1.0. Introduction
Multiple Myeloma (MM) is characterized by monoclonal proliferation of plasma cells in the bone marrow. Based on cytogenetics, disease can be classified as high risk: t(14;16), t(14;20), and/or del 17p13), intermediate risk: t(4;14) and/or (1q) gain, and standard risk: trisomies, t(11;14) and/or t(6;14).[1] Consensus based treatment guidelines recommend 4 cycles of induction therapy such as bortezomib, lenalidomide and dexamethasone (VRd) followed by autologous stem cell transplantation therapy (ASCT) in transplant eligible patients with newly diagnosed multiple myeloma (NDMM).[1] Some guidelines such as mSMART recommend patients with high risk NDMM should be given carfilzomib based triple combination induction regimen. In patients with second or higher relapses, treatment depends on prior therapy, comorbidities (peripheral neuropathy, renal failure), marrow functioning as indicated by blood counts, and rapidity of relapse. Combination therapy incorporating proteasome inhibitors, immunomodulators, steroids and alkylating agents is generally recommended.[1]
Over the past two decades, advancements in MM therapy have markedly improved disease outcome and overall survival (OS). According to national institute of health (NIH) cancer statistics death rates due to MM have declined on average 0.7% each year over 2005–2014.[2] Despite noticeable improvement in disease outcome, MM remains incurable with high rates of relapses, highlighting the unmet need for new treatment strategies. Proteasome inhibitor bortezomib was approved by the US Food and Drug Administration (FDA) for treatment of multiple myeloma in 2003.[3] Despite its notable efficacy there are serious issues of side effects such as peripheral neuropathy (PNP), which has been reported in up to 30% of patients treated with bortezomib based regimens.[4, 5] Risk of PNP with bortezomib has been mitigated with subcutaneous and once weekly administration. [6] In 2012, carfilzomib (CFZ), a novel proteasome inhibitor, was approved for treatment of relapsed and refractory multiple myeloma (RRMM).[7] CFZ is highly selective, irreversible epoxy-ketone molecule that targets chymotrypsin like activity of 20S proteasome leading to cellular apoptosis which is particularly beneficial in malignant cells. CFZ has minimal off target activity causing fewer side effects including lower rates of PNP.[5, 8] Further, CFZ has demonstrated activity in bortezomib resistant cell lines.[7, 9, 10]. Since 2012, CFZ has been studied in various clinical trials. The aim of our study is to conduct comprehensive literature search for efficacy, dosing and toxicity profile of CFZ in both newly diagnosed and relapsed setting. Our secondary aim is to analyze whether CFZ treatment can be extended to the frontline setting.
2.0. Methods
2.1. Literature search
A comprehensive literature search was performed on 6/5/2017 in the following resources: PubMed, EMBASE, Wiley Cochrane library, Scopus, Web of Science, CINAHL, and Clinicaltrials.gov. Search results were not limited to any geographical area or language, in the case English translations were available. However, studies done only after 2007 were included. Example search strategy is provided in appendix 1. Relevant articles from following conference proceedings were also included: the European Hematology Association, the American Society of Hematology, the American Society of Oncology and the American Society of Bone Marrow Transplantation.
2.2. Eligibility criteria
Studies fulfilling the following criteria were included: (1) Phase II or III clinical trials (2) Clinical trials from last 10 years (Jan 2007 till June 2017) (3) Studies that have efficacy outcomes clearly documented and (4) Studies focusing on CFZ as primary drug therapy.
2.3. Study Selection
Relevant studies were reviewed by three independent reviewers (A. M., A. L., V. K.) based on title and abstract. Potentially relevant articles were screened through full text by afore-mentioned reviewers. Any conflicts were resolved with discussion.
2.4. Data extraction and analysis
Data was extracted on pre-specified tables which included following parameters: Author, year, study design, number of patients, median age, MM staging and cytogenetics, CFZ regimen, dose, median number of cycles, and efficacy outcomes (complete response [CR], near complete response [nCR], stringent complete response [sCR], very good partial response [VGPR], partial response [PR], overall response rate [ORR], overall survival [OS] and progression free survival [PFS]). If desired data was not reported in study, we documented it as not specified (NS).. Data was recorded as median or percentage.
3.0. Results
3.1. Search results
The literature search identified a total of 1835 articles. Additional 4 articles were identified through citation analysis. After excluding 630 duplicate studies, remaining 1209 were screened for relevance based on titles and abstracts. 89 studies were found potentially useful for our study after excluding 1120 studies. After reading full texts of remaining articles, further 63 articles were excluded due to one or more of the following reasons: phase 1 study, interim analysis, not focused on CFZ, full text not available, duplicate study, outcome not measured or not a clinical trial. 26 articles met the inclusion criteria, 15 in NDMM group and 11 in RRMM group. Summary of this selection process is given in PRISMA flow chart (Figure 1).
Fig 1.
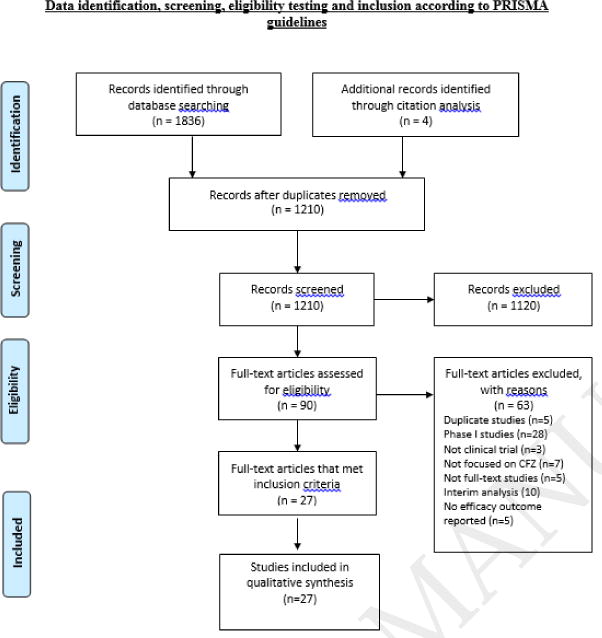
Records identified through PubMed, EMBASE, Cochrane, Web of Science, Scopus, CINAHL and ClinicalTrials.gov database searches. Relevant articles from following conference proceedings were also included: the European Hematology Association, the American Society of Hematology, the American Society of Oncology and the American Society of Bone Marrow Transplantation.
3.2. Study demographics
26 articles included a total of 5980 patients: 4205 in RRMM group and 1775 in NDMM group.
4.0. Group A: Carfilzomib based regimens in newly diagnosed multiple myeloma (Table 1)
Table 1. Carfilzomib based regimens for newly diagnosed multiple myeloma.
| Author, Year, Study Design |
N/RE P |
Medi an age |
ISS STAGING I/II/III/unkn own |
Cytogeneti cs; High Risk/Stand ard Risk/Unkn own |
CFZ Dose mg/m2; No. Of Cycles |
CFZ Regim en |
INDUCTION | ASCT | CONSOLIDATION | ADDITIONAL THERAPY |
OR R (% ) |
O S (% ) |
PFS (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR/nCR/s CR |
VGPR/ PR |
CR/nCR/ sCR |
VGPR/ PR |
CR/nCR/ sCR |
VGPR/ PR |
CR/nCR/ sCR |
VGPR/ PR |
||||||||||
| Carfilzomib based triplet regimens | |||||||||||||||||
| Roussel M,2016,phaseII | 46/46 | 56 | 46/48/6 | 21% high risk | 20/36, induction: 4 consolidation: 4 | KRd | 25.5/NS/25.5 | 58/14 | 69/NS/6 9 | 23.5/5 | 45/NS/4 5 | 43/9.5 | NS | 98 | NS | Not reached | |
| Zimmerman T, 2016,phase II | 75/73 | 59 | (II+III)57 | 35% high risk | 20/36, induction: 4 consolidation: 4 | KRd | NS | NS/NS/2 0 | NS/NS | 73/NS/6 9 | 96/NS | NS/NS/8 2 | NS | 24 M : 99 | 24 M: 97 | ||
| Korde,2013, phase II | 41/38 | 60 | NS | 20/36, median of 9 cycles | CRd | 351 | 50/12 | NS | 68 | 20/8 | NS | 96 | NS | 10 M: 83 | |||
| Jasielec J, 2013, phase II | 53/25 | NS | 33% risk high | 20/36, median of 24 cycles | CRd | NS | 64/NS/552 | NS | 36 M : 76 | 36 M: 96 | |||||||
| Jakubowiak A, 2013, phase I/II | 53/51 | 59 | 40/34/26 | 17/34/2 | 20/27/36 median of 12 cycles | CRd | NS/78/61 | 92/100 | NS | NS/72/62 | 86/100 | NS | 98 | NS | 24 M: 92 | ||
| Sonneveld P, 2012, phase II | 58/40 | 58 | 40/35/25 | NS | 20,27,36, induction: 4 cycles, consolidation: 4 cycles | CTD | 183 | 534 | 253 | 634 | 353 | 704 | NS | 90 | 10 M : 100 | 12 M: 97 | |
| Sonneveld P,2015,phase II | 91/89 | 58 | 42/34/24 | 38/40/22 | 20/27/36/45, induction: 4 consolidation: 4 | KTd | 25/NS/NS | 68/90 | 33/NS/NS | 76/96 | 63/NS/NS | 89/96 | NS | 90 | NS | 36 M: 72 | |
| Wester R, 2016,phase II | 111/111 | 58 | 41/34/23 | NS | 20/27–20/36–20/45–20/56/NS | KTd | 18/NS/18 | 66/94 | 31/NS/31 | 78/95 | 64/NS/NS | 87/95 | NS | 95 | 30 M : 91 | 30 M: 71 | |
| Palumbo A, 2014, phase I/II | 28/25 | 74 | (III)36 | 24/8/NS | 36/45/70, median of 5 cycles | wCCd | 22/NS/NS | 39/83 | NS | 83 | NS | ||||||
| Bringhen S, 2013, phase II | 58/41 | 71 | NS/NS/40 | 35% high risk | 20/36, induction + consolidation: 9 | CCd | NS | 535, sCR: 23 | 77/100 | NS | 12 M : 86 | 12 M: 85 | |||||
| Bringhen S, 2015, phase I/II | 30/28 | 74 | (III)37 | 33% high risk | 45/56/70, median of 8 cycles | wCCd | 17/42/17 | 100/92 | NS | ||||||||
| Moreau P,2012,phase I/II | 70/50 | 72 | 34/31/35 | 18/82/9 | 20/27/36/45/ | CMP | 12/NS/NS | 46/32 | NS | 90 | 36 M : 80 | 21 M | |||||
| median of 9 cycles | |||||||||||||||||
| Harper S,2016,phase III | 955/955 | 72 | NS | 20/36/NS | KMP | NS | 22.3 M | ||||||||||
| Bortezomib dose: 1.3/NS | VMP | 22.1 M | |||||||||||||||
| Carfilzomib based quadruplet regimens | |||||||||||||||||
| Forsberg P,2016,phaseI/II | 72/70 | NS | 20/45/56/NS | Car-BiRD | 136 | 59/20 | NS | 28 | 67/5 | 48 | 50/2 | 94 | NS | ||||
| Mark T.M,2014,phase II | 40/36 | NS | (II+III)58 | 62/NS/NS | 20/45/56/NS | Car-BiRD | 2.8/NS/2.8 | 47/36 | NS | 0/NS/23.8 | 57.1/4.8 | 0/NS/42 | 42.1/5.3 | 92 | NS | 16.8 M7 | |
| Mikhael JR,2015,phase I/II | 64/64 | 63 | 36/42/22 | Intermediate risk:9/61, standard:23 | 20/15/27/36/NS | CYKLONE | NS | 56/18 | 18/9 | 5/3/NS | 51/22 | NS | 91 | 24 M : 96 | 24 M: 76 | ||
Abbreviations: N; number of patients, REP; response evaluated patients, CFZ; carfilzomib, ASCT; autologous stem cell transplant, ORR; overall response rate, PFS; progression free survival, CR; complete response, nCR; near complete response, sCR: stringent complete response, VGPR; very good partial response, PR; partial response, NS: not specified, M; months, Yr; year, CTD; carfilzomib, thalidomide and dexamethasone, CRd; carfilzomib, lenalidomide and dexamethasone, CCd; carfilzomib, cyclophosphamide and dexamethasone, Car-BiRd; carfilzomib, clarithromycin, lenalidomide and dexamethasone, wCCd; weekly carfilzomib, cyclophosphamide and dexamethasone, KTd; carfilzomib, thalidomide and dexamethasone, CYCLONE; carfilzomib, cyclophosphamide, thalidomide and dexamethasone, KRd; carfilzomib, revlimid and dexamethasone, KMP; carfilzomib, melphalan and prednisone, VMP; bortezomib, melphalan and prednisone. 1 nCR/CR/sCR, 2After median number of 29 cycles. Not specified as consolidation or induction, 3≥CR, 4 ≥VGPR 5CR/nCR, 6CR/sCR, 7Only for high risk patients.
4.1. Carfilzomib based triplet regimens
4.1.1. Carfilzomib, lenalidomide and dexamethasone (CRd) (5 studies, n=268)
We summarized five studies [11] [12] [13] [14, 15] with total of 268 NDMM patients who were treated with CRd. Roussel et al. (2016)[11] conducted a phase II study (n=46) to assess efficacy of CRd regimen. All patients underwent 4 cycles of CRd induction, 43 patients received ASCT, 41 patients received further 4 cycles of consolidation therapy followed by 1 year of Len maintenance therapy in 27 patients. The dose of CFZ was 20 mg/m2 on D1, D2 of cycle 1 followed by 36 mg/m2 in subsequent cycles. There was no grade ≥ 3 PNP. Cardiovascular AE were reported in 20 patients including 7 cardiac and 13 thrombotic events.[11]
Zimmerman et al. (2016)[12] in their phase I/II trial studied CRd regimen in 75 NDMM patients. 72 patients received 4 cycles of CRd induction followed by ASCT in 71 patients, 4 cycles of CRd consolidation in 66 patients and 10 additional cycles of CRd maintenance in 44 patients. Response was evaluated in 73 patients. VGPR and CR after consolidation were 96% and 73% respectively. After median follow-up of 17.5 months, PFS and OS of 2 years were 97% and 99% respectively. Peripheral neuropathy of only grade 1 or 2 was observed in 39% of patients. Most commonly reported grade ≥3 adverse events (AE) were lymphopenia (28%), neutropenia (18%) and infections (8%).[12]
Korde et al. (2013).[13] conducted a phase II trial on 41 NDMM patients using CRD. Four cycles of induction with CRd were followed by ASCT in eligible patients. After 4 cycles of consolidation therapy, patients received 24 cycles of Len maintenance. Efficacy and toxicity data was evaluated in 38 patients. 25 out of 38 patients completed 8 cycles of CRd. No incidence of peripheral neuropathy was reported. Cardiovascular events of grade ≥3 were reported in 8% of patient population.[13]
Jasielec et al. (2013)[14] conducted a phase II study in 53 NDMM patients with CRd regimen. 53 patients received a median of 24 cycles followed by Len maintenance for a median of 12 months. After 24 cycles of therapy CR and sCR were 64% and 55% respectively. After follow-up of 31 months 3-year PFS and OS were 76% and 96% respectively. Toxicity data was not available. Estimated PFS and OS in patients with high risk cytogenetics were lower than in patients with standard risk cytogenetics 69% vs 88%, and 83% vs 100% respectively.[14]
Jakubowiak et al. (2012)[15] conducted a phase I/II study in 53 NDMM patients with CRd regimen. Patients received 8 cycles of induction therapy followed by 16 cycles of CRd maintenance therapy (n=36) and off protocol single agent Len therapy (n=5). Observed nCR and VGPR after 12+ cycles were 72% and 100% respectively. After follow-up of 13 months, 2-year PFS was 92%. There was no grade ≥ 3 PNP. In 2014, they presented updated follow-up outcomes in a subgroup of 23 elderly patients with median age of 72 years.[16] All patients were given 24 cycles of CRd with CFZ dose of 20 mg/m2 in 2 patients, 27mg/m2 in 4 patients and 36mg/m2 in 17 patients. Reported ≥minimal response was as follows: All patients achieved at least a PR (100%) and 91% achieved at least a VGPR. CR, nCR, and sCR were 79%, 87% and 65% respectively. After follow-up of 30 months estimated PFS and OS were 80% and 100% respectively.
5 studies on CFZ regimen have reported promising efficacy outcomes with VGPR ranging from 20–96%, CR of 45 to 73%, ORR of 96% to 98%, OS of 83% to 97% and 2-year PFS of 92% to 97%. Triple combination (CRd) maintenance was used in Jakubowiak and Zimmerman et al’s study.
4.1.2. Carfilzomib, thalidomide and dexamethasone (KTd): (3 studies, n=260)
We included three studies.[17] [18, 19] (n=260) that tested KTd in frontline setting.
Sonneveld et al. (2012)[19] conducted a phase II trial on 58 patients with KTD to evaluate efficacy of KTD as frontline treatment for NDMM patients who were transplant eligible. Patients received 4 cycles of induction with KTD followed by high dose melphalan (200mg/m2) and ASCT. After SCT patients underwent 4 cycles of KTD consolidation. Patients were divided into two cohorts. Dose of CFZ was 20mg/m2 on D1/D2 of 1st cycle (in both cohorts) followed by 27mg/m2 (1st cohort) and 36mg/m2 (2nd cohort) in subsequent cycles. 37 patients completed KTD induction, 31 patients underwent SCT followed by consolidation in 17 patients. Peripheral neuropathy grade ≥ 2 was observed in 7 (17%) patients. No hematological toxicity was reported.[19]
Sonneveld et al. (in 2015).[17] conducted a phase II trial (n=91) using KTd as induction and consolidation therapy. Patients were divided into four cohorts based on carfilzomib doses. During induction and consolidation therapy, 20 mg/m2 CFZ was infused over 2–10 minutes on D1/D2 of cycle 1 (all 4 dosing cohorts) which was escalated to 27mg/m2 (1st cohort), 36mg/m2 (2nd cohort), 45mg/m2 (3rd cohort) and 56mg/m2 (4th cohort) in the following cycles. After KTd induction therapy eligible patients received melphalan 200mg/m2 and underwent ASCT. Following ASCT patients received 4 cycles of KTd consolidation therapy. There was no grade ≥ 3 PNP. Cardiac and hematologic AE of grade ≥3 were observed in 5 % of patients.[17]
Wester R et al. (2016)[18] (n=111) conducted a phase II trial using KTd. 4 cycles of KTd induction therapy were initiated at CFZ dose of 20mg/m2 on D1/D2 of 1st cycle in four cohorts followed by dose escalation to 27 mg/m2, 36 mg/m2, 45 mg/m2 in 2nd, 3rd and 4th cohort respectively. After 4 cycles of induction, patients received high dose melphalan (HDM) and ASCT followed by 4 cycles of consolidation therapy. Results in general were comparable between all cohorts. Common AEs were non-hematological. Grade ≥ 3 AE included respiratory disorders (15%) and GIT disorders (13%). Cardiac AEs were seen in 4% of patients.[18]
Results of aforementioned three clinical trials on KTd regimen for NDMM could be summarized to as follows: CR ranging from 35 to 64% after consolidation therapy, VGPR ranging from 70 to 89% after consolidation therapy, ORR ranging from 90 to 95% and 1-year, 2-year and 3-year PFS of 97%, 71% and 72% respectively.
4.1.3. Carfilzomib, cyclophosphamide and low dose dexamethasone (CCd) (3 studies/n=116)
There were three studies[20–22] on CCd including 116 NDMM patients.
Palumbo et al. (2014)[20] conducted a phase I/II study in 28 NDMM patients. During phase I portion, CFZ was given to twelve patients at escalating doses of 36mg/m2, 45 mg/m2 and 70 mg/m2 once weekly for 1 cycle to determine maximum tolerated dose (MTD). During phase II, CFZ at 70 mg/m2 was given once weekly for 9 cycles followed by consolidation therapy with 70mg/m2 of CFZ. Incidences of CR and VGPR after 4 cycles on induction therapy were 83 and 22% respectively. ORR was 87%. Common AE of grade ≥ 3 were hematologic that included neutropenia (12%) and anemia (12%).[20]
Bringhen et al. (2013)[22], conducted a phase II study on 58 patients using CCd regimen. CFZ was used at dose of 20 mg/m2 on D1 /D2 of 1st cycle and 36mg/m2 in subsequent 9 cycles, followed by maintenance with CFZ until progression of disease or intolerance due to toxicity. Response was evaluated in 41 patients after at least 4 cycles and toxicity data was evaluated in 51 patients after at least 1 cycle. Commonly observed non-hematological toxicities of grade ≥ 3 were infections (7%), renal (4%), cardiac (4%), and gastrointestinal complications (2%).
Bringhen S et al. (2015)[21], conducted a phase I/II study in 30 patients using CCd regimen. CFZ at doses of 40mg/m2, 56mg/m2 and 70mg/m2 on days 1, 8 and 15 was used in a 3+3 dose escalation scheme in combination with cyclophosphamide and dexamethasone. In 28 response evaluable patients at the end of 9 cycles VGPR and PR were 92% and 100% respectively. Commonly observed grade ≥3 AEs were neutropenia (10%), acute pulmonary edema (8%), fatigue (3%) and nausea (3%). No PNP was observed.
Data on CCd regimen for NDMM was insufficient and heterogenous. Incidences of CR and VGPR after consolidation therapy was reported 17, 53% and 100, 20 % respectively in 2 trials by Bringhen et al.
4.1.4. Carfilzomib, melphalan and prednisone (CMP)
An ongoing phase III trial (NCT01818752)[23] by Harper et al. has compared CMP with VMP (bortezomib, melphalan and prednisone) in 955 transplant ineligible NDMM patients. After follow-up of 54 weeks median PFS was 22.3 months in CFZ group vs 22.1 months in bortezomib group. Incidence of PNP was 2.5% (CFZ group) vs 35% (bortezomib group). Grade ≥ 2 AEs were seen in 74.7% of patients in CFZ group vs 76.2% of patients in bortezomib group.
Moreau P et al. (2015)[8], conducted a phase I/II study with CMP regimen (CFZ, melphalan and prednisone) in 50 patients. Twenty-four patients were enrolled in phase I and were divided into 4 cohorts based on CFZ dose. IV CFZ was given at doses of 20mg/m2 (1st cohort), 27mg/m2 (2nd cohort), 36mg/m2 (3rd cohort), and 45 mg/m2 (4th cohort) for a 42-day cycle. During phase I MTD was defined as 36mg/m2. Further 44 patients were enrolled in phase II portion of the study. The most common hematologic AEs were neutropenia (38%), anemia (35%) and thrombocytopenia (28%).
4.2. Carfilzomib based quadruplet regimens
4.2.1. Car-BiRD (carfilzomib, clarithromycin, lenalidomide and low dose dexamethasone): (2 studies/n= 112)
Forsberg et al. (2016)[24] conducted a phase II trial with Car-BiRd regimen in 72 patients. During induction phase, patients received CFZ and dex followed by ASCT. Following ASCT, 57 patients underwent consolidation with BiRD (Clarithromycin, Len and dex) regimen. After consolidation, 54 patients received 2-year lenalidomide (Len) maintenance. CFZ was used at dose of 20mg/m2 for 30 minutes during D1/D2 of 1st cycle followed by 45mg/m2 which was escalated to 56 mg/m2 after enrolment of 1st 26 patients until maximum response was achieved. Results were evaluated in 70 patients. ORR was achieved in 94% patients. The rate of CR/sCR of 13% with Kd induction improved to 28% with BiRD consolidation and to 48% with Len maintenance. Most of the observed AEs were of grade 1 or 2. PNP was seen in 31% of patients, all grade 1 or 2. Commonly observed grade ≥3 toxicities were infections (17%).[24]
Mark et al. (2014) [25] conducted a phase II trial in 40 NDMM patients with Car-BiRd therapy. Patients received IV CFZ at dose of 20mg/m2 for 30 minutes during D1/D2 of 1st cycle and 45mg/m2 in subsequent cycles. After enrolment of 26 patients, dose was escalated to 56 mg/m2. ASCT was performed in eligible patients and consolidation phase with BiRd was initiated. While transplant ineligible patients were started directly with BiRd therapy followed by Len maintenance. Results were evaluated in 36 patients. Grade ≥2 AEs seen were cardiovascular (5%) and renal (8%).[25] Treatment with Car-BiRD did not yield impressive results especially when compared to other treatment options available. Incidences of CR and VGPR after consolidation therapy were 0%, 28% of CR and 57%, 67% of VGPR in above mentioned two studies.
4.2.2. Carfilzomib, cyclophosphamide, thalidomide and low dose dexamethasone (CYKLONE)
Mikhael et al. (2015)[26] conducted a phase Ib/II trial with CYKLONE (CFZ, cyclophosphamide, thalidomide and dex) regimen in 64 NDMM patients. Four cycles of induction therapy in all patients were followed by SCT in 34 patients. After SCT, patients received 8 cycles of consolidation. Dose of carfilzomib was 15–20mg/m2 in 3 patients, 20–27mg/m2 in 25 patients, 20–36mg/m2 in 29 patients and 20–45 mg/m2 in 7 patients. ORR was 91%. In this regimen, most grade 1 PNP (31%) was said to be primarily related to thalidomide treatment. There was no grade ≥ 3 PNP. Grade ≥1 cardiopulmonary AEs (Cough (6%) and dyspnea (20%)) were observed in 5% of patients.[26] Quadruplet regimens didn’t yield any greater benefit as compared to triplet regimen for treatment of NDMM. In fact, incidence of PR and VGPR were low with Car-BiRD and CYKLONE when compared to KRd or KTd regimens.
5.0. Group B: Carfilzomib based regimens in relapsed and refractory multiple myeloma (Table 2)
Table 2. Carfilzomib based regimens for relapsed and refractory multiple myeloma.
| Author, year, study design |
Number of patients |
Median age |
ISS
staging: I/II/III/unknown (%) |
Cytogenetics: High risk/ Standard risk/unknown or misssing (%) |
CFZ (mg/m2) or control group dose |
Median duration of treatment/ Median number of cycles |
CFZ
or control group regimen |
Median number of prior lines of therapy |
CR/nCR/sCR (%) |
VGPR/PR (%) |
ORR (%) |
OS (months) |
PFS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials on single agent carfilzomib | |||||||||||||
| Jagannath, S., 2012, phase II | 46 | 63.5 | NS | 15.2/71.7/10.9 | 20 | NS/≤ 12 cycles | CFZ alone | 5 | NS | NS/16.7 | 16.7 | NS | 3.5 |
| Siegel, D.S.,2012,phase II | 266 | 63 | 29/38/31/2 | 28/60/12 | 20/27 | 3 months/Maximum number of 12 cycles | CFZ alone | 5 | 0.4/NS/NS | 5.1/18.3 | 23.7 | 15.6 | 3.7 |
| Watanabe, T., 2016, Phase I/II | 50 | 67 | 32/36/22/10 | 32/62/6 | 15/20, 20/27 | NS | CFZ alone | 5 | 0/NS/NS | 4/16 | 20 | Not reached | 5.1 |
| Vij, R., 2012, phase II | 129 | 65 | 73% had stage I or II/17% stage III/10% missing | 14.7/79.8/5.4 | 20/27 | NS/7 | CFZ alone | 2 | 2.4/NS/NS | 20.6/24.6 | 47.6 | NS | 54.3% at 9 months |
| Hajek, R., 2016, phase III | CFZ group (n=157) | 63 | 17/20/42/21 | 14/43/43 | 20/27 | 16.3 weeks/NS | CFZ alone | 5 | 1/NS/NS | 3/15 | 19.1 | 10.2 | 3.7 |
| Control group (n=158) | 66 | 13/26/35/26 | 18/48/34 | Cd1 | 10.7 weeks/NS | Cd1 | 5 | 0/NS/NS | 3/8 | 11.4 | 10 | 3.3 | |
| Carfilzomib based doublet regimens | |||||||||||||
| Berdeja, J.G., 2015, Phase II | 44 | 66 | 46/32/11/11 | 342/39/27 | 20/45 | NS/6 | CFZ+ P | 5 | NS | 33(≥ VGPR)/33 | 67 | 67% at 24 months | 7.7 at median follow up of 17 months |
| Berenson, J.R., 2014, Phase I/II | 116 | 68.5 | 40/43/14/2 | 17/49/34 | 20/70 | 7.7/NS | Kd | 1 | 11/NS/3 | 33/31 | 77 | NS | 12.6 |
| Lendvai, N., 2014, Phase II | 44 | 63 | NS | 45/52/2 | 20/56 | NS | Kd | 5 | 2/NS/NS | 21/31 | 55 | 20.3 | 4.1 |
| Dimopoulos, M.A., 2016, phase III | 464 | 65 | 44% stage I/56% stage II III | 21/61/18 | 20/56 | NS× | Kd | 2 | 11/NS/2 | 42/22 | 77 | Immature at interim analysis | 18.7 |
| 465 | 65 | 44% stage I/56% stage II III | 24/63/13 | 1.3mg/m2 bortezomib | NS× | Vd | 2 | 4/NS/2 | 22/34 | 63 | 9.4 | ||
| Carfilzomib based triplet regimens | |||||||||||||
| Wang, M., 2013, Phase I/II | 523 | 63 | NS | 21.2/76.9/1.9 | 20/27 | NS/9.5 cycles | CRd | 3 | 1.9/NS/3.8 | 36.5/34.6 | 76.9 | NS | 15.4 |
| Stewart, A.K., 2014, Phase III | 396 | 64 | NS | 12/37/50.8 | 20/27 | 88 weeks/NS | KRd | 2 | 17.7/NS/14.1 | 69.9/NS | 87 | 73% at 24m | 26.3 |
| 396 | 65 | 13/42.9/43.9 | Rd4 | 57 weeks/NS | Rd | 2 | 5/NS/4.3 | 40.4/NS | 66.7 | 65% at 24m | 17.6 | ||
Abbreviations:
continued until disease progression, intolerbale toxicity or withdrwal of consent,
84mg dexamethasone and optional cyclophosphamide (1400mg),
mentioned as abnormal and not high risk,
Maximum planned dose cohort,
25mg lenalidomide, 40mg dexamethasone,
NS: not specified, P: panobinostat, CFZ: carfilzomib, m: months, N: number of patients, CR: complete response, nCR: near complete response, sCR: stringent complete response, VGPR: very good partial response, PR: partial response, ORR: overall response rate, OS: overall survival, PFS: progression free survival, Kd: carfilzomib + dexamethasone, KRd/CRd: carfilzomib + lenalidomide + dexamethasone, Vd: bortezomib + dexamethasone, Rd: lenalidomide + dexamethasone
5.1. Single agent carfilzomib (5 studies, n=807)
5.1.1. Phase III (1 study, n=315)
Hajek et al.[27] (2016) compared single agent carfilzomib with best supportive treatment (low dose corticosteroids and optional cyclophosphamide) in heavily pretreated RRMM patients with median 5 prior lines of therapy. A total of 315 patients were randomized into CFZ group (n=157) and control group (n=158). Primary end-point was OS (time form randomization to death from any cause) which was not statistically different in 2 groups (10.2 months vs 10 months in carfilzomib and control group respectively, HR 0.975). The most common grade ≥ 3 AE were anemia (26 vs 31%), neutropenia (8 vs 12%) and thrombocytopenia (24 vs 22%) with CFZ vs control group. Incidence of any grade hypertension (15 vs 6%) and grade ≥ 3 grouped renal failure events were higher in CFZ group than control group (24 vs 9%).[27]
5.1.2. Phase II studies (4 studies, n=491)
Jagannath et al.[10] (2012) performed the first phase II trial on single agent CFZ in 46 RRMM patients who had received median of 5 prior lines of therapy. Median duration of response (i.e. from time of ≥ PR to confirmed disease progression or death from any cause) was 7.2 months. Significant grade 3/4 adverse events were anemia (37%), lymphopenia (28.3%), thrombocytopenia (26%), renal failure (13%) and pneumonia (11%).
Siegel et al.[7] (2012) used 20–27mg/m2 dose of CFZ to study its efficacy in heavily pretreated 266 multiple myeloma patients. Patients had median 5 prior lines of therapy including bortezomib, thalidomide and lenalidomide. Most common toxicities were hematologic (anemia 46% and thrombocytopenia 39%). Incidence of all grade peripheral neuropathy was 12%. CR achieved was 0.4%.
Watanabe et al.[28] (2016) performed phase II study on single agent CFZ with 15/20 and 20/27 mg/m2 dose in 50 patients. Patients had median 5 prior lines of therapy. ORR in patients who received 20/27mg/m2 CFZ was 22.5% comparable to 23.7% in Siegel et al.’s study.[7] 88% experienced at least one grade ≥ 3 AE, the most common of which were lymphopenia (68%), neutropenia (38%), anemia (30%) and 26% of each thrombocytopenia and leucopenia. Incidence of PN was 16% all less than grade 3. 50% of those who developed PN, already had PN at baseline. They reported higher incidence of hypertension (HTN) (20% vs 14.3% in Siegel et al.’s study) with 8% experiencing grade ≥ 3 HTN. 16% discontinued treatment with infection being the most common cause for it. Incidence of grade ≥ 3 cardiac AE was 2 % as compared to 9.5% in Siegel et al.’s study.[7]
Vij et al.[9] (2012) studied efficacy of single agent CFZ in bortezomib naïve patients with less heavily treated MM (1 to 3 prior lines of therapy). They divided total cohort of 129 bortezomib naïve patients in 2 groups. Cohort 1 received 20mg/m2 CFZ for all cycles of treatment. Cohort 2 received 20mg/m2 for cycle 1 and 27mg/m2 for remaining cycles of treatment. Overall incidence of PN was 17% with only one patient experiencing grade 3 PN.
With single agent CFZ in patients with median 5 prior lines of therapy, efficacy outcomes were reported as follows: CR of 0% to 1%, VGPR of 3 to 5.1%, ORR of 16.7% to 23.7% and median PFS ranging from 3.5 to 5.1 months.
5.2. Carfilzomib based doublet regimens
5.2.1. Phase II studies (3 studies, n= 204)
Carfilzomib + panobinostat
Berdeja et al.[29] (2015) performed phase I/II study on combination of CFZ and panobinostat in 44 RRMM patients who had median 5 prior lines of therapy. Patients were treated at maximum tolerable dose of 30mg panobinostat and 20/45mg/m2 of CFZ until disease progression or intolerable toxicity. 59% patients had toxicity related dose reductions for panobinostat. Out of 44 patients, 35 patients discontinued treatment (41% due to disease progression, 20% due to patient’s/physician’s decision, 9% due to treatment related side effects mainly cardiotoxicity and 9 % due to non-treatment related side effects). One patient died due to progressive disease and one due to heart failure.
Carfilzomib + dexamethasone
Berenson et al. (2014)[30] performed phase I/II study on 116 RRMM patients to investigate efficacy of 70mg/m2 once weekly CFZ plus dexamethasone. Berenson et al. evaluated 30-min infusion to investigate whether longer infusion time allows administration of higher doses. ORR was 77% and median PFS 12.6 months. Most common adverse effects of any grade were hematologic (anemia 28%, thrombocytopenia 22% and neutropenia 10%). Common grade ≥3 AE were fatigue 11%, HTN 7%, pneumonia 6% and acute kidney injury 6%. Rates of grade ≥3 dyspnea, cardiac failure, and peripheral neuropathy were 5%, 2%, and 1%, respectively.[30]
Lendvai et al. [31] (2014) studied efficacy and AE profile of 20/56mg/m2 dose of CFZ in 44 heavily pretreated RRMM patients who had median of 5 prior lines of therapy.. Incidences of CR, VGPR, PR, ORR, median OS and median PFS were 2%, 21%, 31%, 55%, 20.3 months and 4.1 months respectively. Most common grade 3/4 AE were hematologic (lymphopenia 43%, thrombocytopenia 32% and 18% for each of anemia, leukopenia and neutropenia). Most common grade 3/4 non-hematologic AE were HTN (25%), pneumonia (18%) and heart failure (20%). Out of 25% (n=11) who developed grade 3/4 HTN, one patient developed nephrotic range proteinuria, another developed thrombotic microangiopathy and 4 developed heart failure. Incidence of PNP was 16%, all grade 1. AEs related discontinuations were most commonly due to decline in left ventricular systolic function (5 patients). 39% patients required dose reductions with HTN being the most common cause.
With CFZ based doublet regimens in patients with 5 prior lines of therapy, efficacy outcomes were as follows: ≥ VGPR ranging from 23 to 33%, ORR ranging from 55 to 67% and median PFS of 4.1 months and 7.7 months (at median follow up of 17 months).
5.2.2. Phase III (1 study, n=929)
ENDEAVOR [carfilzomib plus dexamethasone (Kd) vs bortezomib plus dexamethasone (Vd)]
ENDEAVOR study by Dimopoulos et al. (2016)[32] is the largest (929 patients) multi-center phase 3 trial on RRMM. They did head to head trial on CFZ plus dexamethasone versus bortezomib plus dexamethasone. Results of preplanned interim analysis have been reported. 929 patients were randomized into 2 groups: 464 in CFZ and 465 in bortezomib group. Patients had received median of 2 prior regimens. CFZ was given at doses of 20 and 56 mg/m2 infused over 30-min. In the bortezomib group, 79% received it subcutaneously while 21% intravenously. Primary endpoint was PFS which was double (18.7 months) with CFZ compared to bortezomib (9.4 months). The most common ≥ grade 3 AE in CFZ and bortezomib group respectively were anemia (14% vs 10%), HTN (9% vs 3%), thrombocytopenia (8% vs 9%) and pneumonia (7% vs 8%). The percentage of grade ≥ 2 PN was significantly higher in bortezomib group (32%) than CFZ group (6%). Serious adverse events were more frequently reported in CFZ group (48%) than bortezomib group (36%). Incidence of many AE (noticeably dyspnea, cough, grade 3/4 HTN (9% vs 3%), pyrexia, any grade cardiac failure, any grade renal failure and muscle spasms) was higher with CFZ.
ENDEAVOR subgroup analyses
Impact of prior treatment
Moreau and colleagues[33] performed subgroup analysis of ENDEAVOR to study effect of prior treatment and number of prior lines of therapy. Median PFS for Kd vs Vd in patients who had one prior therapy was 22.2 vs 10.1 months respectively; with ≥2 prior lines of therapy, 14.9 vs 8.4 months respectively; with prior bortezomib exposure, 15.6 vs 8.1 months respectively and with prior lenalidomide exposure, 12.9 vs 7.3 months respectively.
Impact of cytogenetic risk
Chang et al.[34] performed subgroup analysis of ENDEAVOR to evaluate whether cytogenetic risk had an impact on outcome with Kd vs Vd. Median PFS was 8.8 months vs 6.0 months for Kd vs Vd in the high-risk group. In the standard-risk group median PFS was not estimable for Kd vs 10.2 months for Vd.
Impact of age
Ludwig and colleagues[35] did subgroup analysis of ENDEAVOR to study efficacy outcomes in patients who had been grouped by age and received either Kd or Vd. PFS was superior with Kd than Vd in each subgroup (<65 years: not estimable for Kd vs 9.5 months for Vd, 65–74 years: 15.6 months for Kd vs 9.5 months for Vd, ≥75 years: 18.7 months for Kd vs 8.9 months for Vd). Interestingly, the eldest-age subgroup (≥75 years) showed improved survival than two younger-age subgroups.
ENDEAVOR is the only available trial with head to head comparison of CFZ vs bortezomib, reported CR of 11% vs 4%, VGPR of 42% vs 22%, ORR of 77% vs 63%, and median PFS of 18.7 months vs 9.4 months with Kd vs Vd respectively. Subgroup analyses of ENDEAVOR showed that improved survival was irrespective of age, prior transplant status, prior treatment, number of prior lines of therapy or cytogenetics. Inferior outcomes in patients with high risk cytogenetics suggest that poor prognostic outcome of unfavorable cytogenetics couldn’t be overcome by either proteasome inhibitor.
5.3. Carfilzomib based triplet regimens (2 studies, n=448)
5.3.1. Phase II studies (1 study, n= 52)
Carfilzomib, lenalidomide and low dose dexamethasone (CRd)
Wang et al.[5] (2013) did phase I/II study on 52 patients who were given 20–27mg/m2 CFZ for median of 9.5 cycles. Most common grade 3/4 adverse events were hematologic (lymphopenia 48%, neutropenia 33%, thrombocytopenia 19% and anemia 19%). Incidence of overall cardiac adverse events was 19% (16 patients) with 6 patients (7%) experiencing ≥ grade 3 cardiac AE.
5.3.2. Phase III studies (1 study, n= 396)
Carfilzomib + lenalidomide + dexamethasone (KRd) vs lenalidomide and dexamethasone (Rd)
Stewart et al.[36] (2014) compared safety and efficacy of KRd vs Rd in 792 RRMM patients randomized into two groups in 1:1 ratio. The dose of CFZ was 20mg/m2 (initial dose) escalated to 27mg/m2 (target dose) infused over 10 minutes. The trial has reached its primary endpoint at interim analysis. Primary endpoint was PFS which was 26.3 months for KRd vs 17.6 months for Rd. Any grade AE more frequently encountered with KRd group were HTN (14.3 vs 6.9 in Rd), hypokalemia (27.6 vs 13.4), muscle spasms, infection, nasopharyngitis, cough, upper respiratory tract infection, diarrhea and pyrexia. However, rates of discontinuations with these AE were very low (< 1%). Grade ≥ 3 AE were HTN (4.3% for CFZ vs 1.8% for control group), dyspnea (2.8% vs 1.8%) and cardiac failure (3.8% vs 1.8%). Rate of discontinuation was 69.9% and 77.9% for KRd vs Rd respectively. Most of them were due to disease progression (39.8% and 50.1%) or AE (15.3% and 17.7%). Triple combination therapy (CRd) yielded highest efficacy among the trials reported so far for RRMM with CR of 17.7%, VGPR of 70%, ORR of 87% and median PFS of 26.3 months. CRd regimen in trial by Wang et al. resulted in outcomes less impressive than Stewart et al.’s results with CR of 1.9%, VGPR of 36.5%, ORR of 77% and median PFS of 15.4 months. Another randomized phase III trial by Moreau and colleagues[37] comparing KRd with Rd is ongoing.
6.0. Toxicity profile of carfilzomib
Incidence of grade ≥3 AE was as follows: anemia(11–41%), lymphopenia (5.7–50%), thrombocytopenia (4–53%), neutropenia (11–53%), leukopenia (1.4–43%), dyspnea (3.4–11%), pyrexia (1.5–2.4%), URTI (1.8–5.7%), increased creatinine (2.6–2.9%), PN (1–1.1%), hypophosphatemia (1.7–16%), hyponatremia (7.1–11%), pneumonia (8.6–18%), renal failure (3.4–9%), febrile neutropenia (0.8–7%), hypertension (3–25%), heart failure (3.4–20%) and hypokalemia (1.7 to 9.4%).[38–44] Most common grade ≥3 AE were hematologic. ENDEAVOR reported significantly higher incidence of ≥2 PNP in bortezomib group (32%) vs 6% in carfilzomib group. ENDEAVOR trial using serial echocardiograms reported no increased risk of cardiotoxicity and ventricular dysfunction with CFZ vs bortezomib.
7.0. Discussion
CFZ based therapies have been evaluated in RRMM setting, a population that is more difficult to treat than NDMM patients for multiple reasons. Based on literature summarized in this review, it is suggested that CFZ based treatment was very successful in the frontline setting. PN is a crippling condition that can develop as complication of disease itself or its treatment including agents like bortezomib and thalidomide.[45] Reported incidence of bortezomib induced grade ≥ 3 PNP (2–23%)[46] can impair life quality and the best way to preserve quality of life is to prevent development of peripheral neuropathy at the first place without any compromise on the efficacy of the regimen. A phase III trial[23] (ongoing) with head to head comparison of CFZ + MP vs bortezomib + MP, reported no significant difference in PFS with CFZ vs bortezomib. However, observed incidence of PNP was 35.1% with bortezomib vs 2.5% with CFZ.
With ORR approaching higher than 95%, CR rate close to 70 %, 2-year PFS ≥90% and 3-year PFS ≥70%, outcomes reported with CFZ are comparable if not higher to bortezomib based regimens but with much lower incidence of PNP.[23, 44, 47] This supports use of CFZ in frontline setting across the board as well as for high risk disease. From comparison of studies included in our systematic review, we reported best responses with CRd combination therapy with VGPR ranging from 20%–96%, CR of 45% to 73%, ORR of 96% to 98%, OS of 83% to 97% and 2-year PFS of 92% to 97%. Short term follow-up results from a phase I trial of four drug combination using daratumumab + KRd in 22 patients with NDMM produced very promising results with ORR of 100% (5% CR and 86% VGPR) and 100% six month PFS.[48] The idea of quadruplet regimen incorporating monoclonal antibody needs further exploration based on large scale trials with longer follow up. It should be kept in mind that due to population heterogeneity and differences in CFZ dosing, schedule and combination, comparison results can be imprecise. It is important to recognize, many studies in the CFZ group relied on frontline induction and melphalan high dose consolidation (consolidation 1) with autologous stem cell rescue followed by variable cycles (4 and higher) of CFZ with conventional dose consolidation (consolidation 2) intent and then patients were kept on maintenance for a variable duration. Triple combination (CRd) as a maintenance strategy was used in Jakubowiak and Zimmerman et al’s study, this approach is not standard with limited available data on efficacy and safety. Idea of second conventional dose consolidation, either single agent CFZ or combination (CRd based) maintenance therapy needs further exploration in large randomized trials. Despite heavy use and exposure of CFZ, most studies had negligible incidence of grade >3 PNP if at all present. Based on the limited data from two studies where head to head comparison was done, results suggest that CFZ demonstrates higher or equal efficacy to bortezomib with much favorable AE profile.[23, 44]
Car-BiRD regimen has not produced promising results in treatment of NDMM. It could be due to higher percentage (62%) of their patients with high risk cytogenetics and lack of additional cycles of consolidation beyond use of high dose melphalan. In the presence of other options available especially KRd, Car-BiRD doesn’t seem an attractive option for treatment of MM patients. Quadruplet regimens didn’t yield ang greater benefit as compared to triplet regimen for treatment of NDMM. In fact, percentage of patients with PR and VGPR was less with Car-BiRD and CYKLONE when compared to KRd or KTd regimens. Daratumumab + KRd combination generated exceptionally good results with ORR of 100% in phase 1 setting and warrants further testing in larger randomized trials.
Carfilzomib is approved for treatment of RRMM in patients with 1–3 prior lines of therapy, either alone or in combination with lenalidomide ± dexamethasone. Clonal heterogeneity is common in MM. Combination therapy helps decrease tumor burden despite clonal heterogeneity. CFZ based triplet combination therapy produced better outcomes than doublet regimen which in turn had better results than single agent CFZ. Phase III study by Hajek et al. didn’t reach primary endpoint i.e. single agent CFZ has superior OS than supportive treatment in heavily pretreated MM.[27] Though single agent CFZ failed to significantly improve outcomes in these heavily pretreated patients, CFZ remains an active treatment option as combination therapy and in a variety of settings as suggested by ENDEAVOR, ASPIRE and other trials on CFZ in NDMM setting.
Higher incidence of hypertension with CFZ based therapy reported in some studies [27, 28, 32, 49, 50] is note-worthy. Proteasome inhibitors are postulated to cause cardiomyocyte apoptosis through accumulation of pre-apoptotic bodies.
MM patients treated with PI should have their blood pressure (BP) carefully monitored and controlled to alleviate risk of cardiotoxicity. Standard FDA approved dose of CFZ is 20/27mg/m2 infused over 10 minutes. Higher dose 20/56mg/m2 used by Lendvai et al. produced deeper and durable responses but with higher incidence of grade 3 /4 HTN. Berenson et al (2014)[30] studied 70mg/m2 per weekly dose of CFZ while Dimopoulos et al.[44] used 56mg/m2 twice weekly dose. Although cross trial comparisons can be imprecise due to differences in patient population (e.g. median no. of prior lines of therapy was 1 for Berenson’s study vs 2 for ENDEAVOR study), ORR reported in 2 studies were comparable (77% for both). However, median PFS was higher in ENDEAVOR study (18.7 vs 12.6 in Berenson study). An ongoing ARROW trial[51] is comparing 70mg/m2 once weekly dose of CFZ to twice weekly 20/27 mg/m2 dose. Results of ARROW and further large-scale trials are needed to study benefit-to-risk profile of 20/56 and 20/70mg/m2 dosing of CFZ vs standard 20/27mg/m2 dose.
8.0. Conclusion
Our results suggest that CFZ demonstrates comparable efficacy to bortezomib with much favorable AE profile both in NDMM and RRMM. There are only two studies with head to head comparison of CFZ based regimens with bortezomib based regimens.[23, 44] Cross-trial comparisons of studies on CFZ with studies on bortezomib can be imprecise due to significant heterogeneity in patient population, number of prior lines of therapy, dose and schedule of drug used and whether treatment was in conjunction with stem cell transplantation.. KRd and Rd regimen have well documented efficacy for treatment of RRMM. Further large-scale trials are needed to study benefit-to-risk profile of 20–56 and 20–70mg/m2 dose of CFZ vs standard 20–27mg/m2 dose. Reported incidence (3%–25%) of grade ≥3 HTN with CFZ deserves attention and emphasizes the importance of serial BP monitoring before, during and after CFZ infusions. For patients with NDMM, data supporting KRd mainly comes from phase II trials. Deep, rapid and sustainable response using KRd with safer toxicity profile supports extension of KRd therapy to frontline therapy for all risk categories of MM. Role of conventional dose second consolidation after HDCT and autologous stem rescue needs further exploration for safety and efficacy in larger randomized trials. Data from randomized phase III trials is needed for head to head comparison of KRd vs RVd, and KRd vs daratumumab-KRd for NDMM patients.
Acknowledgments
Financial disclosure statement: This work was supported by grant P30 CA023074 (FA) from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
We acknowledge the efforts of all investigators and their teams who worked on the original trials. We sincerely appreciate all myeloma patients and their families, who volunteered and participated in these clinical trials which are mentioned in our manuscript.
Biographies
AM is a medical doctor, who graduated from Rawalpindi Medical College (RMC), Pakistan. Dr. Adeela is working as research volunteer in Hematology/Oncology at University of Arizona Cancer Center (UACC).
AL has her MD and is a graduate from RMC, Pakistan. She is currently working as a research volunteer in Hematology/Oncology at UACC.
VK has his MD and is a graduate from RMC, Pakistan. He is working as a research volunteer in Hematology/Oncology at UACC.
AI has his MD degree from RMC, Pakistan. He is working as a research volunteer in Hematology/Oncology at UACC.
UZ has his MD degree from Punjab medical college (PMC), Pakistan. He is a graduate student of MPH Biostatistics at the Mel & Enid Zuckerman College of Public Health, University of Arizona. He is also a research associate at the Department of Medicine, Hematology/Oncology division at UACC.
IBR: had his MD from Nishtar Medical College, currently working as a Hem/Onc trainee fellow at Mayo Clinic, Department of Hematology and Oncology, Rochester, Minnesota.
AM has his PharmD and MS and is the Clinical Coordinator of Hematology/Oncology UACC.
IA is a chief scientist at Matrix45 and professor (part-time) at University of Arizona in the Department of Pharmacy Practice. He has lectured, consulted, and conducted research throughout the America, Europe, the Asia-Pacific region, and Africa.
FA has his MD, is team leader for Multiple Myeloma program, Clinical Director of the adult Blood and Marrow Transplant Program and the Director of the Stem Cell Harvest Program in the Division of Hematology/Oncology at UACC and is an Assistant Professor of Medicine at the University of Arizona, College of Medicine.
Appendix 1
Example Search Strategy (“carfilzomib”[Supplementary Concept] OR “carfilzomib”[All Fields]) AND (“multiple myeloma”[MeSH Terms] OR (“multiple”[All Fields] AND “myeloma”[All Fields]) OR “multiple myeloma”[All Fields]) AND ((Clinical Trial[ptyp] OR Meta-Analysis[ptyp] OR systematic[sb]) AND “2007/06/28”[PDat] : “2017/06/24”[PDat])
Footnotes
AM (co-first author): literature search, figures, study design, data collection, data analysis, data interpretation, writing, editing
VK (co-first author): literature search, figures, study design, data collection, data analysis, data interpretation, writing, editing
AL (co-first author): literature search, figures, study design, data collection, data analysis, data interpretation, writing, editing
AI: literature search, figures, study design, data collection, data analysis, data interpretation
UZ: data interpretation, writing, editing
AMΔ: data interpretation, writing, editing
IA: data interpretation, writing, editing
IBR: data interpretation, writing, editing:
FA (corresponding author): literature search, study design, data analysis, data interpretation, writing, editing
Conflict of interest statement Authors declare that there is no conflict of interest with this manuscript. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript. For full disclosure, Dr. McBride discloses financial interest from Seattle Genetics in the form of advisory board membership. Dr. Anwer discloses financial interest from Seattle Genetics and Incyte Corporation in the form of advisory board membership and participation in speakers’ program.
All other authors have nothing to disclose.
References
- 1.Strite S, et al. Comparing the efficacy of denosumab versus ZO-ledronic acid (ZA) for prevention of Skeletal-Related events (SRES): A critical appraisal of three pivotal trials. Supportive Care in Cancer. 2015;23(1):S33. [Google Scholar]
- 2.Institute, N.C. Cancer Stat Facts: Myeloma [Google Scholar]
- 3.Kane RC, et al. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8(6):508–13. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 4.Mohty B, et al. Peripheral neuropathy and new treatments for multiple myeloma: Background and practical recommendations. Haematologica. 2010;95(2):311–319. doi: 10.3324/haematol.2009.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, et al. Final results from the phase Ib/II study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone (CRd) in patients with relapsed or progressive multiple myeloma. Journal of Clinical Oncology. 2013;31(15) [Google Scholar]
- 6.Rosinol L, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–96. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 7.Siegel DS, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreau P, et al. Phase 1/2 study of carfilzomib plus melphalan and prednisone in patients aged over 65 years with newly diagnosed multiple myeloma. Blood. 2015;125(20):3100–3104. doi: 10.1182/blood-2015-02-626168. [DOI] [PubMed] [Google Scholar]
- 9.Vij R, et al. An open-label, single-arm, phase2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119(24):5661–5670. doi: 10.1182/blood-2012-03-414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagannath S, et al. An open-label single-arm pilot phase II study (PX-171-003-A0) of low-dose, single-agent carfilzomib in patients with relapsed and refractory multiple myeloma. Clinical Lymphoma, Myeloma and Leukemia. 2012;12(5):310–318. doi: 10.1016/j.clml.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Roussel M, et al. Frontline therapy with carfilzomib, lenalidomide, and dexamethasone (KRd) induction followed by autologous stem cell transplantation, krd consolidation and lenalidomide maintenance in newly diagnosed multiple myeloma (NDMM) patients: primary results of the intergroupe francophone du myelome (IFM) krd phase II study. Blood. 2016;128 Conference: 58th annual meeting of the american society of hematology, ASH 2016. United states. Conference start: 20161203. Conference end: 20161206. [Google Scholar]
- 12.Zimmerman T, et al. Final results of a phase 2 trial of extended treatment (TX) with Carfilzomib (CFZ), Lenalidomide (LEN), and Dexamethasone (KRd) plus autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (NDMM) Blood. 2016;128(22) [Google Scholar]
- 13.Korde N, et al. Phase II clinical and correlative study of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended dosing (CRD-R) induces high rates of mrd negativity in newly diagnosed Multiple Myeloma (MM) patients. Blood. 2013;122(21) [Google Scholar]
- 14.Jasielec J, et al. Predictors of treatment outcome with the combination of carfilzomib, lenalidomide, and low-dose dexamethasone (CRD) in newly diagnosed multiple myeloma (NDMM) Blood. 2013;122(21) [Google Scholar]
- 15.Jakubowiak AJ, et al. Carfilzomib, lenalidomide, low-dose dexamethasone (CRD) in elderly patients with newly diagnosed multiple myeloma (NDMM) Clinical Lymphoma, Myeloma and Leukemia. 2013;13:S41–S42. [Google Scholar]
- 16.Dytfeld D, et al. Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99(9):e162–e164. doi: 10.3324/haematol.2014.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonneveld P, et al. Phase 2 study of carfilzomib, thalidomide, and dexamethasone as induction/consolidation therapy for newly diagnosed multiple myeloma. Blood. 2015;125(3):449–456. doi: 10.1182/blood-2014-05-576256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wester R, et al. Phase 2 study of carfilzomib, thalidomide, and low-dose dexamethasone as induction/consolidation in newly diagnosed, transplant eligible patients with multiple myeloma, the carthadex trial. Blood. 2016;128(22) doi: 10.3324/haematol.2018.205476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonneveld P, et al. Carfilzomib combined with thalidomide and dexamethasone (CTD) is an highly effective induction and consolidation treatment in newly diagnosed patients with multiple myeloma (MM) who are transplant candidate. Blood. 2012;120(21) [Google Scholar]
- 20.Palumbo A, et al. Weekly carfilzomib, cyclophosphamide and dexamethasone (wCCd) in newly diagnosed multiple myeloma patients: A phase I–II study. Blood. 2014;124(21) doi: 10.1182/blood-2014-03-563759. [DOI] [PubMed] [Google Scholar]
- 21.Bringhen S, et al. Weekly carfilzomib in combination with cyclophosphamide and dexamethasone (wccyd) in patients (pts) with newly diagnosed multiple myeloma (NDMM): A phase 1/2 study of the european myeloma network trialist group. Clinical Lymphoma, Myeloma and Leukemia. 2015;15:e70. [Google Scholar]
- 22.Bringhen S, et al. A phase II study with carfilzomib, cyclophosphamide and dexamethasone (CCd) for newly diagnosed multiple myeloma. Blood. 2013;122(21) doi: 10.1182/blood-2014-03-563759. [DOI] [PubMed] [Google Scholar]
- 23.ClinicalTrials.gov. Phase 3 Study of Carfilzomib, Melphalan, Prednisone vs Bortezomib, Melphalan, Prednisone in Newly Diagnosed Multiple Myeloma (CLARION) 2017 Jun; [Google Scholar]
- 24.Forsberg PA, et al. Carfilzomib induction with lenalidomide and clarithromycin consolidation and lenalidomide maintenance (CarBiRD) for multiple myeloma (MM) Blood. 2016;128(22) [Google Scholar]
- 25.Mark TM, et al. Car-bird [carfilzomib, clarithromycin(biaxin®), lenalidomide/(revlimid®), dexamethasone) for newly-diagnosed multiple myeloma. Blood. 2014;124(21) [Google Scholar]
- 26.Mikhael JR, et al. Phase Ib/II trial of CYKLONE (cyclophosphamide, carfilzomib, thalidomide and dexamethasone) for newly diagnosed myeloma. Br J Haematol. 2015;169(2):219–27. doi: 10.1111/bjh.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hájek R, et al. A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS) Leukemia. 2017;31(1):107–114. doi: 10.1038/leu.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe T, et al. A phase 1/2 study of carfilzomib in Japanese patients with relapsed and/or refractory multiple myeloma. British Journal of Haematology. 2016;172(5):745–756. doi: 10.1111/bjh.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berdeja JG, et al. A phase I/II study of the combination of panobinostat (PAN) and carfilzomib (CFZ) in patients (PTS) with relapsed or relapsed/refractory multiple myeloma (MM): Comparison of two expansion cohorts. Blood. 2015;126(23):1825. [Google Scholar]
- 30.Berenson JR, et al. Replacement of bortezomib with carfilzomib for multiple myeloma patients progressing from bortezomib combination therapy. Leukemia. 2014;28(7):1529–1536. doi: 10.1038/leu.2014.27. [DOI] [PubMed] [Google Scholar]
- 31.Lendvai N, et al. A phase 2 single-center study of carfilzomib 56 mg/m2 with or without low-dose dexamethasone in relapsed multiple myeloma. Blood. 2014;124(6):899–906. doi: 10.1182/blood-2014-02-556308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimopoulos MA, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): And randomised, phase 3, open-label, multicentre study. The Lancet Oncology. 2016;17(1):27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 33.Moreau P, et al. Impact of prior treatment on patients with relapsed multiple myeloma treated with carfilzomib and dexamethasone vs bortezomib and dexamethasone in a subgroup analysis of the phase 3 endeavor study ( NCT01568866) Blood. 2015;126(23):729. doi: 10.1038/leu.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chng WJ, et al. Efficacy and safety of carfilzomib and dexamethasone vs bortezomib and dexamethasone in patients with relapsed multiple myeloma based on cytogenetic risk status: Subgroup analysis from the phase 3 study endeavor ( NCT01568866) Blood. 2015;126(23):30. [Google Scholar]
- 35.Ludwig H, et al. Carfilzomib and dexamethasone vs bortezomib and dexamethasone in patients with relapsed multiple myeloma: results of the phase 3 study ENDEAVOR ( NCT01568866) according to age subgroup. Leuk Lymphoma. 2017:1–4. doi: 10.1080/10428194.2017.1298755. [DOI] [PubMed] [Google Scholar]
- 36.Stewart AK, et al. Carfilzomib, lenalidomide, and dexamethasone vs lenalidomide and dexamethasone in patients (Pts) with relapsed multiple myeloma: Interim results from ASPIRE, a randomized, open-label, multicenter phase 3 study. Blood. 2014;124(21) [Google Scholar]
- 37.Moreau P, et al. A randomized, multicenter, phase (Ph) III study comparing carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (Dex) to LEN and Dex in patients (Pts) with relapsed multiple myeloma (MM) Journal of Clinical Oncology. 2011;29(15) [Google Scholar]
- 38.Siegel DS, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–25. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart AK, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–52. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 40.Vij R, et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol. 2012;158(6):739–48. doi: 10.1111/j.1365-2141.2012.09232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman JL. Phase I Study of the Combination of Carfilzomib and Panobinostat for Patients with Relapsed and Refractory Myeloma: A Multiple Myeloma Research Consortium (MMRC) Clinical Trial. 2014 [Google Scholar]
- 42.Bringhen S, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124(1):63–9. doi: 10.1182/blood-2014-03-563759. [DOI] [PubMed] [Google Scholar]
- 43.Vesole DH, et al. Phase I study of carfilzomib, lenalidomide, vorinostat, and dexamethasone in patients with relapsed and/or refractory multiple myeloma. Br J Haematol. 2015;171(1):52–9. doi: 10.1111/bjh.13517. [DOI] [PubMed] [Google Scholar]
- 44.Dimopoulos MA, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 45.Morawska M, et al. Therapy-related peripheral neuropathy in multiple myeloma patients. Hematol Oncol. 2015;33(4):113–9. doi: 10.1002/hon.2149. [DOI] [PubMed] [Google Scholar]
- 46.Schlafer D, et al. Safety of proteasome inhibitors for treatment of multiple myeloma. Expert Opinion on Drug Safety. 2017;16(2):167–183. doi: 10.1080/14740338.2017.1259310. [DOI] [PubMed] [Google Scholar]
- 47.Richardson PG, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679–86. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jakubowiak AJ. Daratumumab (DARA) in combination with carflzomib, lenalidomide, and dexamethasone (KRd) in patients (pts) with newly diagnosed multiple myeloma (MMY1001): An open-label, phase 1b study. 2017 Jun; [Google Scholar]
- 49.Bringhen S, et al. A multicenter, open label phase I/II study of carfilzomib, pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma (MM) patients. Blood. 2016:128. Conference: 58th annual meeting of the american society of hematology, ASH 2016. United states. Conference start: 20161203. Conference end: 20161206. [Google Scholar]
- 50.Papadopoulos KP, et al. A phase 1b/2 study of prolonged infusion carfilzomib in patients with relapsed and/or refractory (R/R) multiple myeloma: Updated efficacy and tolerability from the completed 20/56mg/m2 expansion cohort of PX-171-007. Blood. 2011;118(21) [Google Scholar]
- 51.ClinicalTrials.gov. A Study in Subjects With Relapsed and Refractory Multiple Myeloma Receiving Carfilzomib in Combination With Dexamethasone, Comparing Once-weekly Versus Twice-weekly Carfilzomib Dosing (ARROW) 2017 Apr; [Google Scholar]


