Abstract
Purpose
Effective clinical trial enrollment can be difficult in a protocol designs that contain one treatment arm that is perceived as being more “aggressive” or “favorable.” There have been limited studies focusing on the barriers to enrollment and the efficacy of alternative study design to improve accrual. We analyzed barriers to enrollment, particularly the influence of timing, in context of three prospective randomized oncology trials where one arm was considered more aggressive.
Methods and materials
From June 2011 to March 2015, patients who were enrolled on three prospective institutional protocols (an oligometastatic non-small cell lung cancer (NSCLC) trial, and two proton vs. intensity-modulated radiation therapy (IMRT) trials in NSCLC and esophageal cancer) were screened for protocol eligibility. Eligible candidates were approached about trial participation, and patient characteristics (age, sex, T/N categorization) were recorded along with details surrounding trial presentation (appointment number). Fisher’s exact test, Student’s t tests, and multivariate analysis were performed to assess differences between enrolled and refusal patients.
Results
309 eligible patients were approached about trial enrollment. The enrollment success rate (ESR) during this time span was 52% (n=160 patients). Enrolled patients were more likely to be presented trial information at an earlier appointment (oligomet protocol: 5 vs. 3 appointments (P<0.001), NSCLC protocol: 4 vs. 3 appointments (P = 0.0018), esophageal protocol: 3 vs. 2 appointments (P = 0.0086No other factors or patient characteristics significantly affected ESR.
Conclusion
Improvement in enrollment rates for randomized control trials is possible, even in difficult accrual settings. Earlier presentation of trial information to patients is the most influential factor for success, and may help overcome accrual barriers without compromising trial design.
Keywords: Clinical trials, enrollment barriers, trial accrual
INTRODUCTION
Data from randomized clinical trials is considered the “gold standard” for clinical research and key in advancing changes in oncologic practice. However, enrollment in clinical trials remains low, with an estimated 2%-3% of all oncology patients participating in clinical trials [1, 2]. This number is further reduced when protocol designs include a treatment arm that is perceived, by physicians or patients, as being more “aggressive” or “favorable” than the other. Previous studies have suggested that physician-based factors such as academic versus community settings, oncologic specialty, personal bias [2–5], and patient-based factors such as age, sex, racial profile, performance status, and socioeconomic status can all influence clinical trial accrual [1, 6–8].
Despite these findings, patient accrual has not demonstrably improved within the last decade, leading the Institute of Medicine in 2013 to conclude that “the system for conducting clinical trials in the United States is approaching a state of crisis” [9]. To resolve this crisis, greater insight into the patient-physician interaction and the influence of both parties on trial accrual is necessary.
To better understand the relative significance of factors influencing enrollment in clinical trials, we analyzed barriers to enrollment and the efficacy of alternative study design in what many would consider an idealized recruitment scenario—The University of Texas MD Anderson Cancer Center. We sought to determine our enrollment success and the influence of timing and predetermined treatment bias in trial recruitment; to help facilitate patient discussion and overcome accrual difficulties. This analysis was done in the context of three prospective randomized controlled trials in which one treatment arm was considered more aggressive than the other.
METHODS AND MATERIALS
Patient Populations
From June 2011 to March 2015, the interim accrual patterns of three open prospective radiation-based randomized controlled trials at our institution were recorded (Supplementary Table S1). Two trials were technology-based, comparing the use of proton therapy versus standard-of-care intensity-modulated radiation therapy (IMRT) for either non-small cell lung carcinoma (NSCLC) or esophageal carcinoma; the third trial assessed upfront versus delayed consolidative radiation therapy for oligometastic NSCLC.
Patient screening was evaluated separately for each protocol. Patient characteristics included demographics (age, sex, ethnicity, home location, marital status, and insurance status) and diagnostic information (date of diagnosis, location of diagnosis, pathology, performance status, tumor and nodal categorization, metastatic sites if applicable, and use of induction chemotherapy). Note that analysis was done prior to completion of these studies, and patient inclusion was based only on those screened or accrued until March 2015.
Method for Approaching Patients
Patients were screened by physicians, care providers, and research nurses for eligibility in respective institutional protocols. Once eligibility was confirmed, physicians were notified by research nurses, and initial trial information was presented to patients by a treating physician (surgeon, medical oncologist, or radiation oncologist) either during initial visit, or during subsequent follow-up visits. Research nurses assigned to the protocol of interest were typically available at time of visit for additional discussion immediately following physician-presentation. Alternatively, patients could set up subsequent appointments with research nurses to discuss additional information, obtain handouts, answer questions, and were provided treatment consent forms when appropriate. Patients were enrolled at the time of initial presentation if they desired, or they were allowed to enroll later if further discussion or evaluation was necessary.
Assessment of Enrollment Success Rate
Definition
Enrollment success rates (ESRs) were calculated based on the proportion of eligible patients who enrolled in individual protocols, when accounting for the total eligible patient population. Enrollment dates were also recorded to assess quarterly ESRs per protocol. Reasons for refusal were not recorded unless the patient expressed a preference for a specific treatment.
Influence of timing
To assess the influence of the timing on ESR for eligible patients, we calculated the date from diagnosis to initial protocol presentation to the patient. The date of this appointment, as well as MD Anderson appointment number, was recorded. This appointment number included consultations and follow-up visits with treating oncologic providers (surgeons, medical oncologists, and radiation oncologists) only. Ancillary appointments were not included in the appointment number tally. To assess the effect of timing on protocol enrollment, ESRs were assessed both in relation to induction chemotherapy and in quarterly intervals per protocol.
Accounting for predetermined treatment bias
For the two technology-based trials, patience preference, if any, was recorded. Eligible patients were stratified according to their agreement or refusal to participate. Treatment details were recorded so that the treatment preferences could be matched with actual treatment delivered.
Statistical Analysis
To identify predictors of enrollment success, Univariate comparisons of categorical and continuous variables were conducted via Fisher’s exact tests and Student’s t tests, respectively. Statistical tests were two-sided, and P values of <0.05 were considered to indicate a statistically significant difference. Recursive partitioning analysis was completed for each protocol and for all protocols combined to identify potential influences on ESR. Analyses were done with JMP Pro, Version 12 software (SAS institutes, Cary, NC). Randomization details per protocol can be found in the Supplementary Table 1.
RESULTS
Evaluating Protocol Accrual
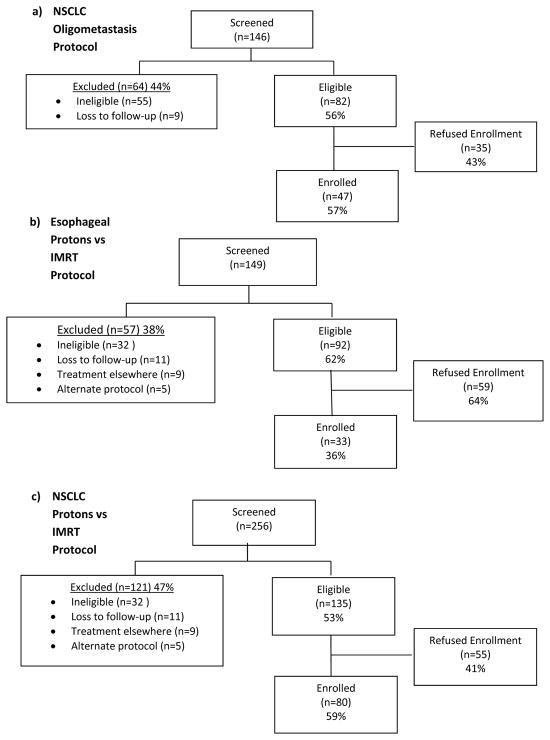
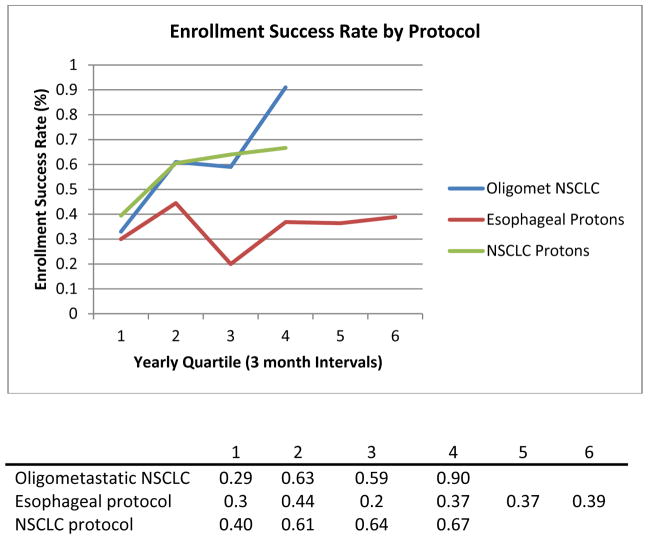
During the study period, 309 eligible patients were approached about trial enrollment. Among eligible patients, the overall enrollment success rate was 52% (ESR of 57% in [oligomet protocol], 36% in [esophageal protocol], and 59% in [NSCLC protocol]) (Figure 1). ESRs assessed quarterly increased in the oligomet protocol (ESRs 33%, 61%, 59%, 91%), while in the technology-based protocols, ESRs remained consistent after the initial accrual quartile (esophageal ESR 30%, 44%, 20%, 37%, 37%; NSCLC ESR 40%, 61%, 64%, 67%) (Figure 2).
Figure 1.
Flow diagram of patients enrolled by individual protocol.
Figure 2.
Enrollment success rate by trial.
Comparing Enrolled versus Non-Enrolled Eligible Patients
Both the oligomet and NSCLC protocols had approximately equivalent numbers of men and women, whereas the esophageal protocol had predominantly men (89%) (Table 1). Patient age (medians and ranges) were consistent among the three protocols, and most eligible patients were white (84%–89%). Tumor and nodal categories were consistent with the eligibility criteria of each individual protocol, with adenocarcinoma the dominant histologic subtype (Table 1).
Table 1.
Characteristics of eligible patients by protocol
| Protocol | ||||
|---|---|---|---|---|
| Immediate vs. Delayed Local Consolidative Therapy for oligometastatic NSCLC | IMRT vs. Proton Beam Therapy for Esophageal Carcinoma | IMRT vs. Proton Beam Therapy for LA-NSCLC | All Protocols | |
| Age at diagnosis, years, median (range) | 66 (42–83) | 64.5 (39–84) | 66 (37–82) | 66 (37 – 84) |
| Sex, no (%) | ||||
| Female | 38 (46) | 10 (11) | 65 (48) | 113 (37) |
| Male | 44 (54) | 82 (89) | 70 (52) | 196 (63) |
| Race, no (%) | ||||
| White | 73 (89) | 77 (84) | 118 (87) | 268 (87) |
| Non-white | 9 (11) | 15 (16) | 17 (13) | 41 (13) |
| Histology, no (%) | ||||
| Adenocarcinoma | 66 (80) | 78 (85) | 86 (64) | 230 (74) |
| Squamous | 16 (20) | 14 (15) | 49 (36) | 79 (26) |
| T-Category | ||||
| Tx | 1 | 0 | 2 | 3 |
| T1 | 27 | 4 | 30 | 61 |
| T2 | 37 | 9 | 51 | 97 |
| T3 | 6 | 78 | 26 | 110 |
| T4 | 11 | 1 | 26 | 38 |
| N-Category | ||||
| Nx | 0 | 4 | 0 | 4 |
| N0 | 29 | 29 | 14 | 72 |
| N1 | 13 | 27 | 16 | 56 |
| N2 | 25 | 25 | 65 | 115 |
| N3 | 15 | 7 | 40 | 62 |
| Performance Status no (%) | ||||
| 0 | 310 | 56 | 75 | 162 (52) |
| 1 | 45 | 34 | 56 | 135 (44) |
| 2 | 6 | 2 | 1 | 9 (3) |
| Days from Dx to Trial Screening, median (range) | 66 (0–370) | 35 (0–1384) | 35 (0–1308) | 38 (0 – 1384) |
Oligomet protocol
In the oligomet trial, eligible patients who enrolled versus those who refused protocol participation did not exhibit statistically significant differences in age, sex, home location, location of initial diagnosis, performance status, or tumor or nodal categories (all P>0.05). Married patients exhibited a borderline significant inverse association with trial participation (86% vs. 55%, P=0.07), and those diagnosed with metastatic disease at MD Anderson versus at another institution) were more likely to refuse enrollment (66% vs 38%, P=0.02) (Table 2). Notably, however, the appointment number at MD Anderson at which eligible patients were approached about protocol participation seemed to greatly influence enrollment. Patients who refused to enroll were approached later in their oncologic treatment course than those who chose to enroll (median 5 appointments vs. 3 appointments, P<0.001). Timing from diagnosis to protocol presentation did not seem to affect enrollment success, however (refusal group 0.4 months vs. enrolled group 0.3 months, P=0.43).
Table 2.
Univariate analysis of factors potentially influencing enrollment in one of three clinical trials Immediate vs. Delayed Local Consolidative Therapy for Oligometastatic NSCL
| Immediate vs. Delayed LCT for Oligometastatic NSCLC | IMRT vs. PBT for Esophageal Cancer | IMRT vs. PBT for Locally Advanced NSCLC | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No. Refused (n=35) | No. Enrolled (n=47) | P Value | No. Refused (n=59) | No. Enrolled (n=33) | P Value | No. Refused (n=55) | No. Enrolled (n=80) | P Value | |
| Categorical Variables, frequency (proportion) | |||||||||
| Sex: Female | 17 (49) | 21 (45) | 0.82 | 6 (10) | 4 (12) | 0.74 | 28 (51) | 37 (46) | 0.6 |
| Race/Ethnicity: White | 30 (86) | 43 (91) | 0.49 | 51 (86) | 27 (82) | 0.56 | 49 (89) | 69 (85) | 0.79 |
| Home location: Texas | 22 (63) | 25 (53) | 0.5 | 29 (49) | 22 (67) | 0.13 | 30 (55) | 48 (60) | 0.6 |
| Marital status: Married | 30 (86) | 31 (66) | 0.07 | 44 (75) | 26 (79) | 0.8 | 37 (67) | 60 (75) | 0.34 |
| Previous Cancer Diagnosis | 9 (26) | 9 (20) | 0.59 | 2 (3) | 3 (9) | 0.34 | 6 (11) | 6 (7.5) | 0.55 |
| Biopsy at MD Anderson | 8 (23) | 8 (17) | 0.58 | 6 (10) | 2 (6) | 0.7 | 9 (16) | 19 (24) | 0.39 |
| Metastasis Diagnosed at MDA | 23 (66) | 18 (38) | 0.02 | -- | -- | -- | -- | -- | -- |
| T status: T 3/4 | 5 (14) | 12 (26) | 0.28 | 54 (92) | 26 (79) | 0.11 | 22 (40) | 30 (38) | 0.86 |
| N status: N+ | 20 (57) | 31 (66) | 0.49 | 39 (66) | 20 (61) | 0.65 | 47 (85) | 74 (93) | 0.25 |
| Symptomatic metastasis | 11 (31) | 12 (26) | 0.62 | — | — | — | — | — | — |
| Approached before/during induction chemo | 21 (64) | 43 (91) | 0.001 | — | — | — | — | — | |
| LCT before chemo start | 13 (39) | 19 (38) | >0.99 | — | — | — | — | — | — |
| Induction chemo | -- | -- | -- | 15 (25) | 8 (24) | 1 | 27 (49) | 32 (450) | 0.38 |
| No RT preference | — | — | — | 19 (36) | 26 (79) | <0.0001 | 26 (47) | 75 (94) | <0.0001 |
| Continuous Variables, median (min-max) | |||||||||
| Age at diagnosis | 64 (50–82) | 66 (44–83) | 0.98 | 65 (39–84) | 62 (49–83) | 0.088 | 67 (43–82) | 66 (37–82) | 0.75 |
| Mo. from diagnosis to metastasis | 0.4 (0–31.8) | 0.3 (0–73.9) | 0.43 | — | — | — | — | — | — |
| Months from diagnosis to trial screen | — | — | — | 1 (0–46) | 1 (0.1–5.4) | 0.86 | 1.1 (0.03–43.6) | 1.25 (0.06–14.4) | 0.69 |
| MDA appt approached (all) | 5 (1–12) | 3 (1–9) | <0.001 | 4 (2–11) | 3 (2–8) | 0.0018 | 3 (1–11) | 2 (1–19) | 0.0086 |
| MDA appt approached, with RT pref | — | — | — | 4 (2–11) | 3 (2–8) | 0.0016 | 3 (1–11) | 4 (2–19) | 0.5 |
| MDA appt approached, no RT pref | — | — | — | 4 (2–11) | 3 (2–8) | 0.53 | 4 (2–9) | 2 (17) | 0.075 |
| ESR by RT preference, frequency (ratio) | |||||||||
| Radiation preference | — | — | — | 40 | 7 | <0.0001 | 29 | 5 | <0.0001 |
| Refused randomization | — | — | — | 9 (9/40) | NA | NA | NA | NA | NA |
| Protons | 24 (24/40) | 3 (3/7) | 0.15 | 24 (24/40) | 3 (3/7) | 0.15 | 22 (22/29) | 3 (3/5) | 0.08 |
| IMRT | 7 (7/40) | 4 (4/7) | 0.04 | 7 (7/40) | 4 (4/7) | 0.04 | 7 (7/29) | 2 (2/5) | 1 |
| Matching betw. RT pref & trtment rec’d | |||||||||
| Proton/Proton | — | — | — | 14 (14/24) | 2 (2/3) | 1 | 15 (15/22) | 2 (2/3) | 1 |
| IMRT/IMRT | — | — | — | 6 (6/7) | 3 (3/4) | 0.27 | 6 (6/7) | 1 (1/2) | 0.42 |
Technology-based protocols
In both technology trials, characteristics of patients who enrolled or did not enroll were similar, with age, sex, ethnicity, home location, tumor and nodal categories not influencing enrollment decisions (Table 2). Marital status did not influence enrollment in either the esophageal protocol (Refusal group 75% married vs Enrolled group 79% married, P=0.8) or the NSCLC protocol (Refusal group 67% vs. Enrolled group 75%, P=0.34).
Because the technology trials compared two different radiation modalities (IMRT vs. proton therapy), patient preference for treatment modality was also recorded. In both of these protocols, enrolled patients were more likely to not have a treatment preference than non-enrolled patients (esophageal 79% vs. 36%, P<0.0001; NSCLC 94% vs. 47%, P< 0.0001) (Table 2). Regardless of preference, however, enrolled patients were more likely to have been presented trial information earlier in the course of treatment than were their counterparts who refused (esophageal 3 vs. 4 appointments, P=0.0018; NSCLC 2 vs. 3 appointments, P=0.0086). (Table 2).
Similar to the results of the univariate analyses, recursive partitioning analysis also showed appointment number to be the strongest predictor of enrollment. For the oligomet protocol, 73% (n=37) of eligible patients who were approached within 4 appointments or less were enrolled vs. 32% (n=10) who were approached after 5 or more appointments. In the technology protocols, 57% (n=95) of patients approached within 4 appointments enrolled versus 28% (n=17) after 5 or more appointments. When data from all three protocols were analyzed together, protocol presentation within the first 4 appointments led to a 61% (n=133) rate of enrollment versus 30% (n=27) at 5 appointments or more (Table 3).
Table 3.
Recursive Partition Analysis
| Protocol | ||
|---|---|---|
| Oligomet NSCLC Protocol | ||
| MDA Appt No. Approached | < 5 | ≥5 |
| Enrollment Rate | 73% | 32% |
| No. of Patients Enrolled | 37 | 10 |
| Technology Trials | ||
| MDA Appt No. Approached | <5 | ≥5 |
| Enrollment Rate | 57% | 28% |
| No. of Patients Enrolled | 95 | 17 |
| All Protocols | ||
| MDA Appt No. Approached | <5 | ≥5 |
| Enrollment Rate | 61% | 30% |
| No. of Patients Enrolled | 133 | 27 |
NSCLC, non-small cell lung cancer.
DISCUSSION
Despite the difficulties in enrolling patients in clinical trials, patient accrual remains pivotal to ensure sufficient high-quality data that will help guide treatment decisions. When accounting for an annual average of 1250 thoracic radiation consultations at our Institution, these three protocols (of ten ongoing thoracic radiation trials) were able to achieve 2.5% enrollment. The overall accrual rate of screened patients was 29% (160 enrolled of 551 screened), which translated to enrollment of 52% of eligible patients (Figure 1). By comparison, reported enrollment rates at other institutions have ranged from 5% to 25% of eligible patients [10–12].
Furthermore, assessment of ESRs for the oligomet and NSCLC technology trial demonstrate increasing ESRs as protocol duration increases (Figure 2), suggesting momentum for successful enrollment. This is also reflected in the ESR trend for the Esophageal protocol where there is a noticeable drop in ESR in quartile 3 (ESR = 20%, 1 of 5 eligible patients enrolled), and a subsequent “rebuilding” of momentum for enrollment success (Supplementary Table 2). This difference in enrollment rates suggests two important and hopeful findings: (1) that enrollment rates are not inherently low and, more importantly, (2) continued institutional efforts can lead to increasing accrual rates throughout the duration of a trial. In other words, only by repeatedly approaching patients, rather than harboring preconceived notions of futility, can lead to improved protocol participation.
Delving further into the influence of earlier protocol presentation to patients, our findings show that earlier presentation to eligible patients forecasted greater enrollment success. When all eligible patients from the three protocols were combined, only 27 of 91 patients were enrolled if they had been approached later than their 4th appointment, compared with 133 of 218 enrolled patients approached before the 5th appointment (Table 3). We hypothesize this difference in ESR is due in large part to the 4th appointment as the first “return visit” where a treatment course is decided, as opposed to the first 3 appointments (1 each with a surgeon, medical oncologist, or radiation oncologist) serving as introductory evaluations and opinions. If providers were to wait until the 5th appointment, a treatment course has likely been presented with group consensus, and patients may be less inclined to deviate from a previously proposed plan.
To our knowledge, this is the first study to show that the timing of protocol presentation to patients can influence trial participation. We hypothesized that earlier presentation allows physicians to overcome implicit bias or reservations patients may have regarding participating in a randomized trial, and it also provides patients with greater initial decision-making capacity in their oncologic care [13–15]. Earlier protocol presentation could suggest to patients greater personal benefit given the belief of physicians in the value of the trial, and thus influence enrollment success [16]. This finding represents a modifable approach for healthcare providers to optimize enrollment of their current available patient pool, without broadening their eligibility criteria for the sake of increased enrollment.
Given the multi-factorial nature of successful patient enrollment in trials, our paper could not address all potential factors that may influence ESR. Physician preference in particular, could influence the timing of protocol presentation to patients, where a principle investigator would have a greater invested interest and effort towards ensuring successful enrollment. Our study did not record physician preference or enthusiasm for the studied protocols, but would be an important direction for future analysis.
Perhaps the most challenging barrier to enrollment however, is that of patient preference, as this can ultimately translate to hesitation towards treatment randomization. Our technology-based protocols were not immune to this, particularly when the perception was that one treatment option (proton therapy) was somehow more advantageous than the other (IMRT), despite the lack of findings from randomized comparisons to support this perception. Within our eligible patient pool, the subset of patients with a noted treatment preference tended to decline participation in trials (Table 2). Furthermore, it should be acknowledged that the focus of our analysis assessed randomization between treatment arms whose course were fairly similar. The ability to generate ESR momentum could be more difficult in treatment arms where there is a significantly more invasive component (surgery vs radiation) and thus ultimately lead to lower accrual numbers compared to arms with less invasive randomizations [17, 18].
Although our findings suggest exciting ways to capitalize on existing patient populations, our study was conducted within a somewhat optimized accrual setting, with resources that may not be available to all healthcare professionals [3]. Not all centers will have dedicated research staff which can help facilitate additional discussion with patients, but we believe that the experience at large academic centers can present a framework for optimizing research in other patient-care settings.
An additional limitation of our study was the heterogeneity within our populations. By nature of the various protocols (oligomet protocol with an induction chemotherapy arm) and the NSCLC technology trial (allowing for solitary brain metastasis), for certain patients, their appointment number could potentially be relatively high. There was a single patient with 19 appointments who enrolled on the NSCLC protocol after developing disease recurrence with a solitary brain metastasis. Additionally, this may help to explain the results from our recursive partitioning analysis, in which the experience with the oligomet trial suggested that approaching patients within their first 4 appointments led to nearly a 75% ESR, versus our technology trials with ESRs of 57% at 4 appointments. Regardless of the appointment number, however, the theme remains that presenting the opportunity to participate in trials earlier during treatment can help to increase patient interest and ultimately accrual.
CONCLUSIONS
Our findings demonstrate that even in the face of difficulties in accruing patients to randomized trials, several factors can help to influence ESRs. Notably, high enrollment rates were observed when patients were approached repeatedly and earlier during the course of treatment. At our institution, the timing of protocol participation had the most significant role in helping garner patient accrual, and this approach can allow physicians to optimize accrual within their available patient populations without sacrificing eligibility criteria. We therefore recommend considering this change to trial design to facilitate increased trial enrollment, particularly in randomized controlled trials that compare substantially different treatment regimens.
Supplementary Material
Acknowledgments
We thank Christine Wogan, MS, ELS, for her input in reviewing and editing this manuscript.
FUNDING
Supported in part by Cancer Center Support (Core) grant CA016672 from the US National Cancer Institute to The University of Texas MD Anderson Cancer Center.
Footnotes
DISCLOSURE: The authors have declared no conflicts of interest.
| Conflicts of Interest | Contributions | |
|---|---|---|
| Jennifer Logan | None | Idea conception, data collection + analysis, manuscript writing |
| Chad Tang | None | Data analysis, manuscript editing |
| Zhongxing Liao | None | Data collection, idea conception, manuscript editing |
| Jack Lee | None | Data analysis, manuscript editing |
| John Heymach | None | Data collection, manuscript editing |
| Stephen Swisher | None | Data collection, manuscript editing |
| James Welsh | None | Data collection, manuscript editing |
| Jianjun Zhang | None | Data collection, manuscript editing |
| Steven Lin | None | Data collection, manuscript editing |
| Daniel Gomez | None | Idea conception, data collection, manuscript writing + editing |
References
- 1.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. Jama. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 2.Movsas B, Moughan J, Owen J, et al. Who enrolls onto clinical oncology trials? A radiation Patterns Of Care Study analysis. Int J Radiat Oncol Biol Phys. 2007;68:1145–1150. doi: 10.1016/j.ijrobp.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 3.Fisher WB, Cohen SJ, Hammond MK, et al. Clinical trials in cancer therapy: efforts to improve patient enrollment by community oncologists. Med Pediatr Oncol. 1991;19:165–168. doi: 10.1002/mpo.2950190304. [DOI] [PubMed] [Google Scholar]
- 4.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan CP, Napoles AM, Dohan D, et al. Clinical trial discussion, referral, and recruitment: physician, patient, and system factors. Cancer Causes Control. 2013;24:979–988. doi: 10.1007/s10552-013-0173-5. [DOI] [PubMed] [Google Scholar]
- 6.Wood CG, Wei SJ, Hampshire MK, et al. The influence of race on the attitudes of radiation oncology patients towards clinical trial enrollment. Am J Clin Oncol. 2006;29:593–599. doi: 10.1097/01.coc.0000236213.61427.84. [DOI] [PubMed] [Google Scholar]
- 7.Brooks SE, Muller CY, Robinson W, et al. Increasing Minority Enrollment Onto Clinical Trials: Practical Strategies and Challenges Emerge From the NRG Oncology Accrual Workshop. J Oncol Pract. 2015;11:486–490. doi: 10.1200/JOP.2015.005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baquet CR, Ellison GL, Mishra SI. Analysis of Maryland cancer patient participation in national cancer institute-supported cancer treatment clinical trials. J Clin Oncol. 2008;26:3380–3386. doi: 10.1200/JCO.2007.14.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An American Society of Clinical Oncology and Institute of Medicine W, Institute of M, National Cancer Policy F, Board on Health Care S. Implementing a National Cancer Clinical Trials System for the 21st Century: Second Workshop Summary. Washington (DC): National Academies Press (US) Copyright 2013 by the National Academy of Sciences; 2013. All rights reserved. [PubMed] [Google Scholar]
- 10.Du W, Gadgeel SM, Simon MS. Predictors of enrollment in lung cancer clinical trials. Cancer. 2006;106:420–425. doi: 10.1002/cncr.21638. [DOI] [PubMed] [Google Scholar]
- 11.Horn L, Keedy VL, Campbell N, et al. Identifying barriers associated with enrollment of patients with lung cancer into clinical trials. Clin Lung Cancer. 2013;14:14–18. doi: 10.1016/j.cllc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Fouad MN, Lee JY, Catalano PJ, et al. Enrollment of patients with lung and colorectal cancers onto clinical trials. J Oncol Pract. 2013;9:e40–47. doi: 10.1200/JOP.2012.000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comis RL, Miller JD, Aldigé CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 14.Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 15.Ellis PM, Butow PN, Tattersall MH, et al. Randomized clinical trials in oncology: understanding and attitudes predict willingness to participate. J Clin Oncol. 2001;19:3554–3561. doi: 10.1200/JCO.2001.19.15.3554. [DOI] [PubMed] [Google Scholar]
- 16.Wright JR, Whelan TJ, Schiff S, et al. Why cancer patients enter randomized clinical trials: exploring the factors that influence their decision. J Clin Oncol. 2004;22:4312–4318. doi: 10.1200/JCO.2004.01.187. [DOI] [PubMed] [Google Scholar]
- 17.Wallace K, Fleshner N, Jewett M, et al. Impact of multi-disciplinary patient education session on accruala to a difficult clinical trial: the Toronto experience with surgical prostatectomy versus interstital radiation intervention. J Clin Oncol. 2006;24(25):4158–62. doi: 10.1200/JCO.2006.06.3875. [DOI] [PubMed] [Google Scholar]
- 18.Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;338(10055):2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.