Abstract
Background & Aims
Environmental factors have been identified that affect risk of hepatocellular carcinoma (HCC), but little is known about the effects of sex hormones on liver cancer development or outcome. We investigated whether menopause hormone therapy (MHT) affects risk, age at onset, or outcome of HCC.
Methods
We performed a case–control study of 234 female patients treated for HCC at a tertiary medical center and with 282 healthy women (controls), from January 1, 2004 through May 31, 2015. We collected detailed information on environmental exposures, ages of menarche and menopause, hysterectomies, and uses of birth control and MHT. We performed multivariable logistic and Cox regression analyses to determine the independent effects of factors associated with women on risk and clinical outcome in HCC. The primary outcomes were effect of MHT on HCC risk, the relationship between MHT with hepatitis virus infection on HCC development, and effect of MHT on age at HCC onset or survival after diagnosis of HCC.
Results
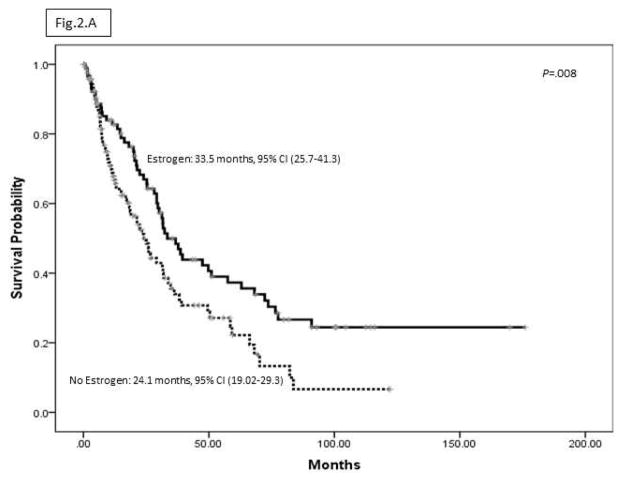
The estimated adjusted odds ratio (AOR) for HCC in women who ever used estrogen was 0.53 (95% CI, 0.32–0.88). This association was supported by the older age of HCC onset among estrogen users (mean, 64.5±0.9 years) vs non-users (mean, 59.2±1.1 years) (P=.001) and the reduced risk of HCC among long-term users (more than 5 years) (AOR, 0.36; 95% CI, 0.20– 0.63). Users of estrogen also had a reduced risk for hepatitis-associated HCC: AOR for users, 4.37 (95% CI, 1.67–11.44) vs AOR for non-users, 17.60 (95% CI, 3.88–79.83). Estrogen use reduced risk of death from HCC (hazard ratio, 0.55; 95% CI, 0.40–0.77) (P=.01). Median overall survival times were 33.5 months for estrogen users (95% CI, 25.7–41.3 months) and 24.1 months for non-users (95% CI, 19.02–29.30 months) (P=.008).
Conclusion
In a case–control study of women with HCC vs female controls at a single center, we associated use of estrogen MHT with reduced risk of HCC and increased overall survival times of patients with HCC. Further studies are needed to determine the benefits of estrogen therapy for women and patients with HCC, and effects of tumor expression of estrogen receptor.
Keywords: liver tumor, mortality, risk factor, reduction
INTRODUCTION
Irrespective of the worldwide variation in the incidence of hepatocellular carcinoma (HCC) 1, HCC is a male-dominant disease 2. In the United States, gender disparity in HCC has been observed not only in disease incidence, 3, 4 but also in etiological factors 5, 6, progression to cirrhosis 7, and survival 8; where male-to-female ratio is 3:1 with poorer prognosis and is commonly associated with chronic viral hepatitis, cigarette smoking, and alcohol consumption. The sex difference has also been observed in transgenic mice with hepatitis B– or C–induced HCC.9, 10
In view of the notable male predominance of HCC, several investigators raised the question about the importance of sex hormones in HCC risk and prognosis. The liver expresses estrogen and androgen receptors, both of which may act as transcription factors and may regulate expression of several regulatory genes involved in several pathways including those associated with cell proliferation and immune response. 11, 12
According to the National Health Statistics Report, in the United States, the percentage of women using contraception increases with age, with 75% of women aged 40–44 years now classified as users 13. The association between contraception and HCC was not shown to be conclusive by a meta-analysis of 12 case-control studies. The null association was later confirmed by a U.S. liver cancer pooling project with an OR of 1.12 (95% CI, .82–1.55) 14. Despite the available literature about the association between contraception and HCC, very little has been published about the association between MHT and risk of HCC.14, 15
This case-control study aimed at integrating clinical and epidemiological data to assess 1) the effect of MHT on HCC risk in females, 2) the relationship between MHT with hepatitis virus infection on HCC development, and 3) the effect of MHT on age at HCC onset or HCC survival.
METHODS
The current investigation is part of an ongoing hospital-based case-control study, which was approved by the Institutional Review Board at The University Texas MD Anderson Cancer Center. Written informed consent for participation was obtained from each participant.
Cases were new patients with pathological or radiological evidence of HCC who were treated at MD Anderson. The control subjects were healthy and genetically unrelated family members (i.e., spouses) of patients at M. D. Anderson who had cancers other than liver, gastrointestinal, lung, or head and neck cancer.
Between January 1, 2004, and May 31, 2015, 234 female patients (cases) with HCC and 282 female controls were eligible for the current investigation. HCC patients and controls were US residents and were interviewed simultaneously in person for demographic features and HCC risk factors with the use of a structured and validated questionnaire.
All participants were asked about their age of menarche, age of menopause, history of hysterectomy, their age when they underwent a hysterectomy, and whether one or both ovaries were removed during their hysterectomy.
Each female was interviewed for ever-use of various birth control types including pills, implant, or injection and the duration of use of various forms of contraception. Participants were also questioned about use of exogenous hormones including estrogen, progesterone, and combined estrogenprogesterone. Methods of use (oral pills, skin patch, injection, and vaginal) and duration of each method were documented. We missed to collect parity information from cases and controls. However, for case patients, we extracted the history of pregnancy, number of pregnancies, and number of children from the institutional epidemiological database of cancer patients. In addition, baseline clinical variables were retrieved from patients’ medical records.
Statistical Methods
Stata software (Stata Corp, College Station, TX) was used for statistical analysis. We performed multivariate unconditional logistic regression analyses. We calculated the adjusted odds ratio (AOR) and 95% confidence interval (CI) values, using maximum likelihood estimation after controlling for confounding effect of demographic and HCC risk factors.
Overall survival (OS) was defined as the time between HCC diagnosis and death or end of follow-up. Median survival was estimated by using the Kaplan-Meier product-limit method, and significant differences between the survival times were determined by using the log-rank test 16. To identify independent prognostic factors for OS, hazard ratios (HR) and 95% CIs were calculated by using Cox proportional hazard models with a backward stepwise selection.
Analysis of covariance was used to analyze patients’ mean age at HCC onset by hormonal exposure. Linear regression models were used to estimate the mean differences in age at HCC onset associated with use of birth control and exogenous hormones after adjusting for other factors associated with age at onset in this study population.
RESULTS
Table 1 shows that cigarette smoking was not associated with HCC risk in women. Consistent with our previous reports race, HCV, HBV, alcohol use, diabetes, hypothyroidism, early adulthood obesity, and positive family history of cancer were significant risk factors for HCC in US females.6, 17, 18
Table 1.
Multivariate-adjusted Odds Ratio (AOR)* and 95% Confidence Interval (CI) for HCC risk factors
| Demographic variables | HCC patients | Controls | AOR (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| N =234 | (%) | N =282 | (%) | |||
| Age (years) | ||||||
| <50 | 41 | (17.5) | 42 | (14.9) | 1 (Reference) | |
| ≥50 | 193 | (82.5) | 240 | (85.1) | 0.76 (0.42–1.36) | .4 |
| Race | ||||||
| White | 171 | (73.1) | 261 | (92.6) | 1 (Reference) | |
| Non-White | 63 | (26.9) | 21 | (7.4) | 0.27 (0.14 – .55) | <.0001 |
| Educational level | ||||||
| < College Education | 174 | (74.4) | 182 | (64.5) | 1 (Reference) | |
| ≥ College Education | 60 | (25.6) | 100 | (35.5) | 1.38 (0.86– 2.21) | .2 |
| HCV (Anti-HCV+)† | ||||||
| No infection | 174 | (74.4) | 256 | (90.8) | 1 (Reference) | |
| HCV infection | 60 | (25.6) | 1 | (0.4) | 71.6 (9.6 – 536.04) | <.0001 |
| HBV | ||||||
| No infection | 224 | (95.7) | 249 | (88.3) | 1 (Reference) | |
| HbsAg | 10 | (4.3) | 1 | (0.4) | 13.95 (1.28–151.58) | .03 |
| Anti-HBc | 26 | 7 | (2.5) | 2.98 (1.1–8.07) | .03 | |
| Unavailable | 0 | 25 | (8.9) | --- | ||
| Cigarette smoking‡ | ||||||
| No smoking | 128 | (54.7) | 191 | (67.7) | 1 (References) | |
| Smokers | 106 | (45.3) | 91 | (32.3) | 1.43 (0.89–2.31) | .1 |
| Alcohol drinking¥ | ||||||
| No drinking | 109 | (46.6) | 198 | (70.2) | 1 (Reference) | |
| Drinkers | 125 | (53.4) | 84 | (29.8) | 2.9 (1.81–4.64) | <.0001 |
| Prior history of diabetes | ||||||
| No diabetes | 179 | (76.5) | 256 | (90.8) | 1 (Reference) | |
| Diabetes | 55 | (23.5) | 26 | (9.2) | 3.84 (1.96–7.5) | <.0001 |
| Prior BMI (age20–40) | ||||||
| Normal/Slim | 153 | (65.9) | 225 | (80.1) | 1 (Reference) | |
| Overweight | 47 | (20.3) | 39 | (13.9) | 1.24 (0.67–2.3) | .5 |
| Obese | 32 | (13.8) | 17 | (6) | 2.35 (1.0–5.18) | .03 |
| Hypothyroidism | ||||||
| No | 176 | (75.2) | 239 | (84.8) | 1 (Reference) | |
| Yes | 58 | (24.8) | 43 | (15.2) | 2.43 (1.43– 4.16) | .001 |
| Family history of cancer | ||||||
| No | 52 | (22.2) | 91 | (32.3) | 1 (Reference) | |
| Yes | 182 | (77.8) | 191 | (67.7) | 1.79 (1.07–2.98) | .03 |
Adjusted for age, race, education level, HCV, HBV, alcohol drinking, cigarette smoking, history of diabetes, family history of cancer, obesity at age (20–40), and hypothyroidism;
Serological evidence of HCV and HBV were not determined in 25 control females;
Smokers are as subjects who had smoked ≥100 cigarettes during their lifetime;
Drinkers were subjects who had consumed at least 4 alcoholic drinks each month for 6 months in their lifetime.
Table 2 shows female characteristics in cases and controls. A significant impact was observed only among estrogen users yielding a 50% reduction in HCC risk compared with non-users; the estimated AOR (95% CI) was .50 (.29–.86) (Table 3). Long-term use of estrogen alone (>5 years) was reported by 64.2% of control subjects and 55.5% of case patients, yielding a significant reduction in HCC risk (AOR = .36, 95% CI [.20–.63]) compared with never-users. Estrogen use was mainly postmenopausal in cases (87/94) and in controls (177/179). Restricted analysis among white cases and controls did not meaningfully change the observed reduced risk of HCC among estrogen users.
Table 2.
Distribution of Female Characteristics by disease status (HCC cases and Healthy controls)
| Demographic variables | HCC patients | Controls | P value | ||
|---|---|---|---|---|---|
| N =234 | (%) | N =282 | (%) | ||
| State of residency | |||||
| Texas | 117 | (50) | 161 | (57.1) | .06 |
| Other states | 117 | (50) | 121 | (42.9) | |
| Marital Status | |||||
| Single | 83 | (35.5) | 25 | (8.9) | <.0001 |
| Married | 151 | (64.5) | 257 | (91.1) | |
| Age of menarche | |||||
| Mean (± SD) | 12.86 ± 1.7 | 12.91 ± 1.7 | .7 | ||
| Hysterectomy | |||||
| No | 122 | (52.1) | 136 | (48.2) | .2 |
| Yes | 112 | (47.9) | 146 | (51.8) | |
| Oophorectomy | |||||
| No | 25 | (22.3) | 46 | (36.7) | .01 |
| Yes | 87 | (77.7) | 100 | (63.3) | |
| One ovary | 14 | (16.1) | 15 | (15) | .4 |
| Two ovaries | 73 | (83.9) | 85 | (85) | |
| Age of Hysterectomy | |||||
| Mean (± SD) | 39.3± 1.1 | 41.4±.8 | .1 | ||
| Menopause | |||||
| No | 35 | (15) | 39 | (13.8) | .4 |
| Yes | 199 | (85) | 243 | (86.2) | |
| Age of menopause† | |||||
| Mean (± SD) | 48.78 ± 5.41 | 49.88 ± 4.48 | .1 | ||
| Prior history of Cancer | |||||
| None | 195 | (83.3) | 282 | (100) | |
| Breast | 20 | (8.5) | 0 | ||
| Endometrial | 6 | (2.6) | 0 | ||
| Cervix | 4 | (1.7) | 0 | ||
| Others | 9 | (3.8) | 0 | ||
Exclude women who had oophorectomy
Table 3.
Association between oral contraceptives and exogenous hormonal replacement with HCC
| Demographic variables | HCC patients | Controls | OR* (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| N =234 | (%) | N =282 | (%) | All | ||
| Contraception Use | ||||||
| No | 88 | (37.6) | 74 | (26.2) | 1 (Reference) | |
| Yes | 146 | (62.4) | 208 | (73.8) | .70 (.42–1.18) | |
| Contraception Duration† | ||||||
| ≤5 years | 51 | (35.7) | 96 | (46.2) | .41 (.22– .79) | .01 |
| 6–10 | 26 | (18.2) | 47 | (22.6) | .54 (.26– 1.15) | .18 |
| >10 | 66 | (46.2) | 65 | (31.3) | 1.38 (.74– 2.60) | .3 |
| Estrogen Use | ||||||
| Never | 140 | (59.8) | 103 | (36.5) | 1 (Reference) | |
| Ever | 94 | (40.2) | 179 | (63.5) | .53 (.32– .88) | .01 |
| Estrogen Alone | 63 | (26.9) | 130 | (46.1) | .50 (.29–.86) | .01 |
| Estrogen & Progesterone | 31 | (13.3) | 49 | (17.4) | .62 (.31–1.27) | .19 |
| Ever Estrogen Duration‡ | ||||||
| ≤5 years | 37 | (44.6) | 64 | (35.8) | .56 (.34–1.02) | .06 |
| 6–10 | 13 | (15.7) | 36 | (20.1) | .32 (.13– .77) | .01 |
| >10 | 33 | (39.8) | 79 | (44.1) | .45 (.24– .85) | .01 |
| Progesterone Use | ||||||
| Never | 196 | (83.8) | 219 | (77.7) | 1 (Reference) | |
| Ever | 38 | (16.2) | 63 | (22.3) | .84 (.47– 1.52) | .57 |
| Progesterone Alone | 7 | (3) | 14 | (5) | 1.22 (.35–4.22) | .75 |
| Progesterone & Estrogen | 31 | (13.2) | 49 | (17.3) | 1.38 (.45–4.29) | .56 |
| Ever Progesterone duration§ | ||||||
| ≤5 years | 21 | (60) | 33 | (52.4) | 1.08 (.51– 2.31) | .84 |
| 6–10 | 4 | (11.4) | 11 | (17.5) | .57 (.14– 2.32) | .43 |
| >10 | 10 | (28.6) | 19 | (30.2) | .57 (.20–1.63) | .30 |
Abbreviations:
AOR, multivariate-adjusted odds for age, race, education level, marital status, HCV, HBV, alcohol drinking, cigarette smoking, history of diabetes, family history of cancer, obesity at age (20–40), hypothyroidism, oophorectomy, and marital status
DuraYon of OC use was unknown in 3 HCC cases
DuraYon of estrogen use was unknown in 11 HCC cases
Duration of progesterone use was unknown in 3 HCC cases
The mean age at HCC onset among case patients who recalled estrogen use (± SE) was 64.5 ± .9 years, significantly higher than the mean age at onset of those with no estrogen use, which was 59.2 ± 1.1 years (P = .001). The mean difference in age at HCC onset between estrogen users and non-users was statistically significant after adjusting for other factors associated with age at HCC onset including smoking, alcohol, obesity, hypothyroidism, diabetes, HCV, HBV, marriage, history of pregnancy, educational level, and family history of cancer. The estimated coefficient was 4.65 (95% CI, 1.59–7.70) (P < .003). Considering the years of estrogen exposure in a continuous variable multiple linear regression analysis showed that predicted mean age at HCC onset increased with duration of estrogen use (Figure 1).
Figure 1.
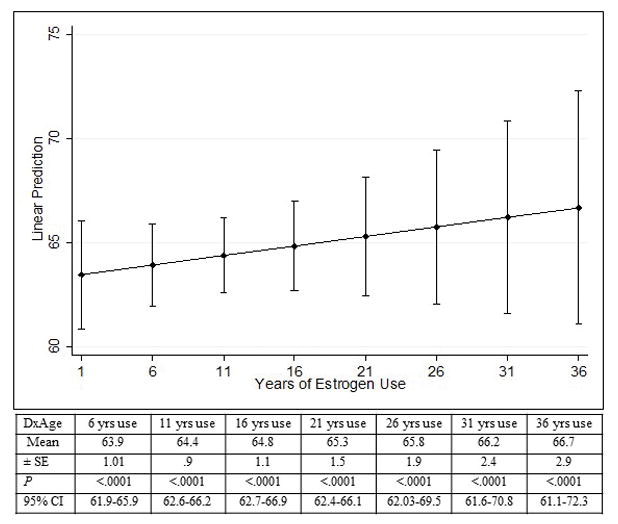
Predicted mean age (years) at HCC onset and duration of estrogen use by linear regression; for example, the predicted mean ages at HCC onset at 6 years, 16 years, and 31 years of estrogen exposure were
As compared to no estrogen users without hepatitis virus infection, the ORs (95% CI) for estrogen use in the absence of hepatitis infection was 0.44 (.27–.74) and for hepatitis virus infection in the absence of estrogen use was 17.60 (3.88–79.83). However, estrogen use attenuated the magnitude effect of hepatitis virus infection on HCC risk, yielding an OR of 4.37 (95% CI, 1.67–11.44).
A total of 39 case patients recalled a prior history of cancer, especially breast cancer (N = 20) (Table 2), whereas additional 3 cases reported a prior history of nonalcoholic steatohepatitis, primary biliary cirrhosis, and autoimmune hepatitis. Restricted analysis among 192 cases and 282 controls without a prior history of cancers or chronic liver diseases did not change the observed reduced risk of HCC among estrogen users.
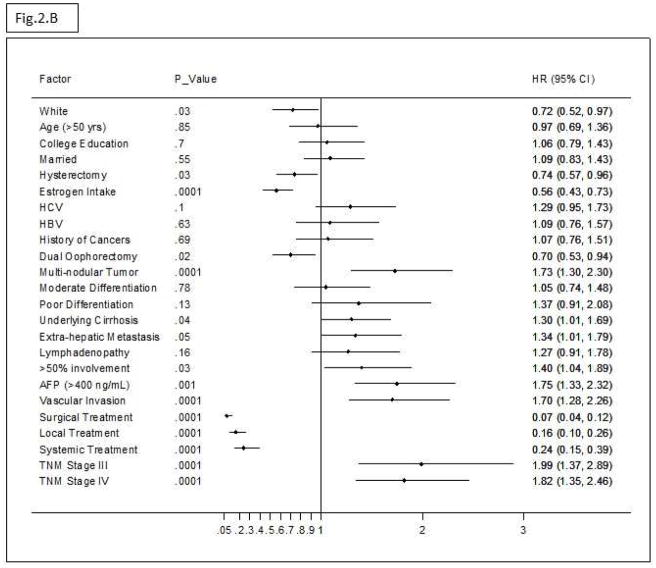
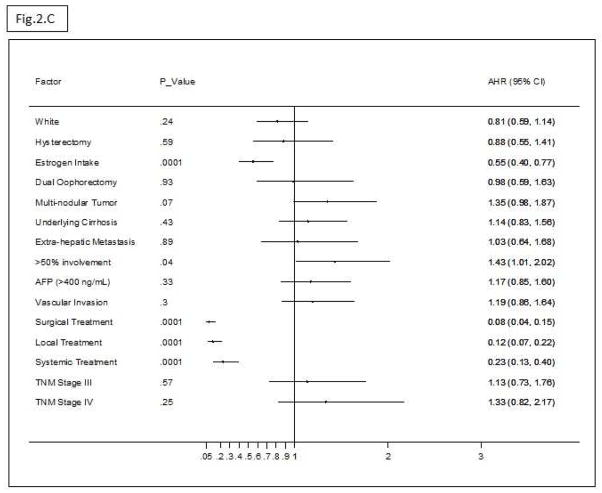
Figure 2A shows that OS of HCC patients was significantly longer among estrogen users than among non-users, P = .008. Figure 2B shows the univariate HRs (95% CI) of estrogen use and the clinical features of HCC at the time of diagnosis. Multivariate Cox regression analysis of the significant factors related to HCC prognosis indicated that estrogen use was significantly associated with 45% reduced mortality (Adjusted Hazard Ratio (AHR) = .55; 95% CI, .40–.77) (P = .01) after controlling for all confounding factors of HCC OS (Figure 2C).
Figure 2.
(A) Median OS (95%CI) by estrogen use.
(B) Univariate HRs (95% CI) of HCC prognostic factors.
(C) Multivariate AHRs (95% CI) of estrogen use (.55, .40–.77) after adjusting for significant confounding factors of survival including race, hysterectomy, oophorectomy, multi-nodular tumor, cirrhosis, extra-hepatic metastasis, >50% liver involvement, AFP, vascular invasion, TNM staging, and treatment type.
Prior history of pregnancy and pregnancy numbers were not significantly associated with HCC prognosis. Among HCC cases, we found that 66 (28.2%) never get pregnant, 142 (60.7%) had ≤ 3 pregnancies, and 26 (11.1%) had > 3 pregnancies. As compared to no pregnancy the HRs (95%) were .91 (.66–1.26), .86 (.62–1.20), and 1.24 (.74–2.10) for history of prior pregnancy, ≤ 3 pregnancies, and > 3 pregnancies respectively.
DISCUSSION
This study demonstrates 50% reduction in HCC risk development among women who used MHT. The observed reduced risk of HCC among estrogen users in this study was supported by three additional findings: 1) The positive correlation between age at HCC onset and duration of estrogen use. The adjusted linear regression analysis revealed significant coefficients indicating that in women with long-term use of estrogen, HCC tended to be diagnosed at an older age. 2) Attenuation of the magnitude of association between hepatitis virus infection and HCC development among estrogen users compared with non-users. 3) OS improvement in women with HCC who used estrogen compared with survival in non-users. The favorable prognostic observation of estrogen use was independent of the significant baseline clinical features of HCC related to HCC outcome.
Very few studies have investigated the association between MHT and HCC. However, the protective effect that we observed with postmenopausal estrogen use in US women agreed with the results from different populations. The multivariate AOR reported by Yu and colleagues 15 was .46 (95% CI, .27–.79). In addition, large nested case-control study within the United Kingdom’s Clinical Practice Research Datalink by McGlynn and colleagues 19 showed that the use of estrogen therapy was associated with a significantly lower risk of HCC (OR=0.44, 95% CI=0.22–0.88).
Findings from population studies with respect to the association between estrogen exposure and other cancers have been contradictory. Although some studies failed to show a significant impact of estrogen on pancreatic 20 or bladder cancers, 21 the preventive effect of estrogen against liver cancer in the current study and in others studies was observed for gastric 22 distal large bowel cancer23, and esophageal cancers 24, with an average risk reduction of 28%, and the estimated ORs (95% CI) were .77 (.64–.92) and .68 (.48–.97), respectively. In contrast, estrogen use was significantly associated with increased risk of breast cancer. 25 Our statistical analysis after excluding HCC women with prior cancers continued to show the protective effect of estrogen use.
Whereas other epidemiological studies focused on the relationship between HCC and some reproductive factors that may modify endogenous levels of female hormones such as age at menarche, age at menopause, hysterectomy, oophorectomy, and parity 14, 15, 26, we observed no significant independent effect of oophorectomy on HCC risk.
There are potential lines of evidence that estrogen may protect against HCC development: the anti-inflammatory effect of estrogen via inhibition of the NF-kB pathway 27 and possible suppression of the release of several pro-inflammatory cytokines that may deregulate the inflammatory and oxidative stress pathways involved in the carcinogenic process 28, 29. Given the key role of IL-6 in carcinogenesis and poor outcome in HCC patients, Naugler et al. 30 showed that estrogen treatment inhibited IL-6 production from Kupffer cells in female mice, leading to a reduction in liver cancer induction. Others have suggested that estrogen may inhibit hepatic tumor growth and progression by acting as a suppressor for alternative activation of tumor-associated macrophages and inhibiting the Jak1-Stat6 signaling pathway 31. A recent report provided new insight into the protection of estrogen in HCC via the regulation of NLRP3 inflammasome by estrogen through the ERβ/MARK pathway 32.
Several systemic reviews have shown a negative impact of tamoxifen treatment in HCC 33–37. A possible explanation for the tamoxifen failure is the lack of defined eligible patients for treatment according to hormonal receptor expression and the possibility that tamoxifen may not be a candidate therapy for patients with variant estrogen receptors.
Given the anti-inflammatory role of estrogen, our finding of the attenuated risk of hepatitis infection among estrogen users versus non-users may not be surprising and is possibly explained by suppression of hepatitis-related hepatic inflammation and steatohepatitis 38 and observed antifibrotic effect of estrogen in animal studies 39, 40
Similar to the natural history of HCC,41, 42 most of our HCC patients presented with advanced-stage disease. In addition, heathy control subjects were selected to represent the population from which case patients were ascertained. Only U.S. patients and controls were included, and the geographic distribution of their residential states was similar. Moreover, age of natural menopause in our controls were similar to the general US population.43 We choose not to use patients with cirrhosis as control subjects. We argue that this may lead to differential selection bias due to the significant association between estrogen and other environmental factors with fibrosis progression.
Given the U.S. Food and Drug Administration contraindication for MHT in patients with active liver diseases 44 we found that majority of women with MHT had preserved liver function at time of HCC diagnosis.
In conclusion, this study provides robust epidemiological evidence for the benefits of postmenopausal use of estrogen replacement against HCC development and has been corroborated by previous studies. However, this study is the first to highlight survival improvement among women with HCC who used estrogen replacement, after controlling for clinical prognostic factors, which raises the questions of whether similar effects can be observed in men who ever experienced hormonal exposure and whether estrogen can be used in targeted therapy for a selected population based on tumor expression and types of estrogen receptors.
Acknowledgments
Funding: Supported by National Institutes of Health NIH R03 grant ES11481 (to MMH), CA-106458 (to MMH), ONYX-33839 (to MMH), and by Sheikh Ahmed Center for Pancreatic Cancer Research (to RAW) at The University of Texas M. D. Anderson Cancer Center.
Abbreviation
- HCC
hepatocellular carcinoma
- MHT
menopausal hormonal therapy
- CI
confidence interval
- AFP
alpha-fetoprotein
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- AOR
adjusted odds ratio
- OS
overall survival
- HR
hazard ratio
- SE
standard error
- AHR
adjusted hazard ratio
Footnotes
The authors disclose no conflicts of interest.
- Study concept and design: Manal Hassan.
- Acquisition of data: Manal Hassan, Gehan Botrus, Reham Abdel-Wahab, Hesham M. Hassabo, Yasmin Abaza, Ahmed Shalaby, Sahin Lacin.
- Analysis and interpretation of data: Manal Hassan, Gehan Botrus, Reham Abdel-Wahab, Hesham M. Hassabo, Yasmin Abaza, Ahmed Shalaby, Sahin Lacin.
- Drafting of the manuscript: Manal M. Hassan, Gehan Botrus, Reham Abdel-Wahab, Robert A. Wolff, Donghui Li, David Tweardy, Alexandria T. Phan, Ernest Hawk, Milind Javle, Ju-Seog Lee, Harrys A. Torres, Asif Rashid, Renato Lenzi, Jeffrey Morris, Yehuda Z. Patt, Christopher I. Amos, and Ahmed O. Kaseb.
- Critical revision of the manuscript for important intellectual content: Manal M. Hassan, Robert A. Wolff, Donghui Li, David Tweardy, Alexandria T. Phan, Ernest Hawk, Milind Javle, Ju-Seog Lee, Harrys A. Torres, Asif Rashid, Renato Lenzi, Jeffrey Morris, Yehuda Z. Patt, Christopher I. Amos, Saira A Khaderi, John A Goss, Prasun K Jalal, and Ahmed O. Kaseb.
- Statistical analysis: Manal Hassan, Reham Abdel-wahab.
- Obtained funding: Manal Hassan, Ahmed Kaseb.
- Administrative, technical, or material support: Manal M. Hassan, Robert A. Wolff, Donghui Li, David Tweardy, Alexandria T. Phan, Ernest Hawk, Milind Javle, Ju-Seog Lee, Harrys A. Torres, Asif Rashid, Renato Lenzi, Jeffrey Morris, Yehuda Z. Patt, Christopher I. Amos, Saira A Khaderi, John A Goss, Prasun K Jalal, and Ahmed O. Kaseb.
- Study supervision: Manal Hassan
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.The Global Burden of Cancer 2013. JAMA Oncol. 2015 doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kew MC. Epidemiology of hepatocellular carcinoma in sub-Saharan Africa. Ann Hepatol. 2013;12:173–182. [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 4.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology. 152:812–820. doi: 10.1053/j.gastro.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan MM, Spitz MR, Thomas MB, El-Deeb AS, Glover KY, Nguyen NT, Chan W, Kaseb A, Curley SA, Vauthey JN, Ellis LM, Abdalla E, Lozano RD, Patt YZ, Brown TD, Abbruzzese JL, Li D. Effect of different types of smoking and synergism with hepatitis C virus on risk of hepatocellular carcinoma in American men and women: case-control study. Int J Cancer. 2008;123:1883–1891. doi: 10.1002/ijc.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan MM, Kaseb A, Li D, Patt YZ, Vauthey JN, Thomas MB, Curley SA, Spitz MR, Sherman SI, Abdalla EK, Davila M, Lozano RD, Hassan DM, Chan W, Brown TD, Abbruzzese JL. Association between hypothyroidism and hepatocellular carcinoma: a case-control study in the United States. Hepatology. 2009;49:1563–1570. doi: 10.1002/hep.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003;23:63–69. doi: 10.1034/j.1600-0676.2003.00811.x. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology. 2001;33:62–65. doi: 10.1053/jhep.2001.21041. [DOI] [PubMed] [Google Scholar]
- 9.FIRMINGER HI, REUBER MD. Influence of adrenocortical, androgenic, and anabolic hormones on the development of carcinoma and cirrhosis of the liver in A x C rats fed N-2- fluorenyldicetamide. J Natl Cancer Inst. 1961;27:559–595. [PubMed] [Google Scholar]
- 10.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 11.Nagasue N, Ito A, Yukaya H, Ogawa Y. Androgen receptors in hepatocellular carcinoma and surrounding parenchyma. Gastroenterology. 1985;89:643–647. doi: 10.1016/0016-5085(85)90463-9. [DOI] [PubMed] [Google Scholar]
- 12.Nagasue N, Ito A, Yukaya H, Ogawa Y. Estrogen receptors in hepatocellular carcinoma. Cancer. 1986;57:87–91. doi: 10.1002/1097-0142(19860101)57:1<87::aid-cncr2820570118>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. Natl Health Stat Report. 2012:1–25. [PubMed] [Google Scholar]
- 14.McGlynn KA, Sahasrabuddhe VV, Campbell PT, Graubard BI, Chen J, Schwartz LM, Petrick JL, Alavanja MC, Andreotti G, Boggs DA, Buring JE, Chan AT, Freedman ND, Gapstur SM, Hollenbeck AR, Hou L, King LY, Koshiol J, Linet M, Palmer JR, Poynter JN, Purdue M, Robien K, Schairer C, Sesso HD, Sigurdson A, Wactawski-Wende J, Zeleniuch-Jacquotte A. Reproductive factors, exogenous hormone use and risk of hepatocellular carcinoma among US women: results from the Liver Cancer Pooling Project. Br J Cancer. 2015;112:1266–1272. doi: 10.1038/bjc.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu MW, Chang HC, Chang SC, Liaw YF, Lin SM, Liu CJ, Lee SD, Lin CL, Chen PJ, Lin SC, Chen CJ. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38:1393–1400. doi: 10.1016/j.hep.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 16.GEHAN EA. A GENERALIZED WILCOXON TEST FOR COMPARING ARBITRARILY SINGLY-CENSORED SAMPLES. Biometrika. 1965;52:203–223. [PubMed] [Google Scholar]
- 17.Hassan MM, Spitz MR, Thomas MB, El-Deeb AS, Glover KY, Nguyen NT, Chan W, Kaseb A, Curley SA, Vauthey JN, Ellis LM, Abdalla E, Lozano RD, Patt YZ, Brown TD, Abbruzzese JL, Li D. Effect of different types of smoking and synergism with hepatitis C virus on risk of hepatocellular carcinoma in American men and women: case-control study. Int J Cancer. 2008;123:1883–1891. doi: 10.1002/ijc.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan MM, Abdel-Wahab R, Kaseb A, Shalaby A, Phan AT, El-Serag HB, Hawk E, Morris J, Singh Raghav KP, Lee JS, Vauthey JN, Bortus G, Torres HA, Amos CI, Wolff RA, Li D. Obesity Early in Adulthood Increases Risk but Does Not Affect Outcomes of Hepatocellular Carcinoma. Gastroenterology. 2015;149:119–129. doi: 10.1053/j.gastro.2015.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGlynn KA, Hagberg K, Chen J, Braunlin M, Graubard BI, Suneja N, Jick S, Sahasrabuddhe VV. Menopausal hormone therapy use and risk of primary liver cancer in the clinical practice research datalink. Int J Cancer. 2016;138:2146–2153. doi: 10.1002/ijc.29960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang B, Lv J, Li Y, Yuan S, Wang Z, He S. Relationship between female hormonal and menstrual factors and pancreatic cancer: a meta-analysis of observational studies. Medicine (Baltimore) 2015;94:e177. doi: 10.1097/MD.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietrich K, Demidenko E, Schned A, Zens MS, Heaney J, Karagas MR. Parity, early menopause and the incidence of bladder cancer in women: a case-control study and meta-analysis. Eur J Cancer. 2011;47:592–599. doi: 10.1016/j.ejca.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:20–38. doi: 10.1158/1055-9965.EPI-11-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long MD, Martin CF, Galanko JA, Sandler RS. Hormone replacement therapy, oral contraceptive use, and distal large bowel cancer: a population-based case-control study. Am J Gastroenterol. 2010;105:1843–1850. doi: 10.1038/ajg.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang BJ, Zhang B, Yan SS, Li ZC, Jiang T, Hua CJ, Lu L, Liu XZ, Zhang DH, Zhang RS, Wang X. Hormonal and reproductive factors and risk of esophageal cancer in women: a meta-analysis. Dis Esophagus. 2015 doi: 10.1111/dote.12349. [DOI] [PubMed] [Google Scholar]
- 25.Anothaisintawee T, Wiratkapun C, Lerdsitthichai P, Kasamesup V, Wongwaisayawan S, Srinakarin J, Hirunpat S, Woodtichartpreecha P, Boonlikit S, Teerawattananon Y, Thakkinstian A. Risk factors of breast cancer: a systematic review and meta-analysis. Asia Pac J Public Health. 2013;25:368–387. doi: 10.1177/1010539513488795. [DOI] [PubMed] [Google Scholar]
- 26.Amr S, Iarocci EA, Nasr GR, Saleh D, Blancato J, Shetty K, Loffredo CA. Multiple pregnancies, hepatitis C, and risk for hepatocellular carcinoma in Egyptian women. BMC Cancer. 2014;14:893. doi: 10.1186/1471-2407-14-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 29.Shi L, Feng Y, Lin H, Ma R, Cai X. Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med. 2014;12:93. doi: 10.1186/1479-5876-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 31.Yang W, Lu Y, Xu Y, Xu L, Zheng W, Wu Y, Li L, Shen P. Estrogen represses hepatocellular carcinoma (HCC) growth via inhibiting alternative activation of tumor-associated macrophages (TAMs) J Biol Chem. 2012;287:40140–40149. doi: 10.1074/jbc.M112.348763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei Q, Guo P, Mu K, Zhang Y, Zhao W, Huai W, Qiu Y, Li T, Ma X, Liu Y, Chen X, Han L. Estrogen suppresses hepatocellular carcinoma cells through ERbeta-mediated upregulation of the NLRP3 inflammasome. Lab Invest. 2015 doi: 10.1038/labinvest.2015.63. [DOI] [PubMed] [Google Scholar]
- 33.Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol. 1997;8:117–136. doi: 10.1023/a:1008285123736. [DOI] [PubMed] [Google Scholar]
- 34.Mathurin P, Rixe O, Carbonell N, Bernard B, Cluzel P, Bellin MF, Khayat D, Opolon P, Poynard T. Review article: Overview of medical treatments in unresectable hepatocellular carcinoma--an impossible meta-analysis? Aliment Pharmacol Ther. 1998;12:111–126. doi: 10.1046/j.1365-2036.1998.00286.x. [DOI] [PubMed] [Google Scholar]
- 35.Nowak AK, Stockler MR, Chow PK, Findlay M. Use of tamoxifen in advanced-stage hepatocellular carcinoma. A systematic review Cancer. 2005;103:1408–1414. doi: 10.1002/cncr.20963. [DOI] [PubMed] [Google Scholar]
- 36.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 37.Di MM, Daniele B, Pignata S, Gallo C, De ME, Morabito A, Piccirillo MC, Perrone F. Is human hepatocellular carcinoma a hormone-responsive tumor? World J Gastroenterol. 2008;14:1682–1689. doi: 10.3748/wjg.14.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di MV, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, Moussalli J, Thabut D, Buffet C, Poynard T. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–1433. doi: 10.1002/hep.20463. [DOI] [PubMed] [Google Scholar]
- 39.Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719–727. doi: 10.1002/hep.510290307. [DOI] [PubMed] [Google Scholar]
- 40.Cengiz M, Ozenirler S, Yilmaz G. Estrogen receptor alpha expression and liver fibrosis in chronic hepatitis C virus genotype 1b: a clinicopathological study. Hepat Mon. 2014;14:e21885. doi: 10.5812/hepatmon.21885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serper M, Taddei TH, Mehta R, D’Addeo K, Dai F, Aytaman A, Baytarian M, Fox R, Hunt K, Goldberg DS, Valderrama A, Kaplan DE. Association of Provider Specialty and Multi-disciplinary Care With Hepatocellular Carcinoma Treatment and Mortality. Gastroenterology. doi: 10.1053/j.gastro.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 43.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randel A. AACE Releases Guidelines for Menopausal Hormone Therapy. American Family Physician. 2012;86:865–867. [Google Scholar]