Abstract
The heart is a large organ containing many cell types, each of which is necessary for normal function. Because of this, cardiac regenerative medicine presents many unique challenges. Because each of the many types of cells within the heart has unique physiological and electrophysiological characteristics, donor cells must be well matched to the area of the heart into which they are grafted to avoid mechanical dysfunction or arrhythmia. In addition, grafted cells must be functionally integrated into host tissue to effectively repair cardiac function. Because of its size and physiological function, the metabolic needs of the heart are considerable. Therefore grafts must contain not only cardiomyocytes but also a functional vascular network to meet their needs for oxygen and nutrition. In this article we review progress in the use of pluripotent stem cells as a source of donor cardiomyocytes and highlight current unmet needs in the field. We also examine recent tissue engineering approaches integrating cells with various engineered materials that should address some of these unmet needs.
Keywords: cardiomyocytes, cardiac progenitors, pluripotent cells, hydrogels, engineered heart tissues
I. Introduction
Despite the promise of regenerative medicine, cardiovascular disease remains the leading cause of death in the United States.1 Cardiovascular regenerative medicine presents many unique challenges. First, unlike other muscles in the body, the human myocardium possesses only limited cell division2 and a limited ability to repair itself after injury. A recent study using apical resection of neonatal mouse hearts suggested that murine hearts have significant regenerative capacity for some days after birth.3 This capacity was lost within the first week of birth, however, and a recent attempt to repeat these studies using a different inbred mouse strain showed only incomplete regeneration of heart tissue.4 These results suggest that many as yet unknown factors may impact the regenerative capacity of the young mouse heart. Spontaneous regeneration of human hearts on a scale that would be required to repair a typical myocardial infarction (MI) has not been observed to date. Indeed, it has been suggested that a typical infarct episode in the human heart might damage as many as one billion cells,5 well beyond even the most optimistic estimates of the heart's ability to replace damaged cells. Carbon-dating experiments indicate that under normal conditions cells within the adult human are renewed at a rate of about 1% per year until age 25 and at only 0.45% per year by age 70,2 suggesting that there is minimal turnover of cells in healthy hearts. By contrast, the heart may be able to activate a program of renewal after injury. One study showed significant cell renewal in mouse hearts following pressure overload or infarct6; however, this type of spontaneous, functional recovery after a major cardiac event has not been observed in adult human hearts. For these reasons, it has long been suggested that the most feasible approach to cardiac regeneration after MI would be the engraftment of cardiomyocytes or cardiac progenitors that have been expanded ex vivo from stem cell populations. Here we review recent progress in the use of both transcription factor–mediated reprogramming within the heart and the isolation of cardiac cells or cardiac progenitors from pluripotent cells types such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). We also examine how bioengineers are using tissue-engineering approaches that involve both cell grafts and hydrogels to improve the integration, differentiation, and survival of cells to be grafted.
II. Characteristics of an Ideal Cell Population for Cardiac Grafts
Cells that are useful as potential donors for cardiac repair should be readily available, be expandable in culture, show an excellent natural ability for selfrenewal, and have contractile and electrophysiological characteristics consistent with their roles within the heart. Cells isolated from unrelated donors raise immunological concerns. In addition, the use of human ESCs raises ethical concerns. Because of this, noncardiac contractile cells such as skeletal muscle cells and/or nonpluripotent stem cells derived from adult tissues were long considered to be the most desirable sources of potential donor cells for cardiac repair. The more recent development of protocols to differentiate large numbers of bona fide cardiac cells from iPSCs has overcome these ethical and immunological concerns, while providing hope that these cells may overcome the problems of functional integration and arrhythmias.
Several protocols for the efficient production of cardiac cells from ESCs have been developed in recent years, and these (or slight modifications of them) have proven to be equally effective for the differentiation of both mouse and human iPSCs. Most notably, coculture of human ESCs (hESCs) with the visceral endoderm-like END2 cell line7 has induced 20–25% cardiac differentiation, whereas protocols using either carefully timed addition of growth factors8 or a combination of growth factor addition and flow cytometry–based selection of cardiac progenitors9 have activated 30% and 40–50% of cardiac cells, respectively. These protocols are, in turn, based on a large body of work using frog, chick, and mouse embryos, as well as ESCs, to elucidate the embryology and molecular genetics of heart induction.
III. Studies Elucidating the Molecular Mechanisms of Cardiac Differentiation
The mammalian heart is made up of cells from at least 3 sources. First, multipotent cardiac progenitors that form during gastrulation give rise to the original linear heart tube and are referred to as the first heart field (FHF). In addition, 2 groups of cells that lie outside this initial heart tube also contribute to the adult heart: the so-called second (or secondary/anterior) heart field (SHF)10–15 and the neural crest.16 We previously reviewed the embryology and molecular genetics of primary (FHF) induction in detail17,18; however, a few features that are particularly relevant to stem cell differentiation of cardiac cells should be mentioned here. Heart formation is a multistep process that begins with the formation of mesoderm during gastrulation. In all vertebrate embryos and in ESCs the activities of transforming growth factor (TGF)-β family members and Wnts are required to form the mesoderm as cells exit the primitive streak (the dorsal lip in amphibian embryos).19–28 Once formed, the mesoderm immediately begins to migrate away from the streak and toward its final position in the embryo, where it will begin to differentiate according to its location within the embryonic axis.29,30 When Wnt signals are depleted from the endoderm of early mouse embryos, multiple beating hearts form all along the embryonic axis,31 suggesting that there is a broad potential for cardiac formation within the mesoderm of the early embryo. These studies also suggest that Wnt signaling from the endoderm actively represses myocardial formation outside of the normal heart field. Thus the migration of mesoderm away from the primitive streak may serve not only to bring cells into their final positions within the embryo but also to protect the future heart field from Wnt signals that are present in the primitive streak.
This finding is consistent with earlier studies of chicks, frogs, mice, and zebrafish demonstrating that the transition from uncommitted mesodermal cell to cardiac progenitor involves both cell migration away from the primitive streak (or its embryological equivalent) and the presence of signals that inhibit Wnt.32–34 In the embryo (and almost certainly in differentiating ESCs) these signals come from the adjacent endoderm. Later, other growth factors including Fibroblast growth factor act on the myocardium to activate cell proliferation.17,18 Thus a hallmark of FHF induction across all vertebrates is the transient activation of Wnt and TGF-β signaling to initiate mesoderm formation from the pluripotent epiblast, followed by a period of Wnt inhibition that is necessary for mesodermal cells to adopt a cardiac fate35,36 (Figure 1). As with mammalian embryos, a combination of Wnt and TGF-β signaling can be used to activate a primitive streak–like activity from ESCs37, and timely modulation of TGF-β and Wnt signaling have proven to be necessary and sufficient to activate the formation of cardiac progenitors and beating cardiomyocytes from both mouse and human ESCs and iPSCs.8,9,14,38–44
Fig. 1.
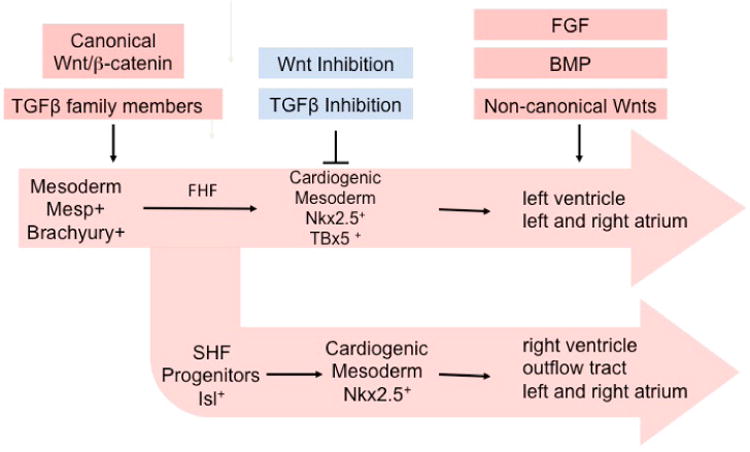
A graphical representation of cardiac differentiation showing the stages of cardiac differentiation, including characteristic markers of each stage. Also noted are key indicators that differentiate between the first heart field (FHF) and second heart field (SHF). Key regulators of each developmental step are indicated. BMP, bone morphogenic protein; FGF, fibroblast growth factor; TGF, transforming growth factor.
As they proliferate and move toward the heart tube, differentiation of cardiomyocytes from the SHF is delayed through a mechanism that is not fully understood. At about embryonic day (E) 8.5, SHF cells begin to contribute to the growing heart by migration through the arterial and venous poles of the heart. The right and left atria contain derivatives from both the FHF and the SHF. By comparison, the left ventricle develops primarily from the FHF and the right ventricle and outflow tract (OFT) primarily from SHF progenitors.13,45,46 Whether there are equivalents to the FHF and SHF when cardiomyocytes are differentiated from ESCs is unclear. Since the major force generating cardiomyocytes of the heart (left ventricular cardiomyocytes) are derived from the FHF, however, understanding the factors that mediate the switch between FHF and SHF development may lead to improved protocols for the in vitro differentiation of cardiac cells enriched for the left ventricular fate.
IV. Cardiac Progenitors
When ESCs are differentiated as embryoid bodies (EBs), they readily form cardiac cells but also produce many other cell types. A major area of research over the past decade has therefore been the search for markers that could identify a cardiac-specific progenitor population (Figure 1). This would allow researchers to isolate, using flow cytometry or another selection method, just those cells that had the potential to differentiate as myocardium.
Genetic fate mapping experiments in the mouse indicate that both the FHF and SHF are derived from a progenitor population expressing Mesp1. Mesp1-expressing mesoderm emerges from the primitive streak of the mouse embryo during early gastrulation (∼E6.5)47,48 and migrates around the epiblast cylinder, coalescing across the anterior midline to form the cardiac crescent at E7.5. By E8.5 the cardiac crescent has undergone a series of morphogenetic changes to form the beating linear heart tube. Mice lacking both Mesp1 and Mesp2 form axial mesoderm at the streak, as assessed by the expression of the pan-mesodermal marker Brachyury/T, but these mice do not form the migratory “mesodermal wings”49 and as such are devoid of most mesodermal lineages. Only 2–3% of cells within a mouse EB typically differentiate as cardiac. When Mesp1 is transiently overexpressed in EBs, this percentage increases to as much as 10%, suggesting that Mesp1 expression encompasses a cardiac progenitor population. Since most mesodermal lineages are marked early on by the expression of Mesp1, however, this proved to be too nonspecific to be an effective marker of cardiac progenitors.50
Some of the earliest markers of the cardiac primordium are the transcription factors Nkx2.5 and Tbx5. Although there is considerable overlap between the expression patterns of these 2 genes, fate mapping reveals that Nkx2.5 is expressed in derivatives of both the FHF and SHF,51 whereas the expression of Tbx5 is biased (but not exclusive) to the SHF.52 Because of this, Nkx2.5 also was examined as a potentially specific marker for cardiac progenitors. Nkx2.5-expressing cells that are isolated either in vivo from mouse embryos or in vitro from differentiating ESCs are bipotential and give rise to both cardiac and smooth muscle cells. In addition, this progenitor population can give rise to multiple cardiac lineages (based on cell shape and action potential morphology), including atrial, ventricular, and conduction system cells.53
During the cardiac crescent and linear heart tube stages, SHF cells, identified by the expression of Islet-1 (Isl1)10,12,14,54 and Fgf1,13 reside outside of the heart tube.12 Initial genetic fate mapping experiments suggested that cells that express Isl1 give rise to both the coronary vasculature and multiple cardiac lineages, including cells in the atrium, ventricles, conduction system, and OFT,12,55,56 with the majority of cells located in the right atrium and OFT and only a small contribution to the left ventricle.55 A recent reassessment of the Isl1 fate map suggests that Isl1 is expressed in all cardiac progenitors.51,57 Not surprisingly, Isl1-expressing cells that are isolated from differentiating hESCs give rise to smooth muscle, cardiac, and endothelial cells.54
One marker that has proven to be extremely useful in identifying and isolating cardiac progenitors is fetal liver kinase-1 (Flk-1). Flk-1 is the major receptor for vascular endothelial growth factor A. Because of this, it was thought that it would primarily mark endothelial and hematopoietic lineages. However, Cremediated fate mapping in mouse embryos revealed, surprisingly, that Flk-1-expressing cells also give rise to several mesodermal lineages,58,59 and Flk-1-expressing cells isolated from differentiating murine ESCs gave rise not only to endothelial cells but also to cardiac and smooth muscle cells.60–62 Differentiating ESCs that are sorted using fluorescence-activated cell sorting based on the simultaneous expression of both Flk-1 and the chemokine receptor CXCR4 comprise a cardiopoietic lineage that is largely depleted of endodermal cell types.63 The combination of selection, based on expression of Flk-1 (KDR in humans), and the addition of procardiogenic growth factors ultimately served as the basis for the efficient differentiation of cardiac cells from hESCs.9
Unfortunately, of the potential cardiac progenitor markers that have been identified to date, no single marker identifies cardiac and only cardiac lineages. Therefore the isolation of highly purified cardiac cells may require further isolation steps based on several lineage markers. For example, sorting based on the expression of several markers might produce a population that is more enriched by cardiac cells or could be used to isolate specific subpopulations within the myocardium. A potential problem with the protocols currently used to isolate cardiac cells from stem cell sources is that these protocols result in the formation of multiple cardiac sublineages, including atrial, ventricular, and conduction system cells,64–66 raising the possibility of arrhythmias if cells with inappropriate electrophysiologies become established within grafts.
V. Progress and New Challenges for Cell-Based Therapies
Recent work by Chong and colleagues67 highlighted concerns about the potential for engrafted cells to cause arrhythmias. Their study demonstrated both the progress that has been made in generating donor cardiac cells from pluripotent cell sources and some important new challenges. They showed the feasibility of large-scale production of cardiomyocytes from hESCs that can be frozen in sufficient numbers (1 × 109 cells) and with sufficient subsequent viability for therapeutic applications in humans. When these cells were injected directly into the infarct zone of a nonhuman primate model of MI, these cells were retained within the heart for at least several weeks (and in the one case for up to 3 months) and showed calcium transients that were synchronized with the rest of the myocardium, suggesting functional engraftment.67 During these studies, they made 2 observations that highlight the next major challenges for this field. First, recovery of ventricular function was not statistically significant. Although this finding may be because of the small group size, it raises the possibility that these cells lacked some of the mechanical properties of mature ventricular cells. Indeed, myofibril alignment, sarcomere registration, and cardiomyocyte diameter suggested that these cells were not fully mature. Advances in the field of physiological maturation of cardiomyocytes were recently reviewed by Yang et al.68 Electrophysiological maturation of these cells was not assessed; however, grafts resulted in arrhythmias not observed in sham-injected animals, suggesting either incomplete electrophysiological maturation, scarring, or the presence of nonventricular cardiac cells within the graft.
One of the great unmet needs in the field of cardiac regeneration is to determine the extent to which the characteristics of a cardiomyocyte are determined by its final position within the heart and to what extent they are determined by extrinsic factors. In short, when does a cardiomyocyte “know” that it will be part of the atrium, ventricle, or conduction system? If a cell fated for the atrium is grafted into the ventricle, what happens to it? Does it change its physiological characteristics according to its new position? Does it die? Does it fail to functionally integrate? Or, does it become a potential source of arrhythmias? Early fate maps of chick embryos suggest that atrial and ventricular fates within the myocardium are sorted out before they exit the primitive streak.69 This occurs during the midblastula phase in the zebrafish.70 Explants of the prospective chick heart field that are grown in isolation differentiate according to their fate. That is, an atrial-specific myosin heavy chain is expressed only in posterior explants and not in anterior explants that are fated to become the ventricle.71 These data suggest that some degree of lineage determination occurs well before cells differentiate as cardiomyocytes. There is also evidence, however, that the fate of cardiac progenitors remains flexible for some time. For example, explants of the future ventricle could be induced to express atrial markers by treating them with retinoic acid72; when cells fated to the atrium were grafted into the ventricle, they changed their rate of beating.73 These early studies suggested that plasticity was maintained throughout the cardiac progenitor phase and ended only after differentiation as beating cardiomyocytes. The implication for regenerative medicine is that transplanting cardiac progenitors, rather than beating cardiomyocytes, may be more therapeutically beneficial. Although, as discussed above, a progenitor population that gives rise exclusively to cardiomyocytes has not yet been identified.
To date, few groups have attempted to differentiate cells of specific cardiac lineages. The underlying assumption has been that cardiac progenitors function as generic cardiac cells that will develop mature electrophysiologies that are appropriate for their ultimate position within the heart. However, this hypothesis has not been directly tested. It was shown recently that a small-molecule inhibitor of the canonical Wnt/β-catenin signaling pathway seems to direct cells with high preference to a ventricular fate.74–76 Interestingly, other small-molecule inhibitors of Wnt and the protein Dkk1 do not have this effect,75 suggesting that our understanding of the molecular genetics of this process is not yet fully understood. The ability to direct myocardial differentiation to specific cardiac cell types may represent an extremely important next step in realizing their full regenerative potential.
VI. Transcription Factor–Mediated Re-Programming
Another area currently being explored in cardiac regenerative medicine is the use of transcription factor–mediated reprogramming of cells. The development of iPSCs,77 which demonstrated that essentially any differentiated cell could be restored to a pluripotent state by activating a small number of transcription factors, renewed interest in the concept that any cell might be converted to a different cell type by ectopic expression of the correct combination of transcription factors. Previous work on genes such as MyoD and Pax6 suggested that at least some cell fates might be controlled by single master regulatory genes. Indeed, overexpression of the Pax6 homolog in drosophila legs and wings was sufficient to induce ectopic eye formation.78 Before that, MyoD was shown to be capable of converting fibroblasts to a myogenic state.79,80 However, further research quickly demonstrated that few cell fates are controlled by the activity of a single master regulatory gene. Current work in the field of transcription factor–mediated reprogramming is focused on identifying minimal sets of transcription factors that might control given cell fates. With regard to the cardiac fate, overexpression of 3 transcription factors (Gata4, Mef2C, and Tbx5) both in isolated mouse cardiac fibroblasts and in vivo can activate many characteristics of cardiomyocytes, including beating, in noncardiomyocytes.81,82 Attempts to repeat this work using tail fibroblasts, however, showed only inefficient reprogramming, as well as the absence of spontaneous action potentials and a contractile phenotype.81,83 In addition, this combination of transcription factors did not induce full reprogramming in human fibroblasts.84 It may be that a different, or expanded, set of transcription factors is required to accomplish full reprogramming in human fibroblasts. The efficiency of this approach also will have to be improved, given that full reprogramming using current protocols is rare and the average infarct in human hearts may involve injury to as many as a billion cells.5 In addition, the current route of delivery of these reprogramming factors in vivo is genetic modification of cells with viruses, and this approach may present some regulatory barriers.
Until recently, it has been assumed that reprogramming approaches would involve the conversion of a generic cell type, such as a fibroblast, to desired cell types; however, using transcription factors to convert cells from one fate to another closely related fate may be possible (and more straightforward). For example, Kapoor et al.85 reported that they were able to convert neonatal rat ventricular cardiomyocytes to a pacemaker-like fate by viral transduction of the transcription factor Tbx18.
These early studies are encouraging but await improvements in efficiency. Also needed are studies to determine the extent to which reprogrammed cells recapitulate normal cardiac mechanics and electrophysiologies.
VII. Engineering Cardiovascular Tissues
Although tremendous progress has been made, there are still many challenges for cardiac regenerative medicine that remain to be solved. Bona fide cardiac cells grafted into a nonhuman primate seemed to be functionally linked to the host myocardium, as demonstrated by synchronized calcium transients. However, they caused arrhythmias and did not statistically improve overall ventricular contractility.67 These findings are possibly due to a failure of these cells to mature in place. In addition, thinning of the ventricular wall after MI often results in remodeling that affects overall heart function (Figure 2). Although this was not assessed by Chong et al.,67 remodeling could also contribute to the poor recovery of contractile function.
Fig. 2.
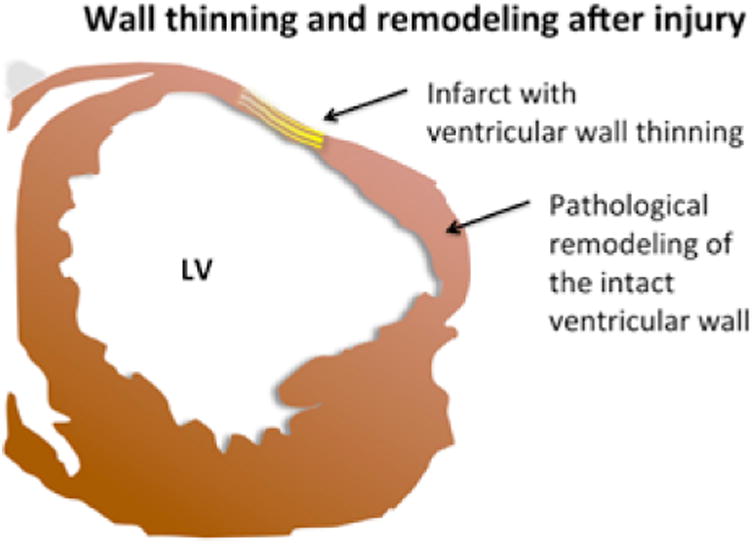
A graphical representation of a typical infarcted left ventricle (LV), showing wall thinning in the damaged area (yellow). Note that while not thinned, the wall adjacent can undergo pathological remodeling that can interfere with normal functioning.
Solutions to the unmet challenges in cardiovascular regenerative medicine may involve the use of engineered materials to enhance cardiac differentiation and electrophysiological maturation and/ or to preserve or replicate the 3-dimensional (3D) structure of the heart following MI. Here we focus specifically on the potential of hydrogels, or the combination of hydrogels and cells, to meet some of these challenges. These could be injected directly into an area of cardiac damage or be used in the context of 3D printing to generate patches.
Using bioinks or other types of scaffolds, bioengineers are attempting to create microenvironments conducive to cardiac differentiation or maturation and/or that maintain the 3D structure of cardiac tissue after MI. Hydrogel scaffolds have facilitated the growth and expansion of vascular tissues within myocardial grafts. In addition, by varying the mechanical and chemical properties of hydrogel scaffolds, they can be designed in ways that allow researchers to test the roles of mechanical stress86 or electrical pacing (reviewed by Vunjak-Novakovic et al.87).
A. Hydrogels
A number of research groups are now exploring the feasibility of using hydrogel/cell combinations as patches, injectable fillers, or printable bioinks. Natural hydrogels include Matrigel (a commercially available combination of laminin, type IV collagen, and heparin sulfate),88 collagen,89 fibrin,90 and alginate.91 Each of these separately has enhanced the retention and integration of injected cells and preserved the normal morphology of the ventricular wall after MI.88 In addition, a number of synthetic hydrogels are being explored.92 However, the cytotoxic and immune potential of these various synthetic compounds is largely unknown but should be explored. For example, injection of a nondegradable synthetic polyethylene glycol (PEG) hydrogel into the infarct zone of a rat MI model resulted in significant infiltration of macrophages, suggesting an immune response.93
The precise physical properties of hydrogels will likely vary somewhat depending on the experimental and therapeutic context; however, there are some general characteristics that would be highly desirable for use in cardiac regenerative therapies.
First, the ability to vary the viscosity of a hydrogel would be highly desirable. Less viscous hydrogels could be injected into the wall of a damaged heart with lower injection pressure so as not to create further damage to the wall of the heart during injection or cause damage to the cells being injected. This would include hydrogels that could be injected in a liquid or semiliquid state but that would become more rigid at body temperature. Alternatively, slightly more viscous hydrogels would be useful for 3D printing of organs or patches. For example, Duan and colleagues94 recently reported the development of bioinks with high viscosity and low stiffness that were practical for 3D printing of structures that encapsulated human aortic valvular interstitial cells (HAVICs). This was accomplished by manipulating the relative amounts of methacrylated hyaluronic acid and methacrylated gelatin. Increased relative methacrylated gelatin resulted in improved cell spreading and maintained fibroblastic phenotype. Using 3D bioprinting Duan et al. produced 3D trileaflet valve conduits with HAV-ICs encapsulated by a hybrid hydrogel. After 7 days of culture, the encapsulated HAVICs showed high cell viability and cell-type appropriate morphologies and expressed all target genes that were tested, including αSMA, Vimentin, Periostin, and collagen.94
Second, for hydrogels that would be injected into heart tissue, having gels with sufficient flexibility so as not to interfere with the contractility of cardiac cells within or near the site of the injection would be desirable.
In addition, temporary hydrogels that could degrade over time would also be highly desirable. In the short run the gel itself could be used to maintain the geometry of the ventricular wall of the heart during repair. This would give cells encapsulated within the gel time to expand and integrate into the host myocardium or provide a scaffold for endogenous stem cells to migrate into the infarct zone.
Finally, hydrogels that could be linked to, or encapsulate, small molecules, peptides, or proteins that enhance growth, differentiation, or physiological function would be tremendously useful. Similarly, attachment of nanoparticles could be used to delivery drugs in a spatially and temporally controlled fashion. For example, Paul et al.95 recently demonstrated the feasibility of using a hydrogel for localized delivery of the angiogenic factor Vegf that was complexed with a functionalized graphene oxide nanosheet.
B. Hydrogels and Cardiac Repair
Pathological remodeling of the ventricular wall is a common and deleterious effect of MI. Not only is there thinning of the wall at the site of the infarct, the intact wall nearby is also susceptible to remodeling that can ultimately lead to heart failure (Figure 2). Several groups have tested the ability of hydrogels to maintain the geometry of the left ventricular wall after MI. Dobner and colleagues93 injected a PEG hydrogel or saline into infarct areas within 2 minutes of coronary artery ligation. At 2 and 4 weeks after ligation, hearts injected with PEG had less ventricular wall thinning and significant reduction in end diastolic diameter increase. By 13 weeks, however, there was no difference in end diastolic diameter increase between the groups treated with PEG and those treated with saline. This suggests that the impact of hydrogel alone was relatively short lived. Alginates96,97 and fibrin98 also have been tested for their ability to moderate ventricular remodeling after infarct, with similar positive effects. Hydrogels derived from decellularized ventricular wall extracellular matrix (ECM) have been studied more recently to determine whether ECM can be used, either as sheets to form patches99 or as fully injectable fillers to repair damage to infarct zones.100,101 Increasing the percentage of native ventricular wall ECM in the injection facilitated the differentiation of encapsulated hESC-derived cardiac progenitors, increasing the expression of cardiac-specific genes. The addition of growth factors to these ECM-derived hydrogels did not further increase cardiac differentiation, suggesting that the matrix alone was sufficient to support cardiac differentiation.101 In a similar study, however, a hydrogel consisting of a hybrid of ventricular ECM, fibrin, and a low dose of TGF-β increased the vascular differentiation of mesenchymal progenitor cells.99 A likely next step would be the use of hydrogels including both cardiac and vascular progenitor cells with or without the addition of growth factors (Figure 3).
Fig. 3.
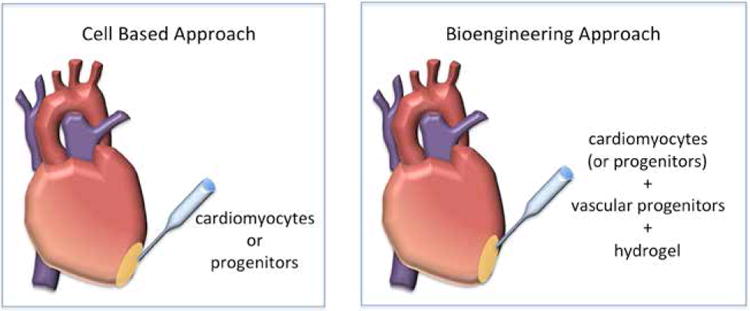
A comparison of cell-based therapies for heart repair with a hypothetical bioengineering approach. In cell-based therapies cells are injected directly into the infarct (yellow). Bioengineering approaches involve injecting cells (or progenitors) of both the cardiac and vascular lineages that have been encapsulated or coated on hydrogels. Hydrogels have been show to effectively preserve the 3-dimensional structure of the ventricular wall after myocardial infarction. They have also been shown to facilitate engraftment of donor cells and promote the physiological maturation of engrafted cells. Finally, hydrogels can be used for localized delivery of growth factors and drugs.
Other approaches used specifically to increase vascular formation within cardiac grafts include work by Cui and Boland,102 who used inkjet printer technology for 2-dimensional printing of a fibrin scaffold that facilitated the growth of vascular structures with only minor deformation. After 21 days of culture, the proliferated cells formed a tubular microvasculature within the fibrin channels, suggesting that inkjet printing technology could be used for the biofabrication of human microvasculature with high resolution.
Similarly, Vollert and colleagues103 fabricated 3D structures with microchannels that served as artificial vessels for the perfusion of engineered heart tissues (EHTs). To accomplish this, thin alginate fibers were embedded in a matrix of cells, fibrin, and thrombin. After polymerization, the fibers were removed using alginate lyase or sodium citrate. These artificial vessels improved the oxygen concentration in the center of the EHT and were ultimately populated by endothelial cells.
By combining approaches similar to those described above, Vukadinovic-Nikolic et al.104 recently generated a large sample of EHT. This cardiac construct consisted of 3 separate layers. The bottom layer was decellularized porcine small intestinal submucosa, the middle layer was a monolayer of rat neonatal cardiomyocytes, and the top layer comprised rat heart endothelial cells. Using this approach, Vukadinovic-Nikolic et al. were able to engineer an artificial tissue of substantial size, with an average beat rate of 208 ± 78 beats/minute on day 3 and 154 ± 48 beats/minute on day 10 compared with 43 ± 27 beats/minute in cardiac cells grown without the other tissues, suggesting that this approach may improve the physiological or electrophysiological maturation of cardiac cells. Rat endothelial cells seeded in the top layer migrated through the cardiac compartment within 7 days and colocalized with the vessel bed of the submucosal layer. These studies demonstrate that effects caused by cell engraftment alone can be greatly enhanced using engineering approaches.
The most ambitious projects in cardiac bioengineering are efforts to completely rebuild hearts either by 3D printing or using decellularized hearts as a scaffold for repopulation by cardiac progenitor cells. In 2008 Ott et al.105 developed a technique to efficiently decellularize rat hearts and subsequently use them as a natural platform to fabricate a beating bioartificial heart. At first, they carried out coronary perfusion with a mild detergent over 12 hours to generate a decellularized construct with a perfusable vascular tree, patent valves, and an intact ECM. They then reseeded the construct with rat neonatal cardiac cells. By day 8, these repopulated structures beat and were able to generate a constant, albeit weak, contractile force (about 2% of the force generated by a healthy adult heart). While far from clinical usefulness, this study showed proof of principle that ECM scaffolds could be used to create bioartificial heart tissue. More recently, Lu and colleagues106 reseeded decellularized mouse hearts with human iPSC-derived cardiovascular progenitors. After 26 days of culture, including the addition of growth factors to promote the differentiation of cardiac tissue and blood vessels, the repopulated hearts showed a spontaneous beat rate of 40–50 beats/minute. This rate was accelerated to 90 beats/minute by perfusing isoproterenol. Subsequent studies showed that the perfused multipotent cardiac progenitors had differentiated into cardiomyocytes, smooth muscle cells, and endothelial cells.
VIII. Conclusions
Work by Chong et al.67 highlights the advances that have been made in cardiac regenerative medicine based on cell-based therapies alone. However, the studies described here also highlight the unique challenges presented by this field. We highlighted several recent studies that demonstrate the potential power of combining cells with engineered materials (Figure 3). These studies suggest that encapsulating cardiac or cardiac progenitor cells within hydrogels may greatly enhance their regenerative capacities. This is accomplished by providing scaffolds that facilitate migration and differentiation, mechanically protect cells, and preserve the 3D structure of the damaged heart tissue during repair and recovery.
References
- 1.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61(6):1–51. [PubMed] [Google Scholar]
- 2.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen DC, Ganesalingam S, Jensen CH, Sheikh SP. Do neonatal mouse hearts regenerate following heart apex resection? Stem Cell Reports. 2014;2(4):406–13. doi: 10.1016/j.stemcr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89(1):151–63. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13(8):970–4. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107(21):2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 8.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR(+) embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 10.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127(6):1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6(5):685–98. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- 12.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5(6):877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1(3):435–40. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 14.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1(2):165–79. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ Res. 2004;95(3):261–8. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- 16.Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev Biol. 1998;196(2):129–44. doi: 10.1006/dbio.1998.8860. [DOI] [PubMed] [Google Scholar]
- 17.Foley A. Cardiac lineage selection: integrating biological complexity into computational models. Wiley Interdiscip Rev Syst Biol Med. 2009;1(3):334–47. doi: 10.1002/wsbm.43. [DOI] [PubMed] [Google Scholar]
- 18.Guzzo RM, Foley AC, Ibarra Y, Mercola M. Signalling pathways in embryonic heart induction. Adv Dev Biol. 2007;18:117–51. [Google Scholar]
- 19.Eisenberg CA, Gourdie RG, Eisenberg LM. Wnt-11 is expressed in early avian mesoderm and required for the differentiation of the quail mesoderm cell line QCE-6. Development. 1997;124(2):525–36. doi: 10.1242/dev.124.2.525. [DOI] [PubMed] [Google Scholar]
- 20.Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79(5):791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 21.Zorn AM, Butler K, Gurdon JB. Anterior endomesoderm specification in Xenopus by Wnt/beta-catenin and TGF-beta signalling pathways. Dev Biol. 1999;209(2):282–97. doi: 10.1006/dbio.1999.9257. [DOI] [PubMed] [Google Scholar]
- 22.Christian JL, McMahon JA, McMahon AP, Moon RT. Xwnt-8, a Xenopus Wnt-1/int-1 related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991;111:1045–55. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- 23.Kofron M, Demel T, Xanthos J, Lohr J, Sun B, Sive H, Osada S, Wright C, Wylie C, Heasman J. Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFbeta growth factors. Development. 1999;126(24):5759–70. doi: 10.1242/dev.126.24.5759. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13(24):3185–90. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimelman D, Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987;51:869–77. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- 26.Pfendler KC, Yoon J, Taborn GU, Kuehn MR, Iannaccone PM. Nodal and bone morphogenetic protein 5 interact in murine mesoderm formation and implantation. Genesis. 2000;28(1):1–14. doi: 10.1002/1526-968x(200009)28:1<1::aid-gene10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Rodaway A, Takeda H, Koshida S, Broadbent J, Price B, Smith JC, Patient R, Holder N. Induction of the mesendoderm in the Zebrafish germ ring by yolk cell-derived TGF-beta family signals and discrimination of mesoderm and endoderm by FGF. Development. 1999;126(14):3067–78. doi: 10.1242/dev.126.14.3067. [DOI] [PubMed] [Google Scholar]
- 28.Rosa F, Roberts AB, Danielpour D, Dart L, Sport MB, Dawid IB. Mesoderm induction in amphibians: the role of TGF-b2-like factors. Science. 1988;239:783–5. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- 29.Tam PP, Beddington RS. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development. 1987;99(1):109–26. doi: 10.1242/dev.99.1.109. [DOI] [PubMed] [Google Scholar]
- 30.Tam PP, Parameswaran M, Kinder SJ, Weinberger RP. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 1997;124(9):1631–42. doi: 10.1242/dev.124.9.1631. [DOI] [PubMed] [Google Scholar]
- 31.Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell. 2002;3(2):171–81. doi: 10.1016/s1534-5807(02)00206-x. [DOI] [PubMed] [Google Scholar]
- 32.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15(3):316–27. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–15. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura T, Sano M, Songyang Z, Schneider MD. A Wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci U S A. 2003;100(10):5834–9. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103(52):19812–7. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104(23):9685–90. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(45):16806–11. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002;16(12):1558–66. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 39.Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol. 2000;44(3):253–65. [PubMed] [Google Scholar]
- 40.Jamali M, Karamboulas C, Rogerson PJ, Skerjanc IS. BMP signaling regulates Nkx2-5 activity during cardiomyogenesis. FEBS Lett. 2001;509(1):126–30. doi: 10.1016/s0014-5793(01)03151-9. [DOI] [PubMed] [Google Scholar]
- 41.Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci U S A. 2007;104(22):9313–8. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takei S, Ichikawa H, Johkura K, Mogi A, No H, Yoshie S, Tomotsune D, Sasaki K. Bone morphogenetic protein-4 promotes induction of cardiomyocytes from human embryonic stem cells in serum-based embryoid body development. Am J Physiol Heart Circ Physiol. 2009;296(6):H1793–803. doi: 10.1152/ajpheart.01288.2008. [DOI] [PubMed] [Google Scholar]
- 43.Willems E, Leyns L. Patterning of mouse embryonic stem cell-derived pan-mesoderm by Activin A/Nodal and Bmp4 signaling requires fibroblast growth factor activity. Differentiation. 2008;76(7):745–59. doi: 10.1111/j.1432-0436.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 44.Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa S, Okano H, Fukuda K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23(5):607–11. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 45.Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238(1):97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- 46.Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128(16):3179–88. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 47.Saga Y, Kitajima S, Miyagawa-Tomita S. Mesp1 expression is the earliest sign of cardiovascular development. Trends Cardiovasc Med. 2000;10(8):345–52. doi: 10.1016/s1050-1738(01)00069-x. [DOI] [PubMed] [Google Scholar]
- 48.Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126(15):3437–47. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- 49.Kitajima S, Takagi A, Inoue T, Saga Y. MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development. 2000;127(15):3215–26. doi: 10.1242/dev.127.15.3215. [DOI] [PubMed] [Google Scholar]
- 50.Bondue A, Lapouge G, Paulissen C, Semeraro C, Iacovino M, Kyba M, Blanpain C. Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification. Cell Stem Cell. 2008;3(1):69–84. doi: 10.1016/j.stem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Ma Q, Zhou B, Pu WT. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev Biol. 2008;323(1):98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spater D, Abramczuk MK, Buac K, Zangi L, Stachel MW, Clarke J, Sahara M, Ludwig A, Chien KR. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat Cell Biol. 2013;15(9):1098–106. doi: 10.1038/ncb2824. [DOI] [PubMed] [Google Scholar]
- 53.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127(6):1137–50. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 54.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ, Chien KR. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460(7251):113–7. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 55.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433(7026):647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Y, Liang X, Najafi N, Cass M, Lin L, Cai CL, Chen J, Evans SM. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev Biol. 2007;304(1):286–96. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amir G, Ma X, Reddy VM, Hanley FL, Reinhartz O, Ramamoorthy C, Riemer RK. Dynamics of human myocardial progenitor cell populations in the neonatal period. Ann Thorac Surg. 2008;86(4):1311–9. doi: 10.1016/j.athoracsur.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 58.Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107(1):111–7. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- 59.Motoike T, Markham DW, Rossant J, Sato TN. Evidence for novel fate of Flk1+ progenitor: contribution to muscle lineage. Genesis. 2003;35(3):153–9. doi: 10.1002/gene.10175. [DOI] [PubMed] [Google Scholar]
- 60.Kattman SJ, Adler ED, Keller GM. Specification of multipotential cardiovascular progenitor cells during embryonic stem cell differentiation and embryonic development. Trends Cardiovasc Med. 2007;17(7):240–6. doi: 10.1016/j.tcm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Christoforou N, Miller RA, Hill CM, Jie CC, McCallion AS, Gearhart JD. Mouse ES cell-derived Cardiac precursor cells are multipotent and facilitate identification of novel cardiac genes. J Clin Invest. 2008;118(3):894–903. doi: 10.1172/JCI33942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson TJ, Faustino RS, Chiriac A, Crespo-Diaz R, Behfar A, Terzic A. CXCR4+/FLK-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem cells. 2008;26(6):1464–73. doi: 10.1634/stemcells.2007-0808. [DOI] [PubMed] [Google Scholar]
- 64.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93(1):32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 65.Kolossov E, Lu Z, Drobinskaya I, Gassanov N, Duan Y, Sauer H, Manzke O, Bloch W, Bohlen H, Hescheler J, Fleischmann BK. Identification and characterization of embryonic stem cell-derived pacemaker and atrial cardiomyocytes. FASEB J. 2005;19(6):577–9. doi: 10.1096/fj.03-1451fje. [DOI] [PubMed] [Google Scholar]
- 66.Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res. 1994;75(2):233–44. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- 67.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114(3):511–23. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Martinez V, Alvarez IS, Schoenwolf GS. Locations of the ectodermal and nonectodermal subdivisions of the epiblast at stages 3 and 4 of avian gastrulation and neurulation. J Exp Zool. 1993;267:431–46. doi: 10.1002/jez.1402670409. [DOI] [PubMed] [Google Scholar]
- 70.Stainier DY, Lee RK, Fishman MC. Cardiovascular development in the Zebrafish. I. Myocardial fate map and heart tube formation. Development. 1993;119(1):31–40. doi: 10.1242/dev.119.1.31. [DOI] [PubMed] [Google Scholar]
- 71.Yutzey K, Gannon M, Bader D. Diversification of cardiomyogenic cell lineages in vitro. Dev Biol. 1995;170(2):531–41. doi: 10.1006/dbio.1995.1234. [DOI] [PubMed] [Google Scholar]
- 72.Yutzey KE, Rhee JT, Bader D. Expression of the atrial-specific myosin heavy chain, AMHC1 and the establishment of anteroposterior polarity in the developing chicken heart. Development. 1994;120:871–83. doi: 10.1242/dev.120.4.871. [DOI] [PubMed] [Google Scholar]
- 73.Satin J, Fujii S, DeHaan RL. Development of cardiac beat rate in early chick embryos is regulated by regional cues. Dev Biol. 1988;129(1):103–13. doi: 10.1016/0012-1606(88)90165-0. [DOI] [PubMed] [Google Scholar]
- 74.Wang J, Chen A, Lieu DK, Karakikes I, Chen G, Keung W, Chan CW, Hajjar RJ, Costa KD, Khine M, Li RA. Effect of engineered anisotropy on the susceptibility of human pluripotent stem cell-derived ventricular cardiomyocytes to arrhythmias. Biomaterials. 2013;34(35):8878–86. doi: 10.1016/j.biomaterials.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 75.Karakikes I, Senyei GD, Hansen J, Kong CW, Azeloglu EU, Stillitano F, Lieu DK, Wang J, Ren L, Hulot JS, Iyengar R, Li RA, Hajjar RJ. Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes. Stem Cells Transl Med. 2014;3(1):18–31. doi: 10.5966/sctm.2013-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weng Z, Kong CW, Ren L, Karakikes I, Geng L, He J, Chow MZ, Mok CF, Keung W, Chow H, Leung AY, Hajjar RJ, Li RA, Chan CW. A simple, cost-effective but highly efficient system for deriving ventricular cardiomyocytes from human pluripotent stem cells. Stem Cells Dev. 2014;23(14):1704–16. doi: 10.1089/scd.2013.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 78.Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267(5205):1788–92. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 79.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 80.Lassar AB, Paterson BM, Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986;47(5):649–56. doi: 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- 81.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–86. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–8. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, Gregoire S, Engels MC, Rajarajan K, Karra R, Abel ED, Wu JC, Milan D, Wu SM. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res. 2012;111(1):50–5. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct Reprogramming of Human Fibroblasts toward a Cardiomyocyte-like State. Stem Cell Rep. 2013;1(3):235–47. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kapoor N, Liang W, Marban E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol. 2013;31(1):54–62. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A. 2012;18(9–10):910–9. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vunjak-Novakovic G, Lui KO, Tandon N, Chien KR. Bioengineering heart muscle: a paradigm for regenerative medicine. Annu Rev Biomed Eng. 2011;13:245–67. doi: 10.1146/annurev-bioeng-071910-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112(9 Suppl):I173–7. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y, Thorn S, DaSilva JN, Lamoureux M, De-Kemp RA, Beanlands RS, Ruel M, Suuronen EJ. Collagen-based matrices improve the delivery of transplanted circulating progenitor cells: development and demonstration by ex vivo radionuclide cell labeling and in vivo tracking with positron-emission tomography. Circ Cardiovasc Imaging. 2008;1(3):197–204. doi: 10.1161/CIRCIMAGING.108.781120. [DOI] [PubMed] [Google Scholar]
- 90.Roura S, Bago JR, Soler-Botija C, Pujal JM, Galvez-Monton C, Prat-Vidal C, Llucia-Valldeperas A, Blanco J, Bayes-Genis A. Human umbilical cord blood-derived mesenchymal stem cells promote vascular growth in vivo. Plos One. 2012;7(11):e49447. doi: 10.1371/journal.pone.0049447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jia J, Richards DJ, Pollard S, Tan Y, Rodriguez J, Visconti RP, Trusk TC, Yost MJ, Yao H, Markwald RR, Mei Y. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014;10(10):4323–31. doi: 10.1016/j.actbio.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, Hubbell JA. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29(18):2757–66. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 93.Dobner S, Bezuidenhout D, Govender P, Zilla P, Davies N. A synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodeling. J Card Fail. 2009;15(7):629–36. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 94.Duan B, Kapetanovic E, Hockaday L, Butcher J. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014;10(5):1836–46. doi: 10.1016/j.actbio.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paul A, Hasan A, Kindi HA, Gaharwar AK, Rao VT, Nikkhah M, Shin SR, Krafft D, Dokmeci MR, Shum-Tim D, Khademhosseini A. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014;8(8):8050–62. doi: 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117(11):1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 97.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, Petnehazy O, Landa N, Feinberg MS, Konen E, Goitein O, Tsur-Gang O, Shaul M, Klapper L, Cohen S. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009;54(11):1014–23. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 98.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44(3):654–60. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 99.Godier-Furnémont AF, Martens TP, Koeckert MS, Wan L, Parks J, Arai K, Zhang G, Hudson B, Homma S, Vunjak-Novakovic G. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc Natl Acad Sci U S A. 2011;108(19):7974–9. doi: 10.1073/pnas.1104619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59(8):751–63. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duan Y, Liu Z, O'Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Res. 2011;4(5):605–15. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30(31):6221–7. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 103.Vollert I, Seiffert M, Bachmair J, Sander M, Eder A, Conradi L, Vogelsang A, Schulze T, Uebeler J, Holnthoner W, Redl H, Reichenspurner H, Hansen A, Eschenhagen T. In vitro perfusion of engineered heart tissue through endothelialized channels. Tissue Eng Part A. 2014;20(3–4):854–63. doi: 10.1089/ten.TEA.2013.0214. [DOI] [PubMed] [Google Scholar]
- 104.Vukadinovic-Nikolic Z, Andrée B, Dorfman SE, Pflaum M, Horvath T, Lux M, Venturini L, Bär A, Kensah G, Lara AR. Generation of bioartificial heart tissue by combining a three-dimensional gel-based cardiac construct with decellularized small intestinal submucosa. Tissue Eng Part A. 2014;20(3–4):799–809. doi: 10.1089/ten.TEA.2013.0184. [DOI] [PubMed] [Google Scholar]
- 105.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 106.Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun. 2013;4:2307. doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]


