Abstract
Background
Patients with combined post- and pre-capillary pulmonary hypertension (CpcPH) due to left heart disease (PH-LHD) have a worse prognosis compared with isolated post-capillary (IpcPH). However, it remains unclear if increased mortality in CpcPH is simply a result of higher total right ventricular (RV) load. Pulmonary effective arterial elastance (Ea) is a measure of total RV afterload, reflecting both resistive and pulsatile components. We aimed to test whether pulmonary Ea discriminates survivors from non-survivors in patients with PH-LHD, and if it does so better than other hemodynamic parameters associated with CpcPH.
Methods and Results
We combined three large heart failure patient cohorts (n=1036) from academic hospitals including patients with PH due to heart failure with preserved ejection fraction (HFpEF) (n=232), reduced ejection fraction (HFrEF) (n=335) and a mixed population (n=469). In unadjusted and two adjusted models, pulmonary Ea more robustly predicted mortality than pulmonary vascular resistance (PVR) and the transpulmonary gradient. Along with pulmonary arterial compliance (PAC), pulmonary Ea remained predictive of survival in patients with normal PVR. The diastolic pulmonary gradient did not predict mortality. Additionally, in a subset of patients with echocardiographic data, Ea and PAC were better discriminators of RV dysfunction than the other parameters.
Conclusions
Pulmonary Ea and PAC more consistently predicted mortality than PVR or TPG across a spectrum of left heart disease with pulmonary hypertension including patients with HFpEF, HFrEF, and pulmonary hypertension with a normal PVR.
Keywords: Pulmonary effective arterial elastance, pulmonary arterial compliance, pulmonary hypertension due to left heart disease, combined post- and pre-capillary pulmonary hypertension, diastolic pulmonary gradient
Introduction
Among patients with pulmonary hypertension due to left heart disease (PH-LHD), individuals with combined post- and pre-capillary pulmonary hypertension (CpcPH) have worse prognosis compared with isolated post-capillary pulmonary hypertension (IpcPH) 1, 2. CpcPH is differentiated from IpcPH hemodynamically by measures that suggest the presence of pulmonary vascular pathology including pulmonary vascular resistance (PVR) ≥ 3 Wood units, transpulmonary gradient (TPG) ≥ 12-15 mmHg and diastolic pulmonary gradient (DPG) ≥ 7mmHg, and each has been associated with worse survival in various heart failure studies 1, 3-5. However, isolated pressure measures (i.e. TPG or DPG) do not incorporate flow (stroke volume), which may limit their prognostic value, and PVR (which does incorporate flow) only accounts for non-pulsatile afterload. Additionally, it remains unclear if patients with CpcPH simply have worse overall hemodynamics and higher total right ventricular (RV) load compared with IpcPH, and therefore, if markers of total RV load better predict mortality 2. In the setting of left heart failure, pulmonary arterial compliance (PAC) is determined by both PVR and left heart filling pressures 6. Consistently, several studies have demonstrated it to be a superior prognostic marker compared with PVR 7-9. Pulmonary effective arterial elastance (Ea) is a “lumped” measure of RV afterload that more fully incorporates resistive, pulsatile and passive components 10, 11. We sought to determine if pulmonary Ea as a measure of total RV load is associated with mortality in PH-LHD and compare its prognostic ability to hemodynamic markers of pre-capillary PH.
Methods
Patient populations
The combined patient cohort (A, B, and C) consisted of 1036 patients from three academic institutions. The data, analytic methods, and study materials will not be made available to other investigators.
Cohort A - Johns Hopkins (HFrEF and HFpEF)
As previously reported 12 this cohort included 1236 patients referred to the Johns Hopkins Hospital Cardiomyopathy Service for evaluation of new cardiomyopathy between 1982 and 1997. Heart failure specialists performed all right heart catheterization (RHC) procedures and interpreted the hemodynamic data. A complete set of hemodynamic data was available in 1174 patients. The patients were followed until death, cardiac transplantation or the end of the study period (January 1, 1998). Participants who underwent transplantation were censored at the time of transplantation. Vital status was obtained from medical records and through a search of the Nation Death Index 13. The Johns Hopkins Hospital Joint Committee on Clinical Investigation approved the study. There were 193 events recorded in 1608 patient-years.
Cohort B - Mayo Clinic Rochester (HFrEF)
This cohort included 490 ambulatory outpatients with HFrEF (LVEF ≤ 40%) referred to the Mayo Clinic (Rochester, MI) for a RHC between 2002 and 2008. Exclusion criteria were previously described14 and included patients with primary parenchymal lung disease, chronic obstructive pulmonary disease, prior valvular surgery, infiltrative, constrictive, or hypertrophic cardiomyopathy, myocardial infarction within 6 months, any history of group 1 (idiopathic, familial, collagen vascular disease, congenital heart disease), group 3 or group 4 (pulmonary thromboembolic disease) PH disease, tachyarrhythmia, chronic kidney disease, history of chest radiation, cardiac or lung transplantation. A complete set of hemodynamic data was available in 478 patients. Patient survival or all-cause mortality status was confirmed through June 30, 2010, using Mayo Clinic (Rochester, MI) electronic medical records, Olmsted County (MI) medical record linkage system and Social Security mortality index. The Mayo Foundation Institutional Research Review Board approved the study and all patients included provided informed consent. There were 140 events recorded in 805 patient-years.
Cohort C - Northwestern University (HFpEF)
Four hundred twenty patients were enrolled prospectively between March 2008 and May 2011 from the outpatient clinic of the Northwestern University HFpEF Program. 293 patients underwent invasive hemodynamic testing and of those 232 subjects met criteria for PH-LHD (defined below). All patients were recruited as outpatients after hospitalization for HF as previously reported15, and all patients were enrolled into the study based on a left ventricular ejection fraction (LVEF) >50% and the presence of Framingham criteria for HF. Although not required for inclusion into the cohort, all patients had evidence of either significant diastolic dysfunction (grade 2 or 3) on echocardiography, evidence of elevated LV filling pressures on invasive hemodynamic testing, or BNP >100 pg/mL. All enrolled patients followed-up in a specialized HFpEF outpatient program. Patients with greater than moderate valvular disease, prior cardiac transplantation, history of reduced LVEF < 40% (i.e., recovered EF), or diagnosis of constrictive pericarditis were excluded. All participants gave written informed consent, and the institutional review board at Northwestern University approved the study. There were 78 events recorded in 1144 patient-years.
Hemodynamic Measures
PH-LHD was defined as a mean pulmonary artery pressure (mPAP) ≥ 25mmHg with a pulmonary artery wedge pressure (PAWP) > 15mmHg. Total RV load was estimated by pulmonary Ea. Historically, systemic Ea was initially derived using parameters from a 3-element arterial Windkessel model16, but later was simplified to end-systolic pressure (ESP) divided by SV which can be readily obtained from a pressure-volume loop10. This simplification was later validated in the RV17. Under normal conditions, the RV ejects into a low impedance circuit and pressure declines throughout ejection18. Therefore, in the setting of normal afterload, ESP is closely related to mPAP. However, with development of PH, the RV pressure volume loop changes shape with pressure rising throughout ejection and peaking near end-systole. Therefore, in PH patients end-systolic pressure (ESP) is more closely approximated by systolic pulmonary artery pressure (sPAP), and Ea can be defined as sPAP/SV 19, 20. Because our cohort included only participants with pulmonary hypertension, this latter definition of Ea (sPAP/SV) was used in all analyses. TPG was calculated as the difference between mPAP and PAWP, and PVR as TPG divided by cardiac output (CO). DPG was calculated as the difference between the diastolic pulmonary artery pressure (dPAP) and PAWP. Pulmonary arterial compliance (PAC) was estimated as stroke volume divided by pulmonary artery pulse pressure. CO was determined by either thermodilution or direct Fick technique in all three cohorts. These calculations are summarized in Supplemental Table 1.
Statistical Analysis
Continuous variables were compared across the 3 cohorts with 1-way ANOVA or Kruskal Wallis if non-normally distributed. Categorical variables were compared with chi-squared test. Hazard ratios of death for PVR, TPG, DPG, PAC and pulmonary Ea were estimated with Cox proportional hazards regression analysis (unadjusted and two adjusted models). We chose to adjust for only age in the first adjusted model given the known impact of aging on PAC and PVR 6. In the second model, we adjusted for age, body mass index (BMI), heart rate, gender and cohort (cohort only in pooled analysis). We did not adjust for race since these data were not available in cohort B. We tested the proportionality assumption using scaled Schoenfeld residuals on time in each individual cohort and for each variable added to the Cox model. Survival was also estimated with the non-parametric methods of Kaplan and Meier and compared using the log-rank test. Qualitative data on RV function were available in cohort B and C. Each subject was classified as having none, mild-moderate, or severe RV dysfunction. Those who had been reported as moderate-severe were considered severe for our analysis. The ability of Ea, PAC, PVR, TPG, and DPG to discriminate “any RV dysfunction” as well as “severe RV dysfunction” was assessed by calculating a C-statistic. A p-value (two-tailed) of <0.05 was considered significant. Medians are presented with interquartile range. Statistical analyses were performed using STATA version 12 (Stata Corp, Texas) and SigmaPlot version 11 (Systat Software Inc).
Results
Study populations
Clinical characteristics and hemodynamics for the combined cohort and each individual cohort is shown Table 1. Cohort A had the youngest patients among the three groups (p<0.001), had a high percentage of non-ischemic cardiomyopathy, and included patients with higher heart rate than the other two cohorts. Cohort C had a higher percentage of female gender and higher BMI than cohorts A and B. In contrast to cohorts A and B, cohort C (HFpEF) also had an overall better hemodynamic profile as evident by the higher CI, PAC, RV stroke work index (RVSWI), left ventricular stroke work index (LVSWI) and lower pulmonary Ea and PVR when compared with the other two groups. Forty percent of cohort A (469 of 1174 subjects), 68% of cohort B (335 of 490 subjects) and 79% of cohort C (232 of 293 subjects) met invasive hemodynamic criteria for PH-LHD. PH-LHD was therefore present in 53% of the combined cohort.
Table 1.
| All Cohorts (n=1036) |
Cohort A (n=469) |
Cohort B (n=335) |
Cohort C (n=232) |
p- value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 57 [45-67] | 49 [34-64] | 59 [49-68] | 63 [57-71] | <0.001 |
| Body Mass Index (kg/m2) | 27.7 [23.9-33.3] | 26.2 [19.1-33.4] | 28.4 [25.0-33.0] | 31.8 [26.0-38.4] | <0.001 |
| Female Gender | 400 (39) | 167 (36) | 89 (27) | 144 (61) | <0.001* |
| Race | |||||
| Black | 256 (37) | 169 (36) | 87 (38) | ||
| Caucasian | 411 (59) | 289(62) | NA | 122 (52) | |
| Other | 32 (4) | 9(2) | 23 (10) | <0.001* | |
| Diagnosis | |||||
| Non-Ischemic | 557 (80) | 423 (90) | NA | 134 (58) | |
| Ischemic | 144 (20) | 46 (10) | NA | 98 (42) | <0.001* |
| Hemodynamics | |||||
| Heart rate (beats per minute) | 81 [69-97] | 92 [75-111] | 75 [66-87] | 71 [63-80] | <0.001 |
| Systemic Blood Pressure | |||||
| Systolic (mmHg) | 121 [106-139] | 118 [91-145] | NA | 124 [112-138] | 0.097 † |
| Diastolic (mmHg) | 75 [66-84] | 78 [62-94] | NA | 70 [60-77] | <0.001 † |
| Mean (mmHg) | 90 [81-102] | 91 [73-109] | NA | 87 [81-97] | <0.001 † |
| Systemic Vascular Resistance (WU) | 18 [13-25] | 21 [12-30] | NA | 12.3 [9.4-15.5] | <0.001 † |
| Left Ventricular Stroke Work Index (mmHg ml/m2) | 1685 [1154-2510] | 1375 [654-2096] | NA | 2595 [1886-3168] | <0.001 † |
| Right Atrial Pressure (mmHg) | 13 [9-18] | 11 [5-17] | 14.7 [10-19] | 15 [12-20] | <0.001 |
| Pulmonary Artery Pressures | |||||
| Systolic (mmHg) | 53 [45-63] | 52 [40-64] | 54 [46-64] | 53 [45-66] | 0.038 |
| Diastolic (mmHg) | 27 [22-31] | 27 [21-33] | 26 [22-31] | 27 [23-33] | 0.051 |
| Mean (mmHg) | 35 [30-42] | 35 [27-43] | 36 [30-42] | 36 [30-43] | 0.130 |
| Pulmonary Artery Wedge Pressure (mmHg) | 25 [20-35] | 25 [19-31] | 24 [20-28] | 25 [21-31] | 0.006 |
| Cardiac Index (L/min/m2) | 2.2 [1.7-2.7] | 1.9 [1.3-2.5] | 2.0 [1.7-2.4] | 2.9 [2.4-3.4] | <0.001 |
| Right Ventricular Stroke Work Index (mmHg ml/m2) | 598 [407-835] | 517 [275-761] | 629 [410-846] | 866 [612-1200] | <0.001 |
| Pulmonary Effective Arterial Elastance (mmHg/ml) | 1.0 [0.7-1.5] | 1.3 [0.6-2.0] | 1.0 [0.8-1.4] | 0.6 [0.5-0.9] | <0.001 |
| Pulmonary Artery Compliance (ml/mmHg) | 2.0 [1.4- 2.9] | 1.6 [0.7-2.6] | 1.9 [1.4-2.5] | 3.2 [2.4-4.7] | <0.001 |
| Pulmonary Vascular Resistance (WU) | 2.3 [1.4-3.5] | 2.4 [0.5-4.3] | 2.8 [1.8-4.1] | 1.7 [1.1-2.5] | <0.001 |
| Transpulmonary Gradient (mmHg) | 11 [7-14] | 9 [3.5-14 .5] | 11 [7.0-15.3] | 10 [8.0-14.0] | <0.001 |
| Diastolic Pulmonary Gradient (mmHg) | 1 [-1.0 – 5.0] | 1 [-3.9 - 5.9] | 1.3 [-1.0 - 5.0] | 0 [0 – 3.0] | 0.078 |
| RA/PCWP | 0.5 [0.3-0.7] | 0.4 [0.2-0.6] | 0.5 [0.4-0.70] | 0.6 [0.4-0.7] | <0.001 |
Data presented as median [interquartile range]
ANOVA or Kruskal Wallis test unless otherwise indicated
Chi-square,
Rank Sum test
() = Percentage
Pulmonary effective arterial elastance is associated with mortality in patients with PH-LHD
In unadjusted and adjusted analyses, pulmonary Ea was significantly associated with increased mortality (adjusted1 HR per 1 mmHg/mL increase in Ea 1.47 [1.31-1.65], χ2=44.3, p<0.001 and adjusted2 HR 1.45 [1.27-1.65], χ2=29.6, p<0.001) (Table 2). Lower PAC, higher PVR, and higher TPG were also associated with increased mortality in age adjusted analyses (PAC: adjusted1 HR per 1mL/mmHg decrease in PAC 1.33 [1.22-1.45], χ2=41.0, p<0.001 and adjusted2 HR 1.30 [1.18-1.43], χ2=29.3 p<0.001; PVR: HR per 1 Wood unit increase in PVR 1.15 [1.10-1.20], χ2=36.5, p<0.001 and adjusted2 HR 1.14 [1.08-1.19], χ2=27.8 p<0.001; TPG: adjusted1 HR per 1mmHg increase in TPG 1.02 [1.00-1.03], χ2=6.5, p=0.01 and adjusted2 HR 1.02 [1.01-1.04], χ2=8.2, p=0.004). Higher PAWP alone also tended to predict mortality: adjusted1 HR 1.02 per 1mmHg increase, χ2=3.9, p=0.052 and adjusted2 HR 1.03 [1.00-1.06], χ2=4.5, p=0.034. The median pulmonary Ea was 1.036 mmHg/mL. Over a median follow up of 2.8 years, mortality was significantly worse in those patients with an Ea higher than the median (p<0.001)(Figure 1 (pooled cohort) and Supplemental Figure 1(individual cohorts)). Similar pooled data relative to median values is shown for PAC and PVR in Supplemental Figure 2.
Table 2.
| All Cohorts | Cohort A | Cohort B | Cohort C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) per unit increase | P-value | x2 | Hazard Ratio (95% CI) per unit increase | P-value | x2 | Hazard Ratio (95% CI) per unit increase | P-value | x2 | Hazard Ratio (95% CI) per unit increase | P-value | x2 | |
| Ea | ||||||||||||
| Unadjusted | 1.41 (1.26-1.59) | <0.001 | 34.0 | 1.26 (1.06-1.51) | 0.009 | 6.9 | 1.65 (1.23-2.22) | 0.001 | 10.9 | 1.60 (1.13-2.27) | 0.008 | 7.0 |
| Adjusted* | 1.47 (1.31-1.65) | <0.001 | 44.3 | 1.25 (1.08-1.49) | 0.012 | 6.3 | 1.64 (1.22-2.20) | 0.001 | 10.9 | 1.44 (0.99-2.10) | 0.053 | 7.8 |
| Adjusted† | 1.45 (1.27-1.65) | <0.001 | 29.6 | 1.30 (1.08-1.56) | 0.005 | 7.8 | 1.72 (1.23-2.42) | 0.002 | 9.8 | 1.47 (0.97-2.23) | 0.069 | 3.3 |
| PAC‡ | ||||||||||||
| Unadjusted | 1.30 (1.19-1.41) | <0.001 | 35.8 | 1.22 (1.03-1.45) | 0.021 | 5.3 | 1.45 (1.18-1.82) | 0.001 | 11.3 | 1.22 (1.06-1.41) | 0.006 | 7.7 |
| Adjusted* | 1.33 (1.22-1.45) | <0.001 | 41.0 | 1.19 (1.02-1.41) | 0.033 | 4.5 | 1.37 (1.11-1.72) | 0.004 | 8.3 | 1.16 (1.01-1.35) | 0.042 | 4.1 |
| Adjusted† | 1.30 (1.18-1.43) | <0.001 | 29.3 | 1.26 (1.04-1.52) | 0.017 | 5.6 | 1.37 (1.09-1.73) | 0.007 | 7.2 | 1.15 (0.99-1.34) | 0.076 | 3.2 |
| PVR | ||||||||||||
| Unadjusted | 1.16 (1.11-1.21) | <0.001 | 40.2 | 1.13 (1.06-1.20) | <0.001 | 12.9 | 1.12 (1.04-1.21) | 0.002 | 9.3 | 1.18 (1.01-1.37) | 0.035 | 4.4 |
| Adjusted* | 1.15 (1.10-1.20) | <0.001 | 36.5 | 1.10 (1.03-1.18) | 0.004 | 8.4 | 1.11 (1.03-1.20) | 0.007 | 7.3 | 1.11 (0.94-1.30) | 0.22 | 1.5 |
| Adjusted† | 1.14(1.08-1.19) | <0.001 | 27.8 | 1.11 (1.04-1.19) | 0.003 | 8.8 | 1.12 (1.03-1.21) | 0.005 | 7.8 | 1.08 (0.92-1.28) | 0.36 | 0.8 |
| TPG | ||||||||||||
| Unadjusted | 1.02 (1.00-1.04) | 0.003 | 8.8 | 1.02 (1.00-1.05) | 0.025 | 5.0 | 1.01 (0.99-1.04) | 0.15 | 2.1 | 1.03 (0.99-1.06) | 0.17 | 1.9 |
| Adjusted* | 1.02 (1.00-1.03) | 0.011 | 6.5 | 1.02 (1.00-1.05) | 0.071 | 3.2 | 1.02 (0.99-1.04) | 0.12 | 2.4 | 1.02 (0.98-1.06) | 0.27 | 1.2 |
| Adjusted† | 1.02 (1.01-1.04) | 0.004 | 8.2 | 1.03 (1.00-1.05) | 0.042 | 4.1 | 1.02 (1.00-1.05) | 0.063 | 3.4 | 1.02 (0.98-1.06) | 0.27 | 1.2 |
| DPG | ||||||||||||
| Unadjusted | 1.01 (0.99-1.03) | 0.30 | 1.0 | 1.02 (1.00-1.05) | 0.095 | 2.6 | 1.00 (0.97-1.03) | 0.90 | 0.1 | 0.98 (0.90-1.06) | 0.55 | 0.3 |
| Adjusted* | 1.01 (0.99-1.03) | 0.29 | 1.0 | 1.02 (0.99-1.05) | 0.12 | 2.2 | 1.01 (0.98-1.04) | 0.44 | 0.6 | 0.98 (0.90-1.06) | 0.66 | 0.2 |
| Adjusted† | 1.01 (0.99-1.03) | 0.17 | 1.9 | 1.02 (1.00-1.05) | 0.099 | 2.7 | 1.01 (0.99-1.04) | 0.36 | 0.8 | 0.99 (0.91-1.07) | 0.78 | 0.1 |
The outcome is all-cause mortality.
Adjusted model accounts for age.
Adjusted model accounts for age, gender, body mass index, heart rate, and cohort.
HRs are per one-unit decrease in PAC. Ea = Pulmonary effective elastance. PAC = Pulmonary artery compliance, PVR = Pulmonary vascular resistance. TPG = Transpulmonary gradient. DPG = Diastolic pulmonary gradient.
Figure 1.
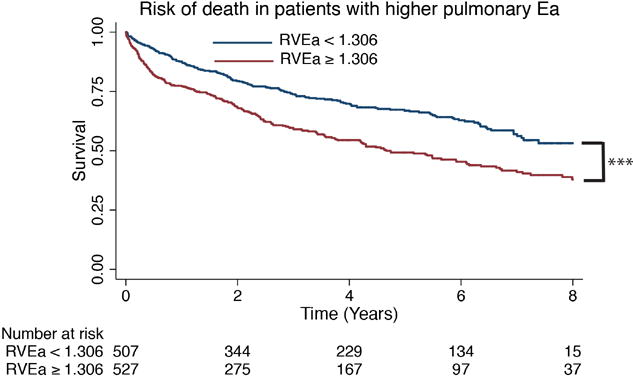
Kaplan-Meier survival curve of patients with heart failure in combined cohorts. In patients with PH-LHD (mPAP ≥25mmHg and PAWP >15mmHg), higher pulmonary Ea discriminated survivors. The median pulmonary Ea for all three cohorts combined was used as a cut-off value. *** P <0.001; Ea = effective arterial elastance.
DPG is not associated with mortality in patients with PH-LHD
DPG was not associated with mortality in unadjusted or adjusted analyses nor was it using the guideline-recommended cutoff of ≥ 7mmHg (p=0.46, Figure 2A). CpcPH defined as DPG ≥ 7 mmHg AND PVR > 3WU trended toward worse survival compared with the rest of the cohort but did not reach statistical significance (p=0.086, Figure 2B).
Figure 2.

Kaplan-Meier survival curve of patients with heart failure in combined cohort. A. In patients with PH-LHD (mPAP ≥25mmHg and PAWP >15mmHg), DPG ≥ 7mmHg did not discriminate survivors. B. Similarly, in patients with PH-LHD DPG ≥ 7 mmHg and PVR >3 WU did not discriminate survivors. DPG = Diastolic pulmonary gradient. PVR = Pulmonary vascular resistance.
Pulmonary Ea and PAC association with RV dysfunction
Qualitative data on RV function determined by echocardiography was available in cohort B and C. In this combined cohort (B and C), pulmonary Ea and PAC discriminated more strongly severe RV dysfunction as well as any RV dysfunction compared with PVR, TPG, and DPG. (Table 3).
Table 3.
| C-statistic for Severe RV dysfunction | C-statistic for Any RV dysfunction | |
|---|---|---|
| Ea | 0.78 | 0.72 |
| PAC | 0.78 | 0.71 |
| PVR | 0.67 | 0.65 |
| TPG | 0.52 | 0.52 |
| DPG | 0.52 | 0.51 |
RV = Right Ventricular, Ea = Pulmonary effective arterial elastance. PAC = Pulmonary arterial compliance, PVR = Pulmonary vascular resistance. TPG = Transpulmonary gradient. DPG = Diastolic pulmonary gradient.
Pulmonary Ea and PAC are associated with mortality in subjects with normal PVR
In the subset of subjects with normal PVR (n=685), 212 had an Ea above the median value for the larger cohort and 217 had a PAC below the median for the larger cohort. Pulmonary Ea (adjusted1 HR 1.31 [1.04-1.66], χ2=5.2 p=0.022 and adjusted2 HR 1.34 [0.99-1.81], χ2=3.6 p=0.057) and PAC (adjusted1 HR 1.20 [1.09-1.33], χ2=13.8, p<0.001 and adjusted2 HR 1.22 [1.08-1.37], χ2=11.0 p=<0.001) remained predictive of mortality when examining the subgroup of patients with PVR ≤ 3WU, whereas PVR did not. PAWP by itself also did not predict mortality in this subset. We then divided the entire combined cohort into tertiles of PVR and plotted pulmonary Ea and PAC as a function of PAWP (Figure 3A and B). Pulmonary Ea increased and PAC decreased as PAWP increased in all PVR tertiles. Data for individual cohorts are shown in Supplemental Figures 3 and 4. As illustrated in these figures, subjects in the two lower PVR tertiles have similar or even higher pulmonary Ea (or lower PAC) than the highest PVR tertile when PAWP is significantly elevated.
Figure 3.
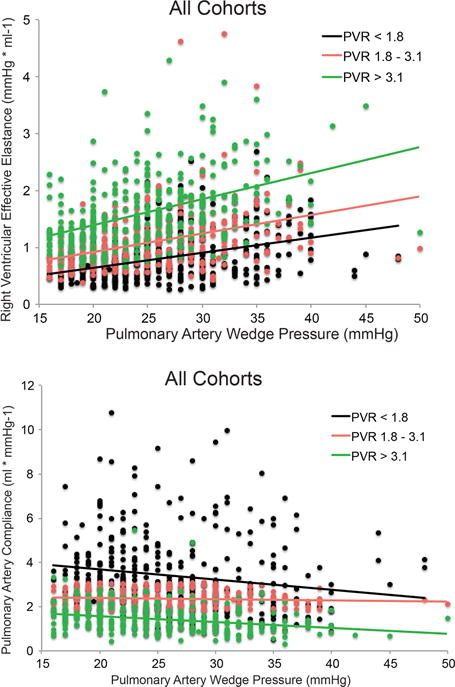
A. Distribution of pulmonary Ea values and their respective regression lines as a function of PAWP into tertiles of PVR in combined cohort of patients with pulmonary hypertension due to left heart disease. Pulmonary Ea increased as PAWP increased in all tertiles of PVR. When compared to the highest PVR tertile, the two lower PVR tertiles can have similar or even higher pulmonary Ea at significantly elevated PAWP levels. B. Distribution of PAC as a function of PAWP into tertiles of PVR in the combined cohorts of patient with pulmonary hypertension due to left heart disease. Similarly, patients in the two lower PVR tertiles had similar or even lower PAC compared to the higher PVR tertile at significantly elevated PAWP.
Discussion
Main Findings
In an analysis of a large combined cohort of patients with PH due to left heart disease, pulmonary Ea as a hemodynamic marker of total RV afterload was associated with mortality in patients with PH-LHD. Pulmonary Ea and PAC more consistently predicted mortality than PVR or TPG across a spectrum of left heart disease with pulmonary hypertension including patients with HFpEF and HFrEF and were more strongly associated with RV dysfunction. Ea and PAC remained predictive of mortality in a subgroup of patients with normal resistive load. Lastly, DPG was not associated with mortality in this large combined cohort, or in individual cohorts of HFrEF or HFpEF.
RV function is a strong predictor of prognosis in both heart failure with preserved and reduced ejection fraction 21, 22. The RV is more afterload sensitive (both acutely and chronically) than the left ventricle 11, 23, and it is therefore not surprising that development of PH is itself associated with worse outcomes. Although PH with a pre-capillary component is associated with a worse prognosis than isolated post-capillary PH, those patients with a pre-capillary component also tend to have worse overall hemodynamics and higher total RV load. The gold standard for assessing RV afterload is pulmonary artery input impedance spectra which allows for assessment of total and individual components of afterload 24. Although resistive load (i.e. PVR) makes up a substantial proportion of total RV load, other components including pulmonary arterial compliance and arterial wave reflections contribute as well. Ea incorporates these other factors and is an attractive hemodynamic marker because it can be derived from a standard right heart catheterization, therefore not requiring more invasive or sophisticated measurement techniques. This study confirms that measures of total RV afterload are more predictive of mortality and RV dysfunction in PH-LHD than measures associated with pre-capillary PH. Ghio and colleagues recently showed significant vasoreactivity and reversibility of the pre-capillary component in those with CpcPH suggesting a significant functional component to the precapillary parameters, including the DPG25. One may speculate that this could explain, in part, why therapies targeting the pre-capillary component in LHD have yielded disappointing results. In a post-hoc analysis of the RELAX study, for example, sildenafil failed to significantly lower total RV afterload26.
In absence of left heart failure and normal PAWP, RV pulsatile load is almost exclusively dependent on resistive load – which is unique compared to the systemic circulation – and therefore is generally constant proportion of total RV load 27. However, in left heart failure, elevated left atrial pressure (i.e. PAWP) has been shown in a number of studies to reduce PAC out of proportion to PVR, thereby increasing RV pulsatile loading 6, 7, 28, 29. Thus, in the pulmonary circulation, compliance is also a ‘lumped’ parameter that is determined by both resistive and pulsatile components of RV load. Similar to the findings in our study, Pellegrini and colleagues showed that PAC remained a predictor of mortality even in subjects with LHD and normal PVR 29. In this situation, pulsatile loading makes up a higher proportion of total RV load. Because the pulmonary resistance-compliance relationship is steep when PVR is borderline or just mildly elevated, modestly lowering PVR in this setting may result in robust reductions in pulsatile load 6. For example, inorganic sodium nitrite, a novel NO-providing agent, increased PAC significantly despite only a modest reduction in resistive load in patients with HFpEF 30. This effect was also in part due to lowering PAWP.
Importantly, the effects of pulsatile loading are reflected in the sPAP not dPAP. Because mPAP reflects both dPAP and sPAP, it is less reflective of pulsatile loading than sPAP. Indeed, in a recent study of subjects with HFpEF, sPAP was the only hemodynamic parameter associated with cardiac events in multivariate analysis 8. In addition, Wong et al showed sPAP and heart rate were the two major determinants of RV myocardial oxygen consumption in a group of patients with idiopathic pulmonary arterial hypertension 9. In our study, Ea – which is estimated by sPAP/SV – and PAC – which is estimated by SV/(sPAP-dPAP) – were more predictive of mortality than measures incorporating mPAP (i.e. PVR). It is also important to consider that Ea can be easily measured non-invasively using echocardiography by simply dividing estimated right ventricular systolic pressure by stroke volume. This could prove to be an attractive, straightforward metric to measure total RV afterload non-invasively and index ventricular function to RV afterload.
Finally, although DPG ≥7mmHg has been suggested as a diagnostic marker of CpcPH, the findings of this work along with other published data 7, 12, 31-33, call into question its use as a prognostic variable in PH-LHD. The European Society of Cardiology/European Respiratory Society (ESC/ERS) PH Guidelines currently define CpcCH as DPG ≥ 7mmHg “and/or” PVR > 3WU. More recently however it has been suggested that this definition should be modified to DPG ≥ 7mmHg “AND” PVR > 3WU because DPG ≥ 7mmHg in the setting of a PVR < 3WU is likely related to measurement error or tachycardia 34. In the current study, the group fitting this definition trended toward worse outcome yet did not reach statistical significance. It remains to be seen if DPG may still be valuable as a marker to identify patients with PH-LHD who are responsive to pulmonary vasodilator therapy. Alternatively, therapies targeting total RV load rather than the pre-capillary component, many of which are aimed at treating the left ventricle in tandem with PA vascular loading 30, may be more appropriate.
Strengths and Limitations
A major strength of our study is the large sample size of the combined cohort but also that results were generally concordant among the three independent cohorts, thereby increasing the validity of our findings across the spectrum of ejection fraction in patients with heart failure and left heart disease. Specifically, cohort B (HFrEF) and cohort C (HFpEF) showed similar results. In addition, each of these 3 cohorts included invasive hemodynamic data, which is the gold standard for diagnosing PH-LHD.
We do acknowledge that our analysis has several limitations. First, our retrospective study was composed of three cohorts from three different hemodynamic laboratories and different heart failure cardiologists performed all measurements and interpreted the hemodynamic tracings. Therefore, variation in data analysis might be present. Despite the different operators and patient characteristics, the fact that our results were generally consistent among all three cohorts is reassuring. Although we controlled for cohort in our pooled analysis, there is still the potential of pooled estimates to accentuate confounding and inflate the effect estimate. Information regarding heart failure therapies including medications, laboratory values, and co-morbidities such as COPD, atrial fibrillation, and renal failure were not available in all cohorts nor where pulmonary vascular pathology samples. Echocardiography was only available in two of the cohorts and only qualitative assessment of RV function was available for our study. Although patients with left heart failure may have elevated PVR, TPG or DPG, significant pulmonary vascular remodeling may be absent in many left heart disease patients based on the relative quick reversal of PH seen after transplant or implant of a mechanical assist device 35. In those heart failure patients with definite pulmonary vascular disease, such as that seen in pulmonary arterial hypertension, it remains possible that pre-capillary markers of PH have additive value. Lastly, because Ea and PAC incorporate the contributions of elevated left heart pressures, they are not likely to be useful in defining pre-capillary disease for diagnostic purposes.
Conclusion
In conclusion, our study shows that in PH due to left heart failure, higher pulmonary effective arterial elastance and lower pulmonary arterial compliance are associated with mortality and RV dysfunction. These parameters were more consistently associated with mortality across the spectrum of heart failure, and even when resistive load was normal. These findings add support to the idea that worse mortality in CpcPH is a result of higher total RV load.
Supplementary Material
Clinical Perspective.
What is new?
Pulmonary effective arterial elastance (Ea) is associated with mortality in pulmonary hypertension due to left heart disease (PH-LHD) in both heart failure with preserved and reduced ejection fraction.
In the setting of PH-LHD, pulmonary Ea and pulmonary arterial compliance (PAC), parameters reflective of total right ventricular afterload, are more consistently associated with right ventricular (RV) dysfunction and prognosis than measures of pre-capillary disease (pulmonary vascular resistance (PVR), transpulmonary gradient, and diastolic pulmonary gradient(DPG)).
The DPG or combination of PVR>3 Wood units AND DPG ≥ 7 mmHg did not predict prognosis in this study.
What are the clinical implications?
This study confirms that measures of total RV afterload are more predictive of mortality and RV dysfunction in PH-LHD than measures associated with pre-capillary PH.
Pulmonary Ea could prove to be an attractive, non-invasive metric to measure total RV afterload and index ventricular function to RV afterload.
These findings add support to the idea that worse mortality in combined post- and pre-capillary PH is a result of higher total RV load. Therapies targeting total RV load rather than the pre-capillary component, many of which are aimed at treating the left ventricle in tandem with pulmonary vascular loading, may prove beneficial.
Acknowledgments
Sources of Funding
Dr. Tampakakis is supported by a Johns Hopkins University Clinician Scientist Award (CSA). The National Institutes of Health supports Dr. Shah (R01 HL107577 and R01 HL127028), Dr. Damico (R01HL132153 and U01HL125175), Dr. Mathai (U01HL125175), and Dr. Kass (R35HL135827).
Footnotes
Disclosures
All authors report no conflicts of interest related to this study.
References
- 1.Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. Journal of the American College of Cardiology. 2013;62:D100–8. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–90. doi: 10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 3.Cappola TP, Felker GM, Kao WH, Hare JM, Baughman KL, Kasper EK. Pulmonary hypertension and risk of death in cardiomyopathy: patients with myocarditis are at higher risk. Circulation. 2002;105:1663–8. doi: 10.1161/01.cir.0000013771.30198.82. [DOI] [PubMed] [Google Scholar]
- 4.Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143:758–66. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 5.Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. The European respiratory journal. 2013;41:217–23. doi: 10.1183/09031936.00074312. [DOI] [PubMed] [Google Scholar]
- 6.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–97. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary Arterial Capacitance Is an Important Predictor of Mortality in Heart Failure With a Preserved Ejection Fraction. JACC Heart failure. 2015;3:467–74. doi: 10.1016/j.jchf.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschauer S, Kammerlander AA, Zotter-Tufaro C, Ristl R, Pfaffenberger S, Bachmann A, Duca F, Marzluf BA, Bonderman D, Mascherbauer J. The right heart in heart failure with preserved ejection fraction: insights from cardiac magnetic resonance imaging and invasive haemodynamics. European journal of heart failure. 2015;66(11):1308–1310. doi: 10.1002/ejhf.418. [DOI] [PubMed] [Google Scholar]
- 9.Wong YY, Westerhof N, Ruiter G, Lubberink M, Raijmakers P, Knaapen P, Marcus JT, Boonstra A, Lammertsma AA, van der Laarse WJ, Vonk-Noordegraaf A. Systolic pulmonary artery pressure and heart rate are main determinants of oxygen consumption in the right ventricular myocardium of patients with idiopathic pulmonary arterial hypertension. European journal of heart failure. 2011;13:1290–5. doi: 10.1093/eurjhf/hfr140. [DOI] [PubMed] [Google Scholar]
- 10.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–21. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 11.Tedford RJ. Determinants of right ventricular afterload (2013 Grover Conference series) Pulmonary circulation. 2014;4:211–9. doi: 10.1086/676020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tampakakis E, Leary PJ, Selby VN, De Marco T, Cappola TP, Felker GM, Russell SD, Kasper EK, Tedford RJ. The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC Heart failure. 2015;3:9–16. doi: 10.1016/j.jchf.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. American journal of epidemiology. 1990;131:160–8. doi: 10.1093/oxfordjournals.aje.a115470. [DOI] [PubMed] [Google Scholar]
- 14.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart failure. 2013;1:290–9. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–79. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. The American journal of physiology. 1983;245:H773–80. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 17.Morimont P, Lambermont B, Ghuysen A, Gerard P, Kolh P, Lancellotti P, Tchana-Sato V, Desaive T, D’Orio V. Effective arterial elastance as an index of pulmonary vascular load. American journal of physiology Heart and circulatory physiology. 2008;294:H2736–42. doi: 10.1152/ajpheart.00796.2007. [DOI] [PubMed] [Google Scholar]
- 18.Maughan WL, Shoukas AA, Sagawa K, Weisfeldt ML. Instantaneous pressure-volume relationship of the canine right ventricle. Circulation research. 1979;44:309–15. doi: 10.1161/01.res.44.3.309. [DOI] [PubMed] [Google Scholar]
- 19.Vanderpool RR, Pinsky MR, Naeije R, Deible C, Kosaraju V, Bunner C, Mathier MA, Lacomis J, Champion HC, Simon MA. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2015;101:37–43. doi: 10.1136/heartjnl-2014-306142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metkus TS, Mullin CJ, Grandin EW, Rame JE, Tampakakis E, Hsu S, Kolb TM, Damico R, Hassoun PM, Kass DA, Mathai SC, Tedford RJ. Heart Rate Dependence of the Pulmonary Resistance x Compliance (RC) Time and Impact on Right Ventricular Load. PloS one. 2016;11:e0166463. doi: 10.1371/journal.pone.0166463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. Journal of the American College of Cardiology. 2001;37:183–8. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 22.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. European heart journal. 2014;35:3452–62. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman JH, Brittain EL, Robbins IM, Hemnes AR. Effect of acute arteriolar vasodilation on capacitance and resistance in pulmonary arterial hypertension. Chest. 2015;147:1080–5. doi: 10.1378/chest.14-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols WW, O’Rourke MF, Hartley C, McDonald DA. McDonald’s blood flow in arteries : theoretic, experimental, and clinical principles. 4th. London New York: Arnold; Oxford University Press; 1998. [Google Scholar]
- 25.Ghio S, Crimi G, Temporelli PL, Traversi E, La Rovere MT, Cannito A, Vizza D, Scelsi L, Raineri C, Guazzi M, Oltrona Visconti L. Haemodynamic effects of an acute vasodilator challenge in heart failure patients with reduced ejection fraction and different forms of post-capillary pulmonary hypertension. European journal of heart failure. 2017 Nov 16; doi: 10.1002/ejhf.1067. [epub ahead or print]. [DOI] [PubMed] [Google Scholar]
- 26.Borlaug BA, Lewis GD, McNulty SE, Semigran MJ, LeWinter M, Chen H, Lin G, Deswal A, Margulies KB, Redfield MM. Effects of sildenafil on ventricular and vascular function in heart failure with preserved ejection fraction. Circulation Heart failure. 2015;8:533–41. doi: 10.1161/CIRCHEARTFAILURE.114.001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saouti N, Westerhof N, Helderman F, Marcus JT, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Right ventricular oscillatory power is a constant fraction of total power irrespective of pulmonary artery pressure. American journal of respiratory and critical care medicine. 2010;182:1315–20. doi: 10.1164/rccm.200910-1643OC. [DOI] [PubMed] [Google Scholar]
- 28.Dupont M, Mullens W, Skouri HN, Abrahams Z, Wu Y, Taylor DO, Starling RC, Tang WH. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circulation Heart failure. 2012;5:778–85. doi: 10.1161/CIRCHEARTFAILURE.112.968511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, Dini FL, Temporelli PL, Vassanelli C, Vanderpool R, Naeije R, Ghio S. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest. 2014;145:1064–70. doi: 10.1378/chest.13-1510. [DOI] [PubMed] [Google Scholar]
- 30.Borlaug BA, Melenovsky V, Koepp KE. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. Circulation research. 2016;119:880–6. doi: 10.1161/CIRCRESAHA.116.309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tedford RJ, Beaty CA, Mathai SC, Kolb TM, Damico R, Hassoun PM, Leary PJ, Kass DA, Shah AS. Prognostic value of the pre-transplant diastolic pulmonary artery pressure-to-pulmonary capillary wedge pressure gradient in cardiac transplant recipients with pulmonary hypertension. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33:289–97. doi: 10.1016/j.healun.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palazzini M, Dardi F, Manes A, Bacchi Reggiani ML, Gotti E, Rinaldi A, Albini A, Monti E, Galie N. Pulmonary hypertension due to left heart disease: analysis of survival according to the haemodynamic classification of the 2015 ESC/ERS guidelines and insights for future changes. European journal of heart failure. 2017;20:248–255. doi: 10.1002/ejhf.860. [DOI] [PubMed] [Google Scholar]
- 33.Mazimba S, Kennedy JL, Zhuo D, Bergin J, Abuannadi M, Tallaj J, Bilchick KC. Decreased Pulmonary Arterial Proportional Pulse Pressure After Pulmonary Artery Catheter Optimization for Advanced Heart Failure Is Associated With Adverse Clinical Outcomes. Journal of cardiac failure. 2016;22:954–961. doi: 10.1016/j.cardfail.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Guazzi M, Naeije R. Pulmonary Hypertension in Heart Failure: Pathophysiology, Pathobiology, and Emerging Clinical Perspectives. Journal of the American College of Cardiology. 2017;69:1718–1734. doi: 10.1016/j.jacc.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 35.Masri SC, Tedford RJ, Colvin MM, Leary PJ, Cogswell R. Pulmonary Arterial Compliance Improves Rapidly After Left Ventricular Assist Device Implantation. ASAIO journal. 2017;63:139–143. doi: 10.1097/MAT.0000000000000467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


