Abstract
Co-inhibitory receptors play a key role in regulating T cell responses and maintaining immune homeostasis. Their inhibitory function prevents autoimmune responses but also restricts the ability of T cells to mount effective immune responses against tumors or persistent pathogens. T cells express a module of co-inhibitory receptors, which display great diversity in expression, structure, and function. Here, we focus on the co-inhibitory receptors Tim-3, Lag-3, and TIGIT and how they regulate T cell function, maintenance of self-tolerance, their role in regulating ongoing T cell responses at peripheral tissues, and their synergistic effects in regulating autoimmunity and antitumor responses.
1 Introduction
Initiation of adaptive T cell responses requires two signals: T cell receptor (TCR) stimulation through antigen recognition in the context of MHC and co-stimulation through interaction of co-receptors on T cells with their ligands on APCs. Many of these receptors are members of the B7 family that either positively or negatively contribute to TCR signaling and thus fine-tune the threshold for T cell activation. Positive co-stimulatory molecules promote T cell proliferation and effector function. The most prominent example of such a molecule is CD28, which allows for proper T cell activation upon recognition of its ligands CD80 or CD86 expressed by mature antigen presenting cells (APCs) (Esensten et al. 2016). Signaling via co-inhibitory receptors counteracts TCR-driven T cell activation and promotes functional inactivation of T cells leading to anergy and a state of tolerance. The best studied co-inhibitory receptor is CTLA-4, which outcompetes CD28 for its ligands and actively delivers inhibitory signals to the T cell to dampen T cell activation (Schildberg et al. 2016). At steady state, co-inhibitory receptors are critical for maintaining immune homeostasis as they counterbalance co-stimulatory signals and prevent excessive effector T cell activation, which would lead to autoimmunity. In addition to regulating effector T cell responses directly, co-inhibitory receptors like CTL-4 also dampen T cells responses indirectly by promoting the suppressive function of regulatory T cells (Tregs) (Wing et al. 2008). Through interaction with their ligands, co-inhibitory molecules can further regulate the ability of APCs to prime effector T cells. For instance, binding of B7 by CTLA-4 leads to back sig-naling into the APC, resulting in reduced cytokine production and induction of IDO and conditions DCs to downmodulate co-stimulatory ligands (Fallarino et al. 2003; Dejean et al. 2009; Grohmann et al. 2002). Co-inhibitory receptors thus regulate T cell responses at three different levels, namely by directly inhibiting effector T cell activation, by promoting the suppressive function of Tregs, and by modulating APC function to prevent T cell activation.
The strict control of T cell responses through co-inhibitory molecules is of utmost importance to a functioning immune system, as dysregulation of T cell responses results in pathology. Failure to keep T cell responses in check causes excessive immune activation and autoimmunity (Linsley et al. 1991; Waterhouse et al. 1995). On the other hand, excessive inhibition of T cell responses predisposes to cancer and allows for pathogen persistence. The importance of co-inhibitory receptors in cancer and chronic viral infection is highlighted by the fact that in these settings, co-inhibitory pathways are being targeted clinically to improve antitumor and antiviral T cell responses (Pauken and Wherry 2015; Chen and Mellman 2013).
T cells express a diverse repertoire of co-inhibitory receptors, which changes depending on the activation status of the T cell and its environment. Which of these receptors regulate T cell responses in any given setting not only depends on the expression pattern of the co-inhibitory receptor but also on where their ligands are expressed. Furthermore, triggers in the tissue microenvironment may dictate the expression and persistence of co-inhibitory molecules on T cells. While CTLA-4 primarily acts as a global switch during the early priming phase in lymphoid organs, other co-inhibitory receptors predominantly regulate effector T cell responses within tissue where effector T cell responses are being executed. This chapter will focus on the co-inhibitory receptors Tim-3, Lag-3, and TIGIT and how they regulate T cell function, especially during ongoing T cell responses.
2 Tim-3
T cell immunoglobulin-3 (Tim-3) is a type I transmembrane protein originally identified as a specific marker for Th1 and Tc1 cells and its expression is regulated by the Th1 transcription factor T-bet (Monney et al. 2002; Anderson et al. 2010) together with another transcription factor NFIL3 (Zhu et al. 2015). Tim-3 is further expressed on Tregs, NK cells, monocytes, macrophages, and DCs. The discovery of Tim-3 also led to the identification of the Tim family of genes, of which 3 proteins (Tim-1, Tim-3, and Tim-4) are conserved between mouse and humans (Meyers et al. 2005). They share a common structure consisting of an N-terminal IgV domain, a mucin stalk containing potential O-linked glycosylation sites, a type I transmembrane domain, and a cytoplasmic tail, which does not harbor any classical inhibitory signaling motifs but contains five conserved tyrosine residues (Meyers et al. 2005).
Initial efforts to determine the ligands of Tim-3 identified the C-Type lectin galectin-9 and a second protein, which was recently characterized as Ceacam1 (Fig. 1a) (Huang et al. 2015; Zhu et al. 2005). Galectin-9 binds to the N-linked sugar moieties in the Tim-3 IgV domain and this interaction triggers cell death in Th1 and Tc1 cells (Zhu et al. 2005; Kang et al. 2015). Ceacam-1 is co-expressed with Tim-3 on T cells and their cis-interaction in required for the inhibitory function of Tim-3, which is compromised in the absence of Ceacam-1 (Huang et al. 2015). Furthermore, the Tim-3—Ceacam-1 trans-interaction also suppresses T cell function. While Ceacam-1 and galectin-9 bind to different regions of the IgV domain of Tim-3, both ligands induce phosphorylation of the same two tyrosine residues required for functional activity of Tim-3 (Huang et al. 2015; Rangachari et al. 2012).
Fig. 1.
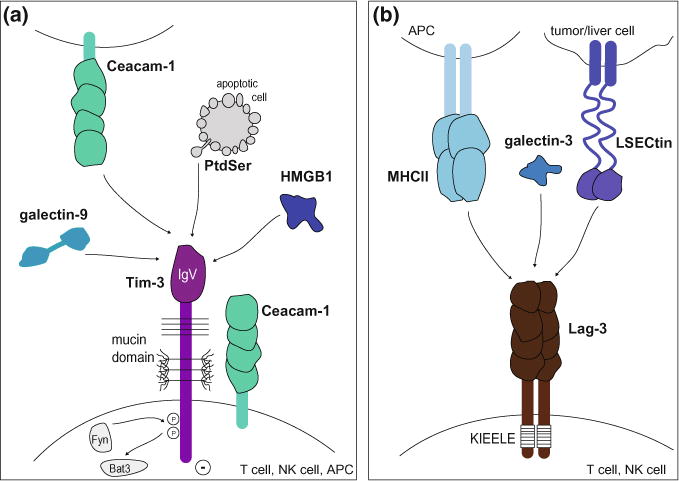
Tim-3 and Lag-3 pathways. a The Tim-3 pathway. Tim-3 is composed of an extracellular IgV domain, a mucin stalk with N- and O-linked glycosylation sites, and an intracellular tail with conserved tyrosine residues. It is expressed on T cells, NK cells, and APCs and binds to cell surface receptors (Ceacam-1 and phosphatidyl serine (PtdSer)) and soluble ligands (galectin-9 and HMGB1). Ligand binding triggers phosphorylation of two conserved tyrosine residues and release of Bat3 from the cytoplasmic tail of Tim-3, allowing Tim-3 to exert its inhibitory function. b The Lag-3 pathway. Lag-3 is composed of four extracellular Ig-like domains, a transmembrane domain, and a cytoplasmic tail containing a unique KIEELE motif. It is expressed on T cells and NK cells and binds to MHC class II on APCs, galectin-3, and LSECtin on tumor cells or liver cells
In addition to galectin-9 and Ceacam-1, Tim-3 has been reported to bind two additional ligands, phosphatidyl serine (PtdSer) and high mobility group protein B1 (HMGB1), which mainly play a role in the action of Tim-3 in innate cells (Fig. 1a) (Chiba et al. 2012). PtdSer is a nonprotein ligand, which is shared between the different Tim family members and is expressed on apoptotic cells (Cao et al. 2007; Santiago et al. 2007a, 2007b). Binding of PtdSer by Tim-3 expressing phagocytic cells can mediate uptake of apoptotic cells (DeKruyff et al. 2010; Nakayama et al. 2009) and perhaps contribute to initiating Tim-3-mediated inhibitory function. Tim-3 binding to HMGB1 has been shown to promote inhibitory function by blocking the transport of nucleic acids to endosomes and thereby interfering with nucleic acid sensing and danger signaling pathways in DCs (Chiba et al. 2012). Whether interactions of Tim-3 and PtdSer or HMGB1 take place in T cells and whether such contacts have functional consequences is still unknown. However, one can imagine that binding of apoptotic cells to Tim-3 in DCs could promote Tim-3 dependent inhibitory function and thus indirectly dampen T cell responses.
2.1 Signaling
Tim-3 acts as a negative regulator of Th1 and CTL responses. However, as mentioned above, the Tim-3 tail does not contain classical inhibitory signaling motifs. Instead, it harbors five conserved tyrosine residues, two of which are phosphorylated and important for binding to the intracellular adapter protein Bat3 (HLA-B associated transcript 3). Phosphorylation of the two tyrosines in the Tim-3 tail promotes downstream inhibitory signals (Rangachari et al. 2012; Lee et al. 2011). In the absence of binding of Tim-3 ligands, Bat3 is bound to the unphosphorylated cytoplasmic tail of Tim-3, recruits Lck, and preserves or may even promote T cell signaling (Rangachari et al. 2012). The interaction of Tim-3 with its ligands (galectin-9 or Ceacam1) induces an intracellular calcium flux and phosphorylation of the two critical tyrosine residues (Y256 and Y263), which releases Bat3 from the cytoplasmic tail of Tim-3 (Zhu et al. 2005; Rangachari et al. 2012). The release of Bat3 allows for the binding of SH2 domain-containing Src kinases and promotion of subsequent negative regulation of TCR signaling (Huang et al. 2015; Rangachari et al. 2012). Since Fyn and Bat3 bind to the same domain of the Tim-3 tail, it is possible that a switch between Tim-3/Bat3 and Tim-3/Fyn triggers the switch of Tim-3 being permissive to TCR signaling to Tim-3 inhibiting upstream TCR signaling (Rangachari et al. 2012). Much less is known about the downstream signaling events of Tim-3 in innate cells and it will be important to determine how the different Tim-3 ligands affect its function depending on the cellular context.
2.2 Tim-3 in Innate Cells
Tim-3 is highly expressed on innate cells and regulates their function in several ways. In human, all mature resting CD56dim NK cells express Tim-3 and its expression on NK cells is induced upon cytokine stimulation (Gleason et al. 2012; Ndhlovu et al. 2012), however, the role of Tim-3 on NK cells is controversial. High expression of Tim-3 is found on effector NK cells that display high cytotoxicity and produce IFN-γ. In this context, ligation of Tim-3 by galectin-9 promotes IFN-γ production by the NK cells and antibody blockade of Tim-3 results in impaired IFN-γ production (Gleason et al. 2012). In contrast, in advanced melanoma patients, Tim-3 marks functionally exhausted NK cells with impaired IFN-γ secretion and cytotoxicity (da Silva et al. 2014). Moreover, in this context Tim-3 expression levels on NK cells correlate with poor prognosis (da Silva et al. 2014; Xu et al. 2015). Furthermore, Tim-3 blockade reverses the exhausted phenotype and restores NK function (da Silva et al. 2014; Xu et al. 2015), suggesting that, similar to its role in CD8+ CTLs, Tim-3 not only marks exhausted NK cells but also contributes to their dysfunction in cancer settings.
DCs and macrophages also constitutively express high levels of Tim-3, which seems to act as a negative regulator of their function. In DCs, Tim-3 binding to HMGB1 inhibits DC activation by interfering with nucleic acid sensing as described above (Chiba et al. 2012). Tim-3 expression on macrophages down-modulates responses to TLR4 stimulation and has a dampening effect during sepsis (Yang et al. 2013). Tim-3 can also suppress the immune response indirectly by promoting generation of myeloid-derived suppressor cells (MDSC) in a Tim-3/galecin-9 dependent manner. Transgenic overexpression of Tim-3 or galectin-9 promotes expansion of MDSCs leading to decreased adaptive immunity as exemplified in accelerated tumor growth and decreased autoimmunity (Dardalhon et al. 2010). Loss of Tim-3 results in reversing the MDSC phenotype further supporting that the interaction between Tim-3/galectin-9 is promoting generation of MDSC (Dardalhon et al. 2010).
Reverse signaling on macrophages through the Tim-3/galectin-9 interaction also seems to have a protective effect in infections with intracellular pathogens. In a mouse tuberculosis model, treatment with Tim-3-Fc fusion protein reduced the bacterial burden in macrophages. This treatment was effective in both WT and Tim-3−/−, but not galectin-9−/− macrophages. Similarly, Tim-3 transgenic but not Tim3−/− CD4+ T cells controlled mycobacterial replication in galectin-9 expressing macrophages (Jayaraman et al. 2010; Sada-Ovalle et al. 2012). The interaction of Tim-3 expressing T cells with galectin-9 expressing macrophages thus controls microbial replication through reverse signaling. This suggests that Tim-3 expressed on effector T cells might directly interact with galectin-9 on macrophages/DCs to control intracellular pathogen, but in return the galectin-9/Tim-3 interaction might inhibit or delete Tim-3 bearing T cells, providing an effective mechanism by which to control effector T cells.
2.3 Role of Tim-3 in Effector Cells
Tim-3 was originally identified as a receptor expressed on Th1 and Tc1 cells, where it acts as a negative regulator of type 1 immunity (Monney et al. 2002). Tim-3 blocking antibodies were shown to exacerbate experimental autoimmune encephalomyelitis (EAE), a mouse model for human multiple sclerosis (MS) (Monney et al. 2002). Furthermore, blockade of the Tim-3 pathway accelerated Th1-driven diabetes in non-obese diabetic mice (Sanchez-Fueyo et al. 2003). In contrast, activation of Tim-3 by administration of its ligand galectin-9 dampened Th1 responses through induction of cell death in Tim-3+ Th1 cells and ameliorated EAE (Zhu et al. 2005).
Tim-3 also plays an important role in the induction of T cell tolerance. Loss of Tim-3 abrogates the induction of antigen-specific tolerance (Sabatos et al. 2003). Furthermore, anti-Tim-3 treatment prevents tolerance induction as its administration accelerated disease in a model of acute GVHD and negatively affected maternal–fetal tolerance resulting in increased risk of miscarriage (Oikawa et al. 2006; Wang et al. 2015).
In addition to expression on IFN-γ expressing Th1 and CD8+ T cells, Tim-3 is also transiently expressed on T cells upon activation, but stable expression is only observed upon sustained stimulation (Zhu et al. 2005; Sanchez-Fueyo et al. 2003). Interestingly, recent studies have investigated the role of Tim-3 in settings of chronic antigenic stimulation such as chronic infections or cancer. Tim-3 is indeed highly expressed on exhausted T cells in HIV-infected patients, where increased frequencies of Tim-3 expressing CD4+ T cells correlated with disease progression (Jones et al. 2008). Similarly, in mice chronic infection with lymphocytic choriomeningitis virus (LCMV) clone 13 induced exhausted CD8+ T cells that co-express Tim-3 and PD-1. These PD-1+ Tim-3+ double positive cells are functionally more deeply impaired than those expressing PD-1 alone (Jin et al. 2010). Tim-3 is also highly expressed on tumor-infiltrating lymphocytes and parallel to its expression pattern during chronic viral infection, Tim-3 is usually co-expressed with PD-1 and marks the most highly dysfunctional T cell subset (Sakuishi et al. 2010). Importantly, in both settings blockade of Tim-3 alone or in combination with PD-1 restores functionality in these cells, resulting in viral control or tumor regression suggesting that Tim-3 is involved in actively enforcing T cell exhaustion (Jin et al. 2010; Sakuishi et al. 2010; Baitsch et al. 2011; Ngiow et al. 2011).
2.4 Role of Tim-3 in Regulatory Cells
Under steady-state conditions, Tim-3 is barely expressed on Foxp3+ Tregs. In contrast, Tim-3 is unregulated on Tregs during an active immune response and is highly expressed on allograft and tissue infiltrating Tregs including tumor-infiltrating Tregs (Gupta et al. 2012; Sakuishi et al. 2013; Yan et al. 2013; Gao et al. 2012). In in vitro suppression assays, Tim-3+ Tregs display enhanced suppressive capacity compared to their Tim-3− counterparts (Gupta et al. 2012; Sakuishi et al. 2013; Gautron et al. 2014). Furthermore, Tim-3 expressing Tregs show increased expression of molecules associated with the suppressive function of Tregs including co-inhibitory receptors such as CTLA-4, Lag-3, and PD-1 as well as higher secretion of suppressive cytokines such as IL-10 and TGF-β (Gupta et al. 2012; Gautron et al. 2014). In tumor settings, the majority of tumor-infiltrating Tregs expresses Tim-3 and seems to represent a specialized tissue resident Treg subset with enhanced suppressive activity. Importantly, tumor-resident Tim-3+ Tregs may play a role in promoting effector T cell dysfunction observed in tumor-infiltrating lymphocytes, as their depletion restores functionality to effector T cells (Sakuishi et al. 2013). In an allograft rejection model, graft infiltrating Tim-3+ Tregs arise from activated, proliferating Tim-3− Tregs. Nevertheless, despite their superior suppressive function, Tim-3+ Tregs were inferior compared to their Tim-3− counterparts in prolonging graft survival in an adoptive transfer model as Tim-3+ Tregs were more short-lived and prone to undergo apoptosis (Gupta et al. 2012). These data suggest that parallel to its function in Th1 cells, Tim-3 might promote cell death in Tim-3 expressing Tregs.
3 Lag-3
Lag-3 (CD223) is expressed on activated CD4+ and CD8+ effector T cells, CD4+Foxp3+ Treg, Tr1 cells, B cells, plasmacytoid DCs, and a subset of NK cells (Triebel et al. 1990; Huang et al. 2004; Kisielow et al. 2005; Workman et al. 2009; Gagliani et al. 2013). Lag-3 is an immunoglobulin superfamily member composed of four extracellular Ig-like domains and a type I transmembrane domain and hence structurally resembles the CD4 co-receptor (Huard et al. 1995). Like CD4, Lag-3 binds MHC class II, but with higher affinity (Huard et al. 1995). Recently, two additional binding partners for Lag-3 have been described, LSECtin and galectin-3 (Kouo et al. 2015; Xu et al. 2014). LSECtin, a member of the DC-sign family, is expressed in the liver and on tumor cells, while galectin-3 is a soluble lectin expressed in a wide variety of cell types including tumor cells (Fig. 1b).
3.1 Signaling
Following TCR engagement, Lag-3 associates with CD3 in the TCR complex and crosslinking of Lag-3 together with CD3 negatively regulates signal transduction leading to reduced T cell proliferation and cytokine production (Hannier et al. 1998). However, the molecular aspects of the inhibitory effects of Lag-3 are still largely unknown, because of lack of a definable motif in the cytoplasmic tail. The cytoplasmic tail of Lag-3 does not contain any inhibitory motifs that are shared with other inhibitory receptors. As a consequence, the exact signaling pathway utilized by Lag-3 is still unclear. Nevertheless, the Lag-3 cytoplasmic tail contains three regions that are conserved between human and mouse and are thus likely involved in signal transduction. The first region contains a serine-phosphorylation site, the second region a single lysine residue within a unique “KIEELE” motif, and the third region contains glutamic acid-proline (EP) repeats (Workman et al. 2002). Among these three regions, the KIEELE motif was shown to be essential for signal transduction and the inhibitory function of Lag-3 (Workman et al. 2002). However, which binding partners interact with Lag-3 and mediate signal transduction is still unknown.
3.2 Role of Lag-3 in Effector Cells
Lag-3 acts as a negative regulator of T cell activation as blockade of Lag-3 or Lag-3 deficiency induces enhanced T cell proliferation and cytokine production (Workman et al. 2004; Workman and Vignali 2003). Lag-3 deficient OVA-specific CD4+ T cells show uncontrolled expansion upon immunization with their cognate antigen (Workman and Vignali 2003). Similarly, increased proliferation of Lag-3 deficient donor T cells causes more severe acute GVHD (Sega et al. 2014).
On CD8+ T cells, Lag-3 expression is induced by T cell activation and, like in CD4+ T cells, blockade of Lag-3 improves CTL proliferation and effector function (Grosso et al. 2007). Importantly, Lag-3 is also highly expressed on exhausted CD8+ T cells in both chronic viral infections and cancer (Blackburn et al. 2009; Richter et al. 2010). As Lag-3 blockade during chronic LCMV infection synergizes with PD-1 blockade to reverse exhaustion and improve viral control, Lag-3 seems to functionally contribute to CD8+ T cell exhaustion (Blackburn et al. 2009). Lag-3 is also co-expressed with PD-1 in tumor-infiltrating lymphocytes in various human tumors and mouse tumor models. While Lag-3 blockade alone might not necessarily be able to reverse the exhausted phenotype in CD8+ T cells, it synergizes with PD-1 blockade to improve tumor control or regression (Woo et al. 2012). Lag-3 hence functionally contributes to T cell exhaustion in both chronic viral infections and cancer.
3.3 Role of Lag-3 in Regulatory Cells
In addition to its expression in effector cells, Lag-3 has been reported to be highly expressed in regulatory IL-10 producing Tr1 cells and Foxp3+ Tregs. In fact, together with CD49b, Lag-3 was shown to identify IL-10 producing Tr1 cells in both mice and humans (Gagliani et al. 2013). While Lag-3 expression correlates with IL-10 levels (Burton et al. 2014), it has not been addressed whether Lag-3 directly contributes to the suppressive function of Tr1 cells.
In Tregs, loss of Lag-3 reduced the suppressive function of Tregs, while forced expression of Lag-3 conferred effector T cells with suppressive capacity (Huang et al. 2004). In line with these results, tumor-infiltrating Lag-3+ Treg display an activated phenotype and produce high amounts of IL-10 and TGF-β1 (Camisaschi et al. 2010). Furthermore, Lag-3 crosslinking of MHC II on DCs was shown to tolerize DCs and thus suppress the priming of effector T cell responses (Liang et al. 2008). Lag-3 thus plays an important role in dampening immune responses by functionally contributing to immune suppression by regulatory T cells.
4 TIGIT
Several groups simultaneously identified TIGIT (T cell immunoglobulin and ITIM domain; also called VSig9, Vstm3, or WUCAM) by bioinformatic analysis as a novel member of the CD28 family (Boles et al. 2009; Levin et al. 2011; Stanietsky et al. 2009; Yu et al. 2009). It acts as a co-inhibitory receptor and is expressed on NK cells and T cells, specifically activated, memory, and follicular T helper cells as well as on a subset of regulatory T cells (Boles et al. 2009; Levin et al. 2011; Stanietsky et al. 2009; Yu et al. 2009; Joller et al. 2011, 2014). TIGIT is composed of one extracellular IgV domain, a type I transmembrane region, and a cytoplasmic tail containing an ITAM and an immunoglobulin tail tyrosine (ITT)-like motif (Boles et al. 2009; Levin et al. 2011; Stanietsky et al. 2009; Yu et al. 2009; Stengel et al. 2012).
TIGIT is part of a complex ligand/receptor network in which it binds with high affinity to CD155 (PVR) and weakly interacts with CD112 (PVRL2, nectin-2) (Fig. 2a) (Levin et al. 2011; Stanietsky et al. 2009; Yu et al. 2009). Both of these ligands are expressed on APCs and a variety of non-hematopoietic cell types including tumor cells and are shared with CD226 (DNAM-1), the co-stimulatory receptor of this network (Bottino et al. 2003; Casado et al. 2009; Mendelsohn et al. 1989). We, in fact, predicted the existence of an inhibitory molecule that parallels CD226, in our in vivo blocking studies (Dardalhon et al. 2005), which was later identified as TIGIT. CD226 binds the two ligands with about 10 times lower affinity than TIGIT, which can inhibit the interaction between CD226 and CD155 in a dose-dependent manner (Levin et al. 2011; Stanietsky et al. 2009; Yu et al. 2009; Lozano et al. 2012). In addition to ligand competition, TIGIT can also directly bind to CD226 in cis and disrupt its homodimerization and hence its co-stimulatory function (Johnston et al. 2014). The network is completed by the co-inhibitory receptors CD96 (Tactile), an additional binding partner for CD155, and CD112R, which interacts with CD112 (Fig. 2a) (Chan et al. 2014; Fuchs et al. 2004; Zhu et al. 2016).
Fig. 2.
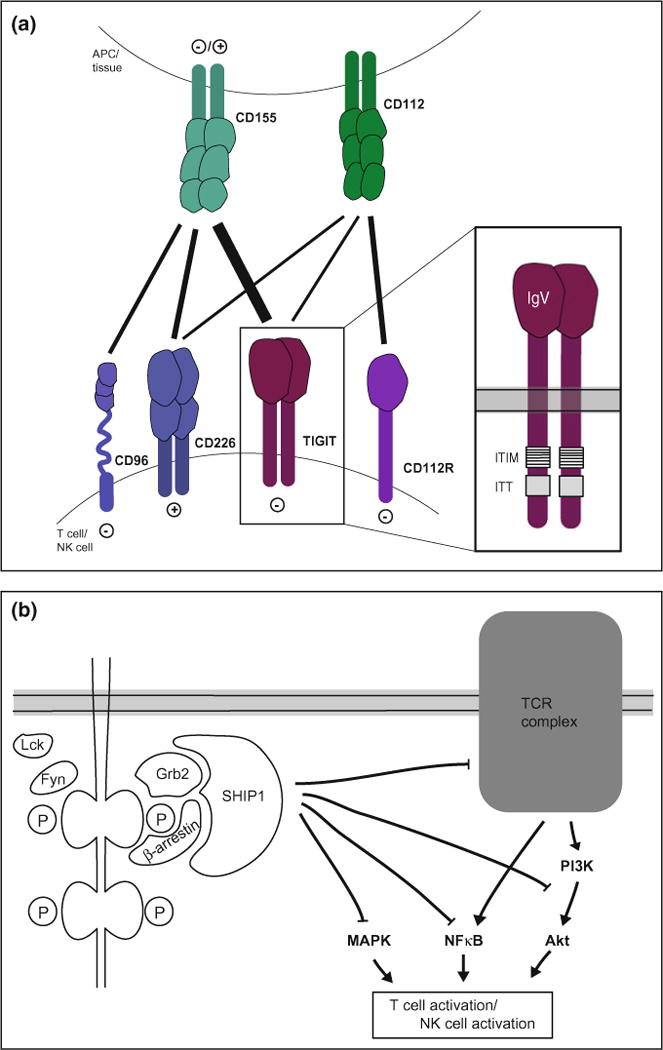
TIGIT pathway. a TIGIT forms part of a complex network where TIGIT, the co-stimulatory receptor CD226, and the co-inhibitory receptors CD96 and CD112R are expressed on T and NK cells and their ligands CD155 and CD112 are expressed on APCs and in tissue. TIGIT is composed of an extracellular IgV domain and a cytoplasmic tail containing an ITIM and ITT-like motif. b Upon ligand binding, the ITIM and ITT-like motifs in the TIGIT tail are phosphorylated and recruit SHIP1 via the adaptor proteins Grb2 or β-arrestin. SHIP1 inhibits signaling through the MAPK, NFκB, and Akt pathways, thus inhibiting activation
4.1 Signaling and Direct Inhibition
Although initial studies suggested that TIGIT only inhibits immune responses in a cell extrinsic manner, subsequent studies clearly demonstrated direct, cell intrinsic inhibitory functions of TIGIT (Levin et al. 2011; Stanietsky et al. 2009; Joller et al. 2011; Lozano et al. 2012). In NK cells, TIGIT directly inhibits NK cytotoxicity, granule polarization, and cytokine secretion (Stanietsky et al. 2009, 2013; Li et al. 2014; Liu et al. 2013). In T cells, TIGIT engagement inhibits their proliferation, cytokine production, and TCR signaling in a cell intrinsic manner (Levin et al. 2011; Joller et al. 2011; Lozano et al. 2012; Inozume et al. 2016).
The cytoplasmic tail of TIGIT contains an ITIM and an ITT-like motif, which are highly conserved between mouse and human and mediate its cell intrinsic inhibitory function (Boles et al. 2009; Levin et al. 2011; Stanietsky et al. 2009; Yu et al. 2009; Stengel et al. 2012). However, there is still some debate as to which of the two motifs is important for signaling and the inhibitory function of TIGIT. In mouse, the two motifs seem redundant as phosphorylation of the tyrosine residue in either the ITIM motif (Y233) or the ITT-like motif (Y227) is sufficient for signal transduction and the inhibitory activity of TIGIT is only abolished when both tyrosine residues are mutated (Stanietsky et al. 2013). Which of the two motifs is important for TIGIT signaling in human cells is still unclear as contradictory reports have been published claiming an essential role for either the ITIM motif (Y231) (Stanietsky et al. 2009) or the ITT-like motif (Y225) (Li et al. 2014; Liu et al. 2013). These differences might stem from the experimental system used as both groups performed their studies in TIGIT overexpressing cell lines. Hence, it will be important to study the relative contribution of ITIM versus ITT-like motifs under physiological conditions in primary human cells.
Engagement of TIGIT in NK cells induces the phosphorylation of the tyrosine residues in its ITIM and ITT-like motifs through the Src-family kinases Fyn and Lck. This allows for binding of the adaptor proteins Grb2 (growth factor receptor-bound protein 2) and β-arrestin 2, which in turn recruit SHIP1 (SH2 domain-containing inositol-5-phosphatase 1) to the cytoplasmic tail of TIGIT (Li et al. 2014; Liu et al. 2013). SHIP1 recruitment prematurely terminates PI3K (phosphoinositide 3-kinase), MAPK (mitogen-activated protein kinase), and NF-κB (nuclear factor-κB) signaling and results in NK cell inhibition marked by reduced cytotoxicity and cytokine secretion (Fig. 2b) (Li et al. 2014; Liu et al. 2013). In T cells, TIGIT signaling has not been studied at the protein level. Nevertheless, TIGIT engagement downregulates transcription of central components of the TCR signaling pathway as well as the TCR complex itself, thereby inhibiting productive T cell activation (Joller et al. 2011). In addition to its inhibitory effects on the TCR signaling pathway, TIGIT engagement promotes T cell survival through the induction of anti-apoptotic molecules (e.g., Bcl-xL) and receptors for pro-survival cytokines such as IL-2, IL-7, and IL-15 (Joller et al. 2011). TIGIT thus not only inhibits T cell activation but also promotes T cell survival and maintenance.
4.1.1 TIGIT in Effector Cells
The direct inhibitory function of TIGIT was first described in NK cells (Stanietsky et al. 2009). Here, TIGIT inhibits NK cytotoxicity and IFN-γ secretion and this TIGIT-mediated inhibition is dominant over co-activation through CD226 (Stanietsky et al. 2009; Stanietsky et al. 2013). In human NK cells, expression of TIGIT correlated with reduced cytokine production, degranulation, and cytotoxicity (Wang et al. 2015). Importantly, TIGIT expression levels on NK cells determine the effectiveness of inhibition of their cytotoxicity (Sarhan et al. 2016). TIGIT-mediated inhibition of NK function thus is an important mechanism for determining the threshold for NK activation. Furthermore, engagement of TIGIT through CD155 plays an important role in dampening NK-mediated immunopathology, as shown in a murine model of acute viral hepatitis (Bi et al. 2014).
In effector T cells, TIGIT can directly inhibit T cell activation and proliferation. Stimulation of T cells in the absence of APCs but in the presence of an agonistic anti-TIGIT antibody inhibits proliferation of mouse as well as human T cells (Levin et al. 2011; Joller et al. 2011). Furthermore, TIGIT stimulation inhibits IFN-γ production in human CD4+ T cells (Lozano et al. 2012). This T cell intrinsic inhibitory role of TIGIT was also confirmed in vivo, as mice with a CD4+ T cell specific loss of TIGIT developed augmented T cell responses marked by increased levels of the pro-inflammatory cytokines IFN-γ and IL-17 upon immunization (Joller et al. 2011). Similarly, stimulation of CD8+ T cell with CD155 expressing melanoma cells inhibits their IFN-γ production in a T cell intrinsic manner, as signaling through CD155 was not required to mediate the effect (Inozume et al. 2016). As seen for CD4+ T cells, in vivo, selective loss of TIGIT on T cells results in increased IFN-γ secretion by CD8+ T cells (Johnston et al. 2014). TIGIT thus acts as a cell intrinsic inhibitor by dampening effector cell activation, by inhibiting their proliferation and thereby limiting the effector cell pool, and finally by reducing effector cell function and cytokine production.
4.1.2 TIGIT in Regulatory Cells
In addition to effector cells, TIGIT is highly expressed in regulatory cells, where it promotes their suppressive function. In Tr1 cells (CD4+Foxp3−IL-10+), induction of the regulatory cytokine IL-10 correlates with TIGIT expression (Burton et al. 2014). TIGIT is also a direct target of Foxp3 and is expressed in a subset of predominantly natural CD4+Foxp3+ Tregs (Joller et al. 2014; Zhang et al. 2013). TIGIT+ Tregs express higher levels of Treg signature genes, such as Foxp3, CD25, and CTLA-4 and show enhanced demethylation in Treg-specific demethylated regions (TSDR) leading to higher lineage stability (Joller et al. 2014; Fuhrman et al. 2015). Interestingly, while TIGIT+ Tregs are highly suppressive and stable, CD226 expression in Tregs is associated with lineage instability and decreased suppressive capacity (Fuhrman et al. 2015). Engagement of TIGIT on Tregs directly induces expression of the suppressive mediators IL-10 and Fgl2 (Joller et al. 2014). TIGIT-dependent induction of Fgl2 confers superior suppressive capacity to TIGIT+ Tregs and most importantly enables them to selectively suppress pro-inflammatory Th1 and Th17 responses but not Th2 responses (Joller et al. 2014). In regulatory cells, TIGIT thus contributes to lineage stability and enhances their inhibitory function through the direct induction of suppressive mediators.
4.2 Indirect Inhibition
Early studies suggested that TIGIT indirectly suppresses immune responses via interacting with CD155 on DCs (Yu et al. 2009). TIGIT engages CD155 as a homodimer, where each TIGIT molecule binds one CD155 molecule, thus assembling into a heterotetramer with a TIGIT homodimeric core (Stengel et al. 2012). TIGIT binding induces phosphorylation of the ITIM motif in the CD155 cytoplasmic tail resulting in recruitment of SHP-2 and phosphorylation of Erk (Yu et al. 2009; Coyne et al. 2007). Overall, this leads to a decrease in IL-12p40 while increasing IL-10 production in DCs (Yu et al. 2009). TIGIT thus inhibits T cell responses indirectly by inducing tolerogenic DCs through engagement of CD155.
More importantly, TIGIT is also able to indirectly dampen immune responses by enhancing and modulating the suppressive function of Tregs. TIGIT expressing Tregs displays an activated phenotype and increased suppressive capacity compared to their TIGIT− counterparts (Joller et al. 2014). TIGIT engagement in Tregs induces suppressive mediators, which dampen T cell proliferation and mediate pleiotropic immunosuppressive effects (Chan et al. 2003). TIGIT-dependent induction of Fgl2 also confers Tregs with the ability to selectively dampen pro-inflammatory type 1 and type 17, but not type 2 immune responses (Joller et al. 2014). TIGIT+ Tregs thus potently suppress activation and proliferation of effector T cells in general, but also shape the effector response by shifting the balance away from pro-inflammatory Th1/Th17 towards more anti-inflammatory, IL-10-dominated responses.
5 Role in Diseases and Therapy
5.1 T Cell Exhaustion
The main function of co-inhibitory receptors is to maintain tolerance under homeostatic conditions and to dampen excessive immune responses in order to prevent immunopathology. In chronic infections, the pathogens and therefore also the antigens persist and constitute a source of persistent antigenic stimulation. This chronic activation is counterbalanced by an upregulation of co-inhibitory receptors, which serve to keep the effector response in check and prevent immunopathology. At the same time, this persistent high expression of co-inhibitory receptors can constrain effector T cells to a degree where they become dysfunctional or exhausted and are no longer able to promote pathogen clearance. Exhausted T cells show impaired effector function (cytokine production, cytotoxicity) and are marked by the sustained expression of multiple inhibitory receptors (reviewed in (Wherry and Kurachi 2015)). PD-1 was the first inhibitory receptor identified to selectively mark exhausted T cells and actively contribute to the dysfunctional state, as its blockade was able to restore function in virus-specific T cells (Barber et al. 2006). It has since become clear that several other co-inhibitory receptors are co-expressed with PD-1 and synergistically act to curb T cell responsiveness and function.
Chronic infection with LCMV serves as the prototypic model for studying T cell exhaustion (Moskophidis et al. 1993). Here, Lag-3, Tim-3, and more recently also TIGIT were shown to be co-expressed with PD-1 on exhausted virus-specific CD8+ T cells (Table 1.) (Jin et al. 2010; Blackburn et al. 2009; Richter et al. 2010; Johnston et al. 2014). The extent of exhaustion seems to correlate with the number of co-inhibitory receptors expressed and while the expression of a single co-inhibitory receptor is not indicative of exhaustion, co-expression of multiple inhibitory receptors is a hallmark of exhausted T cells (Fig. 3.) (Doering et al. 2012). However, it is not clear whether the co-inhibitory molecules are co-expressed on the same dysfunctional T cell or the co-inhibitory molecules are progressively acquired in the population, which leads to the loss of effector functions. The co-expression of inhibitory molecules also bears functional relevance as blockade of a single co-inhibitory receptor does not or only poorly reverse exhaustion, while co-blockade results in synergistic effects. This concept was demonstrated for PD-1 and Lag-3 (Blackburn et al. 2009), PD-1 and Tim-3 (Jin et al. 2010), and PD-1 and TIGIT (Johnston et al. 2014), where simultaneous targeting of the two pathways was able to synergistically reverse exhaustion and improve T cell function (Table 1.). Similar to their cooperative action in enforcing T cell exhaustion during chronic LCMV infection in mice, inhibitory receptors are also found to be co-expressed in chronic viral infections in humans such as HIV, HBV, or HCV infection (Fromentin et al. 2016; Jones et al. 2008; Golden-Mason et al. 2009; McMahan et al. 2010; Wu et al. 2012; Nebbia et al. 2012). In these chronic viral infections, Tim-3 marks virus-specific dysfunctional CD8+ T cells and Tim-3 blockade improves their function (Jones et al. 2008; Golden-Mason et al. 2009; McMahan et al. 2010; Wu et al. 2012; Nebbia et al. 2012). Similarly, TIGIT expression correlates with parameters of HIV disease progression and combinational blockade of TIGIT and PD-L1 restore CD8+ T cell function (Chew et al. 2016). Collectively, these data suggest that Tim-3, Lag-3, and TIGIT act in a cooperative manner with PD-1 and have nonredundant functions. Their divers expression patterns, binding partners, and signaling motifs all contribute to their synergistic effects. However, whether co-blockade of Tim-3, Lag-3, or TIGIT will have synergistic effect with each other independent of PD-1 is currently being tested. As such their therapeutic targeting alone or in combination bears the potential of gradual or site specific restoration of function in exhausted T cells.
Table 1.
Expression pattern and therapeutic effect on Tim-3, Lag-3, and TIGIT in chronic infections and cancer
| Expression | Therapeutic targeting reverses exhaustion | ||||||
|---|---|---|---|---|---|---|---|
| Tim-3 | Lag-3 | TIGIT | Tim-3 | Lag-3 | TIGIT | ||
| Chronic infection | LCMV | x | x | x | With α-PD-1 | With α-PD-1 | With α-PD-1 |
| HIV | x | x | x | x | With α-PD-L1 | ||
| Cancer | CD8+ TILs | x | x | x | With α-PD-1 | with α-PD-1 | With α-PD-1 |
| CD4+ TILs | Not tested | x | Not tested | With α-TIGIT | With α-Tim-3 | ||
| Treg TILs | x | Not tested | x | ||||
Fig. 3.

Co-inhibitory receptors in chronic infections and cancer. Antigen persistence drives T cells into a state of exhaustion/dysfunction characterized by hierarchical loss of cytokine production as well as impairment of cytotoxicity. As T cells enter the state of T cell exhaustion they progressively express PD-1 and upregulate Lag-3, Tim-3, and TIGIT
5.2 Cancer and Checkpoint Inhibitors
Chronic antigen exposure is a key feature shared between persistent infections and cancer. Indeed, tumor-specific T cells resemble the dysfunctional effector T cells present in settings of chronic infection in that they acquire expression of multiple co-inhibitory receptors during tumor progression, leading to their functional exhaustion (Fig. 3). Exhausted tumor-specific T cells express high levels of CTLA-4 and PD-1 and recent immunotherapeutic advances have aimed at targeting these co-inhibitory receptors to reverse their dysfunctional phenotype, reinvigorate tumor-specific T cell responses, and promote tumor elimination. The success of therapies targeting CTLA-4 (Ipilimumab) and PD-1 (Prembrolizumab and Nivolumab) has marked a major breakthrough in cancer therapy (Couzin-Frankel 2013; Gravitz 2013). Despite their successes, response rates for these therapies only range around 20–30% (Hodi et al. 2010; Topalian et al. 2012) and at present, combinatorial approaches are being explored to improve their efficacy. More recently, the list of co-inhibitory receptors expressed on TILs has been extended to include Tim-3, Lag-3, and TIGIT, which might represent additional therapeutic targets for cancer immunotherapy.
Expression of Tim-3 was found on functionally exhausted cells in a broad spectrum of both murine tumor models and cancer patients (melanoma, non-small cell lung cancer, follicular B cell non-Hodgkin lymphoma) (Sakuishi et al. 2010; Gao et al. 2012; Fourcade et al. 2010; Yang et al. 2012). Tim-3 positively correlates with cancer severity and poor prognosis in different cancer settings (Gao et al. 2012; Yang et al. 2012) and identifies a highly exhausted population of CD8+ TILs, which fail to produce IL-2, TNF-α, or IFN-γ (Sakuishi et al. 2010). Similar to observations in chronic infections, Tim-3 is co-expressed with other co-inhibitory receptors, most notably PD-1. While blockade of Tim-3 alone only shows minor effects, co-blockade of Tim-3 with PD-1 is superior at improving antitumor effector function and suppressing tumor growth than blockade of either pathway alone (Sakuishi et al. 2010; Ngiow et al. 2011; Zhou et al. 2011). Similarly, Lag-3 is co-expressed with PD-1 on dysfunctional CD4+ as well as CD8+ TILs and co-blockade of both pathways shows synergistic effects in improving antitumor immunity (Woo et al. 2012; Matsuzaki et al. 2010). TIGIT was shown to negatively regulate antitumor responses as TIGIT deficiency results in significantly delayed tumor growth (Kurtulus et al. 2015). Like Tim-3 and Lag-3, TIGIT is highly expressed on human and murine TILs and shows synergistic effects with PD-1 (Johnston et al. 2014; Kurtulus et al. 2015; Chauvin et al. 2015). In murine tumors, CD8+ TIGIT+ TILs co-express PD-1, Tim-3, and Lag3, where TIGIT marks the most dysfunctional T cell population among the CD8+ TILs (Kurtulus et al. 2015). Importantly, TIGIT was shown to not only synergize with PD-1 but also with Tim-3 as their co-blockade synergistically improved antitumor immunity (Kurtulus et al. 2015). Thus, in both chronic infections and in cancer PD-1, Tim-3, Lag-3, and TIGIT are co-expressed on highly dysfunctional effector T cells and their cooperative action seems to functionally contribute to T cell exhaustion (Table 1).
In addition to their inhibitory function on tumor-infiltrating effector cells, co-inhibitory receptors also play a role in dampening antitumor responses through their action on Tregs. Indeed, tumor-infiltrating Tregs have been shown to express high levels of co-inhibitory receptors. While Tregs in peripheral lymphoid tissues express only moderate levels of TIGIT and are mostly negative for Tim-3, the majority of tumor-infiltrating Tregs express TIGIT and Tim-3 (Yan et al. 2013; Gao et al. 2012; Kurtulus et al. 2015; Sakuishi et al. 2013). Both Tim-3+ and TIGIT+ Tregs have been shown to possess superior suppressive capacity in vitro and express high levels Treg signature genes including Foxp3 (Sakuishi et al. 2013; Gautron et al. 2014; Joller et al. 2014; Sakuishi et al. 2013; Gupta et al. 2012). Furthermore, TIGIT+ and Tim-3+ Tregs both display increased production of suppressive mediators such as IL-10, perforin, or TGF-β, which contribute to their superior suppression (Sakuishi et al. 2013; Joller et al. 2014; Kurtulus et al. 2015). Highly suppressive Tregs are indeed a main driver in actively suppressing antitumor responses (Nishikawa and Sakaguchi 2010) and we found that loss of TIGIT on Tregs but not on CD8+ TILs was able to delay tumor growth and restore CD8+ T cell function (Kurtulus et al. 2015). Hence, expression of TIGIT, and possibly also Tim-3, on Tregs seems to play a dominant role in restraining antitumor responses and may actively promote the dysfunctional phenotype observed in CD8+ TILs.
Taken together, enhanced expression of co-inhibitory receptors directly impairs effector T cell responses and their concerted action synergistically contributes to the dysfunctional T cell phenotype observed within the tumor microenvironment. In addition, these co-inhibitory receptors also contribute to enhanced suppression through Tregs present in the tumor tissue, further dampening the antitumor response. The success of cancer immunotherapy targeting checkpoint inhibitors has demonstrated that the enhanced expression of co-inhibitory receptors on both effector and regulatory T cells represents a central obstacle for tumor elimination. The synergistic effects of co-blockade of several co-inhibitory receptors seen in preclinical cancer models and on patient-derived samples suggest that combination therapy might greatly improve the low response rates observed in current monotherapies and Tim-3, Lag-3, and TIGIT represent the most promising new targets. As these novel checkpoint inhibitors and their ligands show distinct expression patterns, personalized combination therapy bears the potential of yielding optimal results depending on the type of cancer and the tissue affected.
5.3 Autoimmunity
Despite the striking success of therapies targeting the co-inhibitory receptors CTLA-4 and PD-1 in several cancer indications, a sizable portion of patients (10–20%) shows significant side effects, in particular autoimmune syndromes, including colitis, pneumonitis, skin disorders, and hepatitis (Callahan et al. 2016; Robert et al. 2015). Similarly, in certain settings of chronic infections, interference with co-inhibitory pathways results in severe immune-mediated tissue damage that can have detrimental consequences (Frebel et al. 2012; Hafalla et al. 2012; Lazar-Molnar et al. 2010; Vaccari et al. 2012). Indeed, co-inhibitory receptors play a central role in maintaining immune homeostasis and their loss, most notably of CTLA-4 or PD-1, results in spontaneous, severe autoimmunity with loss of CTLA-4 and a milder form of tissue inflammation with loss of PD-1 (Tivol et al. 1995; Waterhouse et al. 1995; Nishimura et al. 1999; Nishimura et al. 2001). Many co-inhibitory pathways, including the Tim-3 and CD226/TIGIT pathways, have been genetically linked to susceptibility to autoimmune diseases and their function in regulating immune responses has been intensively studied in the context of autoimmune diseases (Kasagi et al. 2011; Qu et al. 2009; Wang et al. 2014; Song et al. 2011; Hafler et al. 2009). However, deficiency in Tim-3, Lag-3, or TIGIT alone does not predispose to autoimmunity unless the mice are on a permissive background (Joller et al. 2011; Bettini et al. 2011; Okazaki et al. 2011; Lee and Goverman 2013). While loss of Lag-3 was shown to accelerate type 1 diabetes in NOD mice (Bettini et al. 2011; Okazaki et al. 2011), the function of Tim-3 and TIGIT has most intensively been studied in the context of CNS autoimmunity. Loss or blockade of either Tim-3 or TIGIT resulted in enhanced pro-inflammatory T cell responses leading to exacerbated EAE (Monney et al. 2002; Sanchez-Fueyo et al. 2003; Levin et al. 2011; Joller et al. 2011). The inhibitory function of the receptors is achieved through their direct inhibitory action on effector T cells as well as their ability to indirectly dampen immune responses by promoting Treg-mediated suppression and production of regulatory cytokines. An important common feature among these co-inhibitory receptors is their association with the immunoregulatory cytokine IL-10. In Tregs, IL-10 is almost exclusively found within the Tim-3+, Lag-3+, and TIGIT+ Treg subsets and TIGIT ligation is able to directly induce IL-10 production in Tregs (Sakuishi et al. 2013; Camisaschi et al. 2010; Joller et al. 2014). Furthermore, in Tr1 cells induced to mediate antigen-specific tolerance, Tim-3, Lag-3, and TIGIT correlate with the expression of IL-10 (Burton et al. 2014). In DCs, ligation of CD155 by TIGIT induces IL-10 production and promotes a tolerogenic phenotype (Yu et al. 2009). Finally, dysfunctional CD8+ TILs found in melanoma co-express Tim-3, Lag-3, and TIGIT and show enhanced IL-10 production (Singer et al. 2016; Tirosh et al. 2016). These observations suggest that expression of Tim-3, Lag-3, and TIGIT might be co-regulated to ensure optimal T cell regulation through their cooperative function.
In contrast to CTLA-4 or PD-1, Tim-3 and TIGIT do not act as global inhibitors of immune responses but specifically target certain aspects of the immune response (Fig. 4.). Tim-3 is predominantly expressed on Th1 but not Th2 cells and interaction with its ligands galectin-9 or Ceacam-1 triggers cell death in Th1 and Tc1 cells, thereby specifically dampening Th1 responses (Huang et al. 2015; Zhu et al. 2005; Kang et al. 2015). The specificity of Tim-3-mediated inhibition is also demonstrated by the fact that Tim-3-deficiency regulates Th1- but not Th17-driven EAE (Lee and Goverman 2013). Similarly, TIGIT and its co-stimulatory counterpart CD226 have differential effects on different types of immune responses. While CD226 promotes Th1 and Th17 responses, TIGIT selectively inhibits production of IFN-γ and IL-17 but enhances Th2 cytokines (Burton et al. 2014; Yu et al. 2009; Joller et al. 2011, 2014; Dardalhon et al. 2005; Lozano et al. 2012, 2013). TIGIT selectively inhibits pro-inflammatory Th1 and Th17 responses through its action on multiple cell types. In DCs, TIGIT ligation of CD155 inhibits IL-12 production and thus interferes with polarization of naïve CD4+ T helper cells into Th1 cells during priming (Yu et al. 2009). In effector T cells, loss of TIGIT results in upregulation of the Th1 master transcription factor T-bet and enhanced production of IFN-γ and IL-17 (Joller et al. 2011; Lozano et al. 2012). Finally in Tregs, TIGIT directly induces production of the suppressive mediator Fgl2, which enables TIGIT+ Tregs to selectively suppress Th1 and Th17 responses (Joller et al. 2014). TIGIT and Tim-3 therefore specifically inhibit pro-inflammatory immune responses that drive organ-specific autoimmunity. These co-inhibitory receptors hence seem to play a particularly important role in maintaining peripheral T cell tolerance and preventing autoimmunity.
Fig. 4.
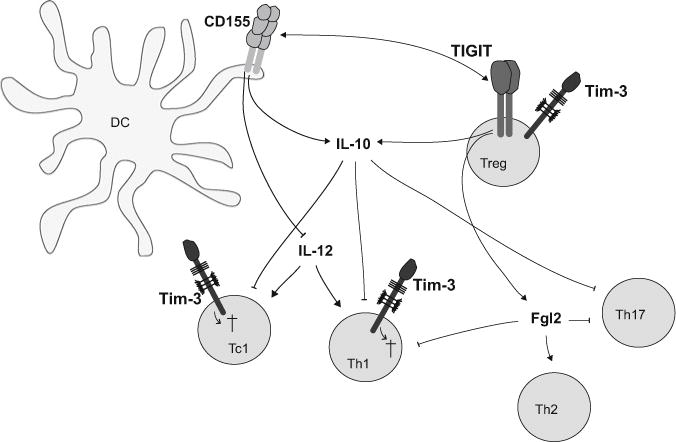
The Tim-3 and TIGIT pathways specifically inhibit pro-inflammatory responses in autoimmunity. Tim-3 is selectively expressed on Th1 and Tc1 cells, which drive tissue inflammation and autoimmunity. Tim-3 regulates their response by inducing apoptotic cell death or dysfunction by binding to its ligands. TIGIT expressed on Tregs induces IL-10 as well as Fgl2, which selectively inhibit pathogenic Th1 and Th17 responses. TIGIT expressing effector and regulatory T cells engage CD155 on APC thereby dampening IL-12 and enhancing IL-10 secretion and thus inhibiting inflammatory responses
6 Conclusion
Co-inhibitory receptors play a pivotal role in maintaining immune homeostasis and preventing autoimmunity while at the same time permitting effective immune responses to control cancer and eradicate pathogens. The family of co-inhibitory receptors has grown from the potent, global inhibitor of immune responses, CTLA-4, to include co-inhibitory receptors (Tim-3, Lag-3, and TIGIT) that show more specialized functions in regulating T cell responses. These co-inhibitory receptors and their ligands are highly expressed at tissue sites, where they regulate ongoing effector T cell responses and maintain tissue tolerance. As such, Tim-3, Lag-3, and TIGIT are highly expressed in T cells that are stimulated by persistent antigen including pro-inflammatory T cells in autoimmune diseases, tumor-infiltrating lymphocytes in cancer, and exhausted virus-specific T cells in chronic infections. In fact, these T cells co-express multiple co-inhibitory receptors that functionally synergize to dampen effector T cell responses to prevent immunopathology. At the same time, the synergistic effects observed, when several co-inhibitory receptors are targeted together, show that the inhibitory functions of Tim-3, Lag-3, and TIGIT are not identical but that they have nuanced functions even when expressed together on the population of “exhausted” or “dysfunctional” T cells. Further knowledge on how expression and specialized function of these receptors synergize and regulate effector functions of T cells will open up new areas for therapeutic targeting of these co-inhibitory receptors in chronic human diseases.
Acknowledgments
This work was supported by the Swiss National Science Foundation (PP00P3_150663/1 to N.J.), the European Research Council (677200 to N.J.), and the National Institutes of Health (P01 AI073748, P01 NS076410, P01 AI039671, and R01 NS045937 to V.K.K.).
Contributor Information
Nicole Joller, Institute for Experimental Immunology, University of Zurich, Zurich, Switzerland.
Vijay K. Kuchroo, Harvard Medical School and Brigham & Women’s Hospital, Evergrande Center for Immunologic Diseases, Boston, MA, USA
References
- Anderson AC, Lord GM, Dardalhon V, Lee DH, Sabatos-Peyton CA, Glimcher LH, Kuchroo VK. T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur J Immunol. 2010;40(3):859–866. doi: 10.1002/eji.200939842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Bettini M, Szymczak-Workman AL, Forbes K, Castellaw AH, Selby M, Pan X, Drake CG, Korman AJ, Vignali DA. Cutting edge: accelerated autoimmune diabetes in the absence of LAG-3. J Immunol. 2011;187(7):3493–3498. doi: 10.4049/jimmunol.1100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, Zhang Q, Liang D, Xiong L, Wei H, Sun R, Tian Z. T-cell Ig and ITIM domain regulates natural killer cell activation in murine acute viral hepatitis. Hepatology. 2014;59(5):1715–1725. doi: 10.1002/hep.26968. [DOI] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8 + T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles KS, Vermi W, Facchetti F, Fuchs A, Wilson TJ, Diacovo TG, Cella M, Colonna M. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur J Immunol. 2009;39(3):695–703. doi: 10.1002/eji.200839116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198(4):557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton BR, Britton GJ, Fang H, Verhagen J, Smithers B, Sabatos-Peyton CA, Carney LJ, Gough J, Strobel S, Wraith DC. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat Commun. 2014;5:4741. doi: 10.1038/ncomms5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity. 2016;44(5):1069–1078. doi: 10.1016/j.immuni.2016.04.023. [DOI] [PubMed] [Google Scholar]
- Camisaschi C, Casati C, Rini F, Perego M, De Filippo A, Triebel F, Parmiani G, Belli F, Rivoltini L, Castelli C. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3 (+) regulatory T cells that are expanded at tumor sites. Journal of immunology. 2010;184(11):6545–6551. doi: 10.4049/jimmunol.0903879. [DOI] [PubMed] [Google Scholar]
- Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Federov A, Federov E, Zencheck WD, Lary JW, Cole JL, Deng H, Xiao H, DiLorenzo TP, Allison JP, Nathenson SG, Almo SC. T cell immunoglobulin mucin-3 crystal structure reveals a novel ligand binding surface. Immunity. 2007;26:311–321. doi: 10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Casado JG, Pawelec G, Morgado S, Sanchez-Correa B, Delgado E, Gayoso I, Duran E, Solana R, Tarazona R. Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines. Cancer Immunol Immunother. 2009;58(9):1517–1526. doi: 10.1007/s00262-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CW, Kay LS, Khadaroo RG, Chan MW, Lakatoo S, Young KJ, Zhang L, Gorczynski RM, Cattral M, Rotstein O, Levy GA. Soluble fibrinogen-like protein 2/fibroleukin exhibits immunosuppressive properties: suppressing T cell proliferation and inhibiting maturation of bone marrow-derived dendritic cells. J Immunol. 2003;170(8):4036–4044. doi: 10.4049/jimmunol.170.8.4036. [DOI] [PubMed] [Google Scholar]
- Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, Ritchie DS, Colonna M, Andrews DM, Smyth MJ. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol. 2014;15(5):431–438. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest. 2015;125(5):2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, Hammond KB, Clayton KL, Ishii N, Abdel-Mohsen M, Liegler T, Mitchell BI, Hecht FM, Ostrowski M, Shikuma CM, Hansen SG, Maurer M, Korman AJ, Deeks SG, Sacha JB, Ndhlovu LC. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog. 2016;12(1):e1005349. doi: 10.1371/journal.ppat.1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13(9):832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Kim KS, Bergelson JM. Poliovirus entry into human brain microvascular cells requires receptor-induced activation of SHP-2. EMBO J. 2007;26(17):4016–4028. doi: 10.1038/sj.emboj.7601831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res. 2014;2(5):410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Schubart AS, Reddy J, Meyers JH, Monney L, Sabatos CA, Ahuja R, Nguyen K, Freeman GJ, Greenfield EA, Sobel RA, Kuchroo VK. CD226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions. J Immunol. 2005;175(3):1558–1565. doi: 10.4049/jimmunol.175.3.1558. 175/3/1558 [pii] [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, Cornejo M, Nishi N, Yamauchi A, Quintana FJ, Sobel RA, Hirashima M, Kuchroo VK. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b + Ly-6G + myeloid cells. J Immunol. 2010;185(3):1383–1392. doi: 10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean AS, Beisner DR, Ch’en IL, Kerdiles YM, Babour A, Arden KC, Castrillon DH, DePinho RA, Hedrick SM. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10(5):504–513. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, Freeman GJ, Casasnovas JM. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol. 2010;184(4):1918–1930. doi: 10.4049/jimmunol.0903059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8 + T cell exhaustion versus memory. Immunity. 2012;37(6):1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44(5):973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–1212. doi: 10.1038/ni1003. 10.1038/ni1003ni1003[pii] [DOI] [PubMed] [Google Scholar]
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8 + T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frebel H, Nindl V, Schuepbach RA, Braunschweiler T, Richter K, Vogel J, Wagner CA, Loffing-Cueni D, Kurrer M, Ludewig B, Oxenius A. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med. 2012;209(13):2485–2499. doi: 10.1084/jem.20121015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromentin R, Bakeman W, Lawani MB, Khoury G, Hartogensis W, DaFonseca S, Killian M, Epling L, Hoh R, Sinclair E, Hecht FM, Bacchetti P, Deeks SG, Lewin SR, Sekaly RP, Chomont N. CD4 + T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog. 2016;12(7):e1005761. doi: 10.1371/journal.ppat.1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J Immunol. 2004;172(7):3994–3998. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- Fuhrman CA, Yeh WI, Seay HR, Saikumar Lakshmi P, Chopra G, Zhang L, Perry DJ, McClymont SA, Yadav M, Lopez MC, Baker HV, Zhang Y, Li Y, Whitley M, von Schack D, Atkinson MA, Bluestone JA, Brusko TM. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J Immunol. 2015;195(1):145–155. doi: 10.4049/jimmunol.1402381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, Di Serio C, Bacchetta R, Andreani M, Brockmann L, Gregori S, Flavell RA, Roncarolo MG. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19(6):739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS ONE. 2012;7(2):e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron AS, Dominguez-Villar M, de Marcken M, Hafler DA. Enhanced suppressor function of TIM-3 + FoxP3 + regulatory T cells. Eur J Immunol. 2014;44(9):2703–2711. doi: 10.1002/eji.201344392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, Niki T, Hirashima M, Blazar BR, Miller JS. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119(13):3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4 + and CD8 + T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravitz L. Cancer immunotherapy. Nature. 2013;504(7480):S1. doi: 10.1038/504S1a. [DOI] [PubMed] [Google Scholar]
- Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097–1101. doi: 10.1038/ni846. 10.1038/ni846 ni846 [pii] [DOI] [PubMed] [Google Scholar]
- Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, Drake CG. LAG-3 regulates CD8 + T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117(11):3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Thornley TB, Gao W, Larocca R, Turka LA, Kuchroo VK, Strom TB. Allograft rejection is restrained by short-lived TIM-3 + PD-1 + Foxp3 + Tregs. J Clin Invest. 2012;122(7):2395–2404. doi: 10.1172/JCI45138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafalla JC, Claser C, Couper KN, Grau GE, Renia L, de Souza JB, Riley EM. The CTLA-4 and PD-1/PD-L1 inhibitory pathways independently regulate host resistance to plasmodium-induced acute immune pathology. PLoS Pathog. 2012;8(2):e1002504. doi: 10.1371/journal.ppat.1002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler JP, Maier LM, Cooper JD, Plagnol V, Hinks A, Simmonds MJ, Stevens HE, Walker NM, Healy B, Howson JM, Maisuria M, Duley S, Coleman G, Gough SC, Worthington J, Kuchroo VK, Wicker LS, Todd JA. CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immun. 2009;10(1):5–10. doi: 10.1038/gene.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161(8):4058–4065. [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of LAG-3 in regulatory T cells. Immunity. 2004;21(4):503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, Clayton KL, Raab M, Chen Q, Beauchemin N, Yazaki PJ, Pyzik M, Ostrowski MA, Glickman JN, Rudd CE, Ploegh HL, Franke A, Petsko GA, Kuchroo VK, Blumberg RS. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517(7534):386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol. 1995;25(9):2718–2721. doi: 10.1002/eji.1830250949. [DOI] [PubMed] [Google Scholar]
- Inozume T, Yaguchi T, Furuta J, Harada K, Kawakami Y, Shimada S. Melanoma cells control antimelanoma CTL responses via interaction between TIGIT and CD155 in the effector phase. J Invest Dermatol. 2016;136(1):255–263. doi: 10.1038/JID.2015.404. [DOI] [PubMed] [Google Scholar]
- Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, Kuchroo VK, Behar SM. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med. 2010;207(11):2343–2354. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107(33):14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26(6):923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186(3):1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, Sharpe AH, Quintana FJ, Mathis D, Benoist C, Hafler DA, Kuchroo VK. Treg Cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205(12):2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CW, Dutta A, Chang LY, Mahalingam J, Lin YC, Chiang JM, Hsu CY, Huang CT, Su WT, Chu YY, Lin CY. Apoptosis of tumor infiltrating effector TIM-3 + CD8 + T cells in colon cancer. Sci Rep. 2015;5:15659. doi: 10.1038/srep15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasagi S, Kawano S, Kumagai S. PD-1 and autoimmunity. Critical reviews in immunology. 2011;31(4):265–295. doi: 10.1615/critrevimmunol.v31.i4.10. [DOI] [PubMed] [Google Scholar]
- Kisielow M, Kisielow J, Capoferri-Sollami G, Karjalainen K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol. 2005;35(7):2081–2088. doi: 10.1002/eji.200526090. [DOI] [PubMed] [Google Scholar]
- Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, Jaffee E. Galectin-3 shapes antitumor immune responses by suppressing CD8 + T Cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol Res. 2015;3(4):412–423. doi: 10.1158/2326-6066.CIR-14-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK, Anderson AC. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125(11):4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR., Jr Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci USA. 2010;107(30):13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Goverman JM. The influence of T cell Ig mucin-3 signaling on central nervous system autoimmune disease is determined by the effector function of the pathogenic T cells. J Immunol. 2013;190(10):4991–4999. doi: 10.4049/jimmunol.1300083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Su EW, Zhu C, Hainline S, Phuah J, Moroco JA, Smithgall TE, Kuchroo VK, Kane LP. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Mol Cell Biol. 2011;31(19):3963–3974. doi: 10.1128/MCB.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D, Hebb L, Wolf A, Bukowski TR, Rixon MW, Kuijper JL, Ostrander CD, West JW, Bilsborough J, Fox B, Gao Z, Xu W, Ramsdell F, Blazar BR, Lewis KE. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41(4):902–915. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xia P, Du Y, Liu S, Huang G, Chen J, Zhang H, Hou N, Cheng X, Zhou L, Li P, Yang X, Fan Z. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J Biol Chem. 2014;289(25):17647–17657. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S, Tybulewicz V, Vignali D, Clynes R. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180(9):5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- Linsley P, Brady W, Urnes M, Grosmaire L, Damle N, Ledbetter J. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhang H, Li M, Hu D, Li C, Ge B, Jin B, Fan Z. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ. 2013;20(3):456–464. doi: 10.1038/cdd.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012 doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano E, Joller N, Cao Y, Kuchroo V, Hafler DA. The CD226/CD155 Interaction Regulates the Proinflammatory (Th1/Th17)/Anti-Inflammatory (Th2) Balance in Humans. J Immunol. 2013 doi: 10.4049/jimmunol.1300945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K. Tumor-infiltrating NY-ESO-1-specific CD8 + T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107(17):7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1 + HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120(12):4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. 0092-8674(89)90690-9[pii] [DOI] [PubMed] [Google Scholar]
- Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med. 2005;11(8):362–369. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–541. doi: 10.1038/415536a415536a. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362(6422):758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113(16):3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119(16):3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J, Kennedy P, Maini MK. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PloS one. 2012;7(10):e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71(10):3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/s1074-7613(00)80089-8. S1074-7613(00)80089-8 [pii] [DOI] [PubMed] [Google Scholar]
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi: 10.1126/science.291.5502.319291/5502/319. [DOI] [PubMed] [Google Scholar]
- Oikawa T, Kamimura Y, Akiba H, Yagita H, Okumura K, Takahashi H, Zeniya M, Tajiri H, Azuma M. Preferential involvement of Tim-3 in the regulation of hepatic CD8 + T cells in murine acute graft-versus-host disease. J Immunol. 2006;177(7):4281–4287. doi: 10.4049/jimmunol.177.7.4281. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, Hioki K, Honjo T. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Experimental Med. 2011;208(2):395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu HQ, Bradfield JP, Grant SF, Hakonarson H, Polychronakos C, Type IDGC. Remapping the type I diabetes association of the CTLA4 locus. Genes and immunity. 2009;10(Suppl 1):27–32. doi: 10.1038/gene.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, Angin M, Wakeham A, Greenfield EA, Sobel RA, Okada H, McKinnon PJ, Mak TW, Addo MM, Anderson AC, Kuchroo VK. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med. 2012;18(9):1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int Immunol. 2010;22(1):13–23. doi: 10.1093/intimm/dxp107. [DOI] [PubMed] [Google Scholar]
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, K-investigators Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4(11):1102–1110. doi: 10.1038/ni988. 10.1038/ni988ni988[pii] [DOI] [PubMed] [Google Scholar]
- Sada-Ovalle I, Chavez-Galan L, Torre-Bouscoulet L, Nava-Gamino L, Barrera L, Jayaraman P, Torres-Rojas M, Salazar-Lezama MA, Behar SM. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with mycobacterium tuberculosis. J Immunol. 2012;189(12):5896–5902. doi: 10.4049/jimmunol.1200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, Anderson AC. TIM3 + FOXP3 + regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2(4):e23849. doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, Gutierrez-Ramos JC, Coyle AJ, Strom TB. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4(11):1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- Santiago C, Ballestros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casanovas JM. Structures of T Cell Immunoglobulin Mucin Protein 4 show a metal-ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007a;27:941–945. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C, Ballestros A, Tami C, Martinez-Munoz L, Kaplan GG, Casanovas JM. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007b;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan D, Cichocki F, Zhang B, Yingst A, Spellman SR, Cooley S, Verneris MR, Blazar BR, Miller JS. Adaptive NK cells with Low TIGIT expression are inherently resistant to myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5696–5706. doi: 10.1158/0008-5472.CAN-16-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 2016;44(5):955–972. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sega EI, Leveson-Gower DB, Florek M, Schneidawind D, Luong RH, Negrin RS. Role of lymphocyte activation gene-3 (Lag-3) in conventional and regulatory T cell function in allogeneic transplantation. PLoS ONE. 2014;9(1):e86551. doi: 10.1371/journal.pone.0086551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Wang C, Cong L, Marjanovic ND, Kowalczyk MS, Zhang H, Nyman J, Sakuishi K, Kurtulus S, Gennert D, Xia J, Kwon JY, Nevin J, Herbst RH, Yanai I, Rozenblatt-Rosen O, Kuchroo VK, Regev A, Anderson AC. A Distinct Gene Module for Dysfunction Uncoupled from Activation in Tumor-Infiltrating T Cells. Cell. 2016;166(6):1500–1511 e1509. doi: 10.1016/j.cell.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YW, Im CH, Park JH, Lee YJ, Lee EY, Lee EB, Park K. T-cell immunoglobulin and mucin domain 3 genetic polymorphisms are associated with rheumatoid arthritis independent of a shared epitope status. Human Immunol. 2011;72(8):652–655. doi: 10.1016/j.humimm.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci USA. 2009;106(42):17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, Enk J, Jonjic S, Mandelboim O. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur J Immunol. 2013;43(8):2138–2150. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel KF, Harden-Bowles K, Yu X, Rouge L, Yin J, Comps-Agrar L, Wiesmann C, Bazan JF, Eaton DL, Grogan JL. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1120606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh I, Izar B, Prakadan SM, Wadsworth MH, 2nd, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin JR, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CG, Kazer SW, Gaillard A, Kolb KE, Villani AC, Johannessen CM, Andreev AY, Van Allen EM, Bertagnolli M, Sorger PK, Sullivan RJ, Flaherty KT, Frederick DT, Jane-Valbuena J, Yoon CH, Rozenblatt-Rosen O, Shalek AK, Regev A, Garraway LA. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352(6282):189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. CTLA-4 deficient mice exhibit massive lymphoproliferation and multi-organ lymphatic infiltration: a critical negative immunoregulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171(5):1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari M, Boasso A, Fenizia C, Fuchs D, Hryniewicz A, Morgan T, Weiss D, Doster MN, Heraud JM, Shearer GM, Franchini G. Fatal pancreatitis in simian immunodeficiency virus SIV(mac251)-infected macaques treated with 2′,3′-dideoxyinosine and stavudine following cytotoxic-T-lymphocyte-associated antigen 4 and indoleamine 2,3-dioxygenase blockade. J Virol. 2012;86(1):108–113. doi: 10.1128/JVI.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ji B, Wang J, Cheng X, Zhou Q, Zhou J, Cao C, Guo Q. Tim-3 polymorphism downregulates gene expression and is involved in the susceptibility to ankylosing spondylitis. DNA and Cell Biology. 2014;33(10):723–728. doi: 10.1089/dna.2014.2456. [DOI] [PubMed] [Google Scholar]
- Wang SC, Li YH, Piao HL, Hong XW, Zhang D, Xu YY, Tao Y, Wang Y, Yuan MM, Li DJ, Du MR. PD-1 and Tim-3 pathways are associated with regulatory CD8 + T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015a;6:e1738. doi: 10.1038/cddis.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, Yin B, Huang J, Mao L, Lu Y, Sun Z. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol. 2015b;45(10):2886–2897. doi: 10.1002/eji.201545480. [DOI] [PubMed] [Google Scholar]
- Waterhouse P, Penninger J, Timms E, Wakeham A, Shahinian A, Lee K, Thompson C, Griesser H, Mak T. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3 + regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol. 2003;33(4):970–979. doi: 10.1002/eji.200323382. [DOI] [PubMed] [Google Scholar]
- Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. 2002;169(10):5392–5395. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172(9):5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182(4):1885–1891. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, Chen Z. Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. European J Immunol. 2012;42(5):1180–1191. doi: 10.1002/eji.201141852. [DOI] [PubMed] [Google Scholar]
- Xu F, Liu J, Liu D, Liu B, Wang M, Hu Z, Du X, Tang L, He F. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014;74(13):3418–3428. doi: 10.1158/0008-5472.CAN-13-2690. [DOI] [PubMed] [Google Scholar]
- Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C, He J, Wu G, Liu X, Zhang Y. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol. 2015;29(2):635–641. doi: 10.1016/j.intimp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang Y, Zhang JP, Liang J, Li L, Zheng L. Tim-3 expression defines regulatory T cells in human tumors. PLoS ONE. 2013;8(3):e58006. doi: 10.1371/journal.pone.0058006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest. 2012;122(4):1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Jiang X, Chen G, Xiao Y, Geng S, Kang C, Zhou T, Li Y, Guo X, Xiao H, Hou C, Wang R, Lin Z, Li X, Feng J, Ma Y, Shen B, Li Y, Han G. T cell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response. J Immunol. 2013;190(5):2068–2079. doi: 10.4049/jimmunol.1202661. [DOI] [PubMed] [Google Scholar]
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Maksimovic J, Naselli G, Qian J, Chopin M, Blewitt ME, Oshlack A, Harrison LC. Genome-wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells. Blood. 2013;122(16):2823–2836. doi: 10.1182/blood-2013-02-481788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, Blazar BR. Coexpression of Tim-3 and PD-1 identifies a CD8 + T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117(17):4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- Zhu C, Sakuishi K, Xiao S, Sun Z, Zaghouani S, Gu G, Wang C, Tan DJ, Wu C, Rangachari M, Pertel T, Jin HT, Ahmed R, Anderson AC, Kuchroo VK. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun. 2015;6:6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Paniccia A, Schulick AC, Chen W, Koenig MR, Byers JT, Yao S, Bevers S, Edil BH. Identification of CD112R as a novel checkpoint for human T cells. J Exp Med. 2016;213(2):167–176. doi: 10.1084/jem.20150785. [DOI] [PMC free article] [PubMed] [Google Scholar]


