Abstract
Background
Randomized trials can compare economic as well as clinical outcomes, but economic data are difficult to collect. Linking clinical trial data with Medicare claims could provide novel information on health care utilization and cost.
Methods
We linked data from Medicare claims of women ≥65 years old who had Medicare fee-for-service coverage with their clinical data from the Women’s Health Initiative (WHI) trials of conjugated equine estrogens plus medroxyprogesterone acetate (CEE+MPA) versus placebo and of CEE-alone versus placebo.
The primary outcome was total Medicare spending during the intervention phase of the trial, and the secondary outcomes were spending on diseases hypothesized a priori to be sensitive to the effects of hormone therapy.
Results
In the CEE+MPA trial, 4,557 participants ≥65 years old were included. Women randomly assigned to CEE+MPA had 4% higher mean Medicare spending overall ($45,690 vs. $43,920, p=0.08), but 0.5% lower spending for hormone sensitive diseases ($3,526 vs. $3,547, p=0.07), with 73% higher spending for coronary heart disease (p=0.045) and 122% higher spending for pulmonary embolism (p=0.026). In the CEE-alone trial, 3,107 participants were included. Total spending among women randomly assigned to CEE was 3.3% higher ($75,411 vs. $72,997, p=0.16), and 1.7% higher spending for hormone sensitive diseases ($5,213 vs. $5,127, p=0.57), but with 39% lower spending for hip fracture (p<0.03).
Conclusions
Menopausal hormone therapy increased spending for some diseases, but decreased spending for others. These offsetting effects led to modest (3% to 4%), non-significant increases in overall spending among women aged 65 years and older.
Introduction
Randomized clinical trials are accepted as the best method to assess health interventions. As planned experiments comparing an intervention with an alternative, randomized trials provide unbiased data to guide clinical practice. The impact of interventions on the cost of health care is increasingly important, so randomized trials have begun to collect economic outcome data, such as utilization of key services and cost, alongside collection of clinical outcome data. The addition of economic outcomes to clinical trials has provided valuable information about a number of cardiac interventions (1–6). Nonetheless, most randomized trials do not collect economic data prospectively, so practical methods to assess this outcome, even after trial completion, could be valuable.
One attractive way to document economic outcomes is to take advantage of administrative claims data about the health care services provided to trial participants. Claims data are collected routinely, are subject to a number of quality control measures, and can be linked to individual participant data from clinical research studies. Medicare claims have been linked to clinical registries to provide follow-up on outcomes such as hospitalizations and death (7), but have not typically been used to examine economic outcomes in clinical trials.
The Women’s Health Initiative (WHI) provides an outstanding resource to examine health outcomes, based on several landmark randomized trials and a large, detailed observational database. Medicare claims for participants aged 65 years and older been linked to data from the WHI studies, and their reliability in identifying several key clinical outcomes has been established (8–11). The linked WHI Medicare data also provide information that would otherwise be unavailable about health care utilization and health care costs among WHI participants. The impact of hormone therapy on economic outcomes has not been documented in a clinical trial, so the purpose of this study was to compare Medicare spending among women aged 65 years and older who were randomized to menopausal hormone therapy or to placebo in the WHI.
Methods
The two parallel WHI hormone trials used different interventions based on whether participants had undergone a hysterectomy prior to randomization. Eligible post-menopausal women with an intact uterus were randomized to either conjugated equine estrogens plus medroxyprogesterone acetate (CEE+MPA) or placebo, whereas women with a prior hysterectomy were randomized to either conjugated equine estrogens or placebo (CEE-alone). The methods and main results of these trials have been previously described (12–18), and are summarized in the Appendix.
The analytic cohort for this study consisted of women aged 65 years and older at randomization who had Medicare Parts A and B coverage, the traditional fee-for-service portion of Medicare. Trial data from WHI participants were securely linked with each participant’s Medicare claims data using social security numbers, dates of birth and, in some cases, dates of death or residential zip codes. Health care spending was obtained from carrier claims (physician and supplier files (Part B)), inpatient and skilled nursing facility claims (Medicare Provider Analysis and Review files (Part A)), outpatient claims (data submitted by institutional outpatient providers, such as hospital outpatient departments and ambulatory surgery centers), hospice, home health, and durable medical equipment claims.
The primary outcome for this study was the cumulative Medicare spending for each participant from date of randomization to the end of the intervention phase of the WHI hormone trial in which she was enrolled. The secondary outcomes were: cumulative spending for disease-specific categories hypothesized a priori to be sensitive to hormone therapy based on the trials’ “global index”: coronary heart disease, stroke, pulmonary embolism, invasive breast cancer, endometrial cancer, colorectal cancer, and hip fracture (see Appendix Table 2 for diagnosis codes); cumulative spending across all disease categories in the global index; and cumulative spending for each participant after the conclusion of the intervention phase of the WHI hormone trial.
We tested for differences in cumulative spending during the intervention phases of the trials using the non-parametric Wilcoxon rank sum test. We also described cumulative total spending over time using an actuarial method adapted for cost data (1), which is analogous to the Kaplan-Meier method for binary endpoints to accommodate variable follow-up with censoring, and used 1,000 bootstrapped samples to obtain 95% confidence limits (CL).
Patients were censored from the analysis at the time of loss of continuous Medicare Parts A and B coverage, or study withdrawal, or death. Follow-up in the intervention phase was defined as from randomization (between 1993 and 1998) to July 7, 2002 for the CEE+MPA trial, and from randomization to February 29, 2004 for the CEE-alone trial. Participants continued to be followed in Medicare data until December 31, 2012. Spending was adjusted to 2016 US dollars using the global Consumer Price Index, but not discounted.
Results
CEE+MPA Trial
In the CEE+MPA trial, 7,303 of the total 16,608 participants were aged 65 years or older at randomization, with 93% enrolled in Medicare. A total of 4,557 participants, 62% of those age-eligible, had Medicare Part A and Part B coverage and formed the analytic cohort (Figure 1). Study participants were generally similar to the remaining age-eligible women randomized in the CEE+MPA trial (Table 1), although they were more likely to be white (92% vs. 85%) and taking aspirin (28% vs. 26%), and less likely to be taking statins (9% vs. 11%) and hormone therapy at study entry (2% vs. 5%). Baseline clinical characteristics of the study participants were also similar between women randomized to hormone therapy or to placebo (Appendix Table 3), although there were small imbalances in history of MI and stroke.
Figure 1.
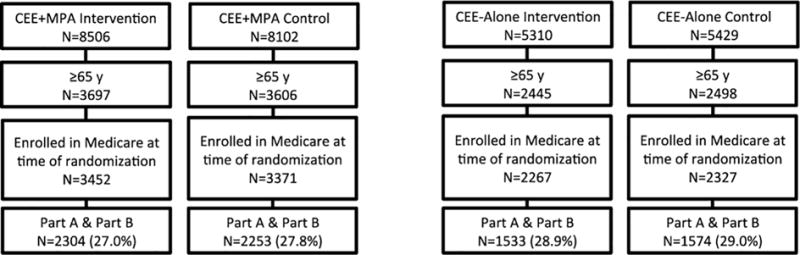
Study Flow
Table 1.
Baseline characteristics of women age ≥ 65 by Medicare fee-for-service coverage (Part A/B) status at the time of entry into the Women’s Health Initiative hormone therapy clinical trials.
| CEE+MPA Trial | CEE-Alone Trial | |||||
|---|---|---|---|---|---|---|
| Has Part A/B (n=4557) |
Does not have Part A/B (n=2746) |
P value | Has Part A/B (n=3107) |
Does not have Part A/B (n=1836) |
P value | |
| Age at screening, y | 70.0±3.7 | 70.1±3.8 | 0.44 | 70.3±3.7 | 70.1±3.7 | 0.044 |
| Age stratum, y | ||||||
| 60-69 | 51.3 | 50.6 | 0.68 | 46.5 | 50.2 | 0.036 |
| 70-79 | 48.7 | 49.4 | 53.5 | 49.8 | ||
| Race | ||||||
| White | 92.0 | 85.4 | <0.0001 | 84.7 | 78.2 | 0.92 |
| Black | 4.2 | 4.9 | 10.7 | 12.6 | ||
| Hispanic | 1.5 | 4.4 | 2.1 | 4.7 | ||
| Asian | 1.3 | 3.6 | 1.1 | 2.2 | ||
| American Indian | 0.1 | 0.3 | 0.4 | 0.6 | ||
| Other | 0.9 | 1.4 | 1.0 | 1.7 | ||
| Years since menopause, y | ||||||
| <10 | 3.2 | 2.8 | 0.60 | 2.3 | 3.5 | 0.05 |
| 10-<20 | 47.5 | 47.0 | 22.1 | 22.7 | ||
| ≥ 20 | 49.3 | 50.2 | 75.6 | 73.8 | ||
| Hormone use | ||||||
| Never | 78.4 | 73.8 | <0.0001 | 54.5 | 50.1 | <0.0001 |
| Past | 19.3 | 20.8 | 38.6 | 38.1 | ||
| Current | 2.3 | 5.4 | 6.9 | 11.8 | ||
| Vasomotor symptoms | 60.7 | 60.1 | 0.59 | 61.9 | 59.9 | 0.18 |
| Body mass index (kg/m2) | 27.9±5.4 | 28.0±5.5 | 0.38 | 29.1±5.5 | 29.3±5.7 | 0.26 |
| Systolic blood pressure (mmHg) | 132.8±18.3 | 132.0±18.0 | 0.12 | 134.3±18.5 | 134.4±17.7 | 0.82 |
| Diastolic blood pressure (mmHg) | 75.2±9.5 | 74.7±9.3 | 0.02 | 75.4±9.6 | 75.3±9.8 | 0.73 |
| Smoking | ||||||
| Never | 52.9 | 52.4 | 0.71 | 55.6 | 53.1 | 0.23 |
| Past | 40.5 | 40.5 | 27.3 | 39.5 | ||
| Current | 6.6 | 7.1 | 7.1 | 7.4 | ||
| Never pregnant | 92.1 | 93.0 | 0.17 | 93.3 | 92.9 | 0.62 |
| Age at time of first birth, y | ||||||
| <20 | 11.9 | 11.7 | 0.99 | 18.6 | 20.0 | 0.06 |
| 20-29 | 75.4 | 75.3 | 74.0 | 72.8 | ||
| ≥ 30 | 12.7 | 13.0 | 7.4 | 7.2 | ||
| Bilateral oophorectomy | 0.3 | 0.4 | 0.71 | 43.9 | 44.2 | 0.85 |
| Medications at baseline | ||||||
| Diabetes class | 4.0 | 4.5 | 0.29 | 7.3 | 7.7 | 0.58 |
| Statin class | 9.0 | 10.6 | 0.033 | 10.8 | 11.1 | 0.79 |
| Salicylate class | 28.0 | 25.5 | 0.021 | 28.3 | 25.5 | 0.034 |
| Past Medical History | ||||||
| Hypertension ever | 34.9 | 35.8 | 0.38 | 44.4 | 45.5 | 0.45 |
| MI ever | 2.6 | 3.1 | 0.20 | 4.0 | 4.9 | 0.13 |
| Angina ever | 5.4 | 6.5 | 0.05 | 9.8 | 9.9 | 0.92 |
| CABG/PCI | 2.2 | 1.9 | 0.36 | 3.5 | 2.9 | 0.24 |
| Stroke | 1.0 | 1.7 | 0.016 | 2.0 | 2.2 | 0.53 |
| Venous thromboembolism | 0.9 | 1.1 | 0.33 | 1.6 | 1.3 | 0.35 |
| Fracture at age 55+ * | 24.7 | 23.8 | 0.46 | 23.9 | 22.5 | 0.29 |
| Family history of breast cancer** | 36.1 | 35.7 | 0.48 | 36.4 | 36.9 | 0.81 |
| Region | ||||||
| Northeast | 24.2 | 23.8 | 23.6 | 23.4 | 0.84 | |
| South | 21.3 | 21.0 | 0.91 | 24.1 | 23.1 | |
| Midwest | 24.3 | 25.0 | 24.3 | 24.9 | ||
| West | 30.2 | 30.2 | 28.0 | 28.6 | ||
| Family Income ≥ $50,000 | 21.6 | 20.7 | 0.34 | 16.2 | 15.1 | 0.32 |
| Education > high school degree or | 71.7 | 72.8 | 0.33 | 65.7 | 66.8 | 0.43 |
| GED | ||||||
Missing 15% data.
Missing 55% data.
The study participants had a median follow-up during the intervention phase of 5.1 years (25th to 75th percentiles = 4.3 to 6.0 years), with 669 women (14.7%) censored due to loss of Medicare fee-for-service coverage (324 assigned to CEE+MPA, 345 assigned to placebo). Overall Medicare spending was slightly (4%), but not significantly, higher among those assigned to CEE+MPA therapy (mean $45,690) than to placebo (mean $43,920, p=0.08, Table 2). Using the actuarial (lifetable) method (Figure 2), cumulative spending at seven years was $59,076 (CL $51,391 to $67,257) in the CEE+MPA group, and $56,567 (CL $49,263 to $64,256) in the placebo group. Spending for the disease-specific categories in the “global index” did not differ significantly (0.5% lower in the CEE+MPA group, p=0.07, Table 2), but spending on these diagnoses comprised only 8% of overall Medicare spending (Figure 3). Spending for pulmonary embolism was 122% higher (p=0.026), and spending for coronary heart disease was 73% higher (p=0.045) in the women assigned to CEE+MPA therapy (Table 2).
Table 2.
Unadjusted total and disease-specific spending, during the intervention phase of the Women’s Health Initiative hormone therapy clinical trials (2016 US dollars).
| With Any Spending (%)
|
Mean Spending
|
|||||
|---|---|---|---|---|---|---|
| CEE+MPA | Placebo | CEE +MPA | Placebo | % Diff | P-value | |
| (n=2304) | (n=2253) | |||||
| Total spending | 98.0 | 97.3 | $45,690 | $43,920 | 4.0 | 0.08 |
| Global index spending | 19.3 | 17.2 | 3,526 | 3,547 | −0.5 | 0.07 |
| Stroke | 8.5 | 7.6 | 773 | 633 | 22.1 | 0.24 |
| Coronary heart disease | 3.9 | 2.8 | 681 | 393 | 73.3 | 0.045 |
| Pulmonary embolism | 1.7 | 1.0 | 169 | 76 | 122.3 | 0.026 |
| Invasive breast cancer | 4.0 | 3.2 | 1,317 | 1,349 | −2.4 | 0.13 |
| Endometrial cancer | 0.8 | 0.8 | 62 | 171 | −63.3 | 0.66 |
| Colorectal cancer | 1.6 | 2.2 | 246 | 509 | −51.6 | 0.18 |
| Hip fracture | 1.9 | 2.3 | 276 | 413 | −33.2 | 0.34 |
| (n=1533) | (n=1574) | |||||
| Total spending | 98.3 | 97.3 | $75,411 | $72,997 | 3.3 | 0.16 |
| Global index spending | 26.0 | 24.8 | 5,213 | 5,127 | 1.7 | 0.57 |
| Stroke | 13.4 | 12.0 | 1,158 | 1,458 | −20.5 | 0.26 |
| Coronary heart disease | 6.6 | 5.1 | 1,125 | 636 | 76.9 | 0.07 |
| Pulmonary embolism | 2.3 | 2.0 | 117 | 146 | −19.8 | 0.47 |
| Invasive breast cancer | 3.6 | 4.5 | 1,484 | 1,600 | −7.3 | 0.22 |
| Colorectal cancer | 2.9 | 2.4 | 888 | 722 | 22.9 | 0.36 |
| Hip fracture | 2.6 | 3.9 | 439 | 723 | −39.3 | 0.029 |
Figure 2.
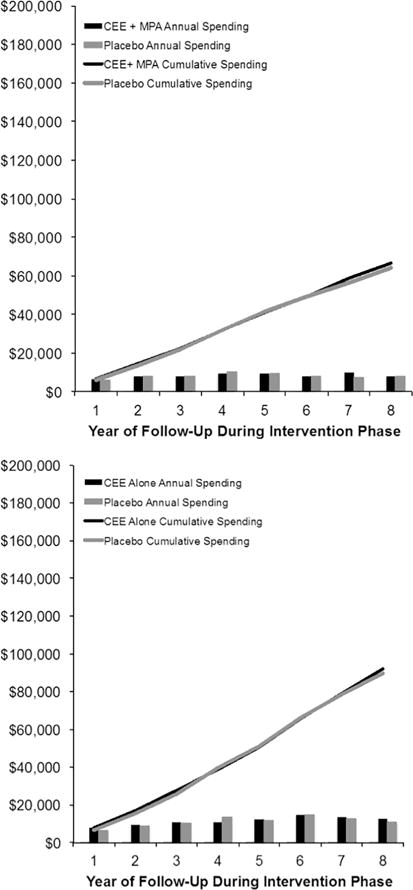
Cumulative costs during the intevention phases of the CEE+MPA trial and CEE-alone trials. The vertical axis indicates cumulative spending in 2016 U.S. dollars, and the horizontal axis indicates the years of follow-up after randomization. The incremental costs in each follow-up year are indicated by the bar graphs at the bottom of the figure. The right panel provides data on the CEE+MPA trial, and the right panel provides data on the CEE-alone trial.
Figure 3.
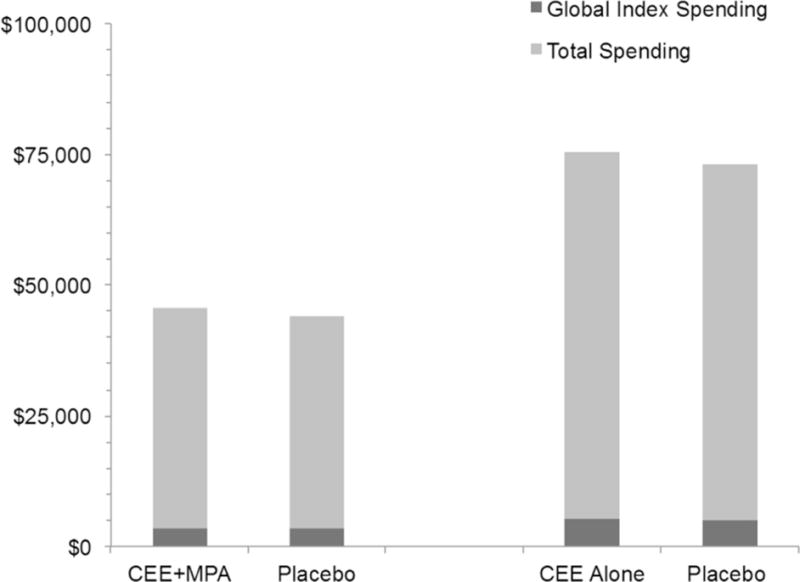
Mean total and hormone sensitive spending during the intervention phases of the CEE+MPA trial (median follow 5.1 years) and the CEE-alone trial (median follow-up 6.7 years). The global index spending includes coronary heart disease, invasive breast cancer, pulmonary embolism, stroke, colorectal cancer, hip fracture and, for the CEE+MPA trial, endometrial cancer.
Combining data from the intervention phase with post-intervention phase provided a median follow-up of 14.4 years (interquartile range 8.6 to 15.8 years). Overall spending was 0.6% higher in the women assigned to CEE+MPA ($191,313 vs. $190,195, p=0.36), with 24% higher spending on breast cancer (Table 3, Figure 4). A landmark analysis of costs after the end of the active intervention phase showed no significant differences in either overall spending or spending on the diseases in the global index of hormone sensitive diseases (Appendix Table 4).
Table 3.
Unadjusted total and disease-specific spending for the combined intervention and post-intervention phases (cumulative follow-up) of the Women’s Health Initiative hormone therapy clinical trials (2016 USD)
| With Any Spending (%)
|
Mean Spending
|
|||||
|---|---|---|---|---|---|---|
| CEE+MPA | Placebo | CEE +MPA | Placebo | % Diff | P-value | |
| (n=2304) | (n=2253) | |||||
| Total spending | 98.3 | 97.5 | $191,313 | $190,195 | 0.6 | 0.36 |
| Global index spending | 43.3 | 42.7 | 11,801 | 13,008 | −9.3 | 0.72 |
| Stroke | 21.7 | 21.3 | 2,407 | 2,588 | −7.0 | 0.54 |
| Coronary heart disease | 9.6 | 10.3 | 1,612 | 1,467 | 9.9 | 0.51 |
| Pulmonary embolism | 4.3 | 4.1 | 535 | 313 | 7.1 | 0.63 |
| Invasive breast cancer | 8.4 | 6.7 | 4,115 | 3,329 | 23.6 | 0.028 |
| Endometrial cancer | 1.3 | 2.0 | 332 | 948 | −65.0 | 0.10 |
| Colorectal cancer | 3.7 | 4.3 | 1,036 | 2,060 | −49.7 | 0.28 |
| Hip fracture | 9.5 | 10.6 | 1,761 | 2,299 | −23.4 | 0.19 |
| CEE-Alone (n=1533) |
Placebo (n=1574) |
CEE-Alone | Placebo | % Dif | P-value | |
| Total spending | 98.5 | 97.6 | $221,628 | $214,830 | 3.2 | 0.40 |
| Global index spending | 44.3 | 44.8 | 12,885 | 11,140 | 15.7 | 0.87 |
| Stroke | 24.6 | 24.3 | 2,791 | 3,029 | −7.9 | 0.97 |
| Coronary heart disease | 12.2 | 10.2 | 2,215 | 1,448 | 52.9 | 0.06 |
| Pulmonary embolism | 4.6 | 5.2 | 312 | 387 | −19.3 | 0.44 |
| Invasive breast cancer | 6.8 | 7.6 | 4,133 | 2,957 | 39.8 | 0.39 |
| Colorectal cancer | 4.4 | 4.5 | 1,448 | 1,304 | 11.0 | 0.99 |
| Hip fracture | 9.7 | 10.4 | 1,983 | 2,013 | −1.5 | 0.60 |
Figure 4.
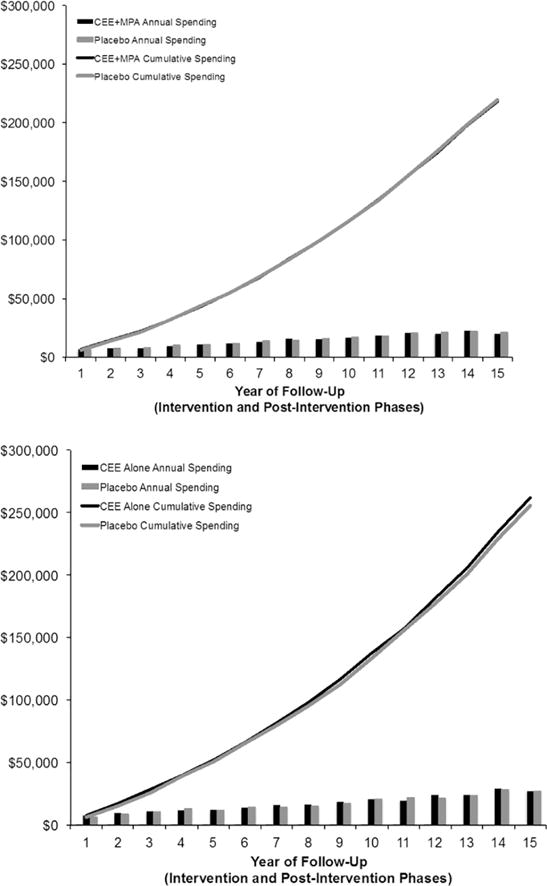
Cumulative spending over total follow-up, including both the intervention phase and pos-intevention phase. Format as in Figure 2.
CEE-Alone Trial
In the CEE-alone trial, 4,943 of the total 10,739 randomized participants were 65 years of age, with 93% enrolled in Medicare. A total of 3,107 women, 62% of those age-eligible, had coverage in Medicare Parts A or B and formed the analytic cohort (Figure 1). Study participants were generally similar to the remaining age-eligible women in the CEE-alone trial (Table 1), apart from being slightly older (70.3 vs. 70.1 years), more likely to be taking aspirin (28% vs. 26%), and less likely to be taking hormone therapy at entry (7% vs. 12%). The baseline characteristics of randomly assigned study participants were quite similar, except more women assigned to CEE were 20 or more years since menopause (Appendix Table 2).
The study participants had median follow-up during the intervention phase of 6.7 years (interquartile range 5.8 to 7.5), with 537 of women (17.3%) censored due to loss of Medicare fee-for-service coverage. (260 assigned to CEE, 277 assigned to placebo). Overall cumulative Medicare spending was slightly (3%), but not significantly higher among participants assigned to CEE therapy (mean $75,411) compared with placebo ($72,997, p=0.16). Using the actuarial method, cumulative spending at seven years was $80,214 (CL $70,707 to $90,053) in the CEE group, and $76,449 (CL $67,407 to $85,749) in the placebo group (Figure 2). Total spending in the global index of disease categories hypothesized to be sensitive to hormone therapy was 1.7% higher (p=0.57) among women assigned to hormone therapy, but this spending comprised only 7% of overall spending (Figure 3). Spending within the individual disease categories was 39% lower for hip fracture (p=0.029) among women assigned to CEE therapy (Table 2).
Cumulative spending over the entire follow-up (median 13.5 years interquartile range 7.6 to 15.7 years), including the intervention and post-intervention phases, was 3.2% higher in the CEE assigned women (p=0.40), with no significant differences in the global index or any disease category (Table 3, Figure 4). In a landmark analysis of costs after the completion of the active intervention phases of the trials, there was no difference in overall spending or spending on the global index of hormone sensitive diseases (Appendix Table 3).
Discussion
Linkage of data from randomized clinical trials with Medicare claims provides additional information about the outcomes of trial participants. In this study, we used linked data to document Medicare spending among age-eligible WHI trial participants, a valuable outcome measure that was not collected as part of the original trial protocol. Hormone therapy increased overall Medicare spending only slightly and non-significantly (by 3% to 4%) during the intervention phases of the WHI clinical trials. Disease-specific spending, however, was more greatly affected by hormone therapy, and generally paralleled the clinical outcomes of hormone therapy. Spending for pulmonary embolism, for example, was increased by 122% during the intervention phase among participants assigned to CEE+MPA therapy, consistent with the 98% increase in pulmonary embolism found in the trial (19), and spending on coronary heart disease was increased by 73%, in line with the 18% increase in these outcomes during the trial. Similarly, spending on hip fracture was decreased by 39% among in women assigned to CEE-alone therapy, reflecting the 33% lower rate of hip fractures in the CEE-alone trial. The mixed effects of hormone therapy on clinical outcomes led to increased spending for some disease categories, and decreased spending for other categories, such that the offsetting effects led to a smaller net impact of hormone therapy on spending overall and for the global index of hormone sensitive diseases.
Another reason for the relatively small, 3% to 4% effects of hormone therapy on overall spending was because over 90% of all spending was for diagnoses that were unlikely to be affected by use of hormone therapy (Figure 3). This result is perhaps unsurprising in a population of women with an average age of 70 years at randomization. Background medical spending, unrelated to medical conditions affected by treatment, formed the vast majority of overall spending in this study, even though participants in WHI were relatively healthy at the time of study entry.
Studies of economic outcomes might reasonably focus on disease-specific spending in order to separate any “signal” due to the intervention targeted to specific conditions from the “noise” of background medical spending for unrelated conditions. Clinical trials of interventions for heart failure, for example, might assess only the costs of managing heart failure, and ignore the costs of “unrelated” conditions. Focusing on disease-specific costs assumes, however, that the intervention has no important adverse clinical effects, such as increasing cardiac arrhythmias or gastrointestinal bleeding. While this assumption may be reasonable, any intervention may have unanticipated, off-target effects (20, 21). In this study, we found that hormone therapy significantly increased some categories of spending (e.g., pulmonary embolism, coronary heart disease), and significantly decreased other categories of spending (e.g., hip fracture), underscoring the complexity of assessing the net economic impact of a therapy.
The design of this study differs considerably from the economic simulation model by Roth and associates (22), which projected the impact on the health care costs of the United States population if the WHI trials had never been performed. Their model assessed outcomes for all post-menopausal women, not just women ≥65 years of age, as in the present study. Roth and associates projected that use of hormone therapy would lead to higher population costs for venous thromboembolism, breast cancer, ischemic heart disease, and stroke. These patterns of spending are similar to those seen in our empirical study of older women in WHI. Roth and associates also calculated that there would be substantial costs of administering hormone therapy, including the costs of the medication itself, and the health care visits needed to initiate and monitor therapy. These additional costs were not captured in our data, since hormone therapy was provided free of charge in the WHI trials, and the clinic visits and tests to monitor therapy were equalized between the two randomized groups by the study protocol to maintain blinding of the treatment assignment.
Linkage of clinical trial data to claims data can provide new information about both clinical and economic outcomes, which can be novel and potentially very valuable. Clinical outcomes can be assessed over extended follow-up, including conditions not documented prospectively in the trial, such as atrial fibrillation. The Medicare data in this study also extended follow-up by two years beyond the end of clinical follow-up, which could facilitate long-term, routine follow-up in many clinical trials. In addition, comparing the trial participants with members of the general population can assess the generalizability of a clinical trial. The potential advantages of using claims data to capture clinical and economic outcomes are accompanied by several limitations. In the fragmented American healthcare system, the multiple sources of health insurance coverage means that there is no comprehensive, universal source of administrative data on healthcare utilization, so only some patients in a trial can be followed using this method. In the WHI, we were able to study only women 65 years of age and older at entry, and we had no claims data from younger women or from older women without fee-for-service Medicare coverage. As a practical matter, linkage of WHI trial data with Medicare data required an Interagency Agreement between the National Institutes of Health (NIH) and the Centers for Medicare and Medicaid Services (CMS), with cumbersome limitations on use of Medicare files due to government regulations and privacy concerns. There is no comprehensive agreement between the NIH and CMS to link trial data and Medicare data, so each trial would need to establish a separate agreement to perform data linkage. Finally, there is an inevitable delay between the collection of CMS data and their release to researchers, which may impede use of Medicare claims as a method to follow patients in pragmatic clinical trials.
Limitations
This study was limited to women aged 65 years and older with fee-for-service Medicare coverage because their claims data were available to document health care utilization and spending. Women 65 years of age and older comprised less than half of all WHI trial participants, however, and our findings may not apply to younger women. Hormone therapy had generally more favorable effects among younger women than among older women, especially in the CEE-alone trial (18), so its effects on health care costs among younger women are uncertain. Furthermore, younger women have lower overall medical costs, so hormone therapy may lead to proportionately larger changes in spending among younger women. Evaluation of this possibility in the WHI participants would be difficult, however, in light of the multitude of health insurance plans used by younger women. The 37% of WHI participants 65 years of age who did not have fee-for-service Medicare coverage were not included in the analytic cohort. These women were largely enrolled in capitated health plans, such as Kaiser Permanente or Group Health Cooperative, whose data on individual health care utilization and costs are not included in CMS research files. While there were some differences in the baseline clinical characteristics of older women according to their type of Medicare coverage (Table 1), we do not believe there were any major differences in outcomes and their attendant costs in these women compared with the analytic cohort.
We estimated spending for broad categories of diseases that were specified a priori as likely to be affected by hormone therapy, such as coronary heart disease, invasive breast cancer, and hip fracture. We did not evaluate, however, spending patterns for other specific conditions that might have been affected by hormone therapy, such as abnormal uterine bleeding, pelvic organ prolapse, urinary incontinence, vaginal discharge, gall bladder disease, atrial fibrillation, or dementia. Examination of Medicare utilization and spending related to these, and other specific conditions, tests, and procedures, was beyond the scope of the present study, but could be evaluated in future studies. Furthermore, most long-term institutional care of patients with dementia is reimbursed by Medicaid, not by Medicare, and hence was not captured in this study.
Medicare spending in this study occurred between 1993 and 2012, so we adjusted all costs to 2016 dollars using the Consumer Price Index. There are other methods to adjust for inflation, but since any form of adjustment is applied to both treated and control patients, comparisons between the randomized groups should not be greatly affected. When we instead used the Gross Domestic Product deflator to adjust for inflation, overall Medicare spending was about 2.5% lower in all groups, so the between group comparisons (Table 2) were virtually unchanged (data not shown). We did not apply discounting to late follow-up costs, but since the cumulative cost curves were virtually superimposable (Figure 2), application of a 3% per year discount did not affect the relative spending patterns in Table 2, even though it lowered mean overall spending by about 34% in all groups (data not shown). Finally, we did not have Medicare Part D data on spending for prescription drugs, but hormone therapy was provided to WHI participants free of charge. Adding a cost of $1 per day for hormone therapy would have increased overall spending in the CEE+MPA assigned women by roughly $1,900, and in the CEE-alone assigned women by about $2,500.
Conclusions
Hormone therapy in the WHI clinical trials had mixed effects on disease– specific spending, increasing some categories (e.g., pulmonary embolism) while decreasing other categories (e.g., hip fracture). The offsetting effects of hormone therapy on different disorders, along with the high proportion of background medical costs, led to modest, statistically non-significant increases in overall Medicare spending among post-menopausal women assigned to hormone therapy. Whether these results apply to younger women is unknown.
Routine linkage of data from participants in randomized trials with their Medicare claims would provide a powerful tool that leverages the national investment in clinical research. We recommend that the administrative barriers to linking these data be addressed by policy makers at the National Institute of Health and the Center for Medicare and Medicaid Services.
Supplementary Material
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. This analysis was also supported by a grant by the Stanford Center for Clinical and Translational Research and Education, Stanford University, Stanford CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: NCT 00000611
References
- 1.Hlatky MA, Rogers WJ, Johnstone I, et al. Medical care costs and quality of life after randomization to coronary angioplasty or coronary bypass surgery. N Engl J Med. 1997;336:92–99. doi: 10.1056/NEJM199701093360203. [DOI] [PubMed] [Google Scholar]
- 2.Mark DB, Hlatky MA, Califf RM, et al. Cost effectiveness of thrombolytic therapy with tissue plasminogen activator as compared with streptokinase for acute myocardial infarction. N Engl J Med. 1995;332:1418–1424. doi: 10.1056/NEJM199505253322106. [DOI] [PubMed] [Google Scholar]
- 3.Cohen DJ, Bakhai A, Shi C, et al. Cost-effectiveness of sirolimus-eluting stents for treatment of complex coronary stenosis. Results from the sirolimus-eluting balloon expandable stent in the treatment of patients with De Novo native coronary artery lesions (SIRIUS) trial. Circulation. 2004;110:508–514. doi: 10.1161/01.CIR.0000136821.99814.43. [DOI] [PubMed] [Google Scholar]
- 4.Sculpher MJ, Smith DH, Clayton T, et al. Coronary angioplasty versus medical therapy for angina. Health service costs based on the second Randomized Intervention Treatment of Angina (RITA-2) trial. Eur Heart J. 2002;23:1291–1300. doi: 10.1053/euhj.2001.3075. [DOI] [PubMed] [Google Scholar]
- 5.Heart Protection Study Collaborative Group. Cost-effectiveness of simvastatin in people at different levels of vascular disease risk: Economic analysis of a randomised trial in 20,536 individuals. Lancet. 2005;365:1779–1785. doi: 10.1016/S0140-6736(05)63014-0. [DOI] [PubMed] [Google Scholar]
- 6.Mihaylova B, Schlackow I, Herrington W, et al. Cost-effectiveness of simvastatin plus ezetimibe for cardiovascular prevention in CKD: Results of the Study of Heart and Renal Protection (SHARP) Am J Kidney Dis. 2015;67:576–584. doi: 10.1053/j.ajkd.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan JM, Peterson ED, Messenger JC, et al. Linking the National Cardiovascular Data Registry CathPCI Registry with Medicare claims data: Validation of a longitudinal cohort of elderly patients undergoing cardiac catheterization. Circ Cardiovasc Qual Outcomes. 2012;5:134–140. doi: 10.1161/CIRCOUTCOMES.111.963280. [DOI] [PubMed] [Google Scholar]
- 8.Hlatky MA, Ray RM, Burwen DR, et al. Use of Medicare data to identify coronary heart disease outcomes in the Women’s Health Initiative (WHI) Circ Cardiovasc Qual Outcomes. 2014;7:157–162. doi: 10.1161/CIRCOUTCOMES.113.000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez MV, Wang PJ, Larson JC, et al. Effects of postmenopausal hormone therapy on incident atrial fibrillation: The Women’s Health Initiative randomized controlled trials. Circ Arrhythm Electrophysiol. 2012;5:1108–1116. doi: 10.1161/CIRCEP.112.972224. [DOI] [PubMed] [Google Scholar]
- 10.Mell MW, Pettinger M, Proulx-Burns L, et al. Evaluation of Medicare claims data to ascertain peripheral vascular events in the Women’s Health Initiative. J Vasc Surg. 2014;60:98–105. doi: 10.1016/j.jvs.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 11.Lakshminarayan K, Larson JC, Virnig B, et al. Comparison of Medicare claims versus physician adjudication for identifying stroke outcomes in the Women’s Health Initiative. Stroke. 2014;45:815–821. doi: 10.1161/STROKEAHA.113.003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 13.The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 14.Canonico M, Plu-Bureau G, O’Sullivan MJ, et al. Age at menopause, reproductive history, and venous thromboembolism risk among postmenopausal women: The Women’s Health Initiative hormone therapy clinical trials. Menopause. 2014;21:214–220. doi: 10.1097/GME.0b013e31829752e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 16.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. The Women’ Health Initiative randomized trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 17.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women. The Women’s Health Initiative: A randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 18.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: The Women’s Health Initiative randomized trials. Jama. 2017;318:927–938. doi: 10.1001/jama.2017.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293:935–948. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 21.Cirillo DJ, Wallace RB, Rodabough RJ, et al. Effect of estrogen therapy on gallbladder disease. JAMA. 2005;293:330–339. doi: 10.1001/jama.293.3.330. [DOI] [PubMed] [Google Scholar]
- 22.Roth JA, Etzioni R, Waters TM, et al. Economic return from the Women’s Health Initiative estrogen plus progestin clinical trial. A modeling study. Ann Intern Med. 2014;160:594–602. doi: 10.7326/M13-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


