Summary
Accelerometry is a reliable tool for gauging the occurrence, amplitude and frequency of tremor. However, there is no consensus on criteria for accelerometric diagnosis of tremor syndromes. We enrolled 20 patients with essential tremor (ET), 20 with dystonic tremor (DT), and 20 with classic parkinsonian tremor (PD-T), all meeting accepted clinical criteria. All the patients underwent dopamine transporter imaging (by means of single-photon emission computed tomography) and triaxial accelerometric tremor analysis. The latter revealed groupwise differences in tremor frequency, peak dispersion, spectral coherence, unilaterality and resting vs action tremor amplitude. From the above, five diagnostic criteria were extrapolated for each condition. Receiver operating characteristic curves, depicting criteriabased scoring of each tremor type, showed negligible declines in specificity for scores ≥4 in patients with ET or DT and scores ≥3 in patients with PD-T, thus providing a simple scoring method (accelerometrically derived) for differential diagnosis of the principal tremor syndromes.
Keywords: accelerometry, diagnosis, dystonic tremor, essential tremor, Parkinson’s disease, tremor
Introduction
Tremor is a common neurological disorder that can prove diagnostically challenging; its assessment largely relies upon the skills of specialists in neurology. At present, the gold standard for diagnosis is based on a combination of validated hallmark criteria, long-term patient monitoring, and response to specific drugs (Deuschl et al., 1998). Use of a dopamine transporter tracer in single-photon emission computed tomography (DAT-SPECT) is also an accepted method for differentiating the tremor of idiopathic Parkinson’s disease (PD-T) from essential tremor (ET) or dystonic tremor (DT) (Sixel-Doring et al., 2011). Kinematic analysis is a further means of studying the distinctive features of tremor syndromes. For more than 50 years, accelerometry has proved useful in assessing movement disorders (Marshall, 1959), mostly for research purposes. Indeed, a number of prior investigations have striven to establish accelerometric criteria for specific types of tremor (Hossen et al., 2013). Nevertheless, consensus on criteria for accelerometric diagnosis of the principal tremor syndromes is lacking at present. The International Parkinson and Movement Disorder Society recently appointed a task force to review the use of transducer-based measures for tremor characterization. The reliability of accelerometry in gauging tremor occurrence, frequency and amplitude was verified (Haubenberger et al., 2016).
Materials and methods
Study subjects
Consecutive outpatients seen at our Movement Disorders Clinic and displaying either ET, DT or classic PD-T were enrolled in the present study, all meeting accepted clinical criteria (Deuschl et al., 1998). Integrity of the nigrostriatal dopaminergic pathway was confirmed by DAT-SPECT in all patients with ET or DT, whereas patients with PD-T showed nigrostriatal degeneration. Exclusion criteria were as follows: historical evidence of dementia, epilepsy, stroke, head injury or substance abuse; dysmetabolic or pharmacological cause of tremor; other degenerative neurological diseases; and ongoing pharmacological treatment of ET or DT.
We enrolled 60 patients with ET, DT or PD-T (n=20 in each group). Patient demographics and clinical data are detailed in Table I. The groups were homogeneous for gender, whereas, as expected, age at the time of the study, age at onset, and disease duration differed between the three conditions. At the time of the study, the patients with DT were much younger (average age, 53.6 years) than those with ET (72.6 years) or PD-T (71.6 years). DT (44.2 years) and ET (55.1 years) presented earlier than PD-T (64.7 years). ET had a longer disease duration (17.5 years) than both DT (9.3 years) and PD-T (6.9 years). A family history of tremor was reported in 60% of the subjects with ET, and 65% of the ET group members responded clinically to alcohol or propranolol. Most of the patients with DT (n=17) displayed segmental dystonia, while the others were characterized as multifocal (n=2) or generalized (n=1) dystonia. However, all the DT patients showed dystonia of the upper limbs with upper limb tremor.
Table I.
Demographic and clinical data.
| ET (20 pts) | DT (20 pts) | PD-T (20 pts) | p value (Kruskal-Wallis test) | ET vs DT (p value) | ET vs PD-T (p value) | DT vs PD-T (p value) | |
|---|---|---|---|---|---|---|---|
| Sex (M/F) | 14/6 | 13/7 | 9/11 | 0.233 | 0.736 | 0.11 | 0.2 |
| Age (years) | 72.6 ± 6.9 | 53.6 ± 22.2 | 71.6 ± 9.2 | 0.001 | 0.001 | 0.925 | 0.001 |
| Age at onset (years) | 55.1 ± 14.1 | 44.2 ± 22.9 | 64.7 ± 8.3 | 0.001 | 0.114 | 0.007 | 0.001 |
| Disease duration (years) | 17.5 ± 14.0 | 9.3 ± 7.4 | 6.9 ± 3.5 | 0.003 | 0.03 | <0.001 | 0.445 |
Legend: pts=patients; ET=essential tremor; DT=dystonic tremor; PD-T= parkinsonian tremor
DAT-SPECT methodology
In accordance with European Association of Nuclear Medicine (EANM) procedural guidelines (Darcourt et al., 2010), intravenous injection of 123I-FP-CIT (DaTscan, 185 MBq; GE Healthcare, Chicago, IL, USA) was performed 30 min after thyroid blockade (oral potassium perchlorate, 400 mg). SPECT was then performed, using a dual-head gamma camera system (E.CAM; Siemens Medical Solutions, Malvern, PA, USA) equipped with high-resolution, low-energy, parallel-hole collimators. Standard parameters were utilized for data acquisition and reconstruction (Darcourt et al., 2010), reorienting transaxial, sagittal and coronal slices in the fronto-occipital plane. Two experienced nuclear medicine physicians interpreted the resulting views as normal or abnormal by combining qualitative assessment with semi-quantitative BasGan v2 freeware-assisted evaluation (Calvini et al., 2007).
Tremor recording and analysis
In the patients with Parkinson’s disease (PD), tremor recording was performed in “off” condition after 12-hour overnight medication withdrawal, in order to rule out levodopa-induced modification of tremor amplitude (Tedeschi et al., 1990). The other patients, i.e. in the ET or DT groups were not receiving pharmacological treatments for tremor, in accordance with the study design. Each patient underwent tremor analysis performed using triaxial accelerometers (SOMNOwatch; SOMNOmedics, Randersacker, Germany). These units (diameter, 45 mm; thickness, 16 mm; weight, 30 g) were positioned on the proximal one-third of the metacarpals bilaterally.
Data were acquired for 30 sec under the following conditions: rest; antigravity posture without and with 1-kg load per hand; action with finger-to-nose maneuvers; rest and antigravity posture of the dominant hand during a distractor task (contralateral entrainment at 1 Hz). Accelerometric signals were digitized (100 Hz) and Fourier transformed through proprietary software (DOMINO light; SOMNOmedics). Thus, from three-dimensional (frequency-intensity-time) software-generated graphs, a single investigator (blinded to diagnosis) was able to deduce the following tremor-related indices: intensity (amplitude), center frequency, frequency dispersion (center frequency range accounting for 66% of total spectrum power) and spectrum coherence (coherence of tremor spectra obtained second by second during data acquisition) (Timmer et al., 1996; Farkas et al., 2006; Scanlon et al., 2013; van der Stouwe et al., 2016).
Statistical analysis
Differences in frequency, amplitude, coherence and peak dispersion measurements in resting and action modes were compared between the groups (ET, DT and PD-T). Continuous non-parametric variables were analyzed with the Kruskal-Wallis test for independent samples, using the Bonferroni correction for multiple significance tests. For pairwise comparisons, the Mann-Whitney U test was applied. The chi-square test served to evaluate the proportion of patients with unilateral tremor, the prevailing amplitude of resting tremor, and gender distributions between the three groups. All statistical computations were performed using standard software (SPSS v21.0 for Macintosh; SPSS, Chicago, IL, USA).
Results
Analysis of the accelerometric data revealed significant group differences (p<0.01) in tremor frequency, peak dispersion, spectral coherence, unilateral tremor, and resting vs action tremor amplitude, as shown in Table II. On comparison of the ET vs DT patient groups, tremor frequency was found to be higher among those with ET, whereas peak dispersion and spectral coherence were higher among those with DT. Of note, the patients with ET and DT did not differ in terms of the presence of unilateral tremor or the amplitude of resting vs action tremor.
Table II.
Accelerometric data.
| ET (20 pts) | DT (20 pts) | PD-T (20 pts) | p value (Kruskal-Wallis test) | ET vs DT (p value) | ET vs PD-T (p value) | DT vs PD-T (p value) | |
|---|---|---|---|---|---|---|---|
| Tremor frequency (Hz)* | 10.6 ± 2.5 | 6.1 ± 2.4 | 5.4 ± 0.8 | <0.001 | <0.001 | <0.001 | 0.12 |
| Peak dispersion (Hz)* | 2.0 ± 0.2 | 4.2 ± 1.1 | 2.7 ± 0.5 | <0.001 | <0.001 | <0.001 | <0.001 |
| Spectral coherence (%)* | 89.7 ± 12.7 | 44.0 ± 16.9 | 75.8 ± 20.9 | <0.001 | <0.001 | <0.001 | <0.001 |
| Unilateral tremor | 8/20 | 6/20 | 16/20 | 0.004 | 0.51 | 0.01 | 0.001 |
| Resting tremor amplitude > action tremor amplitude | 0/20 | 4/20 | 14/20 | <0.001 | 0.35 | <0.001 | 0.001 |
Legend: pts=patients; ET=essential tremor; DT=dystonic tremor; PD-T= parkinsonian tremor.
Tremor frequency, peak dispersion and spectral coherence refer to postural tremor for patients with ET and DT and to resting tremor for patients with PD-T.
Comparison of the ET patients with the PD-T patients revealed significant differences in all the variables. Tremor frequency and spectral coherence were higher in the patients with ET, whereas unilateral tremor and a greater amplitude of resting tremor were more common among those with PD-T, who also showed higher peak dispersion.
Comparison of the accelerometric data recorded in the DT vs the PD-T patients revealed similar tremor frequency in the two groups, whereas the other variables differed significantly. Peak dispersion was higher in the patients with DT; but in the patients with PD-T, spectral coherence was higher, tremor was more often unilateral, and resting tremor amplitude exceeded action tremor amplitude.
The sensitivity and specificity of each variable were calculated, identifying optimal ranges for tremor frequency, peak dispersion and spectral coherence in each group. This enabled extrapolation of five diagnostic criteria for each diagnostic group, as follows: 1) ET: frequency, 5–15 Hz; peak dispersion, ≤2.5Hz; spectral coherence, ≥80%; no unilateral tremor; action amplitude > resting amplitude; 2) DT: frequency, 4–10 Hz; peak dispersion, ≥3Hz; spectral coherence, ≤60%; no unilateral tremor; action amplitude > resting amplitude; and 3) PD-T: frequency, 4–7 Hz; peak dispersion, 2.5–3.5 Hz; spectral coherence, ≥70%; unilateral tremor; resting amplitude >action amplitude.
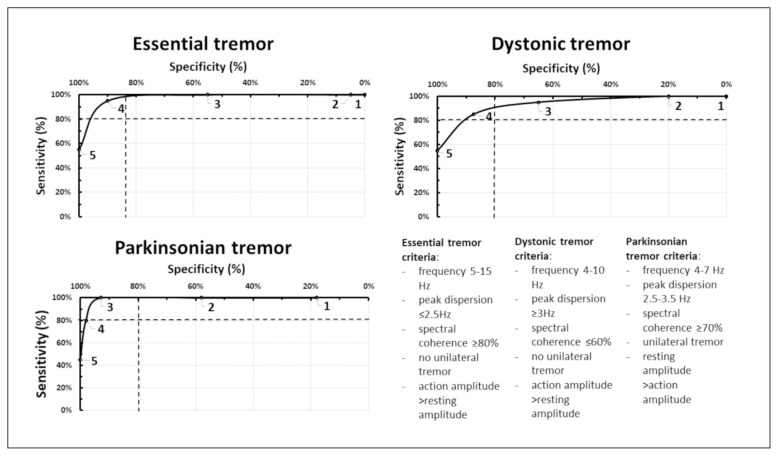
Finally, sensitivity/specificity curves were generated for each diagnostic criterion, selecting the levels at which combined sensitivity/specificity rates in the respective groups were best. Four criteria ultimately emerged for DT (sensitivity, 85%; specificity, 87.5%), four for ET (sensitivity, 95%; specificity, 90%), and three for PD-T (sensitivity, 100%; specificity, 93%) (Fig. 1).
Figure 1.
Receiver operating characteristic curves reflecting predictive accuracies of total scores generated in patients with essential tremor (ET), dystonic tremor (DT), and parkinsonian tremor (PD-T), respectively. Cutoff points for scores of 1–5 are plotted along with logarithmic curve fits. Cutoff points of 4 for DT and ET and 3 for PD-T lie above the intersection of 80% sensitivity and 80% specificity (dashed lines).
Discussion
In this study, we compared accelerometric characteristics of tremor in three groups of patients (ET, DT and PD-T) as a potential basis for differential diagnosis, and discovered significant between-group differences in tremor frequency, peak dispersion, spectral coherence, unilateral tremor and resting vs action tremor amplitude. From the above, five diagnostic criteria were extrapolated for each condition.
First criterion: tremor frequency range. Our data on tremor frequency showed an overlap between the patient subsets and also with data previously reported. In a study by Burne et al. (2002), the range (4–7 Hz) determined for patients with PD-T and that of patients with ET (5–15 Hz) converged to an extent, and both broadly incorporated the range (4–10 Hz) of patients with DT reported by Shaikh et al. (2008). In our patients with ET, mean tremor frequency was 10.6 Hz, exceeding the values obtained by Burne et al. (6 Hz) and van der Stouwe et al. (5.8 Hz) (Burne et al., 2002; van der Stouwe et al., 2016), but falling within the ranges for ET reported by Elble and Deuschl et al. (Elble, 1986; Deuschl et al., 1998). Second criterion: peak dispersion. As expected from electromyographic findings in the context of DT (Jedynak et al., 1991), we observed a broader range of frequencies in patients with DT (peak dispersion, ≥3Hz) than in those with PD-T or ET. Still, frequency dispersion was higher in the PD-T group members (2.5–3.5 Hz) than in the group with ET (<2.5 Hz), as found in an earlier accelerometric study (Farkas et al., 2006).
Third criterion: spectral coherence. Spectral coherence was lower in patients with DT (≤ 60%) than in patients with PD-T (≥70%) or ET (≥80%). This disparity, like the disparity observed in peak dispersion, is attributable to physiological differences in central tremor-related oscillators. In the setting of DT, there are multiple, dynamically diverse oscillators, and this results in a greater likelihood of irregular output (Shaikh et al., 2008). Innate frequencies of individual oscillators depend on synaptic transmission delays and the membrane ion channel kinetics of the neurons within a given oscillator circuit (Deuschl et al., 2001). Accordingly, physiological variations (however small) in two central oscillators may yield differing oscillation frequencies — a broader frequency range reflecting greater disparities. In patients with PD-T or ET, less disparity in synaptic delays and membrane properties of abnormal oscillators may ensure more regular output and less tremor irregularity (Raethjen et al., 2000).
Fourth criterion: unilateral tremor. Unilateral resting tremor is common in patients with PD-T, and it is often the first presenting symptom at the onset of the disease. In fact, all the core features of PD (resting tremor, rigidity and bradykinesia) are more pronounced unilaterally at disease onset and generally persist asymmetrically throughout the course of the illness (Lee et al., 1995). In patients with ET, unilateral tremor is unusual and reportedly occurs at rates of 2–10%, depending on the criteria applied (Thenganatt and Louis, 2012). In patients with DT, unilateral presentation is common in focal forms, whereas bilateral presentation prevails in multifocal, segmental and generalized dystonia (Jedynak et al., 1991). This inconsistency may account for the relative lack of unilateral tremor in our subjects with DT.
Fifth criterion: resting vs action tremor amplitude. In our study, resting tremor amplitude was found to be clearly higher in patients with PD-T and lower in those with ET or DT. Resting tremor is a cardinal feature of PD, occurring in 70–90% of patients. Although the reported prevalence of action tremor in patients with PD is as high as 88–92%, its amplitude is lower than that of resting tremor (Thenganatt and Louis, 2012). On the other hand, resting tremor occurs in 20–30% of patients with ET, even though it shows a lower amplitude than the action tremor seen in these patients (Cohen et al., 2003). Similarly, in patients with DT, the amplitude of action tremor, exacerbated by muscle contraction, exceeds that of resting tremor (Jedynak et al., 1991).
Finally, the total scores computed for the five criteria in each tremor type were plotted to receiver operating characteristic curves that showed negligible specificity declines at the following cutoff points: ≥4 in patients with ET or DT and ≥3 in patients with PD-T. Thus, a simple scoring method for accelerometric parameters, as identified herein, may support the differential diagnosis of PD-T, ET and DT.
Although a transducer-based method is preferable to objectively assess tremor, the use of accelerometry is far from mainstream clinical practice. Our accelerometric criteria and the scoring method we devised will need prospective validation, involving broader, independent sampling from different centers. It must also be underlined that the criteria pertaining to PD-T apply only to patients with classic PD-T (Type I), and not to variants of PD or other tremors. Some patients with PD may display isolated postural and kinetic tremor (Type III) (Deuschl et al., 1998), dystonic tremor due to focal dystonia (Deuschl, 2003), or functional tremor as coexistent features with PD (Parees et al., 2013). In such instances, clinical evaluation should guide instrumental analysis and interpretation of metrics generated. Going forward, other issues left unexplored in the present study should be addressed to improve the overall sensitivity/specificity of total patient scores.
A limit of this study is the divergence between the three groups in terms of age (the patients with DT being notably younger) and disease duration (those with ET being afflicted for the longest time). These differences may both be explained by the younger age at onset of ET and DT, relative to PD-T. However, it is worth considering that in ET patients, tremor frequency can change as the disease progresses (Elble, 1986), and that we considered only patients with a long disease duration. Accordingly, a more homogeneous sample in terms of age and disease duration should be selected for future studies.
In conclusion, accelerometry provides objective and precise linear measurements of tremor occurrence, frequency and amplitude, eliminating potential bias deriving from patient perceptions and clinical oversights. This approach may aid in distinguishing various tremor syndromes through a criteria-based scoring method, although further prospective validation is needed.
References
- Burne JA, Hayes MW, Fung VS, et al. The contribution of tremor studies to diagnosis of parkinsonian and essential tremor: a statistical evaluation. J Clin Neurosci. 2002;9:237–242. doi: 10.1054/jocn.2001.1017. [DOI] [PubMed] [Google Scholar]
- Calvini P, Rodriguez G, Inguglia F, et al. The basal ganglia matching tools package for striatal uptake semi-quantification: description and validation. Eur J Nucl Med Mol Imaging. 2007;34:1240–1253. doi: 10.1007/s00259-006-0357-2. [DOI] [PubMed] [Google Scholar]
- Cohen O, Pullman S, Jurewicz E, et al. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol. 2003;60:405–410. doi: 10.1001/archneur.60.3.405. [DOI] [PubMed] [Google Scholar]
- Darcourt J, Booij J, Tatsch K, et al. EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging. 2010;37:443–450. doi: 10.1007/s00259-009-1267-x. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Raethjen J, Lindemann M. The pathophysiology of tremor. Muscle Nerve. 2001;24:716–735. doi: 10.1002/mus.1063. [DOI] [PubMed] [Google Scholar]
- Deuschl G. Dystonic tremor. Rev Neurol (Paris) 2003;159:900–905. [PubMed] [Google Scholar]
- Elble RJ. Physiologic and essential tremor. Neurology. 1986;36:225–231. doi: 10.1212/wnl.36.2.225. [DOI] [PubMed] [Google Scholar]
- Farkas Z, Csillik A, Szirmai I, et al. Asymmetry of tremor intensity and frequency in Parkinson’s disease and essential tremor. Parkinsonism Relat Disord. 2006;12:49–55. doi: 10.1016/j.parkreldis.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Haubenberger D, Abbruzzese G, Bain PG, et al. Transducer-based evaluation of tremor. Mov Disord. 2016;31:1327–1336. doi: 10.1002/mds.26671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen A, Muthuraman M, Al-Hakim Z, et al. Discrimination of Parkinsonian tremor from essential tremor using statistical signal characterization of the spectrum of accelerometer signal. Biomed Mater Eng. 2013;23:513–531. doi: 10.3233/BME-130773. [DOI] [PubMed] [Google Scholar]
- Jedynak CP, Bonnet AM, Agid Y. Tremor and idiopathic dystonia. Mov Disord. 1991;6:230–236. doi: 10.1002/mds.870060307. [DOI] [PubMed] [Google Scholar]
- Lee CS, Schulzer M, Mak E, et al. Patterns of asymmetry do not change over the course of idiopathic parkinsonism: implications for pathogenesis. Neurology. 1995;45:435–439. doi: 10.1212/wnl.45.3.435. [DOI] [PubMed] [Google Scholar]
- Marshall J. Physiological tremor in children. J Neurol Neurosurg Psychiatry. 1959;22:33–35. doi: 10.1136/jnnp.22.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parees I, Saifee TA, Kojovic M, et al. Functional (psychogenic) symptoms in Parkinson’s disease. Mov Disord. 2013;28:1622–1627. doi: 10.1002/mds.25544. [DOI] [PubMed] [Google Scholar]
- Raethjen J, Lindemann M, Schmaljohann H, et al. Multiple oscillators are causing parkinsonian and essential tremor. Mov Disord. 2000;15:84–94. doi: 10.1002/1531-8257(200001)15:1<84::aid-mds1014>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Scanlon BK, Levin BE, Nation DA, et al. An accelerometry-based study of lower and upper limb tremor in Parkinson’s disease. J Clin Neurosci. 2013;20:827–830. doi: 10.1016/j.jocn.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Jinnah HA, Tripp RM, et al. Irregularity distinguishes limb tremor in cervical dystonia from essential tremor. J Neurol Neurosurg Psychiatry. 2008;79:187–189. doi: 10.1136/jnnp.2007.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixel-Doring F, Liepe K, Mollenhauer B, et al. The role of 123I-FP-CIT-SPECT in the differential diagnosis of Parkinson and tremor syndromes: a critical assessment of 125 cases. J Neurol. 2011;258:2147–2154. doi: 10.1007/s00415-011-6076-z. [DOI] [PubMed] [Google Scholar]
- Tedeschi G, Sasso E, Marshall RW, et al. Tremor in Parkinson disease: acute response to oral levodopa. Ital J Neurol Sci. 1990;11:259–263. doi: 10.1007/BF02333855. [DOI] [PubMed] [Google Scholar]
- Thenganatt MA, Louis ED. Distinguishing essential tremor from Parkinson’s disease: bedside tests and laboratory evaluations. Expert Rev Neurother. 2012;12:687–696. doi: 10.1586/ern.12.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer J, Lauk M, Deuschl G. Quantitative analysis of tremor time series. Electroencephalogr Clin Neurophysiol. 1996;101:461–468. [PubMed] [Google Scholar]
- van der Stouwe AM, Elting JW, van der Hoeven JH, et al. How typical are ‘typical’ tremor characteristics? Sensitivity and specificity of five tremor phenomena. Parkinsonism Relat Disord. 2016;30:23–28. doi: 10.1016/j.parkreldis.2016.06.008. [DOI] [PubMed] [Google Scholar]