Abstract
Because of their central roles in tumor growth and invasion, milligram level amounts of active MMPs are frequently required for cancer research and development of chemical or biological MMP inhibitors. Here we describe methods for functional production of catalytic domains of MMPs (cdMMPs) in E. coli periplasm without refolding or activation process. We demonstrate applications of this straightforward approach for cdMMP-9, cdMMP-14, and cdMMP-14 mutants.
Keywords: periplasmic expression
1 Introduction
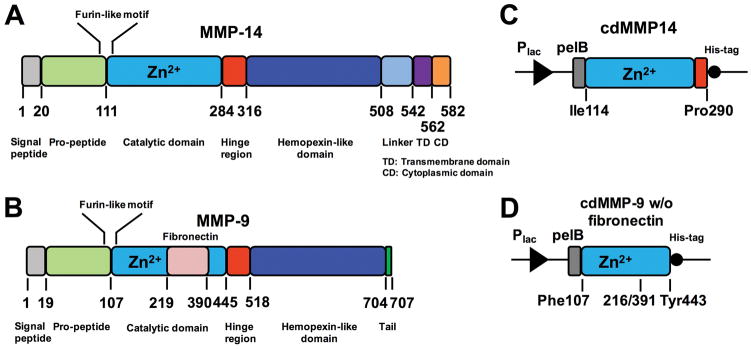
MMPs (matrix metalloproteases) are multi-domain zinc-dependent endopeptidases that share a basic structural organization comprising propeptidic, catalytic, hinge, and hemopexin like domains [1] (Fig 1AB). Besides functioning in normal physiological processes, many MMPs correlate with cancer progression, including cancer cell invasion, proliferation and apoptosis, and tumor angiogenesis and vasculogenesis [2–4]. Taking these preclinical and clinical evidences together, some MMPs such as MMP-2/-9/-14 etc have been considered as important regulatory enzymes for cancer research and predominant therapeutic targets for cancer treatments [5–7]. Therefore, the consistent supply of active MMPs at mg scales is essential for cancer biology research and for the developments of novel diagnostic and therapeutic agents, e.g. identification of physiological substrates of MMPs, characterization of extracellular matrix remolding in vitro and in vivo, and screening for highly selective MMP inhibitors.
Figure 1.
Gene structures for (A) full length MMP-14 and (B) full length MMP-9, and periplasmic expression cassettes for (C) MMP-14 catalytic domain and (D) MMP-9 catalytic domain without fibronectin domain. Plac promoter and pelB leader peptide were used for cdMMP expression.
Recombinant overexpression of many human MMPs and their catalytic domains in E. coli exclusively results in the formation of inclusion bodies [8]. After solubilization and purification, the denatured MMPs can be refolded to their active form via multiple steps of gradient dialysis, which are often labor-extensive and time-consuming. Besides low yields (typically <35%) and uncontrollable lot-to-lot variation, during the refolding process, autoproteolysis occurs at both termini with various degrees thus generating heterogeneous mixtures [9]. Alternatively, MMP with its propeptidic domain, e.g. pro-MMP-14, has been periplasmically expressed in E. coli by fusing with a signal peptide [10]. However, after purification, pro-MMP-14 was treated with 4-aminophynylmercuric acetate (APMA) or trypsin to yield the functional enzyme [11]. As an organomercury compound, APMA is toxic, and complete activation of pro-MMP-14 using APMA is difficult [12]. In addition to cleavage of the propeptide, trypsin treatment also digests the C-terminus of MMP-14 at the putative furin cleavage site (RRKR/YAIQ) resulting in truncated products [11].
Taking advantages of a slow processing rate controlled by secretion machineries and multiple periplasmic molecular chaperons that enhance proper protein folding, we develop a method to directly express functional MMP catalytic domains in the periplasmic space of E. coli [13]. This straightforward method avoids the problematic activation processes and tedious refolding associated with inclusion bodies. Here we demonstrate this method for producing catalytic domains of both a secreted MMP (cdMMP-9) and a membrane-based MMP (cdMMP-14). We further expand this method to produce cdMMP-14 mutants. A facile approach to obtaining milligrams of active cdMMPs is beneficial for MMP related studies, particularly for the development of therapeutic drugs targeting important MMPs for cancer treatments.
2 Materials
2.1 Construction of cdMMP genes
Plasmid pMopac16 [14]
DNA fragments encoding cdMMPs (IDT)
Jude-I [DH10B F′[proAB lacIQ lacZ ΔM15 Tn10(TetR)]] competent cells
2.2 Periplasmic expression and purification of cdMMPs
BL21 [E. coli B F− ompT gal dcm lon hsdSB(rB−mB−) [malB+]K-12(λS)]
2×YT/Chlor: 2×YT (BD Difco), 34 μg/ml chloramphenicol
LB/Chlor agar: LB (BD Difco), 1.5 g/L agar, 34 μg/ml chloramphenicol
Periplasmic buffer: 200 mM Tris-HCl, pH 7.5, 20% sucrose, 50 μg/ml lysozyme
0.45 μm filter units, 90 mm, 500 ml
Ni-NTA agarose
Ni-NTA column equilibrium buffer: 50 mM Tris-HCl, pH 8.0, 300 mM NaCl
Ni-NTA column washing buffer: 50 mM Tris-HCl, pH 8.0, 300 mM NaCl, 20 mM imidazole
Ni-NTA column elution buffer: 50 mM Tris-HCl, pH 8.0, 300 mM NaCl, 200 mM imidazole
Ultrafiltration centrifugal units, 3 kDa MWCO
SnakeSkin dialysis tubing, 3.5 kDa MWCO, 16 mm
Dialysis buffer: 50 mM Tris HCl, pH 7.5, 150 mM NaCl, 5 mM CaCl2, 0.1 mM ZnCl2
2.3 Characterizations of cdMMPs
2.3.1 Activity measurements by FRET
Peptide M-2350: Mca-Lys-Pro-Leu-Gly-Leu-Dap(Dnp)-Ala-Arg-NH2 (Bachem)
Peptide XV: QXL®520-γ-Abu-Pro-Glu-Gly-Leu-Dab(5-FAM)- Ala-Lys - NH2 (AnaSpec Inc)
GM60001 (EMD Millipore)
nTIMP-2, prepared as details described in Ref #15.
Black flat-bottom polystyrene NBS 96-well microplate
20% DMSO
Assay buffer: 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM CaCl2, 0.1 mM ZnCl2
2.3.2 Analysis by gel permeable chromatography
Ovalbumin (43 kDa)
Lysozyme (14 kDa)
Superdex 75 10/300 GL size-exclusion column (GE Healthcare)
Equilibrium buffer: 50 mM HEPES pH 7.5, 150 mM NaCl
3 Methods
3.1 Construction of cdMMP genes
Synthesize DNA fragments encoding catalytic domains of human MMP-14 (Ile114-Pro290) and human MMP-9 (fibronection domain removed, Ile107-Val216 fused with Glu391-Tyr443) (Fig 1CD).
Following standard molecular biology techniques, clone cdMMP genes to the periplasmic expression plasmid pMopac16 [14], which carries a Lac promoter, a pelB leader peptide, and a C-terminal His-tag (Fig 1CD).
Construct cdMMP-14 mutant genes by overlapping PCRs, in which the site-specific mutagenesis is introduced by overlapped primers.
Confirm all cloning results by DNA sequencing (see Note 1). Transform constructed cdMMP periplasmic expression plasmids to Jude-I component cells. Incubate on LB/Chlor agar plates.
3.2 Periplasmic expression and purification of cdMMPs
Inoculate a single colony of Jude-I or BL21 cells carrying pMopac-cdMMP to 5 ml 2×YT/Chlor. Culture at 30 °C overnight (see Note 2).
Add 5 ml overnight pre-culture to 500 ml 2×YT/Chlor. Cultures overnight at 30 °C without IPTG (see Note 3).
Measure OD600. Centrifuge cells at 4,500 ×g, 4 °C for 15 min. Decant supernatant.
Add periplasmic buffer (see Note 4) at a volume of 20 μl per OD600. Suspend collected cells by vortex.
Incubate for 5 min at room temperature with gentle shaking.
Add ice-cold DDW at volume of 20 μl per OD600. Incubate on ice for 10 min with gentle shaking.
Centrifuge 4,500 ×g, 4 °C for 15 min. Filtrate supernatant through 0.45 μm membranes. Osmotic shocked supernatants are ready for cdMMP purification.
Purify cdMMPs by Ni-NTA resin. Use 20 and 200 mM imidazole in 50 mM Tris-HCl, pH 8.0, 300 mM NaCl as the washing and elution buffers.
Concentrate eluted samples using ultrafiltration units (3 kDa MWCO) by centrifugation at 4,000×g 4 °C for 15 min.
Dialyze concentrated cdMMP samples at 4 °C overnight (see Note 5).
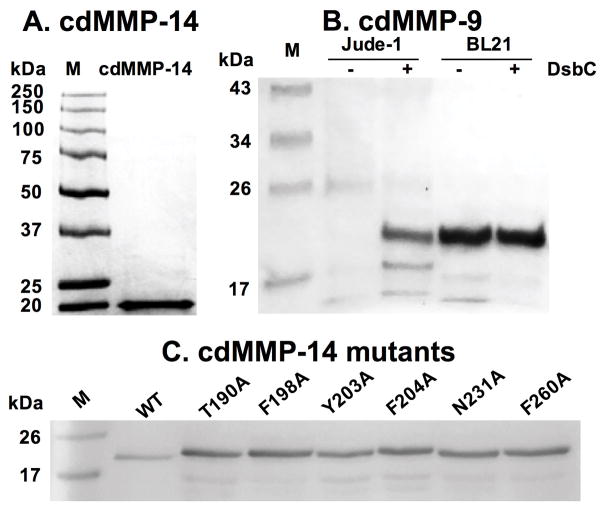
In the next day, measure cdMMP concentrations. Analyze by SDS-PAGE (typical results shown in Fig 2). Store in 20 % glycerol at −80 °C.
Figure 2.
SDS-PAGE of purified (A) cdMMP-14 (B) cdMMP-9 and (C) cdMMP-14 mutants.
3.3 Characterizations of cdMMPs
Km and kcat values of purified cdMMPs and their mutants can be measured (Subheading 3.3.1). In addition to enzymatic kinetics, the periplasmic preparation without purification can be directly applied for activity tests (Subheading 3.3.2), which are particularly useful in screening of MMP inhibitors [16].
3.3.1 Enzymatic kinetics measurement
Dissolve fluorogenic substrate peptide M-2350 in 20% DMSO to concentrations of 62.5–2000 μM.
Add 50 μl 1–10 nM cdMMP to a 96-well black assay plate.
To start the reaction, add 1 μl substrate peptide stock solutions into wells to give final concentrations of 1.25–40 μM.
Monitor the hydrolysis of peptide M-2350 with excitation at 328 nm and emission at 393 nm.
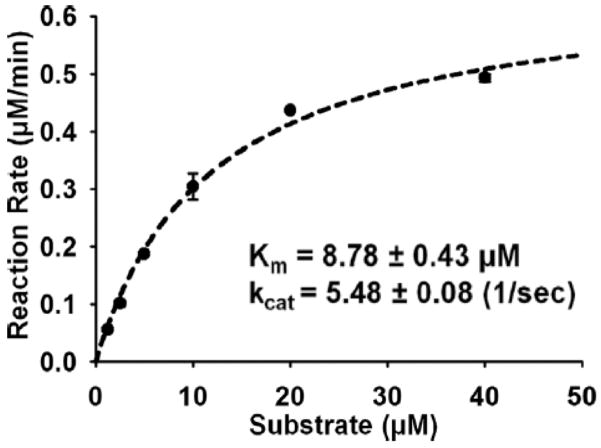
Triplcate the experiments. Fit Michaelis-Menten equation to find Km and kcat (Fig 3).
Figure 3.
Enzyme kinetics of cdMMP-14. 1–10 nM cdMMP-14 and 1.25–40 μM FRET peptide M-2350 were used for measurements of Km and kcat.
3.3.2 Periplasmic FRET assays
Suspend 2–3 OD600 overnight cultures (Subheading 3.2) in 100 μl periplasmic buffer. Incubate on ice for 5 min.
Add same volume ice-cold DDW. Incubate on ice for 10 min to release periplasmic fraction.
Centrifuge 13,000 ×g, RT for 2 min.
Add 50–100 μl periplasmic fraction to a 96-well black assay plate.
To start the reaction, add peptide XV to a final concentration of 1 μM (see Note 6). Monitor hydrolysis with excitation at 490 nm and emission at 520 nm.
For inhibitor screening, add 0–1000 nM compound inhibitors (e.g. GM60001) or 0–8,000 nM Fabs (e.g. 3A2, 17) into the periplasmic fraction before adding the peptide substrate (see Note 7).
3.3.3 Analysis by gel permeable chromatography
Equilibrate superdex 75 10/300 GL size-exclusion column with 50 mM HEPES pH 7.5, 150 mM NaCl.
Inject 200 μl 500 μg/ml cdMMP-9 at a flow rate of 0.5 ml/min.
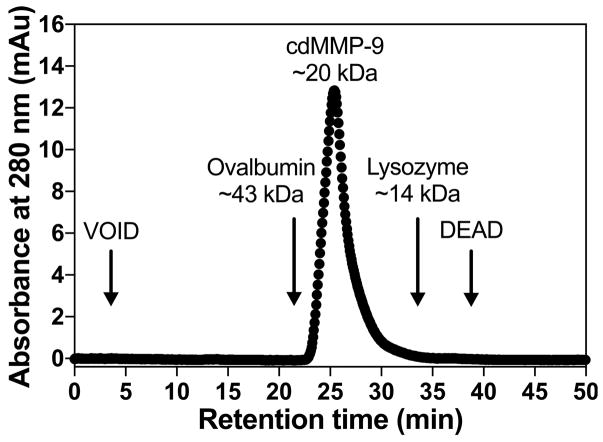
Monitor chromatograms of absorbance at 280 nm (Fig 4).
Estimate the molecular mass of cdMMP-9 based on the retention times of ovalbumin (43 kDa) and lysozyme (14 kDa).
Figure 4.
Gel permeation chromatography result of purified cdMMP-9. Chromatograms were obtained by monitoring absorbance at 280 nm. The molecular mass of cdMMP-9 was estimated by its retention time and comparison with these of standard molecular mass markers, e.g. ovalbumin (43 kDa) and lysozyme (14 kDa).
Acknowledgments
This work was supported by National Science Foundation the Faculty Early Career Development (CAREER) Program 1453645 and National Institutes of Health Grant R01 GM115672 to X.G.
Footnotes
In addition to DNA sequencing, correct in-frame cdMMP clones can also be identified by measuring their proteolytic activities (16, also see Subheading 3.3.2)
Expression of cdMMP-9 in Jude-I resulted in truncated products likely due to endogenous proteases. Using protease deficient host BL21 reduced the amount of unwanted truncations. In addition, although cdMMPs do not have disulfide bonds, we found that co-expression of molecular chaperon DsbC (a disulfide isomerase) significantly improved the yields of cdMMPs (Fig 2B), a similar observation for TIMPs [15].
Although cdMMPs are regulated under a Lac promoter, experimental results indicate that the highest activity was achieved without IPTG induction [13].
EDTA treatment, a step of standard periplasmic fraction protocol [18], should be avoided due to its ability to chelate Ca2+ and Zn2+ which are essential for MMP structure and activity.
Dialysis is required to remove imidazole (a weak inhibitor of MMPs) for downstream applications.
Certain FRET substrates (e.g. M-2350 peptide, Bachem) cannot be used for periplasmic activity assays due to its proteolysis by homologous endopeptidases present in E. coli periplasm.
Other than adding purified Fabs into periplasmic fraction, the Fab clone of interest can also be co-expressed with cdMMP for inhibitor screening [13].
References
- 1.Sela-Passwell N, Rosenblum G, Shoham T, et al. Structural and functional bases for allosteric control of MMP activities: can it pave the path for selective inhibition? Biochim Biophys Acta. 2010;1803(1):29–38. doi: 10.1016/j.bbamcr.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Jiang A, Lehti K, Wang X, et al. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc Natl Acad Sci USA. 2001;98(24):13693–13698. doi: 10.1073/pnas.241293698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tetu B, Brisson J, Wang CS, et al. The influence of MMP-14, TIMP-2 and MMP-2 expression on breast cancer prognosis. Breast Cancer Res. 2006;8(3):R28. doi: 10.1186/bcr1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mimori K, Ueo H, Shirasaka C, et al. Clinical significance of MT1-MMP mRNA expression in breast cancer. Oncol Rep. 2001;8(2):401–403. doi: 10.3892/or.8.2.401. [DOI] [PubMed] [Google Scholar]
- 5.Genís L, Gálvez BG, Gonzalo P, et al. MT1-MMP: universal or particular player in angiogenesis? Cancer Metastasis Rev. 2006;25(1):77–86. doi: 10.1007/s10555-006-7891-z. [DOI] [PubMed] [Google Scholar]
- 6.Morrison CJ, Butler GS, Rodríuez D, et al. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol. 2009;21(5):645–653. doi: 10.1016/j.ceb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Devy L, Dransfield DT. New Strategies for the next generation of matrix-metalloproteinase inhibitors: selectively targeting membrane-anchored MMPs with therapeutic antibodies. Biochem Res Int. 2011;2011:191670. doi: 10.1155/2011/191670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo HM, Kim JH, Hwang IK, et al. Refolding of the catalytic and hinge domains of human MT1-mMP expressed in Escherichia coli and its characterization. Mol Cells. 2002;13(1):118–124. [PubMed] [Google Scholar]
- 9.Lichte A, Kolkenbrock H, Tschesche H. The recombinant catalytic domain of membrane-type matrix metalloproteinase-1 (MT1-MMP) induces activation of progelatinase A and progelatinase A complexed with TIMP-2. FEBS Lett. 1996;397(2–3):277–282. doi: 10.1016/s0014-5793(96)01206-9. [DOI] [PubMed] [Google Scholar]
- 10.Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol. 2004;22(11):1399–1408. doi: 10.1038/nbt1029. [DOI] [PubMed] [Google Scholar]
- 11.Will H, Atkinson SJ, Butler GS, et al. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271(29):17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- 12.Knäuper V, Will H, López-Otin C, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271(29):17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 13.Nam DH, Ge X. Direct production of functional matrix metalloproteinase-14 without refolding or activation and its application for in vitro inhibition assays. Biotechnol and Bioeng. 2016;113(4):717–723. doi: 10.1002/bit.25840. [DOI] [PubMed] [Google Scholar]
- 14.Hayhurst A, Happe S, Mabry R, et al. Isolation and expression of recombinant antibody fragments to the biological warfare pathogen Brucella melitensis. J Immunol Methods. 2003;276(1–2):185–196. doi: 10.1016/s0022-1759(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 15.Lee KB, Nam DH, Nuhn J, et al. Direct expression of active human tissue inhibitors of Metalloproteinase by periplasmic secretion in Escherichia coli. Microb Cell Fact. 2017 doi: 10.1186/s12934-017-0686-9. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam DH, Ge X. Development of periplasmic FRET-based screening method for protease inhibitory antibodies. Biotechnol and Bioeng. 2013;110(11):2856–2864. doi: 10.1002/bit.24964. [DOI] [PubMed] [Google Scholar]
- 17.Nam DH, Rodriguez C, Remacle AG, et al. Active-site MMP-selective antibody inhibitors discovered from convex paratope synthetic libraries. Proc Natl Acad Sci USA. 2017;113(52):14970–14975. doi: 10.1073/pnas.1609375114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman ER, Hayhurst A, Lingerfelt BM, et al. 2,4,6-Trinitrotoluene detection using recombinant antibodies. J Environ Monit. 2003;5(3):380–383. doi: 10.1039/b302012f. [DOI] [PubMed] [Google Scholar]