Abstract
Impulsivity associated with abnormal dopamine (DA) function has been observed in several disorders, including addiction. Choice impulsivity is the preference for small, immediate rewards over larger rewards after a delay, caused by excessive discounting of future rewards. Addicts have abnormally high discount rates and prefer the smaller rewards sooner. While impulsivity has been inversely correlated with DA D2 receptor (D2R) availability in the midbrain and striatum, it is difficult to mechanistically link the two, due to the diverse neuroanatomical localization of D2Rs, which are found throughout the brain, in many types of neurons and neuronal subcompartments. To determine if ventral tegmental area (VTA) D2R hypofunction is linked to impulsivity, we knocked down D2 receptors from the VTA, using an adeno-associated viral (AAV) vector that delivers short hairpin RNAs (shRNA) targeted against the D2R. The D2R knockdown is restricted to neurons whose cell bodies reside in the VTA, leaving postsynaptic D2Rs intact in the striatum, prefrontal cortex, and other mesocorticolimbic structures. Rats were trained in a delay-discounting task to assess impulsive choice until a stable discounting curve was obtained, and then received bilateral VTA infusions of the D2R shRNA or a scrambled control virus. Over the next six weeks, the discounting curve of the VTA D2R knockdown rats shifted to the left, indicating a preference for the smaller, immediate reward, whereas the curve for control rats remained stable and unchanged. Together these results demonstrate that a decrease in VTA D2 receptors enhances choice impulsivity.
Keywords: D2 autoreceptor, VTA, rat, delay-discounting, impulsivity
1. INTRODUCTION
Dopamine (DA) release from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) is critical for calculating the value of rewards and determining the appropriate response for obtaining them [1]. For example, DA plays a role in delay-discounting, in which increasing the interval between the onset of work (e.g. lever press) and onset of reward delivery results in decreasing value of the reward, which decays in a hyperbolic fashion as the interval increases [2]. When DA transmission is disrupted, preference shifts towards smaller, more immediate rewards and away from larger rewards with longer delays. Conversely, increasing DA via amphetamine leads to increased willingness of the subjects to wait for larger rewards [3]. The selection of smaller immediate rewards represents an operational measure for choice impulsivity, while the preference for delayed, but more profitable reinforcers indicates self-control [4]. Mesolimbic dopamine mediates many aspects of choice calculations, including the reinforcing value of the reward, and the ability to attend to the task over time. Several studies also implicate dopamine in impulsivity (see [5] for review). Imaging studies in rats and humans suggest that impulsive subjects have less DA D2 receptor (D2R) binding sites and decreased DA release in the striatum [6–8]. Further, the decline in reinforcing strength of a reward in delay discounting is exaggerated in impulsive subjects (i.e. the delay-discounting curve shifts to the left, [9]). Drug and alcohol abusers, who score consistently higher in impulsivity tests, also have decreased D2R binding in the striatum [1]. In particular, these individuals reliably prefer smaller, immediate rewards over larger delayed rewards [10]. While it is clear that D2R expression is correlated with states of impulsivity, it is not known if low D2R levels predispose the subject to impulsivity or is simply a compensatory change resulting from other alterations in impulsive subjects.
D2Rs are located both pre- and postsynaptically, and these receptor subpopulations have entirely different functions. The presynaptic and dendrodendritic D2Rs on DA neurons act as autoreceptors, in which their activation inhibits adenylyl cyclase activity, thus limiting DA synthesis and release [11,12]. Thus, while a D2R antagonist will block postsynaptic D2R sites, its activity at presynaptic D2Rs will increase DA neurotransmission at postsynaptic DA receptors, primarily D1 and D2 [13,14]. However, since the D2 autoreceptor is found in much lower amounts on DA terminals relative to the postsynaptic D2R in the striatum, the previously mentioned imaging studies likely do not reflect changes in D2 autoreceptors [6], and thus it is unknown to what extent these may also contribute to impulsive choice behaviors.
Recently, we have developed a viral approach to manipulate D2R subpopulations in adult wild type rats, using RNA interference to knockdown the D2Rs. Adeno-associated virus serotype 10 (AAV10) infects the neuronal cell bodies at the injection site [15,16]. Thus, when injected directly into the striatum they induce a knockdown in the postsynaptic D2R, which is found primarily on indirect medium spiny neurons [17]. However, when injected into the midbrain, these viruses will knock down the receptor from neurons whose cell bodies reside in this region, predominantly from DA neurons, including the D2 autoreceptors on the DA terminals projecting to a variety of regions including the NAc [18]. We have previously characterized the phenotype of substantia nigra D2 autoreceptor knockdown rats, and found they demonstrated behaviors consistent with hyperdopaminergia, altered responses to psychostimulants and decreases in DA reuptake [18]. In the present study, we have sought to determine the role of VTA D2Rs in regulating impulsivity in rats trained in a delay-discounting task.
2. MATERIAL AND METHODS
2.1 Subjects
A cohort of sixteen male (n = 16) Sprague–Dawley (Harlan, Indianapolis, IN) rats were housed individually on a 12/12-h light/dark cycle (experiments conducted during the light period) with free access to water in the home cage. A food restriction protocol (10 g/day) was employed to facilitate lever press training. Thereafter, subjects’ body weights increased during the course of experimentation commensurate with age from start to completion of the experiment. A separate cohort of rats (n=5) underwent surgery, their brains were removed 2–4 weeks after viral infusion, and VTA punches were collected and processed for quantitative PCR to determine the extent of D2R knockdown. All animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York, and with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
2.2 Viruses
All viruses have been previously described [18]. Briefly, a mixture of D2 short hairpin (shRNA) viruses are co-infused into the VTA (ML: ±1.1, AP: −4.7, DV: −8.5, in mm from bregma), with each shRNA targeting a different location on the D2 mRNA. The control virus consisted of a scrambled (SCR) shRNA that does not pair with any known RNA sequence in the rat genome. Viruses were prepared as previously described [18,19] by triple transfection of AAV2/10 rep/cap, pHelper and shRNA plasmids, which provide the capsid, helper virus and vector genomes, respectively [20]. The AAV plasmid contains two inverted terminal repeats (ITRs) flanking a transgene consisting of the cytomegalovirus (CMV) promoter driving expression of enhanced green fluorescent protein (EGFP), and a combined intron/polyA sequence derived from SV40. This is followed by the mouse U6 promoter which drives expression of an shRNA specific to either the rat D2R mRNA or a non-specific scrambled shRNA sequence. The shRNA sequence consists of a sense, a loop containing an XhoI site, the antisense segment, followed by a TTTTTT terminator sequence. All sequences were screened to ensure lack of homology with other RNA sequences in the rat.
2.3 Quantitative Real-Time PCR
A cohort of rats was unilaterally infused with 1.0 μl of shD2R virus (n = 5) into the VTA. The rats were sacrificed between 2–4 weeks post-infusion and 2 mm coronal brain punches were taken from both the ipsi- and contra-lateral sides. Total RNA was isolated using an RNeasy Micro Kit (Qiagen, Hilden, Germany). Total RNA (500 ng) was reverse transcribed into cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA) and real-time qPCR using a Bio-Rad Miniopticon Real-Time PCR system (Bio-rad, Hercules, CA). cDNAs were analyzed with sybr assays for: rat Enolase 2 (ENO2), rat D2 dopamine receptor (DRD2), rat beta-actin (BACT) and EGFP. The following primer sets were used. ENO2: ACGTCTGGCGAAGTACAACC (forward), GTCGGGACAGCAAGAAAGAG (reverse), BACT: CATCCTGCGTCTGGACCTGG (forward), TAATGTCACGCACGATTTCC (reverse). DRD2 was quantified using the Bio-Rad PrimePCR™ SybrR® Assay (qRnoCID0001883). The stably expressing housekeeping gene beta-actin was used as a reference and relative quantification of gene expression levels was assessed using the comparative CT method (2−ΔΔCT method) between the gene of interest and Eno2 [21]. The GFP copy number was generated by running a standard curve with linearized CMV-EGFP-pACP plasmid and calculating a total copy number per 1 μl of cDNA. Data were analyzed by student’s t-test and p < 0.05 were considered significant.
2.4 Apparatus
Experimental sessions were conducted in commercially available chambers within sound-attenuating, ventilated enclosures (Coulbourn Instruments Inc., Allentown, PA, USA) as described previously [22]. Each chamber contained a working area of 30.5 cm by 24.5 cm by 21.0 cm, a grid floor, and a 45-mg pellet dispenser with a pellet receptacle that was centered between response levers, above which were stimulus lights. A 28 V houselight was mounted on the rear aluminum wall of the chamber. Chambers were connected to a computer running Graphic State 3.03 software and an interface (Coulbourn Instruments Inc.) to control experimental events and record data.
2.5 Procedure
Initially rats were trained to press on either of two levers for delivery of food pellets (45-mg dustless precision pellets; BioServ Inc., Frenchtown, New Jersey, USA) under a fixed ratio (FR) 1 schedule. Training continued over 35 sessions until stable responding levels were achieved. The delay-discounting task used in the current study was similar to the procedure described by Evenden and Ryan [23] with minor modifications [22]. Sessions consisted of five 12-minute blocks. Each block began with 2 forced trials followed by 10 choice trials. The onset of the houselight signaled the beginning of each trial. During a forced trial a light over one of the levers was illuminated and a response on that lever delivered the reinforcer (one food pellet immediately or three food pellets either immediately or after a delay) that was associated with that lever during subsequent choice trials in that block. During choice trials lights were illuminated over both levers and response on either lever produced a reinforcer. After food pellet presentation signaled by a brief food receptacle light flash (0.1 s), the receptacle and stimulus lights would extinguish until the start of the next trial.
For all rats, responses on the left lever resulted in a brief receptacle light flash (0.1 s) followed by the delivery of one food pellet. Following the delivery of the food pellet was a timeout period that lasted for a variable period (equal to 60 s minus the delay), so the period of time between the end of one response period and the beginning of the subsequent response period was always the same. During the timeout period, all lights were off and responding had no programmed consequence. Each response period lasted for a maximum of 10 s (limited hold); if a response on the active lever did not occur during that time, the stimulus light(s) were extinguished, and a 60-second timeout period was initiated. Sessions were conducted daily at approximately the same time and lasted 60 min. The block of trials began with an increased delay to the three-food-pellet alternative which increased across the five blocks of twelve trials (i.e., 0, 5, 10, 20, 40 s). Stable responding was defined as obtaining at least 80% choice for the larger reinforcer during the 0-s delay block and no increasing or decreasing trends in delay-discounting functions via consecutive 4 days visual inspection. Sessions without any delay in all 5 blocks were conducted every 5–7 sessions to confirm that switching the choices within the sessions was due to delay and not to others factors. No-delay sessions, with both reinforcers available immediately after a response, occurred daily until responding for the larger reinforcer in the last block of the session (previously associated with the longest delay) was greater than 80%. However, in most cases animals adapted to the no-delay contingency within one session (these data were not shown). Delays were reintroduced in the next session. Training in the delay-discounting task continued for six weeks.
2.6 Surgery
Subjects were matched for baseline performance within 4 consecutive days, divided into two groups randomly, and anesthetized with ketamine hydrochloride (80mg/kg, i.p.) and xylazine hydrochloride (10mg/kg, i.p.). Rats were fitted into a digital stereotaxic apparatus (Stoelting Co., Wood Dale, IL), the scalp was shaved, cleaned and an incision made along the center. Two holes were marked and drilled above the VTA using the following coordinates: AP: −4.7, ML: ±1.1, DV: −8.5 (mm from bregma). The coordinates were determined using the stereotaxic atlas of Paxinos & Watson [24]. A 10μl Hamilton syringe was used to deliver 0.5μl of viral vector or control virus at a flow rate of 0.25μl/min with a 5-minute waiting period after the completion of each injection to allow for maximal diffusion of the virus from the tip of the syringe. The syringe was slowly retracted and the incision was then closed with Webcryl 3.0 dissolvable sutures. Rats were relocated to a tub-style cage placed on top of a heating pad for recovery. Rats were administered ketoprofen (5mg/kg, s.c.) immediately following surgery, as well as 24- and 48-hours post-surgery.
2.7 Experimental Design
After acquisition training on the delay-discounting task, rats (n = 16) were randomly selected to infuse either shD2R or a scrambled (shSCR) control virus into the VTA. Subjects were allowed four days to recuperate after surgery, before re-entering the delay-discounting task. The delay-discounting task was conducted daily for the following six weeks. Data from the same day of the week were analyzed and compared to baseline data. At the end of the behavioral study all rats were transcardially perfused with 10% formalin, the brains collected, sectioned and immunofluorescence for EGFP was performed as previously described [18,25]. One rat from each group was removed due to misplaced viral infusions.
2.8 Data Analysis
All data were collected and analyzed using Graphpad Prism 6.0. The % responding for the larger reinforcer was determined and the subsequent area under the curve (AUC) was calculated for each animal. Baseline and six week post-infusion data were analyzed by repeated measures two-way ANOVA, with the time delay and virus as factors, and baseline/week 6 and time delay as repeated factors. Significant effects were probed using Dunnett’s post-hoc tests to determine if any difference existed between this control session (baseline data from rats prior to shSCR viral infusions) and data generated from shSCR treated rats six weeks later as well as both baseline and six week shD2R data. The AUC data were analyzed by repeated measures two-way ANOVA, with weeks post-infusion and virus as factors. Previous studies indicate that the shD2R knockdown virus may take several weeks to take effect, therefore we conducted planned comparisons at 4, 5, and 6 weeks post-viral infusions, consisting of two-tailed t-tests between the shSCR and shD2R virus groups.
3. RESULTS
3.1 Site specific depletion of D2R from VTA neurons
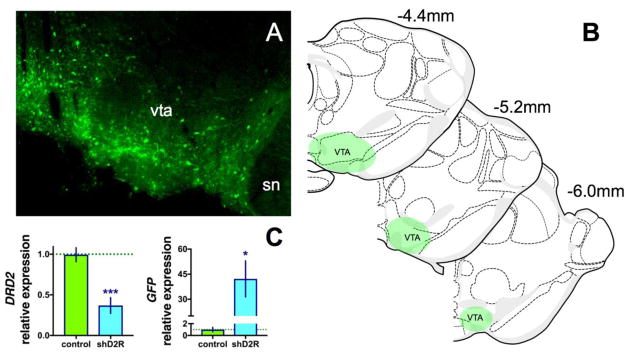
AAV10 transduces primarily the neuronal cell bodies at the site of the injection (Cearley and Wolfe, 2006), thus by injecting into the VTA the shRNA will be restricted to neurons in this region, including DA neurons containing D2 autoreceptors in dendrodendritic and terminal compartments. In previous studies, we demonstrated that infusion of the D2R shRNA viruses infused into the dorsal striatum (DS) significantly decreases both D2R mRNA and protein levels in the DS [17] while infusing the virus into the substantia nigra (SN) resulted in a ~90% decrease in DRD2 mRNA in the SN with no alteration in postsynaptic D2R in the DS [18]. Using these same viruses, we infused the VTA and observed GFP+ neurons throughout the VTA (Figure 1a and b). We also demonstrate that D2R mRNA levels are likewise significantly decreased after D2R shRNA-AAV infusions into the VTA (Figure 1c). Real-time qPCR analysis revealed an ~65% decrease in DRD2 levels compared to the contralateral side. The mean relative expression of DRD2 was 0.994 ± 0.09 SEM relative units for the contralateral control side, and 0.369 ± 0.10 relative units on the virus infused side (student’s two tailed t-test, p < 0.001). There was an ~45 fold increase in GFP mRNA copy number on the virus infused side (mean of 42.6 ± 10.8 relative copy number) compared to the non-infused side (mean of 1 ± 0.38, p < 0.05).
Fig. 1. Extent of viral transduction and knockdown.
A representative immunofluorescence image of the VTA in a rat receiving the scrambled (shSCR) virus (a), and the extent of transduction through the anterior-posterior axis (b). D2 dopamine receptor (DRD2) and green fluorescent protein (GFP) mRNA levels relative to actin (c). *p<0.05. shD2R, short hairpin dopamine D2 receptor; SN, substantia nigra; VTA, ventral tegmental area.
3.2 Effect of VTA D2R knockdown on delay-discounting behaviors
Delay discounting was measured as % responding for the larger reinforcer. In Fig. 2a, baseline data from two groups of rats on the day prior to surgery is represented, along with the % responding curve from six weeks post-viral infusion with either shD2R or shSCR control viruses. These data demonstrate remarkably stable responding between baseline and six weeks after shSCR control viral infusion. However, in rats treated with the shD2R virus in the VTA, we observed a progressive shift in the delay-discounting curves, indicating an increased preference for the smaller reward without delay, over time. For rats designated to receive shSCR viral infusions, prior to surgery the overall mean % responding on the larger lever for the entire 1 hour session ± SEM, was 72.20 ± 8.58, while the shD2R group was 67.34 ± 12.54 (both n = 7). Six weeks after surgery, the shSCR treated rats were still responding at this level, demonstrating an overall mean % responding on the larger lever of 71.32 ± 11.41. However, at this time the % responding had fallen considerably in the shD2R treated rats, resulting in a mean of 49.4 ± 16.42%. A two-way ANOVA was conducted on the influence of virus conditions (shSCR or shD2R baselines and 6 weeks) and delay on % responding for the larger lever. All effects were statistically significant with α < 0.05. The main effect for virus yielded an F ratio of F(3, 18) = 3.884, p = 0.0265, while the main effect of delay was F(4,24) = 57.98, p<0.0001. The interaction was significant, F(12, 72) = 2.725, p = 0.0043. A Dunnett’s post-hoc analysis was conducted in which each virus group/time was compared to the shSCR baseline data (i.e. pre-viral infusion). Neither the shD2R group baseline results nor the six-week post-shSCR infusion differed from this shSCR baseline control group. However, six weeks after infusion of the shD2R virus into the VTA there was a significant decrease in % responding on the larger lever with the addition of a 5, 10, 20 and 40 s delay, indicating a shift in preference to the lever delivering a smaller reward without delay.
Fig. 2. Effects of VTA shD2R knockdown on delay discounting.
Rats were trained in the task, baseline data collected, and then infused with virus. The mean delay-discounting curve for both groups at baseline and six weeks after shD2R or shSCR control viral infusion (a). The mean area under the curve (AUC) ± SEM from each group every week from the entire study (b). Asterisks designate significance at *p<0.05, **p<0.01, and ****p<0.0001.
The AUC from each individual rat’s delay-discounting curve was quantified and the weekly data analyzed (Fig. 2b) to determine the onset of the shift in responding. The mean AUC for the control shSCR group remained extremely consistent throughout the experiment resulting in a mean AUC ± SEM of 0.65 ± 0.07 at baseline and 0.62 ± 0.07, 0.65 ± 0.07, 0.62 ± 0.05, 0.60 ± 0.07, 0.67 ± 0.09, and 0.62 ± 0.06 at weeks one through six, respectively. However, for the shD2R virus treated rats, the AUC decreased from a baseline of 0.571 ± 0.07 to 0.461 ± 0.08, 0.647 ± 0.08, 0.460 ± 0.062, 0.407 ± 0.07, 0.347 ± 0.08 and 0.343 ± 0.05 for weeks one through six post-virus infusion, respectively. Overall, there was a significant effect of virus, F(1, 2) = 5.800, p = 0.033, but the effect of week did not achieve significance, F(6, 72) = 1.942, p = 0.0855. There was also no significant interaction, F(6, 72) = 1.750, p = 0.1218. Planned comparisons using the Dunnet’s post-hoc tests revealed that the mean shD2R were significantly lower than SCR controls at week 4, 5 and 6 post-viral infusion into the VTA.
4. DISCUSSION
Low D2R expression in the striatum has been linked to higher levels of impulsivity in humans [26,27] and potentially increased risk of drug abuse (review [28]). Further, D2 autoreceptors in the midbrain have been linked with higher trait impulsivity [29] and increased steepness in delay-discounting curves [30,31]. However, these studies have largely been retrospective and have not defined whether down-regulation of striatal D2Rs is predisposing or causative of an impulsive phenotype, or if impulsive behaviors and/or repeated drug use lead to compensatory striatal D2R down-regulation. Further, the aforementioned imaging studies that support this hypothesis cannot readily distinguish D2R subpopulations, particularly given the relatively low levels of presynaptic D2Rs compared to the more abundant postsynaptic D2R population on medium spiny neurons. Yet D2 autoreceptors are highly involved in regulating DA release and reuptake in the striatum. Evidence for genetic influence on impulsive behaviors has been shown in healthy volunteers where polymorphisms in the D2R gene were associated with self-report measures of impulsivity [32]. Our results clearly demonstrate that viral knockdown of D2Rs from the VTA results in a substantial shift in the delay-discounting performance of the rats with time.
The delay-discounting task specifically measures an animal’s choice impulsivity, and the extent to which it is able to withstand a delay in order to make an advantageous response. The role of D2Rs in delay discounting, particularly in animal models, has been unclear, with some studies demonstrating an inverse relationship between the steepness of discounting curves and striatal D2R levels [30], while D2R antagonists either had no effect or shifted the discounting curve to the left (suggesting increased impulsivity, [5]). Our results clearly demonstrate that viral knockdown of D2Rs from the VTA results in a substantial shift in the delay-discounting performance of the rats with time. The shSCR infused control rats exhibited a remarkably stable delay-discounting curve over the length of the study (greater than two months). However, rats infused with shD2R virus in the VTA exhibited a leftward shift in the delay-discounting curve, characterized by an overall preference for the smaller reward with shorter delay indicating reduced waiting capacity, and thus impulsive choice. This shift in preference was progressive and developed over time, with the AUC decreasing within one week of viral infusion, and achieving significance within four weeks.
While several pharmacological studies have sought to determine if D2R agonists or antagonists alter impulsivity, even when the ligands are microinfused into the striatum they bind to both pre- and postsynaptic D2R populations. Likewise, global D2R knockouts lack both pre- and postsynaptic D2R, while conditional knockouts (e.g. DAT-Cre x floxed-D2R knockouts) lack D2 autoreceptors from both the VTA and substantia nigra. Therefore, many of these genetic approaches are likely to have developmental compensatory adaptations from the lack of D2R early in life. A primary benefit of a viral manipulation is the ability to restrict the knockdown to the VTA, while sparing nigral D2R, as well as postsynaptic D2R in projection regions, such as the PFC and NAc. Further, the viruses can be infused into adult animals which overcomes developmental compensation commonly seen in transgenic mice.
The improved spatial and temporal resolution of viral manipulation may provide important evidence to understand the underlying roles of the VTA D2R subpopulations in impulsivity. Recently we showed that viral knockdown of the D2R from the SN of rats resulted in profound neurochemical alterations within two weeks of virus infusion, including changes in DA release and reuptake, primarily a decrease in DA per pulse and Vmax of the DA transporter (DAT) in the dorsal striatum. Further, the rats displayed an increase in locomotor activity consistent with a hyperdopaminergic state. While this increase in activity was apparent within two weeks post-SN infusion, it did not reach significance until four weeks post-infusion [18]. Likewise, in our current study we did not see a significant alteration in the discounting curve until four weeks post-infusion. Together, these data indicate that many of the behavioral alterations observed are not a direct result of the acute effects of midbrain D2R genetic depletion on neurochemistry, but rather require a continued exposure and possibly adaptations to the altered neurochemistry. Since shD2R virus infused into the VTA leads to a comparable decrease in D2R mRNA as in our previous SN study [18] and DAT and D2 autoreceptors co-localize in mesostriatal presynaptic terminals [33], it is likely that VTA shD2DR will also alter regulation of DA neurotransmission in the mesostriatal pathway, resulting in increased DA levels in the NAc. Accumulating evidence indicates that impulsive behavior involves abnormal DA transmission and is not only top-down controlled by cortical areas, but also modulated at subcortical level [34]. Increased DA levels in the medial prefrontal cortex have been associated with rats’ performance in a delay-discounting task [35]. However, it is difficult to speculate how D2R knockdown from the VTA may change mesocortical DA signaling in the PFC, and contribute to the delay-discounting phenotype, as cortical regions have less DAT expression [36,37].
Future studies should be conducted to determine how shD2R knockdown in the VTA and potential neurochemical alterations in DA dynamics impact postsynaptic systems. Indeed, VTA DA neurons project widely throughout the brain and current genetic and viral techniques have not defined the role of specific projections to these behaviors. It should be noted that within both the SN and VTA, D2Rs are also found on resident GABA neurons, although relatively higher amounts are found on DA neurons, with some estimates of ~1000:1 mRNA levels between DA and GABA neurons [38]. The viruses used in this study transduce both DA and GABA neurons, and thus D2Rs will be knocked down in both populations. Activation of VTA-GABA D2Rs by DA enhances the neuronal firing rates of these GABA neurons. Approximately 80% of VTA-GABA neurons are thought to be inhibitory interneurons. Therefore, when DA is introduced into the VTA there will likely be a primary effect of decreasing DA activity by activating inhibitory D2Rs on the DA neurons, and indirect inhibition by activating excitatory D2Rs on GABA neurons, which will inhibit the DA neurons further. Conversely, D2R knockdown in both populations should block the overall effect DA on VTA DA neurons, specifically by a primary decrease in autoreceptor inhibition by knocking down the D2 autoreceptor, and additionally decreased GABA activation by DA. Further studies, using subtype-specific viruses would be useful to understanding the proportional contribution of each mechanism.
Midbrain DA function is critical in many other systems including maintenance of working memory, the regulation of emotion, executive function and cognitive control [39]. Disruptions in VTA DA projections impair spatial working memory [40] and contribute to the manifestation of depressive-like behavior in rats [41]. Further, the D2R pathway connecting the VTA and the basolateral amygdala modulates fear and anxiety [42]. Thus, VTA D2R likely play a role in other DA-dependent phenotypes. In delay-discounting tasks, impulsive subjects express reduced waiting capacity and altered time perception [43]. Temporal control requires intact DA signaling in corticostriatal systems [44]. Low-frequency delta/theta activity can index time-based decision-making processes [45]. In line with this, both Parkinson’s disease patients and mice with disrupted mesocortical DA show deficits in temporal processing, which can be compensated by stimulation of medial frontal cortex D1R at these frequencies [46]. VTA DA projections to the PFC, in turn, have been shown to influence temporal control via D1Rs [47]. These findings indicate that VTA DA projections are heavily involved in higher order executive functions, with a potential critical role of VTA D2 autoreceptors. Thus, it is likely that the shift in delay-discounting observed with VTA D2R knockdown will impact several other DA related behaviors. Our viral approach presents one means to tease the role of VTA D2Rs in these respective phenotypes in future experiments.
In summary, in this study we have demonstrated conclusively that decreasing VTA D2R levels leads to impaired temporal discounting. These results indicate that adaptations in the mesolimbic dopaminergic circuitry which produce a decrease in VTA D2Rs can lead to an impulsive phenotype, and may mechanistically explain some of the lack of inhibitory control in maintaining abstinence in substance use disorder, or the increased risk [48] of impulse control disorders in patients with Parkinson’s disease.
Acknowledgments
This work was supported by the National Institutes of Health grants K01DA024763, R21DA043190 (CEB), R01DA034806 (JXL) and the D’Youville College Faculty Research Grant (KAB-S). We thank Dr. Rodrigo España for his assistance.
Abbreviations
- AAV
adeno-associated virus
- AUC
area under the curve
- BACT
beta-actin mRNA
- CMV
cytomegalovirus
- D2R
dopamine D2 receptor
- D1R
dopamine D1 receptor
- DA
dopamine
- DRD2
D2R mRNA
- DS
dorsal striatum
- EGFP
enhanced green fluorescent protein
- ENO2
Enolase 2 mRNA
- FR
fixed ratio
- ITR
inverted terminal repeats
- SCR
scrambled
- shRNA
short hairpin RNA
- SN
substantia nigra
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trifilieff P, Ducrocq F, van der Veldt S, Martinez D. Blunted Dopamine Transmission in Addiction: Potential Mechanisms and Implications for Behavior. Semin Nucl Med. 2017;47:64–74. doi: 10.1053/j.semnuclmed.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Mazur JE. Quantitative analyses of behavior, Vol. 5. The effect of delay and of intervening events on reinforcement value. Lawrence Erlbaum Associates; Hillsdale, NJ: 1987. [Google Scholar]
- 3.de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 4.Bizot J, Le Bihan C, Puech AJ, Hamon M, Thiébot M. Serotonin and tolerance to delay of reward in rats. Psychopharmacology. 1999;146:400–412. doi: 10.1007/pl00005485. [DOI] [PubMed] [Google Scholar]
- 5.Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Annals of the New York Academy of Sciences. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkow ND, Fowler JS, Wang G-J, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 8.Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Laane K, et al. Nucleus Accumbens D2/3 Receptors Predict Trait Impulsivity and Cocaine Reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters J, Büchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci. 2009;29:15727–15734. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu J-M, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological Reviews. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 13.Anzalone A, Lizardi-Ortiz JE, Ramos M, De Mei C, Hopf FW, Iaccarino C, et al. Dual Control of Dopamine Synthesis and Release by Presynaptic and Postsynaptic Dopamine D2 Receptors. Journal of Neuroscience. 2012;32:9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bello EP, Mateo Y, Gelman DM, Noaín D, Shin JH, Low MJ, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britt JP, McDevitt RA, Bonci A. Use of channelrhodopsin for activation of CNS neurons. Curr Protoc Neurosci. 2012;Chapter 2(Unit2.16–2.16.19) doi: 10.1002/0471142301.ns0216s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Molecular Therapy. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Blume LC, Bass CE, Childers SR, Dalton GD, Roberts DCS, Richardson JM, et al. Striatal CB1 and D2 receptors regulate expression of each other, CRIP1A and δ opioid systems. Journal of Neurochemistry. 2013;124:808–820. doi: 10.1111/jnc.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budygin EA, Oleson EB, Lee YB, Blume LC, Bruno MJ, Howlett AC, et al. Acute Depletion of D2 Receptors from the Rat Substantia Nigra Alters Dopamine Kinetics in the Dorsal Striatum and Drug Responsivity. Front Behav Neurosci. 2016;10:248. doi: 10.3389/fnbeh.2016.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, et al. Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front Behav Neurosci. 2013;7:173. doi: 10.3389/fnbeh.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao X, Li J, Samulski RJ. Production of High-Titer Recombinant Adeno-Associated Virus Vectors in the Absence of Helper Adenovirus. Journal of Virology. 1998;72:2224–2232. doi: 10.1111/j.1749-6632.1995.tb44735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Siemian JN, Xue Z, Blough BE, Li J-X. Comparison of some behavioral effects of d- and l-methamphetamine in adult male rats. Psychopharmacology. 2017;234:2167–2176. doi: 10.1007/s00213-017-4623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- 25.Gompf HS, Budygin EA, Fuller PM, Bass CE. Targeted genetic manipulations of neuronal subtypes using promoter-specific combinatorial AAVs in wild-type animals. Front Behav Neurosci. 2015;9:152. doi: 10.3389/fnbeh.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volkow ND, Wang G-J, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2014;76(Pt B):498–509. doi: 10.1016/j.neuropharm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532–532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballard ME, Mandelkern MA, Monterosso JR, Hsu E, Robertson CL, Ishibashi K, et al. Low Dopamine D2/D3 Receptor Availability is Associated with Steep Discounting of Delayed Rewards in Methamphetamine Dependence. Int J Neuropsychopharmacol. 2015;18:pyu119. doi: 10.1093/ijnp/pyu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joutsa J, Voon V, Johansson J, Niemelä S, Bergman J, Kaasinen V. Dopaminergic function and intertemporal choice. Transl Psychiatry. 2015;5:e520. doi: 10.1038/tp.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamidovic A, Dlugos A, Skol A, Palmer AA, de Wit H. Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: an exploratory study with d-amphetamine in healthy participants. Experimental and Clinical Psychopharmacology. 2009;17:374–383. doi: 10.1037/a0017840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J Comp Neurol. 1997;388:211–227. [PubMed] [Google Scholar]
- 34.Jupp B, Caprioli D, Saigal N, Reverte I, Shrestha S, Cumming P, et al. Dopaminergic and GABA-ergic markers of impulsivity in rats: evidence for anatomical localisation in ventral striatum and prefrontal cortex. Eur J Neurosci. 2013;37:1519–1528. doi: 10.1111/ejn.12146. [DOI] [PubMed] [Google Scholar]
- 35.Winstanley CA, Theobald DEH, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006;16:106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- 36.Freed C, Freed C, Revay R, Revay R, Vaughan RA, Vaughan RA, et al. Dopamine transporter immunoreactivity in rat brain. J Comp Neurol. 1995;359:340–349. doi: 10.1002/cne.903590211. [DOI] [PubMed] [Google Scholar]
- 37.Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv Pharmacol. 1998;42:171–174. doi: 10.1016/s1054-3589(08)60720-6. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9327871&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 38.Steffensen SC, Taylor SR, Horton ML, Barber EN, Lyle LT, Stobbs SH, et al. Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels. Eur J Neurosci. 2008;28:2028–2040. doi: 10.1111/j.1460-9568.2008.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bissonette GB, Roesch MR. Development and function of the midbrain dopamine system: what we know and what we need to. Genes, Brain and Behavior. 2016;15:62–73. doi: 10.1111/gbb.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martig AK, Mizumori SJY. Ventral tegmental area disruption selectively affects CA1/CA2 but not CA3 place fields during a differential reward working memory task. Hippocampus. 2011;21:172–184. doi: 10.1002/hipo.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter C, von Rumohr A, Mundt A, Petrus D, Klein J, Lee T, et al. Lesions of dopaminergic neurons in the substantia nigra pars compacta and in the ventral tegmental area enhance depressive-like behavior in rats. Behavioural Brain Research. 2007;184:133–141. doi: 10.1016/j.bbr.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 42.de Oliveira AR, Reimer AE, de Macedo CEA, de Carvalho MC, de Silva MAS, Brandão ML. Conditioned fear is modulated by D2 receptor pathway connecting the ventral tegmental area and basolateral amygdala. Neurobiology of Learning and Memory. 2011;95:37–45. doi: 10.1016/j.nlm.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends in Cognitive Sciences. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Narayanan NS. Medial frontal ~4-Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol. 2015;114:1310–1320. doi: 10.1152/jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Rijn H, Kononowicz TW, Meck WH, Ng KK, Penney TB. Contingent negative variation and its relation to time estimation: a theoretical evaluation. Front Integr Neurosci. 2011;5:91. doi: 10.3389/fnint.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim Y-C, Han S-W, Alberico SL, Ruggiero RN, De Corte B, Chen K-H, et al. Optogenetic Stimulation of Frontal D1 Neurons Compensates for Impaired Temporal Control of Action in Dopamine-Depleted Mice. Curr Biol. 2017;27:39–47. doi: 10.1016/j.cub.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narayanan NS, Land BB, Solder JE, Deisseroth K, Dileone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci USa. 2012;109:20726–20731. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leeman RF, Beseler CL, Helms CM, Patock-Peckham JA, Wakeling VA, Kahler CW. A brief, critical review of research on impaired control over alcohol use and suggestions for future studies. Alcoholism: Clinical and Experimental Research. 2014;38:301–308. doi: 10.1111/acer.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]