Abstract
Background
Prior studies suggest low-risk STEMI patients undergoing primary PCI can be considered for early discharge. We describe the implementation of a STEMI risk score to decrease cost while maintaining optimal patient outcomes.
Methods and Results
We determined the impact of risk-guided STEMI care on healthcare value through the retrospective application of the Zwolle Risk Score to 967 patients receiving primary PCI between 2009 and 2011. Of these patients, 540 (56%) were categorized as low-risk, indicating they may be safely triaged directly to a telemetry unit, rather than the intensive care unit and targeted for early discharge. We subsequently developed and implemented a modified Zwolle Risk Calculator into the electronic medical record to support application of the fast-track protocol for low-risk STEMI patients. Among 549 prospective STEMI patients, 62% were low-risk and the fast-track protocol was followed in 75% of cases. Prospective results confirmed lower rates of complications (low-risk 8.3% vs. high-risk 38.7%; p<0.001) and in-hospital mortality (low-risk 0.4% vs. high-risk 12.5%; p<0.001) in the low-risk cohort. Low-risk patients had a shorter median length of stay (LOS) (median and [25th, 75th percentiles]: low-risk 2 [2,3] vs. high-risk: 3 [2, 6]; p<0.001) and lower overall costs (low-risk $6,720 [$5,280–$9,030] vs. high-risk $11,783 [$7,953–$25,359]; p<0.001). Low-risk patients treated on-protocol had shorter median LOS (on-protocol 2 [1,2] vs. off-protocol 2 [2,3]; p<0.001) and hospital costs (on-protocol $6,090 [$4,730, $7,356] vs. off-protocol $11,783 [$7,953, $25,359]; p<0.001) than those treated off-protocol On-protocol low-risk patients in the prospective cohort also had lower costs and shorter LOS than low-risk patients in the retrospective cohort (p<0.001 for both).
Conclusions
In our study, risk-guided triage and discharge after primary PCI for STEMI improved healthcare value by reducing costs of care without compromising quality of care or patient outcomes.
Journal subject terms: Revascularization, Health Services, Cost Effectiveness
Keywords: STEMI, value based care, risk stratification, electronic medical record, evidence-based medicine
Introduction
There is increasing pressure to improve the value of healthcare, as defined by optimal patient health outcomes at lower cost.1 Common, high-cost healthcare conditions may offer significant opportunities to achieve this goal. Care of patients with ST elevation myocardial infarction (STEMI) represent one such condition, with over 250,000 patients suffering a STEMI in the U.S. annually,2 at an estimated cost of nearly $20,000 a case.3
Although STEMI is a life-threatening cardiovascular emergency, much of the associated clinical risk is mitigated by successful primary percutaneous coronary intervention (PCI).4 As such, some patients may not require a high-acuity of care or lengthy hospitalization following successful PCI. Prior studies have demonstrated the feasibility of identifying low-risk patients who may be safely triaged to lower acuity telemetry care and early discharge.5–8 The implications of risk-guided patient triage following primary PCI on costs of care and long-term patient outcomes have not been described.
We first sought to determine the potential impact of risk-guided STEMI care on healthcare value using a retrospective comparison of actual costs with estimated costs if low-risk patients were triaged to telemetry and discharge within 48 hours. We then prospectively evaluated the impact of an electronic medical record (EMR) integrated risk-calculator to support the identification and standardized care of low-risk patients following successful primary PCI for STEMI.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. Contact the authors to obtain these materials.
We designed the project in three phases: 1) retrospective risk score validation, 2) EMR tool design and integration and 3) prospective application of the EMR risk-tool in providing triage and discharge guidance to providers at the point of care. The project was undertaken at the Minneapolis Heart Institute at Abbott Northwestern Hospital (MHI-ANW), which supports a large regional STEMI program that utilizes a standardized protocol and integrated transfer system for primary PCI from 31 hospitals and 10 clinics throughout Minnesota and Wisconsin.9–11
In the first phase of the study, we retrospectively applied the Zwolle Risk Score to all patients presenting to our regional STEMI system between January 2009 and December 2011. This risk score incorporates 6 clinical and angiographic variables (age, ischemic time, Killip class, number of vessels with coronary artery disease, if the involved vessel led to an anterior infarction and TIMI flow following PCI), with each variable being assigned a weighted point value. Scores of 0-3 are categorized as low-risk for 30-day mortality or post-discharge out of hospital cardiac arrest, while scores ≥4 are considered high-risk5 (Table 1).
Table 1.
Zwolle Risk Score. The left two columns contain the clinical variables and associated weighted points for each. The Zwolle Risk Score is calculated by summing the points total for each variable. The relative risk of death at 30-days based on a given score are located in the right two columns. Low-risk patients are defined by a score of ≤3. Reprinted with permission from De Luca, et al.5
| Zwolle Risk Score for STEMI | Zwolle Risk Score for STEMI | ||
|---|---|---|---|
| Points | |||
| Killip Class | Risk Score | RR [95% CI] of death by 30-D | |
| 1 | 0 | ||
| 2 | 4 | ||
| 3-4 | 9 | 0-1 | 0.03 [0.008–0.13] |
| TIMI flow post | |||
| 3 | 0 | 2 | 0.09 [0.02–0.37] |
| 2 | 1 | ||
| 0-1 | 2 | 3 | 1.04 [0.44–2.45] |
| Age | |||
| <60 | 0 | 4 | 1.40 [0.5–3.98] |
| ≥60 | 2 | ||
| 3-vessel disease | 5 | 2.48 [0.96–6.42] | |
| No | 0 | ||
| Yes | 1 | 6 | 2.52 [0.75–8.46] |
| Anterior infarction | |||
| No | 0 | 7 | 5.99 [1.98–18.1] |
| Yes | 1 | ||
| Ischemia time (>4 Hours) | ≥8 | 32.1 [18.6–55.8] | |
| No | 0 | ||
| Yes | 1 | ||
| Total score | 16 | ||
Clinical and administrative data including baseline demographic information, cardiac risk profiles and pre-PCI clinical presentation (cardiac arrest, cardiogenic shock and Killip Class) were collected. Clinical outcomes including post-PCI triage location (telemetry bed vs. intensive care), hospital length of stay (LOS), ejection fraction and in-hospital, 30-day and 1-year mortality were assessed. We also assessed post-PCI major adverse cardiovascular events (MACE) including stroke, myocardial infarction and death at either 30-days or 1-year, as well as rates of peri-procedural complications (red blood cell transfusion, bleeding within 72 hours, development of cardiogenic shock, development of new heart failure and new need for dialysis) as per National Cardiovascular Data Registry (NCDR) definitions. Costs of care, measured as median total variable cost, were obtained from comprehensive data within Allina Health’s Enterprise Data Warehouse. The warehouse, which integrates data from hospitals and clinics, is a single shared electronic data repository, which supports the comprehensive analytical need to identify, measure and improve clinical quality, financial outcomes, and reporting.
In phase 2, the MHI-ANW System’s Level 1 Myocardial Infarction-STEMI Committee reviewed the retrospective data and developed an implementation strategy for risk guided STEMI care. The committee determined that out of hospital cardiac arrest, while not in the original score, should constitute a high-risk factor and that patients with this clinical presentation should be admitted to the Cardiac Intensive Care Unit (CICU), regardless of traditional Zwolle Risk Score. The modified Zwolle Risk Score, incorporating out of hospital cardiac arrest as an automatic high-risk feature, was added to the EMR at MHI-ANW, enabling risk-based guidance in the post-procedural acuity of care and discharge targets following primary PCI for STEMI. The score was calculated upon the completion of primary PCI, while the patient was still in the catheterization laboratory. STEMI patients who did not undergo PCI or died in the catheterization laboratory were excluded. Patients with a low-risk score were recommended to be transferred to a telemetry floor and be discharged within 48 hours. High-risk patients were recommended to be transferred to the CICU, without recommendation made on timing of discharge.
While the decision support tool recommended triage to either telemetry or the CICU, the final decision on admission location and discharge date was at the discretion of the primary provider. Completion of the risk score and adherence to the protocol by each physician was tracked and incorporated into providers’ quality metrics, which was linked to an annual quality component of their salary. The program was launched at MHI-ANW in July 2013.
In phase 3, following development and implementation of an EMR based risk-calculator and protocol for low-risk STEMI patients, we prospectively evaluated the impact on eligible STEMI patients between July 2013 and December 2014. The same data measures collected for the retrospective cohort in phase 1 were prospectively collected following implementation of the risk-calculator.
Descriptive statistics are displayed as means and SDs for continuous variables; number and percentage with characteristic are given for categorical variables. When continuous variables had skewed distributions (cost data and LOS), data are summarized with medians and 25th and 75th percentiles. Categorical variables were analyzed using Pearson’s chi-square or Fisher’s exact tests. Continuous variables were analyzed using ANOVA for normally distributed variables or Kruskal-Wallis tests for continuous variables with non-normal distribution. A value of p<0.05 was considered significant, and p-values are two-sided where possible. All statistical calculations and plots were done with Stata 14.1 (College Station, TX). Institutional IRB approval was obtained for this study.
Results
Retrospective Comparison of Actual Care with Risk-Guided Care
A total of 967 consecutive STEMI patients (all triaged to the CICU) were retrospectively assessed with the Zwolle Risk Score, 540 (56%) of who were classified as low-risk. Compared with high-risk patients, low-risk patients were younger (57.2 ± 12.8 vs. 69.8 ± 12.3; p<0.001) and had lower rates of hypertension (57.1% vs. 66.0%; p=0.005), previously known CAD (28.2% vs. 37.8%; p=0.002), prior PCI (19.1% vs. 26.2%; p=0.009) and prior CABG (5.0% vs. 11.5%; p<0.001), however, were more likely to be either current (67.2% vs. 46.0%; p<0.001) or former smokers (61.0% vs. 50.6%; p=0.001). Pre-PCI, the low-risk group had lower rates of cardiac arrest (5.0% vs. 15.0%; p<0.001) and cardiogenic shock (0.4% vs. 15.1%; p<0.001) (Table 2). Following PCI, low-risk patients had a significantly higher median ejection fraction (50.7% ± 12.8 vs. 43.0% ± 12.3; p<0.001) (Table 2) and lower rates of procedural complications (any complication 6.5% vs. 17.1%, p<0.001) including post-procedural cardiogenic shock (0.3% vs. 5.1%, p<0.001), need for red blood cell transfusion (2.3% vs. 11.0%, p<0.001) and new need for dialysis (0% vs. 1.7%, p=0.027) (Figure 1). These patients also experienced lower rates of composite MACE at 30-days (0.9% vs. 13.8%, p<0.001) and 1-year (5.0% vs. 17.8%, p<0.001), as well as in-hospital (0% vs. 11.9%, p<0.001), 30-day (0.2% vs. 12.9%, p<0.001) and 1-year mortality (3.9% vs. 16.4% p<0.001) (Table 2). Compared with high-risk patients, low-risk patients had a lower median LOS (median and [25th, 75th percentiles]; 2 [2, 4] vs. 3 [2, 4]; p<0.001) (Table 2) and lower median total hospital costs (median and [25th, 75th percentiles]; $13,648 [$9,856, $19,234] vs. $15,223 [$10,312, $24,827]); p=0.011). (Table 3).
Table 2.
Pre-procedure clinical characteristics, post-procedure ejection fraction, length of stay, complications and mortality for retrospectively studied high-risk (n=427) vs. low-risk (n=540) STEMI patients, prospectively studied high-risk (n=176) vs. low risk (n=286) STEMI patients and prospectively studied on protocol low-risk (n=177) vs. off protocol low-risk (n=109) STEMI patients.
| Retrospective Analysis | Prospective Patients | Prospective Low-Risk Patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low-Risk n=540 | High-Risk n=427 | P-value | Low-Risk n=286 | High-Risk n=176 | P-value | On Protocol (n=177) | Off Protocol (n=109) | P-Value | |
| Age (Years), mean(SD) | 57.2 ± 12.8 | 69.8 ± 12.3 | <0.001 | 61.8 ± 13.4 | 66.3 ± 12.3 | <0.001 | 61.4 ± 12.4 | 62.5 ± 15.0 | 0.47 |
| Males, (%) | 398 (73.8) | 293 (68.6) | 0.074 | 210 (73.4) | 121 (68.8) | 0.28 | 133 (75.1) | 77 (70.6) | 0.40 |
| Hypertension, (%) | 308 (57.1) | 278 (66.0) | 0.005 | 151 (53.0) | 105 (61.1) | 0.092 | 90 (50.9) | 61 (56.5) | 0.36 |
| Dyslipidemia, (%) | 298 (55.8) | 257 (61.3) | 0.086 | 166 (58.5) | 101 (58.7) | 0.96 | 100 (56.8) | 66 (61.1) | 0.48 |
| Diabetes, (%) | 97 (18.0) | 98 (23.1) | 0.052 | 53 (18.6) | 44 (25.6) | 0.077 | 29 (16.4) | 24 (22.2) | 0.22 |
| History of Smoking, (%) | 329 (61.0) | 213 (50.6) | 0.001 | 196 (68.8) | 107 (61.5) | 0.11 | 121 (68.8) | 75 (68.8) | 0.99 |
| Current Smoker, (%) | 221 (67.2) | 98 (46.0) | <0.001 | 105 (36.8) | 55 (31.6) | 0.25 | 68 (38.6) | 37 (33.9) | 0.43 |
| History of CAD, (%) | 152 (28.2) | 161 (37.8) | 0.002 | 79 (27.7) | 51 (29.3) | 0.71 | 46 (26.0) | 33 (30.6) | 0.40 |
| Previous PCI, (%) | 103 (19.1) | 111 (26.2) | 0.009 | 63 (22.2) | 34 (19.5) | 0.50 | 39 (22.2) | 24 (22.2) | 0.99 |
| Previous MI, (%) | 102 (18.9) | 100 (23.7) | 0.072 | 56 (19.8) | 36 (20.8) | 0.79 | 35 (19.9) | 21 (19.6) | 0.96 |
| Previous CABG, (%) | 27 (5.0) | 49 (11.5) | <0.001 | 15 (5.3) | 11 (6.3) | 0.63 | 7 (4.0) | 8 (7.4) | 0.21 |
| Cardiac Arrest, (%) | 27 (5.0) | 64 (15.0) | <0.001 | 0 (0) | 59 (33.5) | <0.001 | ----- | ----- | ----- |
| Cardiogenic Shock, (%) | 2 (0.4) | 63 (15.1) | <0.001 | 0 (0) | 21 (11.9) | <0.001 | ----- | ----- | ----- |
| Killip Class 0-1, (%) 2-4, (%) |
538 (100) 0 (0) |
308 (72.1) 119 (27.9) |
<0.001 |
286 (100) 0 (0) |
132 (75.0) 44 (25.0) |
<0.001 |
----- | ----- | ----- |
| Ejection Fraction, mean(SD) | 50.7 ± 12.7 | 43.0 ± 13.6 | <0.001 | 54.0 ± 10.6 | 45.3 ± 15.5 | <0.001 | 55.6 ± 9.7 | 51.3 ± 11.5 | <0.001 |
| Median LOS, (25th, 75th percentile) | 2 (2, 4) | 3 (2, 6) | <0.001 | 2 (2, 3) | 3 (2, 6) | <0.001 | 2 (1, 2) | 2 (2, 3) | <0.001 |
| Zwolle Risk Score, mean (SD) | 1.87 ± 0.98 | 6.33 ± 3.62 | <0.001 | 1.78 ± 1.14 | 4.93 ± 3.75 | <0.001 | 1.77 ± 1.20 | 1.80 ± 1.04 | 0.86 |
| In-Hospital Death, (%) | 0 (0) | 51 (12.0) | <0.001 | 1 (0.4) | 22 (12.5) | <0.001 | 0 (0) | 1 (0.9) | 0.38 |
| 30 Day Outcomes | |||||||||
| MI, (%) | 3 (0.6) | 2 (0.5) | 1.00 | 1 (0.4) | 2 (1.1) | 0.56 | 0 (0) | 1 (0.9) | 0.38 |
| Stroke, (%) | 1 (0.2) | 4 (0.9) | 0.18 | 1 (0.4) | 2 (1.1) | 0.56 | 1 (0.6) | 0 (0) | 1.00 |
| Death, (%) | 1 (0.2) | 55 (12.9) | <0.001 | 4 (1.4) | 27 (15.3) | <0.001 | 1 (0.6) | 3 (2.8) | 0.16 |
| MACE, (%) | 5 (0.9) | 59 (13.8) | <0.001 | 5 (1.8) | 30 (17.1) | <0.001 | 2 (1.1) | 3 (2.8) | 0.37 |
| 1 Year Outcomes | |||||||||
| MI, (%) | 4 (0.7) | 4 (0.9) | 0.74 | 7 (2.5) | 3 (1.7) | 0.75 | 2 (1.1) | 5 (4.6) | 0.11 |
| Stroke, (%) | 2 (0.4) | 4 (0.9) | 0.41 | 1 (0.4) | 2 (1.1) | 0.56 | 1 (0.6) | 0 (0) | 1.00 |
| Death, (%) | 21 (3.9) | 70 (16.4) | <0.001 | 11 (3.9) | 30 (17.1) | <0.001 | 4 (2.3) | 7 (6.4) | 0.075 |
| MACE, (%) | 27 (5.0) | 76 (17.8) | <0.001 | 17 (5.9) | 34 (19.3) | <0.001 | 7 (4.0) | 10 (9.2) | 0.077 |
Figure 1. Retrospective Analysis: Complications and Mortality in Low-risk vs. High-risk Patients.
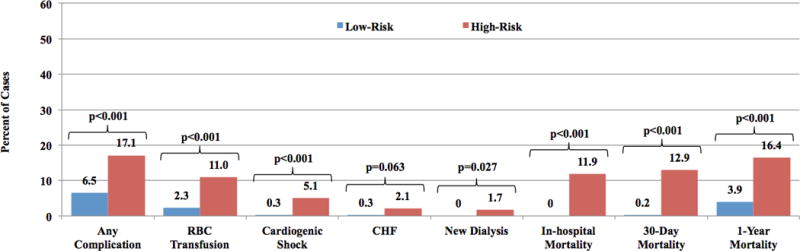
Comparison of complications and mortality for high-risk (n=427) and low-risk (n=540), retrospectively studied STEMI patients. Any complication is defined as a composite of red blood cell transfusion, cardiogenic shock, congestive heart failure and new need for dialysis. Mortality was measured in-hospital and at 30-days and 1-year. RBC= red blood cell; CHF= congestive heart failure
Table 3.
Top: Comparison of median direct patient costs for high-risk (n=427) and low-risk (n=540), retrospectively studied STEMI patients. Bottom: Comparison of median direct patient costs for high-risk (n=176), on protocol low-risk (n=177) and off protocol low-risk (n=109), prospectively studied STEMI patients. Amounts in parenthesis represent 25th and 75th percentiles.
| Retrospective Cohort | Low-Risk (n=540) | High-Risk (n=427) | P-Value |
|---|---|---|---|
| Patient Costs | $13,648 ($9,856, $19,234) | $15,223 ($10,312, $24,827) | 0.011 |
| Prospective Cohort | Low-Risk On Protocol (n=177) | Low-Risk Off Protocol (n=109) | High-Risk (n=176) | P-Value |
|---|---|---|---|---|
| Patient Costs | $6,090 ($4,730, $7,356) | $8,412 ($6,728, $10,920) | $11,783 ($7,953, $25,359) | <0.001 |
Evaluation of Risk-Guided STEMI Care
Following implementation of the modified Zwolle Risk Calculator into the EMR, 549 patients were prospectively assessed, of which 462 met inclusion criteria. Of these, 286 (61.9%) were categorized as low-risk and 176 (38.1%) high-risk. Providers followed the recommended protocol in 75% of the cases, with 177 (61.9%) of the low-risk patients admitted to telemetry and 168 (95.5%) of the high-risk patients admitted to the CICU (Figure 2). As in the retrospective cohort, compared to high-risk patients, low-risk patients were younger (61.8 ± 13.4 vs. 66.3 ± 12.3; p<0.001) and had lower rates of pre-PCI cardiac arrest (0% vs. 33.5%, p<0.001) and cardiogenic shock (0% vs. 11.9%, p<0.001), though the groups were otherwise similar (Table 2). Post-PCI, low-risk patients had a higher median ejection fraction (54.0% ± 10.6 vs. 45.3% ± 15.5; p<0.001), fewer complications (8.3% vs. 38.7%; p<0.001) and shorter LOS (median and [25th, 75th percentiles]; 2 [2, 3] vs. 3 [2, 6]; p<0.001) (Table 2). In-hospital mortality was higher in the high-risk group (12.5% vs. 0.4%; p<0.001), as were total hospital costs ($11,783 [$7,953–$25,359] vs. $6,720 [$5,280–$9,030]; p<0.001). At 30-days, low-risk patients were less likely than high-risk patients to suffer composite MACE (1.8% vs 17.1%, p<0.001), including death (1.4% vs 15.3%, p<0.001). Similar findings were found at 1-year follow up (Table 2).
Figure 2. Prospective Study Flow Chart Criteria.
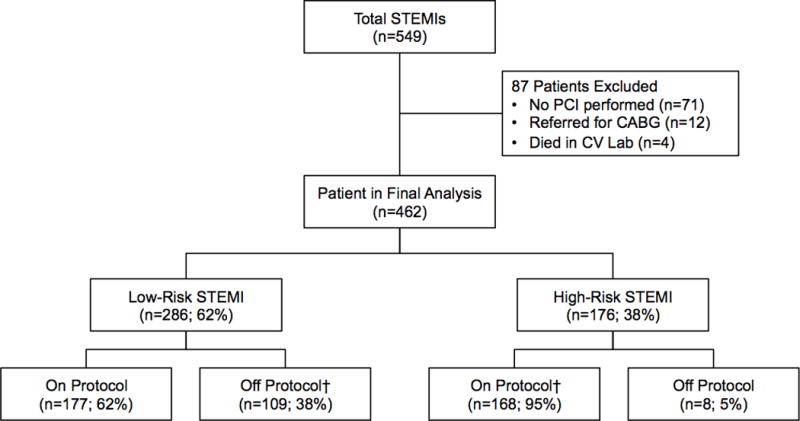
Prospective cohort flow chart
† Transfer to CCU include reasons such as MD request, PCI complication, severe LV systolic dysfunction
Comparison of low-risk, on protocol (transferred to telemetry) vs. off protocol (transferred to CICU) patients demonstrated no difference in baseline demographic or clinical characteristics, though following PCI, low-risk on-protocol patients did have a higher median ejection fraction (55.6% ± 9.7 vs. 51.3% ± 11.5; p<0.001) (Table 2) and overall lower composite procedural complication rate compared with those treated off protocol (4.3% vs. 14.9%; p=0.003), including bleeding at 72 hours (2.5% vs. 8.9%, p=0.018), though need for blood transfusion did not meet statistical significance (1.2% vs. 3.0%, p=0.31) (Figure 3). There was no difference between low-risk on and off protocol patients for in-hospital, 30-day or 1-year mortality or in 30-day or 1-year MACE (Table 2 and Figure 4).
Figure 3. Prospective Analysis: Complications and Mortality in Low-risk Telemetry and CCU vs. High-risk Patients.
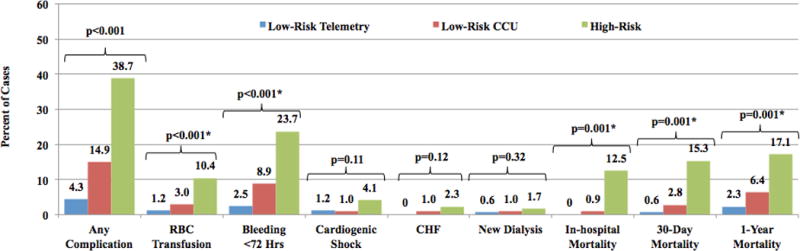
Comparison of complications and mortality for high-risk (n=176), on protocol low-risk (n=177) and off protocol low-risk (n=109), prospectively studied STEMI patients. Any complication is defined as a composite of red blood cell transfusion, cardiogenic shock, congestive heart failure and new need for dialysis. Mortality was measured in-hospital and at 30-days and 1-year. RBC= red blood cell; CHF= congestive heart failure.
* No statistical difference between low-risk telemetry and low-risk CCU patients
Figure 4.
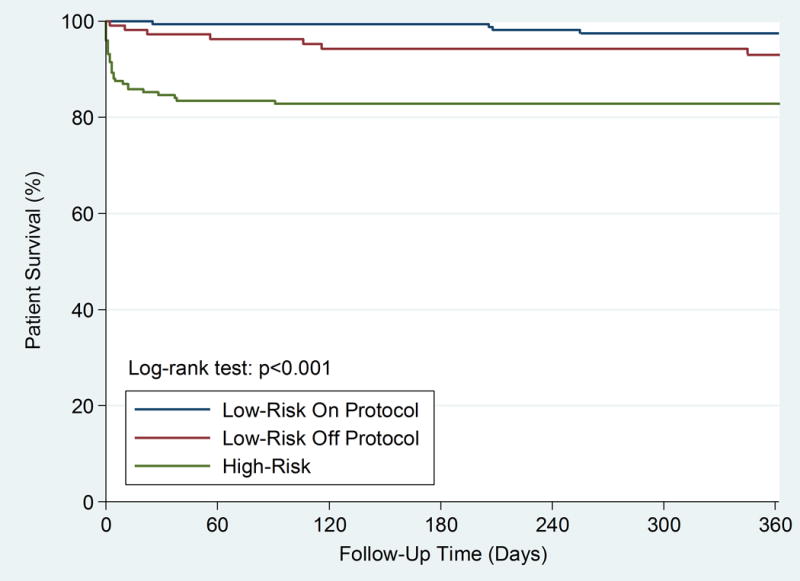
Kaplan-Meier survival analysis comparing high-risk (n=176), on protocol low-risk (n=177) and off protocol low-risk (n=109) patients in the prospective cohort
There was only 1 in-hospital death in the low-risk cohort. A patient initially admitted to the CICU with a stroke who subsequently suffered an in-hospital STEMI therefore a low-risk “off-protocol.” Following PCI, the patient returned to the CICU, but was ultimately placed on palliative care and died. At 30-days, there were 2 additional deaths in the low-risk off protocol group. One patient suffered an in-stent thrombosis 7 days after PCI, with subsequent death and another had an unknown cause of death, 22 days post-STEMI. In the low-risk on protocol group at 30-days, there was only 1 death in a patient who died of a Type A dissection 26 days post-STEMI.
Regarding resource utilization, the median LOS was shorter for low-risk patients both on and off protocol compared with high-risk patients, with low-risk on protocol patients having the shortest LOS (median and [25th, 75th percentiles]; low-risk on protocol: 2 [1, 2]; low-risk off protocol: 2 [2, 3]; high-risk: 3 [2, 6]; p<0.001 between all groups) (Table 2). The distribution of LOS in the 3 groups is shown in Supplemental Figure 1. A total of 54 (30.5%) low-risk patients were discharged earlier than 48 hours with no mortality at 1-year. Significant differences in median total hospital costs were also noted between the three groups, with the lowest cost also noted in the low-risk on protocol patients (median and [25th, 75th percentiles]; low-risk on protocol: $6,090 [$4,730–$7,356]; low-risk off protocol $8,412 [$6,728–$10,920]; high-risk: $11,783 [$7,953–$25,359]; p<0.001 across all groups) (Table 3).
Effect of Protocol Adherence on Low-Risk Patients
In the retrospective cohort, no low-risk patients were triaged to telemetry. There was no in hospital mortality in either cohorts and no significant differences in clinical characteristics, mortality or MACE at 1-year. Both mean and median LOS were significantly shorter in the low-risk on protocol prospective cohort compared to the low-risk retrospective cohort (mean 2.1 ± 2.5 vs. 3.5 ± 4.4, p<0.001) (Supplemental Table 1). Median total variable costs were also significantly less for low risk individuals in whom the protocol was utilized compared with the retrospective low risk cohort (median and [25th, 75th percentiles]; $6,090 [$4,730–$7,356] vs. $13,648 [$9,856, $19,234], p<0.001) (Supplemental Table 1). It should be noted that costs decreased for all groups between the retrospective to prospective cohorts.
Discussion
We describe a unique care innovation, initially utilizing retrospective application of a risk-tool to assess potential implications of risk-guided STEMI care, followed by integration of the risk-tool in the EMR to support triage and discharge guidance to providers following primary PCI, and finally the prospective evaluation of risk-guided STEMI care on patient outcomes and costs of care. This process provided assurance that application of risk-guided care would safely reduce costs, and therefore successful application at the point of care would improve the value of STEMI care.
Prior work on the feasibility of use of the Zwolle Risk Score has demonstrated its ability to safely reduce LOS, however, these studies were small, retrospective and outside the U.S., limiting their generalizability. One Spanish study examined 276 patients with the Zwolle Risk Score, however, only did so retrospectively.6 A prospective study from the same country looked only at low-risk patients, of which just 54 were targeted for early discharge, with no assessment of effect on cost.12 The Safe-Depart Trial studied prospective implementation of the Zwolle Score in a Canadian hospital, but only 54 patients were enrolled, of whom just 27 had the risk score applied to their care.8 Larger studies of early discharge after STEMI have been performed, but with much larger variations from primary literature based protocols and without cost analyses.7
The study that initially developed the Zwolle Risk Score ENREF 55 did perform a prospective analysis of the model, however, this was only for validation, not as an assessment of feasibility of clinical use of the score, as the results were not used to change patient care. Further, only 58% of patients in the validation cohort received a stent, with the remainder undergoing only angioplasty, the standard of care at that time. The cost-effectiveness analysis in the original analysis, was done based upon cost estimates, rather than true changes in cost. We describe the successful integration of the Zwolle Risk Score into a large regional STEMI system, rather than as a stand-alone research protocol, allowing for generalizability to real-world clinical settings. In addition, our study demonstrates true, rather than estimated cost savings associated with this value-based intervention.
When developing systems to improve the value of care, healthcare providers and leaders must first ensure that changes either maintain or improve the safety and quality of care they provide. To ensure both safety and provider buy in to risk-guided STEMI care, we emphasized efforts to exclude potentially high-risk patients from a low-risk pathway. This included addition of patients with out of hospital cardiac arrest to the Zwolle Risk Score. Our prospective analysis of this modified Zwolle Risk Score demonstrated several important findings. First, based on both retrospective and prospective analysis, low-risk STEMI patients are common, representing over 50% of eligible individuals in our study. Second, it validated the modified score’s ability to accurately characterize risk post-PCI, with only 1 in-hospital death in the low-risk cohort, occurring in an off protocol patient, already admitted to the hospital for a different, high-risk diagnosis, stroke.
Total protocol adherence (sending low-risk patients to telemetry and high-risk patients to the CICU) was 75%, but was only 62% for low-risk patients. While exact reasons for protocol deviation were not recorded, bleeding represents an apparent contributing factor to low adherence, with higher complication rates for low-risk off protocol patients triaged to the CICU driven largely by bleeding events. Clinical factors not captured by the risk calculator also likely contributed to protocol deviation, highlighted by the only in-hospital death in the prospective low-risk group attributed to an inpatient STEMI patient, already admitted with a stroke. These factors suggest that further refinements of the risk-tool may be possible, incorporating other clinical factors identified by providers in making treatment decisions. This also suggests that clinical expertise will remain important as providers apply clinical judgment in the application of risk-based care guidance. Furthermore, full adoption of risk-guided treatment decisions may not reflect optimal care and the potential for negative consequences of adherence to clinical decision support requires attention. Despite this, provider confidence in the protocol’s ability to improve quality continued to grow after the study period, with CICU triage for STEMI patients declining to 33.0% by 2017.
This finding also highlights the risk of using process measures as surrogates for outcomes measures. Standardized processes, while helpful, do not always capture the complexity of a given patient, nor do they necessarily correlate with improved outcomes.1 Of greater importance than provider adherence in this study is the improved clinical efficiency and low complication rate, indicative of improved value to both the health system and the patient. Given this and the results our study achieved, the Level 1 Myocardial Infarction STEMI Committee at our institution set a protocol adherence goal for providers at 75%, to allow for appropriate clinical deviation from the protocol, while still encouraging high value care decision making.
Increasingly, changes in care must demonstrate an ability to improve value, either achieving quality improvement that was not previously recognized, or decreasing cost while maintaining or improving on current quality outcomes. Comparison of low-risk patients treated on vs. off-protocol demonstrates that adherence to triage recommendation could save over $2,300 per patient. Based on the initial protocol adherence rate of 62% for the low-risk patients, extrapolation to the entire Allina system (700 total STEMI patients per year) would achieve savings of $623,844 annually. Protocol adherence of 85% in the low-risk patients across the system would save $855,270 a year. Ongoing work to track physician level protocol adherence and evaluation of variance is vital to both achieving this goal and understanding what other factors contribute to the differences between low-risk patients on and off protocol. Such future work will drive improvements in both protocol implementation and patient safety, honing our understanding of what currently unaccounted for factors lead to adverse events.
Imperative to achieving this goal and monitoring future tests of change is a robust data tracking mechanism. Our shared EMR and Enterprise Data Warehouse provided accurate, real-time clinical, financial and outcomes data, which served as the foundation for the retrospective analysis and provided a platform to deploy the prospective standardized early discharge protocol. Without this tool, successfully identifying low-risk STEMI patients, improving clinical efficiency and realizing reduced health care costs would have required a vastly increased amount of time and staff hours. In order to address these and similar challenges, all healthcare systems should invest in the personnel and infrastructure required to adequately understand and monitor their own data.
While this current initiative focuses on post-PCI care for STEMI, numerous other risk calculators and models exist across the spectrum of cardiovascular disease.13–15 Within acute myocardial infarction, expansion of risk models to the triage of patients presenting with non-ST Elevation Myocardial Infarction and Unstable Angina for early discharge may be able to realize similar reductions in LOS and total costs, without compromising quality of care or patient outcomes. Similarly, as percutaneous approaches to structural and valvular heart disease increase, risk-based care delivery strategies may help to achieve higher value care. The current work serves as a model for these types of future endeavors to reduce costs of care while maintaining optimal patient outcomes.
There are limitations to our study, including its single center design, which may limit generalizability. We also employed just one risk score (Zwolle), while others, such as the CADILLAC Score, have also been described for the purpose of identifying patients for early discharge.13 The prior validation of the Zwolle Risk Score and the ubiquitous nature of the EMR across the health system, however, provide the framework for this model to be adopted in other institutions. Our institution also integrates quality metrics into providers’ salary, which likely contributed to the rapid early uptake of the protocol, which may not occur at institutions without similar mechanisms in place. The use of total variable costs may also limit generalizability of realized cost savings, as differences in direct and indirect costs across institutions may vary. Further, the large number of STEMI patients treated by our regional STEMI program allowed large cost savings to be realized quickly, a component which may take longer in smaller networks.
The rapidly evolving healthcare landscape and demand for efficient, high quality cardiovascular treatment, necessitates the use of appropriate risk stratification for the triage of STEMI patients. This work demonstrates the potential value of the Zwolle Risk Score in STEMI to reduce costs, while maintaining high quality of care and lays the framework for future work in other patient populations and clinical conditions.
Supplementary Material
What is Known
STEMI is a life threatening and expensive medical condition, however, much of the risk is mitigated by prompt revascularization.
Risk models exist to be able to predict which patients can bypass the ICU following revascularization and be targeted for early discharge from the hospital
What the Study Adds
We demonstrate the feasibility of implementation of an evidence-based risk stratification tool into the electronic medical record (EMR) for triaging patients after revascularization for STEMI
Use of an EMR based Zwolle Risk Calculator is safe and results in shorter lengths of stays and lower hospital costs for STEMI patients.
Acknowledgments
Sources of Funding: Joseph E. Ebinger, MD was provided funding by the NIH 5 T32HL116273 05.
Footnotes
Disclosures: None
References
- 1.Porter ME. What is value in health care? The New England journal of medicine. 2010;363:2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 2.Ward MJ, Kripalani S, Zhu Y, Storrow AB, Dittus RS, Harrell FE, Jr, Self WH. Incidence of emergency department visits for ST-elevation myocardial infarction in a recent six-year period in the United States. The American journal of cardiology. 2015;115:167–70. doi: 10.1016/j.amjcard.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afana M, Brinjikji W, Cloft H, Salka S. Hospitalization costs for acute myocardial infarction patients treated with percutaneous coronary intervention in the United States are substantially higher than Medicare payments. Clinical cardiology. 2015;38:13–9. doi: 10.1002/clc.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 5.De Luca G, Suryapranata H, van ’t Hof AW, de Boer MJ, Hoorntje JC, Dambrink JH, Gosselink AT, Ottervanger JP, Zijlstra F. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation. 2004;109:2737–43. doi: 10.1161/01.CIR.0000131765.73959.87. [DOI] [PubMed] [Google Scholar]
- 6.Tralhao A, Ferreira AM, Madeira S, Borges Santos M, Castro M, Rosario I, Trabulo M, Aguiar C, Ferreira J, Almeida MS, Mendes M. Applicability of the Zwolle risk score for safe early discharge after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. Rev Port Cardiol. 2015;34:535–41. doi: 10.1016/j.repc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Jones DA, Rathod KS, Howard JP, Gallagher S, Antoniou S, De Palma R, Guttmann O, Cliffe S, Colley J, Butler J, Ferguson E, Mohiddin S, Kapur A, Knight CJ, Jain AK, Rothman MT, Mathur A, Timmis AD, Smith EJ, Wragg A. Safety and feasibility of hospital discharge 2 days following primary percutaneous intervention for ST-segment elevation myocardial infarction. Heart. 2012;98:1722–7. doi: 10.1136/heartjnl-2012-302414. [DOI] [PubMed] [Google Scholar]
- 8.Kotowycz MA, Cosman TL, Tartaglia C, Afzal R, Syal RP, Natarajan MK. Safety and feasibility of early hospital discharge in ST-segment elevation myocardial infarction--a prospective and randomized trial in low-risk primary percutaneous coronary intervention patients (the Safe-Depart Trial) Am Heart J. 2010;159:117 e1–6. doi: 10.1016/j.ahj.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Henry TD, Sharkey SW, Burke MN, Chavez IJ, Graham KJ, Henry CR, Lips DL, Madison JD, Menssen KM, Mooney MR, Newell MC, Pedersen WR, Poulose AK, Traverse JH, Unger BT, Wang YL, Larson DM. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation. 2007;116:721–8. doi: 10.1161/CIRCULATIONAHA.107.694141. [DOI] [PubMed] [Google Scholar]
- 10.Henry TD, Unger BT, Sharkey SW, Lips DL, Pedersen WR, Madison JD, Mooney MR, Flygenring BP, Larson DM. Design of a standardized system for transfer of patients with ST-elevation myocardial infarction for percutaneous coronary intervention. Am Heart J. 2005;150:373–84. doi: 10.1016/j.ahj.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 11.Miedema MD, Newell MC, Duval S, Garberich RF, Handran CB, Larson DM, Mulder S, Wang YL, Lips DL, Henry TD. Causes of delay and associated mortality in patients transferred with ST-segment-elevation myocardial infarction. Circulation. 2011;124:1636–44. doi: 10.1161/CIRCULATIONAHA.111.033118. [DOI] [PubMed] [Google Scholar]
- 12.Azzalini L, Sole E, Sans J, Vila M, Duran A, Gil-Alonso D, Santalo M, Garcia-Moll X, Sionis A. Feasibility and safety of an early discharge strategy after low-risk acute myocardial infarction treated with primary percutaneous coronary intervention: the EDAMI pilot trial. Cardiology. 2015;130:120–9. doi: 10.1159/000368890. [DOI] [PubMed] [Google Scholar]
- 13.Sharkawi MA, Filippaios A, Dani SS, Shah SP, Riskalla N, Venesy DM, Labib SB, Resnic FS. Identifying patients for safe early hospital discharge following st elevation myocardial infarction. Catheter Cardiovasc Interv. 2017;89:1141–1146. doi: 10.1002/ccd.26873. [DOI] [PubMed] [Google Scholar]
- 14.Ebinger J, Henry TD. From D2B to B2D: Value based STEMI care! Catheter Cardiovasc Interv. 2017;89:1147–1148. doi: 10.1002/ccd.27137. [DOI] [PubMed] [Google Scholar]
- 15.Ebinger JE, Porten BR, Strauss CE, Garberich RF, Han C, Wahl SK, Sun BC, Abdelhadi RH, Henry TD. Design, Challenges, and Implications of Quality Improvement Projects Using the Electronic Medical Record: Case Study: A Protocol to Reduce the Burden of Postoperative Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2016;9:593–9. doi: 10.1161/CIRCOUTCOMES.116.003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


