Abstract
Preeclampsia (PE) is characterized by elevated tumor necrosis factor alpha (TNF-α), anti-angiogenic factors such as sFlt-1, increased uterine artery resistance (UARI), and decreased of nitric oxide during pregnancy. Previously we showed that 17-hydroxyprogesterone caproate (17-OHPC) administered into RUPP rats on day 18 of gestation improved hypertension without improving pup weight. We hypothesized that earlier administration of 17-OHPC on day 15 of gestation could improve pathophysiology of PE and fetal outcomes in response to placental ischemia. Carotid catheters were inserted on day 18, mean arterial blood pressure (MAP) and samples were collected on day 19. MAP in normal pregnant (NP) rats was 102± 2, 105±2 in NP+ GD15 17-OHPC, 127 ± 2 in RUPP and 112±1 mmHg in RUPP+GD15 17-OHPC, p<0.05. Pup weight and litter size were improved from 1.9±0.05, 10.1±1.4 in RUPP to 2.1±0.07 grams and 13.2±0.6 in RUPP+ GD15 17-OHPC, p<0.05. UARI was 0.8±0.03 in RUPP which was decreased to 0.6±0.04 in RUPP+ GD15 17-OHPC, p<0.05. Plasma TNF-α levels were 164±34 in RUPP and blunted to 29±9 pg/ml in RUPP+ GD15 17-OHPC, p<0.05. Plasma nitrate-nitrite levels were 10.8±2.3 in RUPP rats and significantly increased to 25.5±5.2 μM in RUPP+GD15 17-OHPC, p<0.05. sFlt-1 levels were 386±141 in RUPP rats, which was reduced to 110.2 ± 11 in RUPP+17-OHPC, p<0.05. These data indicate that GD15 17-OHPC improves pathophysiology in RUPP rats, possibly via improving sFlt-1 reduced NO during pregnancy.
Keywords: Preeclampsia, 17-hydroxyprogesterone caproate, nitric oxide, pregnancy, inflammation
INTRODUCTION
Preeclampsia (PE) is a pregnancy disorder occurring in about 2% to 8% of women, and is associated with an increase in morbidity and mortality to both mothers and fetuses1–4. PE is identified by high blood pressure and abnormal uterine artery Doppler sonography in the presence or absence of proteinuria near the 20th week of gestation. Although the pathophysiologic mechanisms of PE remain unknown, the placenta is implicated in being the central culprit in this disease stimulating circulating factors responsible for hypertension and impairing maternal endothelium-dependent responses4–8. Mediators of endothelial dysfunction such as increased production of the vasoconstrictor endothelin-1 (ET-1), anti-angiogenic factor soluble vascular endothelial growth factor receptor 1 (sFlt-1), and decreased of vasodilators such as nitric oxide (NO) are thought to play a role in the development of hypertension and possibly reduced fetal weight in preeclamptic women. In addition, a significant increase in inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin (IL-6) have been observed in PE and proposed to stimulate factors that play a role in vasoconstriction and hypertension during pregnancy9–14. In fact, studies performed by our laboratory have shown that the normal pregnant (NP) recipient rats implanted with a mini osmotic pump infusing either TNF-α or IL-6 exhibit many characteristics of PE15, 16.
Studies in our preeclamptic women and normal pregnant patients indicate that PE is associated with a progesterone deficiency17. Our in vitro studies have shown that progesterone suppresses ET-1 levels in response to human umbilical venous endothelial cells (HUVEC) cells cultured with either serum from RUPP or preeclamptic patients17. Furthermore, we have published that 17-OHPC, progesterone derivative, administered on day 18 of gestation (GD18) attenuates many clinical characteristics of preeclampsia in the reduced uterine perfusion pressure (RUPP) rat model. In fact, administration of GD18 17-OHPC improves hypertension and vasoconstrictors in rat models of elevated cytokines, either TNF alpha or IL-6, during pregnancy 17–20. Taken together, these findings suggest that 17-OHPC has both anti-inflammatory and antihypertensive effects, possibly by decreasing vasoconstrictor profiles stimulated by inflammatory mediators, and thus improves maternal outcomes in our rat models of PE. However, our previous studies have not shown any effect of 17-OHPC in fetal weight. This may be due to the late term administration of 17-OHPC (GD18) to RUPP rats.
Importantly, many patients are diagnosed as early as 20 week gestation by abnormal Doppler sonography, having no treatment options available to improve uterine restriction or blood flow. Interestingly this time frame also coincides with significant elevation in sFlt-1. Associated with these increases is the elevation in blood pressure which has been coined the “chicken and the egg debate”21. We have recently shown that hypertension caused by infusion of sFlt-1 is associated with endothelial dysfunction characterized by increased ET-1 and decreased NO22. Although our previous study didn’t show profound changes in placental ET-1 with 17-OHPC, we do not know the effect it has on sFlt-1 reduced NO during pregnancy. Therefore, in the present study, we hypothesized that early administration of 17-OHPC on day 15 of gestation (GD15) after placental ischemia is induced could decrease sFlt-1 as a mechanism to improve NO and UARI and thus allow for better blood flow and fetal outcomes during pregnancies stricken with placental ischemia. Doppler sonography will be performed on rats to measure uterine artery resistance index as an indicator of improved blood flow which would allow for improved fetal growth in RUPP rat model of PE. This study could thereby establish proof of concept further supporting the addition of 17-OHPC to the current treatment for early preterm PE.
MATERIALS AND METHODS
Sprague Dawley rats purchased Harlan Inc. (Indianapolis, IN) were used in the present study. Rats were housed in a temperature-controlled room (23°C) with a 12:12 hour light/dark cycle with free access to standard rat chow and water. This study complied with guidelines of the University of Mississippi Medical Center, and the animals were handled according to the guiding principles published in the National Institutes of Health Guide for the Care of Animals and the Institutional Animal Care and Use Committee (IACUC).
Reduction of uterine perfusion pressure (RUPP)
On day 14 of gestation, rat dams weighting approximately 200–250g were exposed to 2% isoflurane and randomly assigned to either RUPP or normal pregnant (NP) control groups. Following a midline incision, pregnant rats in the RUPP group had the lower abdominal aorta isolated and a silver clip (0.203 mm ID) placed around the aorta above the iliac bifurcation. To prevent augmentation of blood flow to the uterus via the ovarian arteries, silver clips (0.100 mm ID) were also placed on the branches of both ovarian arteries that supply the uterus 23. Also analgesics were used to provide comfort for the surgical rats include 0.25% sensor care administered topically or 5mg/kg Carprofen administered via subcutaneous injection, once daily for 2–3 days following surgical procedure.
Administration of 17-hydroxyprogesterone caproate (17-OHPC)
The 17-OHPC (Marty’s Compounding Pharmacy, Jackson, MS) was diluted in normal saline and administered intraperitoneally as 0.5 cm3 solution of 3.32 mg/kg 17-OHPC to pregnant rats on day 15 of gestation. This dose is equivalent to a typical human dose for the prevention of preterm labor previously and has been shown to be effective in RUPP rats to lower blood pressure when administered on day 18 of gestation 18.
Measurement of mean arterial blood pressure
Pregnant rats were catheterized on day 18 of gestation under anesthesia using isoflurane (Webster, Sterling MA) delivered by an anesthesia apparatus (Vaporizer for Forane Anesthetic, Ohio Medical Products, Madison, WI). On day 19 of gestation, mean arterial blood pressure (MAP) was analyzed after placing the rats in individual restraining cages. MAP was recorded continuously for one hour after a 30 minutes stabilization and calculated by MAP=SBP+2(DBP)/3, as described previously 24.
Determination of uterine artery resistance index
On day 18 of gestation, Power Doppler velocimetry measurements were performed on anesthetized pregnant dams at an imaging station with a Vevo 770 unit (Visual sonics) using a 30 Hz transducer and an insonating angle <30° as previously described2. The peak systolic flow velocity (PSV) and end diastolic flow velocity (EDV) were recorded using the uterine artery Doppler waveform and the index was calculated using the following formula: UARI= (PSV−EDV)/PSV 24.
Determination of TNF-α
Plasma collected from all pregnant rats was measured for tumor necrosis factor alpha (TNF-α) levels using commercial ELISA kits from R&D Systems (Quantikine) according the manufacturer’s instructions. The minimal detectable levels for TNF-α was<5 pg/mL, with inter- and intra-assay variability of 10% and 5.1%, respectively.
Determination of sFlt-1
Plasma collected from all pregnant rats was measured for sFlt-1 levels using commercial ELISA kits from R&D Systems (Quantikine) according the manufacturer’s instructions. The minimal detectable levels for sFlt-1 was 15.2 pg/mL, with inter- and intra-assay variability of 8.4% and 7.2%.
Determination of circulating nitrate-nitrite bioavailability
Plasma nitrate-nitrite (NOx) levels were evaluated using commercial Nitrate-Nitrite Colorimetric Assay Kit from Cayman Chemical following instructions outlined by the manufacturer. The inter-assay coefficient of variation is 3.4% while intra-assay coefficient of variation is 2.7%.
Statistical Analysis
All data are expressed as mean ± standard error means (SEM). Comparisons between groups were assessed by two-way ANOVA with Bonferroni’s multiple comparisons test as post hoc analysis. A value of P < 0.05 was considered statistically significant. Significant changes in pup weight and litter size were only seen with student T test.
RESULTS
Early administration of 17-OHPC improved hypertension in RUPP rats
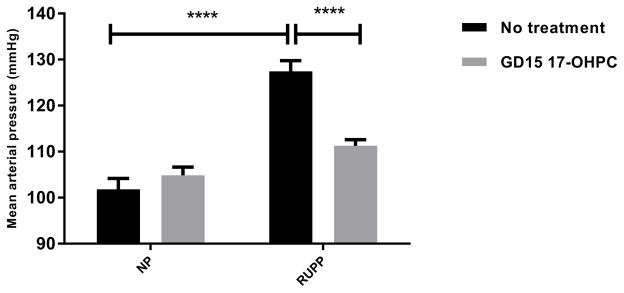
MAP in normal pregnant (NP) (n=10) was 102 ± 2 mmHg, 105±2 mmHg in NP+ GD15 17-OHPC (n=7), and increased to 127±2 mm Hg in RUPP (n=14, p < 0.05, which was improved to 111 ±1 mmHg in RUPP+ GD15 17-OHPC (n=17, P < 0.05, Figure 1). Importantly, with 17-OHPC given in early gestation (GD15) blood pressure was blunted to a greater degree than seen in our previous study with 17-OHPC administrated in late gestation, day 18 of gestation (GD18), in RUPP rats.
Figure 1.
Early administration of 17-OHPC improves blood pressure in RUPP rats. Data are shown as means ± S.E.M. (n=10–17/group). Two-way ANOVA and Bonferroni as post hoc analysis were performed to generate p values. *P <0.05, two-way ANOVA
Early administration of 17-OHPC decreased plasma TNF-α levels in RUPP rats
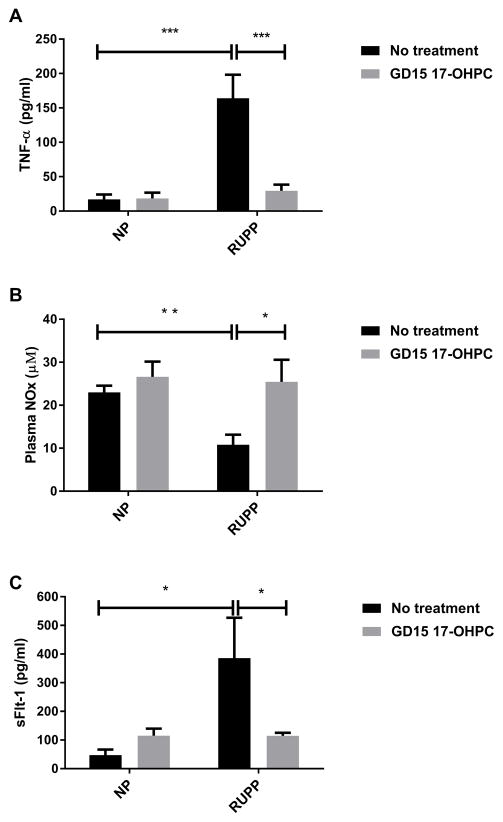
To determine whether GD15 17-OHPC could reduce inflammation while decreasing blood pressure in response to placental ischemia, we analyzed circulating levels of TNF-α in plasma collected on day 19 of gestation. Plasma levels of TNF-α were 17±7 pg/ml in NP (n=6), 18±8 pg/ml in NP+ GD15 17-OHPC (n=6), 164±34pg/ml in RUPP rats (n=7) which was blunted to 29±9 pg/ml (n=7,) in RUPP+ GD15 17-OHPC (n=7, P<0.05, Figure 2A).
Figure 2.
Early administration of 17-OHPC decreases circulating factors in RUPP rats. (A) Bar graph showing the TNF-α in RUPP rats. (B) Bar graph showing the plasma NOx in RUPP rats. (C) Bar graph showing the sFlt-1 levels. Data are shown as means ± S.E.M. (n=6–7/group). Two-way ANOVA and Bonferroni as post hoc analysis were performed to generate p values. *P <0.05, two-way ANOVA
Early administration of 17-OHPC increased plasma levels of nitrate-nitrite in RUPP rats
To determine whether GD 15 17-OHPC increased nitric oxide bioavailability in RUPP rats, we analyzed circulating nitrate-nitrite (NOx). Previously, we have demonstrated that plasma NOx was decreased in RUPP compared to NP.18 In this current study, plasma nitrate-nitrite (NOx) was 23.0±1.6 in NP (n=9), 26.6+3.5 in NP+ GD15 17-OHPC (n=4), 10.8±2.3 in RUPP rats (n=13) which improved to 25.5±5.2 μM in RUPP+ GD15 17-OHPC (n=5), P<0.05, Figure 2B.
Early administration of 17-OHPC decreased sFlt-1 levels in RUPP rats
As we have published previously, sFlt-1 levels were increased in response to placental ischemia during pregnancy. sFlt-1 levels were 58.0±16.7 in NP (n=5), 115± 25.5 in NP+17-OHPC, 386±141 in RUPP rats, which was significantly reduced to 110.2 ± 11 in RUPP+17-OHPC (n=7, p<0.05, Figure 2C).
Early administration of 17-OHPC reduced Uterine Artery Resistance Index
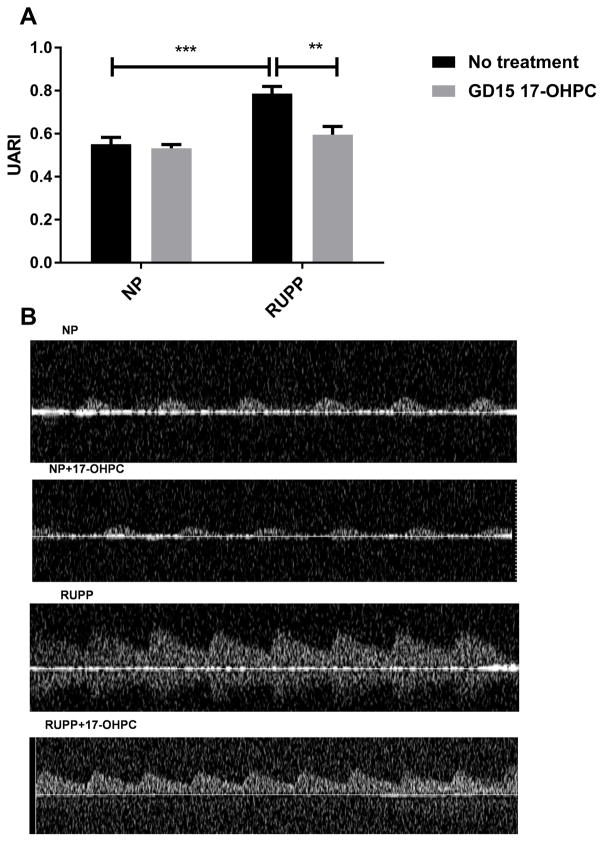
As we have demonstrated previously, UARI was increased in response to placental ischemia during pregnancy18, similar to that seen in PE. In this study, UARI was 0.5±0.03 in NP (n=5), 0.5± 0.02 in NP+ GD15 17-OHPC, 0.8±0.03 in RUPP rats (n=4) and 0.6±0.04 in RUPP+ GD15 17-OHPC (n=5, P <0.05, Figure 3).
Figure 3.
Early administration of 17-OHPC improves uterine artery resistance index in RUPP rats. (A) Bar graph showing the UARI in pregnant rats. (B) Representative Doppler ultrasound waveforms for each group. Data are shown as means ± S.E.M. (n=4–5/group). Two-way ANOVA and Bonferroni as post hoc analysis were performed to generate p values. *P <0.05 versus NP group, two-way ANOVA.
Early administration of 17-OHPC improved pup weight and litter size in RUPP rats
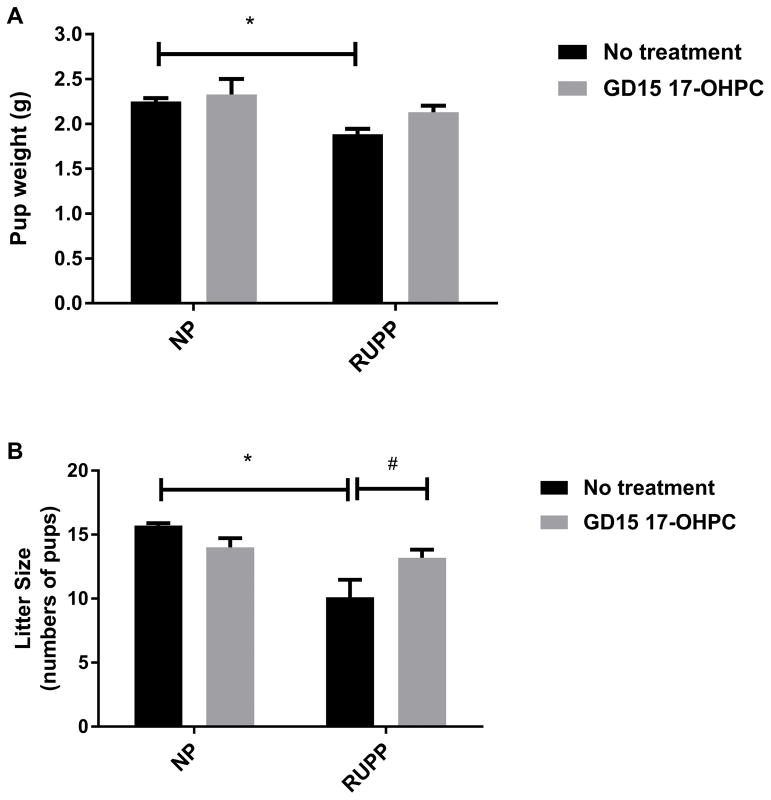
Consistent with PE, we have shown the decrease in pup weight in response to chronic placental ischemia in the RUPP rat. Pup weight was 2.3±0.04 in NP (n=10), 2.3±0.2 in NP+ GD15 17-OHPC (n=7) and 1.9± 0.05 grams in RUPP rats (n=14, P < 0.05). Interestingly, the pup weight was significantly improved to 2.1±0.07 grams in RUPP rats treated with GD15 17-OHPC compared to untreated RUPP rats (n=15, P < 0.05, Figure 4A). In addition, litter size was 15.7±0.2 in NP (n=7), 14.0±0.7in NP+ GD15 17-OHPC (n=7) which was significantly lower in RUPP rats (10. 1± 1.4, n=10) and GD15 administration of 17-OHPC improved litter size to 13.2±0.60 in RUPP+ GD15 17-OHPC (n=16) compared to RUPP rats (n= 14, P<0.05, Figure 4B).
Figure 4.
Early administration of 17-OHPC improves fetal weight and litter size in RUPP rats.(A) Bar graph showing the pup weight in pregnant rats. (B) Bar graph showing litter size in pregnant rats. Data are shown as means ± S.E.M. (n=7–15/group). Two-way ANOVA, Bonferroni as post hoc analysis and T test were performed to generate p values. *P <0.05, two-way ANOVA, # P<0.05, T test.
DISCUSSION
Clinical trials using either oral or vaginal progestogens for PE complication have been debated 25, however there is no effective treatment approved for PE, except the early delivery of placenta. There are not data support the hypothesis that women receiving 17-OHPC for prevention of preterm labor have a lower incidence of preeclampsia25. 17-OHPC is only given to women to prevent subsequent preterm birth, however, importantly these women may not go on to develop preeclampsia but instead have an increased time to deliver. Importantly, previous studies have shown that circulating progesterone levels and urine metabolites of progesterone are decreased in IUGR26 and 17-OHPC may only benefit pregnant women with progesterone deficiency. Our ongoing clinical studies are investigating the potential use of 17-OHPC for the management of severe PE diagnosed prior to 34 weeks gestation.
Since the role of progestogens in this disease is still not fully understood much more research should be performed to better understand the efficacy of progesterone supplementation to improve pregnancy outcomes in response to placental ischemia. Indeed, we have recently published that 17-OHPC administrated during late gestation to RUPP rats reduces inflammation, renal and placental ET-1, blood pressure, UARI, and increases vascular eNOS expression and NO bioavailability 17, 18, 20. Importantly, our findings in the current study demonstrate that early administration of 17-OHPC on day 15 of gestation after placental ischemia has an even greater effect to improve many characteristics of preeclampsia in RUPP rats, further reducing hypertension compared to GD18 administration. Furthermore, we show that early administration of 17-OHPC also reduced signals of inflammation and UARI. Moreover, 17-OHPC increased NO bioavailability, pup weight and litter size. Reduction in fetal weight is an important consequence of PE that was only improved with early administration and not late administration of 17-OHPC.
Interestingly, an important finding of this current study is that UARI significantly reduced with GD15 17-OHPC in RUPP rats. Even though the UARI during the first or second trimester of pregnancy may not be the best to predict complications during pregnancy, Doppler is useful as a primary indicator of placental ischemia and predictor of women that will go on to have troubled pregnancies such as preeclampsia, these data demonstrating that 17-OHPC can improve this parameter within 3 days of injection and lead to improved fetal weight is extremely important. This preclinical data indicate that if 17-OHPC is administered midgestation following unusual Doppler assessment then potentially blood flow, antiangiogenic factors and fetal weight could be improved to some degree in women that may go on to develop PE.
Numerous studies have shown decreased nitric oxide (NO) is associated with preeclampsia 27–29. NO is an important vasodilator required to maintain the physiologic alteration during normal pregnancy such as an increased blood volume that is accommodated within cardiovascular system by systemic vasodilation 30. In fact, animals and human studies have shown a deficiency of NO bioavailability during PE 31. In agreement with these findings, in this study we demonstrated that plasma levels of NO were reduced in RUPP rats compared to control normal pregnant rats and that 17-OHPC improved NO in RUPPs.
Furthermore, we have recently shown that hypertension caused by infusion of sFlt-1 is associated with endothelial dysfunction characterized by increased ET-1 and decreased NO. In this study we show that 17 OHPC can reduce sFlt-1 which could allow for the increase in bioavailable nitric oxide. In turn this would allow for the observed reduction in UARI, a possible indicator of endothelial dysfunction, and thus allowed for increase blood and nutrient delivery to the fetus. Therefore we believe this could be the mechanisms for improved blood pressure and fetal weight observed in this study.
Proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and IL-6 have also been associated with PE 12. Our laboratory has shown that TNF-alpha infusion into pregnant rats promotes sFlt-1 and hypertension during pregnancy16. Indeed, others have shown that in the vasculature inflammation contributes to endothelial dysfunction, a hallmark feature of PE 32. Our findings demonstrate that TNF-α was increased in RUPP rats compared to normal pregnant rats and this increase was attenuated with early administration of 17-OHPC in RUPP rats. Previously we have published that 17-OHPC given on late gestation (GD 18) to RUPP rats decreased pro-inflammatory cytokines such as TNF-α and IL-6 18–20. Together, these results suggest that earlier intervention with a drug (17-OHPC), could improve TNF-α, sFlt-1, NO and fetal growth while reducing the maternal pathophysiology associated with placental ischemia or PE during pregnancy.
In conclusion, although we have shown that GD18 administration of 17-OHPC attenuates pathophysiology of placental ischemia observed in RUPP rats, there were no fetal improvements in those studies. New to this study is that early administration (GD15) of 17-OHPC not only improves inflammation and sFlt-1, but it also reduced UARI and increased NO. This could be the mechanism for the further reduction in blood pressure compared with that seen with GD18 administration. Importantly fetal characteristics such as fetal weight and litter size were significantly increased suggesting earlier intervention with 17-OHPC could have beneficial effects to not only improve maternal outcome but that of the baby, via improvements in the sFlt-1/NO pathway, and should be considered for the addition to the management of PE clinically.
PERSPECTIVES
Previous clinical trials using either oral or vaginal progestogens for PE complication were inconclusive concerning the role of progesterone in this important hypertensive disorder during pregnancy. It is well known that sFlt-1 plays a crucial role in the pathophysiology of PE. Our findings suggest that early administration of 17-OHPC improves important maternal and fetal outcomes during PE that may be stimulated by the increase in sFlt-1. Therefore, the discovery that 17 OHPC reduced sFlt-1 leads to a better understanding of mechanisms by which this drug can improve clinical characteristic of PE in response to placental ischemia and may offer greater positive benefits for both mother and child.
NOVELTY AND SIGNIFICANCE.
What is new?
17-OHPC administered on day 15 of gestation improved hypertension by suppressing TNF-alpha and sFlt-1 levels while improving pup weight, litter size, uterine artery resistance index (UARI) and nitric oxide (NO) bioavailability in response to placental ischemia during pregnancy.
What is relevant?
17-OHPC administered on day 15 suppresses sFlt-1, inflammation and increases NO levels as mechanisms to improve uterine artery resistance index, intrauterine growth restriction (IUGR) while lowering maternal blood pressure in rat models of PE, thereby establishing proof of concept to support the addition of 17-OHPC to the current treatment for early preterm PE.
Summary
17-Hydroxyprogesterone caproate (17-OHPC) is not used in the management or treatment of PE but is routinely used for prevention of preterm labor. Our study illustrates that early administration of 17-OHPC on day 15 of gestation improves intrauterine growth restriction, NO bioavailability, UARI, possibly via reducing sFlt-1 levels and inflammation while lowering blood pressure in response to placental ischemia. These data suggest that this hormone could be administered early in gestation to patients, diagnosed between 22–30 weeks, and potential increase time to delivery and improve both fetal and maternal outcomes observed in response to placental ischemia.
Acknowledgments
SOURCES OF FUNDING
This work was supported by National Institutes of Health grants RO1HD067541 and T32HL105324 and the department of Pharmacology, University of Mississippi Medical Center.
Footnotes
DISCLOSURES
None
References
- 1.World health organization. [Acessed february 6, 2015];Maternal mortality fact sheet no. 348. 2012 Available from: http://www.Who.Int/mediacentre/factsheets/fs348/en/
- 2.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nature clinical practice. Nephrology. 2005;1:98–114. doi: 10.1038/ncpneph0035. quiz 120. [DOI] [PubMed] [Google Scholar]
- 3.George EM, Granger JP. Recent insights into the pathophysiology of preeclampsia. Expert review of obstetrics & gynecology. 2010;5:557–566. doi: 10.1586/eog.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 5.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: Linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. American journal of physiology. Heart and circulatory physiology. 2008;294:H541–550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 7.Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: A lesson in integrative physiology. Acta physiologica. 2013;208:224–233. doi: 10.1111/apha.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan LJ, Morton JS, Davidge ST. Vascular dysfunction in preeclampsia. Microcirculation. 2014;21:4–14. doi: 10.1111/micc.12079. [DOI] [PubMed] [Google Scholar]
- 9.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. American journal of reproductive immunology. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 10.Lamarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva ginecologica. 2010;62:105–120. [PMC free article] [PubMed] [Google Scholar]
- 11.Granger JP. Inflammatory cytokines, vascular function, and hypertension. American journal of physiology. Regulatory, integrative and comparative physiology. 2004;286:R989–990. doi: 10.1152/ajpregu.00157.2004. [DOI] [PubMed] [Google Scholar]
- 12.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Current hypertension reports. 2007;9:480–485. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 13.Hod T, Cerdeira AS, Karumanchi SA. Cold Spring Harbor perspectives in medicine. 2015. Molecular mechanisms of preeclampsia; p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang A, Rana S, Karumanchi SA. Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology. 2009;24:147–158. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- 15.Lamarca B, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, Granger J. Hypertension in response to il-6 during pregnancy: Role of at1-receptor activation. International journal of interferon, cytokine and mediator research : IJIM. 2011;2011:65–70. doi: 10.2147/IJICMR.S22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy SR, LaMarca BB, Parrish M, Cockrell K, Granger JP. Control of soluble fms-like tyrosine-1 (sflt-1) production response to placental ischemia/hypoxia: Role of tumor necrosis factor-alpha. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;304:R130–135. doi: 10.1152/ajpregu.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiprono LV, Wallace K, Moseley J, Martin J, Jr, Lamarca B. Progesterone blunts vascular endothelial cell secretion of endothelin-1 in response to placental ischemia. American journal of obstetrics and gynecology. 2013 doi: 10.1016/j.ajog.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JN, Jr, LaMarca B. 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension. 2015;65:225–231. doi: 10.1161/HYPERTENSIONAHA.114.04484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaral LM, Kiprono L, Cornelius DC, Shoemaker C, Wallace K, Moseley J, Wallukat G, Martin JN, Jr, Dechend R, Lamarca B. Progesterone supplementation attenuates hypertension and the autoantibody to the angiotensin ii type i receptor in response to elevated interleukin-6 during pregnancy. American journal of obstetrics and gynecology. 2014 doi: 10.1016/j.ajog.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keiser SD, Veillon EW, Parrish MR, Bennett W, Cockrell K, Fournier L, Granger JP, Martin JN, Jr, Lamarca B. Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy. American journal of hypertension. 2009;22:1120–1125. doi: 10.1038/ajh.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karumanchi SA, Bdolah Y. Hypoxia and sflt-1 in preeclampsia: The “chicken-and-egg” question. Endocrinology. 2004;145:4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 22.Murphy SR, LaMarca B, Cockrell K, Arany M, Granger JP. L-arginine supplementation abolishes the blood pressure and endothelin response to chronic increases in plasma sflt-1 in pregnant rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;302:R259–263. doi: 10.1152/ajpregu.00319.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin ii synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension. 2001;38:742–745. doi: 10.1161/01.hyp.38.3.742. [DOI] [PubMed] [Google Scholar]
- 24.Tam Tam KB1GE, Cockrell K, Arany M, Speed J, Martin JN, Jr, Lamarca B, Granger JP. Endothelin type a receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. American journal of obstetrics and gynecology. 2011 Apr;204(4):330.e331–334. doi: 10.1016/j.ajog.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meher S, Duley L. Progesterone for preventing pre-eclampsia and its complications. The Cochrane database of systematic reviews. 2006:CD006175. doi: 10.1002/14651858.CD006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pecks U, Rath W, Kleine-Eggebrecht N, Maass N, Voigt F, Goecke TW, Mohaupt MG, Escher G. Maternal serum lipid, estradiol, and progesterone levels in pregnancy, and the impact of placental and hepatic pathologies. Geburtshilfe und Frauenheilkunde. 2016;76:799–808. doi: 10.1055/s-0042-107078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. The journal of obstetrics and gynaecology research. 2010;36:239–247. doi: 10.1111/j.1447-0756.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 28.Shaamash AH, Elsnosy ED, Makhlouf AM, Zakhari MM, Ibrahim OA, HMEL-d Maternal and fetal serum nitric oxide (no) concentrations in normal pregnancy, pre-eclampsia and eclampsia. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2000;68:207–214. doi: 10.1016/s0020-7292(99)00213-1. [DOI] [PubMed] [Google Scholar]
- 29.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 30.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. The New England journal of medicine. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 31.Serrano NC, Casas JP, Diaz LA, Paez C, Mesa CM, Cifuentes R, Monterrosa A, Bautista A, Hawe E, Hingorani AD, Vallance P, Lopez-Jaramillo P. Endothelial no synthase genotype and risk of preeclampsia: A multicenter case-control study. Hypertension. 2004;44:702–707. doi: 10.1161/01.HYP.0000143483.66701.ec. [DOI] [PubMed] [Google Scholar]
- 32.Kharfi A, Giguere Y, Sapin V, Masse J, Dastugue B, Forest JC. Trophoblastic remodeling in normal and preeclamptic pregnancies: Implication of cytokines. Clinical biochemistry. 2003;36:323–331. doi: 10.1016/s0009-9120(03)00060-2. [DOI] [PubMed] [Google Scholar]