Abstract
An outbreak of reproductive failure in a pig farm in Taiwan was investigated. Coinfection with porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) was diagnosed in a stillborn pig by histopathology, polymerase chain reaction, and immunohistochemistry, and should be considered as a cause of reproductive failure.
Résumé
Échec de reproduction associé à la coinfection par le circovirus porcin de type 2 et le virus du syndrome dysgénésique et respiratoire du porc. On a fait enquête sur une éclosion d’échecs de reproduction dans une ferme porcine à Taiwan. La coinfection par le circovirus porcin de type 2 (PCV2) et le virus du syndrome dysgénésique respiratoire du porc (SDRP) a été diagnostiqué chez un porc mort-né par histopathologie, amplification en chaîne par polymérase et immunohistochimie et elle devrait être considérée comme la cause de l’échec de reproduction.
(Traduit par Isabelle Vallières)
Porcine circovirus types 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) are economically significant pathogens in the swine industry worldwide. Both viruses are associated with reproductive failure characterized by mummified fetuses, late-term abortions, stillbirths, and premature farrowings (1,2). PCV2 may be associated with reproductive failure at all stages of gestation and may cause embryonic death (3). By contrast, PRRSV causes abortion or stillbirth during late gestation (2). Herds affected by PCV2-associated reproductive failure are typically composed of a high number of gilts which are probably PCV2-seronegative (4–6). The high seroprevalence of PCV2 in sows is believed to prevent most breeding herds from developing pronounced disease (1). In contrast to PRRSV, which is endemic at the global level and results in significant reproductive loss (2), the prevalence of PCV2-associated reproductive failure under field conditions is variable among studies, ranging from 1 to 25% (3,7,8). Also, some studies have stated that sows may be subclinically infected with PCV2 and may have given birth to viremic presuckling piglets (9,10). Whether transplacental infection occurs in the PCV2-seropositive sow population is still controversial (11–13).
The most common lesion observed in PCV2-infected fetuses is myocarditis (4,5). Lymphoid depletion, interstitial pneumonia, hepatic congestion with hepatocellular loss, and nonsuppurative hepatitis with periacinar necrosis have also been reported (14). Fetal lesions caused by PRRSV may include vasculitis, myocarditis, and encephalitis (15). The diagnostic criteria for PCV2-associated reproductive failure include clinical signs of late-term abortions and stillbirths, and the presence of heart lesions and high concentrations of PCV2 in the myocardial lesion and other fetal tissues (16). Regarding the diagnosis of PRRSV, 1 study suggested that the combination of pathological lesions and presence of PRRSV in the lesions is highly suggestive of PRRSV-associated fetal death (17); however, there is no gold standard for detecting PRRSV in fetuses (18). Both PCV2 and PRRSV are transmitted vertically and horizontally in utero (2,13). However, coinfections with these 2 viruses in fetuses from the same litter have rarely been reported and were only diagnosed by using polymerase chain reaction (PCR) (3,5,8). To date, no information is available regarding the association between the viruses and the corresponding lesions in the case of coinfection.
This case report describes reproductive failure associated with coinfection of PCV2 and PRRSV based on positive PCR and detection of both viral antigens in microscopic lesions by immunohistochemical (IHC) staining.
Case description
The affected herd was from a 450-sow, farrow-to-finish, closed farm in Northern Taiwan, with a history of infections with PCV2, PRRSV, and Mycoplasma hyopneumoniae. The facility was under veterinary care, the housing was adequate, and the animals were humanely cared for. The farm consists of a breeding barn, a boar barn which also houses the gilts for acclimation, a gestation barn, 2 farrowing barns, a nursery barn, 2 growing-finishing barns, and a sales barn. This farm had 3 dogs, and a few feral dogs and cats were occasionally found on the farm. This farm operated on a continuous farrowing program on a weekly basis. All 3-week-old piglets were vaccinated for PCV2 (Fostera PCV; Zoetis, Madison, New Jersey, USA). For PRRSV vaccination (AMERVAC PRRS; Laboratorios Hipra, Amer, Spain), the replacement gilts were inoculated twice before breeding during acclimation, and the sows were inoculated after every farrowing. Other vaccines administered for reproductive management included the commercially available classical swine fever virus (CSFV; SUIGEN Hog Cholera Cell Cultured Live Vaccine; SBC Virbac Biotech, Kaohsiung City, Taiwan), Japanese encephalitis virus (JEV; NISSEIKEN Japanese Encephalitis Live Virus Vaccine; NISSEIKEN, Tokyo, Japan), porcine parvovirus (PPV; Suvaxyn P, Zoetis), and pseudorabies virus (PRV; SUIGEN Swine Pseudorabies Gene Deleted Live Vaccine; SBC Virbac Biotech) vaccines.
An increase in the incidence of late-term abortions, stillbirths, and premature farrowings was noted in this farm from November 2016 to February 2017. Abortion was defined as expulsion of fetuses before 110 d of gestation. Stillbirth indicated the delivery of a fully developed but nonviable offspring on or after 110 d of gestation. Expulsion of live piglets by gilts or sows between 110 and 113 d was considered premature farrowing. A month before this outbreak, the producer became aware that increased numbers of pigs in the herd were anorexic, lethargic, depressed, or pyrexic.
The abortion rate (i.e., number of abortions/number of females served) abruptly increased from 1.1% in October 2016 to the highest rate of 15.6% in December 2016. Subsequently, it decreased to 4.9% in February, although it was still unacceptable. Stillbirth rate (i.e., number of stillbirths/number of total pigs born) and mummy rate (i.e., number of mummified fetuses/number of total pigs born) were not available from the producer. During these 4 mo, records showed that only 8 of a total of 38 cases of abortions, stillbirths, and premature farrowings occurred in the gilts. The etiologies of 6 litters of 7 to 13 mummified fetuses, abortuses, and stillbirths, of varied crown-rump lengths from 3 gilts and 3 sows were investigated. According to the breeding record, gestational ages ranged from 82 to 114 d. These gilts and sows showed no signs of illness other than having reproductive failure during the outbreak.
Abortuses and stillbirths from these 6 litters were necropsied, and gross examination was performed on-site on every fetus from each litter on the same date the abortions occurred. The fetuses had variable crown-rump length ranging from 5 to 27 cm, which corresponded to approximate gestational ages of 40 to 110 d (19). No abnormal external appearances, except for meconium staining and mummification in some fetuses, were observed. The gross and microscopic lesions are summarized in Table 1. Excessive serosanguineous fluid was detected in the thoracic and abdominal cavities in fetuses of all 6 litters. Other gross lesions included marked cardiomegaly with ventricular dilatation, pale areas in the myocardium in cross-section, congested liver and meninges, and patchy pulmonary hemorrhage. Specimens of brain, gastrointestinal tract, heart, kidney, liver, lung, lymph node, placenta, spleen, thymus, tonsil, and umbilical cord were collected from 1 fetus of each litter, fixed in neutral buffered 10% formalin, and processed routinely for histopathological examination on the day after collection.
Table 1.
Summary of clinical history, pathological lesions, and agents identified from 6 litters with abortion and stillbirth.
| Litter | Parity | Gestational age (days) | Number of fetusesa/ mummies/ alive piglets | Gross lesionb | Microscopic lesionc | Presence of protozoa-like organism in myocardiocytes | Agents identified by PCR |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 82 | 7 fetuses | Cardiomegaly, pleural and abdominal effusion | Endocardial hemorrhage, myocardial edema and fibrosis | Yes | PCV2 |
| 2 | 2 | 114 | 4 fetuses/ 7 mummies/ 3 live piglets | Mummification, pleural and abdominal effusion | d | d | PCV2 + PRRSV |
| 3 | 1 | 98 | 10 fetuses | Cardiomegaly, congested meninges, pleural and abdominal effusion, pulmonary hemorrhage | Interstitial pneumonia, meningitis, myocardial edema and fibrosis | Yes | PCV2 |
| 4 | 2 | 113 | 7 fetuses/ 5 mummies | Cardiomegaly, congested liver, congested meninges, mummification, pleural and abdominal effusion, pulmonary hemorrhage | Choroid plexitis, interstitial pneumonia, meningitis, myocardial edema and fibrosis, myocarditis, vasculitis | Yes | PCV2e + PRRSVe |
| 5 | 1 | 103 | 11 fetuses | Cardiomegaly, congested liver, congested meninges, pleural and abdominal effusion, pulmonary hemorrhage | Interstitial pneumonia, meningitis, myocardial fibrosis | No | No |
| 6 | 1 | 112 | 11 fetuses/ 2 mummies | Cardiomegaly, congested liver, mummification, pleural and abdominal effusion, pulmonary hemorrhage | Endocardial hemorrhage, meningitis, myocardial fibrosis | No | No |
Fetuses included abortuses, stillbirths, and dead neonatal piglets.
Gross examination was performed on all fetuses.
Histopathological examination was performed on 1 fetus from each litter which had the most prominent gross lesions.
Histopathological examination was not performed due to severe autolysis and mummification of the fetuses.
The antigen was also detected by immunohistochemical staining.
PCR — polymerase chain reaction; PCV2 — porcine circovirus type 2; PRRSV — porcine reproductive and respiratory syndrome virus.
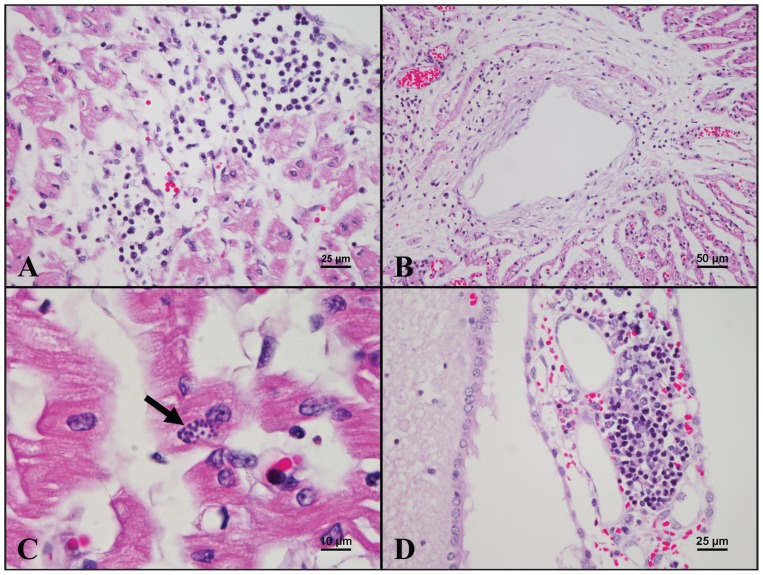
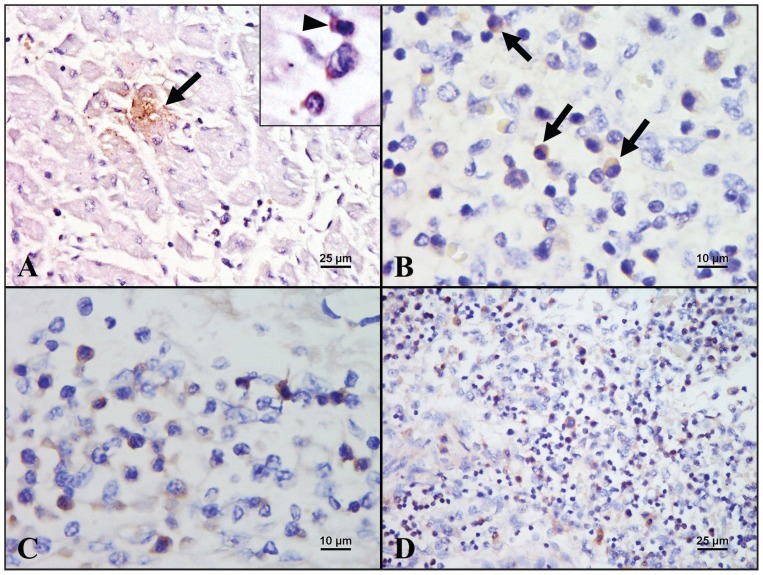
Histopathologically, the heart of 1 fetus from litter 4 had multifocal moderate mononuclear myocarditis (Figure 1A), edema, and fibrosis. In some areas, the myocardiocytes were reduced in size and exhibited cytoplasmic vacuolization. Vasculitis, characterized by infiltration of lymphocytes, macrophages, and plasma cells in the vessel wall (Figure 1B), and thrombus formation were sometimes noted in the same fetal heart. A few protozoa-like organisms were occasionally detected within the cytoplasm of myocardiocytes without associated inflammation in the same fetal heart and also in fetuses from another 2 litters (Figure 1C). Endocardial hemorrhage was observed in 2 other litters. Mononuclear choroid plexitis (Figure 1D) and meningitis, and cerebral edema were found in the brain from the fetus with myocarditis. Marked generalized hepatic lipidosis and congestion were also noted in most fetuses. Furthermore, hemorrhage was commonly observed in the cortex of the kidney and the lung. The IHC staining with Dako EnVision system using in-house anti-PCV2 antibody and anti-PRRSV antibody obtained from the Laboratory of Molecular Pathobiology, School of Veterinary Medicine, National Taiwan University, was performed on the fetus with prominent cardiac lesions from litter 4. PCV2 antigen was detected within the cytoplasm of myocardiocytes and adjacent mononuclear inflammatory cells (Figure 2A), as well as in the mononuclear cells scattered in the lymphoid tissues (Figure 2B). PRRSV antigen was detected within the cytoplasm of the mononuclear inflammatory cells in the heart (Figure 2C), liver, lung, lymph node (Figure 2D), meninges, and spleen.
Figure 1.
Microscopic lesions of a fetus from litter 4 having concurrent infection of porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV). A — The myocardium is infiltrated by mononuclear inflammatory cells [hematoxylin and eosin (H&E), 400×]. B — The vessel wall is infiltrated by mononuclear inflammatory cells, and perivascular fibrosis is apparent (H&E, 200×). C — A cluster of oval, basophilic tachyzoite-like structures (arrow) is observed in the cytoplasm of a myocardiocyte (H&E, 1000×). D — Mononuclear inflammatory cells infiltrate the choroid plexus (H&E, 400×).
Figure 2.
Results of immunohistochemical staining (counterstained with hematoxylin) for porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) performed in a fetus from litter 4. A — PCV2 antigen is detected within the cytoplasm of myocardiocyte (arrow) and lymphocyte (inset, arrowhead) (400×). B — PCV2 antigen is detected in the mononuclear cells (arrows) scattered in the lymphoid tissue (1000×). C — PRRSV antigen is detected in the lymphocytes and macrophages scattered in the myocardium (1000×). D — PRRSV-positive mononuclear cells are present in the lymph node (400×).
Body fluids, fetal tissues (brain, heart, kidney, liver, lung, lymph node, spleen, thymus, and tonsil), mummified fetuses from 6 litters, and the serum of 1 sow (the sow with litter 1) were also collected and frozen for laboratory tests. Multiplex PCR assays for detection of 6 swine abortifacient viruses, namely CSFV, JEV, PCV2, PPV, PRRSV, and PRV, and 2 additional PCR assays for detecting the encephalomyocarditis virus and Menangle virus were performed on pooled frozen fetal tissues, fetal body fluids, mummies from each litter, and serum from the sow, as previously described (20–22). Both positive and negative controls were included in each PCR assay of the multiplex PCR and worked appropriately. The samples were PCR-negative for all the tested viruses except for PCV2 and PRRSV. The fetal tissues from litters 2 and 4 were PCR-positive for both PCV2 and PRRSV, whereas PCV2 alone was detected in litters 1 and 3. The PCR amplicons with expected sizes for PCV2 and PRRSV were confirmed by sequencing. To detect protozoan pathogens, PCR assays were performed on the fetal hearts from the 3 affected litters by using self-designed primers for Toxoplasma gondii (obtained from the Laboratory of Molecular Pathobiology, School of Veterinary Medicine, National Taiwan University) and primers for Neospora caninum (23). The results were negative for both pathogens. Bacterial culture was performed on the fetal lungs, livers, stomach contents, and placenta from litter 1, and no pathogenic bacteria were isolated.
Coinfection of PCV2 and PRRSV was identified in 1 litter (litter 4). Coinfection of these 2 pathogens was suspected in another litter (litter 2); however, severe autolysis and mummification of the fetuses precluded histopathological examination and IHC. PCV2 infection alone was highly suspected in 2 other litters (litters 1 and 3) based on the presence of cardiac lesions and positive PCR result. However, IHC was not performed to validate the presence of PCV2 antigen in the lesion for these 2 litters. Specific lesions or pathogens were not identified in the remaining 2 litters.
Discussion
The first case of coinfection of PCV2 and PRRSV in stillborn pigs and nonviable neonates was reported in Canada by using PCR (5); however, only PCV2 antigen was associated with the lesions in the affected hearts by IHC staining. The PCR and IHC staining are the mainstay in routine diagnosis. In contrast to PCR, IHC staining also provides cellular detail and histological architecture while detecting the viral antigen in the same tissue section. In the present case, coinfection of PCV2 and PRRSV was diagnosed in stillborn pigs based on the clinical signs and the detection of both viral antigens in cardiac lesions and lymph node through IHC staining and PCR. Although the contributions of PCV2 and PRRSV to reproductive failure in this case are unknown, the concurrent presence of both viruses may reflect a possible interaction between these 2 pathogens. Whether PRRSV enhances the replication of PCV2 in utero or vice versa remains to be investigated.
In this case, the lesions found in different organs may be induced by 1 or both viruses. While mononuclear meningitis, choroid plexitis, vasculitis and interstitial pneumonia are all consistent with previously reported fetal lesions induced by PRRSV (14,24), cardiomegaly with myocarditis is more commonly reported in PCV2-infected fetuses (4,5). The vasculitis in the heart in this case could be caused by PRRSV. Alternatively, although endothelial tropism of PCV2 is more commonly reported in growing and adult pigs (25,26), vasculitis in fetuses induced by PCV2 has also been described (27), suggesting that PCV2 potentially contributed to the vascular lesion. Nonetheless, the endothelial tropism of PCV2 in fetuses remains to be determined. Notably, the mononuclear cells infiltrating the choroid plexus resembled hematopoietic cells, and this phenomenon has been previously observed in pigs experimentally inoculated with PRRSV (28). Although the precise mechanism underlying extramedullary hematopoiesis (EMH) in that study was not elucidated, it has been suggested that the niche environment created by inflammation may support EMH (28). The interstitial pneumonia observed in this study was very mild. A possible explanation for this finding is that fetal macrophages have a reduced capacity to respond to inflammatory stimuli (29).
Most previous studies reported that herds affected by PCV2-associated reproductive failure typically consisted of a high number of gilts, or were PCV2-seronegative herds (4–6). In this case, PCV2 affected the reproductive performance of not only gilts but also of multiparous sows. This phenomenon was observed in a study performed earlier in the same farm (30), and also in other farms occasionally (11). It has been suggested that PCV2-associated reproductive failure is rare in the field because most of the sow population is infection-immune at sexual maturity (13). However, a recent study showed that sows with high serum PCV2-specific IgG level still had PCV2 viremia and delivered PCV2-positive mummified fetuses, stillbirths, and seropositive viable neonates, suggesting that antibody titers against PCV2 may not prevent or reduce PCV2-associated reproductive failure (11). Since the study had only tested for total antibody and did not differentiate neutralizing antibody from non-neutralizing antibody, the total antibody level did not necessarily correlate with the protective effect against the disease. Another study showed that even though sows had high PCV2-specific antibody titers, including both total antibody and neutralizing antibody, they still delivered viremic viable piglets (12). In addition, the concept of cell-associated PCV2 viremia has been suggested (13), which may explain the failure of antibody to prevent the disease. Additional studies are required to clarify the roles of humoral and cellular immune responses against PCV2 in fetal infection and the effect of PCV2 on the reproductive performance of multiparous sows.
The identity of the protozoa-like organism was not confirmed in this case. Toxoplasma gondii is the primary differential diagnosis because it is the most commonly reported protozoan that causes reproductive failure in pigs (31). PCR and histopathology are insensitive methods to detect Toxoplasma because the concentration of protozoal cysts in tissues from pigs is very low (32), which correlates with the histopathological findings in this case and possibly explains the negative PCR results. Nevertheless, other diagnostic techniques were not available at the time of investigation, and the identity of the protozoa-like organism and its role as a cofactor in reproductive failure remain unknown.
This study has several limitations. Serum was only collected from the sow of the first litter because all dams showed no clinical signs during this outbreak of reproductive failure. Moreover, only 1 set of PCR primers was used to detect PRRSV; therefore, false negative results may have occurred due to PRRSV strain variation. Owing to the lack of gross and microscopic evidence supporting bacterial infection, routine bacterial culture was only performed for the first litter. Further testing for abortifacient bacteria, such as Brucella spp. and Leptospira spp., was not performed. Toxoplasma gondii tachyzoite antigen ELISA may increase the likelihood of diagnosing the protozoa-like organism in this case. Noninfectious causes including mycotoxin and environmental and nutritional factors were not investigated in the present case.
Based on the history of late-term pregnancy losses, pathological lesions, and positive results of PCR and IHC, stillbirth in this case appeared to be caused by coinfection with both PCV2 and PRRSV. Multiplex PCR with sample pooling is a useful tool for detecting coinfections, and reduces the cost and effort for initial screening during an outbreak of abortion and stillbirth. Immunohistochemistry further characterizes the role of each pathogen in different histopathological lesions in cases of reproductive failure with coinfections. As for the control strategies implemented as a result of the diagnostic investigation, a batch farrowing program with an all-in-all-out system could potentially decrease the chance of disease transmission during the outbreak. The presence of PCV2-associated reproductive failure in this conventional pig farm may stimulate the producer’s interest in implementing a vaccination program against this agent; however, a comprehensive and peer-reviewed study is warranted to elucidate the effect of vaccination against PCV2-associated reproductive failure.
Acknowledgments
We thank Drs. Hue-Ying Chiou and Wen-Ta Li for assistance with the histopathologic examinations. We also thank Drs. Hui-Wen Chang, Yang-Chang Tu, and Hsuan Kuo for assistance with the laboratory work. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Segalés J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012;164:10–19. doi: 10.1016/j.virusres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Karniychuk UU, Nauwynck HJ. Pathogenesis and prevention of placental and transplacental porcine reproductive and respiratory syndrome virus infection. Vet Res. 2013;44:95. doi: 10.1186/1297-9716-44-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Jung K, Chae C. Prevalence of porcine circovirus type 2 in aborted fetuses and stillborn piglets. Vet Rec. 2004;155:489–492. doi: 10.1136/vr.155.16.489. [DOI] [PubMed] [Google Scholar]
- 4.West KH, Bystrom JM, Wojnarowicz C, et al. Myocarditis and abortion associated with intrauterine infection of sows with porcine circovirus 2. J Vet Diagn Invest. 1999;11:530–532. doi: 10.1177/104063879901100608. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor B, Gauvreau H, West K, et al. Multiple porcine circovirus 2-associated abortions and reproductive failure in a multisite swine production unit. Can Vet J. 2001;42:551–553. [PMC free article] [PubMed] [Google Scholar]
- 6.Pittman JS. Reproductive failure associated with porcine circovirus type 2 in gilts. J Swine Health Prod. 2008;16:144–148. [Google Scholar]
- 7.Maldonado J, Segalés J, Martínez-Puig D, et al. Identification of viral pathogens in aborted fetuses and stillborn piglets from cases of swine reproductive failure in Spain. Vet J. 2005;169:454–456. doi: 10.1016/j.tvjl.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Salogni C, Lazzaro M, Giacomini E, et al. Infectious agents identified in aborted swine fetuses in a high-density breeding area: A three-year study. J Vet Diagn Invest. 2016;28:550–554. doi: 10.1177/1040638716656024. [DOI] [PubMed] [Google Scholar]
- 9.Wang ND, Li JJ, Wang AB, et al. Vertical transmission of PCV2b to fetuses in sows intramuscularly infected with PCV2b. Pol J Vet Sci. 2016;19:471–476. doi: 10.1515/pjvs-2016-0059. [DOI] [PubMed] [Google Scholar]
- 10.Chae C. Porcine circovirus type 2 and its associated diseases in Korea. Virus Res. 2012;164:107–113. doi: 10.1016/j.virusres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Dias AS, Gerber PF, Araújo AS, Auler PA, Gallinari GC, Lobato ZIP. Lack of antibody protection against Porcine circovirus 2 and Porcine parvovirus in naturally infected dams and their offspring. Res Vet Sci. 2013;94:341–345. doi: 10.1016/j.rvsc.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Gerber PF, Garrocho FM, Lana AMQ, Lobato ZIP. Fetal infections and antibody profiles in pigs naturally infected with porcine circovirus type 2 (PCV2) Can J Vet Res. 2012;76:38–44. [PMC free article] [PubMed] [Google Scholar]
- 13.Pensaert MB, Sanchez RE, Jr, Ladekjær-Mikkelsen A-S, Allan GM, Nauwynck HJ. Viremia and effect of fetal infection with porcine viruses with special reference to porcine circovirus 2 infection. Vet Microbiol. 2004;98:175–183. doi: 10.1016/j.vetmic.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Opriessnig T, Langohr I. Current state of knowledge on porcine circovirus type 2-associated lesions. Vet Pathol. 2013;50:23–38. doi: 10.1177/0300985812450726. [DOI] [PubMed] [Google Scholar]
- 15.Rossow KD, Laube KL, Goyal SM, Collins JE. Fetal microscopic lesions in porcine reproductive and respiratory syndrome virus-induced abortion. Vet Pathol. 1996;33:95–99. doi: 10.1177/030098589603300115. [DOI] [PubMed] [Google Scholar]
- 16.Segalés J, Allan GM, Domingo M. Porcine circovirus diseases. Anim Health Res Rev. 2005;6:119–142. doi: 10.1079/ahr2005106. [DOI] [PubMed] [Google Scholar]
- 17.Novakovic P, Harding JC, Al-Dissi AN, Ladinig A, Detmer SE. Pathologic evaluation of type 2 porcine reproductive and respiratory syndrome virus infection at the maternal-fetal interface of late gestation pregnant gilts. PLoS One. 2016;11:e0151198. doi: 10.1371/journal.pone.0151198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson JE, Yaeger MJ, Christopher-Hennings J, Lager K, Yoon KJ. A comparison of virus isolation, immunohistochemistry, fetal serology, and reverse-transcription polymerase chain reaction assay for the identification of porcine reproductive and respiratory syndrome virus transplacental infection in the fetus. J Vet Diagn Invest. 2002;14:8–14. doi: 10.1177/104063870201400103. [DOI] [PubMed] [Google Scholar]
- 19.Njaa BL, editor. Kirkbride’s Diagnosis of Abortion and Neonatal Loss in Animals. 4th ed. Ames, Iowa: Wiley-Blackwell; 2012. p. 222. [Google Scholar]
- 20.Zeng Z, Liu Z, Wang W, Tang D, Liang H, Liu Z. Establishment and application of a multiplex PCR for rapid and simultaneous detection of six viruses in swine. J Virol Methods. 2014;208:102–106. doi: 10.1016/j.jviromet.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Kassimi LB, Gonzague M, Boutrouille A, Cruciere C. Detection of encephalomyocarditis virus in clinical samples by immunomagnetic separation and one-step RT-PCR. J Virol Methods. 2002;101:197–206. doi: 10.1016/s0166-0934(01)00439-6. [DOI] [PubMed] [Google Scholar]
- 22.Bowden TR, Bingham J, Harper JA, Boyle DB. Menangle virus, a pteropid bat paramyxovirus infectious for pigs and humans, exhibits tropism for secondary lymphoid organs and intestinal epithelium in weaned pigs. J Gen Virol. 2012;93:1007–1016. doi: 10.1099/vir.0.038448-0. [DOI] [PubMed] [Google Scholar]
- 23.Müller N, Zimmermann V, Hentrich B, Gottstein B. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J Clin Microbiol. 1996;34:2850–2852. doi: 10.1128/jcm.34.11.2850-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halbur P, Paul P, Frey M, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 25.Opriessnig T, Janke BH, Halbur PG. Cardiovascular lesions in pigs naturally or experimentally infected with porcine circovirus type 2. J Comp Pathol. 2006;134:105–110. doi: 10.1016/j.jcpa.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Szeredi L, Dán Á, Solymosi N, Cásgola A, Tuboly T. Association of porcine circovirus type 2 with vascular lesions in porcine pneumonia. Vet Pathol. 2012;49:264–270. doi: 10.1177/0300985811406888. [DOI] [PubMed] [Google Scholar]
- 27.Szeredi L, Cságola A, Dán Á, Dencső L. Vascular lesions and pneumonia in a pig fetus infected by porcine circovirus type 2. Acta Vet Hung. 2015;63:215–222. doi: 10.1556/004.2015.019. [DOI] [PubMed] [Google Scholar]
- 28.Johns JL, Christopher MM. Extramedullary hematopoiesis: A new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Pathol. 2012;49:508–523. doi: 10.1177/0300985811432344. [DOI] [PubMed] [Google Scholar]
- 29.Rowland RR. The interaction between PRRSV and the late gestation pig fetus. Virus Res. 2010;154:114–122. doi: 10.1016/j.virusres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang KH. Investigation of the causes of abortion and stillbirth in swine breeding herds [masters thesis] Taipei, Taiwan: National Taiwan University; 2013. [Google Scholar]
- 31.Dubey JP. Toxoplasmosis in pigs — the last 20 years. Vet Parasitol. 2009;164:89–103. doi: 10.1016/j.vetpar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Dubey JP. Toxoplasmosis of Animals and Humans. 2nd ed. Boca Raton, Florida: CRC Press; 2010. pp. 145–159. [Google Scholar]