Abstract
Paxillin is a group III LIM domain protein that is best characterized as a cytoplasmic scaffold/adaptor protein that functions primarily as a mediator of focal adhesion. However, emerging studies indicate that paxillin’s function s are far broader. Not only does paxillin appear to regulate cytoplasmic kinase signaling, but it also cycles between the cytoplasm and nucleus, and may be an important regulator of mRNA trafficking and subsequent translation. Herein, we provide some insights suggesting that paxillin, like its relative Hic-5, has nuclear binding partners and mediates critical processes within the nucleus, at least in part functioning as coregulator of nuclear receptors and nuclear kinases to mediate genomic signaling.
Keywords: Paxillin, Nuclear, Androgen, Coregulator, Cancer
1. Introduction
Paxillin, first identified as a vinculin-binding focal adhesion protein, demonstrates versatile functions at the plasma membrane and within the cytoplasm. Being a major substrate of Src tyrosine kinase, paxillin plays a critical role in regulating focal adhesion assembly and organization [1, 2]. Within focal adhesion complexes, paxillin serves as a bridge that connects integrins with Focal Adhesion Kinase(FAK), mediating integrin bidirectional signaling that then allows cells to sense and respond to extracellular stimuli. Besides its function in focal adhesions, paxillin also serves as a scaffold protein that regulates spatial and temporal organization of cytoskeleton and cytoplasmic signalosomes.
Given its importance in maintaining cellular structure and interactions, paxillin would be expected to be important for normal organ development and function. Emerging evidence indicates that paxillin participates in many developmental and physiological processes. Paxillin, which is known to mediate fibronectin receptor signaling, is a critical modulator of the development of several mesodermal-derived organs. For example, the paxillin knockout mouse is embryonic lethal very early in embryogenesis due to poor early development of the heart and somites [3]. Furthermore, recent studies in zebrafish suggest that double mutants of two paxillin genes, pxna and pxnb, leads to defects in axial and skeletal muscle development as well as in the cardiovascular system. Specifically, paxillin together with its binding partner FAK are critical players in the maintenance of cardiac contractility, with failing of this orchestrated interplay resulting in heart failure [4]. In addition to its effects in the heart, paxillin is part of myosin regulatory light chain signaling in response to neurostimulation by force development in tracheal smooth muscle. Paxillin is also important for skin fibroblast morphology, with its levels declining during skin aging [5, 6]. Finally, paxillin has been implicated in various diseases, including Alzheimer’s disease [7] as well as many kinds of cancers [8–10].
While paxillin’s roles in the aforementioned processes have been primarily linked to its function in the membrane and cytoplasm, emerging evidence indicates that paxillin may also signal in the nucleus to mediate important processes. This review will focus on paxillin’s role as a liaison that connects extranuclear and nuclear signaling, as well as its actions in the nucleus to regulate genomic signaling in a variety of models.
2. Paxillin structure
2.1 LIM domains
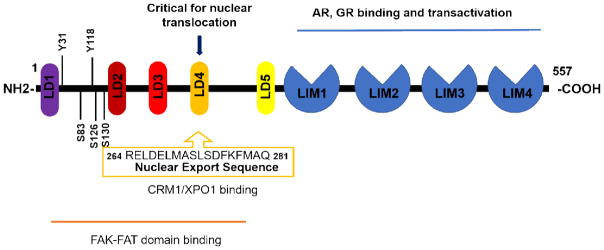
As a multiple domain adaptor protein, paxillin has two major sets of motifs: four LIM domains in the carboxyl terminal half of the protein and 5 LD domains close to the amino terminus (Fig. 1). LIM domains are cysteine-rich protein regions that contain two contiguous zinc-fingers, separated by a two amino acids spacer.
Figure 1. Schematic structure of paxillin with highlights of nuclear function related domains.
Paxillin contains five LD domains on the N-terminal half and four LIM domains on the C-terminal half of the protein. The shown N-terminal domains are critical for FAK binding and nuclear translocation, with highlights of critical serine/tyrosine phosphorylation sites as well as the NES sequence within LD4 domain. The LIM domains on the C terminal half are related to paxillin’s binding and transactivating of AR and GR.
Unlike metalloproteinases or helix–loop–helix transcription factors, which also contain zinc finger motifs, LIM domain-containing proteins are not classified by similar functions, but are instead separated by the subtype of domain structure. There are 14 types of LIM domain proteins falling into four groups. Some are LIM domain only (LMO) proteins, whose functions are considered to be primarily transcriptional within the nucleus [11]. In contrast, the remaining LIM proteins are composed of other functional domains such as PDZ or LD regions in addition to LIM domains. These more complex proteins are thought to be primarily cytoplasmic [12]. Paxillin belongs to the group III LIM proteins, together with several other members, including zyxin and testin [13].
Most LIM domain-containing proteins have essential functions in cytoskeletal organization, cell fate determination, and differentiation through their interactions with other adaptor proteins or with DNA. In paxillin, the LIM2 and LIM3 domains have been identified as focal adhesion targeting motifs, and phosphorylation of these LIM domains is specific and critical for paxillin localization to focal adhesions [14]. Notably, in other LIM domain-containing proteins, the zinc finger motifs can mediate DNA binding of many transcription factors. As mentioned, group I LIM family members, LIM Homeobox (LHX) and LMO proteins, are well known to be localized in the nucleus and to participate in tissue specific gene regulation. LHX3, a neuroendocrine transcription factor, is expressed in nuclei of adult human pituitary cells, where it induces transcription of the glycoprotein alpha-subunit promoter to promote expression of pituitary-derived glycopeptides [15]. LMO1/2, engages with a large array of proteins, including LIM domain-binding protein 1 (LDB1), stem cell leukemia protein (SCL), and E-protein, to form a transcriptional complex that plays roles in the transcriptional regulation of normal and malignant hematopoiesis [16]. Zyxin, one of the LIM group III proteins, is an important component of focal adhesion plaques, like paxillin, but has also been shown to shuttle between the cytoplasm and nucleus. Although there is no conventional nuclear localization sequence identified, in vitro evidence suggests that zyxin interacts with several nuclear proteins, including transcription factors, to regulate gene expression [17, 18]. Taken together, it is intriguing to speculate that many if not all LIM domain-containing proteins may, in addition to their extranuclear effects, modulate gene transcription in the nucleus.
2.2 LD motif
Near the amino terminus of paxillin, there are five tandem LD motifs (Fig.1). LD motifs contain sequences rich in leucine and aspartate. The LD motif is a major targeting sequence for many protein-protein interactions, forming a scaffolding surface that can coordinate large sets of enzymatic reactions between interacting molecules within a protein complex. LD motifs contain many phosphorylation sites that are crucial for protein activation and signal transduction. For example, one major focal adhesion molecule, Focal Adhesion Kinase (FAK), while interacting with paxillin through paxillin’s LD2 domain, also binds with Crk-associated substrate (p130Cas) to regulate its downstream effects. In this situation, paxillin is therefore serving as both kinase and scaffold protein in regulating focal adhesion assembly [19]. More specifically, the Focal Adhesion Targeting (FAT) homology domain in FAK binds hydrophobically through its HP1 (Hydrophobic patch 1) and HP2 (Hydrophobic patch 2) sites to paxillin LD motifs, LD2 and LD4, under normal conditions [20, 21]. However, in disease conditions such as lung cancer, paxillin can be mutated such that it exhibits a disordered intra-molecular regulatory region that results in masking of one of the LD motifs and therefore preferential binding of FAT with the other LD domain, leading to signaling and adhesion changes that may promote tumor growth [22]. Using similar binding machinery, Cerebral Cavernous Malformations 3 (CCM3), which is a frequently mutated protein in cerebral cavernous malformation disease, binds to paxillin via its LD1, LD2, and LD4 motifs to colocalize in mouse cerebral pericytes and possibly regulate cell adhesion [23].
Furthermore, the LD1 motif of paxillin is sufficient to bind to the mitogen-activated protein kinase kinase (MEKK2) amino terminal region, thus relieving MEKK2 auto-inhibition and triggering its activation [24]. Lastly, a recent study suggests that FAK binding to LD domains of paxillin plays a key role in paxillin shuttling between cytoplasm and nucleus [25]. While some interactions with LD1, LD2, and LD4 are characterized, the binding partners of LD3 and LD5 are not well known.
2.3 Nuclear Export Sequence(NES)
As we will discuss, paxillin appears to have nuclear as well as extranuclear actions. However, despite reports of paxillin located in the nucleus, no apparent nuclear localization signal has been reported. Instead, a nuclear export signal (NES) was identified. Initial evidence suggesting the existence of an NES in paxillin was discovered in fibroblasts treated with the nuclear export inhibitor leptomycin B, a treatment that led to paxillin retention within the nucleus [26]. A study by Dong et al proposed that the LD4 motif may consist of a potential leucine-rich NES sequence. Specifically, phosphorylation of the Ser272 within the LD4 motif is critical for blocking paxillin nuclear export, as well as for reducing G protein-coupled receptor kinase-interacting protein (GIT1) binding, without altering FAK1 affinity [27]. While these studies were suggestive, identification of the protein crystallography structure of the paxillin NES sequence (264RELDELMASLSDFKFMAQ281) together with the nuclear export protein CRM1/XPO1 provided the final proof that the NES in paxillin is utilized for paxillin shuttling through nuclear pore [28]. Again, the lack of a conventional NLS (nuclear localization signal) within paxillin protein suggests that paxillin may initially enter the nucleus via a non-conventional NLS or by association with other NLS-containing proteins. For instance, cell adhesion kinase beta/proline-rich tyrosine kinase 2, which is a non-receptor tyrosine kinase member of FAK family that is localized at the perinuclear region and shuttles between cytoplasm and nucleus, has been shown to bind with paxillin’s relative Hic-5 and facilitates its nuclear transportation [29].
3. The paxillin superfamily and their functions in nucleus
The paxillin superfamily includes three main members: paxillin, Hic-5 and Leupaxin. Similar to paxillin, Hic-5 is a group III LIM protein that consists of four LD motifs and four LIM domains that are highly conserved within the paxillin superfamily. Hic-5 localizes and functions in both focal adhesions and in the nucleus. At focal adhesions, it acts as a scaffold/adaptor protein that appears to participate in skin fibroblast contractility, hypertrophic scar tissue formation, platelet aggregation and breast stromal extracellular matrix remodeling [30–32]. Interestingly, in these focal adhesions, Hic-5 shares some binding partners and has some overlapping functions with paxillin. However, unlike the global paxillin knockout mice, Hic-5 deficient mice are viable with no obvious histological abnormalities and only minor vascular defects [33] that includes altered vasculature recovery after injury and enhanced stretch induced vascular smooth muscle cell apoptosis. Together, these observations suggest that Hic-5 may play a less critical role than paxillin in organ development, or that the deficiency of Hic-5 may be compensated by other members in the paxillin family. In the nucleus, Hic-5 was initially characterized as a glucocorticoid receptor (GR) coactivator that binds with GR at its tau2 transcriptional activation domain [34]. Hic-5 has been shown to selectively regulate certain sets of GR target gene expression, perhaps in part by inhibiting GR interaction with several chromatin remodeling enzymes such as chromodomain-helicase DNA-binding protein 9 (CHD9) and Braham homologue (BRM), which ultimately leads to chromatin remodeling and selective GR binding to DNA [35]. In addition to GR, Hic-5 also interacts with androgen receptor (AR) to modulate AR actions. As a stromal specific coactivator of AR, Hic-5 affects androgen-induced keratinocyte growth factor expression in prostate stromal cells, which then alters the stromal microenvironment to favor tumor growth [36].
Leupaxin, another member of paxillin family, is enriched in cells of leukocyte lineage, but is also broadly distributed in other tissues. A recent study in hepatocellular carcinoma suggests that leupaxin serves as a coactivator of beta-catenin by assisting in the recruitment of the coactivator complex consisting of steroid receptor coactivator 1 (SRC-1) and P300 to enhance beta-catenin’s transcriptional activity [37]. Evidence also suggests that leupaxin shuttles between cytoplasm and nucleus, perhaps interacting with the AR in a ligand-dependent pattern to regulate AR-dependent transcription in prostate cancer cells [38].
4. Paxillin actions in the nucleus
Base on the nuclear actions of other paxillin superfamily proteins, as well as on reports that paxillin can be found in the nucleus, it is not surprising that recent studies strongly support a role for paxillin itself in nuclear signaling.
The first evidence that paxillin cycles between cytoplasm and nucleus in a physiologically relevant scenario is from a study of paxillin interactions with an mRNA binding protein, polyadenylation binding protein1 (PABP1), in fibroblast cells [39]. Paxillin directly associates with PABP1 through the amino-terminal, LD-domain-rich region (residues 54–313), co-localizing in the endoplasmic reticulum and in the nucleus, as well as at the tips of lamellipodia. This association is necessary for efficient nuclear export of PABP1, and facilitates transport of mRNA from nucleus to sites of protein synthesis that are occurring at or near the leading edge during cell migration [40]. A recent study suggests that the paxillin protein binds with embryonic PolyAdenylation Binding Protein (ePABP) on polyadenylated Mos (a germ cell specific Raf) mRNA upon androgen stimulation, which induces Mos protein translation and subsequent oocyte maturation (meiotic re-entry) in Xenopus laevis model [41]. Notably, this paxillin-ePABP interaction appears to be enhanced by phosphorylation of serine residues also located within the amino-terminal half of paxillin (Fig.1).
Besides its function as a binding partner of PABP, paxillin also interacts with nuclear receptors within the nucleus and functions as an AR and GR coactivator similar to its family member Hic-5 [42], in prostate cancer cell lines and prostate tissue. Studies from the DeFranco group have revealed that both Hic-5 and paxillin interact with steroid receptors using their carboxyl-terminal LIM domains. However, paxillin appears to potentiate AR and GR transactivation through the same carboxyl-terminal domain, whereas the receptor coactivation domain of Hic-5 seems to be located in its amino-terminal region [34, 43].
With these studies in mind, our group then demonstrated that paxillin regulates both cytoplasmic kinase signaling as well as nuclear transcription. In fact, we found that paxillin serves as a liaison between non-genomic steroid signaling in the cytoplasm and genomic steroid signaling in the nucleus. This pathway was first discovered in the aforementioned study of oocyte maturation (meiotic resumption) in Xenopus laevis, where we showed that paxillin is an essential regulator of meiosis in Xenopus laevis oocytes. Specifically, we found that paxillin functions to enhance androgen-induced translation of the Mos protein in Xenopus oocytes, which then leads to activation of the MAPK cascade and subsequent meiotic resumption. Once extracellular signal-regulated kinase (Erk) is activated, it regulates the phosphorylation of serine residues on paxillin, which then acts in a positive feedback mechanism (likely through interactions with PABP) to enhance MAPK signaling and eventually oocyte maturation [41, 44]. Interestingly, we went on to demonstrate that paxillin similarly plays an important role in extranuclear androgen-mediated MPAK activation in somatic cells. In prostate cancer cells, androgen binds to membrane-localized ARs to promote the MMP-mediated release of membrane-bound EGF receptor (EGFR) ligands, which then transactivate the EGFR. Activated EGFR further induces extracellular signal-regulated kinases 1 and 2 (ERK1/2) signaling via Src-mediated tyrosines 31/118 phosphorylation on paxillin [45]. ERK1/2, which is still complexed with paxillin, then mediates phosphorylation of serines 83/126/130 on paxillin. These results are reminiscent of studies by Ishibe and colleagues, who demonstrated that hepatocyte growth factor (HGF) receptor signaling relies on a similar mechanism to regulate cell spreading, migration and tubulogenesis [46, 47]. Furthermore, these protein complex formations with EGFR, paxillin, and ERK1/2, were confirmed to be occurring in living cells using a novel method of fluorescence photolithography [48]. Interestingly, we found that, once phosphorylated on serines 83/126/130, phosphoserine paxillin is then able to translocate into the nucleus, where it can enhance AR and ERK-mediated transcription. In fact, Chromatin Immunoprecipitation (ChIP) studies using an anti-paxillin antibody demonstrated that, upon androgen or ERK activation, paxillin was targeted to the promoter regions of AR and ERK-dependent genes, respectively. In sum, our studies suggest that extranuclear androgen actions via the AR are inextricably linked to nuclear AR actions in a serial fashion, with paxillin serving as a mediator of both processes (Fig. 2). Both extranuclear and nuclear actions of paxillin appear to be involved in growth, migration, and invasion of prostate cancer cells both in-vitro and in mouse xenografts [49]. Finally, expression of paxillin and nuclear paxillin are increased in human prostate cancer versus benign prostate tissue, confirming that paxillin may be important in cancer and may also serve as a biomarker of prostate cancer [49].
Figure 2. Model of paxillin mediated steroid signaling.
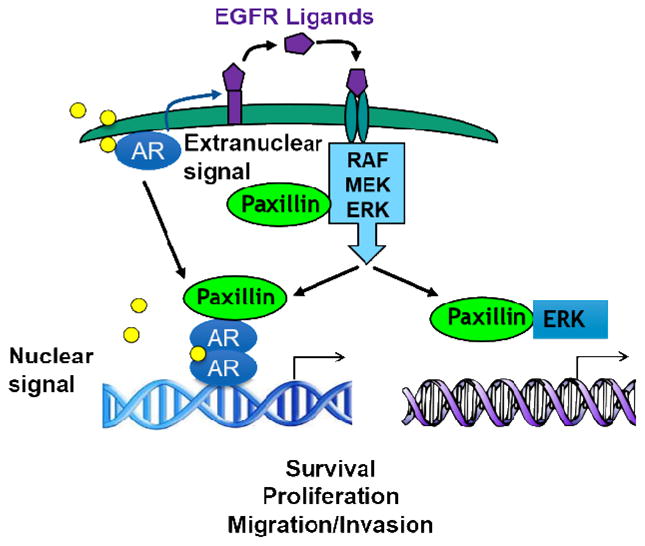
In somatic cells, androgen binds to membrane-localized ARs to promote the MMP-mediated release of membrane-bound EGF receptor (EGFR) ligands, which then transactivate the EGFR. Activated EGFR further induces Erk1/2 signaling, which then regulates serine phosphorylation of paxillin. Phosphorylated paxillin is then able to translate into the nucleus, where it either binds with AR or associate with ERK to induce gene expression ultimately results in cell survival, proliferation, migration and invasion.
Tying many of the aforementioned actions together, we have also recently shown that paxillin similarly regulates AR signaling in granulosa cells within ovary, where it mediates androgen-induced suppression of pro-apoptotic protein expression, as well as mediates androgen-triggered translation of follicle stimulation hormone (FSH) receptor protein, both of which ultimately lead to enhanced ovarian follicle growth and development [50]. It is intriguing to postulate that paxillin and PABP1 are working together to promote androgen-induced FSH receptor expression, much like they work together to enhance translation in other models mentioned above.
Besides mediating nuclear effects of AR and ERK in the prostate and ovary, paxillin also plays critical roles in the nucleus of other cell types and disease processes. In a model of pulmonary hypertension, hypoxic exposure and platelet derived growth factor (PDGF)-BB induce Y13 and Y118 phosphorylation of paxillin and its subsequent localization into the nucleus in a time dependent fashion, leading to increased proliferation and decreased apoptosis of pulmonary arterial smooth muscle cells [51]. In addition, paxillin’s nuclear actions modulate expression of the parental imprint genes H19 and IGF2. In this model, paxillin promotes an interaction between an enhancer region and the IGF2 promoter, leading to increased IGF2 expression. In the meantime, paxillin suppresses an enhancer/promoter interaction within the H19 gene, leading to decreased gene expression. Ultimately, these changes may play a role in cell proliferation and fetal development [27, 52].
Taken together, the aforementioned studies, plus others, demonstrate that paxillin exhibits versatile functions in the nucleus, ranging from nuclear receptor coactivation to facilitation of mRNA translocation, all contributing to enhanced genomic signaling and downstream physiological processes.
5. Therapeutic potentials by disruption of nuclear targeting of paxillin
Paxillin overexpression or dysregulation has been implicated in numerous cancers and other diseases. However, most of the studies have focused on the focal adhesion or scaffolding functions of paxillin in the cytoplasm. Since little has been known about its nuclear actions until recent years, effort at targeting only its nuclear actions have been limited. As mentioned, multiple studies now suggest that paxillin’s nuclear functions are critical for cell proliferation, as well as steroid-dependent cancer progression. For instance, depletion of paxillin by shRNA leads to decreased S phase cell population and increased early apoptosis in colorectal carcinoma cells [53]. Additionally, the paxillin-ERK1/2-cyclin D1 pathway is essential for the PDGF-dependent pulmonary artery smooth muscle cell proliferation and vascular remodeling underlying pulmonary hypertension [51]. Moreover, as mentioned, our group has shown that paxillin modulates AR and ERK dependent gene expression in prostate cancer cells and promotes prostate cancer xenograft growth in vivo. In addition, paxillin expression is elevated in human prostate cancer tissue compared to normal prostate [49]. Likewise, in breast cancer patients, paxillin’s expression is upregulated and correlates with HER2 overexpression [54].
With this in mind, it is intriguing to speculate that specifically targeting nuclear actions of paxillin, while sparing its important structural functions outside the nucleus, might be a useful approach toward slowing proliferation in cancer cells – especially hormone related malignancies such as prostate cancer and breast cancer, which compose a large subset of life threatening diseases all over the world. Although hormone deprivation therapy is usually used as the first line treatment in the advanced disease group, in many cases, tumors gradually evolve to the resistance subtype, resulting in more rapid disease progression. With emerging studies on steroid receptors characteristics, especially the relationship and significance of extranuclear and intranuclear signaling [55] (Fig.2), potential therapies that target the nuclear portion of steroid receptors, prevent steroid receptors nuclear import, or block the activation of coactivator/corepressor, are gaining more attention.
Presence of certain nuclear specific proteins are often related to poor prognosis and drug resistance. One frequently mutated gene, the truncated form of Erb2, is present in the nucleus and leads to ErB2 kinase inhibitor resistance in breast cancer [56]. Likewise, a major AR splicing variant- AR-V7, which lacks the ligand binding domain and is constitutively activated in the nucleus, has been implicated as a biomarker of enzalutamide resistance and poor prognosis among prostate cancer patients [57]. These evidences suggest that discovering efficient methods that target not only the steroid receptors directly, but also the nuclear coregulators, will be critical for next generation of therapies.
Although paxillin’s potential as drug target has been studied for years, the currently available inhibitors are either Src inhibitors or pan-tyrosine kinase inhibitors, most of which have limitations of specificity and efficacy. For instance, Imatinib, the first targeted tyrosine kinase inhibitor that is used in treatment of Philadelphia chromosome (Ph+)-positive chronic myelogenous leukemia, actually enhances tyrosine phosphorylation of p130Cas, FAK, and paxillin in glioblastoma multiforme tumor cells. Imatinib also induces cell migration and invasion in vitro [58]. Thus, a paxillin-specific inhibitor may be a helpful addition when using this or other kinase inhibitors. A pharmacological study from the Ginsburg group using large scale library screening identified one paxillin inhibitor that blocked alpha4 integrin-paxillin binding and reduced mononuclear leukocytes accumulation in inflammation sites [59]. More recently, a study from the Yates group discovered a small molecule inhibitor, JP-153, that abrogates the interaction between paxillin and the FAT domain in FAK, thus breaking down their extranuclear complex formation and paxillin activation, and possibly paxillin nuclear translocation. This compound exhibits inhibitory effects on VEGF induced retinal angiogenesis [60]. Interestingly, a transcriptomic study revealed that a lncRNA-PXN-AS1 is present in hepatocellular carcinoma cells. This natural paxillin anti-sense molecule is alternatively spliced by oncofetal splicing factor, MBNL3, which results in a splice variant that upregulates paxillin expression [61]. Studies of lncRNA-PXN-AS1 and MBNL3 may open new venues for paxillin targeting therapy.
As a key component in the focal adhesion complex, the normal function of paxillin in regulating focal adhesion should be taken into consideration during drug design. Thus, targeting the tyrosine residues critical for paxillin initial activation, for example pY118, may hinder its focal adhesive function. In contrast, designing inhibitors that could prevent serine phosphorylation of paxillin and therefore nuclear entry and subsequent DNA or transcription factor binding, may serve as a more specific target, and therefore might be more beneficial for patients with advanced hormone related cancers.
Conclusions
Paxillin and its family members are complex proteins that play major roles in modulating signaling throughout cells. When paxillin is outside the nucleus, it functions to modulate cell-cell interactions, regulate cytoskeletal changes, and modulate kinase signaling. Changing in the latter process then leads to serine phosphorylation of paxillin, allowing it to enter the nucleus where it can regulate transcription, translocation of mRNAs from the nucleus back into the cytoplasm, and subsequent translation of mRNAs into proteins. Some evidence suggests that paxillin expression, activation, and nuclear localization are upregulated in cancer cells, which may play a critical role in tumor progression. If so, then nuclear paxillin, may serve as an important biomarker for diagnosis, prognosis, and treatment of some cancers.
Acknowledgments
This work was supported by the National Institutes of Health grant R01GM101709.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glenney JR, Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. The Journal of cell biology. 1989;108(6):2401–8. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner CE, Glenney JR, Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. The Journal of cell biology. 1990;111(3):1059–68. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22(3):901–15. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirth S, Buhler A, Buhrdel JB, Rudeck S, Dahme T, Rottbauer W, Just S. Paxillin and Focal Adhesion Kinase (FAK) Regulate Cardiac Contractility in the Zebrafish Heart. PloS one. 2016;11(3):e0150323. doi: 10.1371/journal.pone.0150323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Q, Chen S, Chen Y, Lyga J, Santhanam U. Critical Role of Paxillin in Aging of Human Skin. Journal of Investigative Dermatology. 2012;132(4):1290–1293. doi: 10.1038/jid.2011.456. [DOI] [PubMed] [Google Scholar]
- 6.Skoczynska A, Budzisz E, Podgorna K, Rotsztejn H. Paxillin and its role in the aging process of skin cells. Postepy higieny i medycyny doswiadczalnej (Online) 2016;70(0):1087–1094. doi: 10.5604/17322693.1221385. [DOI] [PubMed] [Google Scholar]
- 7.Caltagarone J, Hamilton RL, Murdoch G, Jing Z, DeFranco DB, Bowser R. Paxillin and hydrogen peroxide-inducible clone 5 expression and distribution in control and Alzheimer disease hippocampi. Journal of neuropathology and experimental neurology. 2010;69(4):356–71. doi: 10.1097/NEN.0b013e3181d53d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CC, Wu DW, Lin PL, Lee H. Paxillin promotes colorectal tumor invasion and poor patient outcomes via ERK-mediated stabilization of Bcl-2 protein by phosphorylation at Serine 87. Oncotarget. 2015;6(11):8698–708. doi: 10.18632/oncotarget.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueno H, Tomiyama A, Yamaguchi H, Uekita T, Shirakihara T, Nakashima K, Otani N, Wada K, Sakai R, Arai H, Mori K. Augmentation of invadopodia formation in temozolomide-resistant or adopted glioma is regulated by c-Jun terminal kinase-paxillin axis. Biochemical and biophysical research communications. 2015;468(1–2):240–7. doi: 10.1016/j.bbrc.2015.10.122. [DOI] [PubMed] [Google Scholar]
- 10.Deng B, Zhang S, Miao Y, Han Z, Zhang X, Wen F, Zhang Y. Adrenomedullin expression in epithelial ovarian cancers and promotes HO8910 cell migration associated with upregulating integrin alpha5beta1 and phosphorylating FAK and paxillin. Journal of experimental & clinical cancer research : CR. 2012;31:19. doi: 10.1186/1756-9966-31-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews JM, Lester K, Joseph S, Curtis DJ. LIM-domain-only proteins in cancer. Nature reviews Cancer. 2013;13(2):111–22. doi: 10.1038/nrc3418. [DOI] [PubMed] [Google Scholar]
- 12.Smith MA, Hoffman LM, Beckerle MC. LIM proteins in actin cytoskeleton mechanoresponse. Trends in cell biology. 2014;24(10):575–83. doi: 10.1016/j.tcb.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Q, Zhao Y. The diverse biofunctions of LIM domain proteins: determined by subcellular localization and protein—protein interaction. Biology of the Cell. 2007;99(9):489–502. doi: 10.1042/BC20060126. [DOI] [PubMed] [Google Scholar]
- 14.Brown MC, Perrotta JA, Turner CE. Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Molecular biology of the cell. 1998;9(7):1803–16. doi: 10.1091/mbc.9.7.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloop KW, Meier BC, Bridwell JL, Parker GE, Schiller AM, Rhodes SJ. Differential activation of pituitary hormone genes by human Lhx3 isoforms with distinct DNA binding properties. Molecular endocrinology (Baltimore, Md) 1999;13(12):2212–25. doi: 10.1210/mend.13.12.0395. [DOI] [PubMed] [Google Scholar]
- 16.Porcher C, Chagraoui H, Kristiansen MS. SCL/TAL1: a multifaceted regulator from blood development to disease. Blood. 2017;129(15):2051–2060. doi: 10.1182/blood-2016-12-754051. [DOI] [PubMed] [Google Scholar]
- 17.Degenhardt YY, Silverstein S. Interaction of zyxin, a focal adhesion protein, with the e6 protein from human papillomavirus type 6 results in its nuclear translocation. Journal of virology. 2001;75(23):11791–802. doi: 10.1128/JVI.75.23.11791-11802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi J, Kloeker S, Jensen CC, Bockholt S, Honda H, Hirai H, Beckerle MC. Members of the Zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. The Journal of biological chemistry. 2002;277(11):9580–9. doi: 10.1074/jbc.M106922200. [DOI] [PubMed] [Google Scholar]
- 19.Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(23):10678–82. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoellerer MK, Noble ME, Labesse G, Campbell ID, Werner JM, Arold ST. Molecular recognition of paxillin LD motifs by the focal adhesion targeting domain. Structure (London, England : 1993) 2003;11(10):1207–17. doi: 10.1016/j.str.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Gao G, Prutzman KC, King ML, Scheswohl DM, DeRose EF, London RE, Schaller MD, Campbell SL. NMR solution structure of the focal adhesion targeting domain of focal adhesion kinase in complex with a paxillin LD peptide: evidence for a two-site binding model. The Journal of biological chemistry. 2004;279(9):8441–51. doi: 10.1074/jbc.M309808200. [DOI] [PubMed] [Google Scholar]
- 22.Neerathilingam M, Bairy SG, Mysore S. Deciphering Mode of Action of Functionally Important Regions in the Intrinsically Disordered Paxillin (Residues 1–313) Using Its Interaction with FAT (Focal Adhesion Targeting Domain of Focal Adhesion Kinase) PloS one. 2016;11(2):e0150153. doi: 10.1371/journal.pone.0150153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Ji W, Zhang R, Folta-Stogniew E, Min W, Boggon TJ. Molecular recognition of leucine-aspartate repeat (LD) motifs by the focal adhesion targeting homology domain of cerebral cavernous malformation 3 (CCM3) The Journal of biological chemistry. 2011;286(29):26138–47. doi: 10.1074/jbc.M110.211250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahle MP, Cuevas BD. Interaction with the Paxillin LD1 Motif Relieves MEKK2 Auto-inhibition. Journal of molecular signaling. 2015;10:4. doi: 10.5334/1750-2187-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sathe AR, Shivashankar GV, Sheetz MP. Nuclear transport of paxillin depends on focal adhesion dynamics and FAT domains. Journal of cell science. 2016;129(10):1981–8. doi: 10.1242/jcs.172643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods AJ, Roberts MS, Choudhary J, Barry ST, Mazaki Y, Sabe H, Morley SJ, Critchley DR, Norman JC. Paxillin Associates with Poly(A)-binding Protein 1 at the Dense Endoplasmic Reticulum and the Leading Edge of Migrating Cells. Journal of Biological Chemistry. 2002;277(8):6428–6437. doi: 10.1074/jbc.M109446200. [DOI] [PubMed] [Google Scholar]
- 27.Dong JM, Lau LS, Ng YW, Lim L, Manser E. Paxillin nuclear-cytoplasmic localization is regulated by phosphorylation of the LD4 motif: evidence that nuclear paxillin promotes cell proliferation. The Biochemical journal. 2009;418(1):173–84. doi: 10.1042/BJ20080170. [DOI] [PubMed] [Google Scholar]
- 28.Fung HYJ, Fu SC, Chook YM. Nuclear export receptor CRM1 recognizes diverse conformations in nuclear export signals. eLife. 2017;6:e23961. doi: 10.7554/eLife.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoto H, Sasaki H, Ishino M, Sasaki T. Nuclear translocation of cell adhesion kinase beta/proline-rich tyrosine kinase 2. Cell structure and function. 2002;27(1):47–61. doi: 10.1247/csf.27.47. [DOI] [PubMed] [Google Scholar]
- 30.Varney SD, Betts CB, Zheng R, Wu L, Hinz B, Zhou J, Van De Water L. Hic-5 is required for myofibroblast differentiation by regulating mechanically dependent MRTF-A nuclear accumulation. Journal of cell science. 2016;129(4):774–787. doi: 10.1242/jcs.170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osada M, Ohmori T, Yatomi Y, Satoh K, Hosogaya S, Ozaki Y. Involvement of Hic-5 in platelet activation: integrin alphaIIbbeta3-dependent tyrosine phosphorylation and association with proline-rich tyrosine kinase 2. The Biochemical journal. 2001;355(Pt 3):691–7. doi: 10.1042/bj3550691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deakin NO, Turner CE. Distinct roles for paxillin and Hic-5 in regulating breast cancer cell morphology, invasion, and metastasis. Molecular biology of the cell. 2011;22(3):327–41. doi: 10.1091/mbc.e10-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim-Kaneyama JR, Takeda N, Sasai A, Miyazaki A, Sata M, Hirabayashi T, Shibanuma M, Yamada G, Nose K. Hic-5 deficiency enhances mechanosensitive apoptosis and modulates vascular remodeling. Journal of molecular and cellular cardiology. 2011;50(1):77–86. doi: 10.1016/j.yjmcc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Guerrero J, Hong H, DeFranco DB, Stallcup MR. Interaction of the tau2 transcriptional activation domain of glucocorticoid receptor with a novel steroid receptor coactivator, Hic-5, which localizes to both focal adhesions and the nuclear matrix. Molecular biology of the cell. 2000;11(6):2007–18. doi: 10.1091/mbc.11.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee BH, Stallcup MR. Glucocorticoid receptor binding to chromatin is selectively controlled by the coregulator Hic-5 and chromatin remodeling enzymes. The Journal of biological chemistry. 2017;292(22):9320–9334. doi: 10.1074/jbc.M117.782607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heitzer MD, DeFranco DB. Hic-5/ARA55: a prostate stroma-specific AR coactivator. Steroids. 2007;72(2):218–20. doi: 10.1016/j.steroids.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Shi J, Wu WJ, Hu G, Yu X, Yu GS, Lu H, Yang ML, Liu B, Wu ZX. Regulation of beta-catenin transcription activity by leupaxin in hepatocellular carcinoma. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37(2):2313–20. doi: 10.1007/s13277-015-4060-4. [DOI] [PubMed] [Google Scholar]
- 38.Kaulfuss S, Grzmil M, Hemmerlein B, Thelen P, Schweyer S, Neesen J, Bubendorf L, Glass AG, Jarry H, Auber B, Burfeind P. Leupaxin, a novel coactivator of the androgen receptor, is expressed in prostate cancer and plays a role in adhesion and invasion of prostate carcinoma cells. Molecular endocrinology (Baltimore, Md) 2008;22(7):1606–21. doi: 10.1210/me.2006-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woods AJ, Roberts MS, Choudhary J, Barry ST, Mazaki Y, Sabe H, Morley SJ, Critchley DR, Norman JC. Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells. The Journal of biological chemistry. 2002;277(8):6428–37. doi: 10.1074/jbc.M109446200. [DOI] [PubMed] [Google Scholar]
- 40.Woods AJ, Kantidakis T, Sabe H, Critchley DR, Norman JC. Interaction of paxillin with poly(A)-binding protein 1 and its role in focal adhesion turnover and cell migration. Mol Cell Biol. 2005;25(9):3763–73. doi: 10.1128/MCB.25.9.3763-3773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miedlich SU, Taya M, Young MR, Hammes SR. Paxillin and embryonic PolyAdenylation Binding Protein (ePABP) engage to regulate androgen-dependent Xenopus laevis oocyte maturation - A model of kinase-dependent regulation of protein expression. Molecular and cellular endocrinology. 2017;448:87–97. doi: 10.1016/j.mce.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heitzer MD, DeFranco DB. Hic-5, an adaptor-like nuclear receptor coactivator. Nuclear receptor signaling. 2006;4:e019. doi: 10.1621/nrs.04019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasai M, Guerrero-Santoro J, Friedman R, Leman ES, Getzenberg RH, DeFranco DB. The Group 3 LIM domain protein paxillin potentiates androgen receptor transactivation in prostate cancer cell lines. Cancer Res. 2003;63(16):4927–35. [PubMed] [Google Scholar]
- 44.Rasar M, DeFranco DB, Hammes SR. Paxillin regulates steroid-triggered meiotic resumption in oocytes by enhancing an all-or-none positive feedback kinase loop. The Journal of biological chemistry. 2006;281(51):39455–64. doi: 10.1074/jbc.M608959200. [DOI] [PubMed] [Google Scholar]
- 45.Sen A, O'Malley K, Wang Z, Raj GV, Defranco DB, Hammes SR. Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. The Journal of biological chemistry. 2010;285(37):28787–95. doi: 10.1074/jbc.M110.134064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishibe S, Joly D, Zhu X, Cantley LG. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Molecular cell. 2003;12(5):1275–85. doi: 10.1016/s1097-2765(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 47.Ishibe S, Joly D, Liu ZX, Cantley LG. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Molecular cell. 2004;16(2):257–67. doi: 10.1016/j.molcel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Singhai A, Wakefield DL, Bryant KL, Hammes SR, Holowka D, Baird B. Spatially defined EGF receptor activation reveals an F-actin-dependent phospho-Erk signaling complex. Biophysical journal. 2014;107(11):2639–51. doi: 10.1016/j.bpj.2014.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen A, De Castro I, Defranco DB, Deng FM, Melamed J, Kapur P, Raj GV, Rossi R, Hammes SR. Paxillin mediates extranuclear and intranuclear signaling in prostate cancer proliferation. The Journal of clinical investigation. 2012;122(7):2469–81. doi: 10.1172/JCI62044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, Barad D, Gleicher N, Hammes SR. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(8):3008–13. doi: 10.1073/pnas.1318978111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veith C, Zakrzewicz D, Dahal BK, Balint Z, Murmann K, Wygrecka M, Seeger W, Schermuly RT, Weissmann N, Kwapiszewska G. Hypoxia- or PDGF-BB-dependent paxillin tyrosine phosphorylation in pulmonary hypertension is reversed by HIF-1alpha depletion or imatinib treatment. Thrombosis and haemostasis. 2014;112(6):1288–303. doi: 10.1160/TH13-12-1031. [DOI] [PubMed] [Google Scholar]
- 52.Marasek P, Dzijak R, Studenyak I, Fiserova J, Ulicna L, Novak P, Hozak P. Paxillin-dependent regulation of IGF2 and H19 gene cluster expression. Journal of cell science. 2015;128(16):3106–16. doi: 10.1242/jcs.170985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin H, Zhang Q, Wang X, Li T, Wan Y, Liu Y, Zhu J. Role of paxillin in colorectal carcinoma and its relationship to clinicopathological features. Chinese medical journal. 2014;127(3):423–9. [PubMed] [Google Scholar]
- 54.Vadlamudi R, Adam L, Tseng B, Costa L, Kumar R. Transcriptional up-regulation of paxillin expression by heregulin in human breast cancer cells. Cancer Res. 1999;59(12):2843–6. [PubMed] [Google Scholar]
- 55.Levin ER, Hammes SR. Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nature reviews Molecular cell biology. 2016;17(12):783–797. doi: 10.1038/nrm.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia W, Liu Z, Zong R, Liu L, Zhao S, Bacus SS, Mao Y, He J, Wulfkuhle JD, Petricoin EF, Osada T, Yang XY, Hartman ZC, Clay TM, Blackwell KL, Lyerly HK, Spector NL. Truncated ErbB2 Expressed in Tumor Cell Nuclei Contributes to Acquired Therapeutic Resistance to ErbB2 Kinase Inhibitors. Molecular cancer therapeutics. 2011;10(8):1367–1374. doi: 10.1158/1535-7163.MCT-10-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sprenger C, Uo T, Plymate S. Androgen receptor splice variant V7 (AR-V7) in circulating tumor cells: a coming of age for AR splice variants? Annals of oncology : official journal of the European Society for Medical Oncology /ESMO. 2015;26(9):1805–7. doi: 10.1093/annonc/mdv311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frolov A, Evans IM, Li N, Sidlauskas K, Paliashvili K, Lockwood N, Barrett A, Brandner S, Zachary IC, Frankel P. Imatinib and Nilotinib increase glioblastoma cell invasion via Abl-independent stimulation of p130Cas and FAK signalling. Scientific reports. 2016;6:27378. doi: 10.1038/srep27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kummer C, Petrich BG, Rose DM, Ginsberg MH. A small molecule that inhibits the interaction of paxillin and alpha 4 integrin inhibits accumulation of mononuclear leukocytes at a site of inflammation. The Journal of biological chemistry. 2010;285(13):9462–9. doi: 10.1074/jbc.M109.066993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toutounchian JJ, Pagadala J, Miller DD, Baudry J, Park F, Chaum E, Yates CR. Novel Small Molecule JP-153 Targets the Src-FAK-Paxillin Signaling Complex to Inhibit VEGF-Induced Retinal Angiogenesis. Molecular pharmacology. 2017;91(1):1–13. doi: 10.1124/mol.116.105031. [DOI] [PubMed] [Google Scholar]
- 61.Yuan JH, Liu XN, Wang TT, Pan W, Tao QF, Zhou WP, Wang F, Sun SH. The MBNL3 splicing factor promotes hepatocellular carcinoma by increasing PXN expression through the alternative splicing of lncRNA-PXN-AS1. Nature cell biology. 2017;19(7):820–832. doi: 10.1038/ncb3538. [DOI] [PubMed] [Google Scholar]