Abstract
Women with polycystic ovarian syndrome (PCOS) have evidence of subclinical cardiovascular disease (CVD). However, insulin resistance, an important factor in the development of CVD in adults, is common in adolescents with PCOS, yet data in adolescents are limited. Therefore, we sought to measure insulin resistance and CVD markers in obese youth with and without PCOS. Thirty-six PCOS and 17 non-PCOS adolescent girls who were obese, sedentary, and non-hypertensive were recruited from clinics located within the Children's Hospital Colorado. Following 3 days of controlled diet and restricted exercise, fasting plasma samples were obtained prior to a hyperinsulinemic euglycemic clamp. PCOS girls were more insulin resistant than controls (glucose infusion rate 5.24±1.86 mg/kg/min vs 9.10±2.69; p<0.001). Girls with PCOS had blood pressure in the normal range, but had greater carotid intima–media thickness (cIMT) (0.49±0.07 mm vs 0.44±0.06; p=0.038), beta stiffness index (5.1±1.3 U vs 4.4±0.9; p=0.037), and reduced arterial compliance (1.95±0.47 mm2/mmHg × 10−1 vs 2.13±0.43; p=0.047). PCOS girls had a normal mean lipid profile, yet had a more atherogenic lipoprotein cholesterol distribution and had persistent elevations of free fatty acids despite hyperinsulinemia (68±28 μmol/mL vs 41±10; p=0.001), both potential contributors to CVD. Free fatty acid concentrations correlated best with all CVD markers. In summary, adolescent girls with PCOS have greater cIMT and stiffer arteries than girls without PCOS, perhaps related to altered lipid metabolism, even when clinical measures of blood pressure and cholesterol profiles are ‘normal’. Therefore, management of adolescent PCOS should include assessment of CVD risk factor development.
Keywords: carotid intima–media thickness, cholesterol, obesity, pediatrics, polycystic ovarian syndrome, risk factors
Introduction
Polycystic ovarian syndrome (PCOS) is the most common endocrine disorder among women of reproductive age, affecting approximately 6–10% of women in the United States.1 Women with PCOS have higher rates of cardiovascular disease (CVD).2–5 Numerous studies report more atherogenic CVD markers early in PCOS, which is significant as CVD is the leading cause of mortality in women.6,7 PCOS is associated with multiple abnormalities including obesity, hypertension, dyslipidemia, and insulin resistance (IR).1 The prevalence of metabolic syndrome, which is associated with IR, is increased among both adult women and adolescent girls with PCOS.8 IR confers a significantly increased risk of CVD.9 The risk of CVD among women with PCOS is further increased by the presence of additional independent risk factors for CVD, such as hypertension, dyslipidemia, and obesity, which, as stated above, are frequently encountered with this condition.9
Women as young as 20–30 years old with PCOS have been shown to have metabolic abnormalities and evidence of accelerated CVD.2,10–14 Compared to weight-matched controls, women with PCOS in their 30s had elevated carotid intima–media thickness (cIMT) and a fourfold increase in the prevalence of coronary artery calcification.10,15 Arterial compliance and endothelial function are also altered in adults with PCOS.9,16–18 Women in their 40s with PCOS have early cardiac disease with reduced left ventricular ejection fraction, increased left ventricular mass, diastolic dysfunction, and dyslipidemia.19–24 In women younger than 40 years, PCOS is thought to be the most common cause of dyslipidemia, demonstrated by higher triglycerides and low-density lipoprotein cholesterol (LDL-C) with lower high-density lipoprotein cholesterol (HDL-C).23,25–27 These CVD risk markers may translate into clinical pathology, as a meta-analysis demonstrated post-menopausal women with pre-menopausal PCOS had a 2.02 relative risk of non-fatal coronary heart disease or stoke compared to non-PCOS controls.28 Further, the risk of cardiovascular events remained increased by 55% in studies that adjusted for body mass index (BMI).28
CVD risk data in adolescents with PCOS is more limited. The prevalence of hypertension in obese adolescents with PCOS was reported to be as high as 27%.8 Adolescents with PCOS were found to have elevated concentrations of plasminogen activating inhibitor-1, atherogenic lipid profiles and no overnight decrease in blood pressure (BP), which is associated with future risk of hypertension.11,12,29,30 However, it is not clear what influences the development of early and perhaps more aggressive CVD in PCOS.
Symptoms of PCOS typically begin in adolescence, but little is known about CVD at this stage. A better understanding of PCOS development in adolescence is needed, so more effective therapeutics can be initiated at the inception to prevent the development and progression of CVD. Adolescents with PCOS are at increased risk of developing CVD, but it is unclear if CVD risk is related to IR, to underlying contributors of IR such as elevated BMI, to hyperandrogenism or to a combination of these factors. Data also remain limited with respect to subclinical CVD using imaging or circulating endothelial function biomarkers in adolescents with PCOS. The objective of this study was to evaluate adolescents with PCOS for evidence of early CVD risk and related contributors such as IR or lipid measures.
Research and design methods
Study population
All participants (females 12–21 years of age) were recruited from polycystic ovarian syndrome, endocrine, and weight management clinics at the Children's Hospital Colorado, a tertiary care referral center in Aurora, Colorado, and enrolled as part of the PCOS or RESISTANT cohorts.31 Screening included a history, physical examination, pubertal Tanner staging and fasting laboratory testing, as previously described.32 Inclusion criteria for all participants included Tanner stage >1 and sedentary status (<3 hours of regular exercise per week) to minimize pubertal and training effects. Exclusion criteria for all participants included weight >250 pounds (113 kg), resting BP >140/90 mmHg, hemoglobin <9 mg/dL, serum creatinine >1.5 mg/dL, smoking, presence of diabetes, medications affecting IR (oral or inhaled steroids, metformin, thiazolidinediones, atypical antipsychotics), antihypertensive medications, statins, pregnancy, breastfeeding, or plans to alter exercise or diet during the study. PCOS was diagnosed per NIH criteria with adolescent adaptation: oligomenorrhea defined as less than eight menses a year, at least two years after menarche and clinical or biochemical signs of hyperandrogenism. Control participants were required to have regular menses and no biochemical features of PCOS. This study was approved by the University of Colorado Anschutz Medical Campus Institutional Review Board and the Children's Hospital of Colorado Scientific Advisory Review Committee. Informed consent was obtained from all participants 18–20 years of age; parental consent and participant assent was obtained for all participants less than 18 years of age.
The study day was preceded by 3 days of restricted physical activity and a fixed-macronutrient, weight-maintenance diet (55% carbohydrates, 30% fat, 15% protein).
Vascular and cardiac measures
All vascular and cardiac measures were performed fasting in the morning in the resting state. BP was recorded after resting for 15 minutes and the average of three readings was recorded. The cIMT, carotid artery beta stiffness index and compliance, and distension were assessed with ultrasound, as previously described.33 Briefly, far-wall cIMT was measured from a longitudinal two-dimensional (2D) B-mode image obtained with a Vivid 7 (General Electric, Waukesha, WI, USA) ultrasound system and analyzed using Vascular Analysis Tools software version 5.0 (MIA, Coralville, IA, USA), a computerized program that can measure IMT. The cIMT was defined as the distance from the leading edge of the lumen–intima interface to the leading edge of the media–adventitia interface. Because the near-wall IMT cannot be precisely measured on a consistent basis, lumen diameter was measured as the distance between the vessel far-wall boundary (corresponding to the interface between the lumen and intima) and a near-wall boundary (corresponding to the interface of the adventitia and media).34 All cIMT measurements were made at end diastole. The average of four separate lumen and IMT measurements was utilized for analysis. Carotid distension was defined as the difference between the diastolic and systolic carotid diameter measurements. The beta stiffness index was calculated as ln[(systolic BP/diastolic BP)/ (carotid distension/diastolic carotid diameter)]. Compliance was calculated as (carotid distension/diastolic carotid diameter)/[(2 × (systolic BP – diastolic BP)) × 3.1467 × (diastolic carotid diameter)2]. All images were coded by number, blinded to group assignment, and analyzed by the same individual with extensive experience in reading arterial imaging (U.T.).
Echocardiography
Resting supine 2D and tissue Doppler echocardiography was performed by a research sonographer using a Vivid 7 (General Electric) ultrasound system to exclude left ventricular systolic dysfunction (ejection fraction <50%), regional wall motion abnormalities, pericardial disease, or significant valvular pathology. Image analysis was completed with EchoPAC software (General Electric). Left ventricular dimensions and ejection fraction were obtained by standard m-mode and 2D volumetric method-of-discs analysis.35 Left ventricular mass was calculated as left ventricular mass (LVM) = 0.8 × 1.05 × [(IVSd + PWd + LVIDd)3 – LVIDd3)].35 Indexed LVM (LVMI) was calculated as LVM/height2.7.36 Using traditional pulse wave blood and tissue Doppler, the mitral inflow peak E and A wave velocities, deceleration time, and myocardial systolic (S′) and early diastolic (E′) velocities at the lateral and septal mitral valve annuli were measured using standard protocols.37
Speckle tracking was performed with EchoPAC software to assess cardiac strain. Global longitudinal strain curves were obtained from each of the three standard apical views. The parasternal short axis view at the papillary muscles was used to obtain circumferential strain. In each view, the endocardium was traced in end-systole. The epicardium was traced by defining the myocardial thickness such that the entire myocardium was included while excluding the pericardium. The software then generated strain curves by tracking and averaging the relative speed and location of defined patterns or ‘speckles’ within each segment. Only those segments that had an adequate number of traceable speckles were included. In order to obtain valid results, adequate tracking in at least four of the six segments had to be verified; otherwise, values from that view were discarded. Per standard techniques, peak strain was measured on strain curves at the time of aortic valve closure.38 Global circumferential strain was obtained from the curves in the short axis view that measured global strain as if the entire left ventricle was one segment (rather than an average of the individual segments). Global longitudinal strain was calculated as an average of the maximum global strain from the three apical views.
Following the vascular and cardiac measures, participants were then admitted to the inpatient Colorado Clinical Translational Research Center for an isocaloric meal, followed by 12 hours of overnight monitored fasting. Blood pressure was measured at admission (after being supine for 15 minutes) and every 2 hours during their admission. Blood pressure dipping was defined as having a difference of greater than 10 mmHg between the morning BP during vascular testing and the overnight nadir BP, recorded during the overnight admission.
The following morning, a three phase hyperinsulinemic euglycemic clamp (fasting, 10 mU/m2/min and 80 mU/m2/ min of insulin) was performed to estimate adipose and whole body insulin sensitivity, as previously described.39
At the start of the clamp procedure, fasting blood samples were obtained for concentrations of glucose, insulin, free fatty acids (FFAs), leptin, adiponectin, glycerol, hemoglobin A1c, estradiol, progesterone, DHEA-sulfate, anti-mullerian hormone, sex hormone binding globulin (SHBG), total testosterone, total cholesterol, HDL-C, LDL-C, triglycerides, highly sensitive C-reactive protein (hsCRP), C-peptide, and a complete blood count. Following the fasting blood draw, two intravenous lines were placed: one for blood sampling from a heated antecubital vein and one for an infusion of insulin and 20% dextrose in the contralateral arm. Primed, continuous low-dose insulin (10 mU/m2/min), followed by primed continuous high-dose insulin (80 mU/m2/min) was infused intravenously, with doses based on the physiologic IR of puberty,39 coupled with a variable infusion of 20% dextrose to keep blood sugars constant at 95 mg/dl. Plasma glucose was assayed at the bedside using a Yellow Springs Instrument (YSI Life Sciences, Yellow Springs, OH, USA). The glucose infusion rate, GIR (mg/kg/min), was measured based on steady-state measurements during the final 30 minutes of the hyperinsulinemic clamp. Fat free mass was determined from dual-energy X-ray absorptiometry, performed by standard methods, as previously described.39 During the last 30 minutes of the clamp, four samples, each 10 minutes apart, were drawn for glucose, FFA and insulin concentrations.
To describe the interaction between FFAs across the different insulin concentrations, the 50% inhibitory concentration (IC50) was calculated. The FFAs and log insulin were plotted, and the slope of the line used to calculate the insulin concentration at the location on the curve equal to 50% suppression of the baseline FFAs, similar to that described previously.40
Physical activity
A 3-day pediatric activity recall (3DPAR) was completed.41 Participants also wore an Actigraph GT3X accelerometer (ActiGraph Corp, Pensacola, FL, USA) for 7 days. The ActiGraph data were corrected for wear time and categorized into the following age appropriate activity levels: sedentary, light, lifestyle, moderate, vigorous and very vigorous.42
Dietary intake
Customary macronutrient pattern was ascertained by diet interview using a SEARCH food frequency questionnaire, modified to incorporate common food choices among ethnically and regionally diverse youth aged 10–19 years.43
Other variables
Weight and height were measured using standard methods, and BMI, defined as weight (kg) divided by height (m2), was calculated. Minimal waist circumference was measured. Hip circumference was performed at the level of the greater trochanter.
Laboratory analyses
All lab assays below except for the lipoprotein analysis were performed at the University of Colorado Clinical-Translational Research Center Laboratory or the Children's Hospital clinical lab. Serum insulin, leptin, and adiponectin were analyzed with radioimmunoassay (Millipore, Billerica, MA, USA); plasma glycerol (R-Biopharm, Marshall, MI, USA) and FFAs (Wako Chemicals, Inc., Richmond, VA, USA) were analyzed enzymatically. Hemoglobin A1c was measured by DCCT-calibrated ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Hercules, CA, USA). Estradiol and progesterone were measured via chemiluminescent immunoassay (Beckman Coulter, Brea, CA, USA). Urine micro-albumin was measured with nephelometry (Seimens, Tarrytown, NY, USA). Complete blood count was measured by Automated Coulter (Beckman Coulter, Brea, CA, USA). Total cholesterol, HDL-C, and triglyceride assays were performed enzymatically on a Hitachi 917 autoanalyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). LDL-C was calculated by the Friedewald equation for individuals with triglycerides <4.52 mmol/L and by Lipid Research Clinics Beta Quantification for those with triglycerides ≥4.52 mmol/L.44,45 Apolipoprotein B was measured with a turbidometric assay (Beckman Coulter). Highly sensitive-CRP was measured via immunoturbidi-metric assay (Beckman Coulter). C-peptide was measured via chemiluminescent immunoassay (DiaSorin, Stillwater, MN, USA).
DHEA-sulfate, anti-mullerian hormone and SHBG were measured via chemiluminescent immunoassay and total testosterone by LC/MS/MS, all by Esoterix (Calbassas Hills, CA, USA). Free androgen index (FAI) was calculated from total testosterone and SHBG.
Lipoprotein analysis was performed in the laboratory of Dr Robert Eckel using fast protein liquid chromatography (FPLC). Individual participant's ethylenediaminetetraacetic acid (EDTA) plasma samples (250 μL) were chromatographed using two Superose 6 columns in series as previously reported.29,46 Forty-nine 0.5-mL fractions were collected. Cholesterol was measured in each fraction using a commercially available kit (Cayman Chemical Company, Ann Arbor, MI, USA) following procedures outlined in the package insert. The cholesterol content in each fraction obtained from the FPLC was expressed as percent of the total cholesterol in all subfractions to adjust for differences in total cholesterol levels between participants adapting the methodology previously used in DCCT/EDIC.47 This was calculated by summing the cholesterol for an individual in all 49 fractions and expressing the result for each fraction as the cholesterol in that fraction divided by the summed cholesterol and multiplied by 100.
Statistical analyses
Variables were examined for normality using the Shapiro-Wilk test of normality. Continuous normally distributed data are presented as mean ± standard deviation and categorical data are presented as frequency (percentage). Differences in clinical, laboratory, clamp, exercise, echo-cardiographic, and vascular parameters between PCOS and control participants were examined using unpaired Student's t tests. Differences in categorical variables were evaluated using chi-squared tests. Associations between FFAs, LDL-C, hsCRP, GIR, GIR per fat free mass, and vascular risk markers, including cIMT, beta stiffness index, compliance and distension, left ventricular mass (measured and indexed), and cardiac strain were examined using Pearson correlation coefficients. Univariable and multivariable logistic regression (controlling for BMI percentile) was performed to identify associations with cIMT and IR. A p-value of <0.05 was considered statistically significant. Although a large number of comparisons were performed, these were exploratory in nature. We considered our major outcomes, cIMT and IR, as our primary outcomes and therefore multiple correction was not performed. Statistical Analysis Software (SAS, version 9.4; SAS Institute, Cary, NC, USA) was used for all analyses.
To test the significance of differences in cholesterol distributions between groups of participants, a difference plot was generated by subtracting the mean percent cholesterol value of each fraction in one group from the mean percent cholesterol value in the same fraction of the second group and determining the 95% confidence interval (CI) for this difference.46 A difference in fractional cholesterol content between groups is significant (p<0.05) when the 95% CI does not cross the zero line.
Results
Fifty-three participants, 36 obese PCOS and 17 obese controls, met our inclusion criteria and were included in the study. Table 1 displays the demographic and body composition characteristics of our study population. Significant differences were noted in terms of weight, BMI, and ethnicity. Although the PCOS group had a greater BMI, no significant differences were identified in body composition measured by waist-to-hip ratio or overall percent fat. Per study design, and as expected for girls with PCOS, total testosterone, FAI, and anti-mullerian hormone were significantly higher when compared to controls. Both groups were equally sedentary per the ActiGraph data and the 3-day physical activity questionnaire. Both groups also had a similar dietary intake of both micro and macronutrients including total calories, total fat, carbohydrate, protein, saturated fat, and fructose (data not shown), according to the dietary recall questionnaire.
Table 1.
Demographic and body composition characteristics of study participants.
| Obese PCOS (n=36) | Obese controls (n=17) | p-value | |
|---|---|---|---|
| Age, years | 14.81 ± 1.56 | 13.94 ± 1.75 | 0.077 |
| Weight, kg | 93.70 ± 11.26 | 84.59 ± 9.92 | 0.006 |
| Body mass index, kg/m2 | 35.60 ± 3.66 | 32.16 ± 2.57 | 0.001 |
| Body mass index, Z score | 2.22 ± 0.26 | 2.08 ± 0.22 | 0.061 |
| Body mass index percentile | 98.4 ± 1.4 | 97.8 ± 1.1 | 0.119 |
| Ethnicity | 0.043 | ||
| Non-Hispanic white | 13 (36.1%) | 6 (35.3%) | |
| Hispanic | 19 (52.8%) | 4 (23.5%) | |
| Black | 3 (8.3%) | 6 (35.3%) | |
| Other | 1 (2.8%) | 1 (5.9%) | |
| Waist-to-hip ratio | 0.89 ± 0.08 | 0.90 ± 0.09 | 0.514 |
| Fat, % | 42.89 ± 3.00 | 42.47 ± 4.84 | 0.745 |
| Free androgen index | 11.32 ± 7.11 | 3.64 ± 1.96 | <0.0001 |
| Total testosterone, ng/mL | 47±20 | 33±19 | 0.015 |
| Anti-mullerian hormone, ng/mL | 6.48 ± 3.17 | 4.21 ± 2.33 | 0.013 |
Continuous variables are presented as mean ± standard deviation; categorical variables as frequency (percentage).
A p-value in bold font indicates a significant difference between groups.
PCOS, polycystic ovarian syndrome.
Cardiac and vascular markers are displayed by group in Table 2. No significant differences were identified in terms of systolic BP, diastolic BP, non-dipper status during monitored admission, or classification of pre-hypertensive status. Urine microalbumin:creatinine ratio, a significant correlate of BP and a marker of microvascular damage, was not significantly different among groups. Compared to obese adolescents without PCOS, cIMT was significantly higher in those with PCOS (p=0.038). Although no differences were detected in carotid distention, the carotid artery beta stiffness index was significantly higher and carotid artery compliance was significantly lower in obese adolescents with PCOS (p=0.037, p=0.047, respectively). No echocardiographic differences were noted between the groups (full data not shown).
Table 2.
Cardiac and vascular markers separated by group.
| Obese PCOS (n=36) | Obese controls (n=17) | p-value | |
|---|---|---|---|
| Systolic BP, mmHg | 114± 7 | 113 ± 7 | 0.621 |
| Diastolic BP, mmHg | 65 ± 7 | 66 ± 5 | 0.368 |
| Non-dipper | 9 (25.0%) | 1 (5.9%) | 0.082 |
| Pre-hypertensive | 6 (16.7%) | 2 (11.8%) | 0.299 |
| Urine microalbumin:creatinine ratio, mcg/mg creatinine | 25.22 ± 47.82 | 28.80 ± 393.50 | 0.817 |
| cIMT, mm | 0.49 ± 0.07 | 0.44 ± 0.06 | 0.038 |
| Carotid distention, mm | 0.82 ± 0.19 | 0.87 ± 0.17 | 0.275 |
| Beta stiffness index, U | 5.1 ± 1.3 | 4.4 ± 0.9 | 0.037 |
| Carotid compliance, mm2/mmHg × 10−1 | 1.95 ± 0.47 | 2.13 ± 0.43 | 0.047 |
| LV mass index, g/m2.7 | 31.94 ± 6.10 | 31.86 ± 13.83 | 0.977 |
| Fractional shortening, % | 35.19 ± 6.56 | 33.01 ± 6.83 | 0.270 |
| Global longitudinal strain, % | −17.48 ± 2.17 | −16.80 ± 3.22 | 0.370 |
| Global circumferential strain, % | −22.58 ± 4.81 | −22.59 ± 3.54 | 0.994 |
Continuous variables are presented as mean ± standard deviation; categorical variables as frequency (percentage).
A p-value in bold font indicates a significant difference between groups.
PCOS, polycystic ovarian syndrome; BP, blood pressure; cIMT, carotid intima–media thickness; LV, left ventricular.
Table 3 lists the metabolic and lipid markers. Adolescents with PCOS had a significantly higher C-peptide concentration compared to those without PCOS (p=0.002). GIR was significantly lower in those with PCOS (p<0.0001). This difference remained significant after correcting for lean mass (p<0.0001). The 10 and 80 phase FFA and insulin were significantly higher in obese adolescents with PCOS than those without PCOS (p=0.0004, p=0.029, respectively, for 10 and p=0.001, p=0.014, respectively, for 80). The IC50 value for FFAs was also significantly higher in those with PCOS (p=0.001). Although total cholesterol and triglycerides were not significantly different, those with PCOS had lower HDL-C and higher LDL-C and apolipoprotein B levels in comparison to those without PCOS (p=0.040, p=0.044, p=0.019, respectively). Absolute differences in FPLC lipoprotein cholesterol distribution by PCOS status is displayed in Figure 1. Adolescents with PCOS had LDL and very-low-density lipoprotein (VLDL) cholesterol distributions that were increased when compared to those without PCOS. Moreover, girls with PCOS also had less cholesterol distribution in HDL fractions than controls and a trend for more cholesterol in HDL in two of the last five fractions.
Table 3.
Metabolic and lipid markers separated by group.
| Obese PCOS (n=36) | Obese controls (n=17) | p-value | |
|---|---|---|---|
| Hemoglobin A1c, % | 5.4 ± 0.3 | 5.4 ± 0.2 | 0.913 |
| C-peptide, ng/mL | 3.4 ± 0.9 | 2.5 ± 0.7 | 0.002 |
| Total cholesterol, mg/dL | 165 ± 32 | 154 ± 33 | 0.257 |
| Triglyceride, mg/dL | 126 ± 53 | 108 ± 50 | 0.249 |
| HDL-C, mg/dL | 36 ± 6 | 42 ± 9 | 0.040 |
| LDL-C, mg/dL | 115 ± 29 | 97 ± 26 | 0.044 |
| Apolipoprotein B, mg/dL | 95.13 ± 20.24 | 78.45 ± 17.10 | 0.019 |
| Fasting glucose, mg/dL | 85 ± 5 | 85 ± 5 | 0.950 |
| Glucose infusion rate, mg/kg/min | 5.24 ± 1.86 | 9.10 ± 2.69 | <0.0001 |
| Glucose infusion rate fat free mass, mg/lean kg/min | 9.76 ± 3.43 | 16.15 ± 4.33 | <0.0001 |
| Fasting free fatty acids, μmol/L | 630 ± 134 | 622 ± 1685 | 0.889 |
| 10 phase free fatty acids, μmol/L | 267 ± 148 | 143 ± 71 | 0.0004 |
| 80 phase free fatty acids, μmol/L | 62 ± 31 | 32 ± 14 | 0.001 |
| Fasting insulin, μIU/mL | 27 ± 12 | 20 ± 9 | 0.126 |
| 10 phase insulin, μIU/mL | 59 ± 23 | 42 ± 21 | 0.029 |
| 80 phase insulin, μIU/mL | 266 ± 55 | 209 ± 50 | 0.014 |
| Free fatty acid IC50, μIU/mL | 68 ± 28 | 41 ± 10 | 0.001 |
Continuous variables are presented as mean ± standard deviation.
A p-value in bold font indicates a significant difference between groups.
PCOS, polycystic ovarian syndrome; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; IC50, insulin concentration needed for 50% suppression from fasting free fatty acids concentration.
Figure 1.
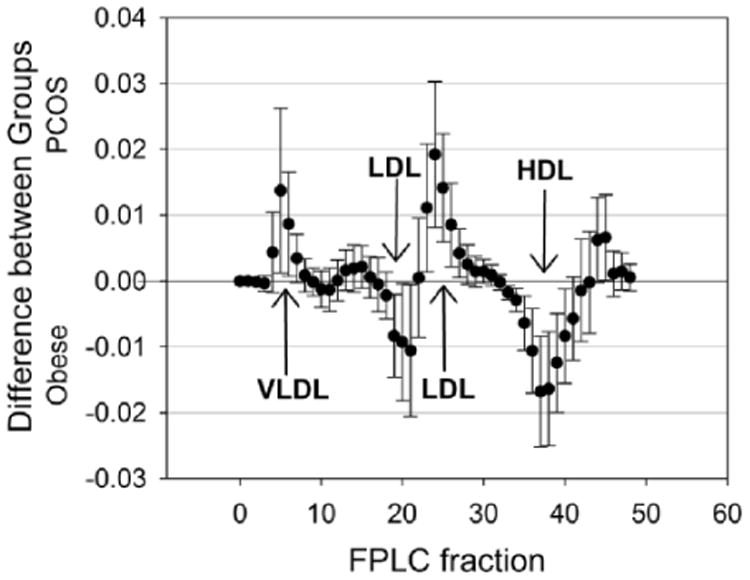
Differences in FPLC lipoprotein distribution by PCOS status (PCOS minus control). A mean above zero indicates more cholesterol in PCOS participants and a mean below zero indicates less. Fractions where the confidence interval does not cross zero are statistically significant. Arrows indicate areas of fractions in which statistically significant differences exist. FPLC, fast protein liquid chromatography; PCOS, polycycstic ovarian syndrome; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; VLDL, very low-density lipoprotein.
Inflammatory and adipokine markers are displayed in Table 4. No significant differences were noted among hsCRP, white blood cell count, or leptin. Baseline adiponectin levels were significantly lower in those with PCOS (p=0.001) and platelet counts were significantly higher (p=0.033).
Table 4.
Inflammatory and adipokine markers separated by group.
| Obese PCOS (n=36) | Obese controls (n=17) | p-value | |
|---|---|---|---|
| Highly sensitive C-reactive protein, mg/dL | 4.0 ± 3.4 | 2.4 ± 5.0 | 0.183 |
| White blood cell count, 103/mL | 7.95 ± 1.50 | 6.87 ± 2.84 | 0.156 |
| Platelets, 103/mL | 280 ± 38 | 253 ± 48 | 0.033 |
| Leptin, ng/mL | 36 ± 15 | 37 ± 15 | 0.821 |
| Adiponectin, ng/mL | 5.5 ± 2.0 | 9.3 ± 3.3 | 0.001 |
Continuous variables are presented as mean ± standard deviation.
A p-value in bold font indicates a significant difference between groups.
PCOS, polycystic ovarian syndrome.
Free fatty acid concentrations during this highest dose of insulin were univariately associated with multiple measures of CVD risk including carotid compliance (r = −0.32, p = 0.049), carotid beta stiffness index (r = 0.45, p = 0.004), LV mass (r = 0.40, p = 0.012), and LV mass index (r = 0.33, p = 0.043) in the entire cohort (Table 5). Cardiac specific measures related to inflammation (measured by hsCRP vs fractional shortening (r = −0.45, p = 0.0006)) and IR (measured by GIR per fat free mass vs LV mass (r = −0.34, p = 0.019), vs LV mass index (r = −0.32, p = 0.028), and vs global longitudinal strain (r = −0.31, p = 0.038)). No associations were identified between GIR, total testosterone, and FAI and vascular measures, even with controlling for BMI percentile (full data not shown).
Table 5.
Correlation of metabolic and inflammatory measures with cardiac and vascular markers.
| r | p-value | |
|---|---|---|
| Free fatty acids | ||
| Carotid distension, mm | −0.12 | 0.489 |
| Carotid compliance, mm2/mmHg × 10−1 | −0.32 | 0.049 |
| Beta stiffness index, U | 0.45 | 0.004 |
| cIMT, mm | 0.08 | 0.639 |
| LV mass index, g/m2.7 | 0.33 | 0.043 |
| Fractional shortening, % | 0.07 | 0.684 |
| Global longitudinal strain, % | 0.10 | 0.547 |
| Global circumferential strain, % | 0.05 | 0.752 |
| Highly sensitive C-reactive protein | ||
| Carotid distension, mm | −0.11 | 0.430 |
| Carotid compliance, mm2/mmHg × 10−1 | −0.18 | 0.192 |
| Beta stiffness index, U | 0.03 | 0.804 |
| cIMT, mm | 0.14 | 0.321 |
| LV mass, g | −0.04 | 0.755 |
| LV mass index, g/m2.7 | 0.05 | 0.746 |
| Fractional shortening, % | −0.45 | 0.0006 |
| Global longitudinal strain, % | −0.16 | 0.243 |
| Global circumferential strain, % | 0.04 | 0.774 |
| Glucose infusion rate per fat free mass | ||
| Carotid distension, mm | 0.01 | 0.928 |
| Carotid compliance, mm2/mmHg × 10−1 | 0.18 | 0.241 |
| Beta stiffness index, U | −0.25 | 0.095 |
| cIMT, mm | −0.06 | 0.679 |
| LV mass, g | −0.34 | 0.019 |
| LV mass index, g/m2.7 | −0.32 | 0.028 |
| Fractional shortening, % | −0.04 | 0.772 |
| Global longitudinal strain, % | −0.31 | 0.038 |
| Global circumferential strain, % | −0.04 | 0.770 |
Data are Pearson correlation coefficients.
A p-value in bold font indicates a significant association.
cIMT, carotid intima–media thickness; LV, left ventricular.
Discussion
We found that relatively healthy obese adolescents with PCOS had more atherogenic lipoprotein lipid profiles on FPLC, greater cIMT and arterial stiffening when compared to obese youth with regular menses, despite having mean standard lipid panels and blood pressures that were in the normal range. Although the PCOS group had a significantly higher LDL-C, it would not meet clinical criteria for pharmacologic management. The FPLC lipoprotein profile was overall more atherogenic as the adolescents with PCOS had increased dense LDL fractions and decreased less dense HDL fractions. The girls with PCOS had significant peripheral IR and also evidence of adipose IR, with decreased suppression of FFAs during hyperinsulinemia. Thus, even at an average age of 15 years, girls with PCOS have more atherogenic markers of CVD risk compared to those without PCOS.
The elevated FFA concentrations in the PCOS girls seem particularly important, as the FFAs during the high dose of the insulin clamp were most strongly related to both cardiac and vascular markers. Elevated FFAs have been associated with markers of CVD risk as well as CVD outcomes. We found that fasting FFAs were not different between the groups; however, FFA concentrations during two levels of hyperinsulinemia were higher in PCOS compared to controls. This finding implies that girls with PCOS have post-prandial elevations in FFAs throughout the day which could contribute to CVD risk. Acute experimental elevations of FFAs have been shown to increase inflammatory substances (vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and plasminogen activator inhibitor-1 (PAI-1)), which are implicated in the development of arterial stiffening.48,49 Elevated fasting FFAs are associated with brachial arterial stiffness measured by pulse wave velocity and with an increased risk incident of cardiac failure.50,51 FFAs during hyperinsulinemia also correlated well with coronary artery calcification in adults with type 1 diabetes.52 Chronic daytime elevations of FFAs in girls with PCOS could lead to increased vascular-specific inflammatory markers and the development of arterial stiffening and thickening of the intimal and medial layers of the arterial wall.
Variations in the lipoprotein subfraction cholesterol distribution by FPLC with type 1 diabetes offer markers of CVD risk beyond the standard fasting lipid profile. We have previously shown alteration in these profiles for both adults and youth with type 1 diabetes, who have an increased risk of CVD mortality.46,53 The adult women with type 1 diabetes had a shift of LDL fractions to the denser more atherogenic type.46 In youth with type 1 diabetes, LDL was shifted towards denser fractions, with the adolescent females having a more ‘male’ atherogenic profile.53 Further, profiles were worse in those participants with more IR. More recently, we have demonstrated that these youth with type 1 diabetes also have markers of early CVD risk, including increased cardiac strain and vascular stiffening.31 Lower levels of less dense HDL particles were associated with hypertension in a large cohort of adults without diabetes, as well as the severity of coronary artery disease in patients presenting with angina.54,55 Thus, the profile of increased cholesterol distribution in VLDL and LDL, and decreased distribution in more buoyant HDL, is associated with markers and outcomes of CVD, and is consistent with our CVD findings in youth with PCOS.
Carotid intima–media thickness in women and girls with PCOS is often, but not always, elevated and can be decreased with different therapies. Greater cIMT has been well demonstrated in older women with PCOS.56–58 Additionally, cIMT was associated with an unhealthier lipid profile and higher arterial BP.59 In addition to greater cIMT in older women with PCOS, several studies have shown that younger women with PCOS also had greater cIMT, regardless of obesity status.15,56–58,60 However, two recent studies in adolescents with PCOS did not find differences in cIMT in youth with PCOS compared to controls.61,62 One possibility for this discrepancy is that measures of cIMT can vary widely among different sonographers and readers. All of our cIMT measures were performed by two calibrated research sonographers, and interpreted by one pediatric cardiologist who was blinded to the group status. Thus, it is possible that our study has less variability. One year of lifestyle intervention with weight loss in adolescents with PCOS was associated with improvements in both BP and a decrease in cIMT.63 Treatment with oral contraceptives worsened cIMT in women in their 20s with PCOS, whereas combination therapy with oral contraceptives and metformin decreased cIMT.64 More recently, it has been demonstrated that a 6-month structured exercise program results in a reduction of cIMT, whereas those on oral contraceptives had no change in measurements.65 Based on these studies, impacts of PCOS therapies on cIMT and other markers of CVD risk need to be considered in order to reduce the development of CVD. By design, our groups were sedentary, and accelerometer and questionnaire data showed that they were equally inactive; therefore the role of exercise habits on cIMT could not be assessed in our cohort.
In women with PCOS, the role of hyperandrogenism per se in CVD development has been difficult to distinguish from the effect of obesity and IR. CVD in PCOS may be related to IR, as cardiac and endothelial dysfunction, greater cIMT, and dyslipidemia are associated with IR in adults.10,19,20,26 A longitudinal study of obese teens without PCOS found that IR at age 13 years, independent of BMI, was the best indicator of development of CVD markers by age 19 years.66 In addition, markers of CVD risk such as elevated BP and endothelial dysfunction have also been demonstrated to be associated with androgen concentrations, independent of obesity in adult women.16,17,67 Androgens may have a direct effect on BP, as evidenced by higher average BP in males than females, and a correlation between androgens and BP in healthy women without PCOS.68,69 Androgens are thought to increase angiotensinogen and contribute to hypertension via modulation of volume status.70 In women with PCOS, plasma androgen concentrations were the best predictor for hypertension, although 65% of subjects in this study were lean, and obesity was not adequately controlled for.67 Further, androgens may also contribute to the dyslipidemia typical of PCOS, as evidenced by more atherogenic lipid profiles in men versus women.71–73
Finally, markers of inflammation have been shown to be increased in women and adolescents with PCOS and were assessed in our study.74,75 We found that hsCRP was related to cardiac measures, suggesting a role of inflammation in CVD risk, as has been demonstrated by previous studies in adults.76 Although hsCRP and white blood cell count were not statistically significant in this study, they trended higher in those with PCOS, and platelets were significantly higher in the PCOS girls, all consistent with a tendency towards a more inflammatory state.
Strengths and Limitations
There are several strengths and limitations to our study. Strengths include use of the gold standard measurement for peripheral and adipose insulin sensitivity with a three phase hyperinsulinemic euglycemic clamp. We used the NIH definition of PCOS, which is the most conservative diagnostic criteria for PCOS. We also controlled for variations in physical activity, diet and menstrual stage, all known to influence insulin sensitivity. Our exclusion criteria eliminated those with significant complications of PCOS, including hypertension, diabetes and morbid obesity. Therefore, our study population was comprised of relatively healthy obese girls. One of the limitations of the study was that the PCOS group was significantly more obese than the control group. However, the difference was not clinically large, as the PCOS group was slightly older and thus the BMI-Z scores are more similar. Further, in terms of specific adiposity, the percent body fat and waist-to-hip ratio did not differ between groups. In addition, our sample size was relatively small, particularly in the non-PCOS obese group. The control group was recruited from the same referral clinics as the PCOS group, which may represent referral bias. Lastly, as multivariable analyses were performed, collinearity may be present between related measures.
Conclusions
In conclusion, we found that healthy obese adolescents with PCOS had a more atherogenic lipid profile by FPLC and greater cIMT and arterial stiffening compared to obese adolescents without PCOS. Because their standard lipid profiles and blood pressures were not clinically abnormal, more attention should be placed on more sensitive early CVD risk factor markers in adolescents with PCOS, as evidenced by the significant differences detectable when compared to controls as early as 15 years of age. One potential area of CVD risk factor regression may be interventions that improve FFA profiles.
Acknowledgments
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: KJN: NCRR K23 RR020038-01, NIH/NCRR Colorado CTSI Co-Pilot Grant TL1 RR025778, NIH/NIDDK 1R56DK088971-01, JDRF5—2008-291, ADA 7-11-CD-08; MCG: AHA 13CRP 14120015, Thrasher Pediatric Research Foundation Mentored Pilot Grant, NIH/NCRR Colorado CTSI Co-Pilot Grant TL1 RR025778, Pediatric Endocrinology Society Fellowship, Endocrine Society Fellowship in Women's Health, NIDDK T32 DK063687, BIRCWH K12HD057022. This research was also supported by Adult CTRC NIH Grant M01-RR00051, Pediatric CTRC NIH Grant 5MO1-RR00069 and NIH/NCRR Colorado CTSI Grant UL1 RR025780.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Blank SK, Helm KD, McCartney CR, et al. Polycystic ovary syndrome in adolescence. Ann N Y Acad Sci. 2008;1135:76–84. doi: 10.1196/annals.1429.005. [DOI] [PubMed] [Google Scholar]
- 3.Barcellos CR, Rocha MP, Hayashida SA, et al. Prevalence of abnormalities of glucose metabolism in patients with poly-cystic ovary syndrome. Arq Bras Endocrinol Metabol. 2007;51:601–605. doi: 10.1590/s0004-27302007000400015. [DOI] [PubMed] [Google Scholar]
- 4.Ehrmann DA, Barnes RB, Rosenfield RL, et al. Prevalence of impaired glucose tolerance and diabetes in women with poly-cystic ovary syndrome. Diabetes Care. 1999;22:141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 5.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 6.Heron M. Deaths: Leading causes for 2004. National Vital Statistics Reports. 2007;56:1–96. [PubMed] [Google Scholar]
- 7.Elci E, Kaya C, Cim N, et al. Evaluation of cardiac risk marker levels in obese and non-obese patients with polycystic ovaries. Gynecol. 2016 Jul 16; doi: 10.1080/09513590. Endocrinol Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 9.Meyer C, McGrath BP, Cameron J, et al. Vascular dysfunction and metabolic parameters in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4630–4635. doi: 10.1210/jc.2004-1487. [DOI] [PubMed] [Google Scholar]
- 10.Vural B, Caliskan E, Turkoz E, et al. Evaluation of metabolic syndrome frequency and premature carotid atherosclerosis in young women with polycystic ovary syndrome. Hum Reprod. 2005;20:2409–2413. doi: 10.1093/humrep/dei100. [DOI] [PubMed] [Google Scholar]
- 11.Rossi B, Sukalich S, Droz J, et al. Prevalence of metabolic syndrome and related characteristics in obese adolescents with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4780–4786. doi: 10.1210/jc.2008-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: Roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86:66–71. doi: 10.1210/jcem.86.1.7123. [DOI] [PubMed] [Google Scholar]
- 13.Shroff R, Kerchner A, Maifeld M, et al. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab. 2007;92:4609–4614. doi: 10.1210/jc.2007-1343. [DOI] [PubMed] [Google Scholar]
- 14.Lewy VD, Danadian K, Witchel SF, et al. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138:38–44. doi: 10.1067/mpd.2001.109603. [DOI] [PubMed] [Google Scholar]
- 15.Vryonidou A, Papatheodorou A, Tavridou A, et al. Association of hyperandrogenemic and metabolic phenotype with carotid intima-media thickness in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:2740–2746. doi: 10.1210/jc.2004-2363. [DOI] [PubMed] [Google Scholar]
- 16.Kelly CJ, Speirs A, Gould GW, et al. Altered vascular function in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:742–746. doi: 10.1210/jcem.87.2.8199. [DOI] [PubMed] [Google Scholar]
- 17.Paradisi G, Steinberg HO, Hempfling A, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. 2001;103:1410–1415. doi: 10.1161/01.cir.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 18.Kravariti M, Naka KK, Kalantaridou SN, et al. Predictors of endothelial dysfunction in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:5088–5095. doi: 10.1210/jc.2005-0151. [DOI] [PubMed] [Google Scholar]
- 19.Orio F, Jr, Palomba S, Spinelli L, et al. The cardiovascular risk of young women with polycystic ovary syndrome: An observational, analytical, prospective case-control study. J Clin Endocrinol Metab. 2004;89:3696–3701. doi: 10.1210/jc.2003-032049. [DOI] [PubMed] [Google Scholar]
- 20.Yarali H, Yildirir A, Aybar F, et al. Diastolic dysfunction and increased serum homocysteine concentrations may contribute to increased cardiovascular risk in patients with poly-cystic ovary syndrome. Fertil Steril. 2001;76:511–516. doi: 10.1016/s0015-0282(01)01937-9. [DOI] [PubMed] [Google Scholar]
- 21.Tiras MB, Yalcin R, Noyan V, et al. Alterations in cardiac flow parameters in patients with polycystic ovarian syndrome. Hum Reprod. 1999;14:1949–1952. doi: 10.1093/humrep/14.8.1949. [DOI] [PubMed] [Google Scholar]
- 22.Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. 2005;90:5711–5716. doi: 10.1210/jc.2005-0011. [DOI] [PubMed] [Google Scholar]
- 23.Talbott E, Guzick D, Clerici A, et al. Coronary heart disease risk factors in women with polycystic ovary syndrome. Arterioscler Thromb Vasc Biol. 1995;15:821–826. doi: 10.1161/01.atv.15.7.821. [DOI] [PubMed] [Google Scholar]
- 24.Conway GS, Agrawal R, Betteridge DJ, et al. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;37:119–125. doi: 10.1111/j.1365-2265.1992.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 25.Wild RA. Polycystic ovary syndrome: A risk for coronary artery disease? Am J Obstet Gynecol. 2002;186:35–43. doi: 10.1067/mob.2002.119180. [DOI] [PubMed] [Google Scholar]
- 26.Robinson S, Henderson AD, Gelding SV, et al. Dyslipidaemia is associated with insulin resistance in women with polycystic ovaries. Clin Endocrinol (Oxf) 1996;44:277–284. doi: 10.1046/j.1365-2265.1996.674495.x. [DOI] [PubMed] [Google Scholar]
- 27.Wild RA, Rizzo M, Clifton S, et al. Lipid levels in polycystic ovary syndrome: Systematic review and meta-analysis. Fertil Steril. 2011;95:1073–1079.e1–11. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 28.De Groot PC, Dekkers OM, Romijn JA, et al. PCOS, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis. Hum Reprod Update. 2011;17:495–500. doi: 10.1093/humupd/dmr001. [DOI] [PubMed] [Google Scholar]
- 29.Glueck CJ, Morrison JA, Friedman LA, et al. Obesity, free testosterone, and cardiovascular risk factors in adolescents with polycystic ovary syndrome and regularly cycling adolescents. Metabolism. 2006;55:508–514. doi: 10.1016/j.metabol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Zachurzok-Buczynska A, Szydlowski L, Gawlik A, et al. Blood pressure regulation and resting heart rate abnormalities in adolescent girls with polycystic ovary syndrome. Fertil Steril. 2011;96:1519–1525. doi: 10.1016/j.fertnstert.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 31.Bjornstad P, Truong U, Pyle L, et al. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: A RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) Study. J Diabetes Complications. 2016;30:1103–1110. doi: 10.1016/j.jdiacomp.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maahs DM, Nadeau K, Snell-Bergeon JK, et al. Association of insulin sensitivity to lipids across the lifespan in people with Type 1 diabetes. Diabet Med. 2011;28:148–155. doi: 10.1111/j.1464-5491.2010.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreau KL, Donato AJ, Seals DR, et al. Arterial intimamedia thickness: Site-specific associations with HRT and habitual exercise. Am J Physiol Heart Circ Physiol. 2002;283:H1409–1417. doi: 10.1152/ajpheart.00035.2002. [DOI] [PubMed] [Google Scholar]
- 34.Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 35.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 36.De Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 37.Quinones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 38.Sun JP. Myocardial Imaging: Future Applications of Speckle Tracking Echocardiography. 1st. Malden, MA: Blackwell Publishing; 2007. [Google Scholar]
- 39.Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95:513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergman BC, Howard D, Schauer IE, et al. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab. 2012;97:1663–1672. doi: 10.1210/jc.2011-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weston AT, Petosa R, Pate RR. Validation of an instrument for measurement of physical activity in youth. Med Sci Sports Exerc. 1997;29:138–143. doi: 10.1097/00005768-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 43.Mayer-Davis EJ, Nichols M, Liese AD, et al. Dietary intake among youth with diabetes: The SEARCH for Diabetes in Youth Study. J Am Diet Assoc. 2006;106:689–697. doi: 10.1016/j.jada.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 45.Hainline A, Jr, Miller DT, Mather A. The Coronary Drug Project. Role and methods of the Central Laboratory. Control Clin Trials. 1983;4:377–387. doi: 10.1016/0197-2456(83)90023-5. [DOI] [PubMed] [Google Scholar]
- 46.Maahs DM, Hokanson JE, Wang H, et al. Lipoprotein sub-fraction cholesterol distribution is proatherogenic in women with type 1 diabetes and insulin resistance. Diabetes. 2010;59:1771–1779. doi: 10.2337/db09-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purnell JQ, Hokanson JE, Marcovina SM, et al. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: Results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998;280:140–146. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathew M, Tay E, Cusi K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol. 2010;9:9. doi: 10.1186/1475-2840-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krauzova E, Kracmerova J, Rossmeislova L, et al. Acute hyperlipidemia initiates proinflammatory and proatherogenic changes in circulation and adipose tissue in obese women. Atherosclerosis. 2016;250:151–157. doi: 10.1016/j.atherosclerosis.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 50.Djousse L, Benkeser D, Arnold A, et al. Plasma free fatty acids and risk of heart failure: The Cardiovascular Health Study. Circ Heart Fail. 2013;6:964–969. doi: 10.1161/CIRCHEARTFAILURE.113.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabara Y, Takahashi Y, Setoh K, et al. Synergistic association of elevated serum free fatty acid and glucose levels with large arterial stiffness in a general population: The Nagahama Study. Metabolism. 2016;65:66–72. doi: 10.1016/j.metabol.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Schauer IE, Snell-Bergeon JK, Bergman BC, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60:306–314. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cree-Green M, Maahs DM, Ferland A, et al. Lipoprotein subfraction cholesterol distribution is more atherogenic in insulin resistant adolescents with type 1 diabetes. Pediatr Diabetes. 2016;17:257–265. doi: 10.1111/pedi.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Li S, Xu RX, et al. Distribution of high-density lipoprotein subfractions and hypertensive status: A cross-sectional study. Medicine. 2015;94:e1912. doi: 10.1097/MD.0000000000001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu RX, Li S, Li XL, et al. High-density lipoprotein subfractions in relation with the severity of coronary artery disease: A Gensini score assessment. J Clinical Lipidol. 2015;9:26–34. doi: 10.1016/j.jacl.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Calderon-Margalit R, Siscovick D, Merkin SS, et al. Prospective association of polycystic ovary syndrome with coronary artery calcification and carotid-intima-media thickness: The Coronary Artery Risk Development in Young Adults Women's study. Arterioscler Thromb Vac Biol. 2014;34:2688–2694. doi: 10.1161/ATVBAHA.114.304136. [DOI] [PubMed] [Google Scholar]
- 57.Teng HW, Chien YW, Hsu MI, et al. The relationship between carotid intima-media thickness and endogenous androgens in young women with polycystic ovary syndrome in Taiwan. Gynecol Endocrinol. 2013;29:238–241. doi: 10.3109/09513590.2012.736553. [DOI] [PubMed] [Google Scholar]
- 58.Allameh Z, Rouholamin S, Adibi A, et al. Does carotid intima-media thickness have relationship with polycystic ovary syndrome? Int J Prev Med. 2013;4:1266–1270. [PMC free article] [PubMed] [Google Scholar]
- 59.Macut D, Antic IB, Bjekic-Macut J. Cardiovascular risk factors and events in women with androgen excess. J Endocrinol Invest. 2015;38:295–301. doi: 10.1007/s40618-014-0215-1. [DOI] [PubMed] [Google Scholar]
- 60.Guleria AK, Syal SK, Kapoor A, et al. Cardiovascular disease risk in young Indian women with polycystic ovary syndrome. Gynecol Endocrinol. 2014;30:26–29. doi: 10.3109/09513590.2013.831835. [DOI] [PubMed] [Google Scholar]
- 61.Deveer M, Deveer R, Basaran O, et al. Serum copeptin, pentraxin 3, anti-Mullerian hormone levels with echocardiography and carotid artery intima-media thickness in adolescents with polycystic ovary syndrome. J Clin Med Res. 2015;7:989–994. doi: 10.14740/jocmr2375w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughan KS, Tfayli H, Warren-Ulanch JG, et al. Early bio-markers of subclinical atherosclerosis in obese adolescent girls with polycystic ovary syndrome. J Pediatr. 2016;168:104–111.e1. doi: 10.1016/j.jpeds.2015.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lass N, Kleber M, Winkel K, et al. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab. 2011;96:3533–3540. doi: 10.1210/jc.2011-1609. [DOI] [PubMed] [Google Scholar]
- 64.Kaya MG, Yildirim S, Calapkorur B, et al. Metformin improves endothelial function and carotid intima media thickness in patients with PCOS. Gynecol Endocrinol. 2015;31:401–405. doi: 10.3109/09513590.2015.1006188. [DOI] [PubMed] [Google Scholar]
- 65.Orio F, Muscogiuri G, Giallauria F, et al. Oral contraceptives versus physical exercise on cardiovascular and metabolic risk factors in women with polycystic ovary syndrome: A randomized controlled trial. Clin Endocrinol (Oxf) 2016;85:764–771. doi: 10.1111/cen.13112. [DOI] [PubMed] [Google Scholar]
- 66.Sinaiko AR, Steinberger J, Moran A, et al. Influence of insulin resistance and body mass index at age 13 on systolic blood pressure, triglycerides, and high-density lipoprotein cholesterol at age 19. Hypertension. 2006;48:730–736. doi: 10.1161/01.HYP.0000237863.24000.50. [DOI] [PubMed] [Google Scholar]
- 67.Chen MJ, Yang WS, Yang JH, et al. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49:1442–1447. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 68.Mantzoros CS, Georgiadis EI, Young R, et al. Relative androgenicity, blood pressure levels, and cardiovascular risk factors in young healthy women. Am J Hypertens. 1995;8:606–614. doi: 10.1016/0895-7061(95)00051-P. [DOI] [PubMed] [Google Scholar]
- 69.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 70.Quan A, Chakravarty S, Chen JK, et al. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol. 2004;287:F452–459. doi: 10.1152/ajprenal.00188.2003. [DOI] [PubMed] [Google Scholar]
- 71.Wild RA, Painter PC, Coulson PB, et al. Lipoprotein lipid concentrations and cardiovascular risk in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1985;61:946–951. doi: 10.1210/jcem-61-5-946. [DOI] [PubMed] [Google Scholar]
- 72.Chen MJ, Yang WS, Yang JH, et al. Low sex hormone-binding globulin is associated with low high-density lipoprotein cholesterol and metabolic syndrome in women with PCOS. Hum Reprod. 2006;21:2266–2271. doi: 10.1093/humrep/del175. [DOI] [PubMed] [Google Scholar]
- 73.Fruzzetti F, Perini D, Lazzarini V, et al. Adolescent girls with polycystic ovary syndrome showing different phenotypes have a different metabolic profile associated with increasing androgen levels. Fertil Steril. 2009;92:626–634. doi: 10.1016/j.fertnstert.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: A systematic review and metaanalysis. Fertil Steril. 2011;95:1048–1058.e2. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cinar M, Aksoy RT, Guzel AI, et al. The predictive role of serum cystatin C levels in polycystic ovary syndrome in adolescents. J Pediatr Adolesc Gynecol. 2016;29:353–356. doi: 10.1016/j.jpag.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 76.Munoz-Durango N, Fuentes CA, Castillo AE, et al. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: Molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17070797. [DOI] [PMC free article] [PubMed] [Google Scholar]


