Abstract
Purpose
We previously reported using statins was correlated with improved metastasis free survival in aggressive breast cancer. The purpose of this study was to examine the effect of statins on metastatic colonization by triple negative breast cancer (TNBC) cells.
Experimental Design
TNBC cell lines were treated with simvastatin and then studied for cell cycle progression and proliferation in vitro, and metastasis formation in vivo, following injection of statin-treated cells. Reverse-phase protein assay (RPPA) analysis was performed on statin-treated and control breast cancer cells. RNA interference targeting FOXO3a was used to measure the impact of simvastatin on FOXO3a-expressing cells. The prognostic value of FOXO3a mRNA expression was examined in 8 public breast cancer gene expression data sets including 1,479 patients.
Results
Simvastatin increased G1/S phase arrest of the cell cycle and inhibited both proliferation and migration of TNBC cells in vitro. In vitro pretreatment and in vivo treatment with simvastatin reduced metastases. Phosphorylated FOXO3a was downregulated after simvastatin treatment in (RPPA) analysis. Ectopic expression of FOXO3a enhanced mammosphere formation and migratory capacity in vitro. Knockdown of FOXO3a attenuated the effect of simvastatin on mammosphere formation and migration. Analysis of public gene expression data demonstrates FOXO3a mRNA downregulation was independently associated with shorter metastasis-free survival in all breast cancers, as well as in TNBC breast cancers.
Conclusions
Simvastatin inhibits in vitro endpoints associated with metastasis through a FOXO3a mechanism and reduced metastasis formation in vivo. FOXO3a expression is prognostic for metastasis formation in patient data. Further investigation of simvastatin as a cancer therapy is warranted.
Keywords: Triple Negative Breast Cancer, Metastasis, Simvastatin, FOXO3a
Introduction
Metastasis is the most common cause of death among women with breast cancer. Triple-negative breast cancers (TNBC) comprise 15% of breast cancers and have the poorest survival outcome of all breast cancer subtypes due to the high propensity for metastatic progression and absence of specific targeted treatments [1]. Breast cancer metastases develop from dissemination of primary tumor cells into distant organs. The primary tumor cells most likely to metastasize are hypothesized to be cancer stem cells (CSCs). CSCs have unlimited self-renewal potential, can generate non-CSC progeny, are resistant to conventional therapies, and are capable of migration [2, 3]. In xenograft models of breast cancer, breast CSCs (i.e., cells expressing CD44) displayed increased metastasis compared to CD44− breast cancer cells [4, 5].
Recently, Ginestier et al. showed statins (3-hydroxyl-3-methyl glutaryl coenzyme A reductase inhibitors) regulate breast CSCs through inhibition of RhoA [6]. Statins are widely used to reduce cholesterol and lipoprotein levels and thereby reduce mortality from cardiovascular disease; recently, statins have also been studied for their impact on cancer. A Danish study comparing local recurrence rates for stage I–III breast cancer between simvastatin users and nonusers showed a significant reduction in recurrence rates in the statin users after 10 years of follow-up [7]. A retrospective study showed statin use improved disease free survival in inflammatory breast cancer (IBC) patients [8]. We showed previously that simvastatin radiosensitized TNBC cell lines in vitro [9]. Statins have also been shown to have clinical benefits in lung, prostate, and colon cancers [10–12]. The molecular mechanisms underlying the antitumor effects of statins have been studied extensively. Statins decrease EGFR dimerization [13], increase inducible reactive nitric oxide levels [14], reduce metalloproteinase levels [15], decrease synthesis of inflammatory cytokines [16], and reduce VEGF secretion in breast cancer models [17]. Statins effect on metastasis and its underlying mechanisms are unknown.
Herein we determined the effect of statins specifically on TNBC metastasis and observed inhibition of metastasis by statins. Further, we identified FOXO3a as a potential mediator of TNBC metastasis using in vitro and in vivo models, and show that statin therapy regulates FOXO3a activation, suggesting a potential mechanism for simvastatin’s anti-metastatic effects.
Materials and Methods
Cell Culture and Drugs
SUM 149 and SUM 159 breast cancer cell lines were obtained from Asterand (Detroit, MI) and passaged in the laboratory for fewer than 6 months after receipt. Both types of cells were cultured in Ham’s F12 medium supplemented with 10% fetal bovine serum, 1 μg/mL hydrocortisone, 5 μg/mL insulin, and 1% antibiotic-antimycotic. MDA-MB-231 cells were obtained from ATCC and were cultured in α-media supplemented with 10% FBS, 1 μg/ml hydrocortisone, 1 μg/ml insulin, 12.5 ng/ml epidermal growth factor, sodium pyruvate, nonessential amino acids, 2 mM glutamine, and 1% antibiotic-antimycotic. Simvastatin (Sigma) was dissolved in DMSO at a stock concentration of 5 mM and stored at −80°C, and a final concentration of 2.5μM was used in this study. DMSO alone was used as a control.
Mammosphere Formation Assay
Mammosphere formation has been used as a measure of the self-renewal capacity of breast CSCs and correlates closely with tumorigenicity [18]. Treated and control cells were grown in standard mammosphere medium (serum-free, growth-factor-enriched medium) in low attachment plates at a concentration of 20,000 cells/mL. For secondary mammosphere assay, cells from primary mammospheres were dispersed with 0.05% trypsin, seeded in ultra-low attachment plates (20,000 cells/mL) in mammosphere medium, incubated for 7 days, and counted.
Aldefluor Assay
To further investigate the self-renewal capacity of cells, we used the Aldefluor assay following the manufacturer’s guidelines (StemCellTechnologies, Vancouver, Canada). Briefly, 5 × 105 cells were suspended in Aldefluor assay buffer containing ALDH substrate and incubated for 30 min at 37°C. As a negative control for each sample, cells were incubated with 50 mmol/L specific ALDH inhibitor diethylamino benzaldehyde (DEAB). Aldefluor fluorescence was excited at 488 nm, and fluorescence emission was detected using a Beckman Coulter machine. The data files were analyzed using FlowJo software (Treestar, Ashland, OR). For sorting, gates were established using ALDH-stained cells treated with DEAB as negative controls and taking the high negative and positive cells.
Cell Cycle Distribution and Cell Proliferation Assays
For assessment of cell cycle distribution, cells were fixed dropwise with 70% ice-cold ethanol overnight at 4°C. Then cells were suspended in 100 μL of phosphate-citrate buffer (0.19 M Na2HPO4, 4 mM citric acid), incubated for 30 min at room temperature, and resuspended in PBS containing 10 μg/mL propidium iodide and 10 μg/mL RNase A. The propidium iodide-stained cell samples were analyzed using FACS Calibur (Becton-Dickinson, San Jose, CA), and the percentage of cells in each phase of the cell cycle (G1, S, and G2/M) was analyzed with CELLQuest (Becton-Dickinson). For proliferation assay, pre-treated or DMSO treated cells were seeded at a density of 1.0 × 104 in 6 cm plates. After the specified number of days (24h-8 days), cells were trypsinized and viable cells counted with a Cellometer automated cell counter.
Migration Assays
Cell migration assays were performed using a Boyden chamber containing 24-well Transwell plates (Corning Inc.) with 8-μm pores on the membrane. All experiments were performed in duplicate and repeated three times. Approximately 5 × 104 cells in 200 μL culture medium supplemented with 10% FBS were seeded into the upper chamber. The lower chamber was filled with 500 μL complete medium (with 10% FBS) as a chemoattractant plus or minus simvastatin. After 24 hours of incubation at 37°C in a 5% CO2 atmosphere, the membranes containing the cells were fixed and stained with crystal violet. The lower surfaces of the membranes were photographed at ×100 magnification. Five random fields were photographed for each chamber to determine the migration.
In Vivo Studies
Four-week-old female SCID/Beige mice (Harlan, USA) were housed and used in accordance with guidelines of The University of Texas MD Anderson Cancer Center under an Institutional Animal Care and Use Committee-approved protocol (ACUF 07-08-07213). Mice were anesthetized through intraperitoneal injection of a cocktail containing ketamine (100 mg/kg), xylazine (2.5 mg/kg), and acepromazine (2.5 mg/kg) in sterile saline solution, and fur at the surgical site was removed. The number-4 inguinal glands were cleared of mammary epithelium, and green fluorescent protein (GFP)-luciferin-labeled control SUM 149 cells and simvastatin (1.25 μM)-treated GFP-luciferin-labeled SUM 149 cells (8.0 × 105 in 15 μL of PBS) were injected into the cleared fat pads. Transplants were allowed to grow until tumors reached a volume of 500 mm3. Tumor growth was monitored weekly with caliper measurements. Tumors were resected under the above-mentioned institutional guidelines. A portion of each tumor was formalin-fixed, paraffin-embedded, sectioned, and stained with hematoxylin and eosin. After tumor resection, mice were injected weekly with D-luciferin (Biosynth) and imaged weekly for metastasis for 6 weeks after resection. Portions of xenografts were formalin-fixed, paraffin-embedded, sectioned, stained with hematoxylin and eosin.
To further investigate in vivo metastasis, we used a tail-vein-injection metastasis model. GFP-labeled SUM 149 or MDA-MB-231 cells were treated with simvastatin (1.25 μM) in vitro. Twenty-four hours later, 1.0 × 106 GFP-labeled simvastatin-treated or DMSO-treated (control) SUM 149 or MDA-MB-231 cells were injected via tail vein into 4-week-old female SCID/Beige mice. In a separate in vivo study, GFP-labeled SUM 149 cells were injected into the tail vein without in vitro treatment. Starting one week following injection, mice from one group started receiving water containing simvastatin. Simvastatin was dissolved into the drinking water (0.06 mg/mL). A mouse on average drank 25 mL of water per day and the average weight of the mice was 20 grams. Therefore the final simvastatin dosing was 15 mg/kg/day. New water was exchanged weekly. Mice were euthanized 8 weeks after injection for SUM 149 and 6 weeks for MDA-MB-231, and lung and brain metastatic colonization were assessed by fluorescent stereomicroscopy. All staining studies were performed with standard protocols, and staining was analyzed by a pathologist specialized in breast cancer.
RPPA Analysis
Cell proteins were denatured by 1% SDS (with beta-mercaptoethanol) and diluted in five 2-fold serial dilutions in dilution buffer (lysis buffer containing 1% SDS). Serial diluted lysates were arrayed on nitrocellulose-coated slides (Grace Biolab) by Aushon 2470 Arrayer (AushonBioSystems). A total of 5808 array spots were arranged on each slide including the spots corresponding to positive and negative controls prepared from mixed cell lysates or dilution buffer, respectively. Each slide was probed with a validated primary antibody plus a biotin-conjugated secondary antibody. All sample analysis was performed at the MD Anderson RPPA Core Facility.
Immunoblotting
For immunoblotting, cells were lysed in 1x RIPA lysis buffer containing 1 μM PMSF, and 40 μg of protein was electrophoresed on SDS-polyacrylamide gels with a concentration gradient of 4% to 20% (Invitrogen). Membranes were incubated with primary antibodies, anti-FOXO3a, anti-phospho FOXO3a (Ser 253), and anti-CDKN1Bkip1(Cell Signaling Technology). Actin antibody was used as a loading control.
FOXO3a Knockdown and Overexpression
SignalSilence siRNA targeting FOXO3a and nontargeting siRNA were purchased from Cell Signaling. SUM 149 cells (100,000/dish) were plated on 35 mm dishes. The following day, the cells were transfected with the siRNAs using X-tremeGENE siRNA Transfection Reagent (Roche) according to the manufacturer’s protocol. Forty-eight hours after transfection, cells were washed collected for analysis of FOXO3a levels by western blotting and assayed for migration and mammosphere formation. Stable overexpression of FOXO3a in SUM 159 cell line was conducted using overexpression plasmids purchased from Systems Biosciences according to the manufacturer’s instruction. Briefly, overexpression FOXO3a plasmids were packaged along with plasmids pRSV-Rev, pMDLg-pRRE and pCMV-VSVG in 293 T cells. Cell lines were then transduced as we described previously [19].
Gene expression data analysis
We analyzed FOX03A mRNA expression in clinical samples in 8 public gene expression data sets selected as follows: pre-treatment sample of primary breast cancer, with at least one probe set representing FOX03A, and with the following clinicopathological annotations (pathological axillary lymph node status pN, tumor size pT, and grade, and follow-up in term of metastatic relapse). Data sets were collected from the National Center for Biotechnology Information (NCBI)/Genbank GEO and ArrayExpress databases, and authors’ website (Supplementary Table 1). The final pooled data set included 1,479 non-metastatic, invasive breast cancers. Their clinicopathological characteristics are summarized in Supplementary Table 2. Data analysis required pre-analytic processing. First, we normalized each data set separately using quantile normalization for the available processed data from the Agilent-based sets, and Robust Multichip Average (RMA) [20] with the non-parametric quantile algorithm for the raw data from the Affymetrix-based data sets. Normalization was done in R using Bioconductor and associated packages. Hybridization probes were then mapped across the different technological platforms. We used SOURCE (http://smd.stanford.edu/cgi-bin/source/sourceSearch) and EntrezGene (Homo sapiens gene information db, release from 09/12/2008, ftp://ftp.ncbi.nlm.nih.gov/gene/) to retrieve and update the Agilent annotations, and NetAffx Annotation files (www.affymetrix.com; release from 01/12/2008) to update the Affymetrix annotations. The probes were then mapped based on their EntrezGeneID. When multiple probes mapped to the same GeneID, we retained the one with the highest variance in a particular dataset. To avoid biases related to immunohistochemistry (IHC) analyses across different institutions and thanks to the bimodal distribution of respective mRNA expression levels, estrogen receptor (ER), progesterone receptor (PR), and ERBB2 expressions (negative/positive) were defined at the mRNA level using gene expression data of ESR1, PGR, and ERBB2 respectively, as previously described [21]. Before analysis of FOXO3A expression, expression data were standardized within each data set as previously described FOXO3A [22] expression in tumors (T) was measured as discrete value after comparison with mean expression in normal breast samples (NB): downregulation, thereafter designated “down” was defined by a T/NB ratio ≤0.5 and no downregulation (“not down”) by a T/NB ratio >0.5.
Statistical Analysis
All data in graphs are presented as mean (standard deviation). For in vitro studies, all data are represented in graphs as means ± SEM. A p-value inferior or equal to 0.05 in a paired two-sided test was considered statistically significant. For RPPA analysis, we used analysis-of-variance models to estimate 1) the overall effects of each treatment on each protein, and 2) the overall effects of all treatments combined on each protein. All treatment effects were computed using control as a reference. These results were averaged across all cell lines. Then the same analyses were repeated for each cell line, to determine which treatments worked the best for which cell line. Statistical analyses were conducted using SAS 9.4 for Windows (SAS Institute Inc., Cary, NC). Correlations between tumor groups and clinicopathological features were analyzed using the Fisher’s exact test. Metastasis-free survival (MFS) was calculated from the date of diagnosis until the date of distant relapse, and the follow-up was measured from the date of diagnosis to the date of last news for event-free patients. Survivals were calculated using the Kaplan-Meier method and curves were compared with the log-rank test. Univariate and multivariate survival analyses were done using Cox regression analysis (Wald test). Variables tested in univariate analyses included patients’ age at time of diagnosis (≤ 50 years vs >50), pathological type, axillary lymph node status (pN: negative vs positive), tumor size (pT: pT1 vs pT2–3), and grade (1 vs 2–3), ER, PR and ERBB2 statutes, and FOXO3A expression-based group (“down” vs “not down”). Variables with a p-value <0.05 in univariate analysis were tested in multivariate analysis. Statistical analysis was done using the survival package (version 2.37) in the R software (version 2.15.2; http://www.cran.r-project.org/). We followed the reporting REcommendations for tumor MARKer prognostic studies (REMARK criteria) [23]. Results with P-values less than 0.05 were regarded as significant.
Results
Simvastatin Inhibits Breast Cancer Metastatic Related Endpoints In vitro
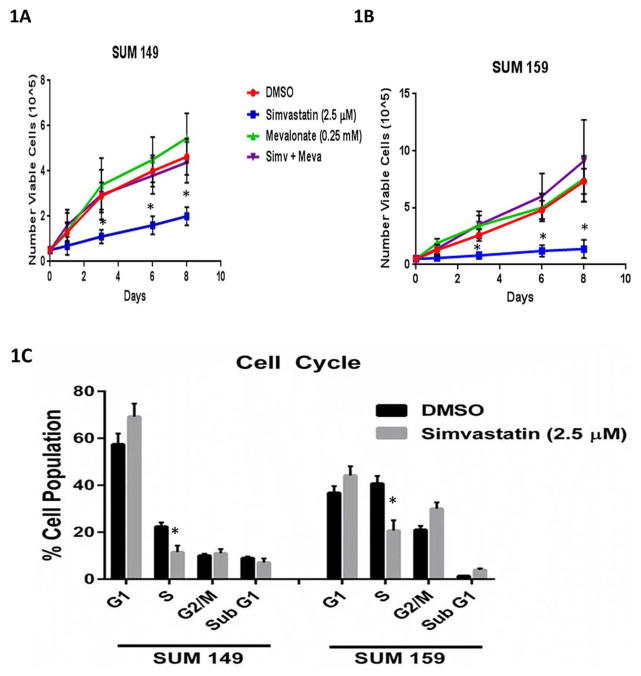
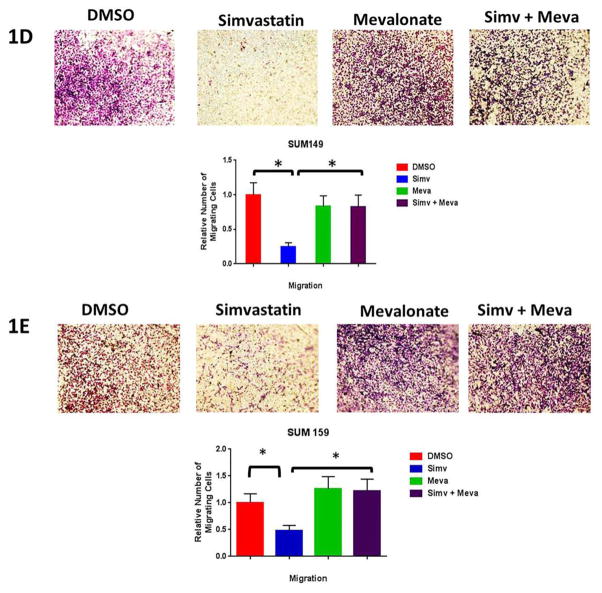
We analyzed the effects of simvastatin on cell proliferation, cell cycle distribution, and cell migration in TNBC cell lines. After 24 hours of pre-treating of SUM 149 and SUM 159 cells with simvastatin, we observed a significant decrease in cell proliferation as early as 48 hours and as long as 8 days (Fig 1A, 1B). Simvastatin decreased the S-phase fraction and increased G1/S arrest in both SUM 149 and SUM 159 TNBC cell lines (Fig 1C, Supplementary Fig S1). Next we used the Boyden chamber assay to measure migration of vehicle (DMSO) - or simvastatin-treated TNBC cell lines in vitro. Simvastatin significantly reduced the number of SUM 149 cells (Fig 1D) and SUM 159 cells that migrated through the pores in the membrane (Fig 1E). The migratory ability of simvastatin pre-treated SUM 149 and SUM 159 cells was rescued by co-treatment with mevalonate, the organic compound downstream of simvastatin inhibition. Similar results were observed in another TNBC cell line MDA-MB-231 (Supplementary Figure S2). These results demonstrate simvastatin inhibited surrogates of metastasis in vitro in multiple TNBC cell lines. As CSCs have been hypothesized as the metastatic initiating cells [2], we used two in vitro stem cell surrogate assays to test for the effect of simvastatin on CSC properties. The mammosphere assay developed by Dontu et al for normal mammary gland has been adapted for cancer cell lines as an assay that correlates to cancer cell self-renewal capacity. This has been validated in particular in TNBC cell lines [6, 18]. Confirming the findings of Ginestier et al [6], we found that primary sphere formation in TNBC cell lines SUM 149 and SUM 159 cells pretreated with simvastatin were reduced 78% and 75%, respectively, compared to DMSO-treated control cells (p<0.01) (Supplementary Figure S3, A,B). The addition of mevalonate (10 mM) rescued mammosphere formation back to control levels. Similarly, secondary sphere formation was inhibited (P<0.01) in simvastatin-treated cells compared to DMSO-treated cells for both cell lines (Supplementary Figure S3A) Moreover, the spheres were smaller in simvastatin-treated cells compared to DMSO-treated control cells (Supplementary Figure S3, A, B). The Aldefluor assay is an alternative surrogate assay to test for stemness [4]. We found that the proportions of ALDH-positive cells among simvastatin-treated SUM 149 and SUM 159 cells were 23% and 70%, respectively, relative to the proportions of ALDH-positive cells among DMSO-treated control cells (P<0.05) (Supplementary Figure S3, C, D). The proportions of ALDH-positive cells were also rescued to the levels seen in DMSO-treated control cells after administration of mevalonate (Supplementary Figure S3C). Thus, simvastatin reduced two independent in vitro surrogates for self-renewal capacity in TNBC cell lines through inhibition of the mevalonate pathway.
Figure 1. Simvastatin reduces percentage of cycling cells, proliferation, and migration.
Cell proliferation assay conducted to assess proliferation rates in (A) SUM 149 or (B) SUM 159 cell populations pretreated for 24h with 2.5 μM simvastatin, 10 mM mevalonate, both, or vehicle (DMSO). (C)SUM 149 and SUM 159 cells were profiled for their cell cycle pattern after they were pretreated 2.5 μM simvastatin of vehicle for 24 hours and stained with PI. (D) SUM 149 and (E) SUM 159 cells migrated through an 8 μM pore in the presence of 2.5 μM simvastatin, 10 mM mevalonate, both, or vehicle (DMSO). The number of cells that passed through the transwell membrane was counted. Significant differences are shown as follows: *, P < 0.05 for unpaired 2-tailed Student’s t-test (n = 3). Abbreviations: PI, propidium iodide.
Simvastatin Inhibits Tumor Formation and Metastasis In vivo
Considering the retrospective studies of reduced metastasis in patients on statins, presumably for dyslipidemia, we speculated that primary tumors that develop in the presence of statins had suppressed metastatic phenotypes and were less able to metastasize either due to less ability to transition out of the primary site or less ability to colonize once in the circulation. To examine both aspects of this metastatic potential of TNBC cells exposed to simvastatin versus control cells, we used two separate in vivo models, an orthotopic xenograft model and an experimental metastasis model via tail vein injection. Twenty weeks after orthotopic injection of pre-treated cells, tumors developed in 67% of the mice injected with simvastatin-treated SUM 149 cells compared to 95% of the mice injected orthotopically with DMSO-treated SUM 149 cells (Fig 2A, p=0.04). Primary tumors were resected at 20 weeks and mice were followed for an additional 8 weeks to evaluate the formation of distant metastasis. Of the mice in which tumors developed, 27% of the mice injected with simvastatin-treated SUM 149 cells had developed metastasis in distant organs compared to 79% of the mice injected with DMSO-treated SUM 149 cells (Supplementary Figure S4A, P=0.017). Metastasis free survival was thus significantly longer in the simvastatin group (Supplementary Figure S4B, p<0.01) despite no change in the primary tumor growth rate (Supplementary Figure S4C). In the tail-vein-injection model, the control group developed distant metastases (either lung or brain) at a higher rate than the simvastatin pre-treated group (86% control, 22% simvastatin) (Fig 2A, P=0.04, Supplementary Figure S5), confirming that the establishment of colonies within the lung or brain was significantly inhibited by simvastatin pre-treatment of the cell lines. These studies demonstrate that a short exposure to simvastatin altered the metastatic potential of the tumor cells.
Figure 2. Simvastatin inhibits metastasis in both orthotopic and tail-vein in vivo experiments.
(A) GFP labeled SUM 149 cells were treated in vitro with simvastatin (1.25 μM) and injected either orthotopically or into the tail vein of mice and tumor metastasis was assessed. For the in vivo treatment, mice injected with SUM 149 cells and simvastatin given ad libitum at weekly starting one week following injection at a dose of 15 mg/kg/day (B) GFP labeled MDA-MB-231 cells were treated in vitro with simvastatin (1.25 μM) and injected into the tail vein of mice. Following 6 weeks lungs were assessed for number of metastasis.
We confirmed these findings in a second triple negative breast cancer cell line, MDA-MB-231. Unlike SUM 149 which develops very few metastasis following tail vein injection, MDA-MB-231 cells injected via tail vein grow extensive metastasis in the lung but not in the brain. Repeating the design of the SUM 149 experiment, we treated MDA-MB-231 GFP labeled cells in vitro with simvastatin for 24 hours prior to tail vein injection. Following 6 weeks after injection, all animals developed metastases, however the MDA-MB-231 cells that were treated with simvastatin had a mean number of metastasis per lungs of 28.4 (SEM=11.9) compared with a mean of 139.5 (SEM=36.1) in the DMSO treated MDA-MB-231 cells (p=0.009) (Fig. 2B).
We next sought to determine if the effect of statins in vivo could be maintained post-inoculation. We tested a more clinically relevant treatment model where statin treatment would start subsequent to shedding of circulating metastatic tumor cells. GFP-labeled SUM 149 cells were injected via tail vein as previously described. After one week, one group of mice (n=10) started treatment with simvastatin given ad labitum in the drinking water (15 mg/kg/day). In the group receiving normal water, the percentage of mice that developed metastasis in the lung or brain was 70%, similar to the percentage of mice in the previous tail-vein experiment. In the group receiving oral simvastatin, the percentage of mice that developed metastasis was 20% (p=0.06) (Fig. 2A).
Simvastatin regulates FOXO3a in TNBC cells
To identify pathways targeted by simvastatin we utilized RPPA to compare protein activation state in simvastatin-treated versus untreated SUM 149 and SUM 159 cells. We found that phosphorylated FOXO3a and Akt were two of several proteins that were significantly down regulated (Supplementary Table 3). FOXO3a is a known tumor suppressor that is targeted for degradation by phosphorylation (27). Akt is activated by phosphorylation following growth factor binding, such as epidermal growth factor (EGF), to its cell membrane receptor. Following activation Akt regulates FOXO3a through phosphorylation leading to subsequent nuclear exclusion and degradation [24]. The possibility of increasing expression of a tumor suppressor is appealing so we examined the relative expression of both phosphorylated and total FOXO3 aproteinsin SUM 149 cells treated with simvastatin or vehicle control. We found that simvastatin inhibited phosphorylation of FOXO3a, and simvastatin maintained the level of total FOXO3a protein in the presence of EGF (Fig 3A). In order to further examine the function role of FOXO3a protein in TNBC metastasis, we evaluated its expression in two additional TNBC cell lines. We found that unlike SUM 149 which had a high level of FOXO3a expression, SUM 159 and MDA-MB-231 had low protein expression of FOXO3a (Fig 3A). We looked to determine how simvastatin regulates FOXO3a in different breast cancer cell lines that express varying levels of FOXO3a. In order to appreciate the changes of FOXO3a regulation in western blotting we used a super sensitive developing solution to detect phosphorylated FOXO3a in SUM 159 and MDA-MB-231 cells which express low protein levels of FOXO3a compared with SUM 149. We found FOXO3a can still be regulated by EGF and simvastatin in both SUM 159 and MDA-MB-231 cells. EGF increased the expression of phosphorylated FOXO3a in both cell lines. In contrast simvastatin treatment reduced the levels of protein expression of phosphorylated FOXO3a. These changes at very low protein concentrations led to an increase in total FOXO3a expression (Fig 3A). We also tested an ER+ breast cancer cell line, MCF7. MCF7 expressed high protein levels of both phosphorylated and total FOXO3a similar to SUM 149 (Fig 3A). Also, simvastatin could inhibit MCF7 proliferation (Supplementary Figure S5A) and mammosphere formation (Supplementary Figure S5B). We used the FOXO3a high-expressing SUM 149 and low/non-expressing SUM 159 cell lines for further loss and gain of function experiments to examine whether simvastatin suppresses metastases by upregulating FOXO3a. A model of our working hypothesis is shown in Figure 3B.
Figure 3. Simvastatin regulates the expression of FOXO3a.
(A) Immunoblot with anti-pFOXO3a and anti-FOXO3a antibodies in SUM 159, MDA-MB-231, MCF 7, and SUM 149 cells treated with 2.5μM simvastatin or vehicle control. Cells were treated with EGF 10 ng/mL for 15 minutes following 24 hours of simvastatin treatment or DMSO treatment. Beta-actin was used as a loading control. (B) A model of how simvastatin regulates FOXO3a.
Next, we stably expressed FOXO3a in the low-expressing SUM 159 using a lentiviral overexpression vector. Protein expression of FOXO3a and its downstream target CDKN1B (p27) in these cells was increased compared with SUM 159 cells stably transfected with empty vector (Fig 4A). Ectopic expression of FOXO3a significantly inhibited mammosphere formation (Fig 4B, p=0.005) and migration of SUM 159 cells (Fig 4C p=0.014) in line with the effects observed with simvastatin treatment.
Figure 4. FOXO3a is a mediator of mammosphere and migration in TNBC cells.
(A) Western blot assay to identify the overexpression efficiency of the plasmids for FOXO3a in SUM 159 cells. (B) Mammosphere formation presented as the average number of spheres per 40,000 cells plated relative to the control plasmid ± SD; representative data are shown. (C) Representative images of the Transwell assays (magnification, × 40). Quantification of the numbers of migrating cells relative to the control plasmid is presented as mean ± SD. (D)Immunoblot with anti-FOXO3a antibodies in SUM 149 cells transfected with either siRNA targeting FOXO3a or scrambled control siRNA for 48 hours. Beta-actin was used as a loading control. (E) SUM 149 cells transfected with either siFOXO3a or scrambled siControl exposed to simvastatin (2.5 μM) treatment (24 h) and seeded in self-renewal promoting suspension culture conditions. Mammosphere formation presented as the average number of spheres per 40,000 cells plated ± SEM; representative data are shown. (F) SUM 149 cells transfected with either siFOXO3a or scrambled siControl migrated through an 8 μM pore in the presence of 2.5 μM simvastatin or vehicle (DMSO). The number of cells that passed through the transwell membrane was counted. Significant differences are shown as follows: *, P < 0.05 for unpaired 2-tailed Student’s t-test (n = 3).
To mimic the effect of high FOXO3a phosphorylation leading to increased degradation, we knocked down FOXO3a in the high expressing SUM 149 cell and examined the effect of simvastatin on these cells lacking FOXO3a. We tested for increased self-renewal and migration in vitro following RNA interference targeting FOXO3a in a metastatic TNBC breast cancer cell line, SUM 149. RNA interference of FOXO3a in SUM 149 inhibited the expression of FOXO3a (Fig. 4D). Knockdown of FOXO3a significantly increased the mammosphere-forming efficiency of SUM 149 cells compared to control cells (Fig. 4E). While pretreatment with simvastatin significantly inhibited mammosphere formation in SUM 149 cells transfected with control siRNA- consistent with our earlier findings (Fig. 1) - simvastatin had no effect on the FOXO3a-knockdown cells (Fig 4E). Similarly, knockdown of FOXO3a resulted in increased migration of SUM 149 cells, and the treatment of these cells with simvastatin was unable to inhibit migration (Fig 4F). These results suggest that not only does FOXO3a regulate migration and self-renewal, but that simvastatin inhibition of these in vitro endpoints is mediated through regulation of FOXO3a.
FOXO3a Predicts For Distant Metastasis Free Survival in Breast Cancer Patients
To determine whether FOXO3a expression correlates with prognosis in patients with breast cancer, we analyzed FOXO3A mRNA expression in 8 public gene expression data sets, including 1,479 breast cancers clinically annotated. FOXO3A expression was heterogeneous with a range of intensities over 11 units in log2 scale (Supplementary Figure S6): 759 tumors (51%) showed downregulation when compared to normal breast (“FOXO3A-down” group), and 720 (49%) did not (“FOXO3A-not down” group). We searched for correlations between FOXO3A expression status (down- versus not down- groups) and clinicopathological variables (Supplementary Table 4). No correlation was found with patients’ age, grade, pN, pT, and PR status, whereas FOXO3A downregulation was associated with ductal type, ER-positive status and ERBB2-negative status (Fisher’s exact test). Regarding survival, 1,069 patients remained metastasis-free during a median follow-up of 93 months (range, 1 to 299) and 410 displayed metastatic relapse. The 5-year MFS rate was 77% [95 CI, 0.75–0.79]. In univariate analysis (Table 1), axillary lymph node involvement, large tumor size, high grade, ER-negative status, PR-negative status, and ERBB2-positive status were associated with poor MFS, as was the FOXO3A-down group (p=0.028, Wald test; HR = 1.24 [1.02–1.51], and p=0.028, log-rank test, Figure 6). In multivariate analysis (Table 1), all these features, except ER status, remained associated with poor MFS, including the FOXO3A-down group (p=0.024, Wald test; HR = 1.29 [1.03–1.60]). The MFS analysis was done in each molecular subtype separately. As shown in Figure 5, FOXO3A expression influenced MFS in the TN subtype and the HR+/ERBB2- subtype, but not in the ERBB2+ subtype. These results suggest that FOXO3a may have a prognostic value for breast cancer metastasis, which is consistent with our mechanistic findings.
Table 1.
Univariate and multivariate analyses for MFS in breast cancer
| Characteristics | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| N | HR [95 CI] | p-value | N | HR [95 CI] | p-value | ||
| F0X03A expression-based group | down vs. no down | 1479 | 1.24 [1.02–1.51] | 2.82E-02 | 1228 | 1.29 [1.03–1.6] | 2.40E-02 |
| Age, years | > 50 vs. <= 50 | 1120 | 0.97 [0.77–1.21] | 0.76 | |||
| Pathological type | ILC vs. IDC | 480 | 1.30 [0.74–2.27] | 0.213 | |||
| MIX vs. IDC | 0.66 [0.27–1.63] | ||||||
| other vs. IDC | 0.53 [0.25–1.15] | ||||||
| Pathological grade | 2–3 vs. 1 | 1437 | 2.89 [2.06–4.06] | 7.56E-10 | 1228 | 2.08 [1.45–2.99] | 6.42E-05 |
| Pathological axillary lymph node status, pN | positive vs. negative | 1309 | 1.63 [1.32–2.01] | 5.29E-06 | 1228 | 1.35 [1.08–1.70] | 9.87E-03 |
| Pathological tumor size, pT | pT2–3 vs. pT1 | 1260 | 1.64 [1.32–2.04] | 8.81E-06 | 1228 | 1.41 [1.12–1.77] | 3.12E-03 |
| ER status | positive vs. negative | 1479 | 0.58 [0.47–0.71] | 1.11E-07 | 1228 | 0.88 [0.67–1.17] | 0.40 |
| PR status | positive vs. negative | 1479 | 0.57 [0.47–0.69] | 8.40E-09 | 1228 | 0.70 [0.54–0.91] | 7.26E-03 |
| ERBB2 status | positive vs. negative | 1479 | 1.58 [1.20–2.08] | 1.04E-03 | 1228 | 1.37 [1.01–1.86] | 4.51E-02 |
Figure 5. Low FOXO3a mRNA expression levels are associated with poor metastasis-free survival in breast cancer patients.
Kaplan-Meier curves showing low FOXO3A mRNA levels (red curves) were associated with shorter metastasis-free survival in all breast cancers (top, left), in TN breast cancers (top, right), and in HR+/ERBB2− breast cancers (bottom, left). No correlation is found in ERBB2+ breast cancers (bottom, right).
Discussion
Our results show that TNBC cells pre-exposed to simvastatin had less metastasis-associated in vitro characteristics and formed less metastasis in vivo, and that such reduction of metastasis in vivo was correlated with intact FOXO3a signaling. We further showed that FOXO3A mRNA downregulation is associated with shorter metastasis-free survival in clinical data.
In the current proposed clinical model, the use of statins is for primary tumor prevention and treatment [25], but our study suggests simvastatin should be further considered for metastasis prevention. The anti-tumor effects of statins were observed over 20 years ago [26, 27]. Subsequent research confirmed statins inhibit breast cancer proliferation in vitro and tumor growth in vivo [28–32]. The association between statin use in patients and breast cancer incidence has not been as clear. Undela et al. investigated the relationship of statin use and breast cancer risk in 24 observational studies, reporting data from more than 2.4 million participants, including 76,759 breast cancer cases. Their findings showed no association of statin use with reduced risk of breast cancer [33]. To move forward with any clinical trials of the efficacy of statins in treating metastasis, biomarkers are necessary to separate patients into groups that would be predicted to be responsive to the effects of the drug.
FOXO3a has been previously described as a tumor suppressor in various tumors, including breast cancer, and is regulated by Akt, a pathway commonly deregulated in breast cancer [24]. FoxO3 acts as a transcription factor and triggers apoptosis through induction of death genes such as FasL and Bim1 [34]. Ni et al showed that FOXO3a silencing promoted invasiveness of renal cancer cells and metastasis of renal cancer cells in vivo [35]. Furthermore, Gopinath et al demonstrated a functional requirement for FOXO3a as a regulator of the Notch signaling pathway, a pathway critical for the self-renewal and maintenance of CSCs [36]. Our data further showed that FOXO3a mRNA downregulation was associated in uni- and multivariate analyses with shorter metastasis-free survival in breast cancer patients, implying FOXO3a could be useful as a marker for metastatic outcomes in breast cancer patients. A recent study by Jiang and colleagues showed high FOXO3a expression was significantly correlated with long-term survival [37]. Whether it may be predictive of response to statin therapy remains to be examined. Conceivably, that those with low FOXO3a may have a favorable response to statins when it can be induced by the drug but could be resistant if baseline low expression is irreversible. Whether there is a role in cells that already express FOXO3a to gain further benefit through simvastatin exposure would also be an important avenue for study in clinical trials. We showed that simvastatin decreased the phosphorylation of FOXO3a and increased total FOXO3a expression in SUM 159 and MDA-MB-231 cell lines and decreased phosphorylated FOXO3a expression and maintained total FOXO3a expression in SUM 149. It may be that SUM 149 maintains FOXO3a in the phosphorylated/inactive form in the cytoplasm but SUM 159 and MDA 231 degrades the phosphorylated FOXO3a more rapidly. It will be important in future studies to measure both total and phosphorylated FOXO3a when looking at human samples. The results of our study suggest a model wherein simvastatin treatment leads to increased activity of FOXO3a and finally inhibition of metastasis (Fig 3B). Our observation that simvastatin decreased the rate of G1-to-S-phase progression is consistent with previous studies showing statins increase the expression of cell cycle inhibitors, which have been shown to decrease the self-renewal capacity of CSCs [38–40]. FOXO3a also mediates decreased transcription of CDKN1B [41], and therefore the increased activity of FOXO3a through simvastatin treatment could be the mechanism underlying the cell cycle arrest that we observed. This inhibition of the G1-to-S-phase transition could be the mechanism underlying the decreased CSC self-renewal capacity observed in our study.
Our data are consistent with data from previous statin studies showing that statins can inhibit self-renewal capacity. Ginestier et al showed that simvastatin targeted CSCs through RhoA/CDKN1B signaling [6]. Similarly, Ni et al. observed downregulation of FOXO3a increased metastasis and predicted worse metastasis-free survival in patients with renal cell carcinoma [35]. This paper distinguishes itself from Ginestier et al because they looked at tumor initiation and this study focused on metastasis initiation. This study supports the correlation between tumor initiating cells and metastasis formation. This study’s focus was not on treatment of metastasis and as such these results suggest statins could be used for preventing metastasis. Consistent with the clinical data in IBC showing benefits only in non-metastatic, i.e. too late in those who develop metastasis in spite of statins [9].
The major strengths of our study are the use of two in vivo assays to study metastasis formation, the use of a large-scale screening tool such as RPPA, and the results of multivariate prognostic analyses in a large series of clinical breast cancer samples. This study would relate more directly to a trial treating patients after primary treatment for breast cancer to treat circulating or disseminated tumor cells and prevent metastasis. The mammosphere and Aldefluor assays both measure tumor initiation capacity but are only surrogates for CSC likeness, but are consistent with already published data examining limiting dilution [42].
In conclusion, we showed that simvastatin inhibits breast cancer cells’ ability to form metastases in spontaneous and experimental metastasis models. Furthermore, for the first time, we showed that FOXO3a in breast cancer is regulated by simvastatin and that FOXO3a mRNA expression levels in breast cancer patients correlated with metastasis-free survival. Our findings underscore the potential of statins for breast cancer therapy.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
The aggressive breast cancer subtype, triple-negative breast cancer (TNBC), metastasizes at a high rate and is notoriously resistant to standard treatments. Cholesterol lowering drugs, statins, have been reported to improve breast cancer survival outcomes, and also statins inhibit the growth of breast cancer in in vitro and in vivo studies. However, the effects of statins specifically on TNBC metastasis and the mechanism of action have not been explored. We show that simvastatin inhibits breast cancer metastasis formation in vivo, and is dependent on FOXO3a activation. In patients high FOXO3a levels predicted for longer distant free metastasis survival. Simvastatin warrants further investigation as a metastasis prevention agent in TNBC.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health R01CA138239-01 and 1R01CA180061-01, the State of Texas Grant for Rare and Aggressive Breast Cancer Research Program, and an institutional research grant from The University of Texas MD Anderson Cancer Center. The Research Animal Support Facility-Houston, Small Animal Imaging Facility, Flow Cytometry and Cellular Imaging Facility, and Research Histopathology Facility are supported in part by the National Institutes of Health through MD Anderson Cancer Center Support (core) Grant CA016672. Lacerda and Debebare recipients of Susan G. Komen for the Cure® Postdoctoral Fellowships (PDF12226438, KG101478, and KG111387, respectively). Adam Wolfe is recipient of a National Center for Clinical and Translational Science Grant TL1-TR000369 fellowship.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Foulkes WD, I, Smith E, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Woodward WA, Sulman EP. Cancer stem cells: markers or biomarkers? Cancer Metastasis Rev. 2008;27(3):459–70. doi: 10.1007/s10555-008-9130-2. [DOI] [PubMed] [Google Scholar]
- 3.Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15(4):338–44. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, Dalerba P, Adorno M, Lobo N, Bueno J, Dirbas FM, Goswami S, Somlo G, Condeelis J, Contag CH, Gambhir SS, Clarke MF. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci U S A. 2010;107(42):18115–20. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginestier C, Monville F, Wicinski J, Cabaud O, Cervera N, Josselin E, Finetti P, Guille A, Larderet G, Viens P, Sebti S, Bertucci F, Birnbaum D, Charafe-Jauffret E. Mevalonate metabolism regulates Basal breast cancer stem cells and is a potential therapeutic target. Stem Cells. 2012;30(7):1327–37. doi: 10.1002/stem.1122. [DOI] [PubMed] [Google Scholar]
- 7.Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sorensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103(19):1461–8. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brewer TM, Masuda H, Liu DD, Shen Y, Liu P, Iwamoto T, Kai K, Barnett CM, Woodward WA, Reuben JM, Yang P, Hortobagyi GN, Ueno NT. Statin use in primary inflammatory breast cancer: a cohort study. Br J Cancer. 2013;109(2):318–24. doi: 10.1038/bjc.2013.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacerda L, Reddy JP, Liu D, Larson R, Li L, Masuda H, Brewer T, Debeb BG, Xu W, Hortobagyi GN, Buchholz TA, Ueno NT, Woodward WA. Simvastatin radiosensitizes differentiated and stem-like breast cancer cell lines and is associated with improved local control in inflammatory breast cancer patients treated with postmastectomy radiation. Stem Cells Transl Med. 2014;3(7):849–56. doi: 10.5966/sctm.2013-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9(1):10–9. [PubMed] [Google Scholar]
- 11.Bansal D, Undela K, D’Cruz S, Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One. 2012;7(10):e46691. doi: 10.1371/journal.pone.0046691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23(34):8606–12. doi: 10.1200/JCO.2005.02.7045. [DOI] [PubMed] [Google Scholar]
- 13.Mantha AJ, McFee KE, Niknejad N, Goss G, Lorimer IA, Dimitroulakos J. Epidermal growth factor receptor-targeted therapy potentiates lovastatin-induced apoptosis in head and neck squamous cell carcinoma cells. J Cancer Res Clin Oncol. 2003;129(11):631–41. doi: 10.1007/s00432-003-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotamraju S, Williams CL, Kalyanaraman B. Statin-induced breast cancer cell death: role of inducible nitric oxide and arginase-dependent pathways. Cancer Res. 2007;67(15):7386–94. doi: 10.1158/0008-5472.CAN-07-0993. [DOI] [PubMed] [Google Scholar]
- 15.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23(5):769–75. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 16.Hakamada-Taguchi R, Uehara Y, Kuribayashi K, Numabe A, Saito K, Negoro H, Fujita T, Toyo-oka T, Kato T. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ Res. 2003;93(10):948–56. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- 17.Alber HF, Dulak J, Frick M, Dichtl W, Schwarzacher SP, Pachinger O, Weidinger F. Atorvastatin decreases vascular endothelial growth factor in patients with coronary artery disease. J Am Coll Cardiol. 2002;39(12):1951–5. doi: 10.1016/s0735-1097(02)01884-3. [DOI] [PubMed] [Google Scholar]
- 18.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klopp AH, Lacerda L, Gupta A, Debeb BG, Solley T, Li L, Spaeth E, Xu W, Zhang X, Lewis MT, Reuben JM, Krishnamurthy S, Ferrari M, Gaspar R, Buchholz TA, Cristofanilli M, Marini F, Andreeff M, Woodward WA. Mesenchymal stem cells promote mammosphere formation and decrease E-cadherin in normal and malignant breast cells. PLoS One. 2010;5(8):e12180. doi: 10.1371/journal.pone.0012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of Clinical Investigation. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D, Bertucci F. Prognostic and predictive value of PDL1 expression in breast cancer. 2015:2015. doi: 10.18632/oncotarget.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK) British Journal of Cancer. 2005;93(4):387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27(16):2312–9. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist. 2006;11(3):306–15. doi: 10.1634/theoncologist.11-3-306. [DOI] [PubMed] [Google Scholar]
- 26.Keyomarsi K, Sandoval L, Band V, Pardee AB. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991;51(13):3602–9. [PubMed] [Google Scholar]
- 27.Wejde J, Blegen H, Larsson O. Requirement for mevalonate in the control of proliferation of human breast cancer cells. Anticancer Res. 1992;12(2):317–24. [PubMed] [Google Scholar]
- 28.Seeger H, Wallwiener D, Mueck AO. Statins can inhibit proliferation of human breast cancer cells in vitro. Exp Clin Endocrinol Diabetes. 2003;111(1):47–8. doi: 10.1055/s-2003-37501. [DOI] [PubMed] [Google Scholar]
- 29.Issat T, Nowis D, Legat M, Makowski M, Klejman MP, Urbanski J, Skierski J, Koronkiewicz M, Stoklosa T, Brzezinska A, Bil J, Gietka J, Jakobisiak M, Golab J. Potentiated antitumor effects of the combination treatment with statins and pamidronate in vitro and in vivo. Int J Oncol. 2007;30(6):1413–25. [PubMed] [Google Scholar]
- 30.Koyuturk M, Ersoz M, Altiok N. Simvastatin induces apoptosis in human breast cancer cells: p53 and estrogen receptor independent pathway requiring signalling through JNK. Cancer Lett. 2007;250(2):220–8. doi: 10.1016/j.canlet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Kang S, Kim ES, Moon A. Simvastatin and lovastatin inhibit breast cell invasion induced by H-Ras. Oncol Rep. 2009;21(5):1317–22. doi: 10.3892/or_00000357. [DOI] [PubMed] [Google Scholar]
- 32.Klawitter J, Shokati T, Moll V, Christians U. Effects of lovastatin on breast cancer cells: a proteo-metabonomic study. Breast Cancer Res. 2010;12(2):R16. doi: 10.1186/bcr2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat. 2012;135(1):261–9. doi: 10.1007/s10549-012-2154-x. [DOI] [PubMed] [Google Scholar]
- 34.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 35.Ni D, Ma X, Li HZ, Gao Y, Li XT, Zhang Y, Ai Q, Zhang P, Song EL, Huang QB, Fan Y, Zhang X. Downregulation of FOXO3a promotes tumor metastasis and is associated with metastasis-free survival of patients with clear cell renal cell carcinoma. Clin Cancer Res. 2014;20(7):1779–90. doi: 10.1158/1078-0432.CCR-13-1687. [DOI] [PubMed] [Google Scholar]
- 36.Gopinath SD, Webb AE, Brunet A, Rando TA. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Reports. 2014;2(4):414–26. doi: 10.1016/j.stemcr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y, Zou L, Lu WQ, Zhang Y, Shen AG. Foxo3a expression is a prognostic marker in breast cancer. PLoS One. 2013;8(8):e70746. doi: 10.1371/journal.pone.0070746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray-Bablin J, Rao S, Keyomarsi K. Lovastatin induction of cyclin-dependent kinase inhibitors in human breast cells occurs in a cell cycle-independent fashion. Cancer Res. 1997;57(4):604–9. [PubMed] [Google Scholar]
- 39.Cheng T. Cell cycle inhibitors in normal and tumor stem cells. Oncogene. 2004;23(43):7256–66. doi: 10.1038/sj.onc.1207945. [DOI] [PubMed] [Google Scholar]
- 40.Chappell J, Dalton S. Altered cell cycle regulation helps stem-like carcinoma cells resist apoptosis. BMC Biol. 2010;8:63. doi: 10.1186/1741-7007-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li CJ, Chang JK, Chou CH, Wang GJ, Ho ML. The PI3K/Akt/FOXO3a/p27Kip1 signaling contributes to anti-inflammatory drug-suppressed proliferation of human osteoblasts. Biochem Pharmacol. 2010;79(6):926–37. doi: 10.1016/j.bcp.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.