Abstract
Astrocytes, once believed to serve only as “glue” for the structural support of neurons, have been demonstrated to serve critical functions for the maintenance and protection of neurons, especially under conditions of acute or chronic injury. There are at least seven distinct mechanisms by which astrocytes protect neurons from damage; these are (1) protection against glutamate toxicity, (2) protection against redox stress, (3) mediation of mitochondrial repair mechanisms, (4) protection against glucose-induced metabolic stress, (5) protection against iron toxicity, (6) modulation of the immune response in the brain, and (7) maintenance of tissue homeostasis in the presence of DNA damage. Astrocytes support these critical functions through specialized responses to stress or toxic conditions. The detoxifying activities of astrocytes are essential for maintenance of the microenvironment surrounding neurons and in whole tissue homeostasis. Improved understanding of the mechanisms by which astrocytes protect the brain could lead to the development of novel targets for the development of neuroprotective strategies.
1. Introduction: Brain Injury and Cellular Responses
Mechanisms causing damage to the central nervous system (CNS) are numerous and complex, ranging from those associated with age-related neurodegeneration to the acute mechanisms of traumatic brain injury (TBI), ischemic stroke, and radiation exposure. In all cases, however, astrocytes play a central role in the compensatory responses that nature has designed to protect against the loss of terminally differentiated, nonreplicating neurons.
Like aging, acute injuries can result in a long-term progression of pathogenic changes that alter brain functions for years afterwards [1]. Specifically, following an initial TBI, secondary events can occur that extend both the area of as well as the intensity of the injury. Loss of vascular integrity resulting in a breakdown of the blood brain barrier (BBB) causes exposure of the CNS to exogenous immune cell types, abnormal levels of cytokines, and other cellular mediators and ionic disruption that can lead to a cascade of pathogenesis [2–7]. Loss of BBB integrity is also observed following ischemic stroke, radiation exposure, and in certain neurodegenerative disorders, due to the loss of neurovascular functions [8–11]. Secondary damage due to vascular and metabolic imbalances leads to increased glutamate release and subsequent excitotoxicity, mitochondrial dysfunction, and excessive production of reactive oxygen species (ROS), as well as disruption of glucose metabolism/release, and further alterations of ion concentrations [12–14]. Glutamate is thought to be a central mediator in this constellation of secondary injury events. An increase of extracellular glutamate activates N-methyl-D-aspartate receptors (NMDARs) in neurons, allowing calcium influx [15]. The resulting calcium excitotoxicity affects mitochondrial functions, causing a disruption of energy balance and production of excessive ROS, ultimately causing acute necrotic cell death and/or delayed apoptotic cell death [15–18]. Further damage can occur due to prolonged neuroinflammatory and related immune responses that exacerbate the injury [19, 20] and may underlie long-term pathogenesis.
Although the initiating events of CNS damage may differ, similar patterns of secondary injuries are observed [10, 21, 22]. This implies that understanding of the mechanisms underlying the CNS response to any injury may allow the development of treatments for other diseases or disorders.
Historically, treatments for acute or chronic damage to the nervous system have focused on neuronal responses and survival. This was due to the neurons' perceived importance in cognition and their postmitotic status which prevents their replacement when damaged [23, 24]. However, more attention is now being paid to the impact of nonneuronal cell types that function to mitigate damage and promote neuronal function and repair following tissue injury. In recent years, there has been a greater appreciation of the role of astrocytes in brain function and survival. The perceived value of astrocytes has risen from their initially defined role of “brain glue” to current findings that astrocytes are critical for modulating synaptic transmissions, managing energy metabolism, water, and ion homeostasis, and protection of neurons from oxidative stress under both mild and catastrophic conditions [25–29]. Here, we review the role of astrocytes in the protection of neurons from the consequences of initial and secondary injury processes (Figure 1).
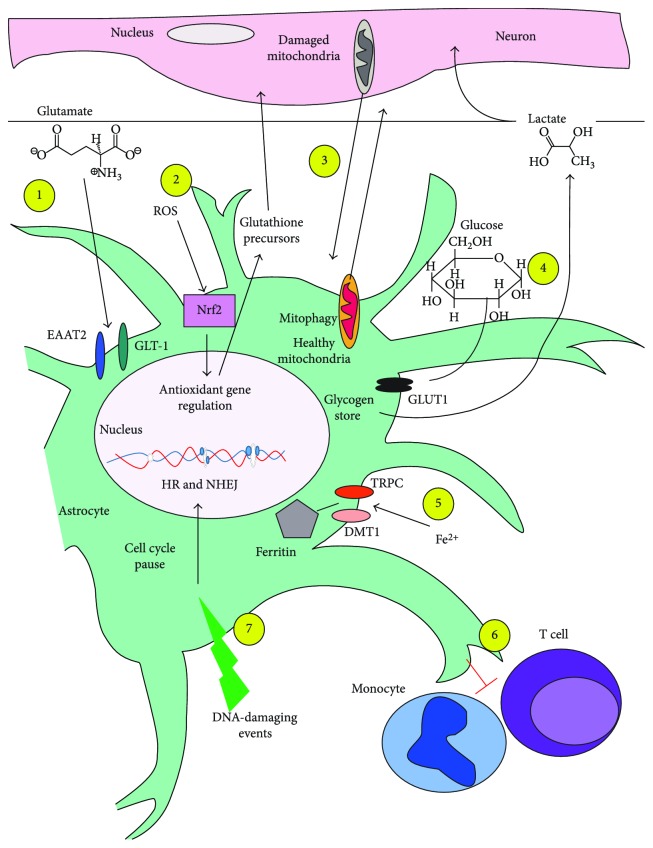
Figure 1.
Schematic of mechanisms of neuroprotective effects of astrocytes. There are at least seven distinct mechanisms by which astrocytes protect neurons from damage. (1) Protection against glutamate toxicity occurs through astrocyte uptake of extracellular glutamate through the excitatory amino acid transporter 2 (EAAT2) and the glutamate transporter 1 (GLT-1). (2) Protection against redox stress through the activation of Nrf2 and regulation of antioxidant genes; protection of the neurons is also advanced by the export of glutathione precursors to help neurons synthesize glutathione. (3) Mediation of mitochondrial repair mechanisms by which astrocytes received damaged mitochondria from neurons for mitophagy and in return deliver healthy mitochondria to the neurons. (4) Protection against glucose-induced metabolic stress, which involves astrocytes taking up extracellular glucose for storage as glycogen; the glycogen can be released to neurons as lactate for their metabolism at a later time. (5) Protection against iron toxicity, in which astrocytes sequester free iron for storage in complex with ferritin. (6) Modulation of the immune response in the brain occurs by astrocyte inhibition of both T cell and monocyte activation; the mechanisms for these actions are not fully known. (7) Maintenance of tissue homeostasis in the presence of DNA damage, where astrocytes can effectively repair their DNA through both homologous recombination and nonhomologous end joining, following pause of the cell cycle.
2. Astrocytes: Origin, Morphology, and Activation
Astrocytes are members of a larger family of nonneural, glial cells which include oligodendrocytes and Schwann cells, both of which form myelin and microglia, which are specialized macrophages that aid in immunity. Astrocytes and the other cells of the glial family are defined, in part, by their inability to produce an action potential upon stimulation [30]. Astrocytes are embryonically derived from progenitor cells of neuroepithelium which differentiate to function in their traditional roles as support cells. They provide nutrients and remove end products of metabolism [31]. Astrocytes exhibit spongiform morphology, with processes in close contact with neuronal synapses and other components of the CNS [32]. Recent advances in our understanding of astrocytes, discussed below, reveal the astrocyte to have essential roles in synaptic function and nervous system repair [33, 34].
Astrocytes, the most abundant nonneuronal cell type in the brain, consist of two main subclasses: protoplasmic and fibrous [35]. Protoplasmic astrocytes display a stellate appearance in the grey matter, and fibrous astrocytes primarily exist as long, thin, fibrocyte-like cells in the white matter of the CNS [36]. Each subtype has a distinctive profile of gene expression, as reflected in their expression of specific receptors and proteins [37, 38]. These two types of astrocytes display differences in their development and their expression of receptors and proteins [37, 38]. However, both subtypes express glial fibrillary acidic protein (GFAP), the main astrocytic intermediate filament, as well as calcium-binding S100B protein (S100B) [39, 40].
Activation of astrocytes can occur in response to a variety of injuries to the brain and in response to inflammation or pathological neurodegeneration [35]. The activated state, astrogliosis or reactive astrogliosis, is believed to have multiple functions in the brain and has been the topic of controversy for over 20 years [32, 35, 41]. While in some cases, astrocyte activation has been linked to repair and return to homeostasis, and in other cases, astrocyte activation has been related to the formation of scar tissue and the inhibition of neuronal axon outgrowth [35]. Induction of the reactive state of astrocytes can occur through multiple mechanisms including the presence of amyloid beta peptides (Aβ peptides), to neuronal damage or neurodegeneration, the release of proinflammatory cytokines by microglia and macrophages, or in response to acute injury to cells of the CNS [42–44]. The time course of astrocyte reactivity is heterogeneous and may depend on the location and type of injury [45]. In certain murine models of mild CNS injury, astrocyte reactivity is transient [46]. However, other studies indicate long-lasting increases in astrocyte reactivity occurring after either moderate or severe CNS injury from TBI or by radiation [47, 48]. Mild perturbations of the CNS can be adequately repaired, and homeostasis can be maintained with cooperation among glial cells. However, under more severe conditions, astrocytes remain in a state of reactivity indicating an inability to adequately repair. Similarly, astrocytes in postmortem Alzheimer's patients appear to maintain themselves in a continuous reactive state, consistent with chronic inflammation observed in this disease [49]. Thus, astrocyte reactivity persistence may indicate the presence of unresolved dysfunction in the CNS.
The primary alterations in the transformation of normal astrocytes to reactive astrocytes include hypertrophy of their main cellular processes, proliferation, and alterations in protein expression [32, 50, 51]. Fibrous and protoplasmic astrocytes display differences in the length of their processes following mechanical injury. In a murine model of axonal injury, fibrous astrocytes displayed condensed, retracted processes [46]. In contrast, protoplasmic astrocytes displayed increased length and branch complexity of their processes after injury [32, 52]. This may be a reflection of their functions within the brain, but more research is required to understand the significance of these changes. Of greater interest are their different sensitivities to damage. Research of brain ischemia and cortical lesions has shown that protoplasmic astrocytes may either die or differentiate into fibrous astrocytes after brain injury caused by ischemia and cortical lesions [52, 53]. This suggests that the differences between astrocyte types are fluid and dependent on environmental conditions. Significantly, protoplasmic astrocytes promote the differentiation of neural stem cell (NSC) into neurons via their secretion of brain-derived neurotrophic factor (BDNF) secretion [54]. Also, while both protoplasmic and fibrous astrocytes aid in motor neuron neurite outgrowth, protoplasmic astrocytes produced factors in the extracellular matrix that aided in axonal growth of V2a interneurons, while extracellular matrix produced by fibrous astrocytes had more factors that inhibited axon growth of V2a interneurons, suggesting that the actions of the protoplasmic and fibrous astrocytes are selective for specific neurons [55]. Thus, the differentiation or death of protoplasmic astrocytes may have a significant impact on replenishing neurons and regrowth of neuronal axons in the CNS following injury depending upon the site of injury.
Reactive astrocytes perform a variety of tasks in response to injury which can be beneficial or deleterious to the surrounding neurons, depending on the circumstances of the injury. Reactive astrocytes can form scars after CNS trauma. In some cases, scars can be viewed as initially beneficial since they limit immune cell invasion, decrease neuroinflammation, and maintain ion homeostasis in damaged brain tissue [56, 57]. Ablation of proliferating reactive astrocytes after moderate closed cortical impact (CCI) in mice produced increased inflammation and neuronal death, suggesting that the overall value of astrocyte reactivity is for the protection of neurons postinjury [58]. Evidence indicates interference in the development of the astroglial scar results in increased neuronal cell death and decreased modulation of inflammation [59]. However, there is controversy over its long-term impact of the scar tissue on repair and functional recovery [60, 61]. Prior evidence suggests that glial scar formation prevents or inhibits axonal regrowth of neurons [62]. This has been attributed to astrocyte expression of chondroitin sulfate proteoglycan, a known inhibitor of neuronal axons during embryogenesis [63]. However, in murine models where astrocyte scar formation is impaired, there was demonstrated to be less neuronal axon regrowth and remodeling [64, 65]. Using transgenic murine models, one research group demonstrated that the formation of an astrocytic scar actually improved neuron axonal regrowth, provided that brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT3) were added [64]. Together, these studies suggest that, in contrast to initial hypotheses, the presence of astrocytic scars alone does not prevent axonal regrowth, but rather that the lack of adequate growth factors may be the problem.
The beneficial nature of gliosis may become detrimental when damage is too severe for homeostasis to be reestablished. For the purposes of this review, we will focus mostly on the mechanisms by which astrocytes protect neurons under basal conditions and after injury. This will involve focusing on the astrocyte's ability to collect and transport vital nutrients, neurotransmitters, and ions in the brain, to release antioxidants during redox stress, to repair mitochondria and DNA after injury.
3. Astrocyte Defense against Glutamate Toxicity
Glutamate is the most abundant excitatory neurotransmitter in the brain, with actions mediated through a diverse family of receptors to modulate synaptic transmission and aid in plasticity [66, 67]. In normal synaptic communication, neurons release measured quanta of glutamate into the synaptic cleft. However, following physical trauma, radiation exposure, and chronic neurodegenerative disorders, including Alzheimer's disease, excessive glutamate is released or fails to be taken up for days after injury [68–71]. The cause of glutamate dysregulation in TBI and neurodegeneration is not completely understood, but elevations in free glutamate are linked to poor clinical outcome [71]. Recent evidence indicates that glutamate is released by dying or damaged neurons, possibly via the cystine glutamate antiporter [72, 73]. Excessive extracellular glutamate leads to excitatory neuronal cell death attributed to overstimulation of NMDAR and subsequent overproduction of ROS in neurons [74, 75].
Under conditions of normal neuronal activity, astrocytes are responsible for the uptake of excess glutamate from the synaptic cleft. Following uptake, astrocytes process the glutamate into glutamine and return it to neurons for reuse [76]. Consistent with this role, astrocytes highly express the excitatory amino acid transporter 2 (EAAT2) and the glutamate transporter 1 (GLT-1) which are responsible for the active uptake of glutamate [77]. Glutamate homeostasis is a critical function of astrocytes in the brain, as demonstrated experimentally by the neurotoxicity that results from inhibition of the astrocyte glutamate transporters [78, 79].
Following tissue injury, astrocytes can actively take up excessive glutamate from the extracellular (nonsynaptic) space and buffer its potential excitotoxic actions on neurons. The reduction of extracellular glutamate by astrocytes decreases the subsequent lesion size, mitigates neuronal death, and improves CNS function postinjury [80].
Under conditions of severe injury, the extent of damage and types of injury to the astrocytes themselves can impact the ability of astrocytes to protect neurons from glutamate toxicity [81]. For example, astrocytes injured by radiation or more severe forms of TBI display reduced glutamate uptake activity as compared to the uninjured condition, allowing increased neuronal uptake of glutamate and a greater extent of neuronal cell death and seizure activity [70, 81, 82]. The mechanism for radiation inhibition of astrocyte uptake of glutamate is thought to be related to ROS inhibition of the astrocytic glutamate transporter via oxidation of protein sulfhydryl groups critical for function [83, 84]. At least three potential mechanisms have been proposed for increased extracellular glutamate levels and subsequent excitotoxicity in TBI [85, 86]. These mechanisms may occur in tandem and are not exclusive. In the first potential mechanism, tumor necrosis factor-α (TNF-α), the proinflammatory factor released during brain damage, downregulates glutamate uptake by astrocytes and suppresses conversion of glutamate to glutamine [87]. In the second possible mechanism, TBI- and ischemia-induced efflux of glutamate from injured astrocytes may occur in response to thrombin, which is released after BBB disruption [88]. In a third potential mechanism, ischemia and glucose deprivation may induce altered glutamate release by astrocytes [89]. Under normal circumstances, glutamate uptake occurs against its gradient and must be actively transported into the astrocyte via EAATs. However, under acidic conditions, as occurs with hypoxia, this transporter is reversed and expels glutamate [89]. Thus, more severe neuronal injuries and/or chronic disruptions lead to cell death when the astrocytes themselves exacerbate glutamate imbalance as they fail to maintain homeostasis.
4. Redox Stress Reduction by Astrocytes
Basal levels of ROS in the brain can result from normal cellular functions and metabolic activity. While the production of ROS is a natural consequence of mitochondrial respiration, overproduction of ROS following injury exceeds the capacity of natural cellular antioxidant mechanisms, resulting in the pathological modification of proteins, lipids, and nucleic acids [90–93]. To combat these processes, the brain utilizes multiple pathways for antioxidant defense including superoxide dismutase (SOD), catalase and glutathione detoxification pathways, and thioredoxin detoxification pathways [94]. These mechanisms are utilized to different degrees by different cell types.
A hallmark of glutamate excitotoxicity is increased intracellular redox stress. Excessive glutamate activation of NMDAR causes Ca2+ influx into the cytosol of neurons [95]. The excessive intracellular Ca2+ can translocate into the mitochondrial matrix where it leads to the collapse of mitochondrial membrane potential with loss of ATP production and, ultimately, cell death [22, 74]. To prevent this, many cell types upregulate uncoupling proteins (UCPs), which aid in removal of intracellular Ca2+ and prevention of Ca2+ entry into the mitochondria [96, 97]. UCPs decrease the levels of hydrogen protons in the mitochondrial intermembrane space and therefore the mitochondrial electrochemical proton gradient, by leaking them into the mitochondrial matrix [98, 99]. Since the electrochemical proton gradient is necessary for ATP synthase function, a decrease in hydrogen protons decreases ATP production [100]. The increase of hydrogen protons in the mitochondrial matrix also causes diminished entry of positively charged molecular calcium [101]. In the short term, the activity of UCP may benefit the neurons for immediate survival, but in the long term, it is detrimental, since this process inhibits ATP production [102, 103]. Catastrophic calcium entry due to acute or chronic brain injury can overcome the UCP system, leading to the production of ROS which causes further mitochondrial dysfunction and cell death [22, 104–106]. This mitochondrial membrane depolarization and increase in ROS induced by high Ca2+ levels can cause apoptosis by facilitating the release of cytochrome C through the mitochondrial transition pore and activation of caspase 3 [107, 108].
Astrocytes normally display a higher basal level of glutathione (0.91 ± 0.08 mM) as compared to neurons (0.21 ± 0.02 mM), suggesting that under normal conditions, they are capable of detoxification of higher amounts of reactive oxygen and nitrogen species [109, 110]. Astrocytes also have a greater inducible expression of glutathione in response to oxidative stress [111, 112]. The ROS-inducible transcription factor nuclear factor E2-related factor 2 (Nrf2) regulates the glutathione system, as well as the thioredoxin system and SOD [113–115]. Under basal conditions, Nrf2 is constitutively produced and ubiquitinated for degradation by binding to the Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm [116]. Under conditions of increased oxidative stress, Keap1 binding to Nrf2 is inhibited [117], allowing Nrf2 to escape degradation and instead to translocate to the nucleus where it interacts with the antioxidant response element (ARE) in gene promoters that activate the expression of oxidative stress response genes. Previous research indicated that astrocytes display higher basal and stimulated levels of ARE binding by NRF2 as compared to neurons [118].
Interestingly, Nrf2-induced expression and downstream upregulation of antioxidant defenses in astrocytes confer enhanced resistance to oxidative stress for both astrocytes and neurons [119, 120]. As stated above, the enhanced Nrf2 within astrocytes effectively upregulates antioxidant genes for the protection of the astrocytes [121]. However, Nrf2 expression in astrocytes was also demonstrated to increase neuronal survival in a murine model of amyotrophic lateral sclerosis (ALS) and in vitro in acute hydrogen peroxide exposure [122, 123]. The mechanism by which Nrf2 upregulation in astrocytes allows protection of neurons is complex, and further research is required for a full understanding. However, two mechanisms have been proposed for astrocyte protection of neurons in response to ROS. In the first mechanism, Nrf2 induces glutathione secretion from astrocytes into the extracellular matrix where it is cleaved to one of its precursors (CysGly, γGluCys, or cysteine) which are then taken up and used by neurons for glutathione resynthesis for their own detoxification [21, 124, 125]. In the second mechanism, the increased levels of Nrf2 induce the upregulation of the EAAT3 in astrocytes. As described above, this neurotransmitter transporter is critical for the removal of extracellular glutamate which after injury can induce neuronal excitotoxicity. Thus, the removal of extracellular glutamate protects neurons via a second independent mechanism [126]. This redox buffering capacity of astrocytes was demonstrated to be necessary for neuronal homeostasis under normal basal conditions [127].
5. Astrocyte Defense against Mitochondrial Dysfunction in Neurons
As describe in Section 4, brain injury can lead to Ca2+-induced mitochondrial dysfunction, including overproduction of ROS, loss of mitochondrial membrane potential and pH gradient, and failure to generate required amounts of ATP [128]. Recently, the transfer of mitochondria from one cell type to another has been described as a mechanism for the replacement and repair of damaged mitochondria. The benefits of mitochondrial transfer were initially shown in cell culture studies in which human mesenchymal stem cells (hMSC) repaired the aerobic respiration of A549-transformed lung epithelial cells that contained mutated mitochondria [129]. Mutant A549 cells which received mitochondria from hMSCs displayed improved ATP production, increased lactate uptake, and higher levels of oxygen consumption, a marker of electron transport chain activity [129]. This study provided compelling evidence for mitochondrial transfer and demonstrated the benefits of this activity as an effective means for protecting vulnerable cell types. The mechanisms by which mitochondria and other organelles are trafficked between different cell types are still not well understood. One proposed mechanism for organelle transfer involves the creation of tunneling nanotubes (TNTs) [130, 131]. TNTs are created by a cell after it is subjected to stress and has been demonstrated to occur during neuronal development [130, 132]. Of special interest, neurons are capable of guiding the formation of astrocyte TNTs during periods of high synaptic activity and thus, high energy demand [132]. Transference of healthy mitochondria from astrocytes to neurons in a murine model of stroke was observed in vivo [133]. Further, it was noted in this model that astrocytes only transferred healthy mitochondria to damaged neurons in a calcium-dependent manner, suggesting neuronal activity was necessary for transference [133]. Conversely, in a separate model, it was demonstrated that retinal ganglion cells are capable of shedding damaged mitochondria and that the shed mitochondria were shown to be taken up by adjacent astrocytes where they underwent mitophagy [134]. Thus, evidence suggests that mitochondrial transfer provides means to deliver healthy mitochondria to injured neurons and for the elimination of damaged mitochondria involved in the overproduction of ROS.
6. Astrocyte Protection against Glucose-Induced Metabolic Stress
The brain is highly metabolically active, utilizing fully 25% of the body's glucose [28]. Accordingly, efficient glucose uptake and distribution throughout the brain is critical for cognition and survival. Disruptions in the delivery of glucose to the brain induce neuronal cell death. Under normal conditions, the BBB acts as a selective barrier to control entry of glucose into the brain; however, this barrier is often disrupted in brain injury [135]. Endothelial cells of the BBB and astrocytes express glucose transporter 1 (GLUT1), a facilitated glucose transporter, to aid in glucose entry into the brain [136]. Astrocytic endfeet encircle endothelial cells of the BBB and mediate the uptake of glucose [137–139]. Once past the BBB, glucose is taken up by all cell types of the CNS. In astrocytes, glucose is converted into glycogen and stored [140]. In times of need, astrocytes mobilize their glycogen to make lactate available for neuronal use. This is especially important when energy demand is high but neuronal glucose supply is low, such as under hypoglycemic conditions [141–143]. While neurons express glucose transporter 3 (GLUT3), a high affinity glucose transporter, they have been shown to prefer lactate as an energy substrate during times of high synaptic activity [144–146]. Glutamate induces the rapid uptake of glucose in to astrocytes. Because extracellular glutamate is released during neurotransmission, this indicates that glutamate-stimulated glycogen production in astrocytes is linked to neuronal activity [147, 148].
Insulin and insulin-like growth factor 1 (IGF-1) increase glycogen storage in astrocytes but fail to impact glucose transport across the astrocyte cell membrane [149]. However, selective ablation of insulin receptors in mouse astrocytes in vivo results in significantly lower cerebral glucose levels [150]. This indicates a central role for astrocytes in monitoring neuronal metabolic activity and maintaining whole brain energy balance in a manner that is responsive to insulin release in the blood, but in a manner that is different from the regulation that occurs in other tissues.
Acute brain damage, including radiation, TBI, and ischemic stroke, can produce sudden damage to the BBB which can lead to a disruption in the supply of glucose as well as imbalances in extracellular ions. Of particular importance in BBB permeability is increased extracellular potassium that must be removed from the extracellular space [151, 152]. The increase in extracellular potassium may be due to multiple factors including direct cellular injury and secondary mechanisms that compromise potassium buffering by astrocytes [153–155]. While glial cells are capable of buffering normal increases in extracellular potassium, they become overwhelmed under conditions of more severe injuries and the potassium overload can cause death of neurons [155, 156]. Both initial disruption of the BBB and the need to maintain ion homeostasis produce a rapid depletion of glucose and metabolic emergency [151, 152, 157]. Hypo- and hyperglycemic conditions both induce greater cell death in neurons than astrocytes [158–160]. Astrocyte survival in hypoglycemic conditions may rely on several factors including glycogen storage within the astrocytes, alternative energy metabolism of fatty acids, and utilization of antioxidant systems to manage increased oxidative stress [161–163]. In vitro research also demonstrates that astrocytes can improve neuronal survival under situations of glucose disruption by upregulating their respective monocarboxylate transporters (MTCs) which transfers lactate from astrocytes to neurons [164, 165].
While astrocytes may increase their release of lactate after TBI, there is some controversy regarding the possible benefit of this release, as neurons appear less capable of taking up the lactate depending on their level of damage [166, 167]. Increased release of lactate by astrocytes may contribute to lactic acidosis which can exacerbate ischemia-induced oxidative stress [168]. High lactate levels in the cerebrospinal fluid (CSF) of TBI patients have been linked worse clinical outcomes, which is blamed on neuronal mitochondrial dysfunctions, neuronal inability to uptake lactate, and subsequent necrosis in the brain [169]. Increased lactate was also seen in patients after they had seizures caused by severe TBI, with astrocytes potentially releasing lactate as an energy source for these overactive neurons [170, 171]. Under normal homeostasis and conditions of mild-to-moderate injury, astrocytes act to maintain neuronal survival by providing energy resources and maintaining the energy balance of the extracellular environment of the brain, but these actions can produce further damage if the CNS is already severely compromised.
7. Astrocyte Mitigation of Iron Toxicity
Astrocytes are responsible for the transfer through the BBB of a variety of nutrients required for brain tissue homeostasis, including iron [172]. Iron performs multiple functions within the brain, serving as an essential cofactor in several enzymatic reactions including those involved in the remyelination of neurons after injury [173, 174]. Iron levels are tightly regulated in the brain via specific transport proteins and metabolic pathways, but dysregulation can occur under pathological conditions [175]. Iron deficiency in the brain, due to causes such as dietary insufficiency or anemia, can produce cognitive impairments [176, 177]. However, an excess of iron, due to TBI, hemorrhagic stroke, or neurodegenerative diseases, causes neurotoxicity [175, 177, 178].
When present at high levels, ferrous iron (Fe2+) interacts with hydrogen peroxide to generate toxic levels of hydroxyl radicals through the Fenton reaction [179]. Neuronal susceptibility to iron-mediated necrotic, apoptotic, and autophagic cell death is likely due to their inability to effectively combat redox stress [180]. This is in marked contradiction to astrocytes which are highly effective at detoxification of ROS [181, 182, 183]. Excess iron induces lipid peroxidation, protein and DNA oxidation, and cell death in neurons [175, 184]. Disruptions in free iron handling within the CNS have been observed after acute injuries such as TBI as well as in chronic neurodegenerative disorders [185, 186]. Iron and other transition metals within the brain bind to Aβ a peptide that accumulates in Alzheimer's disease, causing greater neuronal death and toxicity than Aβ alone [187, 188]. Similarly, in a murine model, it was demonstrated that TBI results in an increase in iron deposition in the brain starting as early as four hours postinjury and extending for at least three weeks after initial damage [189]. These findings support the proposal that acute deregulation of iron homeostasis may participate in long-lasting pathogenic effects that underly neuronal damage and death [185, 190] with associated cognitive impairment.
Astrocytes utilize several distinctly different mechanisms to directly regulate free iron in the CNS. As discussed above, astrocytes utilize parallel mechanisms including increased expression of Nrf2, glutathione, and catalase to combat redox stress that is likely one of the consequences of excessive free iron [183, 191]. Astrocytes may also protect neurons from iron-induced cell damage under normal and pathological conditions by sequestering free iron through transient receptor potential canonical (TRPC) channels and divalent metal transporter (DMT1), respectively [192, 193]. TRPC channels are best known for their proposed role in calcium influx after activation, though they transport multiple cation types across the cell membrane [194, 195]. In a cell culture model, it was demonstrated that overexpression of TPRC6 can increase basal levels of intracellular iron and iron presence after stimulation, suggesting that iron transfer through TRPC channels may occur under basal conditions [196]. In contrast, DMT1 expression is controlled by proinflammatory cytokines. The proinflammatory cytokine tumor necrosis factor alpha (TNF-α), lipopolysaccharide, and interleukin-6 (IL-6) increase DMT1 expression in astrocytes while simultaneously decreasing ferroportin 1 (FPN-1) expression [197, 198]. FPN-1 is an iron efflux transporter so the result of this activity then is to increase total iron uptake and storage in astrocytes after injury. Excess iron in the microenvironment of astrocytes upregulates the expression of ferritin, a rapidly inducible protein which binds and neutralizes ferrous iron, thus preventing its effects on oxidative stress [199]. Ferritin functions by first converting ferrous iron to its less reactive state of ferric iron then nucleating this ferric iron (Fe3+) and storing it within ferritin's iron core [200]. Together, the upregulation of iron transporters plus the upregulation of ferritin allows astrocytes to act as iron stores, resulting in reduced free ferrous iron in the microenvironment where it may contribute to neuronal toxicity.
8. Modulation and Regulation of Immune Responses in the CNS
Immunological activity in the CNS is relevant for the prevention of pathogenic infection as well as responses to injury such as stroke and TBI when the BBB is compromised [201]. Astrocytes play a complex role in responding to such CNS insults, and their inflammatory status as well as regulation of immune cells is controversial. Astrogliosis is the defensive reaction of astrocytes to trauma, ischemic damage, inflammation, or pathological neurodegeneration [35]. During astrogliosis, astrocytes increase at the site of the lesion, exhibit altered morphology with increased thicknesses of cellular processes, and display changes in gene expression related to altered function [32, 35]. The increase in astrocytes at the site of injury is believed to be due to proliferation of astrocytes adjacent to the lesion and not due to astrocyte migration from neighboring areas of the brain [35]. Astrocytes can be activated to a proinflammatory or anti-inflammatory phenotype with an associated alteration in their secretome [202–205]. The overall “defensive response” of astrocytes following injury is highly complex and has been shown in some studies to exacerbate inflammation while generally, it is found to mitigate it [35].
The proinflammatory activation of astrocytes and their expression of proinflammatory cytokines are dependent upon the nature of the stimulation they receive and their location in the brain [206]. The activation of proinflammatory astrocytes can occur through multiple mechanisms including interactions with microglia, in response to cytokines such as IL-1, IL-6 oncostatin M, leukemia inhibitory factor (LIF), and transforming growth factor-α (TGF-α), in response to overt physical damage following brain injury, from interaction with Aβ plaques, or as a result of activation of the calcium-dependent phosphatase calcineurin [35, 41, 207]. Under normal circumstances, astrocytes aid in the morphological and physiological development of neurons and synaptogenesis [208]. However, cell culture studies suggest that inappropriate activation or overactivation of astrocytes can induce the production of TNF-α and other cytotoxic factors that inhibit neurite growth and synapse formation [209]. Additionally, exposure of astrocytes in cell culture to cytokines, such as interferon-γ (IFN-γ), can induce their production of nitric oxide which drives the formation of its toxic metabolite, peroxynitrite [210]. In cell culture, this does not harm astrocytes but can lead to mitochondrial dysfunction and eventual cell death in cocultured neurons [211].
Astrocytes can also modulate the immune system to reduce inflammation. Normal human astrocytes were shown to suppress both monocyte and T cell activation in cell cultures [205]. It was found that astrocytes reduced monocyte activation, not by secreting IL-10, but by blocking CD80 induction on the monocytes through an undefined mechanism [205]. Astrocytes can also function in a manner to recruit and direct white blood cells, both leukocytes and monocytes, to an area of injury, while at the same time protecting healthy tissue from inflammatory consequences of white blood cell invasion [56, 212, 213]. Importantly, ablation of activated astrocytes in a murine model of spinal cord injury resulted in greater inflammation, increased neuronal degeneration, and negatively impacted subsequent motor function, suggesting that the activated astrocytes control the extent and location of inflammation following injury [214]. The mechanisms of suppression of inflammation by astrocytes require further investigation.
9. Tissue Homeostasis under Conditions of DNA Damage
DNA repair and synthesis are necessary for normal tissue homeostasis. DNA repair in neurons has been demonstrated to occur in a nonuniform and, in some cases, inefficient manner. Due to a decreased antioxidant response, neurons display increased chromosomal and mitochondrial DNA lesions that can result in cell death [215]. As compared to astrocytes, neurons are slower in rejoining DNA double strand breaks following radiation exposure, and they display greater cell death after episodes of DNA damage [216]. Interestingly, DNA damage in neurons can induce the production of cell cycle enzymes, cyclin B, cyclin E, and proliferating cell nuclear antigen (PCNA) [217, 218]. But this cell cycle progression precedes apoptotic cell death rather than survival and proliferation in neurons [219, 220]. Administration of cell cycle inhibitors after TBI was shown to decrease neuronal cell death [221]. The reason for this response could be related to the hypothesis that slower cycling cells repair DNA more efficiently [222]. In contrast, a murine model of stroke indicated that a pause of several days occurred in the cell cycle of astrocytes before they continue to proliferate after exposure to injury [223]. Such differences in cell cycle control may mean the difference between life and death at the cellular level. An ability to repair effectively before allowing for cell proliferation may explain astrocyte survival postinjury [222].
Neurons do display a limited ability to repair DNA in both wild type and 7,8-dihydro-8-oxoguanine glycosylase (OGG1) deficient mice in response to ischemia [224]. OGG1, a DNA glycosylase involved in base excision repair, protects neuronal mitochondrial DNA from oxidative damage under ischemic conditions [224]. However, the effectiveness of this repair has been called into question and may depend on the location of DNA damage within the chromosome of the mitochondria. Studies of DNA damage from radiation in NTERA-2-derived neurons showed that DNA was efficiently repaired for transcribed genes but inefficiently repaired in nontranscribed areas, suggesting that chromosomal organization plays an important role in the effectiveness of DNA repair mechanisms in neurons [225].
In contrast to neurons, astrocytes display robust DNA repair capacities for both nuclear and mitochondrial DNA [226]. In cell culture assays, astrocytes exposed to menadione, an agent which induces oxidative stress, displayed a lower mitochondrial DNA strand break frequency and more efficient DNA repair as compared to all other cell types of the brain [227]. The mechanisms induced to repair DNA in astrocytes are multifaceted and include upregulation of both primary double strand break pathways: nonhomologous end joining and homologous recombination [228, 229]. Accordingly, astrocytes are better able to protect themselves after DNA damage as compared to neurons. To do this, they utilize a hierarchy of mechanisms. Protection of astrocyte DNA enables prevention of mutations and subsequent loss of function or induction of cell transformation and carcinogenesis. This resilience allows astrocytes to respond to and aid in the protection of neurons and other cell types after brain damage.
10. Conclusions
Astrocytes are highly involved in the maintenance and protection of the CNS microenvironment under normal and pathophysiological conditions. Brain damage can begin with mechanical damage to cells, as in TBI, or through oxidative stressors, as in radiation or in neurodegenerative diseases. While the cause of the damage differs, the consequences are similar with an unbalance of extracellular nutrients and ions, damage to the BBB, and excessive release of excitatory neurotransmitters. The resulting conditions can damage mitochondria leading to the production of dangerous levels of ROS that will in turn exacerbate DNA damage and increase inflammation, ultimately leading to cell death. Astrocyte maintenance of the ionic and metabolic environment protects neurons through multiple mechanisms. Astrocytes take up and sequester excess neurotransmitters, ions, and metabolic products to restore the homeostatic balance. Astrocytes also take up and process damaged mitochondria from neurons and transfer healthy mitochondria back to injured neurons. Astrocytes are capable of producing a robust antioxidant response to protect themselves and also neurons, through the release of glutathione precursors to neurons. Their role in scar formation allows astrocytes to regulate and contain the immune responses in a manner that controls neuroinflammation. Further understanding of the endogenous protective mechanisms provided by astrocytes may provide new insights that could lead to the development of novel treatment options for the protection of susceptible cells, such as neurons, under conditions of acute injury or pathology.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper. The authors would also like to thank Drs. Kimberly Byrnes and David Mears for critical reading of this manuscript.
References
- 1.Furuse M., Nonoguchi N., Kawabata S., Miyatake S. I., Kuroiwa T. Delayed brain radiation necrosis: pathological review and new molecular targets for treatment. Medical Molecular Morphology. 2015;48(4):183–190. doi: 10.1007/s00795-015-0123-2. [DOI] [PubMed] [Google Scholar]
- 2.Shapira Y., Setton D., Artru A. A., Shohami E. Blood-brain barrier permeability, cerebral edema, and neurologic function after closed head injury in rats. Neurosurgical Anesthesia. 1993;77:141–148. doi: 10.1213/00000539-199307000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Shi L., Adams M. M., Long A., et al. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiation Research. 2006;166(6):892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 4.Katayama Y., Becker D. P., Tamura T., Hovda D. A. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. Journal of Neurosurgery. 1990;73(6):889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 5.Tariot P., Farlow M. R., Grossberg G. T., et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil. JAMA. 2004;291(3):317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 6.Blyth B. J., Farhavar A., Gee C., et al. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. Journal of Neurotrauma. 2009;26(9):1497–1507. doi: 10.1089/neu.2008.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lotocki G., de Rivero Vaccari J. P., Perez E. R., et al. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. Journal of Neurotrauma. 2009;26(7):1123–1134. doi: 10.1089/neu.2008.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahles T., Luedike P., Endres M., et al. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38(11):3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 9.Marques F., Sousa J., Sousa N., Palha J. Blood–brain-barriers in aging and in Alzheimer’s disease. Molecular Neurodegeneration. 2013;8(1):p. 38. doi: 10.1186/1750-1326-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., Chen P., Haimovitz-Friedman A., Reilly R. M., Wong C. S. Endothelial apoptosis initiates acute blood–brain barrier disruption after ionizing radiation. Cancer Research. 2003;63(18):5950–5956. [PubMed] [Google Scholar]
- 11.Garwood C. J., Ratcliffe L. E., Simpson J. E., Heath P. R., Ince P. G., Wharton S. B. Review: astrocytes in Alzheimer’s disease and other age-associated dementias: a supporting player with a central role. Neuropathology and Applied Neurobiology. 2017;43(4):281–298. doi: 10.1111/nan.12338. [DOI] [PubMed] [Google Scholar]
- 12.Pandya J. D., Pauly J. R., Nukala V. N., et al. Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. Journal of Neurotrauma. 2007;24(5):798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- 13.Radak D., Katsiki N., Resanovic I., et al. Apoptosis and acute brain ischemia in ischemic stroke. Current Vascular Pharmacology. 2017;15(2):115–122. doi: 10.2174/1570161115666161104095522. [DOI] [PubMed] [Google Scholar]
- 14.Vijayan M., Reddy P. H. Stroke, vascular dementia, and Alzheimer’s disease: molecular links. Journal of Alzheimers Disease. 2016;54(2):427–443. doi: 10.3233/JAD-160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicole O., Ali C., Docagne F., et al. Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. The Journal of Neuroscience. 2001;21(9):3024–3033. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stout A. K., Raphael H. M., Kanterewicz B. I., Klann E., Reynolds I. J. Glutamate-induced neuron death requires mitochondrial calcium uptake. Nature Neuroscience. 1998;1(5):366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- 17.Pivovarova N., Nguyen H. V., Winters C. A., Brantner C. A., Smith C. L., Andrews S. B. Excitotoxic calcium overload in a subpopulation of mitochondria triggers delayed death in hippocampal neurons. Journal of Neuroscience. 2004;24(24):5611–5622. doi: 10.1523/JNEUROSCI.0531-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ankarcrona M., Dypbukt J. M., Bonfoco E., et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15(4):961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 19.Caso J. R., Pradillo J. M., Hurtado O., Lorenzo P., Moro M. A., Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115(12):1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 20.Kyrkanides S., Moore A. H., Olschowka J. A., et al. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Molecular Brain Research. 2002;104(2):159–169. doi: 10.1016/S0169-328X(02)00353-4. [DOI] [PubMed] [Google Scholar]
- 21.Abramov A., Canevari L., Duchen M. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. Journal of Neuroscience. 2003;23(12):5088–5095. doi: 10.1523/JNEUROSCI.23-12-05088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong Y., Gu Q., Peterson P. L., Muizelaar J. P., Lee C. P. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. The Journal of Neurotrauma. 1997;14(1):23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- 23.Greenwood P. M., Parasuraman R. Neuronal and cognitive plasticity: a neurocognitive framework for ameliorating cognitive aging. Frontiers in Aging Neuroscience. 2010;2(150) doi: 10.3389/fnagi.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrup K., Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nature Reviews Neuroscience. 2007;8(5):368–378. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- 25.Park M. H., Lee S. M., Lee J. W., et al. ERK-mediated production of neurotrophic factors by astrocytes promotes neuronal stem cell differentiation by erythropoietin. Biochemical and Biophysical Research Communications. 2006;339(4):1021–1028. doi: 10.1016/j.bbrc.2005.10.218. [DOI] [PubMed] [Google Scholar]
- 26.Pfrieger F., Barres B. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277(5332):1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 27.Katz D., Kimelberg H. Kinetics and autoradiography of high affinity uptake of serotonin by primary astrocyte cultures. The Journal of Neuroscience. 1985;5(7):1901–1908. doi: 10.1523/JNEUROSCI.05-07-01901.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bélanger M., Allaman I., Magistretti P. J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabolism. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Sofroniew M. V., Vinters H. V. Astrocytes: biology and pathology. Acta Neuropathologica. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. Journal of Neurophysiology. 1966;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- 31.Weber B., Barros L. F. The astrocyte: powerhouse and recycling center. Cold Spring Harbor Perspectives in Biology. 2015;7(12) doi: 10.1101/cshperspect.a020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilhelmsson U., Bushong E. A., Price D. L., et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín E. D., Fernández M., Perea G., et al. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2006;55(1):36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- 34.Okada S., Nakamura M., Katoh H., et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nature Medicine. 2006;12(7):829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 35.Pekny M., Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiological Reviews. 2014;94(4):1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 36.Shehab S. A. S., Cronly-Dillon J. R., Nona S. N., Stafford C. A. Preferential histochemical staining of protoplasmic and fibrous astrocytes in rat CNS with GFAP antibodies using different fixatives. Brain Research. 1990;518(1-2):347–352. doi: 10.1016/0006-8993(90)90996-O. [DOI] [PubMed] [Google Scholar]
- 37.Miller R., Raff M. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. The Journal of Neuroscience. 1984;4(2):585–592. doi: 10.1523/JNEUROSCI.04-02-00585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marin-Padilla M. Prenatal development of fibrous (white matter), protoplasmic (gray matter), and layer I astrocytes in the human cerebral cortex: a golgi study. The Journal of Comparative Neurology. 1995;357(4):554–572. doi: 10.1002/cne.903570407. [DOI] [PubMed] [Google Scholar]
- 39.Saur L., Baptista P. P. A., de Senna P. N., et al. Physical exercise increases GFAP expression and induces morphological changes in hippocampal astrocytes. Brain Structure and Function. 2014;219(1):293–302. doi: 10.1007/s00429-012-0500-8. [DOI] [PubMed] [Google Scholar]
- 40.Brozzi F., Arcuri C., Giambanco I., Donato R. S100B protein regulates astrocyte shape and migration via interaction with Src kinase. Journal of Biological Chemistry. 2009;284(13):8797–8811. doi: 10.1074/jbc.M805897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamanian J. L., Xu L., Foo L. C., et al. Genomic analysis of reactive astrogliosis. Journal of Neuroscience. 2012;32(18):6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J., Akama K. T., Krafft G. A., Chromy B. A., van Eldik L. J. Amyloid-β peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Research. 1998;785(2):195–206. doi: 10.1016/S0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 43.Yong V. W., Moumdjian R., Yong F. P., et al. γ-interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(16):7016–7020. doi: 10.1073/pnas.88.16.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ovsepian S. V., O'Leary V. B. Neuronal activity and amyloid plaque pathology: an update. Journal of Alzheimer's Disease. 2016;49(1):13–19. doi: 10.3233/JAD-150544. [DOI] [PubMed] [Google Scholar]
- 45.Sun D., Jakobs T. C. Structural remodeling of astrocytes in the injured CNS. The Neuroscientist. 2012;18(6):567–588. doi: 10.1177/1073858411423441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun D., Lye-Barthel M., Masland R. H., Jakobs T. C. Structural remodeling of fibrous astrocytes after axonal injury. Journal of Neuroscience. 2010;30(42):14008–14019. doi: 10.1523/JNEUROSCI.3605-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villapol S., Byrnes K. R., Symes A. J. Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte–vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Frontiers in Neurology. 2014;5(82) doi: 10.3389/fneur.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiang C. S., McBride W. H., Withers H. R. Radiation-induced astrocytic and microglial responses in mouse brain. Radiotherapy and Oncology. 1993;29(1):60–68. doi: 10.1016/0167-8140(93)90174-7. [DOI] [PubMed] [Google Scholar]
- 49.Serrano-Pozo A., Muzikansky A., Gómez-Isla T., et al. Differential relationships of reactive astrocytes and microglia to fibrillar amyloid deposits in Alzheimer disease. Journal of Neuropathology & Experimental Neurology. 2013;72(6):462–471. doi: 10.1097/NEN.0b013e3182933788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bardehle S., Krüger M., Buggenthin F., et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nature Neuroscience. 2013;16(5):580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 51.Choi S. S., Lee H. J., Lim I., Satoh J. I., Kim S. U. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One. 2014;9(4, article e92325) doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kajihara H., Tsutsumi E., Kinoshita A., Nakano J., Takagi K., Takeo S. Activated astrocytes with glycogen accumulation in ischemic penumbra during the early stage of brain infarction: immunohistochemical and electron microscopic studies. Brain Research. 2001;909(1-2):92–101. doi: 10.1016/S0006-8993(01)02640-3. [DOI] [PubMed] [Google Scholar]
- 53.Lukaszevicz A.-C., Sampaïo N., Guégan C., et al. High sensitivity of protoplasmic cortical astroglia to focal ischemia. Journal of Cerebral Blood Flow & Metabolism. 2002;22(3):289–298. doi: 10.1097/00004647-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y., Wang L., Long Z., Zeng L., Wu Y. Protoplasmic astrocytes enhance the ability of neural stem cells to differentiate into neurons in vitro. PLoS One. 2012;7(5, article e38243) doi: 10.1371/journal.pone.0038243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson R. E., Lake A., Kenny P., et al. Different mixed astrocyte populations derived from embryonic stem cells have variable neuronal growth support capacities. Stem Cells and Development. 2017;26(22):1597–1611. doi: 10.1089/scd.2017.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bush T. G., Puvanachandra N., Horner C. H., et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23(2):297–308. doi: 10.1016/S0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 57.Leis J. A., Bekar L. K., Walz W. Potassium homeostasis in the ischemic brain. Glia. 2005;50(4):407–416. doi: 10.1002/glia.20145. [DOI] [PubMed] [Google Scholar]
- 58.Myer D. J., Gurkoff G. G., Lee S. M., Hovda D. A., Sofroniew M. V. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129(10):2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 59.Wanner I. B., Anderson M. A., Song B., et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. The Journal of Neuroscience. 2013;33(31):12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohtake Y., Smith G., Li S. Reactive astrocyte scar and axon regeneration: suppressor or facilitator? Neural Regeneration Research. 2016;11(7):1050–1051. doi: 10.4103/1673-5374.187022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rhodes K. E., Fawcett J. W. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? Journal of Anatomy. 2004;204(1):33–48. doi: 10.1111/j.1469-7580.2004.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y., Chen J., Zhang C. L., et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 63.Mckeon R., Höke A., Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Experimental Neurology. 1995;136(1):32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 64.Anderson M. A., Burda J. E., Ren Y., et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Z., Li Y., Cui Y., et al. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014;62(12):2022–2033. doi: 10.1002/glia.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meldrum B. S. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. Journal of Nutrition. 2000;130(4):1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 67.Kettenmann H., Backus K. H., Schachner M. Aspartate, glutamate and γ-aminobutyric acid depolarize cultured astrocytes. Neuroscience Letters. 1984;52(1-2):25–29. doi: 10.1016/0304-3940(84)90345-8. [DOI] [PubMed] [Google Scholar]
- 68.Hynd M., Scott H., Dodd P. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochemistry International. 2004;45(5):583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Yi J.-H., Hazell A. S. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochemistry International. 2006;48(5):394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Sanchez M. C., Benitez A., Ortloff L., Green L. M. Alterations in glutamate uptake in NT2-derived neurons and astrocytes after exposure to gamma radiation. Radiation Research. 2009;171(1):41–52. doi: 10.1667/RR1361.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chamoun R., Suki D., Gopinath S. P., Goodman J. C., Robertson C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. Journal of Neurosurgery. 2010;113(3):564–570. doi: 10.3171/2009.12.JNS09689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soria F. N., Pérez-Samartín A., Martin A., et al. Extrasynaptic glutamate release through cystine/glutamate antiporter contributes to ischemic damage. The Journal of Clinical Investigation. 2014;124(8):3645–3655. doi: 10.1172/JCI71886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki M., Kudo A., Sugawara A., et al. Amino acid concentrations in the blood of the jugular vein and peripheral artery after traumatic brain injury: decreased release of glutamate into the jugular vein in the early phase. Journal of Neurotrauma. 2002;19(2):285–292. doi: 10.1089/08977150252807027. [DOI] [PubMed] [Google Scholar]
- 74.Abramov A. Y., Duchen M. R. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2008;1777(7-8):953–964. doi: 10.1016/j.bbabio.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 75.Ozog M. A., Siushansian R., Naus C. C. G. Blocked gap junctional coupling increases glutamate-induced neurotoxicity in neuron-astrocyte co-cultures. Journal of Neuropathology & Experimental Neurology. 2002;61(2):132–141. doi: 10.1093/jnen/61.2.132. [DOI] [PubMed] [Google Scholar]
- 76.Rothman D. L., Sibson N. R., Hyder F., Shen J., Behar K. L., Shulman R. G. In vivo nuclear magnetic resonance spectroscopy studies of the relationship between the glutamate--glutamine neurotransmitter cycle and functional neuroenergetics. Philosophical Transactions of the Royal Society B. 1999;354(1387):1165–1177. doi: 10.1098/rstb.1999.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Furness D. N., Dehnes Y., Akhtar A. Q., et al. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2) Neuroscience. 2008;157(1):80–94. doi: 10.1016/j.neuroscience.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosenberg P. A., Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neuroscience Letters. 1989;103(2):162–168. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka K., Watase K., Manabe T., et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276(5319):1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 80.Lepore A. C., O'donnell J., Kim A. S., et al. Reduction in expression of the astrocyte glutamate transporter, GLT1, worsens functional and histological outcomes following traumatic spinal cord injury. Glia. 2011;59(12):1996–2005. doi: 10.1002/glia.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ouyang Y.-B., Voloboueva L. A., Xu L. J., Giffard R. G. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. Journal of Neuroscience. 2007;27(16):4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cantu D., Walker K., Andresen L., et al. Traumatic brain injury increases cortical glutamate network activity by compromising GABAergic control. Cerebral Cortex. 2015;25(8):2306–2320. doi: 10.1093/cercor/bhu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Volterra A., Trotti D., Tromba C., Floridi S., Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. The Journal of Neuroscience. 1994;14(5):2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Albrecht J., Talbot M., Kimelberg H. K., Aschner M. The role of sulfhydryl groups and calcium in the mercuric chloride-induced inhibition of glutamate uptake in rat primary astrocyte cultures. Brain Research. 1993;607(1-2):249–254. doi: 10.1016/0006-8993(93)91513-R. [DOI] [PubMed] [Google Scholar]
- 85.Fontana A. C. K., et al. Neuroprotective effects of the glutamate transporter activator (R)-(−)-5-methyl-1-nicotinoyl-2-pyrazoline (MS-153) following traumatic brain injury in the adult rat. Journal of Neurotrauma. 2016;33(11):1073–1083. doi: 10.1089/neu.2015.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dorsett C. R., McGuire J. L., Niedzielko T. L., et al. Traumatic brain injury induces alterations in cortical glutamate uptake without a reduction in glutamate transporter-1 protein expression. Journal of Neurotrauma. 2017;34(1):220–234. doi: 10.1089/neu.2015.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zou J., Wang Y. X., Dou F. F., et al. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochemistry International. 2010;56(4):577–584. doi: 10.1016/j.neuint.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramos-Mandujano G., Vázquez-Juárez E., Hernández-Benítez R., Pasantes-Morales H. Thrombin potently enhances swelling-sensitive glutamate efflux from cultured astrocytes. Glia. 2007;55(9):917–925. doi: 10.1002/glia.20513. [DOI] [PubMed] [Google Scholar]
- 89.Cengiz P., Kintner D. B., Chanana V., et al. Sustained Na+/H+ exchanger activation promotes gliotransmitter release from reactive hippocampal astrocytes following oxygen-glucose deprivation. PLoS One. 2014;9(1, article e84294) doi: 10.1371/journal.pone.0084294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh I. N., Sullivan P. G., Deng Y., Mbye L. H., Hall E. D. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. Journal of Cerebral Blood Flow & Metabolism. 2006;26(11):1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- 91.Gürer G., Gursoy-Ozdemir Y., Erdemli E., Can A., Dalkara T. Astrocytes are more resistant to focal cerebral ischemia than neurons and die by a delayed necrosis. Brain Pathology. 2009;19(4):630–641. doi: 10.1111/j.1750-3639.2008.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sugawara T., Noshita N., Lewén A., et al. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. The Journal of Neuroscience. 2002;22(1):209–217. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kalogeris T., Bao Y., Korthuis R. J. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biology. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kudin A. P., Augustynek B., Lehmann A. K., Kovács R., Kunz W. S. The contribution of thioredoxin-2 reductase and glutathione peroxidase to H2O2 detoxification of rat brain mitochondria. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2012;1817(10):1901–1906. doi: 10.1016/j.bbabio.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 95.Choi D. W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neuroscience Letters. 1985;58(3):293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- 96.Liu D., Chan S. L., de Souza-Pinto N. C., et al. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Medicine. 2006;8(3):389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- 97.Chan S. L., Liu D., Kyriazis G. A., Bagsiyao P., Ouyang X., Mattson M. P. Mitochondrial uncoupling protein-4 regulates calcium homeostasis and sensitivity to store depletion-induced apoptosis in neural cells. The Journal of Biological Chemistry. 2006;281(49):37391–37403. doi: 10.1074/jbc.M605552200. [DOI] [PubMed] [Google Scholar]
- 98.Mao W., Yu X. X., Zhong A., et al. UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Letters. 1999;443(3):326–330. doi: 10.1016/S0014-5793(98)01713-X. [DOI] [PubMed] [Google Scholar]
- 99.Krauss S., Zhang C.-Y., Lowell B. B. A significant portion of mitochondrial proton leak in intact thymocytes depends on expression of UCP2. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):118–122. doi: 10.1073/pnas.012410699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nicholls D. G., Ward M. W. Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends in Neurosciences. 2000;23(4):166–174. doi: 10.1016/S0166-2236(99)01534-9. [DOI] [PubMed] [Google Scholar]
- 101.Horvath T. L., Diano S., Barnstable C. Mitochondrial uncoupling protein 2 in the central nervous system: neuromodulator and neuroprotector. Biochemical Pharmacology. 2003;65(12):1917–1921. doi: 10.1016/S0006-2952(03)00143-6. [DOI] [PubMed] [Google Scholar]
- 102.Reichert S., Kim-Han J., Dugan L. The mitochondrial permeability transition pore and nitric oxide synthase mediate early mitochondrial depolarization in astrocytes during oxygen–glucose deprivation. The Journal of Neuroscience. 2001;21(17):6608–6616. doi: 10.1523/JNEUROSCI.21-17-06608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmed S. M., Rzigalinski B. A., Willoughby K. A., Sitterding H. A., Ellis E. F. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. Journal of Neurochemistry. 2000;74(5):1951–1960. doi: 10.1046/j.1471-4159.2000.0741951.x. [DOI] [PubMed] [Google Scholar]
- 104.Kruman I., Guo Q., Mattson M. P. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. Journal of Neuroscience Research. 1998;51(3):293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 105.Sullivan P. G., Thompson M. B., Scheff S. W. Cyclosporin a attenuates acute mitochondrial dysfunction following traumatic brain injury. Experimental Neurology. 1999;160(1):226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- 106.Bezprozvanny I. Calcium signaling and neurodegeneration. Acta Naturae. 2010;2(1):72–82. [PMC free article] [PubMed] [Google Scholar]
- 107.Brustovetsky N., Brustovetsky T., Jemmerson R., Dubinsky J. M. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. Journal of Neurochemistry. 2002;80(2):207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- 108.Lyng F. M., Maguire P., McClean B., Seymour C., Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiation Research. 2006;165(4):400–409. doi: 10.1667/RR3527.1. [DOI] [PubMed] [Google Scholar]
- 109.Huang J., Philbert M. A. Distribution of glutathione and glutathione-related enzyme systems in mitochondria and cytosol of cultured cerebellar astrocytes and granule cells. Brain Research. 1995;680(1-2):16–22. doi: 10.1016/0006-8993(95)00209-9. [DOI] [PubMed] [Google Scholar]
- 110.Sun X., Shih A. Y., Johannssen H. C., Erb H., Li P., Murphy T. H. Two-photon imaging of glutathione levels in intact brain indicates enhanced redox buffering in developing neurons and cells at the cerebrospinal fluid and blood-brain interface. The Journal of Biological Chemistry. 2006;281(25):17420–17431. doi: 10.1074/jbc.M601567200. [DOI] [PubMed] [Google Scholar]
- 111.Iwata-Ichikawa E., Kondo Y., Miyazaki I., Asanuma M., Ogawa N. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. Journal of Neurochemistry. 1999;72(6):2334–2344. doi: 10.1046/j.1471-4159.1999.0722334.x. [DOI] [PubMed] [Google Scholar]
- 112.Eftekharpour E., Holmgren A., Juurlink B. H. J. Thioredoxin reductase and glutathione synthesis is upregulated by t-Butylhydroquinone in cortical astrocytes but not in cortical neurons. Glia. 2000;31(3):241–248. doi: 10.1002/1098-1136(200009)31:3<241::AID-GLIA50>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 113.Suzuki T., Motohashi H., Yamamoto M. Toward clinical application of the Keap1–Nrf2 pathway. Trends in Pharmacological Sciences. 2013;34(6):340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 114.Rangasamy T., Cho C. Y., Thimmulappa R. K., et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke–induced emphysema in mice. The Journal of Clinical Investigation. 2004;114(9):1248–1259. doi: 10.1172/JCI200421146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang R., Chae S., Lee J. H., Hyun J. W. The cytoprotective effect of butin against oxidative stress is mediated by the up-regulation of manganese superoxide dismutase expression through a PI3K/Akt/Nrf2-dependent pathway. Journal of Cellular Biochemistry. 2012;113(6):1987–1997. doi: 10.1002/jcb.24068. [DOI] [PubMed] [Google Scholar]
- 116.Villeneuve N. F., Lau A., Zhang D. D. Regulation of the Nrf2–Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxidants and Redox Signaling. 2010;13(11):1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang D. D., Lo S. C., Cross J. V., Templeton D. J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Molecular and Cellular Biology. 2004;24(24):10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bell K. F. S., al-Mubarak B., Martel M. A., et al. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nature Communications. 2015;6(1):p. 7066. doi: 10.1038/ncomms8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johnson J. A., Johnson D. A., Kraft A. D., et al. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Annals of the New York Academy of Sciences. 2008;1147(1):61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shih A., Johnson D. A., Wong G., et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. Journal of Neuroscience. 2003;23(8):3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee J.-M., Calkins M. J., Chan K., Kan Y. W., Johnson J. A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. The Journal of Biological Chemistry. 2003;278(14):12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 122.Vargas M. R., Johnson D. A., Sirkis D. W., Messing A., Johnson J. A. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. Journal of Neuroscience. 2008;28(50):13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jimenez-Blasco D., Santofimia-Castaño P., Gonzalez A., Almeida A., Bolaños J. P. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5–Nrf2 pathway. Cell Death & Differentiation. 2015;22(11):1877–1889. doi: 10.1038/cdd.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dringen R., Pfeiffer B., Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. The Journal of Neuroscience. 1999;19(2):562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sagara J., Miura K., Bannai S. Maintenance of neuronal glutathione by glial cells. Journal of Neurochemistry. 1993;61(5):1672–1676. doi: 10.1111/j.1471-4159.1993.tb09802.x. [DOI] [PubMed] [Google Scholar]
- 126.Escartin C., Joon Won S., Malgorn C., et al. Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. The Journal of Neuroscience. 2011;31(20):7392–7401. doi: 10.1523/JNEUROSCI.6577-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schreiner B., Romanelli E., Liberski P., et al. Astrocyte depletion impairs redox homeostasis and triggers neuronal loss in the adult CNS. Cell Reports. 2015;12(9):1377–1384. doi: 10.1016/j.celrep.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 128.Atlante A., Calissano P., Bobba A., Giannattasio S., Marra E., Passarella S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Letters. 2001;497(1):1–5. doi: 10.1016/S0014-5793(01)02437-1. [DOI] [PubMed] [Google Scholar]
- 129.Spees J. L., Olson S. D., Whitney M. J., Prockop D. J. Mitochondrial transfer between cells can rescue aerobic respiration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y., Cui J., Sun X., Zhang Y. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death & Differentiation. 2011;18(4):732–742. doi: 10.1038/cdd.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang X., Gerdes H.-H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death & Differentiation. 2015;22(7):1181–1191. doi: 10.1038/cdd.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun X., Wang Y., Zhang J., et al. Tunneling-nanotube direction determination in neurons and astrocytes. Cell Death & Disease. 2012;3(12, article e438) doi: 10.1038/cddis.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hayakawa K., Esposito E., Wang X., et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535(7613):551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davis C. O., Kim K. Y., Bushong E. A., et al. Transcellular degradation of axonal mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(26):9633–9638. doi: 10.1073/pnas.1404651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hawkins B., Davis T. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological Reviews. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 136.Morgello S., Uson R. R., Schwartz E. J., Haber R. S. The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia. 1995;14(1):43–54. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- 137.Kacem K., Lacombe P., Seylaz J., Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23(1):1–10. doi: 10.1002/(SICI)1098-1136(199805)23:1<1::AID-GLIA1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 138.Forsyth R. J. Astrocytes and the delivery of glucose from plasma to neurons. Neurochemistry International. 1996;28(3):231–241. doi: 10.1016/0197-0186(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 139.Maxwell K., Berliner J. A., Cancilla P. A. Stimulation of glucose analogue uptake by cerebral microvessel endothelial cells by a product released by astrocytes. Journal of Neuropathology and Experimental Neurology. 1989;48(1):69–80. doi: 10.1097/00005072-198901000-00006. [DOI] [PubMed] [Google Scholar]
- 140.Cataldo A. M., Broadwell R. D. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions: I. Neurons and glia. Microscopy Research & Technique. 1986;3(4):413–437. doi: 10.1002/jemt.1060030406. [DOI] [PubMed] [Google Scholar]
- 141.Pellerin L., Pellegri G., Bittar P. G., et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Developmental Neuroscience. 1998;20(4-5):291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 142.Suzuki A., Stern S. A., Bozdagi O., et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wender R., Brown A. M., Fern R., Swanson R. A., Farrell K., Ransom B. R. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. The Journal of Neuroscience. 2000;20(18):6804–6810. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dienel G. A., Cruz N. F., Sokoloff L., Driscoll B. F. Determination of glucose utilization rates in cultured astrocytes and neurons with [14C]deoxyglucose: progress, pitfalls, and discovery of intracellular glucose compartmentation. Neurochemical Research. 2017;42(1):50–63. doi: 10.1007/s11064-015-1650-x. [DOI] [PubMed] [Google Scholar]
- 145.Bouzier-Sore A.-K., Voisin P., Canioni P., Magistretti P. J., Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. Journal of Cerebral Blood Flow & Metabolism. 2003;23(11):1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- 146.Wyss M. T., Jolivet R., Buck A., Magistretti P. J., Weber B. In vivo evidence for lactate as a neuronal energy source. The Journal of Neuroscience. 2011;31(20):7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Heni M., Hennige A. M., Peter A., et al. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS One. 2011;6(6, article e21594) doi: 10.1371/journal.pone.0021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Loaiza A., Porras O., Barros L. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. The Journal of Neuroscience. 2003;23(19):7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Muhič M., Vardjan N., Chowdhury H. H., Zorec R., Kreft M. Insulin and insulin-like growth factor 1 (IGF-1) modulate cytoplasmic glucose and glycogen levels but not glucose transport across the membrane in astrocytes. The Journal of Biological Chemistry. 2015;290(17):11167–11176. doi: 10.1074/jbc.M114.629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.García-Cáceres C., Quarta C., Varela L., et al. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell. 2016;166(4):867–880. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Khanna A., Kahle K. T., Walcott B. P., Gerzanich V., Simard J. M. Disruption of ion homeostasis in the neurogliovascular unit underlies the pathogenesis of ischemic cerebral edema. Translational Stroke Research. 2014;5(1):3–16. doi: 10.1007/s12975-013-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nordal R., Nagy A., Pintilie M., Wong C. S. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clinical Cancer Research. 2004;10(10):3342–3353. doi: 10.1158/1078-0432.CCR-03-0426. [DOI] [PubMed] [Google Scholar]
- 153.Takahashi H., Manaka S., Sano K. Changes in extracellular potassium concentration in cortex and brain stem during the acute phase of experimental closed head injury. Journal of Neurosurgery. 1981;55(5):708–717. doi: 10.3171/jns.1981.55.5.0708. [DOI] [PubMed] [Google Scholar]
- 154.Bellot-Saez A., Kékesi O., Morley J. W., Buskila Y. Astrocytic modulation of neuronal excitability through K+ spatial buffering. Neuroscience & Biobehavioral Reviews. 2017;77:87–97. doi: 10.1016/j.neubiorev.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 155.Antunes A., Schiefecker A., Beer R., et al. Higher brain extracellular potassium is associated with brain metabolic distress and poor outcome after aneurysmal subarachnoid hemorrhage. Critical Care. 2014;18(3, article R119) doi: 10.1186/cc13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bazzigaluppi P., Weisspapir I., Stefanovic B., Leybaert L., Carlen P. L. Astrocytic gap junction blockade markedly increases extracellular potassium without causing seizures in the mouse neocortex. Neurobiology of Disease. 2017;101:1–7. doi: 10.1016/j.nbd.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 157.Yoshino A., Hovda D. A., Kawamata T., Katayama Y., Becker D. P. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Research. 1991;561(1):106–119. doi: 10.1016/0006-8993(91)90755-K. [DOI] [PubMed] [Google Scholar]
- 158.Almeida A., Delgado-Esteban M., Bolaños J. P., Medina J. M. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. Journal of Neurochemistry. 2002;81(2):207–217. doi: 10.1046/j.1471-4159.2002.00827.x. [DOI] [PubMed] [Google Scholar]
- 159.Russell J. W., Golovoy D., Vincent A. M., et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. The FASEB Journal. 2002;16(13):1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 160.Goldberg M., Choi D. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. The Journal of Neuroscience. 1993;13(8):3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Papadopoulos M. C., Koumenis I. L., Dugan L. L., Giffard R. G. Vulnerability to glucose deprivation injury correlates with glutathione levels in astrocytes. Brain Research. 1997;748(1-2):151–156. doi: 10.1016/S0006-8993(96)01293-0. [DOI] [PubMed] [Google Scholar]
- 162.Gotoh H., Nomura T., Ono K. Glycogen serves as an energy source that maintains astrocyte cell proliferation in the neonatal telencephalon. Journal of Cerebral Blood Flow & Metabolism. 2016;37(6):2294–2307. doi: 10.1177/0271678X16665380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Edmond J., Robbins R. A., Bergstrom J. D., Cole R. A., de Vellis J. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. Journal of Neuroscience Research. 1987;18(4):551–561. doi: 10.1002/jnr.490180407. [DOI] [PubMed] [Google Scholar]
- 164.Swanson R. A., Choi D. W. Glial glycogen stores affect neuronal survival during glucose deprivation in vitro. Journal of Cerebral Blood Flow & Metabolism. 1993;13(1):162–169. doi: 10.1038/jcbfm.1993.19. [DOI] [PubMed] [Google Scholar]
- 165.Gao C., Zhou L., Zhu W., et al. Monocarboxylate transporter-dependent mechanism confers resistance to oxygen- and glucose-deprivation injury in astrocyte-neuron co-cultures. Neuroscience Letters. 2015;594(6):99–104. doi: 10.1016/j.neulet.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 166.Lama S., Auer R. N., Tyson R., Gallagher C. N., Tomanek B., Sutherland G. R. Lactate storm marks cerebral metabolism following brain trauma. Journal of Biological Chemistry. 2014;289(29):20200–20208. doi: 10.1074/jbc.M114.570978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rice A. C., Zsoldos R., Chen T., et al. Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Research. 2002;928(1-2):156–159. doi: 10.1016/S0006-8993(01)03299-1. [DOI] [PubMed] [Google Scholar]
- 168.Rossi D. J., Brady J. D., Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nature Neuroscience. 2007;10(11):1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stefani M. A., Modkovski R., Hansel G., et al. Elevated glutamate and lactate predict brain death after severe head trauma. Annals of Clinical and Translational Neurology. 2017;4(6):392–402. doi: 10.1002/acn3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vespa P. M., Miller C., McArthur D., et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Critical Care Medicine. 2007;35(12):2830–2836. doi: 10.1097/00003246-200712000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Angamo E., ul Haq R., Rösner J., et al. Contribution of intrinsic lactate to maintenance of seizure activity in neocortical slices from patients with temporal lobe epilepsy and in rat entorhinal cortex. International Journal of Molecular Sciences. 2017;18(12):p. 1835. doi: 10.3390/ijms18091835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beydoun R., Hamood M. A., Gomez Zubieta D. M., Kondapalli K. C. Na+/H+ exchanger 9 regulates iron mobilization at the blood-brain barrier in response to iron starvation. The Journal of Biological Chemistry. 2017;292(10):4293–4301. doi: 10.1074/jbc.M116.769240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chitambar C. R., Narasimhan J. Targeting iron-dependent DNA synthesis with gallium and transferrin-gallium. Pathobiology. 1991;59(1):3–10. doi: 10.1159/000163609. [DOI] [PubMed] [Google Scholar]
- 174.Schulz K., Kroner A., David S. Iron efflux from astrocytes plays a role in remyelination. The Journal of Neuroscience. 2012;32(14):4841–4847. doi: 10.1523/JNEUROSCI.5328-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gaasch J. A., Lockman P. R., Geldenhuys W. J., Allen D. D., van der Schyf C. J. Brain iron toxicity: differential responses of astrocytes, neurons, and endothelial cells. Neurochemical Research. 2007;32(7):1196–1208. doi: 10.1007/s11064-007-9290-4. [DOI] [PubMed] [Google Scholar]
- 176.Berglund S. K., Torres-Espínola F. J., García-Valdés L., et al. The impacts of maternal iron deficiency and being overweight during pregnancy on neurodevelopment of the offspring. British Journal of Nutrition. 2017;118(07):533–540. doi: 10.1017/S0007114517002410. [DOI] [PubMed] [Google Scholar]
- 177.Youdim M. B. H. Brain iron deficiency and excess; cognitive impairment and neurodegenration with involvement of striatum and hippocampus. Neurotoxicity Research. 2008;14(1):45–56. doi: 10.1007/BF03033574. [DOI] [PubMed] [Google Scholar]
- 178.Zhang Y., Tatsuno T., Carney J. M., Mattson M. P. Basic FGF, NGF, and IGFs protect hippocampal and cortical neurons against iron-induced degeneration. Journal of Cerebral Blood Flow & Metabolism. 1993;13(3):378–388. doi: 10.1038/jcbfm.1993.51. [DOI] [PubMed] [Google Scholar]
- 179.Floyd R. A., Ann Lewis C. Hydroxyl free radical formation from hydrogen peroxide by ferrous iron-nucleotide complexes. Biochemistry. 1983;22(11):2645–2649. doi: 10.1021/bi00280a008. [DOI] [PubMed] [Google Scholar]
- 180.Chen G., Jing C. H., Liu P. P., Ruan D., Wang L. Induction of autophagic cell death in the rat brain caused by iron. The American Journal of the Medical Sciences. 2013;345(5):369–374. doi: 10.1097/MAJ.0b013e318271c031. [DOI] [PubMed] [Google Scholar]
- 181.Regan R. F., Scott Panter S. Neurotoxicity of hemoglobin in cortical cell culture. Neuroscience Letters. 1993;153(2):219–222. doi: 10.1016/0304-3940(93)90326-G. [DOI] [PubMed] [Google Scholar]
- 182.Kress G., Dineley K., Reynolds I. The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. The Journal of Neuroscience. 2002;22(14):5848–5855. doi: 10.1523/JNEUROSCI.22-14-05848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cui Z., Zhong Z., Yang Y., et al. Ferrous iron induces Nrf2 expression in mouse brain astrocytes to prevent neurotoxicity. Journal of Biochemical and Molecular Toxicology. 2016;30(8):396–403. doi: 10.1002/jbt.21803. [DOI] [PubMed] [Google Scholar]
- 184.Ho K., Li L., Zhao L., Qian Z. M. Genistein protects primary cortical neurons from iron-induced lipid peroxidation. Molecular and Cellular Biochemistry. 2003;247(1/2):219–222. doi: 10.1023/A:1024142004575. [DOI] [PubMed] [Google Scholar]
- 185.Wright D. K., Trezise J., Kamnaksh A., et al. Behavioral, blood, and magnetic resonance imaging biomarkers of experimental mild traumatic brain injury. Scientific Reports. 2016;6(1, article 28713) doi: 10.1038/srep28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang X., Michaelis E. Selective neuronal vulnerability to oxidative stress in the brain. Frontiers in Aging Neuroscience. 2010;2:p. 12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Belaidi A. A., Bush A. I. Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. Journal of Neurochemistry. 2016;139(Supplement 1):179–197. doi: 10.1111/jnc.13425. [DOI] [PubMed] [Google Scholar]
- 188.Ghribi O., Golovko M. Y., Larsen B., Schrag M., Murphy E. J. Deposition of iron and β-amyloid plaques is associated withcortical cellular damage in rabbits fed with long-termcholesterol-enriched diets. Journal of Neurochemistry. 2007;99(2):438–449. doi: 10.1111/j.1471-4159.2006.04079.x. [DOI] [PubMed] [Google Scholar]
- 189.Palmer C., Menzies S. L., Roberts R. L., Pavlick G., Connor J. R. Changes in iron histochemistry after hypoxic-ischemic brain injury in the neonatal rat. Journal of Neuroscience Research. 1999;56(1):60–71. doi: 10.1002/(SICI)1097-4547(19990401)56:1<60::AID-JNR8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 190.Lu L., Cao H., Wei X., Li Y., Li W. Iron deposition is positively related to cognitive impairment in patients with chronic mild traumatic brain injury: assessment with susceptibility weighted imaging. BioMed Research International. 2015;2015:7. doi: 10.1155/2015/470676.470676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liddell J. R., Robinson S. R., Dringen R. Endogenous glutathione and catalase protect cultured rat astrocytes from the iron-mediated toxicity of hydrogen peroxide. Neuroscience Letters. 2004;364(3):164–167. doi: 10.1016/j.neulet.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 192.Pelizzoni I., Zacchetti D., Campanella A., Grohovaz F., Codazzi F. Iron uptake in quiescent and inflammation-activated astrocytes: a potentially neuroprotective control of iron burden. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2013;1832(8):1326–1333. doi: 10.1016/j.bbadis.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Codazzi F., Pelizzoni I., Zacchetti D., Grohovaz F. Iron entry in neurons and astrocytes: a link with synaptic activity. Frontiers in Molecular Neuroscience. 2015;8 doi: 10.3389/fnmol.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Song X., Zhao Y., Narcisse L., et al. Canonical transient receptor potential channel 4 (TRPC4) co-localizes with the scaffolding protein ZO-1 in human fetal astrocytes in culture. Glia. 2005;49(3):418–429. doi: 10.1002/glia.20128. [DOI] [PubMed] [Google Scholar]
- 195.Cheng K. T., Ong H. L., Liu X., Ambudkar I. S. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Current Topics in Membranes. 2013;71:149–179. doi: 10.1016/B978-0-12-407870-3.00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mwanjewe J., Grover A. K. Role of transient receptor potential canonical 6 (TRPC6) in non-transferrin-bound iron uptake in neuronal phenotype PC12 cells. Biochemical Journal. 2004;378(3):975–982. doi: 10.1042/bj20031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rathore K. I., Redensek A., David S. Iron homeostasis in astrocytes and microglia is differentially regulated by TNF-α and TGF-β1. Glia. 2012;60(5):738–750. doi: 10.1002/glia.22303. [DOI] [PubMed] [Google Scholar]
- 198.Urrutia P., Aguirre P., Esparza A., et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. Journal of Neurochemistry. 2013;126(4):541–549. doi: 10.1111/jnc.12244. [DOI] [PubMed] [Google Scholar]
- 199.Regan R. F., Kumar N., Gao F., Guo Y. Ferritin induction protects cortical astrocytes from heme-mediated oxidative injury. Neuroscience. 2002;113(4):985–994. doi: 10.1016/S0306-4522(02)00243-9. [DOI] [PubMed] [Google Scholar]
- 200.Harrison P. M., Arosio P. The ferritins molecular properties, iron storage function and cellular regulation. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1996;1275(3):161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 201.Kelso M., Gendelman H. Bridge between neuroimmunity and traumatic brain injury. Current Pharmaceutical Design. 2014;20(26):4284–4298. [PMC free article] [PubMed] [Google Scholar]
- 202.Farina C., Aloisi F., Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in Immunology. 2007;28(3):138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 203.Daginakatte G. C., Gadzinski A., Emnett R. J., et al. Expression profiling identifies a molecular signature of reactive astrocytes stimulated by cyclic AMP or proinflammatory cytokines. Experimental Neurology. 2008;210(1):261–267. doi: 10.1016/j.expneurol.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 204.Yong R. L., Yang C., Lu J., et al. Cell transcriptional state alters genomic patterns of DNA double-strand break repair in human astrocytes. Nature Communications. 2014;5:p. 5799. doi: 10.1038/ncomms6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kostianovsky A. M., Maier L. M., Anderson R. C., Bruce J. N., Anderson D. E. Astrocytic regulation of human monocytic/microglial activation. Journal of Immunology. 2008;181(8):5425–5432. doi: 10.4049/jimmunol.181.8.5425. [DOI] [PubMed] [Google Scholar]
- 206.Sofroniew M. V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends in Neurosciences. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Norris C., Kadish I., Blalock E. M., et al. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. The Journal of Neuroscience. 2005;25(18):4649–4658. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Song H., Stevens C. F., Gage F. H. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nature Neuroscience. 2002;5(5):438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 209.Neumann H., Schweigreiter R., Yamashita T., Rosenkranz K., Wekerle H., Barde Y. A. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. The Journal of Neuroscience. 2002;22(3):854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Stewart V., Land J. M., Clark J. B., Heales S. J. Pretreatment of astrocytes with interferon-α/β prevents neuronal mitochondrial respiratory chain damage. Journal of Neurochemistry. 1998;70(1):432–434. doi: 10.1046/j.1471-4159.1998.70010432.x. [DOI] [PubMed] [Google Scholar]
- 211.Stewart V., Sharpe M. A., Clark J. B., Heales S. J. Astrocyte-derived nitric oxide causes both reversible and irreversible damage to the neuronal mitochondrial respiratory chain. Journal of Neurochemistry. 2000;75(2):694–700. doi: 10.1046/j.1471-4159.2000.0750694.x. [DOI] [PubMed] [Google Scholar]
- 212.Voskuhl R. R., Peterson R. S., Song B., et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. The Journal of Neuroscience. 2009;29(37):11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gadani S. P., Walsh J. T., Smirnov I., Zheng J., Kipnis J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron. 2015;85(4):703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 214.Faulkner J., Herrmann J. E., Woo M. J., Tansey K. E., Doan N. B., Sofroniew M. V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. The Journal of Neuroscience. 2004;24(9):2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Harrison J., Hollensworth S. B., Spitz D. R., Copeland W. C., Wilson G. L., LeDoux S. Oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance. Nucleic Acids Research. 2005;33(14):4660–4671. doi: 10.1093/nar/gki759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gobbel G., Bellinzona M., Vogt A. R., Gupta N., Fike J. R., Chan P. H. Response of postmitotic neurons to X-irradiation: implications for the role of DNA damage in neuronal apoptosis. The Journal of Neuroscience. 1998;18(1):147–155. doi: 10.1523/JNEUROSCI.18-01-00147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang Y., Geldmacher D., Herrup K. DNA replication precedes neuronal cell death in Alzheimer’s disease. The Journal of Neuroscience. 2001;21(8):2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schwartz E. I., Smilenov L. B., Price M. A., et al. Cell cycle activation in postmitotic neurons is essential for DNA repair. Cell Cycle. 2007;6(3):318–329. doi: 10.4161/cc.6.3.3752. [DOI] [PubMed] [Google Scholar]
- 219.Kruman I. I., Wersto R. P., Cardozo-Pelaez F., et al. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41(4):549–561. doi: 10.1016/S0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- 220.Kuan C. Y., Schloemer A. J., Lu A., et al. Hypoxia–ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. Journal of Neuroscience. 2004;24(47):10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.di Giovanni S., Movsesyan V., Ahmed F., et al. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(23):8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tamulevicius P., Wang M., Iliakis G. Homology-directed repair is required for the development of radioresistance during S phase: interplay between double-strand break repair and checkpoint response. Radiation Research. 2007;167(1):1–11. doi: 10.1667/RR0751.1. [DOI] [PubMed] [Google Scholar]
- 223.Kato H., Takahashi A., Itoyama Y. Cell cycle protein expression in proliferating microglia and astrocytes following transient global cerebral ischemia in the rat. Brain Research Bulletin. 2003;60(3):215–221. doi: 10.1016/S0361-9230(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 224.Liu D., Croteau D. L., Souza-Pinto N., et al. Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions. Journal of Cerebral Blood Flow & Metabolism. 2011;31(2):680–692. doi: 10.1038/jcbfm.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nouspikel T., Hanawalt P. C. Terminally differentiated human neurons repair transcribed genes but display attenuated global DNA repair and modulation of repair gene expression. Molecular and Cellular Biology. 2000;20(5):1562–1570. doi: 10.1128/MCB.20.5.1562-1570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Short S. C., Martindale C., Bourne S., Brand G., Woodcock M., Johnston P. DNA repair after irradiation in glioma cells and normal human astrocytes. Neuro-Oncology. 2007;9(4):404–411. doi: 10.1215/15228517-2007-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.LeDoux S. P., Druzhyna N. M., Hollensworth S. B., Harrison J. F., Wilson G. L. Mitochondrial DNA repair: a critical player in the response of cells of the CNS to genotoxic insults. Neuroscience. 2007;145(4):1249–1259. doi: 10.1016/j.neuroscience.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bylicky M., Mueller G., Day R. Radiation resistance of normal human astrocytes: role of non-homologous end joining DNA repair activity. In submission. [DOI] [PMC free article] [PubMed]
- 229.Schneider L., Fumagalli M., d'Adda di Fagagna F. Terminally differentiated astrocytes lack DNA damage response signaling and are radioresistant but retain DNA repair proficiency. Cell Death & Differentiation. 2012;19(4):582–591. doi: 10.1038/cdd.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]