Abstract
Little is currently known of the role(s) of the vasoconstrictor 20-hydroxyeicosatetraenoic acid (20-HETE) in hypertensive pregnancies. We hypothesized that specific inhibition of 20-HETE would attenuate increases in blood pressure in the reduced uterine perfusion pressure (RUPP) rat model of preeclampsia. Specific 20-HETE synthesis inhibitor HET0016 (1mg/kg) was administered daily to RUPP rats from gestational days 14–18. Blood pressure (BP) increased in RUPP rats and was decreased with HET0016 administration. BP was unchanged in NP+HET0016 rats. Fetal death greatly increased in RUPP rats and was reduced in RUPP+HET0016 rats. 20-HETE levels increased modestly in RUPP rats compared to NP and was reduced in both NP+HET0016 and RUPP+HET0016 rats. Furthermore, circulating levels of HETEs, EET, and DHETE were significantly altered between groups. HET0016 shifted CYP metabolism toward EETs, as indicated by a decrease in plasma 20-HETE:EETs in RUPP+HET0016 rats compared to RUPP. In conclusion, 20-HETE inhibition in RUPP rats reduces BP and fetal death, and is associated with an increase in EET/20-HETE ratio.
Keywords: Preeclampsia, 20-HETE, Placental Ischemia, RUPP rat
Introduction
Up to 10% of pregnancies in the United States are afflicted with preeclampsia, a leading cause of maternal and fetal mortality manifesting with late-gestation (>20 weeks) hypertension, abnormal placentation, low birthweight and premature birth, among other complications [1–5]. Preeclampsia is classified as a hypertensive disorder of pregnancy with a multifactorial pathophysiology including immune activation, endothelial dysfunction and vascular resistance [6–8]. The only cure for preeclamptic patients is delivery of the fetoplacental unit. Pharmaceutical treatments to improve the symptoms are targeted at reducing blood pressure and preventing adverse fetal effects and novel targets for beneficial outcomes in preeclamptic pregnancies are needed.
Cytochrome P450 (CYP) metabolites have been implicated in various forms of hypertension in both humans and experimental animal models [9, 10]. 20-hydroxyeicosatetraenoic acid (20-HETE) is an arachidonic acid metabolite whose production is catalyzed by CYP4A and CYP4F enzymes [11]. 20-HETE is a potent vasoconstrictor and genetic polymorphisms of 20-HETE-production enzymes in humans are associated with hypertension [12]. In contrast, another product of CYP enzymes, epoxyeicosatrienoic acids (EETs) are hyperpolarizing vasodilators and have anti-inflammatory properties [13–16]. Alterations in CYP metabolism to favor 20-HETE over EETs are therefore associated with vascular dysfunction [17]. The primary receptor mediating the vascular effects of 20-HETE has long been unknown, however, new important findings from Garcia et al identify a G coupled protein receptor, GPR75 as a potential receptor for 20 HETE [18]. In fact, the authors show that knockdown of GPR75 or GPCR-kinase interacting protein-1 prevented 20-HETE-mediated endothelial growth factor receptor phosphorylation and angiotensin-converting enzyme induction and hypertension in response to 20-HETE. However, very few studies examining a role for 20-HETE or GPR75 receptor have been performed in animal models of preeclampsia.
Total activity of CYP enzymes is increased in pregnancy [19] and several studies have shown that alterations of CYP activity may be present in preeclamptic patients and animal models of preeclampsia [20, 21]. Jiang et al. compared urine samples from normal pregnant and preeclamptic patients and found that DHET levels are decreased in preeclamptic patients, which they concluded indicated a reduction in renal EETs [22]. In addition, CYP4A expression is decreased in a rat model of preeclampsia, the RUPP (reduced uterine perfusion pressure) model, compared to normal pregnant controls [20]. However, 20-HETE levels are not decreased in preeclamptic rats, indicating a shift toward 20-HETE production by CYP enzymes [20]. We recently showed CYP epoxygenase inhibitor, MsPPOH, improved the preeclamptic syndrome in the RUPP rats. The mean arterial pressure (MAP) measured on day 19 of pregnancies with MsPPOH administration decreased from 126 mmHg in RUPP to 111 mmHg in the MsPPOH-RUPP group [21]. However this study was not specific for 20-HETE. Additional studies demonstrate selective pharmacological inhibition of 20-HETE improves hypertension and endothelial function in animal models of experimental hypertension [23–25]. Therefore, the potential for 20-HETE inhibition to improve hypertension and fetal outcomes associated with preeclampsia have not been closely examined. In the current study, we sought to evaluate changes in blood pressure, fetal survival and CYP enzyme metabolism in response to treatment with the 20-HETE-specific synthesis inhibitor N-Hydroxy-N′-(4-butyl-2-methylphenyl) formamidine (HET0016) in a rat model of preeclampsia, the RUPP rat.
Methods
Experimental animals and the RUPP procedure
All experiments involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Mississippi Medical Center in accordance with guidelines set forth by the National Institutes of Health for the care and use of laboratory animals. Female timed-pregnant Sprague-Dawley rats from Harlan Laboratories (Indianapolis, IN) were maintained under a 12 hour light/dark schedule and fed standard laboratory chow. To induce placental ischemia in pregnant rats we performed the RUPP procedure on gestational day 14 (GD14). Briefly, pregnant rats were anesthetized by isofluorane anesthesia and surgical constrictor clips were placed on the lumbar aorta (size of 0.2 mm) and both lateral branches of the ovarian artery (size of 0.1 mm), which served to restrict blood flow and induce ischemia to the placental-fetal unit. The RUPP model of preeclampsia induces many of the characteristics of preeclampsia seen in human patients, including increased blood pressure, immune activation, and endothelial dysfunction, as previously described [26–28]. We injected normal pregnant (NP) and RUPP rats, intraperitoneally, with the 20-HETE-specific synthesis inhibitor HET0016 at a dose of 1mg/kg daily on GD 14–19. The groups were as follows: NP (n=10), NP+HET0016 (n=7), RUPP (n=8) and RUPP+HET0016 (n=5). On GD18, uterine artery resistance index (UARI) was measured by Doppler sonography, as previously described [29]. All experimental animals underwent insertion of indwelling carotid catheters under isofluorane anesthesia on GD18 and blood pressure was measured in conscious animals on GD19 on a Cobe II Transducer CDX Sema pressure transducer. Additionally, on GD19 animals were sacrificed and blood collected into vacutainer tubes containing EDTA and fetal reabsorptions and weights were recorded. Plasma was prepared by centrifugation and excessively hemolyzed plasmas were excluded from analysis.
Quantification of plasma CYP450 metabolites by LC/MS analysis
To prepare plasma for extraction, 300ul of rat plasma was combined with 2.5ml of 95% 0.1M sodium acetate, 5% methanol buffer (pH 7.0), 100ul 10% acetic acid, and 10ul of 0.2ng/ul 20-HETE-d6/11,12-EET-d11 as an internal standard (Cayman Chemical, Ann Arbor, MI). Samples were centrifuged and extracted using Bond Elute Certify Sample Prep columns (pre-washed with methanol followed by 95% 0.1M sodium acetate/5% methanol solution (pH 7.0; (Agilent Technologies, Santa Clara, CA)) before the supernatants were loaded onto columns.). Samples were added to the column and were then washed with 50:50 methanol-water. Eicosanoids were eluted with 75:25 hexane-ethyl acetate solution supplemented with 1% acetic acid. Samples were dried under nitrogen gas and stored at −80°C until analysis.
Nitrogen-dried samples were reconstituted in 30ul acetonitrile and 70ul deionized water and analyzed utilizing a Dionex Ultimate 3000 High-Performance Liquid Chromatography system (Dionex, Banmockburn, IL) prior to analysis on an ABsciex 4000 Q trap tandem mass spectrometer with electrospray ionization (ABsciex, Foster City, CA). Separation of the metabolites was achieved using a reverse phase column (Kinetex 100 × 2.1 mm, 2.6μm; Phenomenex Torrence, CA) and the following mobile phase conditions at a flow rate of 200ul/min: mobile phase A was 90/10/0.1 (water/mobile phase B/acetic acid, v/v/v), and mobile phase B 85/15 (acetonitrile/methanol, v/v). The protocol was as follows: 45% A for 2 min, raise to 54% B in 2 min, 54% B for 4.5 min, raise to 65% B in 5.5 min, hold at 65% for 2 min, raise to 90% B in 1 min, hold at 90% B for 2.5 min, raise to 45% in 0.5 min, and then hold at 45% B for 3 min for equilibration.
Negative ion mode was used for mass spectrometry with the following settings: unit resolution ion spray voltage −4500 V, curtain gas 30, gas 1–50, temp 600°C and gas 2–50. The declustering and exit potential as well as optimum collision energy for each transition was evaluated using commercially-available standards. The transitions monitored for each metabolite measured were: 337–207 (14,15-DHETE); 337–167 (11,12-DHETE); 337–127 (8,9-DHETE); 319–231 (19-HETE); 319–245 (20-HETE); 319–261 (18-HETE); 337–145 (5,6-DHETE); 319–233 (16-HETE); 319–175 (15-HETE); 319–149 (11-HETE); 319–179 (12-HETE); 319–155 (8-HETE); 319–203 (5-HETE); 319–175 (14,15-EET); 319–167 (11,12-EET); 319– 257 (8,9-EET); 319–191 (5,6-EET); and 325–281/307 (d6-20-HETE) and 330-167/312 (d11-11,12-EET) for internal standards.
Final quantitation of all metabolites was based on area ratios of analyte to internal standard and then compared to known concentrations; using d6-20-HETE or d11-11,12-EET internal standards. The concentration range of 0.02 to 20ng/sample of 20-, 15-, 11-, 12-, 8- and 5-HETE, or 0.002 to 2ng/sample of 16-,18-,19-HETE used 2ng of d6-20-HETE as internal standard. Quantitation range of 0.02 to 20ng/sample of the DHETE’s and EET’s used d11-11,12-EET internal standard.
Analyst software version 1.5 (ABSciex) was used for data acquisition, statistical calculations, and quantification. Linear regression analysis using the least-squares method was used to evaluate the calibration curve of each metabolite as a function of its concentration in the plasma samples.
Statistical Analysis
Data are expressed for all groups as mean ± standard error of the mean and standard student’s t test was utilized to determine statistical significance between the control and treated rats among the groups. A P value of less than 0.05 was considered statistically significant.
Results
Blood pressure and fetal death are reduced in RUPP rats treated with HET0016
Blood pressure increased significantly from 104±2 mmHg in NP rats to 122±3 mmHg (*P<0.001) in RUPP rats [Figure 1a]. HET0016 treatment reduced the rise in blood pressure in RUPP rats to 110±2 mmHg, (*P<0.05) and in NP rats HET0016 treatment had no statistically significant effect on blood pressure (110±6 mmHg).
Figure 1. Blood pressure and fetal death in NP and RUPP rats treated with HET0016.
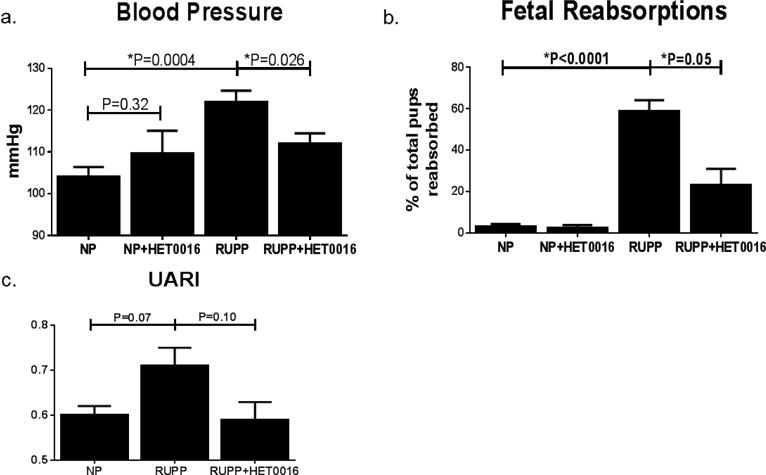
(a). RUPP rats had significantly increased blood pressure compared to NP rats. RUPP rats treated with HET0016 had significantly lower blood pressure than untreated RUPPs. (b). Fetal death was increased with RUPP procedure compared to NP. HET0016 did not adversely affect fetal survival in NP and significantly improved survival in RUPP rats. (c). UARI was modestly increased in RUPP rats and decreased with HET0016 treatment. *P<0.05.
UARI increased in RUPP rats compared to NP (0.71±0.04 vs 0.60±0.02) and was reduced in RUPP+HET0016 rats (0.59±0.04), although these values did not reach statistical significance [Figure 1b]. The percentage of total pups that were reabsorbed on GD19 was drastically increased in RUPP rats to 60±5% (*P<0.0001) compared to NP reabsorptions of 3±1% [Figure 1c]. RUPP rats treated with HET0016 had statistically fewer reabsorptions (23±8%, *P<0.01) than untreated RUPPs. Importantly, HET0016 treatment in NP rats did not increase pup reabsorptions (2±2%), indicating that HET0016 does not increase fetal death in pregnant rats.
CYP enzyme activity was shifted toward the production of EETs in RUPP rats treated with HET0016
Treatment of NP rats with HET0016 modestly reduced circulating 20-HETE levels to 0.77±0.12 ng/ml compared to untreated NP (0.97±0.06 ng/ml), although this did not reach statistical significance [Figure 2a]. Unexpectedly, the RUPP procedure did not significantly increase plasma 20-HETE compared to NP rats (1.03±0.07 ng/ml). To assess the specificity of HET0016 for inhibition of 20-HETE we also measured the combined levels of 5-, 8-, 11-, 12-, 15-, 16-, 18- and 19-HETE [Figure 2b]. Levels of all other HETEs did not significantly change between NP (5.94±0.83 ng/ml) and NP+HET0016 (4.57±0.91 ng/ml), nor RUPP (4.19±0.96 ng/ml) and RUPP+HET0016 (4.20±0.77 ng/ml). While 20-HETE levels were slightly increased in RUPP rats compared to NP, EET levels trended to decrease (0.39±0.05 ng/ml) compared to NP (0.65±0.16 ng/ml) [Figure 2c]. Importantly, HET0016 treatment did not significantly reduce EET levels in either NP+HET0016 (0.49±0.08 ng/ml) or RUPP+HET0016 (0.60±0.17 ng/ml) compared to their untreated counterparts. Plasma levels of 5,6-, 8,9-, 11,12- and 14,15-DHETE, are presented as a measure of the inactive downstream metabolites of EETs, an indirect measure of loss of EET activity, are presented in Figure 2d. We observed no statistically significant differences in DHETE levels between NP (0.25±0.01 ng/ml) and RUPP (0.30±0.05 ng/ml) nor NP+HET0016 (0.21±0.01 ng/ml) and RUPP+HET0016 (0.38±0.02 ng/ml), indicating EET inactivation was unchanged between groups. However, when we assessed circulating 20-HETE:EET in our rat groups treated with HET0016 [Figure 2e], RUPP rats exhibited a modest increase in 20-HETE:EET (0.83±0.08) compared to NP (0.53±0.13). No differences were noted in NP+HET0016 compared to NP (0.46±0.08). However, HET0016 treated RUPP rats had a statistically significantly lower 20-HETE:EET ratio compared to untreated RUPP rats (0.48±0.06, *P<0.01). The change in 20-HETE:EET in RUPP rats indicates a shift of CYP enzyme metabolism from 20-HETE to EET production.
Figure 2. CYP enzyme production of EET’s, HETE’s and DHETE’s in NP and RUPP rats treated with HET0016.
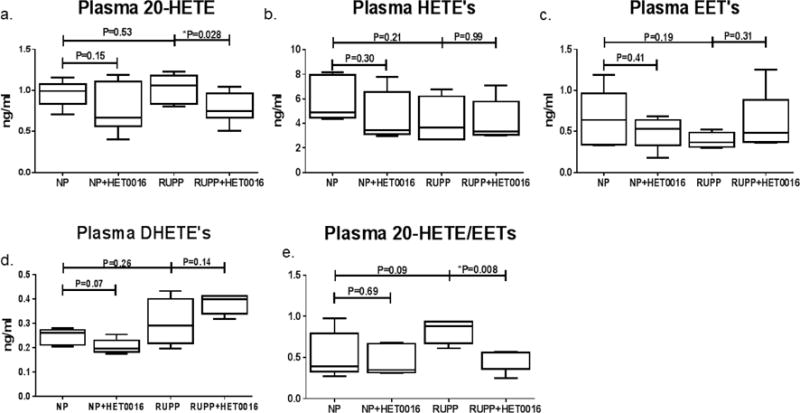
(a). HET0016 treatment significantly reduced 20-HETE levels in RUPP rats. (b). The sum of HETE isoforms 5-, 8-, 11-, 12-, 15-, 16-, 18- and 19-HETE were not altered with HET0016 treatment in either NP or RUPP rats (c). Total EET’s expressed as the sum of 5,6-, 8,9-, 11,12- and 14,15-EET’s were not significantly altered within any of the groups with or without HET0016. (d). The sum of 5,6-, 8,9-, 11,12- and 14,15-DHETE’s, were not altered with HET0016. (e). Ratio of 20-HETE to total EET’s levels is not changed with HET0016 in NP. HET0016 treatment in RUPP rats reduces the ratio of 20-HETE/EET’s. *P<0.05.
Discussion
The purpose of the current study was to examine the effects of 20-HETE-synthesis inhibition on increases in blood pressure and fetal demise associated with the RUPP rat model of preeclampsia. Previous studies examined the role of 20-HETE in the development of various genetic and experimental models of hypertension, and suggest vascular and renal effects of 20-HETE may play a role in the development of hypertensive disorders, which may include preeclampsia [9, 12, 23]. Furthermore, inhibitors specific for 20-HETE, including HET0016, are currently thought to be promising for treatment of hypertension [23, 30]. Our novel findings presented here demonstrate 20-HETE inhibition decreases MAP and fetal demise in association with a decrease in uterine artery resistance in the RUPP rat model of preeclampsia. This decrease in MAP in RUPP rats with HET0016 was in association with a decrease in HETE:EET ratio in the circulation.
Although there is little current data on the role of 20-HETE in pregnancy, Wang and colleagues have found that 20-HETE production is altered throughout pregnancy in rat kidneys [19]. At day 19 of gestation, medullary thick ascending limb production of 20-HETE, which enacts sodium excretion effects, is increased while vascular production of 20-HETE decreases, which are concurrent with a drop in blood pressure and increase in sodium excretion in pregnant rats [19]. This study highlights the fascinating nature of 20-HETE activity as it is pro-hypertensive in vascular beds but renal medullary 20-HETE induces urinary sodium excretion [23, 31, 32]. Urinary excretion rates of EETs are reduced in preeclamptic pregnancies while 20-HETE levels are unchanged [22]. This indicates bioavailable 20-HETE, but not EETs, may be preserved in preeclamptic patients. Our data highlight that plasma 20-HETE:EET ratio is increased in response to placental ischemia in RUPP rats, indicating that the vasoconstrictive effects of 20-HETE may be observed in these rats. Importantly, a reduction of plasma 20-HETE with HET0016 in RUPP rats was associated with a decrease in MAP and uterine vascular resistance, indicating HET0016 inhibited vascular 20-HETE specifically, as a mechanism of reducing vasoconstriction and improving uterine artery resistive index in RUPPs.
EETs and 20-HETE are both arachidonic acid metabolites catalyzed by CYP enzymes and have opposing effects on vascular activity. EETs are a candidate for the endothelial-derived polarizing factor as they have been shown to hyperpolarize endothelial cells and reduce contractility, independent of nitric oxide bioavailability [33–35]. In contrast, 20-HETE prevents activation of Ca2+-activated K+ channels and activates L-type Ca2+ channels in vascular smooth muscle cells and prevents polarization, prolonging contractility [31, 32]. Myometrial resistance arteries respond to EETs with reduced contractile response and increase contractile response when exposed to 20-HETE [36]. Our study is the first to report that blood pressure and fetal demise were improved in preeclamptic rats administered a 20-HETE inhibitor. Llinas et al. has previously shown that the RUPP procedure reduces expression of CYP4A enzymes in the renal tissue compared to normal pregnant rats [20]. A nonspecific CYP enzyme inhibitor, 1-aminobenzotriazol, reduced blood pressure in RUPP rats and was associated with a decrease in tissue 20-HETE levels, but not EET levels [20]. An important point in this report as well as the current study is that the RUPP rat, as it is a late-gestation mechanically-induced model of preeclampsia, does intrinsically possess certain experimental limitations, most notably that the effects of 20-HETE in the development of placental ischemia cannot be evaluated. Therefore, the origin of 20-HETE and EETs dysfunction require the evaluation of these eicosenoids in yet-to-be developed models or studies in human patients. However, as the RUPP rat is the most widely used and accepted animal model of preeclampsia and represents a treatment-oriented model of disease, the therapeutic effects of HET0016 in this model are highly clinically relevant for preeclamptic patients who are started on treatment regimens post-diagnosis. It is also important to note that the inhibitor used in the previous study was less specific for 20-HETE compared to HET0016, therefore, other vasoconstrictive HETEs may have also been reduced in the RUPP rat. Our data suggests that the inhibition activity of HET0016 was restricted to 20-HETE in that plasma levels of other HETEs were not altered by HET0016 in either NP or RUPP rats. Our study demonstrates HET0016 administration decreases 20-HETE levels while preserving EETs.
EETs are metabolized into a less active metabolite, DHETE, by the enzyme soluble epoxide hydrolase (sEH). sEH inhibition has been shown to preserve bioactive EET levels and reduce 20-HETE production in spontaneous hypertensive rats (SHR) [37, 38]. Our studies confirmed that DHETEs were not increased in response to HET0016 in either NP or RUPP groups, and therefore it is unlikely that HET0016 altered the downstream metabolism of EETs into its inactive product. These findings are in line with a previous study by Herse et al. demonstrating 20-HETE levels are not decreased in RUPP rats, but that placental EETs, and importantly, DHETs are increased in RUPP rats [21]. In the previous study, it was observed that in both preeclamptic patients and RUPP rats, CYP enzyme arachidonic acid metabolism is increased and EETs+DHETs are increased and that interruption in this pathway can improve pathology associated with placental ischemia, however this inhibitor was not specific for 20 HETE. However, the data in the current study indicate HET0016-mediated decreases in blood pressure seen in our RUPP rats may, at least in part, be attributed to preserved activity of EETs concurrent with 20-HETE reduction in the maternal vasculature.
In conclusion, 20-HETE inhibition via the specific inhibitor HET0016 reduces blood pressure and fetal demise in a preeclamptic rat model without concurrently reducing circulating EETs. These data indicate that 20-HETE inhibition may represent a novel target for reduction of blood pressure in preeclamptic patients. Importantly, the NP+HET0016 group in this report demonstrates that the pharmacological effects of HET0016 did not have adverse effects on BP or fetal health, as is indicated by a lack of increased fetal demise in this experimental group. Inclusion of control groups with pharmacological treatments in studies of preeclampsia is vital to to demonstrate that off-target effects will not present danger to a fragile feto-placental unit in an otherwise healthy pregnancy. The current data suggests a safety index for HET0016, prompting further studies. The addition of a 20-HETE-specific inhibitor to therapeutic treatment plans in preeclamptic patients may be especially valuable given the teratogenicity of so many anti-hypertensive medications. Further studies are needed to fully evaluate the placental, renal and vascular activity of 20-HETE and EETs in preeclamptic animal models and patients to better define the mechanisms leading to changes in maternal plasma HETE and EET levels in response to HET0016.
Highlights.
20-HETE synthesis is inhibited in RUPP rats via administration of HET0016
Blood pressure and fetal demise are reduced by inhibition of 20-HETE in RUPP rats
HET0016 did not adversely affect normal pregnant rats
HET0016 increased the plasma EET/20-HETE ratio in RUPP rats
Acknowledgments
The authors wish to thank Christine Purser of the University of Mississippi Medical Center’s LC/MS Analytics Core for her contribution to this work.
Funding Sources
This work was supported by NIH grants HL105324, HL124715, HL78147 and HL51971 and HD067541 and AHA grant 14SDG20160020.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All experiments conducted at the University of Mississippi Medical Center, Jackson, MS
The authors declare that there is no conflict of interest involved with this work.
Declaration of Author Contribution
Jessica L Faulkner: Primary author of manuscript, performed experiments, surgeries and data analysis.
Nicole Lee: Collaborator on project, contributed to animal work, performed experiments
Kedra Wallace: Collaborator on project, assisted with data analysis
Lorena M Amaral: Collaborator on project, assisted with animal work and experiments
Mark W Cunningham Jr.- Collaborator on project, assisted with animal work and experiments
Sydney Murphy: Collaborator on project, assisted with experiments and data analysis
Babbette LaMarca: Principal investigator of the laboratory where experiments and animal work, preparation of manuscript and data analysis were performed
All authors of the manuscript have approved this manuscript.
References
- 1.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy, Best practice & research. Clinical obstetrics & gynaecology. 2011;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia, Nature clinical practice. Nephrology. 2005;1(2):98–114. doi: 10.1038/ncpneph0035. quiz 120. [DOI] [PubMed] [Google Scholar]
- 3.Sibai BM, Caritis S, Hauth J, H. National Institute of Child, N. Human Development Maternal-Fetal Medicine Units What we have learned about preeclampsia. Seminars in perinatology. 2003;27(3):239–46. doi: 10.1016/s0146-0005(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 4.Bell MJ. A historical overview of preeclampsia-eclampsia. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN/NAACOG. 2010;39(5):510–8. doi: 10.1111/j.1552-6909.2010.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norwitz ER, Repke JT. Preeclampsia prevention and management. Journal of the Society for Gynecologic Investigation. 2000;7(1):21–36. doi: 10.1016/s1071-5576(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 6.LaMarca B, Cornelius D, Wallace K. Elucidating immune mechanisms causing hypertension during pregnancy. Physiology. 2013;28(4):225–33. doi: 10.1152/physiol.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW, Jr, Wallace K, LaMarca B. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond) 2016;130(6):409–19. doi: 10.1042/CS20150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaMarca B, Cornelius DC, Harmon AC, Amaral LM, Cunningham MW, Faulkner JL, Wallace K. Identifying immune mechanisms mediating the hypertension during preeclampsia, American journal of physiology. Regulatory, integrative and comparative physiology. 2016;311(1):R1–9. doi: 10.1152/ajpregu.00052.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiological reviews. 2002;82(1):131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 10.Waldman M, Peterson SJ, Arad M, Hochhauser E. The role of 20-HETE in cardiovascular diseases and its risk factors. Prostaglandins & other lipid mediators. 2016;125:108–17. doi: 10.1016/j.prostaglandins.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. The Journal of biological chemistry. 2000;275(6):4118–26. doi: 10.1074/jbc.275.6.4118. [DOI] [PubMed] [Google Scholar]
- 12.Wu CC, Gupta T, Garcia V, Ding Y, Schwartzman ML. 20-HETE and blood pressure regulation: clinical implications. Cardiology in review. 2014;22(1):1–12. doi: 10.1097/CRD.0b013e3182961659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Archiv : European journal of physiology. 2010;459(6):881–95. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellien J, Thuillez C, Joannides R. Contribution of endothelium-derived hyperpolarizing factors to the regulation of vascular tone in humans. Fundamental & clinical pharmacology. 2008;22(4):363–77. doi: 10.1111/j.1472-8206.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 15.Larsen BT, Campbell WB, Gutterman DD. Beyond vasodilatation: non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends in pharmacological sciences. 2007;28(1):32–8. doi: 10.1016/j.tips.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Tacconelli S, Patrignani P. Inside epoxyeicosatrienoic acids and cardiovascular disease. Frontiers in pharmacology. 2014;5:239. doi: 10.3389/fphar.2014.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Wu J, Liu H, Lai G, Zhao Y. Disturbed ratio of renal 20-HETE/EETs is involved in androgen-induced hypertension in cytochrome P450 4F2 transgenic mice. Gene. 2012;505(2):352–9. doi: 10.1016/j.gene.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, Gandham SK, Reddy NR, Graves JP, Gruzdev A, Zeldin DC, Capdevila JH, Falck JR, Schwartzman ML. 20-HETE Signals Through G-Protein-Coupled Receptor GPR75 (Gq) to Affect Vascular Function and Trigger Hypertension. Circulation research. 2017;120(11):1776–1788. doi: 10.1161/CIRCRESAHA.116.310525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang MH, Zand BA, Nasjletti A, Laniado-Schwartzman M. Renal 20-hydroxyeicosatetraenoic acid synthesis during pregnancy, American journal of physiology. Regulatory, integrative and comparative physiology. 2002;282(2):R383–9. doi: 10.1152/ajpregu.2002.282.2.R383. [DOI] [PubMed] [Google Scholar]
- 20.Llinas MT, Alexander BT, Capparelli MF, Carroll MA, Granger JP. Cytochrome P-450 inhibition attenuates hypertension induced by reductions in uterine perfusion pressure in pregnant rats. Hypertension. 2004;43(3):623–8. doi: 10.1161/01.HYP.0000117721.83371.9f. [DOI] [PubMed] [Google Scholar]
- 21.Herse F, Lamarca B, Hubel CA, Kaartokallio T, Lokki AI, Ekholm E, Laivuori H, Gauster M, Huppertz B, Sugulle M, Ryan MJ, Novotny S, Brewer J, Park JK, Kacik M, Hoyer J, Verlohren S, Wallukat G, Rothe M, Luft FC, Muller DN, Schunck WH, Staff AC, Dechend R. Cytochrome P450 subfamily 2J polypeptide 2 expression and circulating epoxyeicosatrienoic metabolites in preeclampsia. Circulation. 2012;126(25):2990–9. doi: 10.1161/CIRCULATIONAHA.112.127340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang H, McGiff JC, Fava C, Amen G, Nesta E, Zanconato G, Quilley J, Minuz P. Maternal and fetal epoxyeicosatrienoic acids in normotensive and preeclamptic pregnancies. American journal of hypertension. 2013;26(2):271–8. doi: 10.1093/ajh/hps011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. Journal of cardiovascular pharmacology. 2010;56(4):336–44. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toth P, Csiszar A, Sosnowska D, Tucsek Z, Cseplo P, Springo Z, Tarantini S, Sonntag WE, Ungvari Z, Koller A. Treatment with the cytochrome P450 omega-hydroxylase inhibitor HET0016 attenuates cerebrovascular inflammation, oxidative stress and improves vasomotor function in spontaneously hypertensive rats. British journal of pharmacology. 2013;168(8):1878–88. doi: 10.1111/bph.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speed JS, George EM, Arany M, Cockrell K, Granger JP. Role of 20-hydroxyeicosatetraenoic acid in mediating hypertension in response to chronic renal medullary endothelin type B receptor blockade. PloS one. 2011;6(10):e26063. doi: 10.1371/journal.pone.0026063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction, American journal of physiology. Heart and circulatory physiology. 2008;294(2):H541–50. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 27.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37(4):1191–5. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 28.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods in molecular medicine. 2006;122:383–92. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 29.Faulkner JL, Amaral LM, Cornelius DC, Cunningham MW, Ibrahim T, Heep A, Campbell N, Usry N, Wallace K, Herse F, Dechend R, LaMarca B. Vitamin D supplementation reduces some AT1-AA-induced downstream targets implicated in preeclampsia including hypertension, American journal of physiology. Regulatory, integrative and comparative physiology. 2017;312(1):R125–R131. doi: 10.1152/ajpregu.00218.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edson KZ, Rettie AE. CYP4 enzymes as potential drug targets: focus on enzyme multiplicity, inducers and inhibitors, and therapeutic modulation of 20-hydroxyeicosatetraenoic acid (20-HETE) synthase and fatty acid omega-hydroxylase activities. Current topics in medicinal chemistry. 2013;13(12):1429–40. doi: 10.2174/15680266113139990110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan F, Sun CW, Maier KG, Williams JM, Pabbidi MR, Didion SP, Falck JR, Zhuo J, Roman RJ. 20-Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PloS one. 2013;8(12):e82482. doi: 10.1371/journal.pone.0082482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge Y, Murphy SR, Lu Y, Falck J, Liu R, Roman RJ. Endogenously produced 20-HETE modulates myogenic and TGF response in microperfused afferent arterioles. Prostaglandins & other lipid mediators. 2013;102–103:42–8. doi: 10.1016/j.prostaglandins.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circulation research. 1996;78(3):415–23. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 34.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401(6752):493–7. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 35.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107(5):769–76. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 36.Pearson T, Warren AY, Barrett DA, Khan RN. Detection of EETs and HETE-generating cytochrome P-450 enzymes and the effects of their metabolites on myometrial and vascular function, American journal of physiology. Endocrinology and metabolism. 2009;297(3):E647–56. doi: 10.1152/ajpendo.00227.2009. [DOI] [PubMed] [Google Scholar]
- 37.Koeners MP, Wesseling S, Ulu A, Sepulveda RL, Morisseau C, Braam B, Hammock BD, Joles JA. Soluble epoxide hydrolase in the generation and maintenance of high blood pressure in spontaneously hypertensive rats, American journal of physiology. Endocrinology and metabolism. 2011;300(4):E691–8. doi: 10.1152/ajpendo.00710.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elmarakby AA, Faulkner J, Pye C, Rouch K, Alhashim A, Maddipati KR, Baban B. Role of haem oxygenase in the renoprotective effects of soluble epoxide hydrolase inhibition in diabetic spontaneously hypertensive rats. Clin Sci (Lond) 2013;125(7):349–59. doi: 10.1042/CS20130003. [DOI] [PubMed] [Google Scholar]


