative number of patients with node-positive disease did not differ between borderline types. Of patients with positive nodes, the median number of nodes positive was 3 (range 1 – 21).
The neoadjuvant treatment effect score was determined in 63 of the 66 resected patients; the score was not recorded in the final pathology report in 3 patients (and slides are no longer available for re-review). A partial or complete pathologic response to treatment (< 50% remaining viable tumor cells, scores IIb, III or IV) was found in 37 (56%) of the 66 patients who underwent surgical resection. The percentage of patients with a partial or complete pathologic response was similar across borderline types. Four (6%) of the 66 patients had complete pathologic response (grade IV); one of these patients developed recurrent metastatic pancreatic cancer, one died of metastatic nonsmall cell carcinoma of the lung, and the other 2 patients remain without evidence of disease. The patient who died of metastatic lung cancer was thought to have a separate primary ductal adenocarcinoma of the pancreas based upon the morphology and immunohistochemistry profile of the pancreatic tumor biopsy. The pre-treatment biopsy material of the 2 patients currently without evidence of disease has been re-reviewed by the senior cytopathologist at MDACC. In one patient the diagnosis was confirmed, in the other patient there is no consensus regarding the presence or absence of adenocarcinoma on the pre-treatment biopsy. However, this patient had a serum level of CA19-9 of over 600 U/mL at the start of systemic therapy (with a normal level of serum bilirubin) and it declined to 112.7 U/mL after systemic therapy and to 22.2 U/mL at the time of preoperative restaging following chemoradiation. This patient’s CA19-9 remains within the normal range (21 U/mL) 2 years following pancreaticoduodenectomy.
Follow-up, Survival and Recurrence
Median follow-up for all 160 borderline resectable patients was 15 months (mean 21 months); for censored patients it was 25 months (mean 31 months, minimum 9 months). At the time of last follow-up, 110 (69%) of the 160 patients had died: 29 (44%) of 66 patients who completed all therapy including surgery, and 81 (86%) of 94 patients who did not undergo surgical resection. The overall median survival of all 160 patients was 18 months with a corresponding 5-year survival of 18% (TABLE 3), (FIGURE 3). Patients of borderline types A, B, and C had a median survivals of 21, 16 and 15 months, respectively, with corresponding 5-year survivals of 19%, 19%, and 16%, respectively. The 66 patients who completed all therapy including surgical resection had a median survival of 40 months and a 5-year survival of 36% (type A: 40 months/40%; type B: 29 months/46%, type C: 39 months/19%). In contrast, the 94 patients who did not undergo surgical resection of the primary pancreatic tumor had a median survival of only 13 months (type A: 15 months, type B: 12 months, type C: 13 months, p < 0.001). There were two 5-year survivors among the 94 patients who did not complete all intended therapy to include surgery. Both patients had pretreatment biopsies consistent with pancreatic adenocarcinoma and interpreted at MDACC by our senior-most pathologists; however, the absence of extrapancreatic disease progression in both patients leaves room for continued debate over the diagnosis.
TABLE 3.
Rates of resection, pathologic response and survival for 160 patients with borderline resectable pancreatic cancer
| Type | No. of Patients (%) | Median Survival (Months) | p‡ | ||||
|---|---|---|---|---|---|---|---|
| Total | Resected | Treatment Effect IIb, III or IV* | All Patients | Resected Patients | Patients Who Did Not Undergo Resection | ||
| A | 84 (53) | 32 (38) | 19 (59) | 21 | 40 | 15 | 0.001 |
| B | 44 (28) | 22 (50) | 13 (59) | 16 | 29 | 12 | 0.001 |
| C | 32 (20) | 12 (38) | 5 (42) | 15 | 39 | 13 | 0.009 |
| Total | 160 | 66 (41) | 37 (56) | 18 | 40 | 13 | 0.001 |
Percent of patients of that type that underwent resection; treatment effect not reported in 3 of 66 patients who underwent pancreatectomy
p-value represents comparison between median survivals of resected and non-resected patients
FIGURE 3.
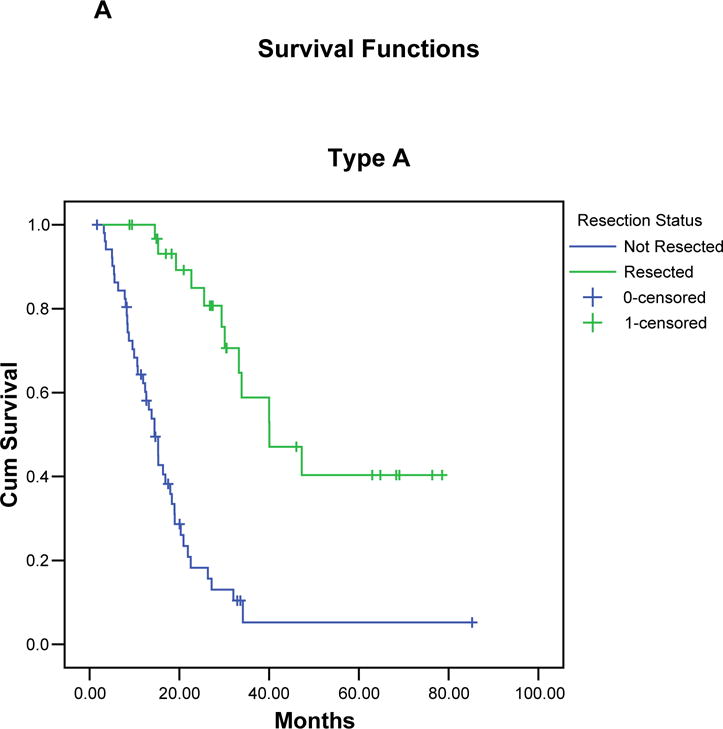
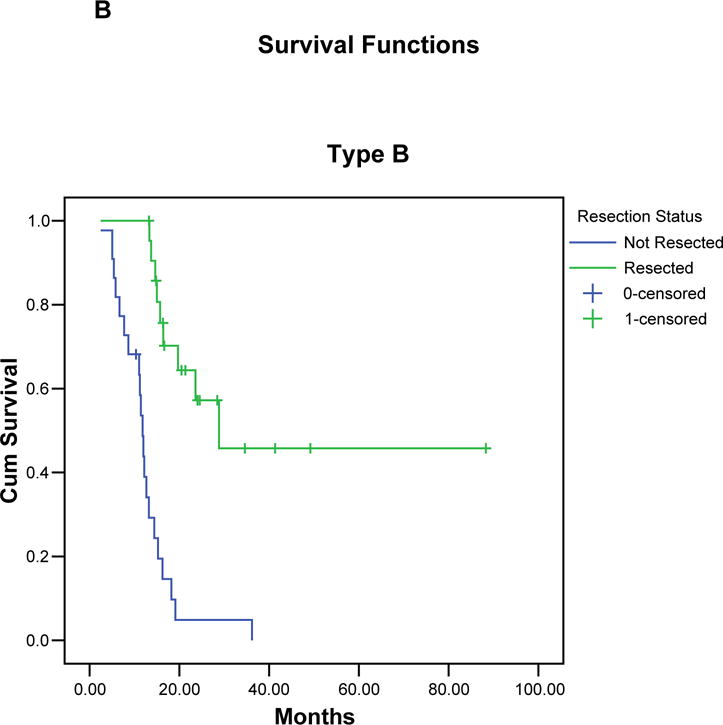
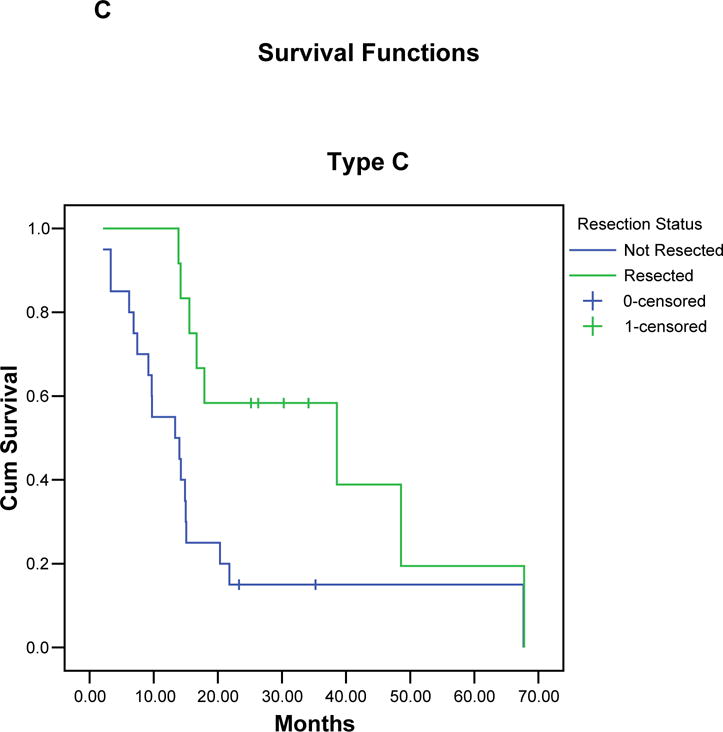
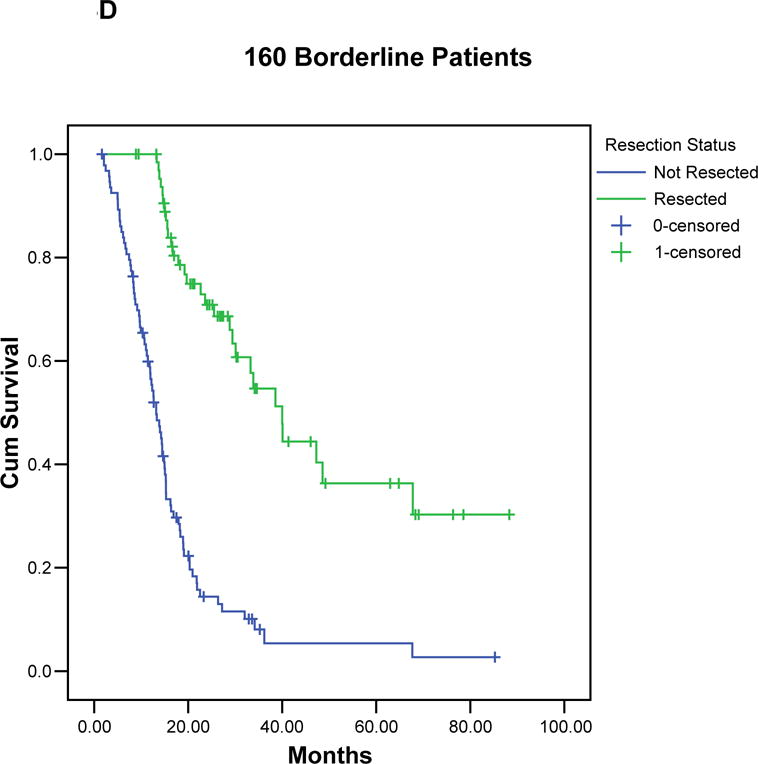
Kaplan-Meier curves illustrating the actuarial survival of patients with resected and unresected pancreatic cancer for each borderline subtype (A-C), and for all 160 patients (D). In each group, patients who completed all therapy including surgery had a significant survival advantage compared to patients who did not undergo surgical resection.
For the 66 patients who completed all therapy, 39 (59%) patients developed recurrent pancreatic cancer which was documented; the median time to progression was 25 months. For these 39 patients, 43 sites of first recurrence were documented in 37 patients (sites of first recurrence unknown in 2 patients) and included distant organs (lung, liver or bone) in 30 (45% of 66), pancreatic bed in 6 (9% of 66) and regional sites (peritoneum or regional lymph nodes) in 7 (11% of 66). Isolated local recurrence was documented in 4 (6%) of the 66 patients who completed all therapy including surgery.
Factors associated with outcome
Evaluable CA19-9 levels at initial referral to MDACC were present in 116 (73%) of the 160 patients. Pre-referral chemotherapy or chemoradiation had been received by X (X%) of the 116 patients and therefore these were also excluded. (Matt, Charlotte, PP also raised the issue of whether it is legit to include pts who presented here after a long interval from diagnosis [? Few months – ie, those that had a laparotomy, stent issuye, etc] – does this lead time bias become a problem??) Serum levels which were not measurable (indicative of individuals with the Lewis a-b- blood group antigen who do not synthesize CA 19-9) and those associated with an elevated serum bilirubin (> 1.5 mg/dL) were excluded from analysis. We found no association between pretreatment serum CA 19-9 level and either the likelihood of undergoing pancreatectomy or overall survival. Of the 116 patients with evaluable CA 19-9 levels, restaging studies were completed in 98 (84%). Both pretreatment and post-treatment (preoperative) serum CA19-9 levels were available in 83 (66%) of the 125 restaged patients. To evaluate the association between the change in serum CA 19-9 over the course of therapy and outcome, we divided 82 of these 83 patients whose CA 19-9 changed (one patient’s serum CA 19-9 did not change) into three groups: 1) patients whose pre-treatment CA 19-9 increased over the course of neoadjuvant therapy (20); 2) patients whose CA 19-9 decreased ≤ 50% from pretreatment to post-treatment levels (14); and 3) patients whose CA 19-9 level decreased > 50% from pre-treatment to post-treatment (48). When compared to the 20 patients who had an increase in their serum CA 19-9 level over the course of induction therapy, patients whose serum CA 19-9 fell were more likely to undergo pancreatectomy (OR = 2.3, p = 0.2, 95% CI [0.6, 9.6] for the 14 patients with a ≤ 50% fall in CA 19-9; OR = 4.7, p = 0.007, 95% CI [1.5, 14.4] for the 48 patients whose CA 19-9 fell > 50%). Similarly, the percent change in CA 19-9 over the course of neoadjuvant treatment was found to be associated with overall survival (p = 0.036). When compared to the 48 patients who had a > 50% decrease in serum CA 19-9, the 14 patients whose CA 19-9 decreased by ≤ 50% had a HR of death of 1.6 (p = 0.27, 95% CI [0.7, 3.6]) and the 20 patients with an increase in serum CA 19-9 had a greater than 2-fold risk of death (HR = 2.4, p = 0.01, 95 % CI [1.2, 4.7]).
Histologic assessment of tumor response to induction therapy was available in 63 of 66 patients. The 26 patients whose tumors demonstrated a minimal pathologic effect from neoadjuvant therapy (treatment score IIa) had over twice the risk of death compared to the 37 patients with a partial or complete pathologic response to treatment (treatment score IIb, III, IV: HR 2.72, p = 0.01, CI [1.26, 5.89]). Although treatment effect was found to be significantly associated with overall survival, the small number of reported patients makes it impossible to draw firm conclusions on the use of treatment effect as a surrogate marker for survival duration. Other factors considered for evaluation included borderline type (A, B or C) and lymph node status. No association between either of these potential covariates and survival could be identified.
DISCUSSION
An important area of clinical investigation by our multidisciplinary working group has been to accurately define the clinical stages of pancreatic cancer using objective, reproducible CT imaging criteria. This allows stage-specific therapy to be applied to patients of adequate performance status and is critical to the creation of eligibility criteria for clinical trials. In this report, we expand upon our previously published definition of borderline resectable pancreatic cancer4 and offer a new, comprehensive classification system for borderline disease. We report our initial institutional experience with the multidisciplinary management of borderline resectable patients illustrating the favorable outcome that can be achieved, using a systematic, multidisciplinary approach, in patients that might otherwise not be considered for potentially curative treatment.
Patients with resectable disease (stage I/II) have a normal tissue plane between the tumor and adjacent arterial structures, and have a patent SMV-PV confluence. Patients with locally advanced disease (stage III) have tumor encasement (defined as > 180°) of adjacent arteries or an occluded SMV-PV with no technical option for resection and reconstruction. Borderline resectable patients are those in the middle: tumor abutment (180° or less) of adjacent arteries or an occluded SMV-PV confluence with an adequate segment of vein above and below the site of tumor involvement to allow for venous resection and reconstruction. In this report, we expanded the definition of borderline resectable disease. In addition to patients with tumor-artery abutment (Type A), we have added those with questionable extrapancreatic metastatic disease (Type B), and a marginal pretreatment performance status (Type C). The MDACC Type B and C subgroups arose out of observations made during our weekly multidisciplinary conferences. Increasingly we have seen these subsets of borderline resectable patients; those with questionable metastatic disease (a group that may become more common as our imaging studies become more sensitive) and the group of patients that may have a resectable primary tumor but have associated medical comorbidities or a performance status that makes up-front surgery of unacceptably high risk. It is our strong belief that this approach to pretreatment clinical staging allows for more accurate administration of stage-specific treatment, minimizes treatment indecision, and avoids unnecessary surgery in those patients who clearly have unresectable tumors. In addition, such a uniform staging system allows physicians to communicate with each other using a standard nomenclature for extent of disease and removes some of the imprecision from the terms “resectable” and “unresectable”.
A continued area of concern in the overall care of patients with pancreatic cancer is that patients may undergo laparotomy for planned pancreatic resection and their tumor is not removed due to local disease extent that could and should have been appreciated on preoperative imaging. This failure to accurately perform and/or interpret CT images results in an inappropriate laparotomy. The extent of this problem remains unknown and is difficult to quantify. In our referral-based population of borderline resectable patients reported herein,, 38 (24%) of 160 patients had undergone a non-therapeutic laparotomy prior to referral, including 18 (27%) of the 66 patients who ultimately underwent successful pancreatic resection at MDACC after induction therapy. Local tumor extent can be accurately determined prior to surgery and thus patients should not be taken to the operating room for a planned pancreatectomy if the surgeon is not equipped to manage tumor-vessel involvement which can almost always be appreciated on currently available cross-sectional imaging. For example, even in this complex group of patients, a gross complete resection was performed in 66 (84%) of 79 patients that were taken to the operating room for planned pancreatectomy; an R0 resection was performed in 62 (83%) of 75 patients.
The oncologic outcomes observed in these patients with borderline disease warrant comment. First, the advanced nature of the disease in borderline resectable pancreatic cancer patients is evidenced by the overall modest resectability rate seen in this group of patients; only 66 (41%) of 160 total patients had their tumors removed. When dealing with such complex surgery, that has a defined risk for perioperative mortality (2 [3%] of 66 in this report), it is critically important to reserve surgery for those patients most likely to benefit. Second, the neoadjuvant treatment approach effectively selects for surgery those patients most likely to benefit and in whom the risk of major surgery can be justified. Indeed, those patients who completed all intended therapy including surgical resection of the pancreatic tumor had a median survival of 40 months. In those patients who were proven to be poor candidates for surgery (largely due to disease progression or poor performance status) at the time of post-treatment, preoperative restaging, median survival was only 13 months. The differentiation of these two distinct groups of patients can not be made at the time of diagnosis, but can be accurately determined at the time of restaging following 4 to 5 months of preoperative treatment, a distinct advantage of a treatment approach that places surgery after induction therapy. Our data illustrate that careful monitoring of borderline resectable patients as defined herein, with attention to performance status, medical comorbidities, tolerance to therapy, serum levels of CA19-9 and serial CT imaging, will allow the multidisciplinary team to accurately determine which patients should or should not be considered for a major abdominal operation.
The relatively small number of patients included in this report precludes a meaningful analysis of prognostic factors which will predict patient outcome. For example, it is difficult to draw any firm conclusions on the use of histologic treatment effect as a surrogate marker for survival duration given the small sample size. Nonetheless, these observations warrant assessment of treatment effect as a prognostic factor in future multimodality treatment programs. In addition, the effect of a prolonged course of neoadjuvant therapy likely accounted for the low frequency of lymph node positive disease as well as the apparent modest effect of node positive disease on survival. Ongoing research with molecular profiling of pancreatic cancer by ourselves and others may provide additional or improved techniques to predict individual response to therapy. Now and in the future, the value of a multidisciplinary working group which reviews in detail all aspects of a patients care while under therapy can not be over-emphasized.
In summary, we have reported our recent experience with a newly described category (clinical stage) of patients with pancreatic cancer termed borderline resectable. The sub-grouping of these patients into MDACC Types A, B, and C based on anatomic and patient factors is particularly useful because it draws special attention to that aspect of the patient’s case which is likely to be the limiting factor in achieving possible cure. This report defines a subgroup of patients who cause significant confusion with regard to stage assignment and treatment; namely those that are not clearly resectable or clearly locally advanced. We hope that others will find this nomenclature useful in clinical practice and especially for the design of clinical trials exploring non-surgical therapies delivered pre- or postoperatively. We believe that our classification system adds further detail to the current AJCC staging system12 and allows these patient subsets of borderline disease to be defined by clinical and radiologic criteria at the time of pretreatment staging.
Acknowledgments
Supported by the Various Donor Fund for Pancreatic Cancer Research, and National Institutes of Health grant CA101936-01 (SPORE in Pancreatic Cancer) at The University of Texas M. D. Anderson Cancer Center.
Footnotes
Presented at the 119th Annual Meeting of the Southern Surgical Society, Hot Springs, VA, December 3, 2007.
BIBLIOGRAPHY
- 1.Raut CP, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Annals of surgery. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff RA, Crane C, Li D, Abbruzzese JL, Evans DB. Neoplasms of the exocrine pancreas. In: Kufe DW, et al., editors. Cancer Medicine. 8th. BC Decker; London: 2006. pp. 1331–1358. [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN) practice guidelines for pancreatic cancer. 2004 [Google Scholar]
- 4.Varadhachary GR, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Annals of surgical oncology. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Oken MM, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology. 1982;5:649–655. [PubMed] [Google Scholar]
- 6.Evans DB, Lee JE, Tamm EP, Pisters PW. Pancreaticoduodenectomy (Whipple Operation) and total pancreatectomy for cancer. In: Fischer JF, editor. Mastery of Surgery. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1299–1317. [Google Scholar]
- 7.Gallagher SF, Zervos EE, Murr MM. Distal Pancreatectomy. In: Von Hoff DD, Evans DB, Hruban RH, editors. Pancreatic Cancer. Jones and Bartlett; Sudbury, MA: 2005. pp. 265–285. [Google Scholar]
- 8.Yen TWF, Abdalla EK, Pisters PW, Evans DB. Pancreaticoduodenectomy. In: Von Hoff DD, Evans DB, Hruban RH, editors. Pancreatic Cancer. Jones and Bartlett; Sudbury, MA: 2005. pp. 265–285. [Google Scholar]
- 9.Tseng JF, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. discussion 949-950. [DOI] [PubMed] [Google Scholar]
- 10.Pisters PW, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Annals of surgery. 2001;234:47–55. doi: 10.1097/00000658-200107000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staley CA, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12:373–380. doi: 10.1097/00006676-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, editors. Exocrine Pancreas. AJCC Cancer Staging Manual. Chicago: 2002. pp. 157–164. [Google Scholar]
- 13.Evans DB, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]


